Summary
We thought that the rate of postoperative pulmonary complications might be higher after pressure-controlled ventilation than after volume-controlled ventilation. We analysed peri-operative data recorded for 109,360 adults, whose lungs were mechanically ventilated during surgery at three hospitals in Massachusetts, USA. We used multivariable regression and propensity score matching. Postoperative pulmonary complications were more common after pressure-controlled ventilation, odds ratio (95%CI) 1.29 (1.21–1.37), p < 0.001. Tidal volumes and driving pressures were more varied with pressure-controlled ventilation compared with volume-controlled ventilation: mean (SD) variance from the median 1.61 (1.36) ml.kg–1 vs. 1.23 (1.11) ml.kg–1, p < 0.001; and 3.91 (3.47) cmH2O vs. 3.40 (2.69) cmH2O, p < 0.001. The odds ratio (95%CI) of pulmonary complications after pressure-controlled ventilation compared with volume-controlled ventilation at positive end-expiratory pressures < 5 cmH2O was 1.40 (1.26–1.55) and 1.20 (1.11–1.31) when ≥ 5 cmH2O, both p < 0.001, a relative risk ratio of 1.17 (1.03–1.33), p = 0.023. The odds ratio (95%CI) of pulmonary complications after pressure-controlled ventilation compared with volume-controlled ventilation at driving pressures of < 19 cmH2O was 1.37 (1.27–1.48), p < 0.001, and 1.16 (1.04–1.30) when ≥ 19 cmH2O, p = 0.011, a relative risk ratio of 1.18 (1.07–1.30), p = 0.016. Our data support volume-controlled ventilation during surgery, particularly for patients more likely to suffer postoperative pulmonary complications.
Keywords: lung protection ventilation, pressure goal, pressure-controlled ventilation, volume-controlled ventilation
Introduction
Mortality and morbidity are reduced in patients with acute respiratory distress syndrome by ventilating their lungs with a combination of relatively small tidal volumes, positive end-expiratory pressure (PEEP) and low plateau pressures [1]. Similar intra-operative ventilatory strategies might reduce postoperative pulmonary complications in populations without acute respiratory distress, whose lungs are often ventilated with higher volumes with little or no end-expiratory pressure [2–8].
Pulmonary ventilation, controlled by volume rather than pressure, might modify the rate of postoperative pulmonary complications, even though studies in patients with acute lung injury have not shown significant differences [9, 10]. Postoperative pulmonary complications are associated with intra-operative PEEP, ventilatory driving pressure and oxygen partial pressure [2, 11–15]. Studies have reported effects of pressure-controlled vs. volume-controlled intra-operative ventilation on physiological variables but not clinical pulmonary outcomes [16, 17].
We assessed whether the rate of pulmonary complications after pressure-controlled ventilation during surgery was higher than that after volume-controlled ventilation.
Methods
The Institutional Review Board at the Massachusetts General Hospital approved the study. We analysed data for adults whose lungs were mechanically ventilated via a tracheal tube during surgery at one of three hospitals in Massachusetts, USA between January 2007 and December 2015. We did not study patients with pulmonary complications in the seven days before surgery, those who were not extubated immediately after surgery, those with ASA physical status 6 and those with missing data.
We categorised patients by the mode of ventilation (pressure or volume control) that was used most during an operation. The primary outcome was major pulmonary complications within seven postoperative days, a composite of re-intubation, pulmonary oedema, pulmonary failure or pneumonia, as defined by the ninth and tenth revisions of the International Classification of Diseases codes and by Current Procedural Terminology codes (see also Supporting Information, Table S1) [2, 17–19]. We regressed mode of ventilation and the following covariates against the primary outcome: sex; age; body mass index; ASA physical status; chronic pulmonary disease; heart failure; the Charlson Comorbidity Index [18]; emergency surgery; high-risk surgery (neurosurgery, general, transplant, thoracic, vascular or burns) [19]; Score for Pre-operative Prediction of Obstructive Sleep Apnoea (SPOSA) [20]; surgical service; duration of surgery; surgical complexity; age-adjusted minimum alveolar concentration of inhalational anaesthetics; opioid dose in morphine equivalents; neuromuscular blocking drug dose; neostigmine dose; volumes of intra-operative intravenous fluids and blood products; PEEP; mean SPO2/ FIO2 ratio; pulmonary compliance.
We used these covariates to calculate a propensity score to minimise the effects of observed confounding by matching patients ventilated with each mode. We performed nearest-neighbour matching with a caliper of 0.1 times the standard deviation of the logit of the estimated propensity score in a 1:1 fashion using a greedy algorithm. We performed a subgroup analysis of patients who had abdominal surgery. We further analysed the risk of high tidal volume ventilation and high driving pressure ventilation.
