Emily Dickinson described it as a funeral in her brain. Ursula K. Le Guin illustrated it as a nightmare from which one occasionally wakes in sleep. And Andrew Solomon described it as not the opposite of happiness, but as the loss of vitality. Metaphors lend clinicians and loved ones a glimpse into the experience of depression, a disease nearly 18% of Americans experience at some point in their lifetime. However, despite the monumental social and economic burden, fast and effective treatments for depression have been lacking.
How do you treat a disease for which you do not fully understand the causes? For centuries, individuals suffering from depression have endured a range of ineffective treatments, from the peculiar to the inhumane: prolonged water immersion, enemas, arsenic, and lobotomy.
Then, in the fall of 1951, everything changed. Patients in a tuberculosis ward at Sea View Hospital on Staten Island who were treated with iproniazid showed unexpected improvements in their mood. Soon, clinicians had a class of medications, monoamine oxidase inhibitors, that could be used to treat depression. With it, the first biological model was born: if increasing monoamines (including serotonin and norepinephrine) leads to improvement in mood, perhaps a deficit of these neurotransmitters is central to the disease process.
Later in the same decade, the first tricyclic antidepressant was discovered. By blocking the reuptake of serotonin and norepinephrine, tricyclic antidepressants seemed to work by the same general mechanism as the monoamine oxidase inhibitors. Subsequent laboratory studies lent further support to a monoaminergic model: for individuals with a history of depression, depriving them of the essential amino acid tryptophan (a necessary precursor of serotonin) could precipitate a new depressive episode (1). Researchers homed in on the role of serotonin and, in 1988, fluoxetine became the first selective serotonin reuptake inhibitor to hit the U.S. market.
The release of Prozac Nation in 1994, in which Elizabeth Wurtzel described her life-saving experience with fluoxetine, sparked a shift in public dialogue about the nature of mental illness and the role of medications. But the hope and hype of the selective serotonin reuptake inhibitors has not been borne out. While the data that these medications work are clear, their limitations are equally clear: they still have adverse effects; they may take weeks to reach full efficacy; and, for a disappointingly large number of individuals, they might not work at all (2). As far as the monoaminergic model may have gotten us, it was not the whole story.
The 1990s and 2000s saw the rise of imaging technologies. With them, clinicians started to think more about circuit level dysfunction. Key studies demonstrated that individuals with depression showed differences in the functional and structural circuits responsible for the regulation of mood and cognition, including the prefrontal cortex (where studies have shown cortical thinning and decreased functional activity), Brodmann area 25, and the hippocampus (3,4). These data inspired investigation into a range of interventional treatment approaches (e.g., deep brain stimulation and repetitive transcranial magnetic stimulation). And while these findings revealed limitations of the basic monoaminergic model, the circuit-level approach on its own also seems to be falling short (5,6).
Over the past 10 to 20 years, a new line of research has combined previous ideas into what may represent a unifying model of depression. This model is predicated on an appreciation of what a healthy, functioning brain looks like.
The human brain may be the most complex machine in the universe. It requires the extraordinary coordination of approximately 1011 neurons, each of which may connect to thousands of other neurons at 1014 synapses. Visually, these connections are stunning (e.g., http://www.gregadunn.com/product/brainbow-hippocampus-in-color/), evoking images of the canopy of a forest, and described with terms like “rich arborization.” But these images are just frozen moments in time. To navigate and respond appropriately to our environment, our brains are constantly and dynamically regulating both the number and strength of these synapses (7). Many growth factors, including brain-derived neurotrophic factor (BDNF), are essential for the process of synaptic plasticity (through which these connections are strengthened or weakened) and thereby for maintaining a healthy, vibrant ecosystem.
If this constant process of remodeling is what allows individuals to engage effectively with the world, Andrew Solomon’s description of depression as the “opposite of vitality” is a powerful metaphor for the molecular correlates of the disease. A range of animal studies have shown that chronic stress, a common animal model of depression, results in decreased expression of BDNF, neuronal atrophy and cell loss in limbic structures (including the hippocampus and prefrontal cortex), decreased synaptic proteins and spine density, and decreased connectivity between regions (3). If the healthy brain looks like a rich forest, the depressed brain appears as if it has been struck by wildfire.
