Abstract
Background.
Tracheal surgery is uncommon, and most of the published literature consists of single-center series over large periods. Our goal was to perform a national, contemporary analysis to identify predictors of major morbidity and mortality based on indication and surgical approach.
Methods.
The Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTSD) was queried for all patients undergoing tracheal resection between 2002 and 2016. We identified 1,617 cases and compared outcomes by indication and approach. We created a multivariable model for a combined end point of mortality or major morbidity. The relationship between volume and outcome was analyzed.
Results.
The cervical approach was used 81% of the time, and benign disease was the indication in 75% of cases. Overall 30-day mortality was 1%, and no significant difference was found between the cervical and thoracic approach (1.1% versus 1.6%, p = 0.57) or between benign and malignant indications (1.1% versus 1.5%, p = 0.61). Independent factors associated with morbidity or mortality included thoracic approach, diabetes, and functional status. Centers were divided into those averaging fewer than four resections per year and those performing at least four per year. The low volume (<4) group had a combined morbidity and mortality of 27%, significantly higher than 17% observed among centers with more than four per year (p < 0.0001).
Conclusions.
STS GTSD participants perform tracheal resection for benign and malignant disease with low early morbidity and mortality. Higher operative volume is associated with improved outcome. Longer follow-up is needed to confirm airway stability and rate of reoperation.
Tracheal resection and reconstruction was pioneered by several surgeons, particularly Drs Hermes Grillo and Joel Cooper at the Massachusetts General Hospital and Dr F. Griffith Pearson at the Toronto General Hospital [1–4]. Their initial work was further developed at a number of international centers with the subsequent publication of a variety of surgical techniques [4–12]. Nevertheless, tracheal surgery remains uncommon relative to other thoracic surgical procedures and few, if any, multicenter reports have been published. The largest series published spans a treatment period of approximately 30 years [9]. As such, the modern postoperative outcomes after tracheal operations remain unclear outside of reference centers.
Therefore, our goal was to perform a contemporary, multi-institutional analysis of tracheal surgical outcomes by using The Society of Thoracic Surgeons General Thoracic Surgery Database (STS GTSD). We hypothesize that outcomes in tracheal resection vary by indication and approach. We also hypothesize that higher volume centers may have improved outcomes.
To analyze these issues, we quantified the volume of tracheal surgery being performed. Second, we analyzed outcomes by approach and indication. Third, we created a multivariable risk model for combined mortality or major morbidity. Finally, we evaluated whether the institutional volume of tracheal resection and reconstruction affected perioperative outcomes.
Patients and Methods
The STS GTSD is a voluntary, prospectively obtained and audited database that has been described elsewhere [13–15]. This study was approved by the institutional review board at Northwestern University. The data for this research were provided by The Society of Thoracic Surgeons National Database Participant User File Research Program. The analysis was performed at the investigators’ institutions.
Patient Population
The STS GTSD was queried for all patients undergoing tracheal resection and reconstruction for either benign or malignant disease between 2002 and 2016. Carinal resections were excluded.
Outcome Definitions
Thirty-day postoperative outcomes were used as defined in the STS GTSD [13–15]. A composite end point of 30-day mortality and the STS-defined variables of major morbidity was used, given the low mortality rate. Major morbidity was defined as the presence of one or more of any of the STS-defined major morbidity criteria.
Selection of Covariates
The study period included five versions (version 2.06 to version 2.3) of the STS GTSD database. We chose to include all variables for the analysis because there are little data on tracheal surgery using large databases. Consistent with previous STS GTSD analyses, for a number of discreet categorical variables, failure to code the presence of a variable was considered to be a negative response [16].