We performed subgroup analyses of the association of postoperative pulmonary complications with mode of ventilation classified by: ASA physical status (< 3 vs. ≥ 3); SPORC (Score for Prediction of Postoperative Respiratory Complications) (< 7 vs. ≥ 7); and PEEP (< 5 cmH2O vs. ≥ 5 cmH2O). We explored whether any association of ventilatory mode and postoperative pulmonary complications interacted with high tidal volumes, high inspiratory plateau pressures and PEEP. We included these interaction terms one by one in the full regression model. We performed several sensitivity analyses, which are described in the online Supporting Information.
We used a mixed-effects logistic regression model with ventilatory mode as the exposure variable, the primary anaesthesia provider as a random effect and other covariates as fixed effect. We used complete cases for the primary analysis. We evaluated the potential for bias arising from missing data by repeating this analysis using multiple imputations by chained equations [20]. We used Stata version 13 or 14 (StataCorp LLC, TX, USA) for all analyses. We considered two-tailed p < 0.05 statistically significant.
Results
Pressure control was used to ventilate the lungs of 18,268 of 109,360 (17%) patients, of whom 18,085 were matched with 18,085 of 91,092 patients ventilated with volume control, within the caliper limit of the propensity score (Fig. 1 and Table 1). The pressures and volumes delivered by the two ventilatory modes were different: pressure-controlled ventilation delivered more varied, as well as higher, driving pressures and tidal volumes than volume-controlled ventilation (Table 2 and Fig. 2).
Figure 1.
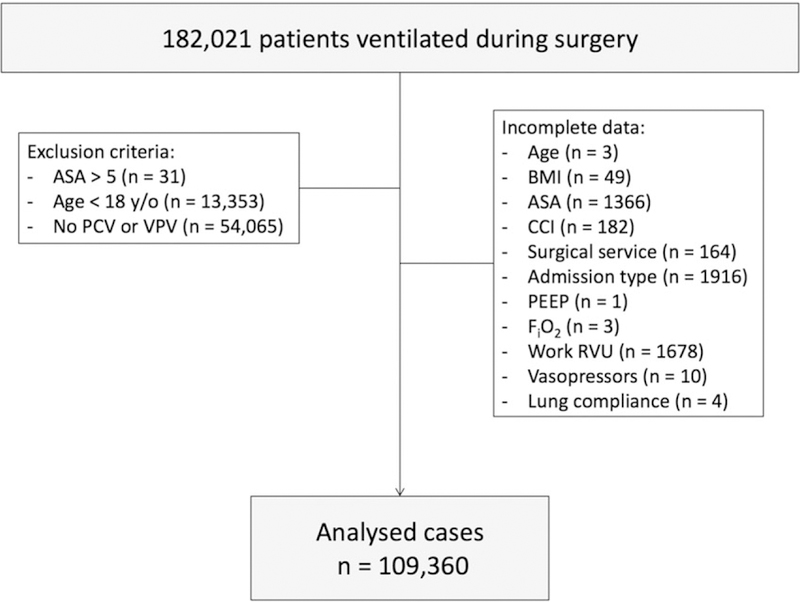
Flow of patients through study. ASA, ASA physical status; BMI, body mass index, CCI, Charlson Comorbidity Index; FIO2, inspired oxygen fraction; PEEP, positive end-expiratory pressure; PCV, pressure-controlled ventilation; RVU, relative value units; VCV, volume-controlled ventilation.
Table 1.
Baseline characteristics of 109,360 surgical patients for whom ventilation was pressure-controlled or volume-controlled. Values are mean (SD), number (proportion) or median (IQR [range]).
