Clostridium difficile is a health care-acquired organism and the causative agent of antibiotic-associated diarrhea. Its spores are implicated in fecal to oral transmission from contaminated surfaces in the health care environment due to their adherent nature. Contaminated surfaces are cleaned using high-strength chemicals to remove and kill the spores; however, despite appropriate infection control measures, there is still high incidence of C. difficile infection in patients in the United States. Our research examined the effect of a high-strength biocide on spores of C. difficile which had been spiked onto a range of clinically relevant surfaces, including isolation gowns, stainless steel, and floor vinyl. This study found that C. difficile spores were able to survive exposure to appropriate concentrations of biocide, highlighting the need to examine the effectiveness of infection control measures to prevent spore transmission and to consider the prevalence of biocide resistance when decontaminating health care surfaces.
KEYWORDS: Clostridium difficile, biocide, public health, spores, surfaces, survival, transmission
ABSTRACT
Clostridium difficile is the primary cause of antibiotic-associated diarrhea globally. In unfavorable environments, the organism produces highly resistant spores which can survive microbicidal insult. Our previous research determined the ability of C. difficile spores to adhere to clinical surfaces, finding that spores had markedly different hydrophobic properties and adherence abilities. Investigation into the effect of the microbicide sodium dichloroisocyanurate on C. difficile spore transmission revealed that sublethal concentrations increased spore adherence without reducing viability. The present study examined the ability of spores to transmit across clinical surfaces and their response to an in-use disinfection concentration of 1,000 ppm of chlorine-releasing agent sodium dichloroisocyanurate. In an effort to understand if these surfaces contribute to nosocomial spore transmission, surgical isolation gowns, hospital-grade stainless steel, and floor vinyl were spiked with 1 × 106 spores/ml of two types of C. difficile spore preparations: crude spores and purified spores. The hydrophobicity of each spore type versus clinical surface was examined via plate transfer assay and scanning electron microscopy. The experiment was repeated, and spiked clinical surfaces were exposed to 1,000 ppm sodium dichloroisocyanurate at the recommended 10-min contact time. Results revealed that the hydrophobicity and structure of clinical surfaces can influence spore transmission and that outer spore surface structures may play a part in spore adhesion. Spores remained viable on clinical surfaces after microbicide exposure at the recommended disinfection concentration, demonstrating ineffectual sporicidal action. This study showed that C. difficile spores can transmit and survive between various clinical surfaces despite appropriate use of microbicides.
IMPORTANCE Clostridium difficile is a health care-acquired organism and the causative agent of antibiotic-associated diarrhea. Its spores are implicated in fecal to oral transmission from contaminated surfaces in the health care environment due to their adherent nature. Contaminated surfaces are cleaned using high-strength chemicals to remove and kill the spores; however, despite appropriate infection control measures, there is still high incidence of C. difficile infection in patients in the United States. Our research examined the effect of a high-strength biocide on spores of C. difficile which had been spiked onto a range of clinically relevant surfaces, including isolation gowns, stainless steel, and floor vinyl. This study found that C. difficile spores were able to survive exposure to appropriate concentrations of biocide, highlighting the need to examine the effectiveness of infection control measures to prevent spore transmission and to consider the prevalence of biocide resistance when decontaminating health care surfaces.
INTRODUCTION
The anaerobic spore-forming Gram-positive bacterium Clostridium difficile is the primary cause of antibiotic-associated diarrhea globally (1). C. difficile asymptomatically forms part of the microbiota of 1% to 3% healthy adults (2, 3); however, if the microbiota of the intestine is disrupted, for example, as a result of broad-spectrum antibiotic treatment, colonization of the colon by vegetative cells of C. difficile can proceed and escalate into the onset of C. difficile infection (CDI) (4). When fulminant infection ensues, the patient will suffer from inflammation and diarrhea. Further complications of CDI include pseudomembranous colitis, sepsis, and the fatal toxic megacolon (5).
Hypervirulent PCR ribotypes such as BI/027/NAP1 have spread intercontinentally and caused epidemics in Western countries, further adding to CDI incidence (6, 7). Many reports highlight the increasing impact of CDI to public health and the associated economic burden. For example, mortality rates in the United States increased from 25 to 57 per million people for the periods 1999 to 2000 and 2006 to 2007, respectively (8). In total, approximately 14,000 deaths occurred in 2007, and this statistic increased still further with an estimated 29,300 deaths in 2011 (9). In 2008 alone, the estimated cost related to CDI within the United States to health care facilities was $4.8 billion, ignoring the additional cost to other facilities such as care homes (10). A similar pattern of statistics can be seen in England, with an increase from 1,149 C. difficile-related deaths in 2001 rising to 7,916 in 2007 (11).
In response to increasing CDI infection rates, stringent infection control procedures were implemented within hospital environments in England, which resulted in a decline in mortality to 1,487 in 2012. This figure surpasses that of methicillin-resistant Staphylococcus aureus (MRSA) and nonspecified Staphylococcus aureus infection mortality (262 in 2012) (12) and thus is still a major source of concern globally. Despite the implementation of appropriate surveillance and infection control procedures, the organism still causes significant levels of morbidity and mortality across nosocomial environments (13).
The incidence of CDI is directly affected by the ability of C. difficile to produce resistant spores which can survive on organic and inorganic surfaces for months and remain viable (6). A major source of CDI and transmission in the health care environment is through the fecal to oral route, often via the contamination of surfaces. As many as 1 × 107 spores per gram feces are released into the environment by infected patients through airborne dispersal and soiling, further adding to the bioburden (14). Possible causes of transmission include inappropriate biocide use, lack of adherence to infection control guidelines, and various standards of practice across health care facilities globally (15–17).
