This study highlights an effective antimicrobial processing approach using a novel combination of lauroyl arginate ethyl (LAE) and two different physical treatments, light (UV-A) and mild heat. Both combinations demonstrated synergistic inactivation against a model Gram-negative bacterium or a Gram-positive bacterium or both by a >5-log reduction. Further mechanistic study revealed that oxidative stress is responsible for synergistic inactivation between LAE and UV-A, while both membrane damage and oxidative stress are responsible for the synergistic combination between LAE and mild heat. The mode of action of LAE was further compared to that of polymyxin B and analyzed using artificial membrane model systems and the addition of antioxidants. The proposed combination of LAE and common physical treatments may improve food preservation, food safety, and current sanitation processes for the food industry and the inactivation of pathogenic strains in biomedical environments.
KEYWORDS: lauric arginate, mild heat, oxidative stress, polymyxin B, synergism, UV-A
ABSTRACT
The need for more effective antimicrobials is critical for the food industry to improve food safety and reduce spoilage of minimally processed foods. The present study was initiated to develop an efficient and novel antimicrobial approach which combines physical treatments (UV-A or mild heat) and generally recognized as safe lauroyl arginate ethyl (LAE) to inactivate surrogate strains, including Escherichia coli and Listeria innocua. Synergistic inactivation of bacteria resulted in an ∼6-log reduction of target bacteria, while individual treatments resulted in <1.5-log inactivation under the same set of conditions. In addition, the synergistic mechanism between LAE and UV-A/mild heat was evaluated by supplementing with a variety of antioxidants for suppressing oxidative stress and measurement of cell membrane damage by nucleic acid release. These results demonstrate that the synergistic antimicrobial activity of LAE and mild physical stresses was suppressed by supplementation with antioxidants. The research also compared LAE with another membrane-targeting lipopeptide antimicrobial agent, polymyxin B, to understand the uniqueness of LAE-induced synergy. Briefly, differences in modes of action between LAE and polymyxin B were characterized by comparing the MIC, damage to liposomes, and oxidative stress generation. These differences in the mode of action between LAE and polymyxin B suggested that both compounds target cell membrane but significantly differ in mechanisms, including membrane disruption and oxidative stress generation. Overall, this study illustrates synergistic antimicrobial activity of LAE with light or mild heat and indicates a novel oxidative stress pathway that enhances the activity of LAE beyond membrane damage.
IMPORTANCE This study highlights an effective antimicrobial processing approach using a novel combination of lauroyl arginate ethyl (LAE) and two different physical treatments, light (UV-A) and mild heat. Both combinations demonstrated synergistic inactivation against a model Gram-negative bacterium or a Gram-positive bacterium or both by a >5-log reduction. Further mechanistic study revealed that oxidative stress is responsible for synergistic inactivation between LAE and UV-A, while both membrane damage and oxidative stress are responsible for the synergistic combination between LAE and mild heat. The mode of action of LAE was further compared to that of polymyxin B and analyzed using artificial membrane model systems and the addition of antioxidants. The proposed combination of LAE and common physical treatments may improve food preservation, food safety, and current sanitation processes for the food industry and the inactivation of pathogenic strains in biomedical environments.
INTRODUCTION
The need for more effective antimicrobials is critical for the food industry to improve food safety and reduce the spoilage of minimally processed foods. In the food sector, microbial spoilage results in wastage of over 30% of fresh produce (1). In addition, according to the Centers for Disease Control and Prevention, every one in six Americans gets sick, including 128,000 hospitalizations and 3,000 deaths each year, as a result of foodborne illness. Foodborne pathogens such as Escherichia coli O157 are among the leading causes of foodborne diseases that require hospitalization (2, 3). Furthermore, the emergence of bacterial resistance against sanitizers commonly used in the food industry enhances the risk of lethal infections. For example, E. coli O157 and Listeria monocytogenes strains have been reported to develop resistance to common sanitizers (4, 5). The prevalence of spoilage/pathogenic foodborne microorganisms and the emergence of sanitizer resistance among many microbes highlight a critical need to develop novel approaches to reduce/eliminate spoilage/pathogenic microorganisms in food systems.
To address the need for more effective antimicrobials, one strategy is to develop a combination of two complementary antimicrobial approaches, such as a combination of mild physical treatment and a bioactive compound. Using these approaches, recent studies have illustrated the potential to synergistically kill a variety of bacteria, including E. coli and Listeria spp. (6–9). This synergistic antimicrobial treatment approach could be promising for food and medical applications since mild physical treatments such as light, mild heat, and ultrasound are commonly used for both food processing and medical applications, and the use of food-grade compounds can reduce regulatory constraints.
Lauroyl arginate ethyl (LAE) is a generally recognized as safe (GRAS) antimicrobial preservative used in the food industry with a maximum allowable concentration of 200 ppm on a per-gram weight basis (10). LAE is potent against a broad spectrum of foodborne and clinical pathogens, including L. monocytogenes, Staphylococcus aureus, E. coli, Salmonella enterica, and Pseudomonas aeruginosa (11, 12). Several studies have demonstrated the mechanism of LAE binding and disruption of the bacterial cell membrane. This binding and disruption leads to rapid bacterial inactivation without any cell lysis (13, 14). In addition, LAE has been reported to have low toxicity, since it can be hydrolyzed in the human body into two natural components, lauric acid and l-arginine, and both components can either enter into the urea cycle or serve as a human dietary component (15). Furthermore, as a cationic surfactant, LAE has been reported to improve the efficiency of removing bacteria from leafy greens at 0.1% concentration (16).
Synergistic applications of LAE with carvacrol have been reported to reduce Salmonella in ground turkey (17). LAE was also studied in combination with leaf oil, eugenol, and cinnamon oil, which showed synergism against L. monocytogenes (14, 18). However, a minimum of 4 h was required to observe the antimicrobial synergy between LAE and essential oils (18). The relatively long treatment time suggests that the combination of LAE and essential oils may not be an ideal processing strategy. In addition, essential oils can impact the flavor of foods (19). Therefore, a more rapid and effective processing technology is evaluated here based on the synergistic combination of LAE with mild physical treatments, including UV-A and mild thermal processing.
The overall motivation in the present study was to discover a novel antimicrobial approach to control spoilage/pathogenic foodborne microorganisms. The novel approach is based on a combination of LAE as a GRAS additive with selected mild physical treatments (i.e., mild heat and light) to achieve the rapid inactivation of E. coli O157:H7 and Listeria innocua. This study also focuses on understanding the mechanism of synergy between LAE and mild physical treatments and compares the mechanistic differences between LAE and a common clinical antibiotic, polymyxin B. The current research illustrates a potential of applying a food-grade antimicrobial compound with multiple physical treatments and evaluates possible synergistic mechanisms between LAE and these physical processes.
RESULTS
Synergistic combination between LAE and light or mild heat with isobolograms.
Isobologram studies were conducted to select synergistic combination between LAE and UV-A light or mild heat using E. coli as a model system. The results presented in Fig. S1 in the supplemental material demonstrate that the combination of LAE (15 ppm) and UV-A is synergistic compared to individual treatments. Furthermore, the synergistic combination requires half the concentration of LAE and a 4-fold lower fluence level of UV-A light to achieve the same 6-log reduction of the inoculated E. coli using individual treatments. Similarly, the results in Fig. S2 demonstrate that the combination of LAE (15 ppm) and mild heat (55°C) is synergistic compared to individual treatments. Furthermore, the synergistic combination requires a 2.3-fold lower concentration of LAE and >27-fold less thermal energy to achieve the same 5-log reduction of the inoculated E. coli using individual treatments. In summary, combinations of LAE and UV-A light/mild heat under the selected conditions demonstrated synergistic inactivation against E. coli.
