The emergence of codling moth populations resistant to commercially applied isolates of CpGV is posing an imminent threat to organic pome fruit production. Very few CpGV isolates are left that are able to overcome the reported types of resistance, emphasizing the demand for new and highly virulent baculoviruses. Here we report the recently discovered CrpeNPV as highly infectious to all types of resistant codling moth populations with a high speed of killing, making it a promising candidate baculovirus in fighting the spread of resistant codling moth populations.
KEYWORDS: CpGV resistance, alphabaculovirus, bioassays, biological control, cell culture, survival time analysis, virulence
ABSTRACT
Cydia pomonella granulovirus (CpGV) is a cornerstone of codling moth (Cydia pomonella) control in integrated and organic pome fruit production, though different types of resistance to CpGV products have been recorded in codling moth field populations in Europe for several years. Recently, a novel baculovirus named Cryptophlebia peltastica nucleopolyhedrovirus (CrpeNPV) was isolated from a laboratory culture of the litchi moth, Cryptophlebia peltastica, in South Africa. Along with CpGV, it is the third known baculovirus that is infectious to codling moth. In the present study, parameters of infectiveness of CrpeNPV, such as the median lethal concentration and median survival time, were determined for codling moth larvae susceptible or resistant to CpGV. In addition, the permissiveness of a codling moth cell line with respect to infection by CrpeNPV budded virus was demonstrated by infection and gene expression studies designed to investigate the complete replication cycle. Investigations of the high degree of virulence of CrpeNPV for codling moth larvae and cells are of high significant scientific and economic value and may offer new strategies for the biological control of susceptible and resistant populations of codling moth.
IMPORTANCE The emergence of codling moth populations resistant to commercially applied isolates of CpGV is posing an imminent threat to organic pome fruit production. Very few CpGV isolates are left that are able to overcome the reported types of resistance, emphasizing the demand for new and highly virulent baculoviruses. Here we report the recently discovered CrpeNPV as highly infectious to all types of resistant codling moth populations with a high speed of killing, making it a promising candidate baculovirus in fighting the spread of resistant codling moth populations.
INTRODUCTION
Codling moth, Cydia pomonella (Lepidoptera: Tortricidae, Linnaeus, 1758), is a serious economic pest of apples, pears, walnuts, and quince production worldwide. The larvae can cause tremendous damage by boring into the fruit, leaving it nonmarketable (2, 3). For the control of C. pomonella, the baculovirus Cydia pomonella granulovirus (CpGV) (genus Betabaculovirus of the virus family Baculoviridae) (4) is registered as a biocontrol agent and is commercially used in nearly all regions of the world where pome fruits are grown (3). CpGV products play a particularly important role in organic apple production (3). A key feature of CpGV is its extremely narrow host range, which comprises larvae of the two tortricid species C. pomonella and the false codling moth Thaumatotibia leucotreta, a pest of economically important crops in Africa (5), making it harmless to nontarget insects. Another important characteristic of CpGV is its high virulence against C. pomonella larvae in early larval instars, particularly neonates, where a single to a few infectious virus particles are sufficient to cause lethal infection (6). The enveloped nucleocapsids (virions) of the baculoviruses are embedded within solid protein matrices, the so-called occlusion bodies (OB), which entirely surround the virions and protect them from harmful abiotic factors such as UV light. Two OB morphologies are known for baculoviruses, subdividing baculovirus isolates into two morphological groups. The granuloviruses (GVs), found exclusively in genus Betabaculovirus, have ovocylindrical OBs and include usually a single virion only, whereas the nucleopolyhedroviruses (NPV), found predominantly in the genus Alphabaculovirus, contain several to many virions (7). The OBs provide the virions with high physical and chemical stability, which allows the spraying of aqueous OB suspensions directly onto plants and fruit using conventional pesticide sprayers. For CpGV, the OBs are applied on entire apple trees, where they are orally ingested from the surface by neonate larvae. Biological control agents that are based on CpGV are applied in at least 34 countries worldwide (8) and represent a cornerstone in the integrated pest management of C. pomonella in apple plantations. To date, different geographic isolates of CpGV are known and are the active ingredients in CpGV-based crop protection products. Different isolates of CpGV were entirely sequenced, and results allowed the classification of the isolates into five different genome groups (namely, groups A to E) (8, 9). The first discovered isolate was the Mexican isolate CpGV-M (genome group A) (10), which was commercially registered in Europe in the early 1990s (2, 3).
After decades of successful application of CpGV-M, the biological control of C. pomonella became hampered by the occurrence of field resistance against CpGV-M, which was reported to have been detected since 2005 (11, 12). Resistant populations of C. pomonella were reported from organic apple orchards in Austria, Czech Republic, France, Germany, Italy, Netherlands, and Switzerland and were characterized by a 1,000-fold- to 100,000-fold-reduced range of susceptibilities to CpGV-M, preventing economic control of C. pomonella and posing a serious threat to organic apple production (11). At least three different types of CpGV resistances have been identified. Type I resistance is targeted against isolate CpGV-M with a sex-linked inheritance located on the Z chromosome (11). A genetically homogeneous laboratory C. pomonella strain carrying this type of resistance was established and named CpRR1 (8). Whole-genome comparisons between CpGV-M and CpGV isolates breaking type I resistance, such as English isolate CpGV-E2 (genome group B), Iranian isolates CpGV-I12 (genome group D) and CpGV-I07 (genome group C), and Canadian isolate CpGV-S (genome group E), revealed a 24-bp insertion in gene pe38 in CpGV-M, which was demonstrated to be the target of type I resistance (8). Different CpGV isolates breaking type I resistance were tested in laboratory and field trials for their potential usage as biological control agents, and several of them are now registered in Europe and North America (13–18).
A second type of resistance (type II) was identified in a genetically homogenous laboratory strain called CpR5M, which derived from a resistant C. pomonella field population in Northwest Germany (11). Larvae of CpR5M exhibited resistance to CpGV-M and also to CpGV-S, CpGV-I12, and CpGV-I07. More recently, a third type of resistance, type III resistance, was postulated for the laboratory strain CpRGO, a homogenous inbred strain of a resistant field population in East Germany (19). CpRGO is characterized by cross-resistance to CpGV-M and CpGV-S (19). So far, the only known commercially applied isolate that can overcome all three types of resistance is CpGV-E2 (13, 19).