Chlorine-releasing agents (CRAs), namely, sodium hypochlorite (NaOCl) and sodium dichloroisocyanurate (NaDCC), are the predominant form of biocide used in health care facilities to disinfect surfaces (18). These microbicides are fast acting in aqueous solutions and are relatively inexpensive (19). Low concentrations of 50 ppm available chlorine have been shown to kill >99% of vegetative bacterial cells in vitro. In addition, when 275 ppm chlorine was applied to a clinical environment, there was a significant reduction in hospital-acquired infections from non-spore-forming bacteria (20, 21). However, the inactivation of spores requires much higher concentrations, with the current recommendation for application of NaDCC in hospitals in England being 1,000 ppm available chlorine for 10 min to deactivate spores of C. difficile and Bacillus species (22, 23). Although the working concentration of NaDCC has been shown to be effective in liquid culture (24), its application to working surfaces is less efficient for the inactivation of spores (25), and this reduced activity is exacerbated by the presence of organic substances, such as bodily fluids and feces, which have a neutralizing effect on the biocide (26). The mechanism of action of chlorine-releasing biocides is poorly understood; however, it has been suggested that their action may be due to strong oxidative ability, their effect on cell membranes, and inhibition of enzymatic reactions (27).
Our previous study showed that adherence of C. difficile spores to inorganic surfaces increased when spores were exposed to sublethal concentrations (500 ppm available chlorine) of sodium dichloroisocyanurate (27). This increase was more pronounced for strain DS1748 (002 ribotype), which is not known to produce an exosporium outer layer (28), and suggests that when spores are exposed to sublethal levels of biocide, they may inadvertently become more adherent to inorganic surfaces. The purpose of the present study was to assess the transfer ability of C. difficile spores from clinical surfaces before and after biocide exposure. The surfaces tested included hospital isolation gowns, hospital-grade stainless steel, and vinyl flooring routinely used within the United States. Spore recovery from spiked clinical surfaces was investigated using a plate transfer assay. Clinical surfaces spiked with spores were exposed to NaDCC to determine sporicidal efficacy, and the presence of spores on each clinical surface before and after NaDCC treatment was examined using scanning electron microscopy.
RESULTS
Transfer of C. difficile spores from liquid form to hospital surgical gowns.
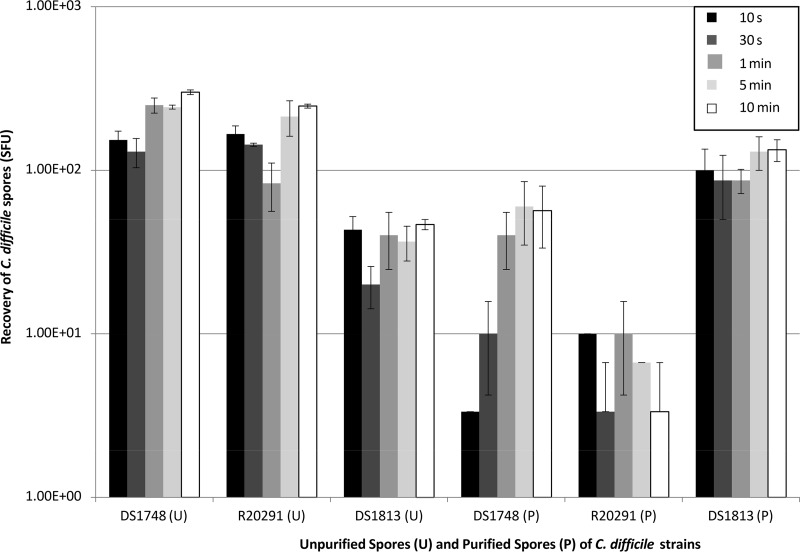
To examine the ability of C. difficile spores (unpurified [U] and purified [P] derived from strains DS1748, R20291, and DS1813) to adhere to, and subsequently transfer from, hospital surgical gowns, spores were applied directly to the surgical gowns in liquid for 10 s, 30 s, 1 min, 5 min, and 10 min before being removed and discarded (Fig. 1; see also Fig. 4A and C). This experiment was designed to mimic the transfer of infectious bodily fluids in the clinical setting and assess the potential for onward transmission to patients. There were no significant differences between the amounts of spores (U and P) recovered from the gowns and the contact times of the spores to the gowns, suggesting that the process of spore transfer between surfaces occurred within the first 10 s of contact with the gown (two-way analysis of variance [ANOVA], P = 0.696). From Fig. 1 it appears as though the recovery of DS1748 P spores increased with contact time; however, this was not statistically significant (one-way ANOVA, P = 0.144). Generally, U spore recovery was significantly higher than that of P spores (two-way ANOVA, P < 0.001); however, the exception to this trend was the increased recovery of DS1813 P spores compared to that of U spores of the same strain (one-way ANOVA, P < 0.001). There were no significant differences in spore recovery between DS1748 and R20291 for either U spores or P spores.
FIG 1.
Recovery of two different C. difficile spore types (unpurified [U] and purified [P]) from spiked hospital surgical gowns. Spores were derived from strains DS1748, R20291, and DS1813, and spores were recovered after being exposed to the gowns at contact times ranging from 10 s to 10 min. Plots represent means ± SEMs (n = 3).
FIG 4.
