Abstract
Occupational lung disease secondary to inhalation of silica particles is variable and potentially life‐threatening. As the artificial stone industry has grown over the last two decades, the development of silicosis has been seen to accelerate and behave differently to chronic silicosis. In this case report, we present two patients who underwent lung transplantation for silicosis at the Alfred Hospital, both with predominantly artificial stone masonry exposure. We have identified the presence of both fibrotic/nodular silicosis and conspicuous alveolar proteinosis within the same lung parenchyma of both patients. We then demonstrate the radiological and histopathological correlates of disease; the first time this has been shown clearly in the literature.
Keywords: Alveolar proteinosis, artificial stone, interstitial lung disease, occupational lung disease, silicosis
Introduction
Artificial stone is a high‐silica‐containing material, which over the last 20 years has become popular for the fabrication of kitchen and bathroom benchtops in Australia. This material contains over 90% silica and since 2010 has been associated with increasing reports of silicosis internationally 1. The typical form of silicosis affecting workers in this industry develops faster and is more rapidly progressive than chronic silicosis 2, 3. This reflects the very high level of respirable silica that workers may be exposed to when processing artificial stone material 4. We describe clinical‐pathological‐radiological correlates for two patients who underwent lung transplantation for artificial stone‐associated silicosis.
Case Reports
Case 1
A 47‐year‐old man who had worked as a stone benchtop fabricator for nine years initially presented for investigation of weight loss and gradually worsening exertional dyspnoea. His work frequently involved cutting and grinding artificial stone without water dust suppression, minimal exhaust ventilation, and only occasional provision of a face mask. One year later, he was referred for consideration of lung transplantation due to progressively worsening respiratory failure. At that time, his respiratory function tests demonstrated forced expiratory volume in 1 s (FEV1) of 1.83 L (40% predicted), forced vital capacity (FVC) of 2.35 L (40%), and single breath carbon monoxide diffusion (TLCO) of 18.1 mL/min/mmHg (54%). A total of 22 months later, his lung function declined to FEV1 1.03 L (29%) and FVC 1.84 L (31%). Autoimmune serology demonstrated a strongly positive homogeneous anti‐nuclear antibody (ANA), titre 1:1280. Extractable nuclear antibodies and other serology were all negative. There were no clinical features of connective tissue disease.
Computed tomography (CT) of his chest at referral for transplant (Fig. 1) demonstrated bilateral upper lobe confluent opacities, traction bronchiectasis, hilar retraction, and volume loss, consistent with progressive massive fibrosis (PMF) (red arrows). Adjacent to these areas, and throughout all other lobes, are diffuse ground glass opacities (green arrows). Bilateral hilum and mediastinum lymph nodes were enlarged, some with peripheral calcification. One year later (and three months prior to transplantation), the upper lobe fibrosis had progressed, as had the diffuse ground glass changes. Of note, this patient had a possible concomitant mild respiratory infection at the time of this most recent CT.
Figure 1.
