Abstract
Objective
The purpose of this study was to evaluate if vitamin D deficiency is associated with increased rates of persistent critical illness, and whether repletion of vitamin D among those who are deficient leads to decreased persistent critical illness.
Design
Retrospective cohort analysis.
Setting
Seven ICUs at the University Medical Center of Graz, Austria, with participants recruited between July 2008 and April 2010. The VITdAL-ICU cohort: five ICUs at the University Medical Center of Graz, Austria, with patients recruited between May 2010 through September 2012.
Participants
628 patients ≥ 20 ng/mL.
Main outcome measures
Development of persistent critical illness
Results
In the retrospective cohort, Vitamin D3 level on admission was not significantly associated with the development of persistent critical illness compared to those discharged alive earlier (RRR: 1.02, 95% CI: 1.00–1.04) or who died (RRR: 1.02, 95% CI: 0.99–1.05). In the VITdAL-ICU trial, supplementation with vitamin D3 did not lead to less persistent illness relative to those discharged alive earlier (RRR: 1.19, 95% CI: 0.76–1.80) or who died (RRR: 1.34, 95% CI: 0.72–2.52).
Conclusion
Vitamin D3 deficiency was not associated with persistent critical illness, nor did supplementation with vitamin D3 mitigate the development of persistent critical illness.
Keywords: Anaesthesia and intensive care, Intensive care, Endocrinology, Statistics, epidemiology and research design
Vitamin D deficiency is associated with disease severity, sepsis, intensive care and hospital length of stay, and mortality.(1–3) Vitamin D deficiency has been hypothesized to worsen critical illness through its mediating effects on the immune, cardiac, and vascular systems.(4) Vitamin D deficiency can be identified either before or very early in a hospitalization making it a readily accessible biomarker to utilize and intervene upon in critically ill people.
A recent study, the VITdAL-ICU randomized clinical trial of vitamin D supplementation in critically ill patients, reported a mortality benefit in a subgroup analysis of patients with severe vitamin D deficiency (defined as ≤ 12 ng/mL) but did not find an overall mortality benefit or reduced hospital length of stay in the primary analysis of all patients with 25-hydroxyvitamin D levels <20 ng/mL (5) These findings have prompted two ongoing large randomized trials to evaluate the role of vitamin D: Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) in patients at risk for the Acute Respiratory Distress Syndrome; and Effect of high-dose vitamin D3 in critically ill patients with severe vitamin D deficiency (VITDALIZE).(6, 7)
In light of the associations of vitamin D deficiency and critical care outcomes, it seems plausible that vitamin D might play a causal role in the development of persistent critical illness. This is particularly true as a major event for many patients with persistent critical illness has been argued to be the development of late septic shock, where vitamin D deficiency’s negative impact on immune and cardiovascular function may contribute.(1, 4, 8) Given vitamin D’s protean effects, we sought to generate hypotheses regarding:
H1: Patients with vitamin D deficiency are more likely to develop persistent critical illness.
H2: The development of persistent critical illness will be mitigated by vitamin D3 supplementation.
We therefore conducted a secondary re-analysis of the de-identified data from a retrospective cohort study conducted at the University Medical Center of Graz in 2014 and the VITdAL-ICU randomized control trial in order to test these hypotheses.(5, 9)
Methods
We conducted a retrospective study in a university hospital from a cohort of patients admitted to the ICU from July 2008 - April 2010 and from May 2010 – September 2012. As these were secondary analyses of de-identified data, they were exempt from human subjects review.
The University Medical Center of Graz is a large tertiary academic center in the southeast of Austria with 1538 beds including 123 ICU beds.
Study Populations
The initial retrospective cohort study was conducted in all adult patients treated in one of seven medical, neurological, neurosurgical, cardiothoracic and mixed surgical ICUs at the University Medical Center of Graz, Austria, between July 2008 and April 2010 who had their 25-hydroxy-vitamin D level measured at least once.(9) Screening for vitamin D deficiency was performed upon request of the treatment team in this time period. The median time in the ICU until the 25(OH)D level was drawn was 2 days (IQR:1,4). 25(OH)D levels and other routine data gathered during hospital stay were extracted from the hospital’s medical documentation system and analyzed in 2014.