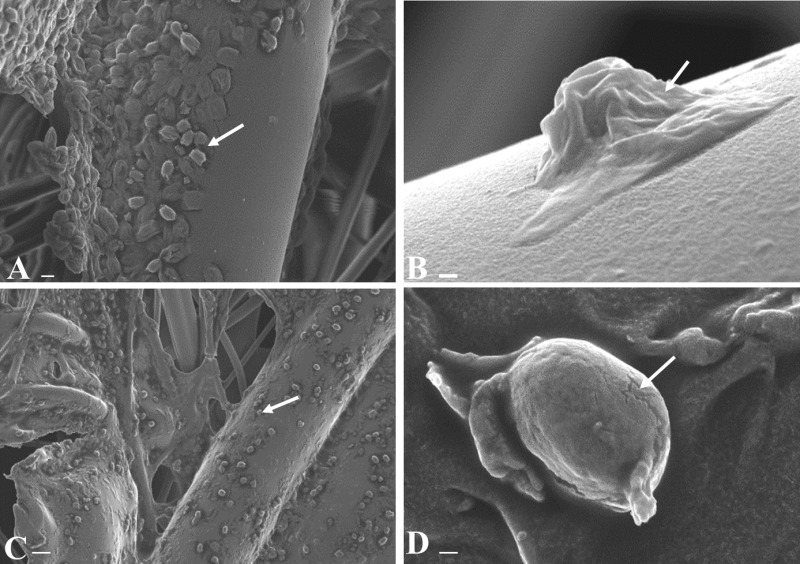
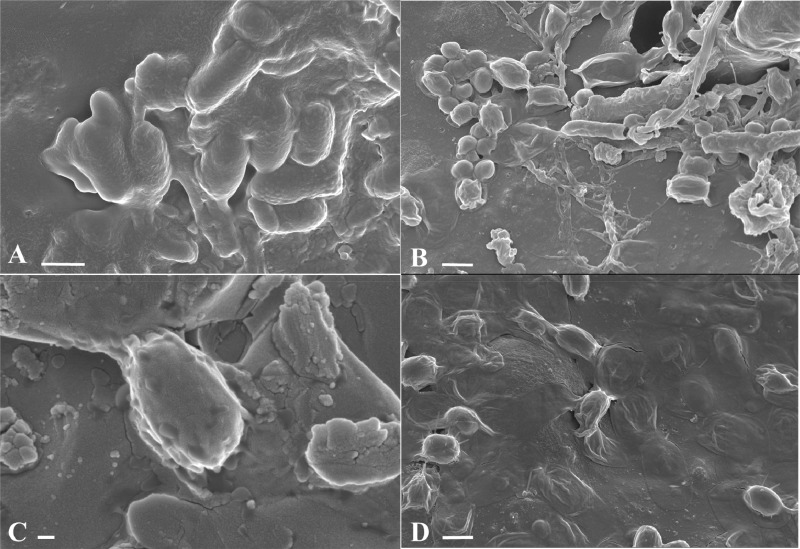
Scanning electron micrographs of C. difficile spores present on spiked hospital surgical gowns before and after treatment with NaDCC at 1,000 ppm for 10 min. Images depict untreated R20291 U spores on surgical gown fibers (A), R20291 P single spore (B), NaDCC-treated R20291 U spores on surgical gown fibers (C), and R20291 U single spore (D). Arrows highlight spores adhered to gown fibers before (A) and after (C) NaDCC treatment, and morphological changes in exosporium before (B) and after (D) NaDCC treatment. Scale bars are 200 nm in panels B and D, 2 μm in panel A, and 10 μm in panel C.
Spore recovery from spiked clinical surfaces after direct contact with hospital gowns.
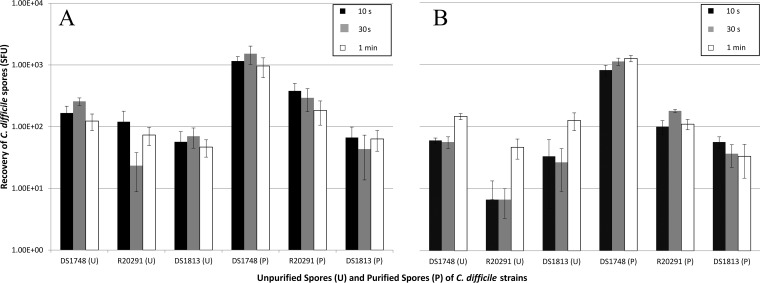
To establish whether hospital-grade stainless steel surfaces and vinyl flooring surfaces act as fomites for C. difficile spore transmission in the clinical setting, sterile sections of hospital surgical gowns were placed in direct contact with hospital-grade stainless steel and vinyl flooring spiked with 1 × 105 spores, and spore recovery from the surgical hospital gowns assessed. The contact times were reduced to 10 s, 30 s, and 1 min due to results presented in Fig. 1 which confirm that the length of contact time had no significant effect on spore recovery. Similarly, there remained no significant difference in spore recovery from steel and vinyl between the contact times used and the amounts of spores recovered from the strains examined (Fig. 2) (two-way ANOVA, P = 0.892 and P = 0.904 for steel and vinyl, respectively). Spore recovery of U DS1748 was significantly higher from both stainless steel surfaces (one-way ANOVA, P = 0.034) and vinyl flooring (one-way ANOVA, P < 0.001) than for other strains. DS1748 P spore recovery was higher on stainless steel (one-way ANOVA, P < 0.001) and vinyl flooring (one-way ANOVA, P < 0.001) than for R20291 and DS1813. DS1748 P spore recovery was approximately 10-fold higher than that of the U Spore equivalent (two-way ANOVA, P < 0.001).
FIG 2.
Transmission ability of two different C. difficile spore types between clinical surfaces. Spores derived from strains DS1748, R20291, and DS1813 were spiked onto hospital stainless steel and vinyl surfaces, and their ability to transfer to hospital surgical gowns was tested. Unpurified (U) and purified (P) spores were recovered via transfer test from hospital-grade stainless steel (A) and hospital vinyl flooring (B) using hospital surgical gowns applied at a pressure of 100 g. Contact times were 10 s, 30 s, and 1 min. Plots represent means ± SEMs (n = 3).
