Abstract
Introduction:
Hidradenitis suppurativa (HS) is a chronic inflammatory disease arising from the hair follicles in apocrine gland-rich areas. It is also one of the most common indications for axillary surgery. Reconstruction of the axillary region after such surgery must be performed meticulously due to its critical location and crucial content. In this report, we present our experience of reconstruction of axillary defects with posterior arm perforator flaps (PAPF) following radical excisions.
Methods:
A total of 14 patients (9 male, 5 female) aged between 16 and 49 years who had presented with HS in the axillary region and, after surgery, underwent reconstruction with either island or skin bridge posterior arm flap between January 2015 and October 2016 were included in the study and evaluated retrospectively. All of the defects were reconstructed with PAPF following wide excision.
Results:
Five of the flaps (over 4 patients) were designed as flaps with skin bridges, while the remaining 12 flaps in 11 patients were raised as island flaps. The flaps had areas ranging from 20 to 84 cm2 (mean 39.5 cm2), depending on the size of the defect after excision. The mean follow-up time after the operation was 6 months. Wound dehiscence was detected in one patient, and another patient developed marginal necrosis during the postoperative period; no other complications were observed.
Conclusion:
Posterior arm perforator flaps can provide sufficient amounts of soft tissue to cover axillary defects and should be considered as the flap of choice in axillary reconstruction.
Keywords: axillary reconstruction, hidradenitis suppurativa, posterior arm perforator flap, tissue defect
Abstract
Introduction:
La maladie de Verneuil (MV), ou hidrosadénite suppurée, est une maladie inflammatoire chronique qui touche les follicules pileux situés près des glandes apocrines. C’est également l’une des indications les plus courantes de chirurgie axillaire. Il faut procéder à une reconstruction méticuleuse de la région axillaire après l’opération en raison de son emplacement difficile et de son contenu crucial. Dans le présent rapport, les auteurs présentent leur expérience de reconstruction des anomalies axillaires à l’aide de lambeaux perforants du bras postérieur (LPBP) après des excisions radicales.
Méthodologie:
Au total, 14 patients (neuf hommes, cinq femmes) de 16 à 49 ans atteints d’une MV dans la région axillaire qui ont subi une reconstruction par lambeau en îlots ou par pont cutané prélevé sur le bras postérieur entre janvier 2015 et octobre 2016 ont participé à l’étude et fait l’objet d’une évaluation rétrospective. Les chirurgiens ont reconstruit toutes les anomalies à l’aide de LPBP après une large excision.
Résultats:
Cinq des lambeaux (sur quatre patients) étaient sous forme de ponts cutanés, et les 12 autres (sur 11 patients) ont été prélevés en îlots. Les lambeaux étaient d’une taille de 20 à 84 cm2 (moyenne de 39,5 cm2), en fonction de la dimension de l’anomalie après l’excision. Le suivi moyen était d’une durée de six mois après l’opération. Un patient a présenté une déhiscence de la plaie et un autre, une nécrose marginale pendant la période postopératoire. Aucune autre complication n’a été observée.
Conclusion:
Le LPBP peut fournir une quantité suffisante de tissus mous pour couvrir les anomalies axillaires. Il faut le considérer comme le lambeau de première intention lors d’une reconstruction axillaire.
Introduction
The axillary region is an anatomically important structure which hosts the shoulder joint and the major vessels and nerves traveling from the thorax to the arm. Although axillary defects are not seen frequently, when needed, reconstruction of this area should be performed meticulously due to its critical location and crucial content.1
Hidradenitis suppurativa (HS) is a chronic inflammatory disease arising from the hair follicles in apocrine gland-rich areas; it is also one of the most common indications for axillary surgery.1 Hidradenitis suppurativa has a serious impact on the quality of patients’ daily life, as it causes both physical and psychological problems. There are several treatment options, depending on the severity of the condition. In the early stages of HS, a conservative approach along with medical treatment is usually effective; however, as the disease is progressive, in its advanced stages, radical excision of the affected glands is the only current cure.1 Defects occurring after such radical excision may heal by secondary intention or be reconstructed with grafts, local flaps, or pedicled flaps. In this study, we present our experience of reconstruction of axillary defects with posterior arm perforator flaps (PAPF) following radical excisions in patients with Hurley grade 3 HS in the axillary region.