Synergistic inactivation of Escherichia coli and Listeria innocua using a combination of mild heat and LAE.
Figure 1a shows the inactivation kinetics of a model Gram-negative bacterium, E. coli, upon treatment with mild heat, LAE, and mild heat plus LAE. The results highlight the synergistic inactivation of E. coli using a combination treatment of mild heat and LAE in contrast to limited antimicrobial activity of mild heat or LAE treatment alone. The results show that the combined treatment of mild heat and LAE inactivated E. coli by 3-log (P < 0.05) within 1 min of treatment and an overall 5-log reduction was observed during 4 min of treatment. In contrast, LAE or mild heat alone failed to demonstrate significant reduction of E. coli within 4 min of treatment. The bacterial count in the case of synergistic treatment was statistically compared to bacterial counts after individual treatments at each time point.
FIG 1.
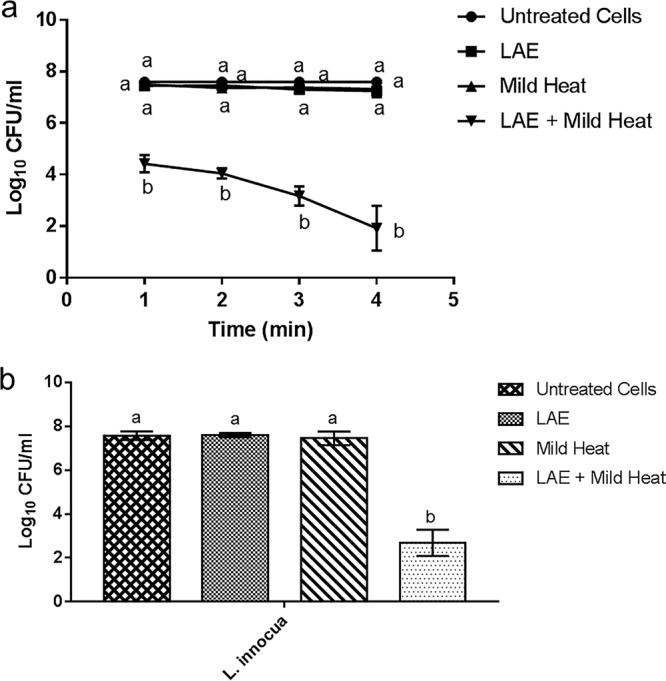
Synergistic inactivation of E. coli O157:H7 and L. innocua using a combination of LAE and mild heat. (a) Inactivation of E. coli O157:H7 treated with the combination of LAE and mild heat for 1, 2, 3, and 4 min. (b) Inactivation of L. innocua treated with the combination of LAE and mild heat for 4 min. The controls included untreated cells and single treatments with LAE or mild heat. Statistical analysis results were compared for each time point/condition. Different letters indicate significant differences among the treatments.
Synergistic combination of LAE and mild heat was also tested against a model Gram-positive bacterium, L. innocua, using the same experimental condition as tested for E. coli. As shown in Fig. 1b, 5-log antimicrobial synergy was achieved when LAE and mild heat were applied simultaneously for 4 min against L. innocua, while the mild heat or the LAE treatment alone resulted in insignificant reduction of the initial bacterial load. In summary, the results indicate significant synergistic inactivation of a model Gram-negative and a Gram-positive bacterium using a combination of mild heat and LAE.
Synergistic inactivation of E. coli and L. innocua using a combination of UV-A and LAE.
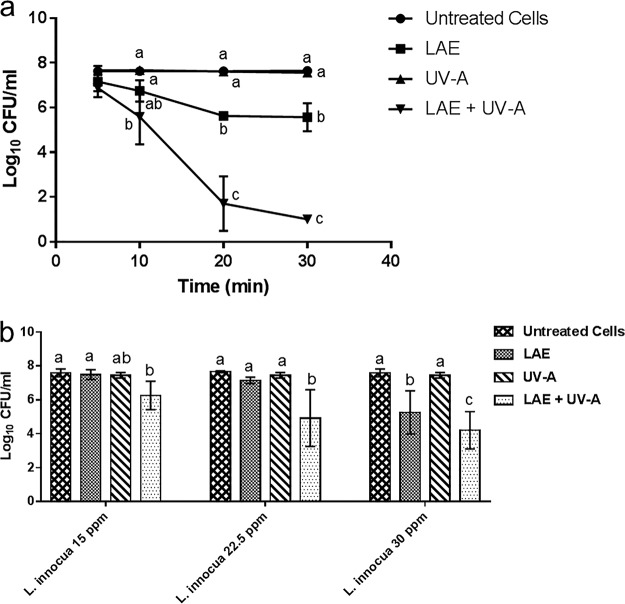
Figure 2a shows the inactivation kinetics of E. coli upon treatment with UV-A, LAE, and both UV-A and LAE at room temperature. The results highlight the synergistic inactivation of E. coli using a combination treatment of UV-A and LAE in contrast to limited antimicrobial activity of UV-A or LAE treatment alone. UV-A plus LAE reduced the E. coli population by 6.5 logs in 30 min. In contrast, UV-A treatment alone did not induce any significant inactivation of the initial inoculum, whereas LAE treatment alone inactivated 2 logs of E. coli after 30 min of incubation.
FIG 2.
Synergistic inactivation of E. coli O157:H7 and L. innocua using a combination of LAE and UV-A. (a) Inactivation of E. coli O157:H7 treated with a combination of LAE and UV-A for 5, 10, 20, and 30 min. (b) Inactivation of L. innocua treated with a combination of LAE (15, 22.5, or 30 ppm) and UV-A for 30 min. The controls included untreated cells and single treatments with LAE or UV-A. Statistical analysis results were compared for each time point/condition. Different letters indicate significant differences among the treatments.
Synergistic combination of UV-A and LAE was also tested against L. innocua for 30 min, with LAE at various final concentrations, i.e., 15, 22.5, or 30 ppm. These concentrations were selected based on similar isobologram studies for E. coli (data not shown). As shown in Fig. 2b, the antimicrobial synergies were not significant compared to individual treatments (LAE or UV-A) at any tested concentrations except for the 22.5-ppm LAE concentration. In summary, the results illustrate significant synergistic inactivation of a model Gram-negative bacterium in contrast to reduced synergism in antimicrobial activity observed in the case of a model Gram-positive bacterium using a combination of LAE and UV-A.
Nucleic acid leakage assay.
Nucleic acid release induced by mild heat and LAE against E. coli is shown in Fig. 3a. Bacterial cells treated only with LAE did not cause release of nucleic acids compared to the blank sample (P > 0.05). In contrast, cells treated with mild heat alone or in combination with LAE significantly increased nucleic acid release over time (4 min) (P < 0.05). The statistical comparison illustrated that the combination of LAE with mild heat significantly (P < 0.05) increased the leakage of nucleic acids compared to mild heat treatment alone.
FIG 3.