Sporicidal efficiency of sodium dichloroisocyanurate.
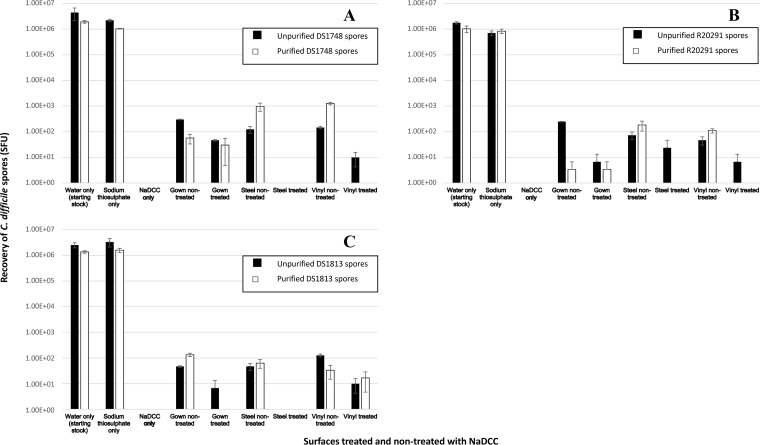
Two types of spore suspension from three C. difficile strains (DS1748, R20291, and DS1813) were exposed to the recommended in-use concentration of NaDCC in solution (1,000 ppm), and spore viability was determined. From Fig. 3 it can be seen that there was no recovery of spores which had been treated in liquid form and then spiked onto gowns. Moreover, recovery of NaDCC-treated U spores from the spiked and directly treated hospital surgical gowns was lower across the three strains tested than for the nontreated spores, with the lowest relative recovery from strain R20291 (Student’s t test, P < 0.005). Scanning electron microscopy images in Fig. 4A and C support this by showing adhered spores on the fibers of the gowns from strain R20291 before and after treatment with the recommended concentration of NaDCC. Interestingly, Fig. 4B shows a single P spore of R20291 with a visible exosporial layer after NaDCC treatment, while Fig. 4D shows a U spore of R20291 after treatment that has no visible evidence of an exosporial layer. These differences in spore exosporium show distinct morphological variations within the R20291 strain but may not necessarily be as a result of NaDCC exposure. It is possible that any damage to the exosporium after NaDCC exposure is not visible via scanning electron microscopy (Fig. 4B); thus, there is a possibility that NaDCC may have chemically altered the exosporium structure without changing the spore’s overall three-dimensional appearance (28).
FIG 3.
Recovery of unpurified and purified C. difficile spores from spiked clinical surfaces after treatment with 1,000 ppm NaDCC for 10 min. Transfer testing was used to recover U and P spores of C. difficile strains DS1748 (A), R20291 (B), and DS1813 (C) from hospital surgical gowns after contact with spores suspended in NaDCC applied to sterile gown, spiked gown exposed to NaDCC, spiked hospital stainless steel, and hospital vinyl flooring exposed to NaDCC. The inoculum was used as the positive control (water only) and was also suspended in sodium thiosulfate to ensure no cross-reactivity. Plots represent means ± SEMs (n = 3).
Decreased sporicidal activity was observed for strains tested with NaDCC on the various clinical surfaces (Fig. 3). Similar results were observed with DS1813 P spores, but not for P spores of DS1748 and R20291. There was detectable recovery of R20291 U spores (∼73 to ∼23 spore-forming units [SFU]) after NaDCC treatment on stainless steel, although this was not significantly different compared to the lack of recovery of the other U strains tested (Mann-Whitney test, P = 0.40). Despite the lack of DS1813 spore recovery from stainless steel surfaces after NaDCC exposure (Fig. 3C), spores were still present on the steel surfaces, indicating lack of viability (Fig. 5A).
FIG 5.
Scanning electron micrographs of C. difficile spores present on spiked hospital stainless steel and floor vinyl before and after treatment with NaDCC at 1,000 ppm for 10 min. Images are NaDCC-treated DS1813 P spores on stainless steel (A), DS1748 U spores on floor vinyl (B), DS1748 U spores on stainless steel (C), and R20291 U spores on floor vinyl (D). Arrows highlight areas in the exosporium layer. Scale bars are 1 μm in panels A, B, and D and 200 nm in panel C.
After NaDCC exposure, no DS1748 or R20291 P spores were recovered from the vinyl flooring, whereas U spores from these strains were recovered (Fig. 3A and B). Scanning electron microscopy results revealed the presence of spores of both types on the vinyl (Fig. 5B and D). The recovery of R20291 U spores significantly decreased (Student's t test; P = 0.001) but not that of DS1748 (Fig. 3A and B). In contrast, the recovery of both U and P spores of DS1813 did not change significantly after NaDCC treatment (Student's t test, P > 0.05 for both U spores and P spores) (Fig. 3C).
DISCUSSION
Gowns have been used by health care professionals to mitigate the risk of transmitting infectious materials between patients, hospital visitors, and other health care workers (29). Many gowns have shown differences in barrier and textile performance, and it is these variations that play a role in the dissemination of microorganisms across health care facilities (30). With the advent of modern technology, single-use isolation gowns made from fluid-resistant materials, such as polypropylene, are now widely used as a form of barrier protection; however, there is some debate as to their efficiency (29, 31, 32). Our results demonstrated that C. difficile spores were able to transfer and adhere to fibers of the polypropylene spun gowns when spiked in a liquid medium. As there was no significant difference between the contact time of the spores and the recovery of spores from the gown, it appears as though the process of spore transfer occurred rapidly, within the first 10 s of contact, when examining spore recovery from spiked liquid, hospital-grade stainless steel, and vinyl flooring. This suggests a clear need to ensure appropriate decontamination of surfaces that a contaminated gown may come into direct contact with in a clinical setting.