Methods
Patients with Hurley grade 3 HS in the axillary region, who underwent reconstruction with either island or skin bridge posterior arm flaps between January 2015 and October 2016, were included in the study and evaluated retrospectively.
Overall, 14 patients (9 male, 5 female) with axillary Hurley grade 3 HS were included in the study. Of these, 5 patients had left-sided, 6 patients had right-sided, and 3 patients had bilateral axillary HS. All of the defects were reconstructed, with 17 PAPF being utilised in total following wide excisions of the involved areas. The mean age of the patients was 31.1 years, with a range of 16 to 49 years. Three of the patients had diabetes mellitus, and 11 of the patients were smokers.
Surgical Technique
All the procedures were performed under general anaesthesia. Patients were placed in the supine position with their involved upper extremity abducted by 90° and a pillow placed under the scapula. Methylene blue dye was injected into each fistula in the affected area with the help of a blunt tip-cannula until the dye overflowed from the fistula tract. The excision material included all macroscopically affected skin, subcutaneous tissues dyed with methylene blue, and the deformed fibrotic HS tissue.
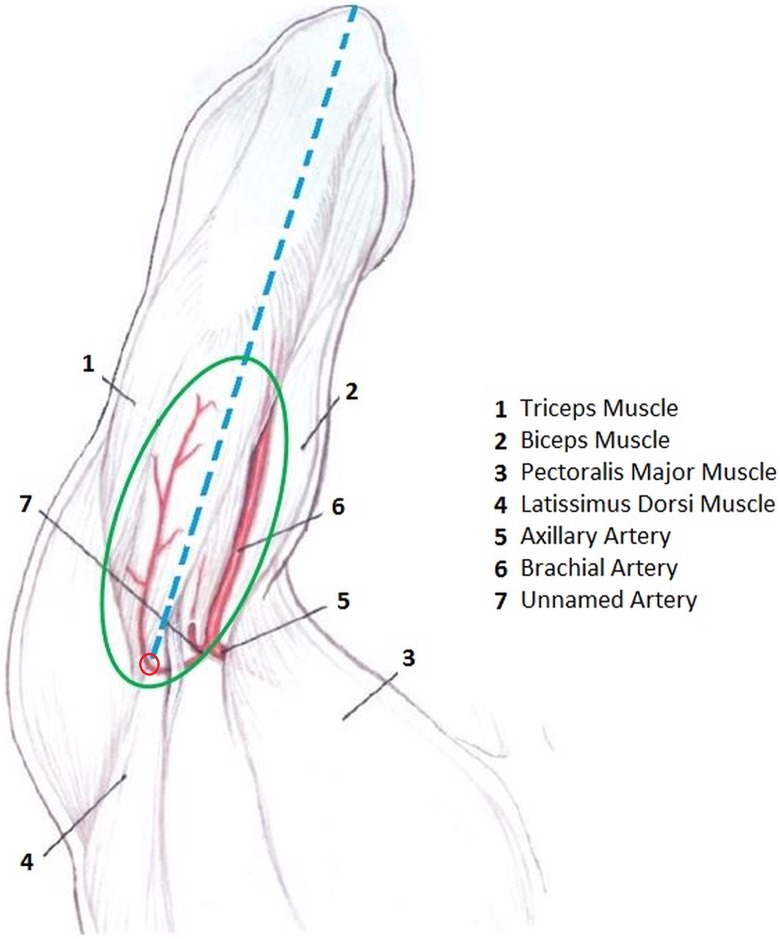
Flaps were designed in an elliptical shape proportionate to the size of the defect, with the long axis of each located in the midline on the posterior aspect of the arm and extending between the olecranon and the posterior axillary line. The width of the flap was determined via pinch test, with the aim of rendering primary closure possible. With the help of a hand-held Doppler device, the perforator artery was marked; in all cases, this was located 1 to 2 cm medial to the long head of the triceps muscle and 3 to 4 cm distal to the axillary crease (Figure 1). The proximal segment of the flap was incised, leaving 3 cm of skin intact laterally on both anterior and posterior borders. The elevation of the flap was initiated distally, and as the dissection proceeded proximally, the posterior brachial nerve and the triceps muscle fascia were included in the flap. After the pedicle was observed entering the flap through the undersurface, the branch supplying the medial head of the triceps was ligated in order to gain extra pedicle length. In order to avert risks caused by inexperience, in the first case and in subsequent relatively large defect cases, the flaps were designed with small skin bridges.
