Abstract
Background
Leventhal’s Self-regulatory Model proposes that somatic characteristics of a health threat (e.g., symptom severity), and prior experience with the threat (e.g., unsuccessful treatment), are determinants of illness perceptions. Chronic lymphocytic leukemia (CLL) is appropriate for test of these postulates, having three phases differing in symptom severity and prior treatment experiences: indolent disease requiring no treatment (active surveillance; AS), symptomatic disease requiring a first treatment (FT), and highly symptomatic disease in those who have relapsed and/or failed to respond to prior treatments (relapsed/refractory; RR).
Purpose
To test symptom severity and prior treatment experiences as determinants of illness perceptions, illness perceptions were characterized and contrasted between CLL groups.
Methods
Three hundred and thirty CLL patients (AS, n = 100; FT, n = 78; RR, n = 152) provided illness perception data on one occasion during a surveillance visit (AS) or prior to beginning treatment (FT, RR).
Results
Analysis of variance with planned comparisons revealed that consequences, identity, and concern were least favorable among RR patients, followed by FT, then AS (ps < .01). AS patients endorsed the lowest levels of coherence (ps < .01), and the most chronic illness timeline (ps < .01). FT patients endorsed the highest levels of personal and treatment control (ps < .01).
Conclusions
Data provide preliminary empirical support for Self-regulatory Model postulates that symptom severity and prior disease experiences influence illness perceptions. Unique knowledge needs for AS patients and elevated psychological/physical symptoms for later-stage CLL patients may warrant clinical attention.
Keywords: Illness perceptions, Chronic lymphocytic leukemia, Active surveillance, Relapsed refractory disease
For adults with chronic leukemia, beliefs about their disease, such as how controllable it is, how well they understand it, and how it effects them emotionally, are associated with the severity of their disease and previous experiences with leukemia treatment.
Introduction
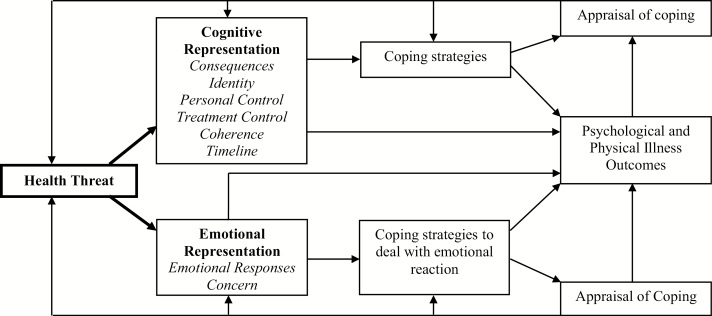
A goal of health psychology is to understand how individual differences in responses (e.g., behavioral, psychological) to chronic illness arise. Leventhal’s Self-regulatory Model of Illness Behavior [1] (Fig. 1) is widely used and highlights mental representations of health threats, or illness perceptions, as central to how individuals understand, cope with, and ultimately respond to disease. According to the model, illness perceptions are generated in response to health threats, such as a new physical symptom or disease diagnosis, and reflect emotional responses to and beliefs about the threat (e.g., consequences, controllability, chronicity) that guide coping and influence psychological and physical health outcomes.
Fig. 1.
Self-regulatory Model of Illness Behavior (adapted from Hagger and Orbell [46]). Bold emphasis is placed on the pathway of interest for the present study.
Limited empirical research has focused on better understanding determinants of illness perceptions [2, 3]. That is, what sources of information do individuals use when forming their perception of a symptom or illness? Although complex, theoretical work of Leventhal et al. [1, 4, 5] highlighted factors such as somatic characteristics of the threat or illness (e.g., symptom severity) and information acquired through prior experience with the threat or illness (e.g., receiving treatment) as central to the formation of illness perceptions. For example, severe abdominal pain would theoretically be perceived as more threatening than a stomachache. Similarly, an individual with chronic abdominal pain, who has received a diagnosis and knows it can be controlled by treatment, would, according to the model, perceive the symptom differently than when it occurred for the first time.
Although direct empirical tests of these postulates have not been a focus of prior research, available illness perception literature (e.g., that documenting relationships between symptom severity measures and illness perceptions) can provide insight into the relationship between theorized determinants and illness perceptions. Regarding symptom severity, Pagels et al. [6] compared illness perceptions between patients with mild-to-moderate (Stages 2–3) and severe (Stages 4–5) chronic kidney disease, finding that, consistent with self-regulatory theory, later-stage patients with greater symptom severity perceived more consequences, experienced more severe symptoms (identity), and endorsed more negative emotional responses than those with mild-to-moderate disease. Single group designs in cancer, osteoarthritis, irritable bowel syndrome, chronic pain, and overactive bladder have corroborated these findings, with greater symptom severity being associated with poorer scores on identity, consequences, emotional responses, and concern, as well as personal and treatment control [7–11].