The ability of microorganisms to travel through fabrics is related to the physicochemical properties of the gowns and the characteristics of the microorganism (30). Another interesting observation from this study is the rapid ability of the spores to move from one hydrophobic surface to another hydrophobic surface, i.e., fluid-resistant gowns and stainless steel, which suggests that the more hydrophobic spores interacted better with the stainless steel surfaces than the gowns (Table 1, Fig. 1). Whether this is related to steel surface structure as opposed to gown structure, or the hydrophobic interactions between (i) the individual strains (which possess various relative hydrophobicity) (Table 1), (ii) the liquid, and (iii) each test surface warrants further investigation at the molecular level. It is also clear that the single-use gowns act as fomites for C. difficile spore transmission. Not only do spores of all strains rapidly attach to the gown fibers from liquid and dry clinical surfaces, but the single-use gowns are then ineffective at trapping spores within their fibers and preventing the onward transmission of spores as demonstrated by spore recovery from the gowns (Fig. 1 and 2). While this ability differs between strains, it does suggest that the adherence ability of the spore to individual gown fibers may be affected by spore hydrophobic properties and exosporium layer, which is known to aid spore adherence on hospital surfaces (Table 1) (28). Results also suggest that C. difficile spores, after microbicidal exposure to NaDCC at the recommended contact time and concentration, can continue to remain viable, adhere, and transmit via hospital gowns (Fig. 4A and C) (1, 28, 33). This highlights the importance of ensuring that single-use surgical isolation gowns are used appropriately in infection prevention and control, i.e., that gowns are adorned upon entering and disposed of when exiting a single room to prevent onward spore transmission and incidence of CDI (34).
TABLE 1.
Clostridium difficile strains used in the present study
| C. difficile strain | PCR ribotype | Source | Exosporium presence | Relative hydrophobicity (%) |
|---|---|---|---|---|
| DS1813 | 027 | Hinchingbrooke | Positive | 77 |
| R20291 | 027 | Stoke-Mandeville | Positive | 62 |
| DS1748 | 002 | Leeds | Negative | 14 |
Despite using recommended concentrations of NaDCC to decontaminate gowns, stainless steel surfaces, and floor vinyl after spore exposure, spores were still visibly attached to each surface and were viable upon culture (Fig. 4 and 5). Decontamination and appropriate cleaning of surfaces is critical in managing the spread of CDI to patients from spores (35). It can be speculated that the hydrophobic properties and weave of the gown fabric may have prevented exposure of spores to NaDCC which explains the increased spore recovery; however, this would need to be examined further by exploring the use of fluorescence-based spore viability tests (36). The smooth surfaces of steel and vinyl would theoretically make NaDCC treatment more effective by increasing the test surface area; however, the occurrence of viable spores on both treated steel and vinyl surfaces conflicts with this hypothesis and clearly evidences spore resistance to NaDCC. This resistance was found for all three strains tested and was not limited to hypervirulent R20291 027 PCR ribotype strains (7) (Table 1). Our results confirm that working concentrations of sporicides (with active concentrations of chlorine) applied at the appropriate contact times may not kill C. difficile spores. The ability of microbicides, such as CRAs, to kill C. difficile spores has been examined previously with similar results (7, 25, 26, 36).
Spores which possess an exosporium-like structure have been demonstrated to have increased adhesion to surfaces in vivo and in vitro, associated with increased hydrophobicity of the spore (28, 33, 37). The exact function of the exosporium-like structures on certain strains of C. difficile spores has yet to be fully elucidated; however, its roles in adhesion to intestinal mucosal cells and in Bacillus spore adhesion have been more clearly defined (33, 37, 38). Our previous study established that exosporium-positive spores (DS1813) were more resistant to NaDCC at sublethal concentrations than exosporium-negative spores (DS1748) (1, 28), which appears to correlate with the theory that the exosporium layer confers a protective barrier to the spore, preventing it from being damaged (39). It has also been hypothesized that exposure of spores to NaDCC at inappropriate concentrations and contact times can alter and increase spore adhesion ability (1). In the present study, while we observed a lack of exosporium-negative DS1748 and exosporium-positive DS1813 spore recovery from hospital stainless steel, scanning electron microscopy (Fig. 5A) revealed the presence of DS1813 spores adhered to the stainless steel surface and the presence of possible damaged spores of DS1748 (Fig. 5C). Indeed, the presence of a small number of spores following NaDCC treatment could still produce recovery of zero viable spores. Moreover, the viability of spores from all strains tested was also observed after NaDCC treatment of vinyl flooring (Fig. 3). This strongly indicates that recovery of spores from stainless steel and vinyl, two very different materials, has been affected by biocide exposure, either due to biocidal killing or reduced spore adherence; however, the exact mechanism of spore adherence and the biocidal activity of NaDCC on the exosporium layer and spore ultrastructure have yet to be determined.
As seen in Fig. 4 and 5, there are exosporium-like projections present on R20291 spores that increase the material surface-spore contact area, which correlates with data from other studies (39). It is possible that these projections increase spore adherence and perhaps biocide resistance by protecting the core from chemical effects. Moreover, the fact that NaDCC was completely effective when spores were exposed in liquid form (Fig. 3) compared to the spore recovery postexposure from spiked surfaces attests to the potential the exosporium has for the protection of the spore from biocide exposure. Interestingly, hypervirulent DS1813 and R20291 strains showed an increased adherence ability throughout this study comparative to that of DS1748, suggesting exosporium-positive spores adhere better and more rapidly with first contact to the test surface (Table 1). Additionally, unpurified R20291 spores were recovered from all surfaces tested after NaDCC exposure, which demonstrates the spore’s ability to remain viable after biocide exposure (Fig. 2 and 3). This concurs with previous studies that have demonstrated CRA resistance in PCR ribotypes 017, 012, and 027 (R20291) (7). Mechanical removal of spores from clinical surfaces has been shown to be effective in studies; however, this may not be appropriate with gowns, as they are designed for single use. Therefore, effective and immediate disposal of surgical gowns after use needs to be considered when preventing the transmission of CDI (6, 25). The impacts of the microbicide on spore structure and resistance warrant further research to fully understand the mechanisms of resistance and to establish up-to-date and effective decontamination protocols. Moreover, our research suggests that the C. difficile exosporium may play a key role in biocide resistance of spores and thus could be a potential target for the development of novel sporicidal disinfectants.