Figure 1.
Regional anatomy and the illustration of the flap design. Blue line: The longitudinal axis of the flap. Red dot: The localization of the perforator artery. Green ellipse: Flap design.
Most of the flaps were designed as island flaps. After the adaptation of the flaps, the skin closure was performed in 2 layers; however, the donor site closure was performed in 3 layers: fascia, subcutaneous tissue, and skin. Patients were discharged on either the first or the second postoperative day. In the bilateral cases, the more affected side was operated on first, and after complete healing was achieved, the contralateral axilla was also treated.
Results
Among these procedures, 5 of the flaps in 4 patients were designed as flaps with skin bridges, while the remaining 12 flaps in 11 patients were raised as island flaps. The flap sizes ranged from a minimum of 20 cm2 to a maximum of 84 cm2 (mean 39.5 cm2). The mean follow-up period was 6 months, with a range of 3 months to 1 year. Wound dehiscence was detected in one patient, and another patient developed marginal necrosis in the postoperative period; otherwise, no complications were observed (Table 1). During the follow-up period, only one case showed recurrence on the lateral side of the flap, and this was immediately excised and closed primarily.
Table 1.
List of the Patients.
| Patient | Gender | Age | Side | Flap Dimensions (cm) | Comorbidity | Flap Type | Complication |
|---|---|---|---|---|---|---|---|
| 1 | Male | 22 | L | 5 × 4 | Smoker (8 pack-year) | Pedicled | None |
| 2 | Male | 16 | L | 7 × 6 | Smoker (1 pack-year) | Island | None |
| 3 | Female | 44 | Bilateral | 10 × 6 | DM | Pedicled Island | Wound dehiscence |
| 9 × 3 | Smoker (20 pack-year) | ||||||
| 4 | Female | 24 | L | 12 × 5 | None | Pedicled | None |
| 5 | Male | 48 | R | 6 × 4 | DM Smoker (30 pack-year) |
Island | None |
| 6 | Female | 49 | R | 7 × 4 | Smoker (30 pack-year) | Island | None |
| 7 | Male | 35 | R | 5 × 4 | Smoker (20 pack-year) | Island | None |
| 8 | Male | 35 | Bilateral | 12 × 7 12 × 6 |
Smoker (15 pack-year) | Pedicled | None |
| 9 | Female | 32 | R | 9 × 6 | Smoker (28 pack-year) | Island | None |
| 10 | Male | 28 | Bilateral | 8 × 5 | DM Smoker (20 pack-year) |
Island | None |
| 11 | Male | 43 | L | 9 × 4 | Smoker (24 pack-year) | Island | None |
| 12 | Male | 19 | L | 8 × 7 | Smoker (3 pack-year) | Island | Marginal necrosis |
| 13 | Female | 24 | R | 7 × 3 | None | Island | None |
| 14 | Male | 17 | R | 7 × 4 | None | Island | None |
Abbreviations: DM, diabetes mellitus; L, left; R, right.