Statistical Analysis
Baseline characteristics and outcomes were summarized by using counts and percentages for categorical variables and mean and SDs for continuous variables. χ2 tests were used to compare categorical variables. Comparisons of continuous variables were performed with the two-sample t test with unequal variances and Satterthwaite’s approximation for degrees of freedom. A multivariable logistic regression model was created to identify baseline characteristics associated with the combined end point of mortality or major morbidity. The initial pool of variables considered consisted of age, sex, race, body mass index (BMI) class, American Society of Anesthesiologists (ASA) class (III, IV, or V versus I or II), Zubrod score (2,3, 4, or 5 versus 0 or 1), hypertension, diabetes, congestive heart failure, coronary artery disease, peripheral vascular disease, renal dysfunction, most recent creatinine level, steroid therapy, prior cardiothoracic operation, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and cerebrovascular disease. Significance level for entry into or exit from the model was less than or equal to 0.2. Surgical indication was included in the model a priori.
Volume and Outcome
The distribution of case volume was right skewed, with one center accounting for 29% of the total cases in the database. To assess the association between surgical volume and outcomes, hospitals were grouped into two categories: hospitals with four or more average annual surgical volume, and average annual volume fewer than four cases. Nine of 107 reporting centers performed at least four cases per year, whereas 64% of centers performed fewer than two cases per year. The combined end point of 30-day mortality or major morbidity was compared in high-and low-volume hospitals by using χ2 tests. The threshold for statistical significance was declared at two-sided 5% level. No adjustments for multiple testing were made so on average, 1 in 20 findings might be spurious.
Results
We identified 1,617 cases from 107 centers, with yearly volume shown in Figure 1 and patient preoperative characteristics in Table 1. Nine centers accounted for 50% of the volume. The cervical approach was used in 81% of cases, and benign disease was the indication in 75% of the cases. Although women made up most of the overall cohort (59%), men were more likely to require a thoracic approach and have a malignant indication. Outcomes by surgical approach and indication are presented in Table 2.
Fig 1.
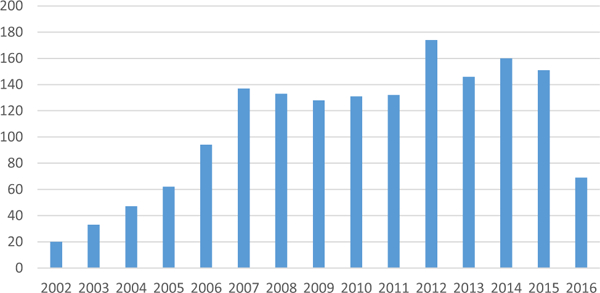
Total case volume per year.
Table 1.
Baseline Characteristics in the Entire Cohort (N = 1,617)
| Variable | No. Available |
Value |
|---|---|---|
| Age at time of operation, years | 1,617 | 50.4 ± 15.5 |
| Male | 1,616 | 669 (41) |
| Race | 1,568 | |
| Asian | 31 (2) | |
| Black | 142 (9) | |
| White | 1,317 (84) | |
| Hispanic | 18 (1) | |
| Native American | 8(1) | |
| Other | 52 (3) | |
| Height, cm | 1,412 | 167.7 ± 12.9 |
| Weight, kg | 1,452 | 84.1 ± 23.4 |
| Body mass index class, kg/m2 | 1,408 | |
| ≤18.5 | 38 (3) | |
| >18.5 and ≤25.0 | 375 (27) | |
| >25.0 and ≤30.0 | 408 (29) | |
| >30.0 and ≤35.0 | 284 (20) | |
| >35.0 | 303 (22) | |
| Smoking history (current + former) | 1,589 | 719 (45) |
| ASA class | 1,582 | |
| I | 18 (1) | |
| II | 432 (27) | |
| III | 887 (56) | |
| IV | 242 (15) | |
| V | 3(0) | |
| Zubrod score | 1,603 | |
| 0 | 151 (9) | |
| 1 | 1,190 (74) | |
| 2 | 143 (9) | |
| 3 | 77 (5) | |
| 4 | 41 (3) | |
| 5 | 1 (0) | |
| Hypertension | 1,412 | 665 (47) |
| Diabetes | 1,554 | 347 (22) |
| Most recent creatinine (mg/dL) level | 1,016 | 1.0 ± 0.9 |
| Congestive heart failure | 1,398 | 69 (5) |
| Coronary artery disease | 1,399 | 203 (15) |
| Peripheral vascular disease | 1,398 | 49 (4) |
| Renal dysfunction | 1,565 | 506 (32) |
| Oral or intravenous steroid within 24 hours | 1,394 | 89 (6) |
| Prior cardiothoracic operation | 1,406 | 336 (24) |
| Weight loss (kg) in past 3 months | 1,308 | 0.4 ± 2.3 |
| Most recent hemoglobin (g/dL) level | 1,028 | 12.9 ± 1.8 |
| COPD | 1,062 | 162 (15) |
| Interstitial lung disease | 1,061 | 4(0) |
| Pulmonary hypertension | 1,046 | 12 (1) |
| Cerebrovascular history | 1,576 | |
| Transient ischemic attack | 51 (3) | |
| Cerebrovascular accident | 71 (5) | |
| Operating room time, minutes | 1,589 | 316.4 ± 132.3 |
| Intraoperative transfusion | 1,498 | 24 (2) |
| Indication | 1,617 | |
| Malignant | 408 (25) | |
| Benign | 1,209 (75) | |
| Approach | 1,604 | |
| Thoracic | 309 (19) | |
| Cervical | 1,295 (81) | |
Values are expressed as mean ± SD or n (%).