MATERIALS AND METHODS
Growth conditions, Clostridium difficile strains, and spore production.
C. difficile cultures were incubated anaerobically at 37°C for 48 h in a BugBox Plus anaerobic workstation (Ruskinn Technology Ltd., United Kingdom) using an 85% nitrogen, 10% carbon dioxide, and 5% hydrogen gas mix. Clinical isolates of C. difficile (PCR ribotypes 027 and 002) used in this study are described in Table 1 and were obtained from the Anaerobic Reference Unit, University Hospital Wales, Cardiff, UK. Unless otherwise stated, all organisms were stored as spores at 4°C. All experiments described were conducted in triplicates. C. difficile spores were produced according to two methods to generate unpurified/crude and purified spore preparations; spores produced via methodology described by Perez et al. (40) were designated unpurified (U) spores due to being harvested via water washing and containing vegetative and spore forms of the organism. These were deemed representative of C. difficile commonly encountered within clinical environments. Spores were produced on reduced brain heart infusion (BHI) agar and BHI broth (Oxoid Ltd., Basingstoke, UK), each supplemented with the germinant 0.1% (wt/vol) sodium taurocholate (28).
Purified spores (P spores) were produced as described by Sorg and Sonenshein (41). Briefly, C. difficile strains were cultured on reduced BHI agar with 5 g/liter yeast extract and 0.1% l-cysteine and were examined after 4 days anaerobic incubation for characteristic colonies. Spores were harvested using sucrose density washing. Spore purity was confirmed via phase-contrast microscopy. Spore concentration was determined via drop count as described by Miles et al. (42), and mean spore-forming units (SFU) per milliliter were calculated (28).
Preparation of clinical surfaces.
Single-use hospital surgical gowns were produced by MediChoice, order no. 77752XL (43), made from fluid-resistant spunbond-meltdown-spunbond (SMS) polypropylene laminate at AAMI PB70:2012 (44) standard at level 2. To test the transfer of spores to and from the gowns, gowns were aseptically cut into 7-cm by 7-cm sections, and testing was performed within a drawn circle of 2 cm in diameter to confer with the surface area of the hospital-grade 2B stainless steel discs and vinyl flooring used in this study.
Spore transfer to hospital surgical gowns.
To test the number of spores transferred to the hospital surgical gown after direct contact, U spores and P spores from strains DS1748, DS1813, and R20291 (Table 1) were produced at 1 × 104 spores/ml. From these, 100 μl was spiked onto the gown surface in triplicate experiments and allowed to remain in static contact for 10 s, 30 s, 1 min, 5 min, and 10 min before being removed and discarded. After contact with spores, each section of gown was aseptically mounted onto a plunger preaffixed with a steel disc so that the disc was aligned with the test area. A plate transfer test was then performed as described by Joshi et al. (28). A force of 100 g was used as a simulated “touch” pressure.
Spore transfer from spiked “high-touch” surfaces to hospital surgical gowns.
To test the number of spores transferred to the surgical hospital gown from dry “high-touch” surfaces (hospital-grade stainless steel and vinyl flooring), U and P spores were produced at concentrations of 1 × 106 spores/ml. Sterilized hospital-grade steel discs and vinyl flooring were inoculated with 100 μl of spores and allowed to dry completely for 120 min in a category 2 biosafety laminar flow cabinet. Sections of gown were then placed in contact with the steel and vinyl under 100 g pressure for 10 s, 30 s, and 1 min, and the gown was then pressed onto the appropriate agar plate for 10 s at 100 g pressure (28). All agar plates were then incubated for 48 h at 37°C under anaerobic conditions. Following incubation, colonies were counted and SFU per milliliter was calculated.
Exposure of spores to sodium dichloroisocyanurate disinfectant.
Spore suspensions (U and P) from strains DS1748, R20291, and DS1813 at a concentration of 1 × 106 spores per ml were exposed to 1,000 ppm NaDCC for 10 min in liquid form (recommended contact time), neutralized with sodium thiosulfate, and deposited onto sterile gowns. Spores were recovered as described previously (1, 22). Second, spores were also spiked onto the gown surface, as described in the spore transfer section above, and spores were spiked onto the surfaces of hospital stainless steel and hospital vinyl flooring for each biological repeat and allowed to dry for 120 min in a category 2 biosafety laminar flow cabinet. The three spiked surfaces were then directly exposed to 100 μl NaDCC at 1,000 ppm for 10 min and neutralized with 1% sodium thiosulfate before plate transfer experiments were performed and spore recovery recorded. Three technical repeats of each experiment were performed. Control experiments in which spores were exposed to sodium thiosulfate, sterile deionized water, and NaDCC alone were also performed.
Scanning electron microscopy.
Gowns, steel, and vinyl were analyzed using scanning electron microscopy for the presence of characteristic spores before and after treatment with NaDCC. Spores which had not been exposed to NaDCC were used as a comparative control. Test surfaces were sputter coated with metal using a gold palladium sputtering target (60% Au and 40% Pd; Testbourne Ltd.) and argon as the sputtering gas. Images were taken on a scanning electron microscope (Zeiss Sigma HD Field Emission Gun Analytical SEM) using an accelerating voltage of 5 kV. Over 100 individual spores were viewed per sample at ×4,890 and ×83,380 magnifications.