Recently, a novel baculovirus, the Cryptophlebia peltastica nucleopolyhedrovirus (CrpeNPV) (genus Alphabaculovirus), was isolated from larvae of Cryptophlebia peltastica (Lepidoptera: Tortricidae, Meyrick 1921), an agricultural pest of litchis and macadamias in South Africa (20). The only other tortricid alphabaculovirus reported to be infectious to C. pomonella larvae, Choristoneura murinana nucleopolyhedrovirus (ChmuNPV) (21), has much lower virulence than CpGV (23).
In the present study, the biological activity of CrpeNPV was evaluated in comparison to CpGV-M and CpGV-E2 in susceptible C. pomonella larvae (CpS), as well as all three types of resistant laboratory strains, CpRR1, CpR5M, and CpRGO, in bioassay experiments. Based on mortality data, CrpeNPV was virulent to all C. pomonella strains, with biological activity similar to that seen with CpGV, offering new opportunities in the microbial control of CpGV-resistant C. pomonella field populations.
In addition, the only cell culture of C. pomonella, Cp14R (24), was demonstrated to be susceptible to CrpeNPV. Initiation of gene expression was detected by the example of six viral genes as well as the formation of new OBs, confirming the completion of the viral replication cycle. The ability of CrpeNPV to infect and replicate in Cp14R cells is opening new opportunities for in vitro and coinfection studies focusing on the interaction of CpGV and CrpeNPV.
RESULTS
Biological activity.
Bioassays were performed to compare the levels of virulence of CrpeNPV, CpGV-M, and CpGV-E2 in neonate larvae of susceptible strain CpS and resistant strains CpRR1 (resistance type I), CpR5M (resistance type II), and CpRGO (resistance type III). Mortality was scored at 7 and 14 days postinfection (dpi), and 50% lethal concentration (LC50) and LC90 values were calculated (Table 1). For CpS, the LC50 values for all three viruses ranged between 1.76 × 10³ and 2.7 × 10³ OB/ml and 0.258 × 10³ to 0.839 × 10³ OB/ml at 7 and 14 dpi, respectively, indicating similar levels of virulence for this strain. Tested with CpRR1, CpGV-M caused low mortality and LC50 as well as LC90 values could not be calculated. In contrast, the LC50 values of CpGV-E2 and CrpeNPV were within a similar range of 2.02 to 3.64 × 10³ OB/ml and 0.272 × 10³ to 1.39 × 10³ OB/ml at 7 and 14 dpi, respectively (Table 1). In bioassays with CpR5M larvae, CpGV-E2 (3.84 × 10³ and 0.298 × 10³ OB/ml at 7 and 14 dpi, respectively) and CrpeNPV (2.19 × 10³ and 0.702 × 10³ OB/ml at 7 and 14 dpi, respectively) showed similar LC50 values, whereas CpGV-M did not cause sufficient mortality for LC50 and LC90 calculation (Table 1).
TABLE 1.
Corrected lethal concentration values determined for neonate larvae of C. pomonella strains CpS, CpRR1, CpR5M, and CpRGOa
| Strain | Isolate | n | 7 dpi |
14 dpi |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% CL) [×103 OBs/ml] |
LC90 (95% CL) [×103 OBs/ml] |
Slopeb | χ2 (df) | LC50 (95% CL) [×103 OBs/ml] |
LC90 (95% CL) [×103 OBs/ml] |
Slopeb (SE) | χ2 (df) | |||
| CpS | CpGV-M | 899 | 1.76 (0.73–4.3) | 28.0 (9.4–340) | 1 A | 22.8 (4) | 0.258 (0.12–0.45) | 4.37 (2.3–13) | 1.04 A | 8.2 (4) |
| CpGV-E2 | 906 | 2.12 (1.3–3.7) | 45.40 (19–190) | 0.96 A | 8.5 (4) | 0.265 (0.19–0.35) | 3.23 (2.4–4.8) | 1.18 A | 4.6 (4) | |
| CrpeNPV | 835 | 2.70 (0.9–7.8) | 28.50 (9.4–440) | 1.25 A | 20.8 (3) | 0.839 (0.43–1.5) | 7.98 (3.8–29) | 1.3 A | 8.4 (3) | |
| CpRR1 | CpGV-M | 1,022 | ND | ND | 0.72 A | 7.7 (4) | ND | ND | 0.45 A | 2.9 (4) |
| CpGV-E2 | 802 | 2.02 (1.3–3.2) | 17.00 (9.2–45) | 1.38 B | 8.9 (4) | 0.272 (0.21–0.34) | 1.99 (1.5–2.9) | 1.48 B | 1.6 (4) | |
| CrpeNPV | 868 | 3.64 (2.0–6.5) | 32.40 (16–110) | 1.35 B | 8.3 (3) | 1.39 (1.1–1.7) | 16.9 (12–25) | 1.18 B | 2.9 (3) | |
| CpR5M | CpGV-M | 843 | ND | ND | 0.19 A | 5.8 (4) | ND | ND | 0.42 A | 12.5 (4) |
| CpGV-E2 | 747 | 3.84 (2.8–5.4) | ND | 0.86 B | 7.5 (4) | 0.298 (0.23–0.38) | 2.54 (0.23–0.38) | 1.38 B | 2.4 (4) | |
| CrpeNPV | 836 | 2.19 (0.18–2.7) | ND | 1.25 B | 5.1 (3) | 0.702 (0.31–1.4) | ND | 1.55 B | 13.6 (3) | |
| CpRGO | CpGV-M | 871 | 5.63 (3.3–11) | ND | 0.43 A | 6.3 (4) | 1.59 (0.26–6.6) | ND | 0.56 A | 17.7 (4) |
| CpGV-E2 | 832 | 0.939 (0.59–1.4) | 7.53 (4.5–17) | 1.42 B | 7.8 (4) | 0.442 (0.35–0.54) | 2.95 (2.3–4.1) | 1.56 B | 3.5 (4) | |
| CrpeNPV | 761 | 9.33 (4.1–36) | ND | 0.89 A | 13.6 (4) | 0.675 (0.41–1.0) | 81.8 (37–270) | 0.62 A | 4.9 (4) | |
Lethal concentration values were corrected using the method described previously by Abbott (39). Mortality rates were scored on days 7 and 14 postinfection. Parallelism of probit lines was tested individually for each C. pomonella strain (CpS [susceptible], CpRR1 [resistance type I], CpR5M [resistance type II], and CpRGO [resistance type III]). CL, confidence limits calculated according to Fieller's theorem; df, degrees of freedom; ND, not defined due to low mortality rates. Slope function was determined following the method of Litchfield and Wilcoxon. Treatment responses were corrected in accordance with the control response using Abbott's formula. All calculations performed with ToxRat software v3.2.1 (ToxRat Solutions GmbH, Alsdorf, Germany).