Case Reports
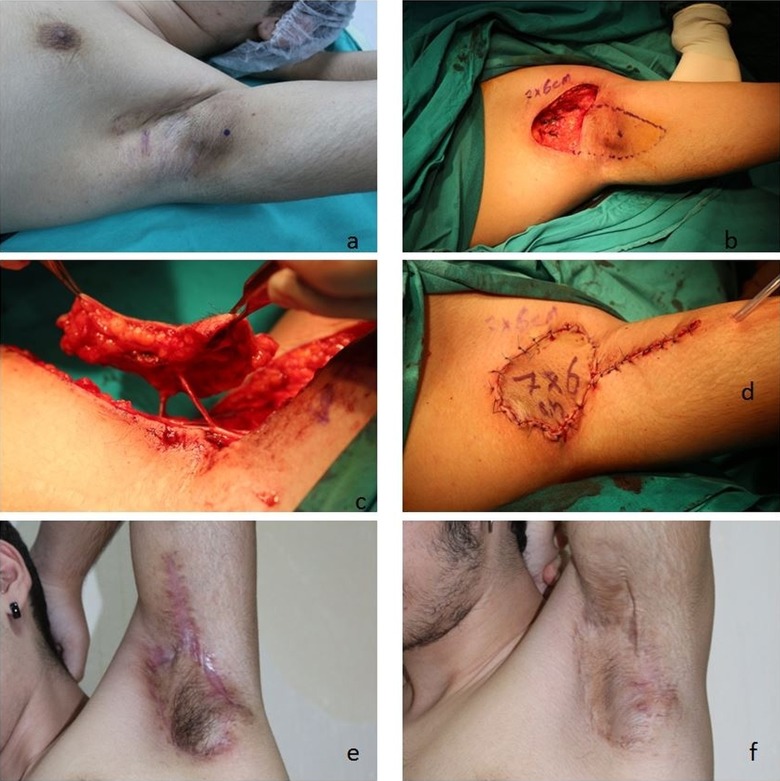
Case 2: A 16-year-old male patient with HS in the left axillary area for 2 years. He had a history of smoking of 1 pack year. The defect formed after excision was reconstructed using a PAPF island flap with a size of 7 × 6 cm (Figure 2).
Figure 2.
Preoperative, perioperative, and postoperative third month and 14th month appearance of patient number 2. The localization of the pedicle has been marked as blue dot in A and B.
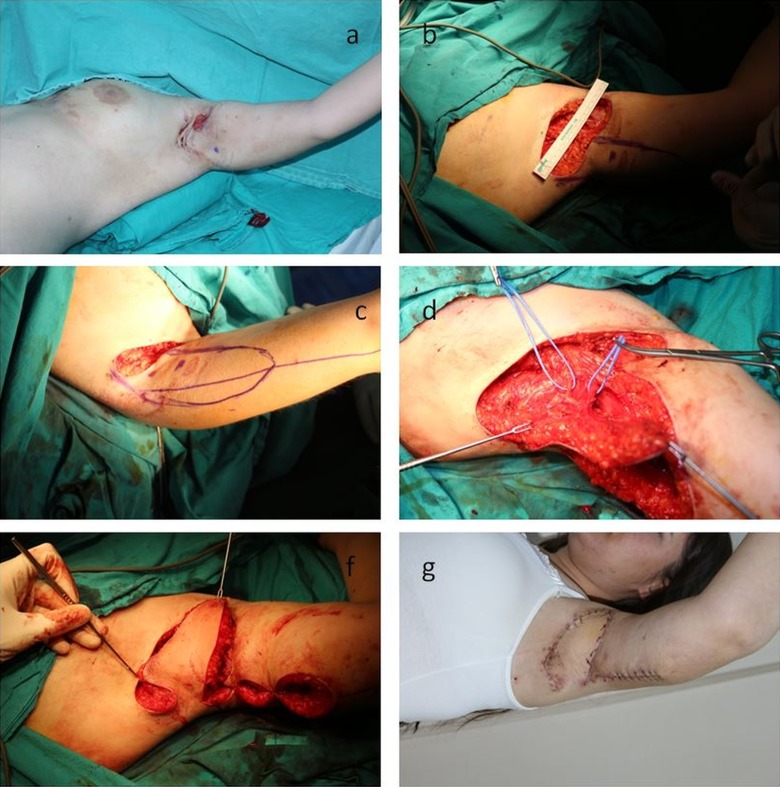
Case 4: A 24-year-old female patient with HS in the left axillary area for 1 year. The defect formed after excision was reconstructed with a skin PAPF flap with skin bridge with a size of 12 × 5 cm (Figure 3).
Figure 3.