Statistical analysis.
Data are expressed as means ± standard errors of the means (SEMs). Paired t tests, one way ANOVA, 2-way ANOVA, and Mann-Whitney U tests were performed using Minitab 17.
ACKNOWLEDGMENTS
We thank Cardiff University Earth and Ocean Sciences for assistance with electron microscopy studies. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
This work was supported by the Society for Applied Microbiology Summer studentship fund and by Robert Burky. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Joshi LT, Welsch A, Hawkins J, Baillie L. 2017. The effect of hospital biocide sodium dichloroisocyanurate on the viability and properties of Clostridium difficile spores. Lett Appl Microbiol 65:199–205. doi: 10.1111/lam.12768. [DOI] [PubMed] [Google Scholar]
- 2.Fekety R, Shah AB. 1993. Diagnosis and treatment of Clostridium difficile colitis. JAMA 269:71–75. doi: 10.1001/jama.1993.03500010081036. [DOI] [PubMed] [Google Scholar]
- 3.Voth DE, Ballard JD. 2005. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson RL, Suda KJ, Evans CT. 2017. Antibiotic treatment for Clostridium difficile-associated diarrhoea in adults. Cochrane Database Syst Rev 3:CD004610. doi: 10.1002/14651858.CD004610.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamont JT, Kelly CP, Bakken JS. 2018. Clostridium difficile infection in adults: clinical manifestations and diagnosis. UpToDate, Waltham, MA. [Google Scholar]
- 6.Hellickson LA, Owens KL. 2007. Cross-contamination of Clostridium difficile spores on bed linen during laundering. Am J Infect Control 35:E2–E33. [Google Scholar]
- 7.Dawson LF, Valiente E, Donahue EH, Birchenough G, Wren BW. 2011. Hypervirulent Clostridium difficile PCR-ribotypes exhibit resistance to widely used disinfectants. PLoS One 6:e25754. doi: 10.1371/journal.pone.0025754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eyre DW, Davies KA, Davis G, Fawley WN, Dingle KE, De Maio N, Karas A, Crook DW, Peto TE, Walker AS, Wilcox MH, EUCLID Study Group. 2018. Two distinct patterns of Clostridium difficile diversity across Europe indicating contrasting routes of spread. Clin Infect Dis 67:1035–1044. doi: 10.1093/cid/ciy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. 2012. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 55:216–223. doi: 10.1093/cid/cis386. [DOI] [PubMed] [Google Scholar]
- 10.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, Wilson LE, Winston LG, Cohen JA, Limbago BM, Fridkin SK, Gerding DN, McDonald LC. 2015. Burden of Clostridium difficile infection in the United States. N Engl J Med 372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubberke ER, Olsen MA. 2012. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 55(suppl 2):S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Office for National Statistics. 2017. Deaths involving Clostridium difficile, England and Wales 1999–2012. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsinvolvingclostridiumdifficilereferencetables. Accessed 20 July 2018.
- 13.Office for National Statistics. 2014. Age-standardised rates for deaths involving Staphylococcus aureus and MRSA and number of deaths by annual registration quarter, England and Wales. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsinvolvingmrsaagestandardisedratesfordeathsinvolvingstaphylococcusaureusandmrsaandnumberofdeathsbyannualregistrationquarterenglandandwales. Accessed 20 July 2018.
- 14.Davies KA, Longshaw CM, Davies GL, Bouza E, Barbut F, Barna Z, Delmée M, Fitzpatrick F, Ivanova K, Kuijper E, Macovei IS, Mentula S, Mastrantonio P, von Müller L, Oleastro M, Petinaki E, Pituch H, Norén T, Nováková E, Nyč O, Rupnik M, Schmid D, Wilcox MH. 2014. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID). Lancet Infect Dis 14:1208–1219. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 15.Best EL, Fawley WN, Parnell P, Wilcox MH. 2010. The potential for airborne dispersal of Clostridium difficile from symptomatic patients. Clin Infect Dis 50:1450–1457. doi: 10.1086/652648. [DOI] [PubMed] [Google Scholar]
- 16.National Institute for Health and Care Excellence. 2014. Infection prevention and control. https://www.nice.org.uk/guidance/qs61. Accessed 20 July 2018.
- 17.U.S. Department of Health and Human Services Centers for Disease Control and Prevention. 2003. Guidelines for environmental infection control in health-care facilities. https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines.pdf. Accessed 20 July 2018.
- 18.World Health Organization. 2004. Practical guidelines for infection control in health care facilities. http://www.wpro.who.int/publications/docs/practical_guidelines_infection_control.pdf. Accessed 20 July 2018.
- 19.Coates D. 1996. Sporicidal activity of sodium dichloroisocyanurate, peroxygen and glutaraldehyde disinfectants against Bacillus subtilis. J Hosp Infect 32:283–294. doi: 10.1016/S0195-6701(96)90039-0. [DOI] [PubMed] [Google Scholar]
- 20.Seymour IJ, Appleton H. 2001. Foodborne viruses and fresh produce. J Appl Microbiol 91:759–773. doi: 10.1046/j.1365-2672.2001.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloomfield SF, Arthur M. 1989. Effect of chlorine‐releasing agents on Bacillus subtilis vegetative cells and spores. Lett Appl Microbiol 83:101–104. doi: 10.1111/j.1472-765X.1989.tb00233.x. [DOI] [Google Scholar]
- 22.Conlon-Bingham G, Aldeyab M, Kearney MP, Scott MG, Baldwin N, McElnay JC. 2016. Reduction in the incidence of hospital-acquired MRSA following the introduction of a chlorine dioxide 275 ppm based disinfecting agent in a district general hospital. Eur J Hosp Pharm Sci Pract 23:28–32. doi: 10.1136/ejhpharm-2014-000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Department of Health and Public Health Laboratory Service Joint Working Group. 1994. Clostridium difficile infection: prevention and management, p 1–49. BAPS Health Publications Unit, Heywood, UK. [Google Scholar]
- 24.Guest Medical. 2017. Guest Medical brochure. http://guest-medical.co.uk/wp-content/uploads/2017/09/62774-Guest-Medical-Brochure17.pdf.