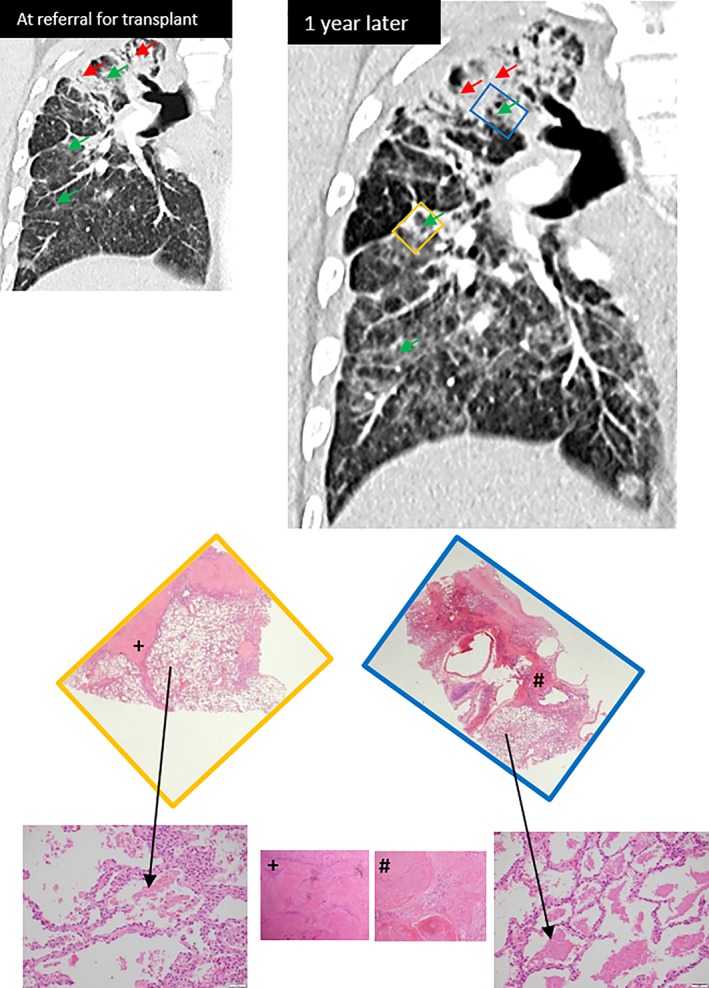
Conspicuous areas of alveolar proteinosis (black arrows) corresponding with areas of ground glass changes on computed tomography chest imaging (green arrows). Adjacent parenchyma demonstrates dense fibrosis with silicotic nodules and progressive massive fibrosis (+ and #).
He underwent right single lung transplantation. The explanted lung demonstrated extensive parenchymal destruction, replaced by hyalinised fibrosis and coalescing “silicotic” nodules. Polarisable flecks of silica were also present. At higher power, there were conspicuous intra‐alveolar macrophages containing fine particular brown material (possibly related to dust particles inhaled other than silica) as well as extensive alveolar dense proteinaceous material. These areas of silicoproteinosis correlate with ground glass changes on CT chest imaging, and are adjacent to areas of fibrosis and nodularity (Fig. 1).
Case 2
A 36‐year‐old non‐smoking man worked as a stonemason for six years fabricating benchtops, primarily from artificial stone material. He typically used high‐powered hand tools to cut the stone without water dust suppression. There was minimal ventilation at the workplace, and he was provided a disposable paper mask. He presented for investigation of worsening dyspnoea and cough.
CT chest at referral for transplant (Fig. 2) showed diffuse micronodularity (red arrows) with a perilymphatic distribution, as well as patchy sub‐pleural consolidative and fibrotic changes (yellow arrows). A repeat CT scan performed six months later (and seven months prior to transplantation) noted progressive upper lobe fibrosis with upward traction of both hilar and worsening sub‐pleural consolidation (blue box). Patchy areas of ground glass changes were also noted throughout, as demonstrated within the right lower lobe (green box). Mediastinal lymph nodes were enlarged but not calcified. Respiratory function testing demonstrated a restrictive ventilatory defect; FEV1 1.29 L (38%), FVC 1.29 L (30%), TLCO of 3.4 mL/min/mmHg (11% predicted), and TLC of 2.7 L (49% predicted). Blood tests revealed a positive ANA (1:1280 nucleolar, >1:1280 homogenous), positive Scl‐70 antibodies, and a normal angiotensin‐converting enzyme level. There were no clinical features of a connective tissue disorder.
Figure 2.