In contrast, perceptions of the timeline of one’s illness, such as being acute versus chronic, have been predominantly unrelated to symptom severity [7, 8, 12–14], and, not surprisingly, stable across time for those with chronic (i.e., permanent) illnesses [15–20]. Exceptions have been observed, however, as in the case of asthma in which some patients adopt a chronic illness model only when symptoms are elevated [21], suggesting that relationships between symptom severity and timeline may vary by disease group. Similar to timeline perceptions, the appraisal of one’s understanding of his/her illness, or coherence, bears little, if any, relationship to symptom severity [6, 8, 13, 14]. Instead, longitudinal studies have observed improvements in coherence with the passage of time and/or receipt of treatment [18, 20, 22, 23], that is, the accumulation of experiences with the illness. As with symptom severity, prior illness experience has not been tested as a determinant of illness perceptions as the model would suggest.
Empirical tests of fundamental postulates of the self-regulatory model are needed and would be clinically useful. Providing empirical support for theorized determinants of illness perceptions may assist in the identification of those vulnerable to developing maladaptive perceptions of their illness and, by extension, may provide a window to addressing problems posed by the illness such as psychological distress or poor treatment adherence. As such, the present study tests the relationship between symptom severity and prior illness experience to illness perceptions. Chronic lymphocytic leukemia (CLL) provided an advantageous paradigm for these tests, having three subgroups distinguished by their differences in symptom severity and treatment exposure. One group consists of patients with asymptomatic or minimally symptomatic early-stage disease who are only monitored (i.e., active surveillance; AS) until symptoms progress sufficiently to require treatment. The duration of surveillance can range from months to years, with approximately 30% of patients never requiring treatment [24]. The second group consists of intermediate- to high-risk patients who are diagnosed with or have progressed and have significant physical symptoms (e.g., fatigue, fever, night sweats, abdominal pain, enlarged lymph nodes) and signs (e.g., enlarged spleen and/or liver, low red blood cell and/or platelet counts) and require initiation of a first CLL treatment (i.e., first treatment; FT) [25]. These patients have greater symptom severity relative to AS patients and are in the midst of initiating treatment (e.g., diagnostics, education, treatment selection) for their illness. The third group consists of patients with significant symptoms/signs who, having initiated a first treatment, either failed to respond (treatment refractory), or responded for a time, but eventually relapsed. These relapsed/refractory (RR) patients experience symptoms similar to or more severe than those initiating a first treatment [26] and have had at least one to several cycles of significant symptoms, treatment, relapse, and retreatment [27, 28]. In sum, three CLL patient groups vary in symptom severity and prior experience with the illness, but it is unknown if variations in these theorized determinants result in differing illness perceptions as the model would suggest.
We tested three hypotheses. First, we hypothesized that identity, consequences, emotional responses, concern, and personal control would covary with symptom severity, such that AS patients report the least threatening perception of CLL along these dimensions, followed by FT, and then RR. Second, we hypothesized that among those initiating treatment (FT and RR groups), treatment control will be higher among those with less severe symptoms, such that FT endorse higher treatment control than RR. AS patients were not administered the treatment control item. Third, we hypothesized that coherence scores will be higher among those with more prior “experience” with CLL (e.g., interaction with medical system, receipt of treatment), such that AS patients report the lowest scores on this dimension, followed by FT, and then RR. Finally, with limited evidence linking symptom severity to timeline perceptions and their stability across time in those with chronic illness [7, 8, 12–20], we did not anticipate group differences for this dimension.
Methods
Design and Participants
A cross-sectional design was used. Three hundred and thirty patients with CLL participated from three intact groups: active surveillance (AS; n = 100), initiating a first treatment (FT; n = 78), and initiating treatment for relapsed/refractory disease (RR; n = 152). Overall, the majority was male (63%), and Caucasian (98%), with a mean age of 62.2 years. Most were partnered (86%) and reported some college education or beyond (70%), with 43.8% reporting an annual household income exceeding $100,000.
Procedures
The Institutional Review Board of a university-affiliated, National Cancer Institute-designated comprehensive cancer center granted ethical approval for all procedures. Eligible patients were adults 18 or older with a physician-confirmed diagnosis of CLL and an Eastern Cooperative Oncology Group performance status of 0–2. Medical inclusion criteria (e.g., normal organ function) were required for patients beginning a treatment. Patients with systemic, life-threatening medical comorbidities, recent major surgery or medical procedures, active or secondary cancers, or severe psychiatric illness were excluded.
AS patients were recruited during routine surveillance appointments, with 126 consented. Of them, 3 were subsequently found to be ineligible, 11 did not participate due to loss of interest, and 12 did not provide illness perception data, resulting in 100 AS participants. Each completed a packet of self-report questionnaires over the telephone with research staff, as described previously [29].
Patients about to receive their first or subsequent treatment were screened for entry into investigational trials (NCT01589302, NCT02296918, NCT02427451, and NCT02518555) of targeted CLL therapies. As they were enrolled, patients completed questionnaire assessments immediately or within the next 2 weeks prior to treatment. A total of 261 were consented with 31 later excluded, resulting in 230 patients initiating a first or subsequent treatment.