These effects are not just seen in animals. Human studies of depression, including postmortem analyses, have demonstrated reduced neuronal size and a loss of synapses in the prefrontal cortex and neuronal atrophy in the hippocampus (3). Genetic studies have further corroborated the role of BDNF. A specific polymorphism in the BDNF gene (Val66Met) results in lower plasma levels in humans (8). Moreover, when this polymorphism was introduced into a mouse model, researchers saw disrupted production of BDNF, reduced spine density in the prefrontal cortex and hippocampus, atrophy of the hippocampus, and depressive-like behaviors (8)—which is to say, introducing this mutation recapitulated the classic correlates of chronic stress.
Recently, one of the most exciting aspects of depression research has been the discovery and characterization of the rapid antidepressant effects of ketamine. As with iproniazid, the story is one of serendipity. Researchers at Yale University were using ketamine—an N-methyl-D-aspartate antagonist historically used as an anesthetic agent—to explore glutamatergic models of depression. They were surprised to find that within hours of receiving a single intravenous dose of ketamine, subjects experienced dramatic improvements in their mood that lasted for up to a week (3), results that have now been replicated many times over.
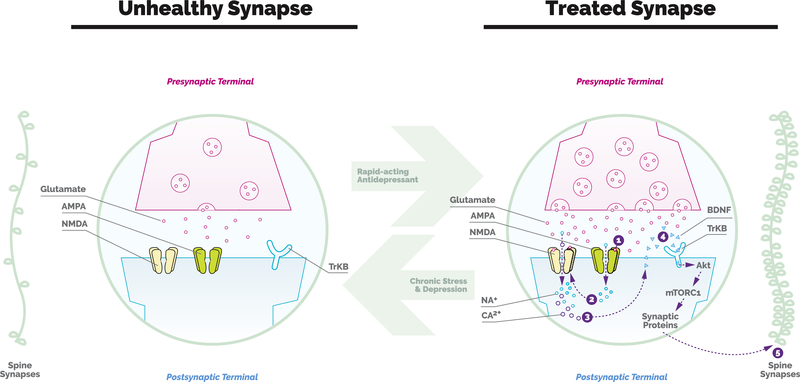
Since this pioneering discovery, researchers have been working hard to elucidate the mechanism of action. As it is currently understood, it appears that low-dose ketamine leads to a rapid and transient burst of glutamate, thereby initiating a cascade of events that ultimately leads to increased BDNF and increased synaptogenesis (Figure 1). If a picture is worth a thousand words, the iconic image from Li et al. (9) beautifully illustrates the story: in a matter of hours, the administration of ketamine leads to the reversal of stress-induced atrophy and synapse loss and a return to a healthy appearing cortex.
Figure 1.
The effects of stress and depression and rapid-acting antidepressants on synaptogenesis. Chronic stress and depression lead to atrophy of spines and synapses in brain regions that are implicated in depression. Preclinical rodent studies have identified reduced production and release of brainderived neurotrophic factor (BDNF) as being integral to this process. Ketamine, a rapid-acting antidepressant, has been shown to rapidly reverse these synaptic deficits through a cascade of signaling events. 1) Subanesthetic doses of ketamine produce a rapid and transient burst of glutamate transmission that activates postsynaptic alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors. 2) and 3) This results in membrane depolarization, opening of N-methyl-D-aspartate (NMDA) receptors, and calcium influx. 4) The influx of intracellular calcium then triggers the release of BDNF. 5) BDNF binding to tyrosine receptor kinase B (TrKB) receptors activates the mammalian target of rapamycin complex 1 (mTORC1) signaling pathway. This leads to an increase in the synthesis of synaptic proteins that ultimately leads to increased spine number and function. NA, sodium; CA2, calcium; Akt, protein kinase B.