Different letters indicate no parallelism of probit lines at either day 7 or 14. The criterion of parallelism was rejected when P values were < 0.05.
For CpRGO and CpGV-M, only the LC50 was calculated with 5.63 × 10³ OB/ml and 1.59 × 10³ OB/ml at 7 and 14 dpi, respectively, which were similar to the LC50 values calculated for CrpeNPV (9.33 × 10³ and 0.675 × 10³ OB/ml) (Table 1). CpGV-E2 appeared to be more virulent than CpGV-M and CrpeNPV for larvae of CpRGO, with LC50 values of 0.939 × 10³ OB/ml (7 dpi) and 0.442 × 10³ OB/ml (14 dpi) (Table 1).
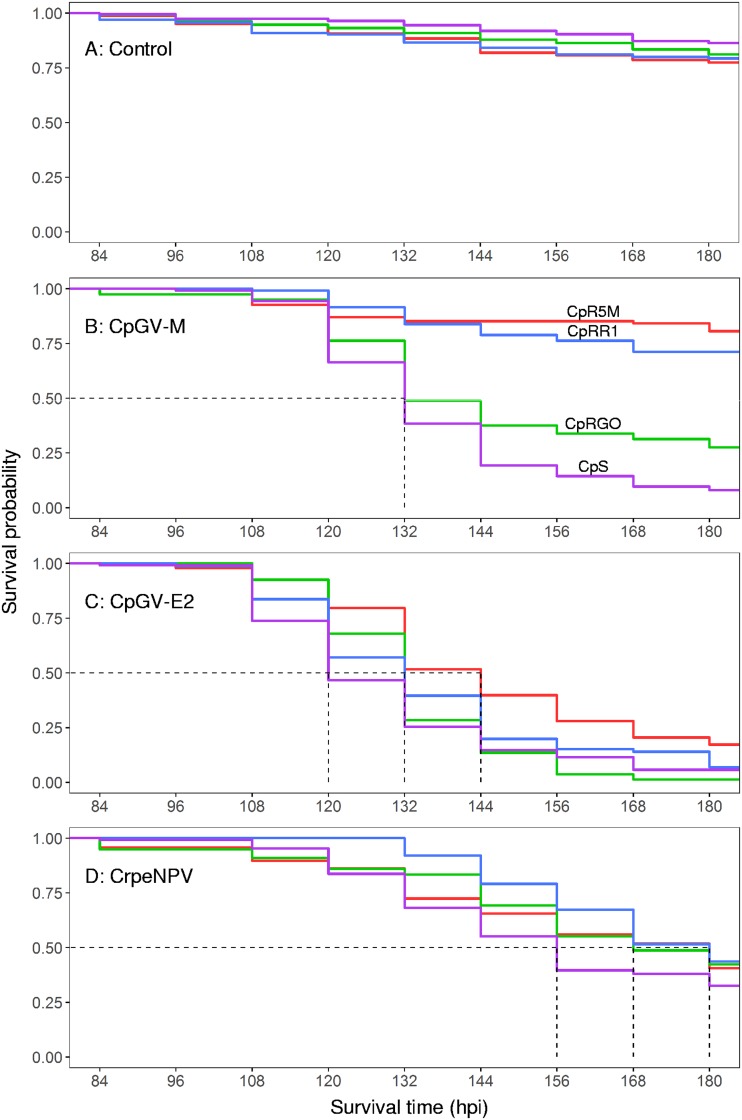
To test the time of response of the CpS, CpRR1, CpR5M, and CpRGO to CpGV-M, CpGV-E2, and CrpeNPV, a survival time analysis was conducted with neonate larvae of all strains for a total duration of 180 h postinfection (hpi) (7.5 dpi). In contrast to the full-range bioassays, larvae were exposed to a fixed concentration of 2.3 × 104 OB/ml. In the uninfected control experiments with CpS, CpRR1, CpR5M, and CpRGO, a steady decrease in survival probability was observed starting at 84 hpi and the levels ranged between 77.4% (95% Cl, 71.6 to 83.8) and 86.2% (95% Cl, 81.5 to 91.2) for CpR5M and CpS, respectively, at 180 hpi (Fig. 1A). No significant differences were detected (χ2 [df = 3, n = 674] = 5.6, P = 0.1). In all C. pomonella strains, mortality did not occur before 84 hpi and the data shown in Fig. 1 do not include data collected prior to this time point. Due to the correction of control mortality by the Henderson-Tilton formula (see below), the survival rates that occurred between 84 and 120 hpi occasionally increased slightly in relation to a prior time point. This was due to higher mortality in uninfected controls, and these values were reset to the survival frequency of the prior data point, since the Kaplan-Meier estimator does not accommodate data representing an increase in the survival rate.
FIG 1.
Survival time analysis of the uninfected control (A) and CpGV-M (B), CpGV-E2 (C), and CrpeNPV (D) tested on laboratory strains of susceptible CpS (purple), resistant type I CpRR1 (blue), type II CpR5M (red) and type III CpRGO (green) neonate larvae.