| Original cohort |
Propensity score-matched cohort |
|||
|---|---|---|---|---|
| Pressure n = 18,268 |
Volume n = 91,092 |
Pressure n = 18,085 |
Volume n = 18,085 |
|
| Age; years | 55.3 (15.9) | 54.5 (16.4) | 55.3 (15.5) | 54.9 (16.1) |
| Sex; female | 9770 (53.5%) | 50,599 (55.5%) | 9673 (53.5%) | 9770 (54.0%) |
| BMI | 29.7 (8.1) | 28.3 (6.8) | 29.7 (8.1) | 29.4 (7.6) |
| ASA | ||||
| < 3 | 11,721 (64.1%) | 63,409 (69.6%) | 11,624 (64.3%) | 11,696 (64.7%) |
| ≥ 3 | 6559 (35.9%) | 27,731 (30.4%) | 6461 (35.7%) | 6389 (35.3%) |
| CCI | 2 (0–4 [0–24]) | 2 (0–3 [0–26]) | 2 (0–4 [0–24]) | 2 (0–4 [0–25]) |
| COPD | 4384 (24.0%) | 14,622 (16.0%) | 4318 (23.9%) | 4060 (22.5%) |
| Heart failure | 1561 (8.5%) | 7057 (7.8%) | 1538 (8.5%) | 1530 (8.5%) |
| High-risk surgery | 9363 (51.2%) | 36,890 (40.5%) | 9178 (50.8%) | 8767 (48.5%) |
| Admission type (%) | ||||
| Ambulatory | 4318 (23.6%) | 19,788 (21.7%) | 4280 (23.7%) | 4654 (25.7%) |
| Inpatient | 3290 (18.0%) | 17,993 (19.7%) | 3251 (18.0%) | 3320 (18.4%) |
| Same day admission | 10,672 (58.4%) | 53,359 (58.6%) | 10,554 (58.4%) | 10,111 (55.9%) |
| PRBC units (%) | ||||
| 0 | 17,665 (96.6%) | 87,855 (96.4%) | 17,476 (96.6%) | 17,451 (96.5%) |
| 1 | 287 (1.6%) | 1433 (1.6%) | 282 (1.6%) | 294 (1.6%) |
| 2 | 217 (1.2%) | 1210 (1.3%) | 216 (1.2%) | 220 (1.2%) |
| > 2 | 111 (0.6%) | 642 (0.7%) | 111 (0.6%) | 120 (0.7%) |
| Neostigmine; mg | 3 (0–4 [0–10]) | 2 (0–3.5 [0–15]) | 3 (0–4 [0–10]) | 2 (0–4 [0–10]) |
| Duration of surgery; min | 155 (98–233 [17–4626]) | 157 (100–238 [8–6529]) | 155 (98–234 [17–4626]) | 148 (92–232 [15–6212]) |
| NDNMBA ED95 | 3 (2–4 [0–29]) | 2 (2–4 [0–44]) | 3 (2–4 [0–29]) | 2 (1–4 [0–42]) |
| Hypotension; min | 0 (0–2 [0–369]) | 0 (0–2 [0–659]) | 0 (0–2 [0–369]) | 0 (0–2 [0–590]) |
| Median PEEP | 4.07 (2.22) | 4.28 (2.04) | 4.08 (2.22) | 4.17 (2.17) |
| Compliance ml.cmH20 | 34.2 (12.2) | 36.8 (10.5) | 34.15 (12.14) | 34.80 (10.76) |
| MAC age adjusted | 0.82 (0.33) | 0.85 (0.30) | 0.82 (0.33) | 0.82 (0.34) |
| Work RVU | 16.97 (10.45) | 17.43 (10.81) | 16.97 (10.44) | 16.39 (10.83) |
| Fluids; ml | 1579 (2242) | 1550 (2316) | 1581 (2242) | 1572 (2264) |
| Morphine equivalent; mg | 3 (0–8 [0–200]) | 4 (0–8 [0–200]) | 3.3 (0–8 [0–200]) | 3.3 (0–8 [0–180]) |
| Noradrenaline equivalent; mg | 0.05 (0–0.29 [0–50.00]) | 0.04 (0–0.27 [0–50.00]) | 0.05 (0–0.28 [0–50.00]) | 0.04 (0–0.26 [0–50.00]) |
| S/F ratio | 62.6 (16.3) | 58.2 (13.1) | 62.5 (16.1) | 61.4 (14.9) |
| Surgical service | ||||
| Anaesthesiology | 276 (1.5%) | 2563 (2.8%) | 276 (1.5%) | 263 (1.5%) |
| Burn | 150 (0.8%) | 1183 (1.3%) | 150 (0.8%) | 166 (0.9%) |
| Cardiac | 25 (0.1%) | 395 (0.4%) | 25 (0.1%) | 35 (0.2%) |
| Emergent-urgent | 587 (3.2%) | 4039 (4.4%) | 587 (3.3%) | 579 (3.2%) |
| General surgery | 2816 (15.4%) | 17,121 (18.8%) | 2816 (15.6%) | 2901 (16.0%) |