- 25.Fawley WN, Underwood S, Freeman J, Baines SD, Saxton K, Stephenson K, Owens RC, Wilcox MH. 2007. Efficacy of hospital cleaning agents and germicides against epidemic Clostridium difficile strains. Infect Control Hosp Epidemiol 28:920–925. doi: 10.1086/519201. [DOI] [PubMed] [Google Scholar]
- 26.Block C. 2004. The effect of Perasafe and sodium dichloroisocyanurate (NaDCC) against spores of Clostridium difficile and Bacillus atrophaeus on stainless steel and polyvinyl chloride surfaces. J Hosp Infect 57:144–148. doi: 10.1016/j.jhin.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 27.Virto R, Mañas P, Álvarez I, Condon S, Raso J. 2005. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl Environ Microbiol 71:5022–5028. doi: 10.1128/AEM.71.9.5022-5028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi LT, Phillips DS, Williams CF, Alyousef A, Baillie L. 2012. Contribution of spores to the ability of Clostridium difficile to adhere to surfaces. Appl Environ Microbiol 78:7671–7679. doi: 10.1128/AEM.01862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutala WA, Weber DJ. 2001. A review of single-use and reusable gowns and drapes in health care. Infect Control Hosp Epidemiol 22:248–257. doi: 10.1086/501895. [DOI] [PubMed] [Google Scholar]
- 30.Kilinc Balci FS. 2016. Isolation gowns in health care settings: laboratory studies, regulations and standards, and potential barriers of gown selection and use. Am J Infect Control 44:104–111. doi: 10.1016/j.ajic.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder GM, Thorn KA, Furuno JP, Perencevich EN, Roghmann MC, Strauss SM, Netzer G, Harris AD. 2008. Detection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci on the gowns and gloves of healthcare workers. Infect Control Hosp Epidemiol 29:583–589. doi: 10.1086/588701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiener-Well Y, Galuty M, Rudensky B, Schlesinger Y, Attias D, Yinnon AM. 2011. Nursing and physician attire as possible source of nosocomial infections. Am J Infect Control 39:555–559. doi: 10.1016/j.ajic.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 33.Husmark U, Rönner U. 1992. The influence of hydrophobic, electrostatic and morphologic properties on the adhesion of Bacillus spores. Biofouling 5:335–344. doi: 10.1080/08927019209378253. [DOI] [Google Scholar]
- 34.Barra-Carrasco J, Paredes-Sabja D. 2014. Clostridium difficile spores: a major threat to the hospital environment. Future Microbiol 9:475–486. doi: 10.2217/fmb.14.2. [DOI] [PubMed] [Google Scholar]
- 35.Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. 2016. Chemical and stress resistances of Clostridium difficile spores and vegetative cells. Front Microbiol 7:1698. doi: 10.3389/fmicb.2016.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laflamme C, Lavigne S, Ho J, Duchaine C. 2004. Assessment of bacterial endospore viability with fluorescent dyes. J Appl Microbiol 96:684–692. doi: 10.1111/j.1365-2672.2004.02184.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart GC. 2015. The exosporium layer of bacterial spores: a connection to the environment and the infected host. Microbiol Mol Biol Rev 79:437–457. doi: 10.1128/MMBR.00050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora-Uribe P, Miranda-Cárdenas C, Castro-Córdova P, Gil F, Calderón I, Fuentes JA, Rodas PI, Banawas S, Sarker MR, Paredes-Sabja D. 2016. Characterization of the adherence of Clostridium difficile spores: the integrity of the outermost layer affects adherence properties of spores of the epidemic strain R20291 to components of the intestinal mucosa. Front Cell Infect Microbiol 6:99. doi: 10.3389/fcimb.2016.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leggett MJ, McDonnell G, Denyer SP, Setlow P, Maillard JY. 2012. Bacterial spore structures and their protective role in biocide resistance. J Appl Microbiol 113:485–498. doi: 10.1111/j.1365-2672.2012.05336.x. [DOI] [PubMed] [Google Scholar]
- 40.Perez J, Springthorpe VS, Sattar SA. 2005. Activity of selected oxidizing microbicides against the spores of Clostridium difficile: relevance to environmental control. Am J Infect Control 33:320–325. doi: 10.1016/j.ajic.2005.04.240. [DOI] [PubMed] [Google Scholar]
- 41.Sorg JA, Sonenshein AL. 2010. Inhibiting the initiation of Clostridium difficile spore germination using analogs of chenodeoxycholic acid, a bile acid. J Bacteriol 192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miles AA, Misra SS, Irwin JO. 1938. The estimation of the bactericidal power of blood. J Hyg (Lond) 38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MediChoice. 2015. MediChoice product catalog. Owens & Minor Inc, Richmond, VA: https://www.owens-minor.com/globalassets/docs/medichoice-product-catalog.pdf. Accessed 20 July 2018. [Google Scholar]
- 44.PB70, A.A.M.I. 2012. Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities. Association for the Advancement of Medical Instrumentation, Arlington, VA. [Google Scholar]