As seen with the lethal concentration bioassay data, the different levels of virulence of CpGV-M for all four laboratory strains of C. pomonella was reflected by the survival time analysis of this study (Fig. 1B). The difference between the treatment groups was statistically significant (χ2 [3, n = 431] = 179, P < 0.05), and two patterns in Kaplan-Meier survival curves were visible. First, the survival probability of CpRR1 was 71.2% (95% confidence interval [CI], 63.5 to 79.9) and of CpR5M was 80.6% (95% CI, 73.4 to 88.4) and did not fall below 70% after 168 hpi, and no significant differences between the two treatments were observed (P = 0.846), indicating similar modes of action of CpGV-M in CpR5M and CpRR1. Second, the survival probability of CpS and CpRGO treated with CpGV-M reached 50% at 132 hpi and further decreased to 8.0% for CpS (95% CI, 4.4 to 14.5) and 27.5% for CpRGO (95% CI, 19.3 to 39.3) at 180 hpi (Fig. 1B). The differences between these two and all other treatments were statistically significant (P < 0.05), although the overall patterns of the two survival curves appeared similar. Based on the results, CpGV-M had three different effects on susceptible strain CpS, resistant type III strain CpRGO, and resistant type I and II strains CpRR1 and CpR5M.
In the survival time analysis performed with CpGV-E2, a median survival time was calculated for CpS (120 hpi) and for CpRR1 and CpRGO (132 hpi) as well as for CpR5M (144 hpi) (Fig. 1C), with significant differences between the treatments (χ2 [3, n = 382] = 27.6, P < 0.05). The pairwise comparison of treatments by the use of a log rank test revealed significant differences between the results of treatment with CpR5M with respect to CpS, CpRR1, and CpRGO (P < 0.05). No differences were observed between the survival curves determined for CpS, CpRR1, and CpRGO. At 180 hpi, strains CpS, CpRR1, and CpRGO had minimal survival rates ranging between 1.2% (95% CI, 0.2 to 8.7) and 7.0% (95% CI, 3.2 to 15.1) (see Table S1 in the supplemental material) whereas CpR5M appeared to be less affected by CpGV-E2, with a minimum survival rate of 17.2% (95% CI, 11.0 to 26.9) (Fig. 1C; see also Table S1).
Slightly different patterns in the Kaplan-Meier survival curves were recorded for CpS, CpRR1, CpR5M, and CpRGO treated with CrpeNPV (Fig. 1D). A steady drop of the probability of survival was recorded. Survival rates appeared to decrease steadily, and median survival times were calculated for CpS (156 hpi) and CpRGO (168 hpi) as well as for CpRR1 and CpR5M at 180 hpi (Fig. 1D). Significant differences between treatments were detected (χ2 [3, n = 424] = 8.9, P = 0.04) but were found only in pairwise comparisons between host CpS and CpRR1 (P = 0.019). According to the survival time analysis, larvae of CpRR1 treated with CrpeNPV did not die before 132 hpi, whereas the probability of survival of CpS had already started to decrease at 108 hpi (Fig. 1D). According to these data, the effects of CrpeNPV were different only on larvae of CpS and CpRR1, with lower and higher survival probabilities, respectively.
Infection of Cp14R cells.
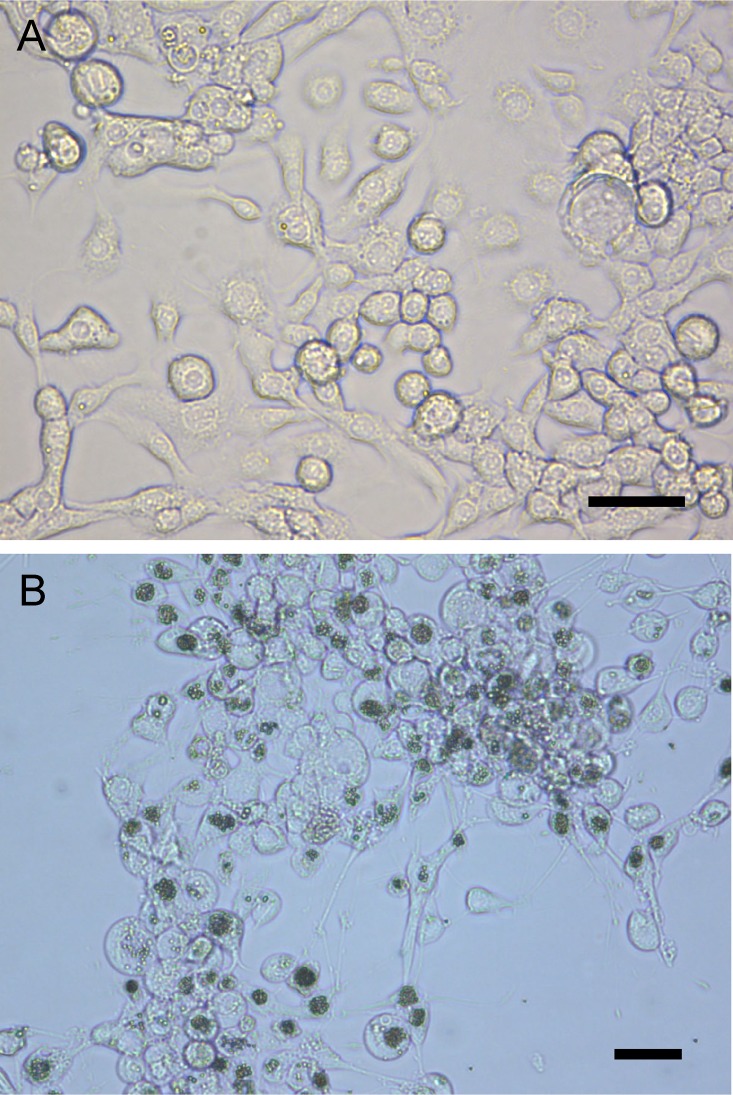
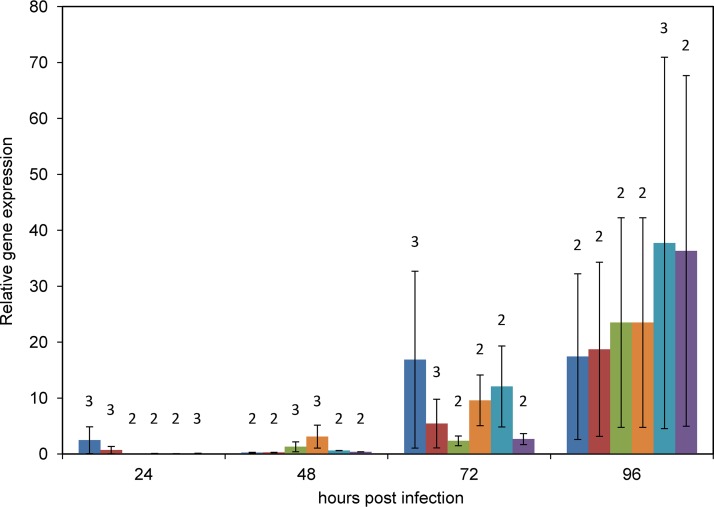
The susceptibility of the C. pomonella cell line Cp14R (24) was tested by exposing Cp14R cells to budded virus (BV) from hemolymph obtained from CrpeNPV-infected C. pomonella fourth instars. Polyhedral OBs started to become visible about 4 dpi (data not shown) and were clearly visible at 7 dpi (Fig. 2). The IZ04 cell culture medium of this infected cell culture was found to be positive for the presence of BV and was titrated by a 50% tissue culture infective dose (TCID50) assay to determine a concentration of PFU per milliliter. The titer of the CrpeNPV BV suspension was 1.54 × 106 PFU/ml, and the suspension was used for the time course experiment to infect Cp14R cells with a multiplicity of infection (MOI) of 2.5. The levels of gene transcripts were calculated in relation to expression of the actin gene. The relative levels of expression of genes polyhedrin (polh), virus protein 39 (vp39), immediate early 1 (ie-1), late expression factor 8 (lef-8), f-protein, and DNA polymerase (dnapol) were calculated in relation