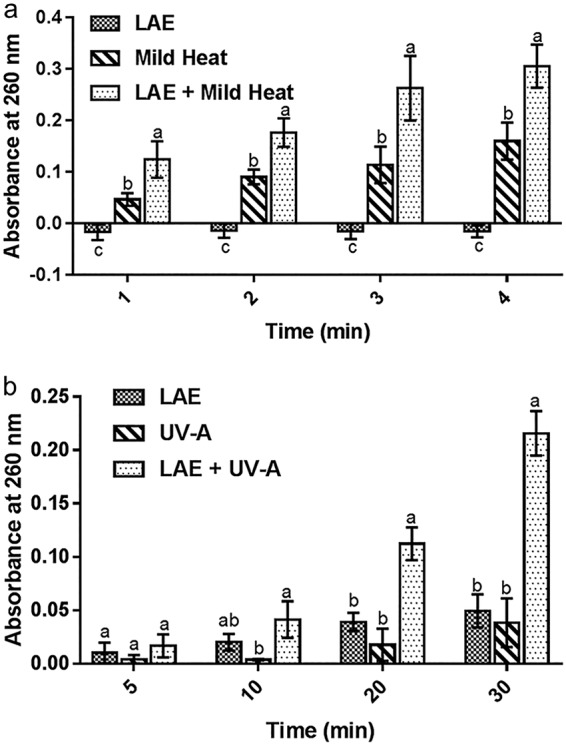
Nucleic acid release from E. coli cells. (a) Nucleic acid leakage measured for individual treatments, i.e., mild heat or LAE, and a combination of mild heat and LAE treatment using absorbance measurement at 260 nm. (b) Nucleic acid leakage measured for individual treatments with UV-A or LAE and for the combination treatment of UV-A plus LAE using an absorbance measurement at 260 nm. Statistical analysis was conducted at each time point.
As shown in Fig. 3b, neither UV-A nor LAE significantly increased nucleic acid leakage during the 30-min treatment against E. coli. In comparison, UV-A plus LAE significantly (P < 0.05) increased nucleic acid release at 20 and 30 min, suggesting increased bacterial cell membrane permeabilization over time. The increased release of nucleic acid absorbance is expected since enhanced inactivation of cells by the combination of LAE and UV-A may result in increased permeability of the bacterial cell membrane (20).
Role of oxidative stress in synergistic inactivation of LAE and UV-A against E. coli.
The results presented in Fig. 4a show that antimicrobial synergy between UV-A and LAE was completely depleted upon treatment with glutathione. Similarly, treatment of cells with other antioxidants, such as ascorbic acid or thiourea, significantly reduced the synergy between UV-A and LAE compared to the controls without antioxidants. These results suggested that oxidative stress is one of the major mechanisms responsible for the observed antimicrobial synergy between UV-A and LAE.
FIG 4.
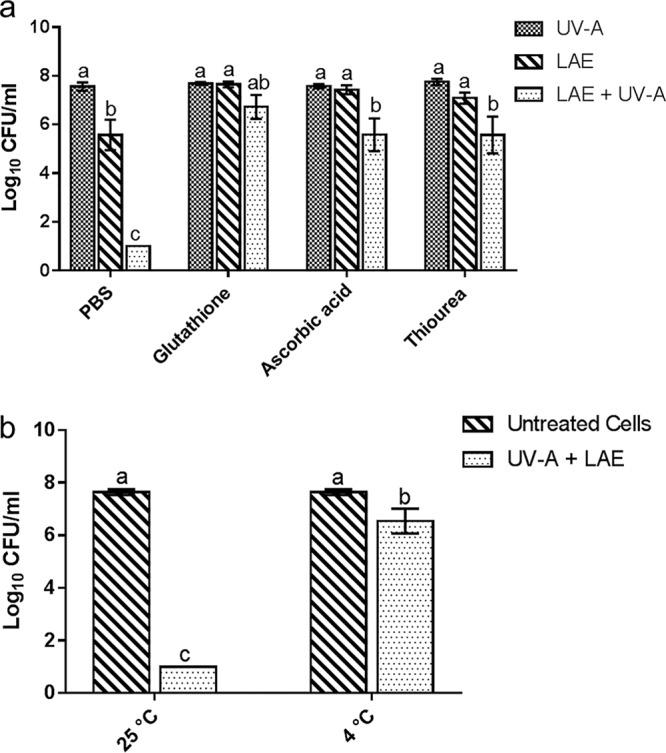
Influence of antioxidants or temperature on the synergistic antimicrobial activity of the combination of UV-A and LAE. (a) Reduction in the synergistic activity of UV-A and LAE upon the addition of glutathione, ascorbic acid, or thiourea at room temperature. (b) Influence of temperature (without antioxidants) on synergistic antimicrobial activity of UV-A and LAE. Different letters indicate significant differences among the treatments.
In addition, antimicrobial synergy between UV-A and LAE was associated with bacterial cell metabolism. As shown in Fig. 4b, antimicrobial synergy was reduced when bacterial cells were treated by the combination of LAE and UV-A at 4°C compared to the same treatment at room temperature. This result suggests that the observed antimicrobial synergy was dependent on the active metabolic state of bacterial cells and that the oxidative stress induced by the synergistic combination of LAE and UV-A was potentially dependent on the metabolic activity of cells.
Investigation of synergistic mechanism between LAE and mild heat against E. coli.
To evaluate the role of oxidative stress in the observed synergistic interactions between LAE and mild heat, the combination of antioxidants with LAE and mild heat was tested using an approach similar to that described for Fig. 5 . As shown in Fig. 5, the synergistic interaction between mild heat and LAE decreased significantly (P < 0.05) in the presence of glutathione compared to the controls without glutathione. The level of bacterial inactivation with glutathione was significantly reduced at 1, 3, and 4 min compared to the controls. Similarly, the level of inactivation of bacteria was also significantly (P < 0.05) reduced in the presence of ascorbic acid at 4 min compared to the controls without ascorbic acid. In contrast to the results obtained with ascorbic acid and glutathione, the addition of thiourea enhanced the antimicrobial synergy between LAE and mild heat at 2, 3, and 4 min. In summary, oxidative stress may have a role in the synergistic antimicrobial activity generated by the combination of mild heat and LAE.
FIG 5.
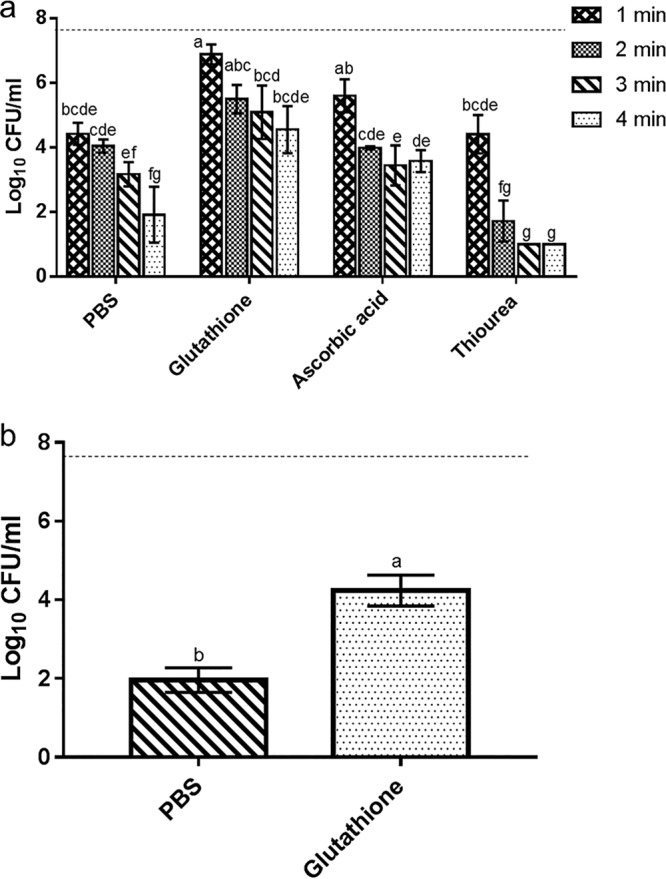
Reduction in the synergistic activity of mild heat- and LAE-treated E. coli for 1 to 4 min (a) or L. innocua for 4 min (b) in the presence of the selected antioxidant. The controls (PBS) were treated with mild heat plus LAE without the presence of any antioxidants. Different letters indicate significant differences among the treatments. The dotted line indicates the inoculum size.