Preoperative, perioperative, and postoperative second week appearance of patient number 4. The localization of the pedicle has been marked as blue dot in A, B, and C.
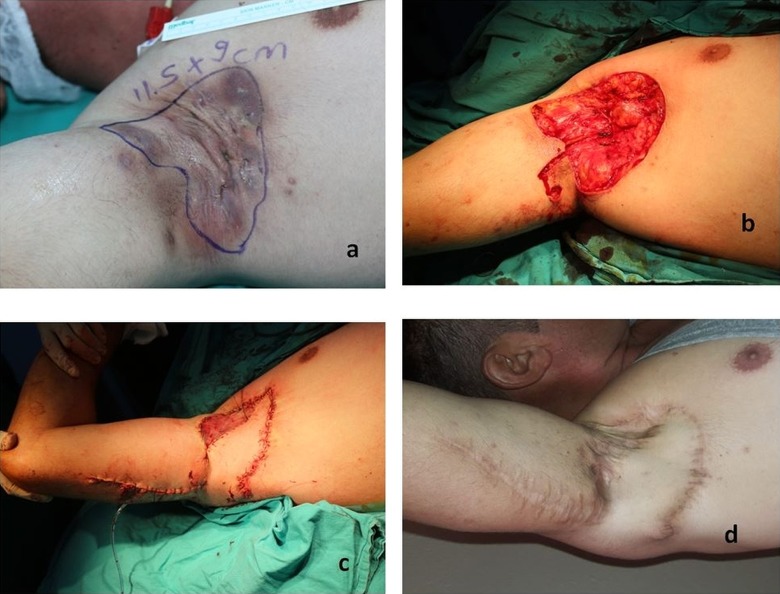
Case 8: A 35-year-old male patient with HS in bilaterally axillary area for 3 years. He had a history of smoking of 15 pack years. The defect formed after excision was reconstructed with a skin PAPF flap with skin bridge with a size of 12 × 7 cm and additional skin graft (Figure 4).
Figure 4.
Preoperative, perioperative, and postoperative 24th month appearance of patient number 8.
Discussion
Hidradenitis suppurativa was first described by Velpau in 1839.2 It was believed to arise from the apocrine glands, and was thus also referred to as apocrinitis3; however, more recent histological studies have shown that HS actually arises from the follicular epithelium.4 The underlying pathological mechanism involves infundibular keratosis, follicular epithelial hyperplasia, cellular debris accumulation, and cyst formation in the hair follicle resulting in occlusion of the follicle, which eventually lead to rupture of the follicle. As a result of follicle rupture, release of keratin and bacteria triggers an immune reaction, and abscess formation, sinus tract formation, scarring, and even contracture formation may then be seen, depending on the severity and the duration of the inflammation.5 The prevalence of HS ranges from 1% to 4% of the population.6 According to the modified Dessau definition, this condition is diagnosed using a combination of diagnostic lesions, topographic features, and the history of the disease.7 Hidradenitis suppurativa is seen in women 3 times more than in men, and it is usually seen in people in their early 20s; however, HS has also been reported in children and postmenopausal women.8 The main risk factors include obesity, smoking, gender, age, and genetic susceptibility. Excessive sweating, stress, tight clothing, friction, using deodorant and other cosmetic products, shaving, consuming fermented beverages, and menstruation are among other potential risk factors.9 Patients with HS have a tendency toward depression, anxiety, and sexual dysfunction when compared to the normal population, and 73.5% of these patients have difficulty in finding a partner.10 Foul odour, discharge, pain, and the need for frequent dressing changes all interfere with daily life for this group of patients.11 Some patients also find it difficult to attend work because of painful lesions.