to the gene expression of the actin housekeeping gene at 6, 12, 24, 48, 72, and 96 hpi, but no transcripts were measured at 6 and 12 hpi for these six genes (Fig. 3). At 24 and 48 hpi, low levels of transcription (less than 1-fold lower than the level measured for the actin housekeeping gene) were detected, except for ie-1 (24 hpi), with a 2.5-fold ± 2.4-fold change in expression, and vp39 (48 hpi), with 3.1-fold ± 2.1-fold change (Fig. 3). The highest relative rate was measured for gene f-protein at 96 hpi, with a 37.7-fold ± 33.2-fold change in expression (Fig. 3). Expression peaked for genes ie-1, lef-8, vp39, and polh at 96 hpi, with mean amounts of transcripts that were between 17-fold and 36-fold higher than those seen with the actin gene (Fig. 3). In mock-infected Cp14R cells, formation of OBs was not observed and no viral gene expression was detected by quantitative PCR (qPCR) analysis, excluding any contamination.
FIG 2.
Uninfected Cp14R cells (A) and Cp14R cells infected with BV from CrpeNPV (B) viewed under the light microscope at 7 dpi. Polyhedral occlusion bodies are visible as dark aggregations within the nuclei of cells. Bars, 30 μm.
FIG 3.
Relative transcription rates of selected CrpeNPV genes after infection of Cp14R cells. Levels of transcripts of ie-1 (blue), lef-8 (red), dnapol (green), vp39 (orange), f-protein (light blue) and polh (purple) genes were measured by reverse transcription-qPCR at 24, 48, 72, and 96 hpi. Analyses of transcription were repeated independently in triplicate. Numbers of replicates (n) with measurable gene transcription are given above the columns. Vertical bars represent standard errors (SE).
DISCUSSION
With CrpeNPV originating from South Africa, a second tortricid-specific baculovirus, infectious to larvae of C. pomonella, was identified. CrpeNPV exhibited high virulence not only for susceptible C. pomonella larvae (CpS) but also, and more importantly, for larvae of CpRR1, CpR5M, and CpRGO, representing C. pomonella strains with all three types of CpGV resistance. Its virulence properties resemble those of resistance-breaking isolate CpGV-E2, which is the only isolate of CpGV that is known to date to be able to overcome type I, II, and III resistance (19) and which is part of the active ingredient currently used in resistance-breaking CpGV products (13). In all full-range bioassay studies conducted, the LC50 concentrations of CrpeNPV and CpGV-E2 were found to be the same in direct comparisons with all C. pomonella strains, except CpRGO. Here, the LC50 of CrpeNPV was comparable to that seen with CpGV-M but was 10-fold higher than that seen with CpGV-E2. Whether the levels of the two LC50 values measured for CrpeNPV and CpGV-M were due to the presence of common pathways to overcome the resistance remains speculative, since the viral factors as well as the host resistance-related factors are unknown. The differences between the median lethal concentrations were most likely due to the different levels of kill speed as demonstrated by the survival time analysis. Here, CrpeNPV showed a steady but low speed of kill, in comparison to CpGV-E2, for CpS, CpRR1, CpR5M, and CpRGO, indicating virulence independent of resistance type.
Comparing the LC50 values determined for CrpeNPV and CpGV, which are baculoviruses of the genera Alphabaculovirus and Betabaculovirus, respectively, it is important to consider the biological properties of their OBs; whereas betabaculoviruses include a single virion per OB, the OBs of alphabaculoviruses can contain several to hundreds of virions. As seen with CrpeNPV, multiple virions are present per OB, although the total number is unknown (20). Since the time response assays were conducted at the same OB concentrations for CrpeNPV and CpGV, the actual amount of infectious virions provided per larva must have been significantly higher for CrpeNPV than for all CpGV isolates. In consequence, it could be speculated that the virion-related virulence of CpGV-E2 is considerably higher than that of CrpeNPV, due to its lower load of virions at the same applied OB concentrations. For an experimental proof, however, the load of virions instead of the level of OBs would need to be adjusted for confirmation.