| Gynaecology | 1372 (7.5%) | 7803 (8.6%) | 1372 (7.6%) | 1452 (8.0%) |
| Neurosurgery | 1265 (6.9%) | 10,009 (11.0%) | 1265 (7.0%) | 1219 (6.7%) |
| Oral/M axi 1 lofaci al | 329 (1.8%) | 2141(2.4%) | 329 (1.8%) | 361 (2.0%) |
| Orthopaedic | 2764 (15.1%) | 16,247 (17.8%) | 2764 (15.3%) | 2841 (15.7%) |
| Otolaryngology | 337 (1.8%) | 410 (0.5%) | 332 (1.8%) | 375 (2.1%) |
| Paediatric surgery | 62 (0.3%) | 293 (0.3%) | 62 (0.3%) | 60 (0.3%) |
| Plastic surgery | 632 (3.5%) | 4927 (5.4%) | 632 (3.5%) | 649 (3.6%) |
| Radiology | 101 (0.6%) | 964 (1.0%) | 101 (0.6%) | 111 (0.6%) |
| Surgical oncology | 986 (5.4%) | 5893 (6.5%) | 986 (5.5%) | 1058 (5.9%) |
| Thoracic | 4280 (23.4%) | 3693 (4.0%) | 4102 (22.7%) | 3591 (19.9%) |
| Transplant | 225 (1.2%) | 1691 (1.9%) | 225 (1.2%) | 231 (1.3%) |
| Urology | 1168 (6.4%) | 7798 (8.6%) | 1168 (6.4%) | 1186 (6.6%) |
| Vascular | 620 (3.4%) | 3172 (3.5%) | 620 (3.4%) | 659 (3.6%) |
| Other | 273 (1.5%) | 755 (0.8%) | 272 (1.5%) | 348 (1.9%) |
| Year of surgery | 2011 (2) | 2012 (2) | 2011 (2) | 2011 (2) |
| SPOSA | 21 (16–26 [2–47]) | 19 (15–25 [1–48]) | 21 (16–26 [2–47]) | 21 (16–26 [2–48]) |
BMI, body mass index; ASA, ASA physical status; CCI, Charlson Comorbidity Index; COPD, chronic obstructive pulmonary disease; MAC, minimum alveolar concentration (of volatile anaesthetic); NDNMBA ED95, non-depolarising neuromuscular blocking agent dose, multiples of 95% effective dose; Hypotension, minutes of mean arterial pressure < 55 mmHg; PEEP, positive end-expiratory pressure; PRBC, packed red blood cell; RVU, relative value units; S/F, mean SpO2/FIO2ratio; SPOSA, Score for Pre-operative Prediction of Obstructive Sleep Apnoea [20].
Table 2.
Intra-operative characteristics for 103,960 adults with ventilation controlled by pressure or volume. Values are median (IQR [range]) or mean (SD).
| Pressure control | Volume control | p value | |
|---|---|---|---|
| Tidal volume; ml.kg–1 | 8.0 (6.7–9.2 [1.5–27.3]) | 8.2 (7.2–9.2 [0.2–29.4]) | < 0.001 |
| Tidal volume variance | 1.61 (1.36) | 1.23 (1.11) | < 0.001 |
| Driving pressure; cmH2O | 15.7 (5.2) | 14.8 (4.3) | < 0.001 |
| Driving pressure variance | 3.9 (3.5) | 3.4 (2.7) | < 0.001 |
| PEEP | 5(3–5 [0–15]) | 5(4–5 [0–17]) | < 0.001 |
| Inspiratory pressure | |||
| Median plateau; cmH2O | 19 (16–23 [5–60]) | 18 (16–22 [5–62]) | < 0.001 |
| Median peak; cmH2O | 19 (16–23 [6–60]) | 20 (17–24 [6–66]) | < 0.001 |
| Compliance; ml.cmH2O–1 | 33 ([25–42 [5–99]) | 36 (29–43 [1–138]) | < 0.001 |
PEEP, positive end-expiratory pressure.
Figure 2.
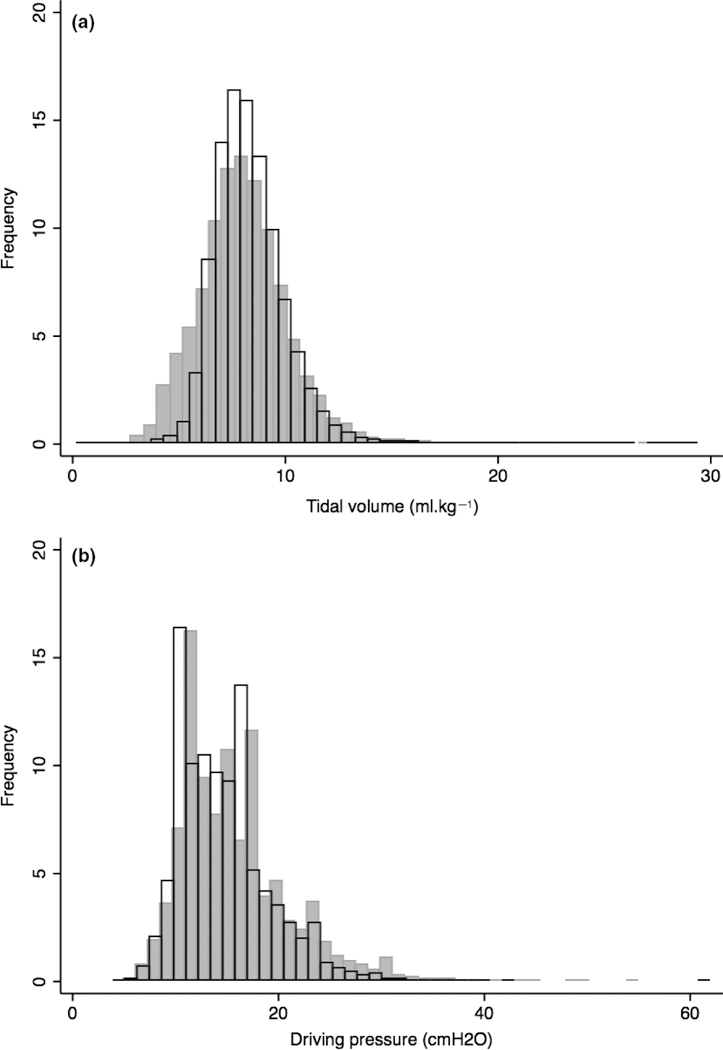
(a) The tidal volumes and (b) the driving pressures delivered by pressure-controlled ventilation ( ) and volume-controlled ventilation (
) and volume-controlled ventilation ( ) during surgery in 18,268 patients and 91,092 patients, respectively.