Inactivation of E. coli by polymyxin B combined with physical treatments (UV-A and mild heat).
Polymyxin B was selected in this study to verify the observed synergistic combination between amino acid-based antimicrobial compounds and mild physical stress. The rationale for selecting polymyxin B is presented in Materials and Methods. Polymyxin B was combined with either UV-A or mild heat to investigate possible antimicrobial synergies. As shown in Fig. 6, the combination of polymyxin B and UV-A at 30 min induced a 1.2-log reduction in the inoculated E. coli population, which is significantly less (P < 0.05) than the synergistic inactivation of bacteria achieved using a combination of LAE and UV-A (>6.5-log reduction) under the same set of experimental conditions. In addition, no antimicrobial synergy between polymyxin B and mild heat was observed at 4 min (P > 0.05). This result is in contrast to the 5.5-log inactivation of E. coli by synergistic treatment with a combination of LAE and mild heat under the same set of experimental conditions. As indicated by the results shown in Fig. 6, permeabilization of the bacterial cell membrane may not be the only factor responsible for antimicrobial synergy between LAE and multiple physical treatments. These observations led to further evaluation of the unique factors that are required for the synergistic antimicrobial effect observed using a combination of LAE with mild physical treatments (UV-A and mild heat) in E. coli. Since polymyxin B alone does not induce any significant bactericidal activity, the data obtained with polymyxin B alone are not presented in Fig. 6.
FIG 6.
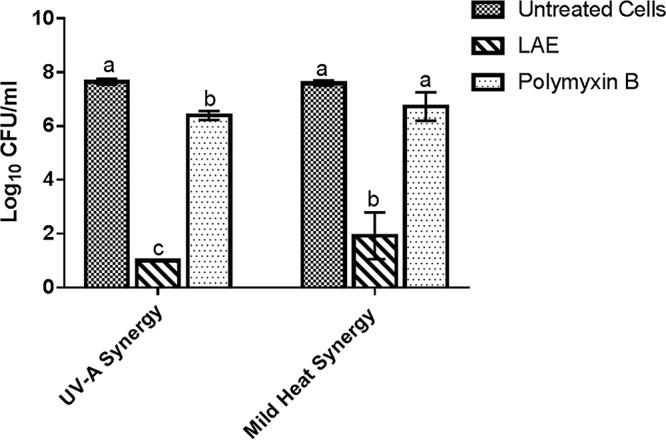
Comparison of microbial inactivation of E. coli O157:H7 cells using synergistic combinations of LAE with physical treatments and of polymyxin B with physical treatments. The control included untreated cells. Different letters indicate significant differences among the treatments.
Comparison between polymyxin B and LAE to understand the uniqueness of LAE-induced synergy.
Since polymyxin B and LAE demonstrated different levels of synergistic interaction with physical treatments (UV-A or mild heat), several experiments were conducted to characterize differences in the antimicrobial mechanisms of LAE and polymyxin B. First, the MICs of these antimicrobial compounds were compared using E. coli O157:H7 as a model target strain. The MIC values for LAE and polymyxin B were 15 and 3.75 μg/ml, respectively. Based on the MIC, polymyxin B can be considered a more potent antimicrobial bacteriostatic compound than LAE. In summary, despite stronger bacteriostatic activity, polymyxin B fails to exhibit an increased level of antimicrobial synergy with physical treatments compared to LAE.
Both LAE and polymyxin B were then analyzed for their mode of action on a model cell membrane using a unilamellar liposome. The zeta potential of the liposomes decreased significantly from −48 mV to −2.01 and −0.93 mV upon incubation with LAE and polymyxin B, respectively. In addition, reduction in interparticle electrostatic repulsions resulted in flocculation of the liposomes, as shown by the particle size distribution curves in Fig. 7 and the value of polydispersity index (PDI) and average peak size (nm) given in Table 1. However, in the presence of LAE, the average volume mean diameter of the unilamellar vesicles did not change significantly (P > 0.05) (Fig. 7). On the other hand, interaction of liposomes with polymyxin B created a significant fraction of colloidal particles (<35 nm, Fig. 7 and Table 1) smaller than intact liposomes. This evidence suggests that polymyxin B caused lysis of the liposomes based on its surfactant properties and potentially promoted the formation of mixed micelles, whereas LAE did not lyse the liposomes. It is important to note that the concentration of both LAE and polymyxin B is greater than the critical micelle concentrations (21, 22). This suggests that LAE binds and potentially inserts itself in a phospholipid membrane, whereas polymyxin B disrupts the bilayer membrane and promotes the formation of mixed micelles. In addition, the levels of oxidative stress generated by LAE and polymyxin B in bacterial cells were investigated. The results showed that LAE is more potent in generating oxidative stress, which may be correlated with its rapid bactericidal activity compared to polymyxin B. Detailed results are shown in the supplemental material and Fig. S3.
FIG 7.
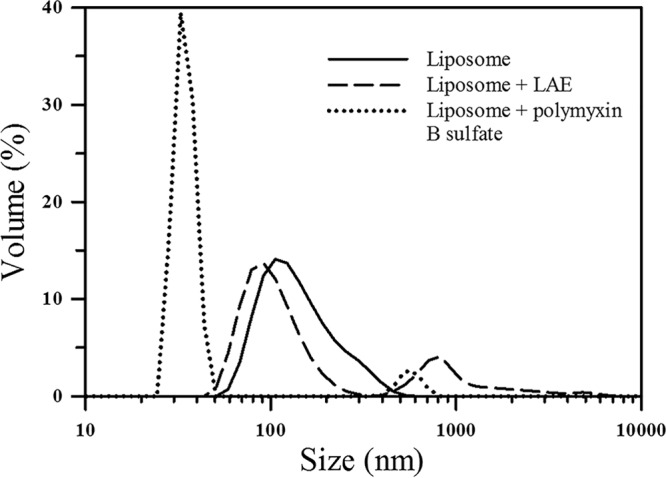
Particle size distribution of model liposomes treated with polymyxin B or LAE; the control included untreated liposome.
TABLE 1.
Size distributions, polydispersity indices, and zeta potentials of model liposomes in the presence or absence of LAE and polymyxin B
| Sample (ppm) | Avg size(s) (nm) | PDIa | Zeta potential (mV) |
|---|---|---|---|
| Liposome | 162.6 | 0.144 | −48.1 |
| Liposome and LAE (15) | Peak 1, 133.4; peak 2, 1,375 | 0.702 | −2.01 |
| Liposome and polymyxin B (15) | Peak 1, 34.9; peak 2, 559.3 | 0.826 | −0.93 |
PDI, polydispersity index.