Measure
Illness perceptions
The Brief Illness Perception Questionnaire (BIPQ [30]) is a nine-item self-report measure used to assess mental representations of illness. The BIPQ uses a single-item scale approach to assess perceptions on a continuous linear 0- to 10-point scale. Five items assess cognitive illness representations: consequences (“How much does your illness affect your life?”), timeline (“How long do you think your illness will last?”), personal control (“How much control do you feel you have over your illness?”), treatment control (“How much do you think your treatment can help your illness?”), and identity (“How much do you experience symptoms from your illness?”). Two items assess emotional representation of illness: concern (“How concerned are you about your illness?”) and emotional responses (“How much does your illness affect you emotionally?”). One item assesses illness coherence, a metacognitive dimension reflecting how well an individual feels they understand their illness. As treatment was not indicated for AS patients at the time of data collection, the treatment control dimension for this group was excluded. Six-week test–retest reliability for the items ranges from 0.42 to 0.75 [30]. Concurrent validity with relevant psychological and biological measures, discriminant validity across illnesses, and predictive validity in different disease groups have been reported [30, 31].
Analytic strategy
First, sociodemographic differences between groups were tested using one-way analysis of variance (ANOVA) for continuous variables (i.e., age) and chi-square tests for nominal variables (i.e., gender, marital status, education level, and household income). Intercorrelations of the illness perception items are reported as well as means, SD, and ranges of all items. Primary analyses testing for group differences in illness perceptions used one-way ANOVA or analysis of covariance as appropriate. Normality and homogeneity of group variances were assessed. Skewed data were log-transformed, and Welch’s ANOVAs were conducted for heteroscedastic variables.
For the six illness perception dimensions for which group differences were hypothesized (i.e., consequences, identity, concern, emotional responses, coherence, and personal control), a priori planned comparisons were used. The first compared AS with FT, and the second compared FT with RR. As the treatment control item was administered to FT and RR groups only, this comparison was made using an independent samples t-test. As there was no a priori expectation of group differences for the timeline dimension, ANOVA followed by the Games–Howell post hoc procedure [32, 33] was used. As timeline comparisons were done post hoc, a Bonferroni corrected p-value of .017 (.05/3) was also established. All analyses were performed using IBM SPSS 20.0 for Windows.
We considered sociodemographic characteristics (age, gender, marital status, education level, and household income) as control variables in group difference analyses. Race was not considered due to lack of variability in the sample. Potential control variables were correlated with each illness perception item, collapsing across groups. Variables significantly associated with an illness perception dimension were included in the respective analyses.
Results
Preliminary, Descriptive, and Correlational Data
Sociodemographic and descriptive characteristics by CLL group are displayed in Table 1 along with results of the ANOVA tests contrasting sociodemographic information between groups. As shown, group differences were found for age, F(2, 323) = 6.615, p = .002, and gender, χ2(2) = 7.69, p = .021, Cramer’s V = .15, with the RR group being significantly older and having a higher percentage of males in comparison to the other groups.
Table 1.
Sociodemographic and descriptive characteristics by chronic lymphocytic leukemia treatment group (N = 330)
| Active surveillance (n = 100) | First treatment (n = 78) | Relapsed/refractory (n = 152) | |
|---|---|---|---|
| Age (years), M (SD) | 61.88 (8.33)a | 59.03 (10.38)a | 64.08 (10.79)b |
| Gender (% male) | 50 (50%)a | 47 (60%)a | 107 (70%)b |
| Married (yes) | 84 (84%)a | 66 (85%)a | 129 (85%)a |
| Race | |||
| Caucasian | 100 (100%) | 76 (97%) | 147 (97%) |
| African American | 0 (0%) | 1 (1%) | 5 (3%) |
| Education | |||
| High school/technical school or below | 21 (21%) | 21 (27%) | 44 (29%) |
| Some college/college graduate | 40 (40%) | 32 (41%) | 58 (38%) |
| Some graduate school/graduate degree | 30 (30%) | 25 (32%) | 46 (30%) |
| Household income (k) | |||
| ≤100 | 41 (41%) | 39 (50%) | 79 (52%) |
| >100 | 44 (44%) | 34 (44%) | 46 (30%) |
| Prefers not to answer/unknown | 15 (15%) | 5 (6%) | 27 (17%) |
Variables with group differences are denoted by superscripts. Similar superscripts denote no difference between groups. Dissimilar superscripts indicate significant group differences (p < .05).
Distributions for all illness perception dimensions were non-normal; thus, analyses were conducted on log-transformed variables. Results did not differ on the basis of transformation, so untransformed results are presented for ease of interpretation. All dimensions met assumptions for homogeneity of variance between groups except for identity, coherence, and timeline. For these exceptions, group differences were confirmed with Welch’s test. Regarding potential covariates, age was significantly associated and was so only with the emotional responses item (r = −.172, p = .002). Thus, age was included as a covariate in the analyses for the latter.
Intercorrelations between illness perception dimensions are displayed in Table 2. Consequences and emotional responses were correlated with the greatest number of other illness perceptions (i.e., five dimensions each). In addition to being positively associated with each other (p < .01), endorsement of greater consequences and negative emotions were both associated with perception of greater symptoms (identity), greater illness concern, lower levels of treatment control, and a more acute illness timeline (ps < .05). The coherence and personal control dimensions displayed the fewest correlations with other illness perceptions (i.e., two dimensions each), each being positively correlated with one other as well as treatment control (ps < .05).
Table 2.