) during surgery in 18,268 patients and 91,092 patients, respectively.
Pulmonary complications were more common after pressure-controlled ventilation in both the unmatched cohort and that matched for propensity score (Table 3). The odds ratio (OR) (95%CI) for pulmonary complications, 1.29 (1.21–1.37), was unaffected by adjustment for anaesthesia provider, 1.28 (1.20–1.37), p = 0.87, or by imputation for missing data, 1.28 (1.21–1.36), p = 0.86.
Table 3.
The odds ratio (OR) for study outcomes in patients receiving pressure-controlled ventilation compared with volume-controlled ventilation. Values are number (proportion).
| Unmatched study cohort | ||||
|---|---|---|---|---|
| Pressure n = 18,268 |
Volume n = 91,092 |
OR 95%CI | p value | |
| Pulmonary complications | ||||
| Any | 3235 (17.7%) | 7080 (7.8%) | 1.29 (1.21–1.37) | < 0.001 |
| Re-intubation | 126 (0.69%) | 532 (0.58%) | 0.96 (0.77–1.20) | 0.7 |
| Pulmonary oedema | 1971 (10.8%) | 4637 (5.1%) | 1.21 (1.12–1.30) | < 0.001 |
| Respiratory failure | 1645 (9.0%) | 2492 (2.7%) | 1.23 (1.13–1.35) | < 0.001 |
| Pneumonia | 724 (4.0%) | 1638 (1.8%) | 1.17 (1.05–1.30) | 0.005 |
| Tidal volume > 12 ml.kg–1 | 638 (3.5%) | 2168 (2.4%) | 1.37 (1.24–1.51) | < 0.001 |
| Driving pressure > 19 cmH2O | 3749 (21%) | 12,589 (14%) | 1.26 (1.20–1.32) | < 0.001 |
| Propensity score-matched cohort | ||||
| n = 18,085 | n = 18,085 | |||
| Any pulmonary complication | 3145 (17.4%) | 2148 (11.9%) | 1.56 (1.47–1.66) | < 0.001 |
Postoperative pulmonary complications were more frequent after pressure-controlled ventilation than volumecontrolled ventilation when we categorised patients by PEEP, ASA physical status, predicted rate of pulmonary complications (SPORC) and ventilatory driving pressures (Table 4). The rates of pulmonary complications were not associated with ventilatory mode when tidal volumes exceeded 12 ml.kg–1.
Table 4.
The odds ratio (OR) for postoperative respiratory complications in patients receiving pressure-controlled ventilation compared with volume-controlled ventilation, according to pre-defined sub-groups of ventilatory pressures and susceptibility to complications. Values are number (proportion).
| Pressure vs. Volume |
Sub-group vs. Sub-group |
|||
|---|---|---|---|---|
| Variable and sub-group | OR (95%CI) | p value | RRR (95%CI) | p value |
| PEEP | ||||
| < 5 (n = 39,015) | 1.40 (1.26–1.55) | < 0.001 | 1.17 (1.02–1.33) | 0.023 |
| ≥ 5 (n = 70,344) | 1.20 (1.11-–1.31) | < 0.001 | ||
| ASA | ||||
| < 3 (n = 75,098) | 1.29 (1.16–1.43) | < 0.001 | 1.06 (0.89–1.16) | 0.82 |
| ≥ 3 (n = 34,261) | 1.27 (1.17–1.37) | < 0.001 | ||
| SPORC | ||||
| < 7 (n = 106,407) | 1.28 (1.20–1.37) | < 0.001 | 1.00 (0.79–1.27) | 1 |
| ≥ 7 (n = 106,407) ≥ 7 (n = 2952) | 1.28 (1.03–1.62) | 0.029 | ||
| Tidal volume | ||||
| < 12 ml.kg–1 (n = 105,012) | 1.32 (1.23–1.41) | < 0.001 | 1.22 (0.95–1.57) | 0.11 |
| ≥ 12 ml.kg–1 (n = 4348) | 1.08 (0.85–1.37) | 0.536 | ||
| Driving pressure | ||||
| < 19 cmH2O (n = 77,424) | 1.37 (1.27–1.48) | < 0.001 | 1.18 (1.07–1.30) | 0.016 |
| ≥ 19 cmH2O (n = 31,936) | 1.16 (1.04–1.30) | 0.011 | ||
ASA, ASA physical status; RRR, relative risk ratio; PEEP, positive end-expiratory pressure; SPORC, Score for Prediction of Postoperative Respiratory Complications [21].