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; No. = number.
Table 2.
Summary of Outcomes by Surgical Approach and Indication
| Surgical Approach |
Surgical Indication |
|||||
|---|---|---|---|---|---|---|
| Variable | Cervical (n = 1,295) |
Thoracic (n = 309) |
p Value |
Benign (n = 1,209) |
Malignant (n = 408) |
p Value |
| Mortality | 15 (1.1) | 5 (1.6) | 0.57 | 14 (1.1) | 6 (1.5) | 0.61 |
| Length of stay, days | 10.3 ± 14.1 | 10.6 ± 12.8 | 0.80 | 10.9 ± 15.1 | 8.9 ± 8.7 | 0.014 |
| 30-Day readmission rate | 65 (5) | 40 (13) | 0.001 | 84 (7) | 21 (5) | 0.20 |
| Major morbidity | ||||||
| Overall complication | 249 (19.2) | 93 (30.1) | 0.001 | 255 (21.1) | 89 (21.8) | 0.78 |
| Atelectasis | 37 (2.9) | 9 (2.9) | 1.00 | 35 (2.9) | 12 (2.9) | 1.00 |
| Pneumonia | 31 (2.4) | 22 (7.1) | 0.001 | 34 (2.8) | 19 (4.7) | 0.08 |
| ARDS | 4 (0.3) | 5 (1.6) | 0.016 | 5 (0.4) | 4 (1.0) | 0.24 |
| Ventilator > 48 hours | 32 (2.5) | 12 (3.9) | 0.18 | 34 (2.8) | 10 (2.5) | 0.86 |
| Pneumothorax | 4 (0.3) | 4 (1.3) | 0.049 | 2 (0.2) | 6 (1.5) | 0.004 |
| PE | 1 (0) | 2 (0.6) | 0.097 | 1 (0.1) | 2 (0.5) | 0.16 |
| Reintubation | 47 (3.6) | 16 (5.2) | 0.25 | 54 (4.5) | 10 (2.5) | 0.08 |
| Unplanned return to OR | 67 (5.2) | 25 (8.1) | 0.056 | 69 (5.7) | 23 (5.6) | 1.00 |
| BPF | 3 (0.2) | 0(0) | 1.00 | 2 (0.2) | 1 (0.2) | 1.00 |
| Tracheostomy | 41 (3.2) | 5 (1.6) | 0.18 | 43 (3.6) | 4 (1.0) | 0.006 |
| Arrhythmia | 30 (2.3) | 22 (7.1) | 0.001 | 29 (2.4) | 23 (5.6) | 0.003 |
| MI | 2 (0.2) | 0(0) | 1.00 | 2 (0.2) | 0(0) | 1.00 |
| DVT | 5 (0.4) | 8 (2.6) | 0.001 | 8 (0.7) | 5 (1.2) | 0.33 |
| UTI | 22 (1.7) | 9 (2.9) | 0.17 | 20 (1.6) | 11 (2.7) | 0.21 |
| Empyema | 0 (0) | 2 (0.6) | 0.037 | 0(0) | 2 (0.5) | 0.06 |
| Recurrent nerve injury | 12 (0.9) | 5 (1.6) | 0.35 | 7 (0.6) | 10 (2.5) | 0.003 |
| New renal failure | 8 (0.6) | 4 (1.3) | 0.26 | 10 (0.8) | 2 (0.5) | 0.74 |
| Anastomotic complication | 3 (0.2) | 3(1) | 0.09 | 1 (0.1) | 5 (1.2) | 0.005 |
| Chylothorax | 3 (0.2) | 2 (0.6) | 0.25 | 2 (0.2) | 3 (0.7) | 0.11 |
| Wound infection | 60 (4.6) | 15 (4.9) | 0.88 | 67 (5.5) | 9 (2.2) | 0.004 |
| Sepsis | 10 (0.8) | 4 (1.3) | 0.33 | 13 (1.1) | 1 (0.2) | 0.21 |
| Delirium | 14 (1) | 5 (1.6) | 0.39 | 14 (1.2) | 5 (1.2) | 1.00 |
Values are expressed as mean ± SD or n (%).
ARDS = acute respiratory distress syndrome; BPF = bronchopleural fistula; DVT = deep vein thrombosis; MI = myocardial infarction; OR = operating room; PE = pulmonary embolism; UTI = urinary tract infection.