The VITdAL cohort has been previously described in detail.(5, 10) The patients were recruited from five ICUs: medical, neurological, cardiothoracic surgery and two mixed-surgery units. Patients were 18 years and older, expected to stay in the ICU for greater than 48 hours and found to have a 25-hydroxyvitamin D level of 20 ng/mL or lower were eligible to participate in the study. Patients were excluded from the trial if they met any of the following criteria: severely impaired gastrointestinal function, other trial participation, pregnant or lactating women; hypercalcemia (total calcium of >10.6 mg/d); tuberculosis, sarcoidosis, nephrolithiasis within the prior year and patients deemed not suitable.
Patients were randomly assigned to either a placebo group or vitamin D3 group in a 1:1 ratio. Patients randomized to the vitamin D3 group received a loading dose of 540, 000 IU of vitamin D3
Key Definitions
Vitamin D deficiency and severe vitamin D deficiency were defined as ≤ 20 ng/mL and <12 ng/mL respectively. The patient’s screening 25-hydroxyvitamin D level was utilized for the analysis.
Persistent critical illness was defined as ICU stay of ≥ 10 days based on population-based data.(11, 12)
Analysis
We present patient characteristics as counts (percentages), means (SDs), or medians (interquartile ranges [IQR]) as appropriate. Charlson comorbidities were tabulated using the method of Deyo. (13, 14) We conducted all analysis with Stata software version 15.1 (StataCorp, College Station, TX).
Multinomial logistic regression identified patient characteristics on ICU admission associated with the development of persistent critical illness. Covariates were chosen a priori.
Results
Retrospective Cohort Results
In the retrospective cohort of 655 patients, 628 were included in the analysis. Patients were excluded if data were missing. Of the 628, 37.4% (n = 235) were female with a median age of 65 years (IQR: 52, 75). The mean SAPS II score and TISS-28 score on admission were 29.4 (SD: 15.7) and 31.7 (SD: 8.8) respectively. The mean 25-hydroxyvitamin D level was 17.4 ng/mL (IQR: 11.2, 25.2). The hospital and ICU mortality were 18.5% and 13.2% respectively. (Table 1)
Table 1:
Patient demographics in the retrospective cohort and the VITdAL-ICU Cohort.
| Patient baseline characteristics | |||
|---|---|---|---|
| Retrospective Cohort | VITdAL ICU Cohort (N= 475) | ||
| N = 628 | Placebo N = 238 | Vitamin D3 N = 237 | |
| Gender: Female n (%) | 235 (37.4) | 83 (35) | 83 (35) |
| Age median (IQR) | 65 (52, 74.5) | 68 (56, 76) | 66 (55, 75) |
| SAPS II at ICU admission, mean (SD) | 29.4 (15.7) | 34.2 (15.7) | 32.4 (15) |
| TISS-28 mean (SD) | 31.7 (8.8) | 38.0 (8.2) | 37.7 (7.6) |
| 25-hydroxyvitamin D (ng/mL) mean (SD) | 19.6 (11.3) | 13.1 (4.3) | 13.0 (4.0) |
| ICU mortality n (%) | 83 (13.2) | 63 (26.5) | 54 (22.8) |
| Hospital mortality n (%) | 116 (18.5) | 84 (35.3) | 67 (28.3) |
| ICU length of stay (d) median (IQR) | 6 (3, 11) | 10.7 (0.1, 154.1) | 9.6 (0.2, 181) |
| Persistent Critical Illness n (%) | 181 (28.8) | 116 (48.7) | 106 (44.7) |