The association of ventilatory mode and rates of postoperative pulmonary complications was modified by three variables, OR (95%CI): tidal volume (within two standard deviations of the mean vs. < 5 ml.kg–1 or > 12 ml.kg–1), 0.65 (0.52–0.81), p < 0.001; driving pressure (within one standard deviation of the mean vs. < 10 cmH2O or > 19 cmH2O), 0.87 (0.77–0.99), p = 0.025; and PEEP (≥ 5 cmH2O vs. < 5 cmH2O), 0.82 (0.73–0.92), p = 0.001 (see also Supporting Information, Table S4 and Fig. S2).
There were 23,222 patients (21.2%) ventilated for abdominal surgery: 3426 (14.8%) with pressure control and 19,796 (85.2%) with volume control (see also Supporting Information, Table S2). The rate of postoperative pulmonary complications was higher after pressure-controlled ventilation than volume-controlled ventilation, 1.56 (1.36–1.80), p < 0.001 (see also Supporting Information, Table S3). Pressure-controlled ventilation was more frequently associated with driving pressures > 19 cmH2O, 1.21 (1.10–1.34), p < 0.001, but not with tidal volumes >12 ml.kg~\ 1.16 (0.96–1.40), p = 0.12 (Table S3).
Discussion
We found that the rate of postoperative pulmonary complications was higher when intra-operative ventilation was controlled by pressure than when it was controlled by volume. Pressure-controlled ventilation resulted in more variable tidal volumes and driving pressures and more frequent delivery of extreme values (> 12 ml.kg–1 predicted body weight and > 19 cmH2O, respectively). The association of pressure-controlled ventilation with pulmonary complications was most marked with low PEEP.
Postoperative pulmonary complications are associated with mortality and morbidity, which contribute to surgical harm and costs [22–24]. Pulmonary postoperative complications are more frequent than any other, even in academic centres that use ‘lung-protective’ strategies [23, 25]. Some trials and systematic reviews have reported no differences in physiological measurements with ventilatory mode while others have [16, 17, 26–31]. Pulmonary system compliance changes during surgery, for instance, with patient positioning, pneumoperitoneum, abdominal content retraction or packing, and fluid infusion or loss. The tidal volumes delivered by pressure-controlled ventilation will therefore vary and may become large after a sudden increase in compliance, while volume-controlled ventilation should deliver more consistent tidal volumes. Driving pressure, which is the inspiratory plateau pressure minus the end-expiratory pressure, may be a more important determinant of lung damage than tidal volume [2, 32, 33]. Pressure-controlled ventilation delivered driving pressures > 19 cmH2O more often than volume-controlled ventilation, either because higher inspiratory pressures were set or lower expiratory pressures were set.
Values of PEEP > 5 cmH2O were associated with fewer postoperative pulmonary complications, which agree with other studies [2, 11, 12, 34]. A plausible mechanism is a reduction in cyclical collapse and opening of lung segments and subsequent inflammation, which would be accompanied by variable tidal volumes with pressure-controlled ventilation [35]. Pulmonary complications were consistently less with volume-controlled ventilation when we categorised patients by ASA physical status and patient susceptibility to postoperative pulmonary complications [19, 36].
Like any observational study, our results might be biased by unmeasured confounding factors and the misclassification of outcomes. In addition, we used a composite outcome to define ‘pulmonary failure’. However, our administrative dataset has been validated, as has the use of this outcome [19, 37], and we have no reason to think that its classification should differ with ventilatory mode [38].
We believe that appropriate PEEP and the min-imisation of driving pressure are key to reducing pulmonary damage [2]. We think that damage associated with pressure-controlled ventilation is mediated through increased strain caused by erratic driving pressures and tidal volumes [1, 32, 33, 39, 40].
In summary, we showed that rates of postoperative pulmonary complications are higher after pressure-controlled ventilation than after volumecontrolled ventilation, in part due to more variable and higher driving pressures and tidal volumes, exacerbated by low or no PEEP. Our data support volume-controlled ventilation during surgery, particularly for patients more likely to suffer postoperative pulmonary complications.
Supplementary Material
In order to ensure the stability of our findings, several additional statistical analyses were performed in addition to those reported in the primary manuscript.