DISCUSSION
An antimicrobial approach based on a synergistic novel combination of LAE with multiple physical treatments was reported in this study. The combination of LAE with mild heat or UV-A light achieved a 5- to 6-log reduction in an E. coli O157:H7 strain in contrast to a 1- to 2-log reduction of bacteria by individual treatments. Combination of LAE with mild heat also demonstrated a 5-log synergistic reduction against L. innocua, a Gram-positive bacterium. To the best of our knowledge, the synergistic combination between an amino acid-derived antimicrobial compound and UV-A or mild heat has not been heavily investigated. Results from prior studies have focused only on synergistic antimicrobial activity using a combination of GRAS polyphenolic compounds and light (6, 7, 9, 23, 24). Furthermore, the results also show the significance of these mechanistic differences in the synergistic antimicrobial activity of these compounds with physical treatments (UV-A or mild heat). The results also illustrate the mechanistic differences in the antimicrobial activity of LAE compared to that of polymyxin B.
Both LAE and polymyxin B are well-established membrane-active antimicrobial agents. The results of this study demonstrate significant differences in the fate of membrane structures upon interaction with these agents. For instance, the results illustrate that both antimicrobial compounds electrostatically interacted with model cell membranes which influenced the net charge on liposomes. However, polymyxin B significantly reduced the size of liposomal particles, suggesting lysis of the membrane, whereas LAE had a minimal effect on the size of model liposomes. These results are in agreement with the literature (13, 14, 25). Despite the membrane activity and bacteriostatic properties of polymyxin B, antimicrobial synergy with light or mild heat was not observed with polymyxin B, in contrast to the results observed with LAE. This comparative evaluation suggested that membrane damage by itself is not a sufficient condition for the synergistic interaction of LAE with light or mild heat.
We proposed here that the oxidative stress may be the key difference between LAE and polymyxin B. Oxidative stress generation has been recently reported as one of the potential pathways that may contribute to the antimicrobial activity of diverse antibiotics (26), although some studies have also presented counterevidence to this hypothesis (27, 28). In the present study, bactericidal activity and oxidative stress generation from LAE or polymyxin B were evaluated. As shown in Fig. S3, the stronger bactericidal activity of LAE compared to polymyxin B was correlated with oxidative stress generation, since LAE bactericidal activity was depleted at lower temperatures and in the presence of antioxidants such as glutathione. A similar finding was reported by other researchers (26), where oxidative damage induced by certain antibiotics was among the lethal factors responsible for cell inactivation. In comparison, no oxidative stress was observed for polymyxin B, even during extended incubation.
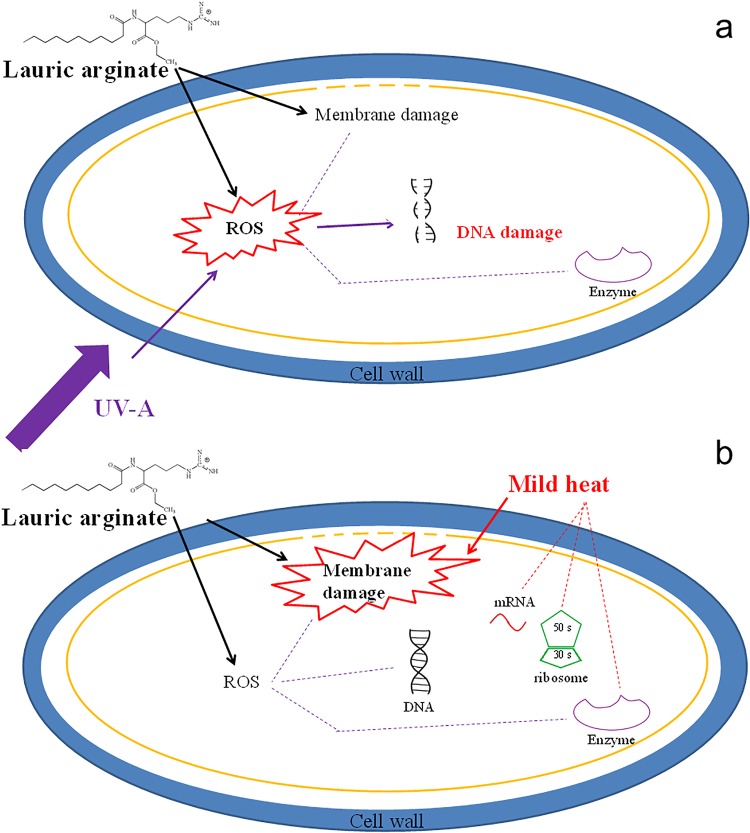
The potential mechanism of antimicrobial synergy between LAE and physical treatments is illustrated in Fig. 8. Figure 8a shows the potential mechanism for the synergistic antimicrobial activity of LAE and UV-A. UV-A alone has been reported to generate reactive oxygen species (ROS), which can damage bacterial DNA, as well as the cell membrane and intracellular proteins (29). As shown in Fig. 8a, oxidative stress generated by a combination of UV-A and LAE is a leading factor for antimicrobial activity. This enhanced oxidative stress can lead to bacterial DNA damage, as well as oxidation of the cell membrane and proteins. This physical perturbation of the bacterial cell membrane plus enhanced oxidation stress may explain the synergistic interaction of bacteria. Interestingly, the synergistic influence of this treatment was dependent on temperature. This suggested the role of the metabolic activity of cells in increasing the susceptibility of cells to oxidative and membrane damage.
FIG 8.
Proposed synergistic antimicrobial mechanisms between LAE and mild physical treatments. (a) The synergistic combination of LAE and UV-A may induce enhanced oxidative stress (ROS), in addition to membrane damage, resulting in enhanced inactivation of bacterial cells. (b) The synergistic combination of LAE and mild heat may induce enhanced membrane damage, in addition to oxidative stress generation. Solid lines indicate pathways evaluated in this study, while dotted lines indicate additional pathways that may contribute to the synergistic inactivation of bacteria.
As illustrated in Fig. 8b, the synergistic mechanism between mild heat and LAE can be attributed to oxidative stress generation by LAE, as well as the dual stress of mild heat and LAE, which induces enhanced membrane damage. The enhanced membrane damage was expected since even under mild heat conditions, bacterial membrane permeation increases, which results in the release of intracellular components (30), and the supplementation of LAE may exacerbate the membrane leakage based on enhanced pore formation. As shown in Fig. 5, oxidative stress-generated antimicrobial synergy can be neutralized with antioxidant supplementation, and the residual synergistic antimicrobial activity can be attributed to enhanced membrane damage induced by LAE and mild heat.
The synergistic combination between LAE and physical treatments may provide an effective, rapid, and low-cost antimicrobial processing approach that can be easily transferred to the food industry or medical applications. Examples may include incorporating LAE into packaging films (31, 32) and coupling LAE with UV-A treatment at room temperature to obtain surface sanitation of fresh produce (33). The application of LAE and mild heat could also be used to surface pasteurize cabbage, tomatoes, and cantaloupes (33–35).