Intercorrelations between illness perception dimensions across chronic lymphocytic leukemia groups (N = 330)
| Illness perception | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. |
|---|---|---|---|---|---|---|---|---|
| 1. Consequences | 1 | .664** | .390** | .034 | .039 | −.194** | −.208** | .531** |
| 2. Identity | 1 | .290** | .063 | .066 | −.198** | −.090 | .392** | |
| 3. Concern | 1 | .009 | −.005 | −.159** | −.018 | .405** | ||
| 4. Coherence | 1 | .136* | −.015 | .201** | −.005 | |||
| 5. Personal control | 1 | −.107 | .249** | .069 | ||||
| 6. Timeline | 1 | −.110 | −.133* | |||||
| 7. Treatment control | 1 | −.144* | ||||||
| 8. Emotional responses | 1 |
*Correlation is significant at the 0.05 level (two tailed).
**Correlation is significant at the 0.01 level (two tailed).
Primary Tests of Group Differences
Identity
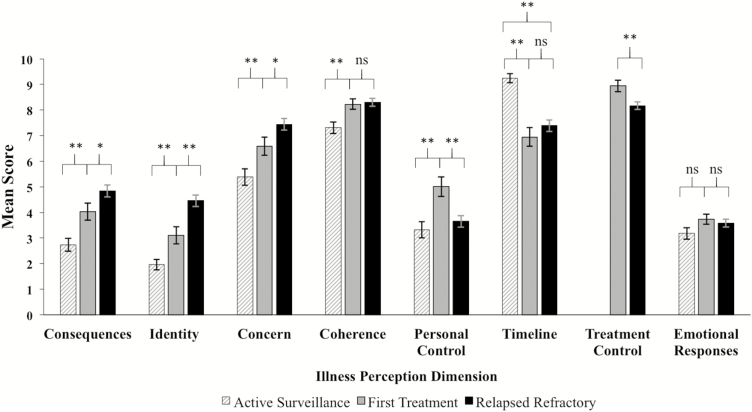
The effect of treatment group on endorsement of CLL symptoms (identity) was significant (see Fig. 2), F(2, 327) = 28.87, p < .001, η2p = .150, as were planned comparisons of AS versus FT, t(327) = 2.93, p < .01, and FT versus RR, t(327) = 3.78, p < .01 groups. Consistent with hypotheses, AS patients reported the fewest symptoms (M = 1.96, SD = 2.05), followed by FT (M = 3.10, SD = 2.74), and then RR (M = 4.46, SD = 2.81). In other respects, these data also provide a “validity check” and confirm the assumption that the groups differed in CLL symptoms.
Fig. 2.
Mean illness perception scores by chronic lymphocytic leukemia treatment group. Error bars denote standard errors of the group mean. The treatment control item was not administered to active surveillance patients. *p < .05, **p < .01.
Consequences
The effect of treatment group on consequences of CLL was also significant, F(2, 326) = 16.93, p < .001, η2p = .094, as were planned comparisons of AS versus FT, t(326) = 3.04, p < .01, and FT versus RR, t(326) = 2.09, p < .05 groups. Consistent with hypotheses, AS patients reported the fewest consequences (M = 2.74, SD = 2.56), followed by FT (M = 4.03, SD = 2.92), and then RR (M = 4.84, SD = 2.89).
Concern
The effect of treatment group on concern about CLL was also significant, F(2, 326) = 14.31, p < .001, η2p = .081, as were planned comparisons of AS versus FT, t(326) = 2.65, p < .01, and FT versus RR, t(326) = 2.08, p < .05 groups. Consistent with hypotheses, AS patients reported the lowest levels of concern (M = 5.38, SD = 3.16), followed by FT (M = 6.58, SD = 3.12), and then RR (M = 7.44, SD = 2.78).
Coherence
The effect of treatment group on coherence was also significant, F(2, 327) = 8.46, p < .001, η2p = .049. Consistent with hypotheses, planned comparisons indicated that AS patients reported lower understanding of CLL (M = 7.31, SD = 2.26) than FT (M = 8.23, SD = 1.77), t(327) = 3.10, p < .01. Contrary to hypotheses, FT (M = 8.23, SD = 1.77) and RR groups (M = 8.30, SD = 1.86) did not differ (p = .793).
Personal control
The effect of treatment group on personal control was also significant, F(2, 323) = 7.49, p = .001, η2p = .045. Contrary to hypotheses, planned comparisons indicated that personal control was highest for FT patients (M = 5.01, SD = 3.36) relative to AS (M = 3.32, SD = 3.10), t(323) = 3.67, p < .01, and RR groups (M = 3.65, SD = 2.77), t(323) = −3.22, p < .01.
Timeline
Contrary to expectations, the effect of treatment group on timeline perceptions was significant, F(2, 321) = 20.24, p < .001, η2p = .112, with AS patients believing that CLL would last the longest (M = 9.24, SD = 1.82), followed by RR (M = 7.39, SD = 2.78), and then FT (M = 6.95, SD = 3.26). Post hoc comparisons indicated that timeline perceptions for AS patients were higher relative to RR (SE = 0.293, p < .001) and FT (SE = 0.414, p < .001); the latter two groups did not differ (SE = 0.436, p = .566).
Treatment control
The effect of treatment group on treatment control perceptions was significant, t(228) = 2.90, p = .004, d = 0.403. Consistent with hypotheses, FT patients believed more strongly that treatment would be helpful (M = 8.94, SD = 1.96) than RR patients (M = 8.14, SD = 1.87).