Benign indications were approached through a cervical incision 88% of the time, whereas resections for malignancy were performed with a cervical approach 54% of the time. Overall 30-day mortality was 1%, and no statistically significant differences were found in mortality between the cervical and thoracic approach to resection (1.1% versus 1.6%) or between malignant and benign indications (1.1% and 1.5%). Benign indications were associated with a significantly longer hospital length of stay than with malignant indications (10.9 ±15.1 days versus 8.9 ± 8.7 days, p = 0.014). The overall 30-day readmission rate was 6.5%. Although no difference was found in readmission rate based on a benign or malignant indication (7% versus 5%, respectively, p = 0.20), there was a more than a doubling of readmissions after a thoracic approach to tracheal resection (13%) compared with a cervical approach (5%, p = 0.001).
The thoracic approach was associated with higher rates of complication (30.1%) than the cervical approach (19.2%, p = 0.001), in particular pneumonia (7.1% versus 2.4%, p = 0.001), arrhythmia (7.1% versus 2.3%, p = 0.001), deep vein thrombosis (2.6% versus 0.4%, p = 0.001), acute respiratory distress syndrome (1.6% versus 0.3%, p = 0.016), pneumothorax (1.3% versus 0.3%, p = 0.049), and empyema (0.6% versus 0%, p = 0.037). The cervical approach was associated with twice the rate of tracheostomy (3.2% versus 1.6%), but this difference was not statistically significant (p = 0.18).
Overall complication rates were similar between the malignant and benign cohorts (21.8% versus 21.1%, p = 0.78); however, some important differences were found with the malignant group experiencing more arrhythmias (5.6% versus 2.4%, p = 0.003), recurrent nerve injuries (2.5% versus 0.6%, p = 0.003), pneumothoraxes (1.5% versus 0.2%, p = 0.004), and anastomotic complications (1.2% versus 0.1%, p = 0.005), as well as a trend in higher incidence of pneumonia (4.7% versus 2.8%, p = 0.08). Benign indications were associated with higher rates of tracheostomy (3.6% versus 1%, p = 0.006) and wound infections (5.5% versus 2.2%, p = 0.004).
A multivariable logistic model was developed to examine the combined end point of mortality or morbidity (Table 3). A Zubrod score of 2 or higher (odds ratio [OR] 2.44, 95% confidence interval [CI]: 1.61 to 3.69, p < 0.001), thoracic approach (OR 1.65, 95% CI: 1.12 to 2.43, p = 0.011), and diabetes (OR 1.54, 95% CI: 1.04 to 2.26, p = 0.030) were all significantly associated with higher odds of 30-day mortality or complication(s). Potentially important trends toward increased odds of 30-day mortality or complications were observed for patients with known coronary artery disease, ASA class of 3 or higher, obesity, pulmonary hypertension, and male sex (Table 3).