| Hospital length of stay (d) median (IQR) | 7 (0, 15) | 19.3 (0.1, 154.1) | 20.1 (0.2, 181) |
| ICU type | |||
| Cardiac Surgery n (%) | 102 (16.2) | 69 (29.0) | 68 (34.1) |
| Medical ICU n (%) | 228 (36.3) | 53 (22.3) | 52 (21.9) |
| Mixed Surgery-Medicine n (%) | 110 (17.5) | 58 (24.4) | 59 (24.9) |
| Neurologic ICU n (%) | 160 (25.5) | 61 (25.6) | 54 (22.8) |
| Neurosurgery ICU n (%) | 28 (4.5) | ||
SAPS II: Simplified acute physiology score; TISS-28: Therapeutic Intervention Score; ICU: Intensive care unit; IQR: interquartile range; d: day
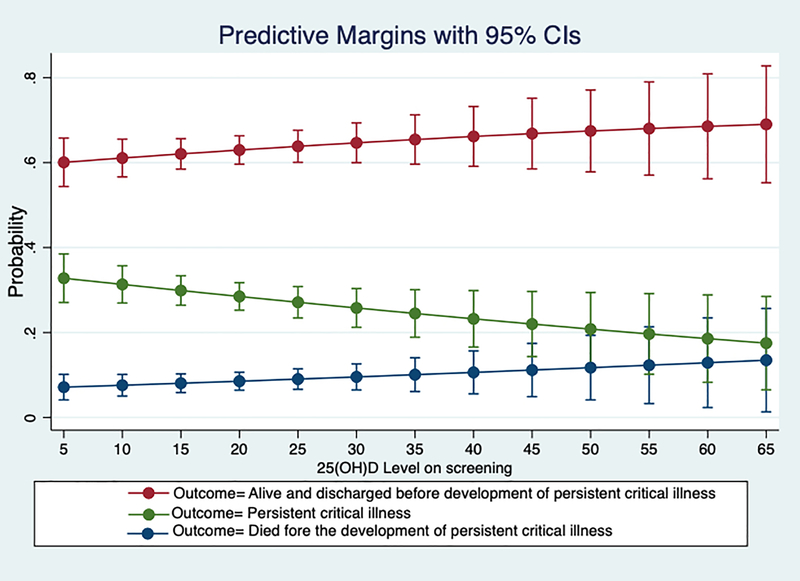
Among the retrospective cohort patients, lower TISS-28 scores and being admitted to a neurosurgical ICU were associated with patients not developing persistent critical illness and remaining alive. Vitamin D3 level on admission was not significantly associated with the development of persistent critical illness compared to those discharged alive earlier or who died before the onset of persistent critical illness. (Table 2 and Figure 1) Across the entire observed range, the incidence of PerCI fell from approximately 30% among those with levels less than 20 to approximately 20% among those with 25OHD levels greater than 50, but these apparent differences were not statistically significant or robust across multiple non-linear specifications of the vitamin D measurements. Vitamin D was also not associated with the development of persistent critical illness compared to those who did not (binary outcome variable, OR: 0.98, 95% CI: 0.96 – 1.00). When dichotomizing vitamin D3 at ≤ 12 ng/mL versus above, there was also no statistically significant association with the development of persistent critical illness compared to those discharged alive earlier or those who died before the onset of persistent critical illness in adjusted multinomial logistic regression; or compared to those who did not develop persistent critical illness in adjusted binary logistic regression. This refutes our hypothesis one (H1).
Table 2:
Logistic regression for the retrospective cohort. The comparison group was those patients who developed persistent critical illness.
| Compared to patients who developed Persistent Critical Illness | ||||
|---|---|---|---|---|
| Alive and discharged before Persistent Critical Illness | Dead before onset of Persistent Critical Illness | |||
| RRR | 95% CI | RRR | 95% CI | |
| 25-hydroxyvitamin D (per ng/mL) | 1.02 | 1.00–1.04 | 1.02 | 0.99– 1.05 |
| SAPS II (per point) | 0.99 | 0.97–1.00 | 0.992 | 0.97–1.01 |
| TISS-28 (per point) | 0.89 | 0.87–0.92 | 1.01 | 0.98–1.06 |
| Age (per year) | 1.01 | 1.00–1.03 | 1.04 | 1.01–1.06 |
| ICU Type | ||||
| Medical | 0.84 | 0.48–1.47 | 0.84 | 1.11–9.18 |
| Mixed Surgical | 1.04 | 0.55–1.98 | 2.10 | 0.64–8.71 |
| Neurologic | 1.03 | 0.52–2.03 | 2.39 | 0.07–8.71 |
| Neurosurgery | 0.07 | 0.02–0.21 | 0.37 | 0.04–3.50 |
SAPS II: Simplified acute physiology score; TISS-28: Therapeutic Intervention Score; ICU: Intensive care unit; RRR: relative risk ratio
Figure 1.
Margins plot of the adjusted association of vitamin D levels and ICU outcomes, from logistic regression of the retrospective cohort.