Tested in larvae of C. peltastica, the biological activity of CrpeNPV was found to be promising, with an LC50 of 6.46 × 103 OB/ml after 7 days postinfection (20). Although it is difficult to compare studies about biological activities due to different methodologies and experimental conditions, the LC50 value for C. peltastica was in the same range as the calculated median lethal concentrations for C. pomonella after 7 dpi in this study.
In terms of resistance management of C. pomonella in apple orchards, CrpeNPV could provide a promising option to combat the menace of CpGV-resistant C. pomonella infestations (12). Improved strategies for avoiding the development of new types of resistance as well as overcoming existing types of resistance could include a more diverse application of baculoviruses, such as the resistance-breaking CrpeNPV and CpGV isolates, in alternating or combined applications.
Interestingly, CrpeNPV is the first alphabaculovirus that is highly infectious to larvae of C. pomonella to be discovered so far despite the intensive research on C. pomonella and the application of CpGV in apple-growing regions in the Americas, Europe, and Asia. This report does not represent the first example of larvae of pest insects that were reported to be coinfected with both an alphabaculovirus and betabaculovirus. One earlier example is that of the summer fruit tortrix, Adoxophyes orana (Tortricidae), mainly a pest in Europe and Japan, found on various fruit types, including apple. In the United Kingdom, larvae of A. orana contained not only the alphabaculovirus Adoxophyes orana nucleopolyhedrovirus (AdorNPV) (25), the baculovirus most closely related phylogenetically to CrpeNPV (20), but also the slow-killing betabaculovirus Adoxophyes orana granulovirus (AdorGV) (26). Currently, a fast-killing isolate of AdorGV from Switzerland (Capex; Andermatt-Biocontrol AG, Switzerland) is registered for the control of A. orana. Another example of larval coinfection derived from the common cutworm Agrotis segetum (Noctuidae), where individual larvae were found to be commonly infected with Agrotis segetum granulovirus (AgseGV) and Agrotis segetum nucleopolyhedrovirus B (AgseNPV-B) (27). Whether coinfections of C. pomonella larvae by CrpeNPV and CpGV are possible and beneficial in terms of increased mortality or speed of kill needs to be evaluated.
Besides its ability to infect C. pomonella larvae, CrpeNPV was able to infect the Cp14R cell line (24), which to date is known to be permissive only to isolates of CpGV. In the present study, gene expression was measurable after 24 hpi, but not at 6 and 12 hpi, indicating a delayed infection in this cell line. The presence of transcripts at earlier time points is likely, but the abundances might be below the level of detection of the applied oligonucleotide combinations and qPCR system. With the applied method, the levels of six viral genes were readily measurable at 72 and 96 hpi but with high standard errors that were due to nonsynchronous infections and a high variations between replicates. Although only two replicates were measurable in some cases, this first initial gene expression analysis allowed a first glimpse at the possibilities of future transcriptome projects using CrpeNPV in the Cp14R cell line. Infectious BVs were verified in the cell culture supernatant, and newly produced OBs were clearly visible under the light microscope after 3 to 4 days postinfection, indicating a complete replication cycle. Whether the virus would remain infectious after serial passages through Cp14R cells and whether accumulation of defective interfering particles in the culture would occur remain unknown. Defective interfering baculoviruses are characterized by significant partial genome deletions, causing loss in virulence of the produced virus stock (28, 29). The phenomenon is well characterized for the most extensively studied baculovirus, Autographa californica multiple nucleopolyhedrovirus (AcMNPV), in cell lines of the fall army worm Spodoptera frugiperda (Sf21), where defective particles occurred after two passages (30). Baculovirus genetic stability in cell cultures is a-factor to consider with regard to the possible large-scale in vitro production of baculoviruses, which would skip the labor-intensive work with insects (22). Given the susceptibility of Cp14R to CrpeNPV, plaque purification of individual genotypes with intragenomic variation, such as was previously detected by complete-genome sequencing (20), is possible. Different plaque-purified genotypes of the Agrotis ipsilon nucleopolyhedrovirus (AgipNPV) and Spodoptera frugiperda multiple nucleopolyhedrovirus (SfMNPV) were demonstrated to have different biological activities with respect to their hosts (31, 32). Whether this is the case for CrpeNPV needs to be evaluated. A model using CrpeNPV and CpGV for studies on interactions between an alphabaculovirus and a betabaculovirus in vitro is available, and the model is further facilitated by the availability of a recombinant CpGV bacmid that expresses green fluorescent protein (GFP) under the control of a heat shock promoter, allowing easy detection of CpGV-infected cells in vitro and in vivo (33). Issues that can be addressed include the ability of these viruses to coinfect cells and whether superinfection exclusion restricts replicates to infection by only one virus at a time (34). In conclusion, the ability of CrpeNPV to infect resistant and susceptible strains of C. pomonella offers new perspectives in biological control of this pest. With only few isolates of CpGV still virulent for different CpGV-resistant C. pomonella types, CrpeNPV should be further evaluated for its potential application in C. pomonella control.
MATERIALS AND METHODS
Codling moth strains.
The following four laboratory strains of C. pomonella were established at the Institute for Biological Control (Julius Kühn Institute) in Darmstadt, Germany: susceptible strain CpS, resistance type I strain CpRR1 (12), resistance type II strain CpR5M (18), and resistance type III strain CpRGO (19) (Table 2). All resistant laboratory strains were derived from C. pomonella field populations that were further selected either by single-pair crossings (CpRR1 and CpRGO) or mass crossings (CpR5M) (18, 19, 35). All strains were reared separately under identical conditions as described previously (1).
TABLE 2.
Laboratory strains of C. pomonella and their corresponding field populationsa
| Laboratory strain | Field population | Resistance type | Reference |
|---|---|---|---|
| CpS | Susceptible | 12 | |
| CpRR1 | DE-BW-FI-03 (CpR) | I | 12 |
| CpR5M | DE-NRW-WE-08 | II | 18 |
| CpRGO | DE-SA-GO-08 | III | 19 |
The origin codes of the field populations are as follows: DE, Germany; BW, Baden-Württemberg; NRW, North Rhine-Westphalia; SA, Saxony. The numbers in the origin codes refer to the analyzed populations.