Table 3.
Summary of Multivariable Logistic Regression Model for Morbidity or Mortality Composite End Point
| Variable | Odds Ratio (95% Confidence Interval) |
p Value |
|---|---|---|
| Zubrod score: 2, 3, 4, or 5 versus 0 or 1 | 2.44 (1.61–3.69) | <0.001 |
| Approach: thoracic versus cervical | 1.65 (1.12–2.43) | 0.011 |
| Diabetes | 1.54 (1.04–2.26) | 0.030 |
| ASA class: III, IV, or V versus I or II | 1.50 (0.95–2.38) | 0.083 |
| Coronary artery disease | 1.52 (0.95–2.41) | 0.081 |
| Obesity: BMI > 30 kg/m2 versus BMI ≤ 30 kg/m2 | 1.33 (0.94–1.87) | 0.11 |
| Most recent creatinine (mg/dL) level | 0.81 (0.63–1.04) | 0.11 |
| Pulmonary hypertension | 2.87 (0.75–10.99) | 0.12 |
| Sex: male versus female | 1.29 (0.92–1.82) | 0.14 |
| Indication: malignant versus benign | 1.19 (0.78–1.80) | 0.42 |
ASA = American Society of Anesthesiologists; index BMI = body mass index.
Nine centers averaged four or more tracheal resections per year, accounting for approximately one-half the volume of cases (Table 4). Thirty-day mortality was not different between high-and low-volume centers (p = 0.80). The higher volume centers (averaging at least four cases per year) had a significantly lower incidence of the 30-day mortality or morbidity composite end point (17%) than lower volume centers (27%; p < 0.001). Patients operated on at higher volume centers were more likely to be women with fewer comorbidities and better functional status undergoing transcervical resections for benign disease (Table 5).
Table 4.
Comparison of Incidence by Average Volume per Year
| Variable | Volume per Year ≥ 4 (n = 9) |
Volume per Year < 4 (n = 98) |
p Value |
|---|---|---|---|
| Total volume, n | 540 | 529 | |
| Total 30-day mortality, n | 7 | 8 | 0.80 |
| Total morbidity, n | 93 | 141 | |
| Total composite end point, n | 94 | 145 | |
| Composite end point incidence, % | 17.4 | 27.4 | <0.001 |
Table 5.
Baseline Characteristics by Facility Volume
| Variable | No. | Overall (n = 1,069) |
Volume per Year < 4 (n = 529) |
Volume per Year ≥4 (n = 540) |
p Value |
|---|---|---|---|---|---|
| Age at time of operation, years | 1,069 | 50.2 ± 15.5 | 51.1 ± 15.8 | 49.3 ± 15.1 | 0.06 |
| Male | 1,068 | 456 (43) | 256 (48) | 200 (37) | <0.001 |
| Race | 1,040 | 0.011 | |||
| Asian | 24 (2) | 15 (3) | 9(2) | ||
| Black | 107 (10) | 68 (13) | 39 (7) | ||
| White | 866 (83) | 406 (79) | 460 (87) | ||
| Hispanic | 1(0) | 1(0) | 0 (0) | ||
| Native American | 7 (1) | 5 (1) | 2 (0) | ||
| Other | 35 (3) | 17 (3) | 18 (3) | ||
| Height, cm | 1,024 | 167.8 ± 13.3 | 168.5 ± 11.8 | 167.1 ± 14.6 | 0.09 |