Acknowledgements
We thank Chloe Gianatasio and Stephanie D. Grabitz for their help with data and statistical analysis. Matthias Eikermann has received grants from Jeffrey and Judy Buzen and Merck & Co., Inc. to conduct research. Marcos F. Vidal Melo received a grant from the National Institute of Health. Aranya Bagchi received a Discovery Award from the United States Department of Defence Peer-Reviewed Medical Research Program and he is a consultant for Lung- pacer Medical, Inc. No other external funding or competing interests declared.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
References
- 1.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database of Systematic Reviews 2013; 2: CD003844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ladha K, Vidal Melo MF, McLean DJ, et al. Intraoperative protective mechanical ventilation and risk of postoperative respiratory complications: hospital based registry study. BMJ 2015; 351: h3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guay J, Ochroch EA. Intraoperative use of low volume ventilation to decrease postoperative mortality, mechanical ventilation, lengths of stay and lung injury in patients without acute lung injury. Cochrane Database of Systematic Reviews 2015; 12: CD011151. [DOI] [PubMed] [Google Scholar]
- 4.Gu WJ, Wang F, Liu JC. Effect of lung-protective ventilation with lower tidal volumes on clinical outcomes among patients undergoing surgery: a meta-analysis of randomized controlled trials. Canadian Medical Association Journal 2015; 187: E101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intra-operative low-tidal-volume ventilation in abdominal surgery. New England Journal of Medicine 2013; 369: 428–37. [DOI] [PubMed] [Google Scholar]
- 6.Wanderer JP, Ehrenfeld JM, Epstein RH, et al. Temporal trends and current practice patterns for intraoperative ventilation at U.S. academic medical centers: a retrospective study. BMC Anesthesiology 2015; 15: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bender SP, Paganelli WC, Gerety LP, et al. Intraoperative lung-protective ventilation trends and practice patterns: a report from the Multicenter Perioperative Outcomes Group. Anesthesia and Analgesia 2015; 121: 1231–9. [DOI] [PubMed] [Google Scholar]
- 8.Ladha KS, Bateman BT, Houle T, et al. Variability in the use of protective mechanical ventilation during general anesthesia. Anesthesia and Analgesia 2017; 10.1214/ANE.0000000000002343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esteban A, Alia I, Gordo F, et al. Prospective randomized trial comparing pressure-controlled ventilation and volume-controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest 2000; 117: 1690–6. [DOI] [PubMed] [Google Scholar]
- 10.Rittayamai N, Katsios CM, Beloncle F, Friedrich JO, Mancebo J, Brochard L. Pressure-controlled vs volume-controlled ventilation in acute respiratory failure: a physiology-based narrative and systematic review. Chest 2015; 148: 340–55. [DOI] [PubMed] [Google Scholar]
- 11.de Jong MA, Ladha KS, Vidal Melo MF, et al. Differential effects of intraoperative positive end-expiratory pressure (PEEP) on respiratory outcome in major abdominal surgery versus craniotomy. Annals of Surgery 2016; 264: 362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang D, Grant MC, Stone A, Wu CL, Wick EC. A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Annals of Surgery 2016; 263: 881–7. [DOI] [PubMed] [Google Scholar]
- 13.Collier B, Vieau C, Lockhart E, et al. Provider bias impacts tidal volume selection and ventilator days in trauma patients. Journal of the American College of Surgeons 2016; 222: 527–32. [DOI] [PubMed] [Google Scholar]
- 14.Sousse LE, Herndon DN, Andersen CR, et al. High tidal volume decreases adult respiratory distress syndrome, atelectasis, and ventilator days compared with low tidal volume in pediatric burned patients with inhalation injury. Journal of the American College of Surgeons 2015; 220: 570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staehr-Rye AK, Meyhoff CS, Scheffenbichler FT, et al. High intraoperative inspiratory oxygen fraction and risk of major respiratory complications. British Journal of Anaesthesia 2017; 119: 140–9. [DOI] [PubMed] [Google Scholar]
- 16.Aldenkortt M, Lysakowski C, Elia N, Brochard L, Tramer MR. Ventilation strategies in obese patients undergoing surgery: a quantitative systematic review and meta-analysis. British Journal of Anaesthesia 2012; 109: 493–502. [DOI] [PubMed] [Google Scholar]
- 17.Sen O, Umutoglu T, Aydin N, Toptas M, Tutuncu AC, Bakan M. Effects of pressure-controlled and volume-controlled ventilation on respiratory mechanics and systemic stress response during laparoscopic cholecystectomy. Springer Plus 2016; 5: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care 2005; 43: 1130–9. [DOI] [PubMed] [Google Scholar]