Viruses.
Two isolates of Cydia pomonella granulovirus (CpGV), namely, Mexican isolate CpGV-M (10) and English isolate CpGV-E2, the latter an in vivo clone of the English isolate CpGV-E (8, 36), and a South African isolate of Cryptophlebia peltastica single nucleopolyhedrovirus (CrpeNPV) (20) were used for all infection experiments. Before each experimental use, a light microscope using an improved Petroff-Hauser and Neubauer hemocytometer was used to determine the concentrations of OBs independently for CpGV and CrpeNPV (37).
Full-range bioassays.
Bioassays were conducted separately for CpGV-M, CpGV-E2, and CrpeNPV in neonate C. pomonella larvae of strains CpS, CpRR1, CpR5M, and CpRGO. CpGV isolates were incorporated into a modified semiartificial diet (38) at the following six concentrations: 102, 3 × 102, 103, 3 × 103, 104, and 3 × 104 OB/ml. CrpeNPV was incorporated into the Ivaldi-Sender diet for bioassays with CpS, CpRR1, and CpR5M at the following five concentrations: 1.28 × 102, 6.4 × 102, 3.2 × 103, 1.6 × 104, and 8 × 104 OB/ml. The bioassays for CpRGO with CrpeNPV were conducted at the same concentrations as for the CpGV isolates. In order to prevent thermal inactivation of the virus, the diet was cooled to 45°C before mixing with the OB suspension was performed. For the control, the OB suspension was replaced by water. The mixtures were poured into 50-well bioassay trays (Licefa, Bad Salzuflen, Germany) for solidification. A single neonate larva was added to each well of the trays with a fine paintbrush. Trays were closed and kept at 22°C at a 16-h/8-h light/dark photoperiod. After 24 h, larval mortality was checked. Larvae that did not survive were assumed to have died from handling and were removed from the analysis. After 7 and 14 days postinfection (dpi), the larval mortality was scored and used for determining the 50% and 90% lethal concentrations (LC50 and LC90, respectively). All calculations were done by probit analysis using ToxRat software (ToxRat Solutions GmbH, Alsdorf, Germany), and mortality data were corrected according to a method previously described by Abbott (39). Each bioassay was repeated in three replicates for CpS, CpRR1, and CpR5M. Bioassays based on strain CpRGO were replicated in duplicate.
Time response assay.
CpGV-M, CpGV-E2, and CrpeNPV were tested individually for laboratory strains CpS, CpRR1, CpR5M, and CpRGO at a single concentration of 2.3 × 104 OB/ml, representing a LC90 of CrpeNPV in CpR5M, determined by pilot tests. For each virus treatment, bioassay trays were prepared as mentioned above. After 72 hpi, mortality was scored every 12 h until 180 hpi. Since the cause of larval death was difficult to determine, especially at the times when the larvae were small in size (between 72 and 120 hpi) and when the deceased larvae were hidden inside the diet, as well as under conditions of occasional outbreaks of infection by a latent CpGV virus in the population, the data were not censored for the Kaplan-Meier analysis. Instead, the survival probabilities of the uninfected controls were analyzed separately and every virus treatment was normalized with its corresponding control by manually applying the Henderson-Tilton formula in Excel (Microsoft) as follows:
where n0control represents the initial number of living larvae in the control at 72 hpi, n1control represents the number of living larvae in the control at a given time point, n0treatment represents the initial number of living larvae in the treatment at 72 hpi, and n1treatment represents the number of living larvae in the treatment at a given time point.
Each virus was tested with CpS, CpRR1, and CpR5M larvae in three independent replicates, whereas CpRGO was tested in duplicate. Statistical analysis and visualization were done with R (version 3.5.1) and RStudio (version 1.1.383). Survival analysis was conducted with R packages “survival” (version 2.38) (40) and “survminer” (version 0.4.3) (41). A test for significant variation between different Kaplan-Meier curves was performed by a log rank test (level of significance, P < 0.05) followed by pairwise log rank tests for comparisons of treatments, with P value adjustments performed according to the Bonferroni method.
Tissue culture infective dose of CrpeNPV.
To obtain sufficient amounts of budded virus (BV) of CrpeNPV, about 50 third to fourth instar CpS were exposed individually overnight (18 h) to small cubes of semiartificial Ivaldi-Sender diet, applied with 104 OB of CrpeNPV. The larvae which ingested most of the provided diet cube were transferred to a virus-free diet the following day. After 5 dpi, larvae were anesthetized with diethyl ether vapor for 2 to 3 min and one of their prolegs was cut off using microscissors to collect hemolymph with a pipette. Hemolymph was transferred into IZD04 cell culture medium containing 50 μg/ml streptomycin and 100 U/ml penicillin and a small crystal of phenylthiourea (1, 24). The suspension was centrifuged at 1,000 × g for 10 min, and the BV-containing supernatant was subjected to filtration using a sterile filter (0.2-μm pore size). A volume of 10 μl of the BV stock was added to one T25 flask of confluent Cp14R cells to initiate infection and BV production. After 7 days, the BV-containing IZD04 medium (4 ml) was removed from the flask, transferred to a 15-ml centrifuge tube, and stored at 4°C.