| Weight, kg | 1,034 | 84.6 ± 23.9 | 87.1 ± 25.6 | 82.1 ± 21.7 | <0.001 |
| Benign indication | 1,069 | 807 (75) | 383 (72) | 424 (79) | 0.020 |
| Cervical approach | 1,069 | 820 (77) | 364 (69) | 456 (84) | <0.001 |
| BMI (obesity: BMI > 30.0) | 1,023 | 0.07 | |||
| ≤18.5 | 28 (3) | 13 (3) | 15 (3) | ||
| >18.5 and ≤25.0 | 271 (26) | 125 (24) | 146 (29) | ||
| >25.0 and ≤30.0 | 294 (29) | 144 (28) | 150 (30) | ||
| >30.0 and ≤35.0 | 207 (20) | 106 (20) | 101 (20) | ||
| >35.0 | 223 (22) | 131 (25) | 92 (18) | ||
| Smoking history | 1,067 | 465 (44) | 257 (49) | 208 (39) | <0.001 |
| ASA class | 1,069 | <0.001 | |||
| 1 | 8(1) | 3(1) | 5(1) | ||
| 2 | 256 (24) | 64 (12) | 192 (36) | ||
| 3 | 622 (58) | 338 (64) | 284 (53) | ||
| 4 | 181 (17) | 123 (23) | 58 (11) | ||
| 5 | 2(0) | 1 (0) | 1 (0) | ||
| Zubrod score | 1,069 | <0.001 | |||
| 0 | 93 (9) | 57 (11) | 36 (7) | ||
| 1 | 803 (75) | 359 (68) | 444 (82) | ||
| 2 | 99 (9) | 67 (13) | 32 (6) | ||
| 3 | 50 (5) | 29 (5) | 21 (4) | ||
| 4 | 23 (2) | 16 (3) | 7(1) | ||
| 5 | 1 (0) | 1(0) | 0 (0) | ||
| Hypertension | 1,044 | 457 (44) | 266 (51) | 191 (36) | <0.001 |
| Diabetes | 1,044 | 251 (24) | 138 (27) | 113 (22) | 0.06 |
| Congestive heart failure | 1,041 | 44 (4) | 32 (6) | 12 (2) | 0.002 |
| Coronary artery disease | 1,041 | 127 (12) | 71 (14) | 56 (11) | 0.13 |
| Peripheral vascular disease | 1,042 | 37 (4) | 27 (5) | 10 (2) | 0.004 |
| Renal dysfunction | 1,037 | 17 (2) | 9 (2) | 8 (2) | 0.78 |
| Last creatinine (mg/dL) level | 997 | 1.0 ± 0.9 | 1.0 ± 1.0 | 0.9 ± 0.7 | 0.21 |
| Steroids | 1,040 | 52 (5) | 36 (7) | 16 (3) | 0.004 |
| Prior cardiothoracic operation | 1,040 | 190 (18) | 127 (25) | 63 (12) | <0.001 |
| Weight loss (kg) in past 3 months | 897 | 0.3 ± 1.7 | 0.3 ± 1.5 | 0.3 ± 1.8 | 0.76 |
| Last hemoglobin (g/dL) level | 1,007 | 12.9 ± 1.8 | 12.8 ± 1.9 | 13.1 ± 1.7 | 0.008 |
| COPD | 1,040 | 160 (15) | 107 (21) | 53 (10) | <0.001 |
| Interstitial lung disease | 1,039 | 4 (0) | 3 (1) | 1 (0) | 0.31 |
| Pulmonary hypertension | 1,025 | 0.24 | |||
| Yes | 11 (1) | 8 (2) | 3 (1) | ||
| No | 768 (75) | 370 (74) | 398 (76) | ||
| Not applicable | 246 (24) | 124 (25) | 122 (23) | ||
| Cerebrovascular history | 1,035 | 0.16 | |||
| No CVD history | 962 (93) | 468 (91) | 494 (94) | ||
| Transient ischemic attack | 23 (2) | 14 (3) | 9 (2) | ||
| Cerebrovascular accident | 50 (5) | 30 (6) | 20 (4) | ||
| OR time, minutesa | 1,069 | 320.6 ± 132.8 | 300.4 ± 128.2 | 340.4 ± 134.4 | <0.001 |
| Intraoperative transfusion | 1,044 | 19 (2) | 12 (2) | 7 (1) | 0.24 |
Calculated as OR exit - OR entry.