Emotional responses
Contrary to hypotheses, groups did not differ in the extent to which they felt CLL affected them emotionally (p = .225).
Discussion
Foundational work in self-regulatory theory [1] highlighted symptoms and prior experiences with health threats as central to the formation of illness perceptions. In an empirical test of this postulate, the present study contrasted illness perceptions between three groups of patients with CLL: active surveillance (AS), initiating a first treatment (FT), and initiating treatment for relapsed/refractory disease (RR). Differing in symptoms and prior disease experiences, these groups provided an ideal context for better understanding factors relevant to patients’ mental representations of illness. Although consequences, identity, and concern were significantly poorer among patients at each successive phase of treatment, personal and treatment control were highest among FT patients. AS patients reported the lowest levels of coherence and the most chronic illness timeline. Despite these differences, groups reported equivalent emotional responses to CLL.
Notably, consequences, identity (symptoms), and concern were significantly poorer among patients at each successive phase of treatment. Mapping onto a clinical picture of increasing symptom severity as CLL patients transition from surveillance to an FT and beyond [26, 34, 35], these findings support Leventhal’s postulate that greater symptom severity influences more negative illness perceptions. Although the illness concern finding is consistent with the self-regulatory model, it is noteworthy that emotional responses, an additional dimension of emotional representations (Fig. 1), did not differ between groups. The concern item in the BIPQ captures worry [31], which is not an emotional response per se, but a chain of thoughts and images, which are laden with negative affect [36]. It could be that worry increases throughout the course of CLL treatment, but not overall rates of negative emotions such as sadness or anger. Van den Broek et al. [35] provide support for this hypothesis, observing differences between CLL treatment groups (surveillance vs. on treatment) on several domains of cancer-specific worry (e.g., personal health, future, cancer recurrence), but no group differences in anxiety or depression. Future work in this population may benefit from use of cancer-specific worry scales when evaluating and monitoring psychological functioning.
Also, consistent with the self-regulatory model, group differences in treatment control mapped onto increasing symptom severity across CLL groups, with FT patients believing more strongly that treatment would be helpful than RR. Although also in line with prior research linking symptom severity and treatment control [7, 9, 10, 12, 13], the treatment history of RR patients is important to consider. Average number of previous CLL therapies for RR patients was 3.5 (SD = 2.6), with some relapsing and/or failing to respond to upwards of 16 prior therapies. Thus, patients conceivably learned that treatment effects do not remain (i.e., relapse) and may be aware that subsequent treatments are less effective in controlling CLL. Nevertheless, treatment control perceptions were high for both groups (FT = 8.9/10; RR = 8.1/10), reflecting that, despite ultimately being incurable, patients in this context had high levels of confidence in the ability of treatment to be helpful for at least some period of time.
Contrary to treatment control findings, personal control did not vary across groups in a manner that would be expected based on symptom severity alone. We hypothesized that personal control would be highest among those with less severe symptoms (i.e., AS patients). Instead, personal control was highest among FT patients, followed by RR and AS groups. It could be that entering a phase of active attempts at managing their disease provides FT patients with more opportunities (or a first opportunity) to mobilize coping behaviors and request information from medical providers about how to best control their symptoms. AS patients are frequently told that their disease requires no immediate action [37]. RR patients, at the other end of the spectrum, may feel less personal control over CLL as a result of their cycling of treatment and relapse. Future longitudinal research documenting changes in personal control as patients’ transition from surveillance to a first treatment and beyond may help clarify the nature of these relationships.
Coherence also differed between groups, with FT and RR patients endorsing greater understanding of CLL than AS. These findings are largely consistent with expectations of self-regulatory theory that those with greater prior experience with a condition would learn from their experiences and thus endorse greater understanding of their condition. Findings are also consistent with prior research [18, 20, 22, 23] and may reflect a general tendency across illnesses to learn more about one’s condition through continued interactions with physicians and treatment experiences.
We anticipated that the timeline dimension would not differ by CLL group. Group differences emerged, however, with AS patients endorsing their illness would last the longest, followed by both treatment groups that did not differ. This finding may be more readily understood in the context of prior research [38–40] criticizing the construct validity of the acute/chronic timeline item (“How long do you think your illness will last?”). The authors have provided evidence that, particularly among older patients and those with advanced disease, the timeline item may elicit responses related to perceived life expectancy rather than permanence of the condition per se. Thus, CLL patients requiring a first or subsequent treatment may have believed that their disease will continue for the rest of their lives but that this will be a shorter period of time. Intercorrelations between illness perception dimensions (Table 2) support this rationale, showing that endorsement of a less chronic illness timeline was associated with greater consequences, symptoms, concern, and negative emotions related to CLL (ps < .05).