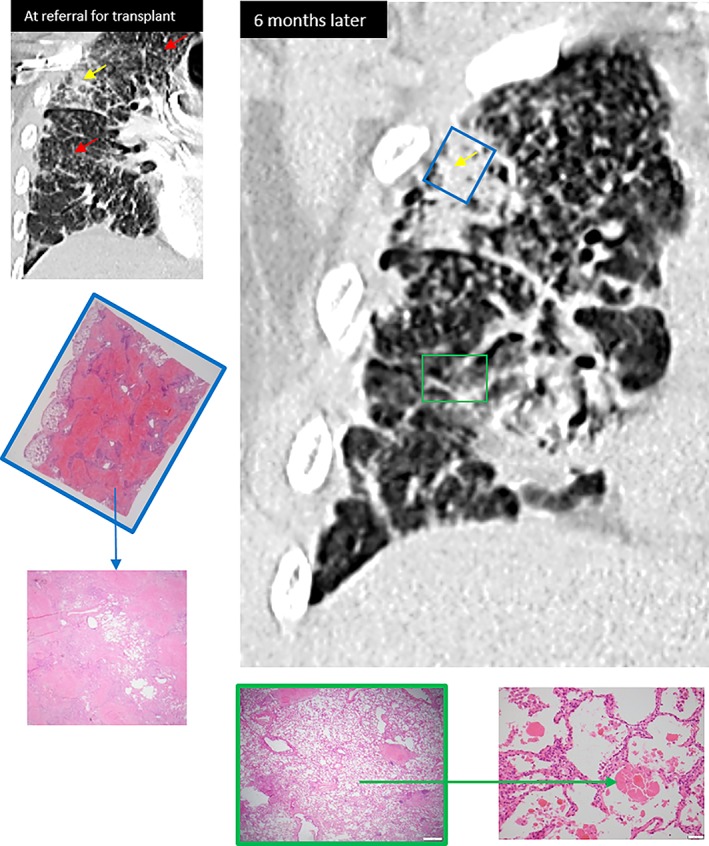
Upper lobe sub‐pleural dense consolidation and fibrosis on computed tomography (CT) chest corresponding with dense conglomerate of nodular and fibrotic silicosis (PMF) on histopathology (blue box and blue arrow). Lower lobe areas of diffuse ground glass changes on CT, corresponding with alveolar proteinosis on histopathology (green box and green arrow).
He underwent bilateral sequential lung transplantation 15 months after presenting with his initial symptoms. The explanted lung tissue demonstrated extensive parenchymal destruction, replaced by hyalinised fibrosis and coalescing “silicotic” nodules, corresponding with areas of dense consolidation and fibrosis on CT chest (blue box). Also present within alveolar spaces were areas of conspicuous proteinaceous material, consistent with silicoproteinosis. These findings correspond with areas of patchy ground glass changes on CT (green box).
Discussion
The commonly described pathological varieties of silicosis are simple silicosis, PMF, and silicoproteinosis 5. Simple silicosis is characterized by discrete, rounded hard silicotic nodules of 3–5 mm diameter in the upper lobes of the lungs. As the disease progresses, nodules coalesce to form fibrous masses (PMF). Silicoproteinosis (also known as acute silicosis) is associated with very high‐intensity silica exposure, develops within a few years of initial silica exposure (median three years), and rapidly progresses to respiratory failure 6. The pathological features are very different to chronic silicosis and are more consistent with primary alveolar proteinosis, with lipoproteinaceous material filling the alveolar spaces.
Accelerated silicosis is a form of silicosis which develops with a shorter duration (less than 10 years) but higher intensity of silica exposure than chronic silicosis. Accelerated silicosis has been poorly described, but is the most typical form associated with artificial stone work 1, 2, 3. Artificial stone contains over 90% silica, which is far higher than naturals stone, such as marble (3% silica) and granite (30% silica) 7. Cutting this material without any dust control can generate respirable crystalline silica (RCS) levels of 44 mg/m3 over 30 min of sampling, which is far higher than internationally recommended silica exposure limits 4. The dust generated contains a high fraction of very small particles <5 μm, which is associated with greater pulmonary inflammatory response than larger particles 7. The dust has also been noted to have metals such as zirconium, titanium, and aluminium in significantly higher percentages than from natural stone 7.
Case series of artificial stone‐associated silicosis have noted a significant risk of disease progression, and unfortunately at this time, there are no available treatment options to influence the progression of disease 2. Lung transplantation may be considered for severe disease, and in 2016, 13 affected workers in Israel had undergone transplantation for this occupational disease 8.