Values are expressed as mean ± SD or n (%).
ASA = American Society of Anesthesiologists; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CVD = cerebrovascular disease; No. = number; OR = operating room.
Comment
This analysis of the STS GTDB represents the largest series of tracheal resections ever published. The series is dominated by cervical approaches (81% versus 75%) to benign stenosis (75% versus 77%) [9]. This is a multi-institutional analysis of tracheal surgery outcomes and examination of a volume–outcome relationship in tracheal surgery.
It is intuitive that a thoracic approach led to a higher rate of morbidity than a cervical incision. The nearly threefold higher incidence (7.1%) of postoperative pneumonia in this group represents an opportunity for preoperative optimization.
The 3.2% to 3.6% incidence of tracheostomy in the benign and cervical groups was higher than the 2.1% reported by Wright and colleagues [9]. The data do not capture whether there was a higher rate of complex resection involving the cricoid or larynx or length of resection in these groups, so it is difficult to know the cause of a higher need for tracheostomy after resection. The extent of resection has been shown to correlate with postoperative anastomotic complications [5, 6, 9]. Because the reported anastomotic complication rate is low (<1% for all groups), we expect that some of the tracheostomies reflect actual anastomotic problems. It is possible that concern for complications with long or complex resections could lead surgeons to protect their reconstruction with a prophylactic tracheostomy.
Tracheal resections for malignancy were associated with a higher rate of specific complications in this series: pneumothorax, arrhythmia, and recurrent nerve injury. Pneumothorax and arrhythmia are more likely because resections for malignancy required a transthoracic approach in 46% of cases. The higher rate of recurrent nerve injury is likely because of the oncologic nature of these procedures that required more aggressive lateral and extraluminal margins.
The overall predictors of mortality and major morbidity derived from the STS GTSD reflect the data collected for all patients in the database. These are valuable for analysis of more commonly performed procedures such as pulmonary resections for cancer but lack the granularity for less common procedures such as tracheal resection [5, 9, 13, 14]. We did confirm the known risk that diabetes confers on tracheal surgery outcomes. We also confirmed the expected increase in adverse outcomes as performance status declines or when comparing a thoracotomy or sternotomy with a collar incision.
It was not surprising to find a volume-outcome relationship with high-volume centers being associated with improved outcomes. The technical details of these operations, anesthetic management, and postoperative care are unique, and accumulated experience may decrease the morbidity at higher volume centers. The low total volume per year and the fact that one-half of the cases were performed at centers doing fewer than four cases per year was novel. One center dominates, but comparable outcomes were also achieved at eight other centers with modest yet consistent (≥4) annual resection volumes. The patients undergoing resection at these higher volume centers were healthier with lower reported comorbid illnesses such as hypertension, congestive heart failure, peripheral vascular disease, or COPD. These differences may explain some of the lower rates of morbidity seen at higher volume centers. In addition, higher volume centers are likely accustomed to identifying problems earlier. The failure to rescue concept may allow centers to salvage patients as well as prevent certain complications. Regionalization may be premature, but the data show that 50% of cases are being done in low volume centers with adverse outcomes in more than 1 in 4 patients.
The heavy right skew and small number of cases at most centers led us to think that many of the normal assumptions in volume-outcome analyses did not apply. We chose four cases per year because of a noticeable difference in the raw data at this number. It may be an adequate volume to maintain skills for tracheal resections. Our analysis of volume and outcome is not risk adjusted, but, as stated previously, the patients at the higher volume centers generally had fewer comorbidities. We think that because 65% of centers perform fewer than two surgeries per year on average, risk adjustment models would be highly unstable and not clinically useful.
There are limitations to this analysis. The database currently does not capture many of the known risk factors for complications, of most importance, length of resection, release maneuvers, prior tracheostomy, laryngotracheal involvement, and prior resection [9]. Longer term follow-up is needed to ensure airway stability and to assess need for reintervention.