In conclusion, antimicrobial synergies against the Gram-negative bacterium E. coli O157:H7 and the Gram-positive bacterium L. innocua were observed between the GRAS antimicrobial compound LAE and two mild physical treatments. Enhanced oxidative stress generation and exacerbated membrane damage resulted in a synergistic mode of action between LAE and mild physical treatments. Mechanistic differences between LAE and polymyxin B were compared based on the MIC, artificial cell membrane disruption, and oxidation stress generation. The synergistic application of mild physical treatments and LAE can be utilized in agriculture, the food industry, and the medical field as an alternative sanitizing approach that is safer, faster, and more efficient than some of the current processes.
MATERIALS AND METHODS
Reagents.
Lauroyl arginate ethyl (LAE; CytoGuard LA20, 20% [vol/vol]) was kindly provided by A&B Ingredients (Fairfield, NJ). Phosphate-buffered saline (PBS) was purchased from Fisher Bioreagent (Pittsburgh, PA). Tryptic soy broth (TSB), tryptic soy agar (TSA), glutathione, ascorbic acid, and l-α-phosphatidylcholine from egg yolk (egg PC) were purchased from Sigma-Aldrich (St. Louis, MO). Polymyxin B sulfate, thiourea, and rifampin were bought from TCI America (Portland, OR). Ultrapure water was filtrated using a Milli-Q filtration system (EMD Millipore, Billerica, MA).
Microbial culture.
A rifampin-resistant L. innocua mutant (ATCC 33090; ATCC, Manassas, VA) and a Shiga toxin knockout rifampin-resistant E. coli O157:H7 mutant (ATCC 700728) were kindly provided by Trevor Suslow (University of California—Davis) and Linda Harris (University of California—Davis). Enumeration of both bacteria was performed on TSA supplemented with rifampin at a final concentration of 50 μg/ml.
Synergistic combination between LAE and light or mild heat based on isobolograms.
Synergistic combination between the selected concentration of LAE and UV-A light was evaluated using isobolograms, as described by Markovsky et al. (36), with some minor modifications. For this analysis, E. coli inactivation was measured by varying the concentration of LAE incubated with bacterial cells for a fixed amount of time without UV-A treatment. Similarly, inactivation of bacterial cells was evaluated with the variation in the total fluence of UV-A exposure of cells without the presence of LAE. Each synergistic combination was selected and tested for bactericidal activity, where LAE was fixed at 15 μg/ml and UV-A treatment for 30 min, corresponding to a fluence of 8.64 J/cm2. Similarly, the synergistic combination between LAE and mild heat was also evaluated using isobolograms. In this analysis, LAE at different concentrations was selected to inactivate E. coli for a fixed amount of time (4 min), whereas for mild heat treatment the temperature was fixed at 55°C, but the incubation time was varied. A synergistic combination was selected and tested for bactericidal activity with an LAE concentration of 15 μg/ml and mild heat treatment for 4 min at 55°C.
Inactivation of E. coli and L. innocua by individual treatments (mild heat or LAE) and their combination.
Overnight cultures of E. coli or L. innocua were grown in TSB for 18 to 22 h at 37 or 30°C, respectively. Both cultures were then centrifuged (1 min, 16,100 × g) and washed twice in sterile PBS to a final concentration of 5.0 × 107 CFU/ml. For mild heating, the solution was heated to 55°C. At this temperature and after a short incubation time, there is minimal deterioration of food quality. The glass scintillation vials (total volume, 20 ml) were preheated in a 55°C water bath for a minimum of 10 min. The E. coli and L. innocua cells were then treated with mild heat, LAE, or mild heat plus LAE for 4 min. Final concentrations of LAE were supplemented at 15 ppm, and the survivor populations were enumerated on rifampin-supplemented TSA plates.
Inactivation of E. coli and L. innocua by individual treatments (UV-A or LAE) and their combination.
Both cell cultures were prepared, centrifuged, washed, and resuspended in PBS, as described earlier. The E. coli or L. innocua cells were then treated at room temperature with UV-A, LAE, or UV-A plus LAE. LAE was supplemented at a final concentration of 15 ppm for testing against E. coli, whereas three different final concentrations (15, 22.5, and 30 ppm) were evaluated for testing against L. innocua. These concentrations were selected based on similar isobologram studies for E. coli (data not shown). The survivor population of E. coli after each treatment was enumerated on rifampin-supplemented TSA at 5, 10, 20, and 30 min, whereas the survivor population of L. innocua was only enumerated after 30 min.
The UV-A treatment was conducted in a UV-A chamber, which consisted of a closed plastic box (Suncast Corporation, Batavia, IL) and four UV-A lamps (320 to 400 nm, 18 W; Actinic BL, Philips, Holland) installed on the underside of the top lid of the chamber (37). Both UV-A-treated and UV-A/LAE-treated samples were set on a plastic rack at the center of the UV-A chamber, with a distance of 0.5 cm to the UV-A lamps. The average light intensity for the combination of four UV-A lamps was 4.8 ± 0.1 mW ⋅ cm−2. Experiments were performed in triplicates.
Nucleic acid leakage assay.
Since LAE was reported as a membrane-disrupting antimicrobial compound (13, 14), damage to bacterial cell membrane was analyzed in the synergistic combination. A disrupted cell membrane structure will usually lead to the diffusion of small molecules, including potassium ions and nucleic acid, from the cytoplasm to the outer environment (20, 38). E. coli was chosen as a model microorganism for the results presented in Fig. 3 through 8. E. coli was selected since it had synergistic interactions with both selected physical treatments, in contrast to Listeria. Briefly, E. coli cells treated under all of the previously described conditions were centrifuged at 16,162 × g for 5 min and 4°C. The supernatant was taken and measured in a Genesys 10S UV-Vis spectrophotometer (Thermo Scientific, Waltham, MA) in UV cuvettes (Brand, Wertheim, Germany) at 260 nm (maximum nucleic acid absorption wavelength). Control bacterial cells without any treatment were also spun down, and the supernatant was used as a blank. All DNA leakage assays were repeated at least three times.
Investigation of the synergistic mechanism between LAE and physical treatments (UV-A and mild heat).
The experiments followed the same scheme described earlier but were supplemented with the antioxidants glutathione, ascorbic acid, and thiourea at final concentrations of 8, 4, and 80 mM, respectively. Antioxidants were applied to evaluate whether oxidative stress generation is one of the mechanisms for the observed antimicrobial synergy.
In order to determine whether the same antimicrobial synergy persisted, UV-A and LAE were combined to treat E. coli according to the same experimental setup at a refrigeration temperature (4°C). Survivor population was enumerated after treatment for 30 min.
Inactivation of E. coli by polymyxin B combined with physical treatments (UV-A and mild heat).
Verification of the observed synergistic combination between LAE and mild physical processing was conducted by replacing LAE with polymyxin B. Polymyxin B was selected due to its structural and mechanistic similarity to LAE: (i) both compounds have a long lipid chain at the N-terminal end, (ii) both compounds are peptides or amino acids in nature and carry a positively charged amino acid(s), and (iii) both compounds have been well documented for their ability to disrupt the bacterial cell membrane. For instance, polymyxin B can interact with lipopolysaccharide on the outer membrane of Gram-negative bacteria through replacement of Ca2+ and Mg2+ and subsequently disrupt both outer (39) and cytoplasmic (40) membranes, resulting in cell lysis (25). Polymyxin B treatments combined with physical treatments (UV-A and mild heat) were conducted as described earlier against E. coli. The survivor population of E. coli was enumerated on TSA agar.
MICs for LAE and polymyxin B.