There are many classifications of HS, although the one developed by Hurley in 1989 is the most commonly used.12 Hidradenitis suppurativa is generally seen in the axillary region; however, inguinal, perianal, gluteal, pubic, scrotal, and postauricular areas, as well as inner thigh, breast, submamarian fold, chest, and scalp may also be affected.11 At the initial onset, the remission rate varies from 11% to 47% based on a course of multiple antibiotherapy for 2.5 months on average; the average time period for relapse is 5 months.13 Recurrent use of multiple antibiotics can lead to resistance to treatment, and the effectiveness of the treatment becomes suboptimal in time.14 Nevertheless, systemic and local antibiotherapies are still effective in the mild and intermediate forms of HS,15 although they do not offer any solution to the fibrotic lesions formed as a result of recurrent inflammatory reactions.16 In the advanced stages of this condition, axial and peripheral arthropathies, peripheral lymphedema, fistulas, and squamous cell carcinomas may also develop.17
The axillary region should be mobile enough to allow the shoulder joint to move without restriction while remaining covered by sufficient quantity of soft tissue to protect the important major vessels and nerves.1 Although axillary defects requiring reconstruction are not seen frequently, the critical anatomy of this transitional area necessitates meticulous functional reconstruction. Hidradenitis suppurativa is one of the most common aetiologies leading to axillary defects.1 Due to the progressive characteristics of the condition, radical excision generally becomes the only option for treatment in the later stages, and the reconstruction of such a complex area is naturally associated with a high rate of complications in the postoperative period. Patients presenting with HS are, however, 4.6% more likely to develop a nonmelanoma skin cancer within or around a HS lesion when compared to the normal population,18 which makes radical excision necessary in the advanced stages. Secondary intention, skin grafts, primary suturing, random local flaps, and pedicled flaps are among the reconstructive options available following radical excision of HS and each method has its own advantages and disadvantages. Recurrence rates are reported as 54% to 70% after primary repair, 0% to 33%19 after skin graft application, and 0% to 6.6%20 after reconstruction with flaps.
The major advantage of secondary intention is that it does not involve donor site morbidity. Depending on the size of the defect, the healing process may take some time, however. Silverberg reported that the average duration of time for secondary wound healing is between 2 and 5 months.21 Dressing changes are usually painful for patients during this period and such wound care requires special attention, as during the period when the defect is left open, it is prone to infections. Primary closure also has the advantage of lacking donor site morbidity, making it the most ideal method for small defects. However, as the size of a defect increases, the suitability of primary closure becomes controversial.
In small defects where the vital structures are not exposed, skin grafts are one of the most commonly used methods for reconstruction of the axilla as donor site morbidity is minimal. Skin grafts are not suitable where the axillary vessels and nerves are exposed, since they cannot provide coverage with sufficient thickness to protect such structures.
Local flaps, including transposition flaps such as Limberg flaps, rotation flaps, or V-Y advancement flaps can also be used in the reconstruction of axilla, and provide both sufficient coverage and good colour matches. However, their limited mobilization capacity makes these flaps unsuitable for the reconstruction of larger defects.1
Pedicled flaps that can be used in the reconstruction of the axilla include thoracodorsal artery perforator flaps, pectoral flaps, and parascapular and scapular perforator flaps.22
The posterior arm flap was first described by Masquelet in 1985,23 and such flaps were used as free flaps in the reconstruction of hand and foot defects.24 It was next described as a pedicled fasciocutaneous transposition flap for axillary reconstruction by Elliot et al in 1992.25 Afterward, its use was reported in the repair of axillary defects, although not very frequently. The pedicle of the flap is defined as the cutaneous branch of the artery supplying the medial head of the triceps muscle, which arises from either the brachial or deep brachial artery.22 The pedicle travels underneath the fibrous band connecting the long head of the triceps muscle and the latissimus dorsi muscle.22 In order to mark the pedicle preoperatively with a hand-held doppler device, the perforator should be looked for at the intersection of these 2 muscles. The pedicle is accompanied by 2 concomitant veins and the posterior cutaneous nerve of the arm,22 which makes the posterior arm flap a sensate one. Release of the fibrous band may provide extra length to the pedicle if needed. After observation of this particular perforator artery in all post-bariatric brachioplasty patients in our clinic, based on examples from the literature, we started to use this flap in the reconstruction of axillary defects following the radical excision of HS lesions. Throughout the case series, we did not encounter anatomical variation in any of our patients. It usually takes no more than 30 minutes to elevate the flap, without the need for a microscope for pedicle dissection. If the flap is elevated as an island flap, in case of a potential damage to the pedicle, the flap itself can also be utilized as a full thickness skin graft. |As the donor site scar remains hidden on the posterior aspect of the arm, the cosmetic result is acceptable without any functional donor site morbidity,22 although in larger PAPF flaps, the scar would be seen under a short-sleeved shirt or T-shirt, much like a brachioplasty scar. In our case series, the largest flap had an area of 84 cm2, and reconstruction of near total defects is possible with a flap this size. Where the defect is larger, there is no restriction of the use of additional local flaps as required. In the postoperative period, splinting for immobilization is not required; this differs from most of the other surgical treatment options. Patients undergoing this procedure were discharged on either their first or second postoperative day, and then followed in the outpatient clinic. For male patients with hair on their posterior arms, although the axilla is reconstructed with similar hairy tissue, we recommend epilation in order to reduce the risk of recurrence. For female patients, the fact that this area has a much lower incidence of hair follicles results in reduced risk of recurrence. In case of a complication leading to the loss of the posterior arm flap, the flaps from thoracic or back regions remain as secondary options for further reconstruction. Two patients demonstrated minor complications in our series. One of them had wound dehiscence and underwent secondary suturation after thorough debridement.