In addition to providing empirical support for components of Leventhal’s model, the patterns of group differences observed here also have clinical implications. As the negative effects of CLL on patients’ lives (consequences), physical symptoms (identity), and illness concern were greater among patients at each successive phase of CLL treatment, it may be important to monitor for levels of/changes in psychosocial and physical distress as patients move through treatment phases, and provide referral to psychological and symptom management services as appropriate. Results also indicated that coherence was poorest among AS patients. These findings are consistent with a prior qualitative report from Evan et al. [37] in which surveillance patients commonly expressed a desire for more information and confusion about their illness and the lack of need for immediate treatment. Thus, surveillance may represent a period of heightened uncertainty as patients face an indolent disease with an undetermined course, and care should be taken to insure that the unique knowledge and communication needs for this population are addressed. Also relevant to the clinical management of surveillance patients were their decreased levels of personal control relative to those initiating a first CLL treatment. This finding is particularly important in light of prior research linking personal control to medication adherence and other self-management behaviors such as appointment attendance, diet, and exercise [41–44]. Although there is no conclusive evidence that positive health behaviors delay progression in CLL, education regarding modifiable lifestyle factors may enhance personal control and reduce risk for development of medical comorbidities (e.g., secondary cancers, cardiovascular disease, diabetes) that may complicate CLL treatment.
A primary strength of the present study is its theory-based analysis of the CLL patient experience, which is particularly appropriate given the unique disease trajectory and limited psychosocial study of CLL relative to other cancers. Furthermore, patients initiating a first or subsequent treatment completed the illness perception assessment before treatment began, preventing the potential confounding physical side effects of anti-cancer therapy (e.g., nausea, fatigue). The group comparison design is an additional strength, as it did not require self-report of symptom severity. Self-regulatory theory indicates that type and severity of symptoms are critical determinants of illness perceptions, yet when considering this question, studies reliant on patients’ self-report encounter methodological issues of common measurement and conceptual overlap. Individuals who perceive more severe symptoms (identity) are likely to report more severe symptoms, complicating inferences about the relationship between severity and illness perceptions. One way to circumvent this is to contrast illness perceptions among groups known or presumed on the basis of prior literature to differ on symptom severity as done here (and corroborated by group differences on the identity item). An additional option for future studies would be to include use of objective disease severity markers (e.g., lymph node volume or hemoglobin counts in CLL) akin to that done previously in a select group of studies from other disease groups [7, 13, 45].
Limitations are also considered. Although the present study focused on symptom severity and prior illness experience as determinants of illness perceptions, additional factors are probably relevant and warrant future study, including social communication (e.g., that from friends, family, media, etc.), history of serious illness in close others, personality, and cultural background [1, 5, 46]. In addition, patients initiating treatment were doing so in the context of clinical trials, which often underrepresent minorities and older adults [47, 48]. Thus, our sample was younger (mean age = 62.2 years) and more likely to be Caucasian (98%) than rates recorded in national CLL samples (median age at diagnosis = 71; 90% Caucasian) [49, 50]. Furthermore, as a low incidence disease, CLL patients are often treated at regional centers, which may produce expectancy effects that differ from those of a community treatment setting. Last, although the cross-sectional design provides a first step in the context of a disease where several years may pass between treatment phases, a longitudinal design, perhaps targeting critical change periods (e.g., patients transitioning from surveillance to a first treatment), would help clarify mechanisms giving rise to group differences.
In conclusion, novel data contrasting illness perceptions from three phases of CLL treatment provided preliminary empirical support for theoretical postulates that symptoms and prior disease experiences influence illness perceptions. Although certain dimensions appeared to map closely onto symptom experiences (i.e., consequences, identity, concern, treatment control), others may have been more influenced by factors such as knowledge acquired through prior experience with the illness (i.e., coherence) or the context of treatment itself (i.e., personal control). Future work is needed, particularly in the form of longitudinal studies and those that examine the influence of multiple theorized determinants (e.g., personality, culture, severe illness in close others) to continue to garner a better understanding of the formation of mental representations of illness.
Funding
This research was supported in part by a Pelotonia Idea Award and a Pelotonia Graduate Fellowship from the Ohio State University Comprehensive Cancer Center and Solove Research Institute, the National Institutes of Health/National Cancer Institute (K05 CA098133, K12 CA133250, R35 CA197734, RO1 CA177292), Pharmacyclics, the Specialized Center of Research from the Leukemia and Lymphoma Society, and the D. Warren Brown Foundation.
Compliance with Ethical Standards
Conflict of Interest K.M. has received research funding from Pharmacyclics. J.W. has received research funding from Morphosys, Karyopharm, Abbvie, Acerta, and served as a consultant for Janssen. J.B. has received research funding from Genentech, Janssen, Acerta, and Pharmacyclics.
Authors’ Contributions All authors were involved in the preparation of this manuscript and read and approved the final version.