- 19.Brueckmann B, Villa-Uribe JL, Bateman BT, et al. Development and validation of a score for prediction of postoperative respiratory complications. Anesthesiology 2013; 118: 1276–85. [DOI] [PubMed] [Google Scholar]
- 20.Shin CH, Grabitz SD, Timm FP, et al. Development and validation of a Score for Preoperative Prediction of Obstructive Sleep Apnea (SPOSA) and its perioperative outcomes. BMC Anesthesiology 2017; 17: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Buuren S Multiple imputation of discrete and continuous data by fully conditional specification. Statistical Methods in Medical Research 2007; 16: 219–42. [DOI] [PubMed] [Google Scholar]
- 22.Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Critical Care Medicine 2011; 39: 2163–72. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the Perioperative Research Network Investigators. JAMA Surgery 2017; 152: 157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arozullah AM, Khuri SF, Henderson WG, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Annals of Internal Medicine 2001; 135: 847–57. [DOI] [PubMed] [Google Scholar]
- 25.Khuri SF, Henderson WG, Daley J, et al. Successful implementation of the Department of Veterans Affairs’ National Surgical Quality Improvement Program in the private sector: the patient safety in surgery study. Annals of Surgery 2008; 248: 329–36. [DOI] [PubMed] [Google Scholar]
- 26.Balick-Weber CC, Nicolas P, Hedreville-Montout M, Blanchet P, Stephan F. Respiratory and haemodynamic effects of volume-controlled vs pressure-controlled ventilation during laparoscopy: a cross-over study with echocardiographic assessment. British Journal of Anaesthesia 2007; 99: 429–35. [DOI] [PubMed] [Google Scholar]
- 27.Cadi P, Guenoun T, Journois D, Chevallier JM, Diehl JL, Safran D. Pressure-controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume-controlled ventilation. British Journal of Anaesthesia 2008; 100: 709–16. [DOI] [PubMed] [Google Scholar]
- 28.Tyagi A, Kumar R, Sethi AK, Mohta M. A comparison of pressure-controlled and volume-controlled ventilation for laparoscopic cholecystectomy. Anaesthesia 2011; 66: 503–8. [DOI] [PubMed] [Google Scholar]
- 29.Gupta SD, Kundu SB, Ghose T, et al. A comparison between volume-controlled ventilation and pressure-controlled ventilation in providing better oxygenation in obese patients undergoing laparoscopic cholecystectomy. Indian Journal of Anaesthesia 2012; 56: 276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogurlu M, Kucuk M, Bilgin F, et al. Pressure-controlled vs volume-controlled ventilation during laparoscopic gynecologic surgery. Journal of Minimally Invasive Gynecology 2010; 17: 295–300. [DOI] [PubMed] [Google Scholar]
- 31.Jiang J, Li B, Kang N, Wu A, Yue Y. Pressure-controlled versus volume-controlled ventilation for surgical patients: a systematic review and meta-analysis. Journal of Cardiothoracic and Vascular Anesthesia 2016; 30: 501–14. [DOI] [PubMed] [Google Scholar]
- 32.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. New England Journal of Medicine 2015; 372: 747–55. [DOI] [PubMed] [Google Scholar]
- 33.Loring SH, Malhotra A. Driving pressure and respiratory mechanics in ARDS. New England Journal of Medicine 2015; 372: 776–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musch G, Venegas JG, Bellani G, et al. Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 2007; 106: 723–35. [DOI] [PubMed] [Google Scholar]
- 35.de Prost N, Costa EL, Wellman T, et al. Effects of ventilation strategy on distribution of lung inflammatory cell activity. Critical Care 2013; 17: R175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nafiu OO, Ramachandran SK, Ackwerh R, Tremper KK, Campbell DA Jr, Stanley JC. Factors associated with and consequences of unplanned post-operative intubation in elderly vascular and general surgery patients. European Journal of Anaesthesiology 2011; 28: 220–4. [DOI] [PubMed] [Google Scholar]
- 37.Grosse-Sundrup M, Henneman JP, Sandberg WS, et al. Intermediate acting non-depolarizing neuromuscular blocking agents and risk of postoperative respiratory complications: prospective propensity score matched cohort study. BMJ 2012; 345: e6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koch CG, Li L, Hixson E, Tang A, Phillips S, Henderson JM. What are the real rates of postoperative complications: elucidating inconsistencies between administrative and clinical data sources. Journal of the American College of Surgeons 2012; 214: 798–805. [DOI] [PubMed] [Google Scholar]
- 39.Neto AS, Simonis FD, Barbas CS, et al. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient aata Analysis. Critical Care Medicine 2015; 43: 2155–63. [DOI] [PubMed] [Google Scholar]
- 40.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. Journal of the American Medical Association 2012; 308: 1651–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In order to ensure the stability of our findings, several additional statistical analyses were performed in addition to those reported in the primary manuscript.