The MICs for LAE and polymyxin B were tested in a 96-well plate according to Clinical and Laboratory Standards Institute recommendations (41). The final concentration of LAE was tested at 7.5, 15, and 30 μg/ml, while the final concentration of polymyxin B was tested at 3.75, 7.5, and 15 μg/ml. The 96-well plate was then incubated at 37°C for 16 to 20 h and observed for turbidity. The MIC was determined as the lowest concentration of an antimicrobial compound that will not lead to visible growth of a microorganism. The MIC experiments were conducted in triplicates.
Preparation of model cell membrane.
Unilamellar liposome as a model cell membrane was created using a dehydration and rehydration method (42). In this method, thin film of egg l-α-phosphatidylcholine was obtained by dissolving the lipid in chloroform and evaporating the solvent under a vacuum using a rotary evaporator. Thin film was further kept under a vacuum to remove all traces of solvents. The dried thin film was rehydrated in PBS to achieve a 1-mg/ml total lipid concentration and then vortexed to prepare multilamellar vesicles. The multilamellar vesicles were extruded 15 times (mini-extruder; Avanti Polar Lipids, Inc.) through 400-nm pores, followed by the use of 200-nm polycarbonate track-etched membranes to obtain unilamellar liposomes of ∼160 nm (Table 1).
Size distribution and zeta potential of model cell membrane.
Size distributions (nm) and zeta potentials (mV) of liposome model cell membranes in the presence or absence of both antimicrobials (LAE and polymyxin B) were acquired using a dynamic light scattering system (Malvern Zetasizer Nano, Westborough, MA). Both treated and untreated liposome solutions were diluted 10-fold in PBS and suspended in a polystyrene cuvette with a standard path length of 10 nm for size distribution and in disposable folded capillary zeta cells for zeta potential measurements. Solutions were equilibrated at room temperature for 2 min before data acquisition. Scattered light was detected at 90° relative to the incident laser (633-nm He-Ne laser) light for 12 to 20 runs of 10 to 20 s each, with a medium viscosity of 0.89 cP and a refractive index of 1.33.
Oxidative stress generation from polymyxin B and LAE against E. coli.
Both polymyxin B and LAE were prepared at a final concentration of 15 μg/ml either in PBS or in PBS supplemented with 10 mM glutathione. Supplementation of antioxidants (e.g., glutathione) can quench oxidative stress potentially generated from antimicrobial compounds, preventing bacterial cells from being inactivated (43). The E. coli cells were then inoculated to a final concentration of 5.0 × 107 CFU/ml. All treatments were incubated at room temperature or 4°C for 30 min or 2 h, followed by enumeration of E. coli survivors on rifampin-supplemented TSA plates.
Statistical analysis.
All experiments were conducted in triplicates. The bacterial population and absorbance at 260 nm were compared statistically, using analysis of variance by SAS 9.4 (SAS Institute, Inc., Cary, NC). Tukey’s honest significant difference test was used to evaluate the significance of the difference among means (P < 0.05).
Supplementary Material
ACKNOWLEDGMENT
This project was funded by grant 2015-68003-23411 from the USDA-NIFA program Enhancing Food Safety through Improved Processing Technologies (A4131).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01033-19.
REFERENCES
- 1.Sperber WH. 2009. Introduction to the microbiological spoilage of foods and beverages, p 1–40. In Compendium of the microbiological spoilage of foods and beverages. Springer, New York, NY. [Google Scholar]
- 2.Pigott DC. 2008. Foodborne illness. Emerg Med Clin North Am 26:475–497. doi: 10.1016/j.emc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2011. CDC estimates of foodborne illness in the United States. https://www.cdc.gov/foodborneburden/pdfs/FACTSHEET_C_IMPROVEMENTS.pdf. [Google Scholar]
- 4.Wang R, Kalchayanand N, King DA, Luedtke BE, Bosilevac JM, Arthur TM. 2014. Biofilm formation and sanitizer resistance of Escherichia coli O157:H7 strains isolated from “high event period” meat contamination. J Food Prot 77:1982–1987. doi: 10.4315/0362-028X.JFP-14-253. [DOI] [PubMed] [Google Scholar]
- 5.Frank JF, Koffi RA. 1990. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J Food Prot 53:550–554. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- 6.Cossu A, Ercan D, Wang Q, Peer WA, Nitin N, Tikekar RV. 2016. Antimicrobial effect of synergistic interaction between UV-A light and gallic acid against Escherichia coli O157:H7 in fresh produce wash water and biofilm. Innov Food Sci Emerg Technol 37:44–52. doi: 10.1016/j.ifset.2016.07.020. [DOI] [Google Scholar]
- 7.de Oliveira EF, Cossu A, Tikekar RV, Nitin N. 2017. Enhanced antimicrobial activity based on a synergistic combination of sublethal levels of stresses induced by UV-A light and organic acids. Appl Environ Microbiol 83:e00383-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Oliveira EF, Tosati JV, Tikekar RV, Monteiro AR, Nitin N. 2018. Antimicrobial activity of curcumin in combination with light against Escherichia coli O157:H7 and Listeria innocua: applications for fresh produce sanitation. Postharvest Biol Technol 137:86–94. doi: 10.1016/j.postharvbio.2017.11.014. [DOI] [Google Scholar]
- 9.Bastarrachea LJ, Walsh M, Wrenn SP, Tikekar RV. 2017. Enhanced antimicrobial effect of ultrasound by the food colorant erythrosin B. Food Res Int 100:344–351. doi: 10.1016/j.foodres.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 10.FDA. 2005. GRAS exemption claim for ethyl-N-lauroyl-l-arginate hydrochloride. U.S. Food and Drug Administration, Bethesda, MD: www.accessdata.fda.gov/scripts/fdcc/?set=GRASNotices&id=164&sort=GRN_No&order=DESC&startrow=1&type=basic&search=164. [Google Scholar]
- 11.Becerril R, Manso S, Nerin C, Gómez-Lus R. 2013. Antimicrobial activity of lauroyl arginate ethyl (LAE), against selected food-borne bacteria. Food Control 32:404–408. doi: 10.1016/j.foodcont.2013.01.003. [DOI] [Google Scholar]
- 12.Porto-Fett ACS, Campano SG, Smith JL, Oser A, Shoyer B, Call JE, Luchansky JB. 2010. Control of Listeria monocytogenes on commercially-produced frankfurters prepared with and without potassium lactate and sodium diacetate and surface treated with lauric arginate using the sprayed lethality in container (SLIC) delivery method. Meat Sci 85:312–318. doi: 10.1016/j.meatsci.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez E, Seguer J, Rocabayera X, Manresa A. 2004. Cellular effects of monohydrochloride of l-arginine, Nα-lauroyl ethyl ester (LAE) on exposure to Salmonella typhimurium and Staphylococcus aureus. J Appl Microbiol 96:903–912. doi: 10.1111/j.1365-2672.2004.02207.x. [DOI] [PubMed] [Google Scholar]
- 14.Ma Q, Davidson PM, Critzer F, Zhong Q. 2016. Antimicrobial activities of lauric arginate and cinnamon oil combination against foodborne pathogens: improvement by ethylenediaminetetraacetate and possible mechanisms. LWT - Food Sci Technol 72:9–18. doi: 10.1016/j.lwt.2016.04.021. [DOI] [Google Scholar]