Ethical Approval All procedures performed were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- 1. Leventhal H, Meyer D, Nerenz D. The common sense representation of illness danger. In: Rachman S, editor. Contributions to Medical Psychology. Vol. 2 New York, NY: Pergamon; 1980:7–30. [Google Scholar]
- 2. Leventhal H, Phillips LA, Burns E. The Common-Sense Model of Self-Regulation (CSM): A dynamic framework for understanding illness self-management. J Behav Med. 2016;39(6):935–946. [DOI] [PubMed] [Google Scholar]
- 3. Lowe R, Norman P. Information processing in illness representation: Implications from an associative-learning framework. Health Psychol. 2017;36(3):280–290. [DOI] [PubMed] [Google Scholar]
- 4. Leventhal H, Nerenz D, Steele D. Illness representations and coping with health threats. In: Baum TS, Singer JE, editors. Handbook of Psychology and Health. Hillsdale, MI: Erlbaum Associates; 1984. [Google Scholar]
- 5. Diefenbach MA, Leventhal H. The common-sense model of illness representation: Theoretical and practical considerations. J Soc Distress Homeless. 1996;5(1):11–38. [Google Scholar]
- 6. Pagels AA, Söderquist BK, Heiwe S. Differences in illness representations in patients with chronic kidney disease. J Ren Care. 2015;41(3):146–155. [DOI] [PubMed] [Google Scholar]
- 7. Edelstein OE, Werner P, Dresner-Pollak R, et al. Illness perceptions among osteoporotic men and women: Correlates and gender differences. J Mens Health. 2012;9(3):168–175. [Google Scholar]
- 8. De Gucht V. Illness perceptions mediate the relationship between bowel symptom severity and health-related quality of life in IBS patients. Qual Life Res. 2015;24(8):1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costa ECV, Vale S, Sobral M, Graça Pereira M. Illness perceptions are the main predictors of depression and anxiety symptoms in patients with chronic pain. Psychol Health Med. 2016;21(4):483–495. [DOI] [PubMed] [Google Scholar]
- 10. Pretorius S, Kinsey D, Alexander T, Glover L, Kraus S, Duggan P. The mediating role of illness perceptions in psychological outcomes in overactive bladder. Int J Urol Nurs. 2014;8(3):151–160. [Google Scholar]
- 11. Westbrook TD, Maddocks K, Andersen BL. The relation of illness perceptions to stress, depression, and fatigue in patients with chronic lymphocytic leukaemia. Psychol Health. 2016;31(7):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chisari C, Chilcot J. The experience of pain severity and pain interference in vulvodynia patients: The role of cognitive-behavioural factors, psychological distress and fatigue. J Psychosom Res. 2017;93:83–89. [DOI] [PubMed] [Google Scholar]
- 13. Dalbeth N, Petrie KJ, House M, et al. Illness perceptions in patients with gout and the relationship with progression of musculoskeletal disability. Arthritis Care Res. 2011;63(11): 1605–1612. [DOI] [PubMed] [Google Scholar]
- 14. Jansen DL, Heijmans MJ, Rijken M, et al. Illness perceptions and treatment perceptions of patients with chronic kidney disease: Different phases, different perceptions? Br J Health Psychol. 2013;18(2):244–262. [DOI] [PubMed] [Google Scholar]
- 15. Dempster M, McCorry NK, Brennan E, Donnelly M, Murray LJ, Johnston BT. Do changes in illness perceptions predict changes in psychological distress among oesophageal cancer survivors? J Health Psychol. 2011;16:500–509. [DOI] [PubMed] [Google Scholar]
- 16. Fischer M, Scharloo M, Abbink J, et al. The dynamics of illness perceptions: Testing assumptions of Leventhal’s common-sense model in a pulmonary rehabilitation setting. Br J Health Psychol. 2010;15:887–903. [DOI] [PubMed] [Google Scholar]
- 17. Foster NE, Bishop A, Thomas E, et al. Illness perceptions of low back pain patients in primary care: What are they, do they change and are they associated with outcome? Pain. 2008;136(1–2):177–187. [DOI] [PubMed] [Google Scholar]
- 18. Lawson VL, Bundy C, Harvey JN. The development of personal models of diabetes in the first 2 years after diagnosis: A prospective longitudinal study. Diabet Med. 2008;25(4):482–490. [DOI] [PubMed] [Google Scholar]
- 19. Rutter CL, Rutter DR. Longitudinal analysis of the illness representation model in patients with irritable bowel syndrome (IBS). J Health Psychol. 2007;12(1):141–148. [DOI] [PubMed] [Google Scholar]
- 20. Tasmoc A, Hogas S, Covic A. A longitudinal study on illness perceptions in hemodialysis patients: Changes over time. Arch Med Sci. 2013;9(5):831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Halm EA, Mora P, Leventhal H. No symptoms, no asthma: The acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129(3):573–580. [DOI] [PubMed] [Google Scholar]
- 22. Janssen V, De Gucht V, van Exel H, Maes S. Changes in illness perceptions and quality of life during participation in cardiac rehabilitation. Int J Behav Med. 2013;20(4):582–589. [DOI] [PubMed] [Google Scholar]
- 23. Bijsterbosch J, Scharloo M, Visser AW, et al. Illness perceptions in patients with osteoarthritis: Change over time and association with disability. Arthritis Rheum. 2009;61(8):1054–1061. [DOI] [PubMed] [Google Scholar]
- 24. Dighiero G. Unsolved issues in CLL biology and management. Leukemia. 2003;17:2385–2391. [DOI] [PubMed] [Google Scholar]