And it is not just ketamine. Perhaps the most compelling aspect of the neurotrophic model is that many of the known treatments for depression seem to act via this pathway. Other rapid-acting antidepressants—including electroconvulsive therapy and intravenous scopolamine— lead to both the same increases in BDNF and new synapse formation. Similarly, when taken over a longer period of time, selective serotonin reuptake inhibitors also lead to increased BDNF, and additional work has confirmed this as the likely mechanism of action for their antidepressant properties (3). Even exercise—shown to be comparable to selective serotonin reuptake inhibitors for treating mild to moderate depression—seems to induce BDNF activity. Considerable work remains to clarify many details of this story (e.g., to what extent do animal findings replicate in humans, and to what extent are neurotrophic changes relevant across a range of interventions?) but, even tempered by antidepressants’ history of previous failures, there is considerable reason for hope.
This research is coming none too soon. While our current first-line treatments may work, they are not good enough. Depression remains the leading cause of disability worldwide, and suicide rates in the United States continue to rise. Disturbingly, despite these statistics, only 15% of people with depression are receiving any treatment at all. For those who do, they may need to wait 6 to 12 weeks to see a response (if there is any at all). Rapid-acting treatments could eliminate this delay, either on their own (if they are shown to be safe and effective for long-term use) or as a means of “jump starting” conventional treatments (either medication or psychotherapy). They might also play a unique role in acute interventions—e.g., by treating patients with suicidal ideation in an emergency setting and thereby avoiding a costly hospitalization.
The discovery of ketamine has also led to a significant shift in the research agenda. Rather than focusing on historical monoaminergic pathways, researchers are now seeking other ways to influence the neurotrophic pathway, including via glutamatergic or cholinergic systems. The article by Mastrodonato et al. in the current issue (10) offers another novel approach: whether ketamine could be given prior to stress to enhance resilience.
So where does this leave us today? For decades, critics have bemoaned the lack of effective medications and argued that neuroscience has not meaningfully advanced the field. After years of struggles, the introduction of powerful new treatments is leading to a dramatic reshaping of the clinical and research landscapes—perhaps, fittingly, a parallel to the transformations we are now able to effect and observe at the molecular level in brains themselves.
Acknowledgments and Disclosures
Clinical Commentaries are produced in collaboration with the National Neuroscience Curriculum Initiative (NNCI). David A. Ross, in his dual roles as co-chair of the NNCI and Education Editor of Biological Psychiatry, manages the development of these commentaries but plays no role in the decision to publish each commentary. The NNCI is supported by National Institutes of Health Grant Nos. R25 MH10107602S1 and R25 MH08646607S1. DMG reports no biomedical financial interests or potential conflicts of interest.
Contributor Information
Danielle M. Gerhard, Department of Psychiatry, Weill Cornell Medical College, New York, New York
David A. Ross, Department of Psychiatry, Yale University, New Haven, Connecticut.
References
- 1.Smith KA, Fairburn CG, Cowen PJ (1995): Relapse of depression after rapid depletion of tryptophan. Lancet 349:P915–P919. [DOI] [PubMed] [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard DM, Wohleb ES, Duman RS (2016): Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov Today 21:454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnsten AF (2015): Stress weakens prefrontal networks: Molecular insults to higher cognition. Nat Neurosci 18:1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtzheimer PE, Husain MM, Lisanby SH, Taylor SF, Whitworth LA, McClintock S, et al. (2017): Subcallosal cingulate deep brain stimulation for treatment-resistant depression: A multisite, randomised, sham-controlled trial. Lancet Psychiatry 4:P839–P849. [DOI] [PubMed] [Google Scholar]
- 6.Yesavega JA, Fairchild JK, Mi Z, Biswas K, Davis-Karim A, Phibbs CS, et al. (2018): Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: A randomized clinical trial. JAMA Psychiatry 75:884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holtmaat A, Svoboda K (2009): Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10:647–658. [DOI] [PubMed] [Google Scholar]
- 8.Dincheva I, Lynch NB, Lee FS (2016): The role of BDNF in the development of fear learning. Depress Anxiety 33:907–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. (2011): Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 69:754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, Denny CA (2018): Ventral CA3 activation mediates prophylactic ketamine efficacy against stress-induced depressive-like behavior. Biol Psychiatry 84:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]