We present the co‐existence of different varieties of silicosis within the same patients with artificial stone‐associated silicosis. Lung transplantation provides the opportunity to histologically examine the entire lung parenchyma, and subsequently describe the clinical‐radiological‐pathological patterns of disease. Within this case series, we have demonstrated the histological presence of both chronic nodular/fibrotic silicosis and silicoproteinosis within the same two patients' lungs. Areas of proteinosis correlate with radiological areas of ground glass changes and may be present within lung parenchyma adjacent to fibrotic areas (Case 1, Fig. 1). These fibrotic areas themselves correspond with radiological areas of dense opacity and fibrosis. Alternatively, as in Case 2 (Fig. 2), silicoproteinosis, and the associated imaging ground glass change, may be seen in areas with no significant nodularity or PMF. The main limitation of these findings relates to time frames between CT imaging and transplantation (three to seven months), which could influence accuracy of histopathological and radiological correlation. Additionally, possible concomitant respiratory infection at the time of CT imaging (as in Case 1) could further confound these findings.
Other case series' in recent years have described clinical‐radiological correlates of silicosis. Grubstein et al. explained the relationship between lung function decline and radiological changes pre‐transplant in 82 artificial stone workers in Israel 8. They mentioned the histological identification of silicoproteinosis in two patients, both with PMF and ground glass changes on high‐resolution CT (HRCT). Souza et al. undertook a comparative study of clinical, histological, and radiological findings in patients with primary alveolar proteinosis and silicoproteinosis 6. Pathology from the 13 sandblasters with silicoproteinosis was derived from bronchoalveolar lavage samples and autopsy specimens 3. Biopsies demonstrated intra‐alveolar periodic acid‐Schiff (PAS)‐positive proteinaceous material, mild peribronchial granulomatous inflammation and a few silica particles. No fibrosis was seen. Bronchoalveolar lavage demonstrated fine granular PAS‐positive material with eosinophils. The predominant finding on HRCT was of dependant consolidation, often with calcification. Centrilobular nodules were also common. These patients did not seemingly have changes of chronic fibrotic silicosis.
These cases have allowed us to demonstrate the correlation between histopathology and radiology from two patients with accelerated silicosis following work with artificial stone materials. Furthermore, we have identified the presence of both silicoproteinosis and chronic fibrotic/nodular silicosis within the same patients' lung. The implications could extend as far as further treatment algorithms for this condition, as more data emerge on the utility of whole lung lavage for silicoproteinosis, something which would otherwise have been dismissed in a patient with predominant fibrotic changes on CT imaging.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Levin, K , McLean, C , Hoy, R . (2019) Artificial stone‐associated silicosis: clinical‐pathological‐radiological correlates of disease. Respirology Case Reports, 7(7), ;e00470. 10.1002/rcr2.470
Associate Editor: Nicole Goh
References
- 1. Leso V, Fontana L, Romano R, et al. 2019. Artificial stone associated silicosis: a systematic review. Int. J. Environ. Res. Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hoy RF, Baird T, Hammerschlag G, et al. 2018. Artificial stone‐associated silicosis: a rapidly emerging occupational lung disease. Occup. Environ. Med. 75:3–5. [DOI] [PubMed] [Google Scholar]
- 3. Paolucci V, Romeo R, Sisinni AG, et al. 2015. Silicosis in workers exposed to artificial quartz conglomerates: does it differ from chronic simple silicosis? Arch. Bronconeumol. 51:e57–e60. [DOI] [PubMed] [Google Scholar]
- 4. Cooper JH, Johnson DL, and Phillips ML. 2015. Respirable silica dust suppression during artificial stone countertop cutting. Ann. Occup. Hyg. 59:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leung CC, Yu IT, and Chen W. 2012. Silicosis. Lancet 379:2008–2018. [DOI] [PubMed] [Google Scholar]
- 6. Souza CA, Marchiori E, Goncalves LP, et al. 2012. Comparative study of clinical, pathological and HRCT findings of primary alveolar proteinosis and silicoproteinosis. Eur. J. Radiol. 81:371–378. [DOI] [PubMed] [Google Scholar]
- 7. Ophir N, Shai AB, Alkalay Y, et al. 2016. Artificial stone dust‐induced functional and inflammatory abnormalities in exposed workers monitored quantitatively by biometrics. ERJ Open Res. 2:00086–02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grubstein A, Shtraichman O, Fireman E, et al. 2016. Radiological evaluation of artificial stone silicosis outbreak: emphasizing findings in lung transplant recipients. J. Comput. Assist. Tomogr. 40:923–927. [DOI] [PubMed] [Google Scholar]