- 25. Hallek M, Cheson BD, Catovsky D, et al. ; International Workshop on Chronic Lymphocytic Leukemia Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pashos CL, Flowers CR, Kay NE, et al. Association of health-related quality of life with gender in patients with B-cell chronic lymphocytic leukemia. Support Care Cancer. 2013;21(10):2853–2860. [DOI] [PubMed] [Google Scholar]
- 27. Shindiapina P, Awan FT. Management of patients with relapsed chronic lymphocytic leukemia. Am J Hematol Oncol. 2016;12(3):25–30. [Google Scholar]
- 28. Chanan-Khan A, Miller KC, Musial L, et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase II study. J Clin Oncol. 2006;24(34):5343–5349. [DOI] [PubMed] [Google Scholar]
- 29. Morrison EJ, Flynn JM, Jones J, Byrd JC, Andersen BL. Individual differences in physical symptom burden and psychological responses in individuals with chronic lymphocytic leukemia. Ann Hematol. 2016;95(12):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception Questionnaire. J Psychosom Res. 2006;60(6):631–637. [DOI] [PubMed] [Google Scholar]
- 31. Broadbent E, Wilkes C, Koschwanez H, Weinman J, Norton S, Petrie KJ. A systematic review and meta-analysis of the Brief Illness Perception Questionnaire. Psychol Health. 2015;30(11):1361–1385. [DOI] [PubMed] [Google Scholar]
- 32. Games PA, Howell JF. Pairwise multiple comparison procedures with unequal N’s and/or variances: A Monte Carlo study. J Educ Stat. 1976;1(2):113–125. [Google Scholar]
- 33. Games PA, Keselman HJ, Rogan JC. Simultaneous pairwise multiple comparison procedures for means when sample sizes are unequal. Psychol Bull. 1981;90(3):594–598. [Google Scholar]
- 34. Levin TT, Li Y, Riskind J, Rai K. Depression, anxiety and quality of life in a chronic lymphocytic leukemia cohort. Gen Hosp Psychiatry. 2007;29(3):251–256. [DOI] [PubMed] [Google Scholar]
- 35. Van den Broek EC, Oerlemans S, Nijziel MR, Posthuma EF, Coebergh JW, van de Poll-Franse LV. Impact of active surveillance, chlorambucil, and other therapy on health-related quality of life in patients with CLL/SLL in the Netherlands. Ann Hematol. 2015;94(1):45–56. [DOI] [PubMed] [Google Scholar]
- 36. Borkovec TD, Robinson E, Pruzinsky T, DePree JA. Preliminary exploration of worry: Some characteristics and processes. Behav Res Ther. 1983;21(1):9–16. [DOI] [PubMed] [Google Scholar]
- 37. Evans J, Ziebland S, Pettitt AR. Incurable, invisible and inconclusive: Watchful waiting for chronic lymphocytic leukaemia and implications for doctor-patient communication. Eur J Cancer Care (Engl). 2012;21(1):67–77. [DOI] [PubMed] [Google Scholar]
- 38. Price A, Goodwin L, Rayner L, et al. Illness perceptions, adjustment to illness, and depression in a palliative care population. J Pain Symptom Manage. 2012;43(5):819–832. [DOI] [PubMed] [Google Scholar]
- 39. Dempster M, McCorry NK. The factor structure of the revised Illness Perception Questionnaire in a population of oesophageal cancer survivors. Psychooncology. 2012;21(5):524–530. [DOI] [PubMed] [Google Scholar]
- 40. Dempster M, Howell D, McCorry NK. Illness perceptions and coping in physical health conditions: A meta-analysis. J Psychosom Res. 2015;79(6):506–513. [DOI] [PubMed] [Google Scholar]
- 41. Chen SL, Tsai JC, Lee WL. The impact of illness perception on adherence to therapeutic regimens of patients with hypertension in Taiwan. J Clin Nurs. 2009;18(5): 2234–2244. [DOI] [PubMed] [Google Scholar]
- 42. Broadbent E, Donkin L, Stroh JC. Illness and treatment perceptions are associated with adherence to medications, diet, and exercise in diabetic patients. Diabetes Care. 2011;34(2): 338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. French DP, Cooper A, Weinman J. Illness perceptions predict attendance at cardiac rehabilitation following acute myocardial infarction: A systematic review with meta-analysis. J Psychosom Res. 2006;61(6):757–767. [DOI] [PubMed] [Google Scholar]
- 44. Aujla N, Walker M, Sprigg N, Abrams K, Massey A, Vedhara K. Can illness beliefs, from the common-sense model, prospectively predict adherence to self-management behaviours? A systematic review and meta-analysis. Psychol Health. 2016;31(8):931–958. [DOI] [PubMed] [Google Scholar]
- 45. Greco A, Steca P, Pozzi R, Monzani D, Malfatto G, Parati G. The influence of illness severity on health satisfaction in patients with cardiovascular disease: The mediating role of illness perception and self-efficacy beliefs. Behav Med. 2015;41(1):9–17. [DOI] [PubMed] [Google Scholar]
- 46. Hagger MS, Orbell S. A meta-analytic review of the Common-Sense Model of illness representations. Psychol Health. 2003;18(2):141–184. [Google Scholar]
- 47. Heller C, Balls-Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemp Clin Trials. 2014;39(2):169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eichhorst BF, Busch R, Stilgenbauer S, et al. ; German CLL Study Group (GCLLSG) First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–3391. [DOI] [PubMed] [Google Scholar]
- 49. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. [DOI] [PubMed] [Google Scholar]
- 50. Shenoy PJ, Malik N, Sinha R, et al. Racial differences in the presentation and outcomes of chronic lymphocytic leukemia and variants in the United States. Clin Lymphoma Myeloma Leuk. 2011;11(6):498–506. [DOI] [PubMed] [Google Scholar]