VITdAL study secondary analysis
In the VITdAL-ICU study, 475 patients participated and were included in our analysis. Of the 475 patients, 238 and 237 patients were in the placebo and treatment groups respectively; 98% and 96% of each arm received the intervention as randomized. In the placebo group and treatment group, 35% (n = 83) were female. The median age was 68 (IQR: 56, 76) and 64 (IQR: 56, 76) and the mean 25-hydroxyvitamin D level was 13.1ng/mL (SD: 4.3) and 13.0 ng/ mL (SD: 4.0) in the placebo and treatment groups respectively. (Table 1)
Among vitamin D deficient patients, regardless if the patient was randomized to treatment or placebo, it did not distinguish between those who would be discharged alive before the onset of persistent critical illness (RRR: 1.19 discharged alive before the development of persistent critical illness compared to those who developed persistent critical illness, 95% CI: 0.76 – 1.80) or who died before the onset of persistent critical illness (RRR 1.34 died before the onset of persistent critical illness compared to those who developed persistent critical illness, 95% CI: 0.72 – 2.52) in intention-to-treat analyses. Group assignment was also not associated with the development of persistent critical illness, measured as a dichotomous variable (OR 0.84 (95% CI: 0.57 – 1.23). Among patients with vitamin D3 at ≤ 12 ng/mL, there was also no association between randomization to treatment versus placebo with the development of persistent critical illness compared to those discharged alive earlier (RRR: 0.92, 95% CI: 0.61 – 1.40) or who died (RRR: 1.62, 95% CI: 0.85 – 3.08) in an intention-to-treat analysis. This refutes hypothesis two (H2).
Discussion
Key Findings
Despite plausible physiologic rationale, vitamin D deficiency was not associated with the development of persistent critical illness in a secondary analysis of a cohort study. In a secondary analysis of a randomized clinical trial, supplementation with vitamin D3 in critically ill vitamin D deficient patients did not mitigate the development of persistent critical illness. In both cases, the best estimate effect was itself quite close to the null, suggesting this lack of association is a truly negative finding, and not solely due to a lack of power in the study design.
Relationship to previous studies
Vitamin D deficiency is associated with sepsis, prolonged ICU stay, acute kidney injury and mortality.(1, 4, 15, 16) Whether supplementation would help mitigate the development or severity of these organ failures has remained unclear. The VITdAL-ICU trial was the first large randomized control trial to explore vitamin D supplementation in critically ill patients and although it reported no difference in the primary outcome, hospital length of stay, it was suggestive of a mortality benefit in the severely vitamin D3 deficient patients (vitamin D3 ≤ 12 ng/mL).(5) Vitamin D deficiency’s role in persistent critical illness was unknown.
Patients with persistent critical illness have prolonged ICU stays.(12, 17, 18) Their ICU courses can be defined by dynamic cascading late organ failures or non-resolving single organ failures. Recently patients with persistent critical illness were described as predominately developing dynamic cascading organ failures which was principally late cardiovascular failure and specifically septic shock.(8) In light of the known relationship between vitamin D deficiency and sepsis, it would seem plausible that vitamin D supplementation could mitigate the development of late septic shock in patients with persistent critical illness. Furthermore, given the dynamic cascade of organ failures described in persistent critical illness, and vitamin D’s association with other organ failures — acute renal failure and acute respiratory distress syndrome (ARDS)— vitamin D supplementation might have helped to mitigate these additional organ failures.(15)
Strength and Limitations
Our study is a secondary analysis and similar to any secondary data analysis is susceptible to the risks criticized as “p-hacking”. In order to minimize the risks of p-hacking in our study, we have been explicit that our examinations were intended to develop hypotheses, not provide a definitive test of them. Second, both cohorts were from a single center in Austria which may limit the generalizability of the findings to other centers and cohorts of patients. However, the cohorts did entail a diverse clinical population from different ICUs. Third, in the VITdAL-ICU trial, despite supplementation, not all participants in the trial arm reached 25-hydroxyvitamin D levels ≥ 30 ng/mL by ICU day 3 and 7.
Conclusion
Vitamin D deficiency is not associated with the development of persistent critical illness and supplementation did not mitigate the development of persistent critical illness in this secondary analysis of an existing cohort study and randomized clinical trial. This suggests that pre-existing vitamin D levels are not a major driver of persistent critical illness and prolonged ICU stay. This is consistent with the argument that a major driver of persistent critical illness is in-ICU events and intercurrent problems, rather than conditions present on admission.
Table 3:
Logistic regression for the VITdAL-ICU cohort. The comparison group was those patients who developed persistent critical illness.