- 15.Ruckman SA, Rocabayera X, Borzelleca JF, Sandusky CB. 2004. Toxicological and metabolic investigations of the safety of N-α-lauroyl-l-arginine ethyl ester monohydrochloride (LAE). Food Chem Toxicol 42:245–259. doi: 10.1016/j.fct.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Huang K, Nitin N. 2017. Enhanced removal of Escherichia coli O157:H7 and Listeria innocua from fresh lettuce leaves using surfactants during simulated washing. Food Control 79:207–217. doi: 10.1016/j.foodcont.2017.03.032. [DOI] [Google Scholar]
- 17.Oladunjoye A, Soni KA, Nannapaneni R, Schilling MW, Silva JL, Mikel B, Bailey RH, Mahmoud BSM, Sharma CS. 2013. Synergistic activity between lauric arginate and carvacrol in reducing salmonella in ground turkey. Poult Sci 92:1357–1365. doi: 10.3382/ps.2012-02620. [DOI] [PubMed] [Google Scholar]
- 18.Ma Q, Davidson PM, Zhong Q. 2013. Antimicrobial properties of lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. Int J Food Microbiol 166:77–84. doi: 10.1016/j.ijfoodmicro.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Burt S. 2004. Essential oils: their antibacterial properties and potential applications in foods - a review. Int J Food Microbiol 94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Cossu A, Ercan D, Tikekar RV, Nitin N. 2016. Antimicrobial effect of photosensitized rose bengal on bacteria and viruses in model wash water. Food Bioprocess Technol 9:441–451. doi: 10.1007/s11947-015-1631-8. [DOI] [Google Scholar]
- 21.Asker D, Weiss J, McClements DJ. 2009. Analysis of the interactions of a cationic surfactant (lauric arginate) with an anionic biopolymer (pectin): isothermal titration calorimetry, light scattering, and microelectrophoresis. Langmuir 25:116–122. doi: 10.1021/la803038w. [DOI] [PubMed] [Google Scholar]
- 22.Yoshino N, Takeshita R, Kawamura H, Murakami K, Sasaki Y, Sugiyama I, Sadzuka Y, Kagabu M, Sugiyama T, Muraki Y, Sato S. 2018. Critical micelle concentration and particle size determine adjuvanticity of cyclic lipopeptides. Scand J Immunol 88:e12698. doi: 10.1111/sji.12698. [DOI] [PubMed] [Google Scholar]
- 23.Wang Q, de Oliveira EF, Alborzi S, Bastarrachea LJ, Tikekar RV. 2017. On mechanism behind UV-A light enhanced antibacterial activity of gallic acid and propyl gallate against Escherichia coli O157:H7. Sci Rep 7:8325. doi: 10.1038/s41598-017-08449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Q, Alborzi S, Bastarrachea LJ, Tikekar RV. 2018. Novel sanitization approach based on synergistic action of UV-A light and benzoic acid: inactivation mechanism and a potential application in washing fresh produce. Food Microbiol 72:39–54. doi: 10.1016/j.fm.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Hancock REW, Wong P. 1984. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother 26:48–52. doi: 10.1128/AAC.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 27.Keren I, Wu Y, Inocencio J, Mulcahy LR, Lewis K. 2013. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 339:1213–1216. doi: 10.1126/science.1232688. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Imlay JA. 2013. Cell death from antibiotics without the involvement of reactive oxygen species. Science 339:1210–1213. doi: 10.1126/science.1232751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamamoto A, Mori M, Takahashi A, Nakano M, Wakikawa N, Akutagawa M, Ikehara T, Nakaya Y, Kinouchi Y. 2007. New water disinfection system using UVA light-emitting diodes. J Appl Microbiol 103:2291–2298. doi: 10.1111/j.1365-2672.2007.03464.x. [DOI] [PubMed] [Google Scholar]
- 30.Ray PH, Brock TD. 1971. Thermal lysis of bacterial membranes and its prevention by polyamines. J Gen Microbiol 66:133–135. doi: 10.1099/00221287-66-2-133. [DOI] [PubMed] [Google Scholar]
- 31.Higueras L, López-Carballo G, Hernández-Muñoz P, Gavara R, Rollini M. 2013. Development of a novel antimicrobial film based on chitosan with LAE (ethyl-Nα-dodecanoyl-l-arginate) and its application to fresh chicken. Int J Food Microbiol 165:339–345. doi: 10.1016/j.ijfoodmicro.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Pattanayaiying R, H-Kittikun A, Cutter CN. 2015. Incorporation of nisin Z and lauric arginate into pullulan films to inhibit foodborne pathogens associated with fresh and ready-to-eat muscle foods. Int J Food Microbiol 207:77–82. doi: 10.1016/j.ijfoodmicro.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 33.Aihara M, Lian X, Shimohata T, Uebanso T, Mawatari K, Harada Y, Akutagawa M, Kinouchi Y, Takahashi A. 2014. Vegetable surface sterilization system using UVA light-emitting diodes. J Med Invest 61:285–290. doi: 10.2152/jmi.61.285. [DOI] [PubMed] [Google Scholar]
- 34.Lamikanra O, Bett-Garber KL, Ingram DA, Watson MA. 2005. Use of mild heat pretreatment for quality retention of fresh-cut cantaloupe melon. J Food Sci 70:C53–C57. doi: 10.1111/j.1365-2621.2005.tb09020.x. [DOI] [Google Scholar]
- 35.Shafiee M, Taghavi TS, Babalar M. 2010. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci Hortic 124:40–45. doi: 10.1016/j.scienta.2009.12.004. [DOI] [Google Scholar]
- 36.Markovsky E, Baabur-Cohen H, Satchi-Fainaro R. 2014. Anticancer polymeric nanomedicine bearing synergistic drug combination is superior to a mixture of individually-conjugated drugs. J Control Release 187:145–157. doi: 10.1016/j.jconrel.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Ercan D, Cossu A, Nitin N, Tikekar RV. 2016. Synergistic interaction of ultraviolet light and zinc oxide photosensitizer for enhanced microbial inactivation in simulated wash-water. Innov Food Sci Emerg Technol 33:240–250. doi: 10.1016/j.ifset.2015.11.015. [DOI] [Google Scholar]
- 38.Yang X, Huang E, Yousef AE. 2017. Brevibacillin, a cationic lipopeptide that binds to lipoteichoic acid and subsequently disrupts cytoplasmic membrane of Staphylococcus aureus. Microbiol Res 195:18–23. doi: 10.1016/j.micres.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Zavascki AP, Goldani LZ, Li J, Nation RL. 2007. Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60:1206–1215. doi: 10.1093/jac/dkm357. [DOI] [PubMed] [Google Scholar]
- 40.Dixon RA, Chopra I. 1986. Polymyxin B and polymyxin B nonapeptide alter cytoplasmic membrane permeability in Escherichia coli. J Antimicrob Chemother 18:557–563. doi: 10.1093/jac/18.5.557. [DOI] [PubMed] [Google Scholar]
- 41.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial disc susceptibility tests; approved standard. CLSI document M02-A10 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 42.Matos C, Moutinho C, Lobão P. 2012. Liposomes as a model for the biological membrane: studies on daunorubicin bilayer interaction. J Membr Biol 245:69–75. doi: 10.1007/s00232-011-9414-2. [DOI] [PubMed] [Google Scholar]
- 43.Goswami M, Mangoli SH, Jawali N. 2007. Effects of glutathione and ascorbic acid on streptomycin sensitivity of Escherichia coli. Antimicrob Agents Chemother 51:1119–1122. doi: 10.1128/AAC.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.