The other patient developed marginal necrosis on the lateral side of the flap, which was treated with local wound management and eventually healed completely.
The flaps from these procedures can look bulky at the initial stage due to the fact that the arm tissue is relatively thicker than the axillary tissue. The flap atrophies with time and the wound edges become level. In unilateral reconstruction cases, minimal asymmetry between the proximal levels of the arms thus develops eventually.
Conclusion
Posterior arm perforator flaps is a reliable flap in terms of anatomical features and circulation.1 It does have a steep learning curve but does not cause any functional donor site morbidity. In addition to ensuring good tissue match, it is also a sensate flap that leaves an acceptable scar on the posterior aspect of the arm. We believe that PAPF should therefore be considered among the primary options during the planning stages of axillary reconstruction.
Footnotes
Level of Evidence: Level 4, Therapeutic
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Schmidt M, Dunst-Huemer KM, Lazzeri D, Schoeffl H, Huemer GM. The versatility of the islanded posterior arm flap for regional reconstruction around the axilla. J Plast Reconstr Aesthet Surg. 2015;68(7):953–959. [DOI] [PubMed] [Google Scholar]
- 2. Velpeau A. In: Dictionnaire de médecine, un repertoire générale des Sciences Medicals sous le rapport. Théorique et Pratique. Vol. 2 2nd ed Paris: Bechet Jeune; 1839:91. [Google Scholar]
- 3. Brunsting HA. Hidradenitis suppurativa; abscess of apocrine sweat glands a study of the clinical and pathologic features with a report of twenty-two cases and a review of the literature. Arch Derm Syphilol. 1939;39(1):108–120. [Google Scholar]
- 4. John H, Manoloudakis N, Stephen Sinclair J. A systematic review of the use of lasers for the treatment of hidradenitis suppurativa. J Plast Reconstr Aesthet Surg. 2016;69(10):1374–1381. [DOI] [PubMed] [Google Scholar]
- 5. von Laffert M, Stadie V, Wohlrab J, Marsch WC. Hidradenitis suppurativa/acne inversa: bilocated epithelial hyperplasia with very different sequelae. Br J Dermatol. 2011;164(2):367–371. [DOI] [PubMed] [Google Scholar]
- 6. Alikhan A, Lynch PJ, Eisen DB. Hidradenitis suppurativa: a comprehensive review. J Am Acad Dermatol. 2009;60(4):539–561. [DOI] [PubMed] [Google Scholar]
- 7. Zouboulis CC, Desai N, Emtestam L. et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol. 2015;29(4):619–644. [DOI] [PubMed] [Google Scholar]
- 8. Esmann S, Dufour DN, Jemec GB. Questionnaire based diagnosis of hidradenitis suppurativa: specificity, sensitivity and positive predictive value of specific diagnostic questions. Br J Dermatol. 2010;163(1):102–106. [DOI] [PubMed] [Google Scholar]
- 9. von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14(5):389–392. [DOI] [PubMed] [Google Scholar]
- 10. Vinding GR, Miller IM, Zarchi K, Ibler KS, Ellervik C, Jemec GB. The prevalence of inverse recurrent suppuration: a population-based study of possible hidradenitis suppurativa. Br J Dermatol. 2014;170(4):884–889. [DOI] [PubMed] [Google Scholar]