| Compared to those who developed Persistent Critical Illness | ||||
|---|---|---|---|---|
| Alive and discharged before Persistent Critical Illness | Dead before onset of Persistent Critical Illness | |||
| RRR | 95% CI | RRR | 95%CI | |
| 25-hydroxyvitamin D (per ng/mL) | 1.03 | 0.98–1.08 | 0.96 | 0.89–1.04 |
| Age (per year) | 1.01 | 1.00–1.03 | 1.03 | 1.01–1.06 |
| SAPs II (per point) | 1.00 | 0.99–1.01 | 1.01 | 0.99–1.02 |
| Charlson Comorbidity Index (per point) | 0.93 | 0.84–1.04 | 1.02 | 0.88–1.18 |
| TISS-28 (per point) | 0.92 | 0.89–0.95 | 1.09 | 1.03–1.14 |
| Treatment | ||||
| Yes (vs Placebo) | 1.19 | 0.79–1.80 | 1.34 | 0.72–2.52 |
| ICU type (vs Medical ICU) | ||||
| Neuro | 1.02 | 0.53–1.99 | 0.53 | 0.20–1.43 |
| Cardiosurgical | 0.73 | 0.40–1.32 | 0.14 | 0.06–0.33 |
| Mixed Surgical ICU | 0.53 | 0.28–0.99 | 0.17 | 0.07–0.41 |
SAPS II: Simplified acute physiology score; TISS-28: Therapeutic Intervention Score; ICU: Intensive care unit; RRR: relative risk ratio
Acknowledgments
The analysis was performed at the University of Michigan in the department of internal medicine, division of pulmonary critical care.
Abbreviations
- ICU
Intensive care unit
- IQR
Interquartile Range
- SD
Standard deviation
- SAPS II
Simplified Acute Physiology Score
- TISS-28
Therapeutic Intervention Scoring System
- VITdAL-ICU
The Correction of Vitamin D Deficiency in Critically Ill Patients ICU trial
References
- 1.de Haan K, Groeneveld AB, de Geus HR, Egal M, Struijs A. Vitamin D deficiency as a risk factor for infection, sepsis and mortality in the critically ill: systematic review and meta-analysis. Crit Care. 2014;18(6):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Upala S, Sanguankeo A, Permpalung N. Significant association between vitamin D deficiency and sepsis: a systematic review and meta-analysis. BMC Anesthesiol. 2015;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P, Eisman JA, Center JR. Vitamin D deficiency in critically ill patients. N Engl J Med. 2009;360(18):1912–4. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. [DOI] [PubMed] [Google Scholar]
- 5.Amrein K, Schnedl C, Holl A, Riedl R, Christopher KB, Pachler C, et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA. 2014;312(15):1520–30. [DOI] [PubMed] [Google Scholar]
- 6.Gov. CT. Vitamin D to Improve Outcomes by Leveraging Early Treatment (VIOLET) [Available from: https://clinicaltrials.gov/ct2/show/NCT03096314.
- 7.gov Ct. The VITDALIZE Study: Effect of High-dose Vitamin D3 on 28-day Mortality in Adult Critically Ill Patients (VITDALIZE) 2018. [Available from: https://clinicaltrials.gov/ct2/show/NCT03188796. [DOI] [PMC free article] [PubMed]
- 8.Viglianti EM, Kramer R, Admon AJ, Sjoding MW, Hodgson CL, Bellomo R, Iwashyna TJ Late organ failures in patients with prolonged intensive care unit stays. J Crit Care. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amrein K, Zajic P, Schnedl C, Waltensdorfer A, Fruhwald S, Holl A, et al. Vitamin D status and its association with season, hospital and sepsis mortality in critical illness. Crit Care. 2014;18(2):R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amrein K, Schnedl C, Berghold A, Pieber TR, Dobnig H. Correction of vitamin D deficiency in critically ill patients - VITdAL@ICU study protocol of a double-blind, placebo-controlled randomized clinical trial. BMC Endocr Disord. 2012;12:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, Bellomo R. Persistent critical illness characterised by Australian and New Zealand ICU clinicians. Crit Care Resusc. 2015;17(3):153–8. [PubMed] [Google Scholar]
- 12.Iwashyna TJ, Hodgson CL, Pilcher D, Bailey M, van Lint A, Chavan S, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med. 2016;4(7):566–73. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. [DOI] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 15.Dancer RC, Parekh D, Lax S, D’Souza V, Zheng S, Bassford CR, et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70(7):617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, Nair P, Eisman JA, Center JR. Vitamin D deficiency in the intensive care unit: an invisible accomplice to morbidity and mortality? Intensive Care Med. 2009;35(12):2028–32. [DOI] [PubMed] [Google Scholar]
- 17.Iwashyna TJ, Hodgson CL, Pilcher D, Orford N, Santamaria JD, Bailey M, et al. Towards defining persistent critical illness and other varieties of chronic critical illness. Crit Care Resusc. 2015;17(3):215–8. [PubMed] [Google Scholar]
- 18.Bagshaw SM, Stelfox HT, Iwashyna TJ, Bellomo R, Zuege D, Wang X. Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med. 2018. [DOI] [PubMed] [Google Scholar]