There is a need to improve the STS GTSD for less-common procedures. In recognition of this, version 2.4 of the GTSD will contain a tracheal surgery module, including data fields specific for factors such as prior intubation, tracheostomy or previous tracheal resection, preoperative airway interventions, intraoperative airway management and cardiopulmonary support strategies, length of resection, use of release maneuvers, need for postoperative airway interventions, and both 30-and 90-day airway status [17].
STS surgeons are consistently reporting 130 to 170 tracheal resections annually with low mortality but with substantial morbidity. There is an opportunity for surgeons to learn from each other through this reporting effort. Planned additions and refinements to the STS GTSD about tracheal resection and reconstruction should provide more procedure-specific and robust outcome data with longer follow-up to enhance the future care for these challenging patients.
References
- 1.Geffin B, Grillo HC, Cooper JD, Pontoppidan H. Stenosis following tracheostomy for respiratory care. JAMA 1971;216: 1984–8. [PubMed] [Google Scholar]
- 2.Grillo HC. The management of tracheal stenosis following assisted respiration. J Thorac Cardiovasc Surg 1969;57: 52–71. [PubMed] [Google Scholar]
- 3.Cooper JD, Grillo HC. The evolution of tracheal injury due to ventilatory assistance through cuffed tubes: a pathologic study. Ann Surg 1969;169:334–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson FG, Todd TR, Cooper JD. Experience with primary neoplasms of the trachea and carina. J Thorac Cardiovasc Surg 1984;88:511–8. [PubMed] [Google Scholar]
- 5.Macchiarini P, Chapelier A, Lenot B, Cerrina J, Dartevelle P. Laryngotracheal resection and reconstruction for postintubation subglottic stenosis. Lessons learned. Eur J Cardiothorac Surg 1993;7:300–5. [DOI] [PubMed] [Google Scholar]
- 6.Bibas BJ, Terra RM, Oliveira Junior AL, et al. Predictors for postoperative complications after tracheal resection. Ann Thorac Surg 2014;98:277–82. [DOI] [PubMed] [Google Scholar]
- 7.Grillo HC, Donahue DM, Mathisen DJ, Wain JC, Wright CD. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486–92; discussion 492–3. [DOI] [PubMed] [Google Scholar]
- 8.Grillo HC, Mathisen DJ, Wain JC. Laryngotracheal resection and reconstruction for subglottic stenosis. Ann Thorac Surg 1992;53:54–63. [DOI] [PubMed] [Google Scholar]
- 9.Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731–9. [DOI] [PubMed] [Google Scholar]
- 10.Grillo HC, Mathisen DJ. Primary tracheal tumors: treatment and results. Ann Thorac Surg 1990;49:69–77. [DOI] [PubMed] [Google Scholar]
- 11.Couraud L, Jougon JB, Velly JF. Surgical treatment of non-tumoral stenoses of the upper airway. Ann Thorac Surg 1995;60:250–9; discussion 259–60. [DOI] [PubMed] [Google Scholar]
- 12.Andrews MJ, Pearson FG. An analysis of 59 cases of tracheal stenosis following tracheostomy with cuffed tube and assisted ventilation, with special reference to diagnosis and treatment. Br J Surg 1973;60:208–12. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez FG, Kosinski AS, Burfeind W, et al. The Society of Thoracic Surgeons Lung Cancer Resection Risk Model: higher quality data and superior outcomes. Ann Thorac Surg 2016;102:370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozower BD, Sheng S, O’Brien SM, et al. STS database risk models: predictors of mortality and major morbidity for lung cancer resection. Ann Thorac Surg 2010;90:875–81; discussion 881–3. [DOI] [PubMed] [Google Scholar]
- 15.The Society of Thoracic Surgeons. STS National Database 2017. Available at http://www.sts.org/national-database. Accessed August 25, 2017.
- 16.Fernandez FG, Kosinski AS, Furnary AP, et al. Differential effects of operative complications on survival after surgery for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1254–64. [DOI] [PubMed] [Google Scholar]
- 17.Hoetzenecker K, Klepetko W, Keshavjee S, Cypel M. Extra-corporeal support in airway surgery. J Thorac Dis 2017;9: 2108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]