- 11. Alavi A, Anooshirvani N, Kim WB, Coutts P, Sibbald RG. Quality-of-life impairment in patients with hidradenitis suppurativa: a Canadian study. Am J Clin Dermatol. 2015;16(1):61–65. [DOI] [PubMed] [Google Scholar]
- 12. Hurley HJ. Axillary hyperhidrosis, apocrine bromhidrosis, hidradenitis suppurativa and familial benign pemphigus: surgical approach. In: Roenigk RK, Roenigk HH, Jr, eds. Dermatologic Surgery: Principles and Practice. 2nd ed New York: Marcel Dekker; 1996:623–645. [Google Scholar]
- 13. van der Zee HH, Boer J, Prens EP, Jemec GB. The effect of combined treatment with oral clindamycin and oral rifampicin in patients with hidradenitis suppurativa. Dermatology. 2009;219(2):143–147. [DOI] [PubMed] [Google Scholar]
- 14. Matusiak Q, Bieniek A, Szepietowski JC. Bacteriology of hidradenitis suppurativa—which antibiotics are the treatment of choice? Acta Derm Venereol. 2014;94(6):699–702. [DOI] [PubMed] [Google Scholar]
- 15. Kohorst JJ, Hagen C, Baum CL, Davis MD. Treatment experience in a local population with hidradenitis suppurativa. J Drugs Dermatol. 2014;13(7):827–831. [PubMed] [Google Scholar]
- 16. Andersen RK, Jemec GB. Treatments for hidradenitis suppurativa. Clin Dermatol. 2017;35(2):218–224. [DOI] [PubMed] [Google Scholar]
- 17. Matusiak L, Bieniek A, Szepietowski JC. Psychophysical aspects of hidradenitis suppurativa. Acta Derm Venereol. 2010;90(3):264–268. [DOI] [PubMed] [Google Scholar]
- 18. Lapins J, Ye W, Nyren O, Emtestam L. Incidence of cancer among patients with hidradenitis suppurativa. Arch Dermatol. 2001;137(6):730–734. [PubMed] [Google Scholar]
- 19. Mandal A, Watson J. Experience with different treatment modules in hidradenitis suppurativa: a study of 106 cases. Surgeon. 2005;3(1):23–26. [DOI] [PubMed] [Google Scholar]
- 20. Varkarakis G, Daniels J, Coker K, Oswald T, Akdemir O, Lineaweaver WC. Treatment of axillary hidradenitis with transposition flaps: a 6-year experience. Ann Plastic Surg. 2010;64(5):592–594. [DOI] [PubMed] [Google Scholar]
- 21. Silverberg B, Smoot CE, Landa SJ, Parsons RW. Hidradenitis suppurativa: patient satisfaction with wound healing by secondary intention. Plast Reconstr Surg. 1987;79(4):555–559. [PubMed] [Google Scholar]
- 22. Guha G, Agarwal AK, Gupta S. et al. Posterior arm flap in management of axillary contracture. Burns. 2013;39(5):972–977. [DOI] [PubMed] [Google Scholar]
- 23. Masquelet AC, Rinaldi S. Anatomical basis of the posterior brachial skin flap. Anat Clin. 1985;7(3):155–160. [DOI] [PubMed] [Google Scholar]
- 24. Masquelet AC, Rinaldi S, Mouchet A, Gilbert A. The posterior arm free flap. Plast Reconstr Surg. 1985;76(6):908–913. [DOI] [PubMed] [Google Scholar]
- 25. Elliot D, Kangesu L, Bainbridge C, Venkataramakrishnan V. Reconstruction of the axilla with a posterior arm fasciocutaneous flap. Br J Plast Surg. 1992;45(2):101–104. [DOI] [PubMed] [Google Scholar]