To estimate the titer of the BV suspension obtained from the cell culture, the 50% tissue culture infective dose (TCID50) was determined in C. pomonella cell line Cp14R (24). Cp14R cells were grown in IZD04 medium to about 60% to 80% confluence in a T20 cell culture bottle. Harvesting of Cp14R cells started with removing the medium and washing the cells with 1 ml phosphate-buffered saline (PBS). The cell monolayer was then treated with 1 ml trypsin (0.05 mg/ml) and incubated for about 10 min. During this incubation step, the cells were detached carefully from the bottom of the flask by tapping it against both hands. When the cells were seen to have detached, the reaction was stopped with 2 ml of IZD04 cell culture medium that included 10% fetal bovine serum. The concentration of living cells was determined by mixing a 10-μl cell suspension with 40 μl IZD04 medium and 50 μl trypan blue, followed by counting of nonblue cells in an improved Neubauer hemocytometer. Based on the measured concentration of cells, the concentration of the stock suspension of cells was adjusted to 4 × 105 cells/ml by dilution with IZD04 medium. From the adjusted stock suspension, a 5-μl volume containing 2 × 10³ cells was seeded in each well of a 60-well microtiter plate. From the freshly prepared BV stock of CrpeNPV, a serial 1:10 dilution ranging from 10−1 to 10−6 was made with IZD04 medium. For each dilution step, a 5-μl volume was added to nine wells of a row. The remaining six wells of the plate served as the uninfected control (sterile IZD04 medium was added). The microtiter plate was placed in an incubator at 26°C and high humidity to avoid evaporation. After 7 dpi, infected wells were scored under the light microscope according to the presence of CrpeNPV OBs inside Cp14R cells. Calculation of the 50% tissue culture infective dose (TCID50) was performed as described previously (37).
Time course experiment.
For the gene expression analysis of CrpeNPV-infected Cp14R cells, 1 × 105 cells were seeded in a total volume of 2 ml IZD04 cell culture medium in each well of a 6-well plate. After 3 days of incubation, the cell culture medium was removed and each well was infected with 500 μl CrpeNPV BV at multiplicity of infection (MOI) of 2.5, which was calculated based on the results of the previously conducted TCID50 experiment. After incubation for 3 h, BV suspensions were replaced with fresh ICD04 medium. For the uninfected control, an additional 6-well plate was set up and treated likewise, except that 500 μl of virus-free medium was used instead of the BV suspension.
After 6, 12, 24, 48, 72, and 96 hpi, all cells of one BV-treated well were harvested. To do so, all medium was removed and the cells were washed twice with 1 ml of 1× PBS buffer. The cells were detached by scratching with the tip of a 1-ml pipette and then collected in 1 ml of 1× PBS buffer and transferred to a 15-ml centrifuge tube. At 96 hpi, all cells of the uninfected control well were collected following the same protocol. Cells were centrifuged at 1,000 × g for 10 min at room temperature, and the cell pellets were stored at −80°C.
RNA isolation and cDNA synthesis.
For RNA isolation, the frozen cells from the time course experiment were thawed and washed once with 1 ml of 1× PBS buffer. RNA was extracted from all samples by following the protocol of a ReliaPrep RNA tissue miniprep kit (Promega GmbH, Germany). RNA was eluted in a final volume of 15 μl of nuclease-free H2O. The RNA concentrations ranged from 13.5 ng/μl (12 hpi) to 82.8 ng/μl (72 hpi) as measured with a NanoDrop 2000c spectrophotometer and were processed for all following steps. To exclude any DNA contamination, the remaining 14 μl of each sample was subjected to an additional DNase I treatment. Each treatment mixture consisted of 14 μl RNA sample, 9.5 μl nuclease-free H2O, 3 μl DNase I buffer (Thermo Fisher Scientific), and 0.5 μl of 5 U DNase I (Thermo Fisher Scientific) and was incubated for 10 min at 37°C. Subsequently, the DNase I activity was stopped by adding 3 μl of 50 mM EDTA followed by an incubation step performed for 10 min at 75°C. Synthesis of cDNA was performed on half (15 μl) of the DNase-treated sample according to the protocol specified for an iScript cDNA synthesis kit (Bio-Rad). For each RNA sample, 15 μl RNA template, 4 μl reaction buffer, and 1 μl reverse transcriptase were combined. To measure any remaining DNA contamination within the RNA samples, second pairs of reaction mixtures were set up that included replacement of the reverse transcriptase by 1 μl of nuclease-free H2O. All samples were incubated in a thermal cycler for 5 min at 25°C, 20 min at 46°C, and 1 min for 95°C. Samples were stored at 4°C until further use.
Gene expression analysis.
The quantitative amount of gene transcripts (relative to that of the actin host housekeeping gene) was measured from cDNA samples for CrpeNPV genes polyhedrin (polh), virus protein 39 (vp39), immediate early 1 (ie-1), late expression factor 8 (lef-8), f-protein, and DNA polymerase (dnapol) (Table 3). Open reading frame-specific oligonucleotide primers were designed based on the whole-genome sequence of CrpeNPV (GenBank accession no. MH394321) (20) (Table 3). Housekeeping gene actin of C. pomonella was used as an internal standard (Table 3). Each qPCR reaction was performed in a total volume of 25 μl containing 1× Maxima SYBR green/Rox qPCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA), a 0.4 pM concentration of each primer, and 2 μl template. As the template, 2 μl of either cDNA or untranscribed RNA (DNA control) was used. Nontarget controls contained 2 μl of nuclease-free H2O instead. Quantitative PCRs were started with an initial denaturation step of 95°C for 5 min, followed by 30 cycles of denaturation (95°C for 1 min), annealing (58°C for 1 min), and elongation (72°C for 1 min). A final elongation step was performed for 5 min at 72°C. Melting curve analysis was done from 40 to 95°C with an increment of 0.5°C each 5 s. All qPCR samples were run in a CFX96 real-time system (Bio-Rad, Hercules, CA, USA). Raw data were loaded into Bio-Rad CFX Manager 2.0 software (Bio-Rad, Hercules, CA, USA) and exported to Excel (Microsoft) for normalization with the housekeeping gene expression of actin and analysis of relative expression levels.
TABLE 3.
Oligonucleotide primers used for the relative quantitation of viral and host transcripts by qPCRa
| Target gene |
Position in genome |
Primer name | Primer sequence (5′ to 3′) | Size (bp) |
|---|---|---|---|---|
| polh | 367–389 | prCrpeNPV_polh_fwd | GTTAACATGCGCCCGACTAG | 159 |
| 506–525 | prCrpeNPV_polh_rev | GCGCTTGGCCAGACTAATAC | ||
| ie-1 | 22001–22020 | prCrpeNPV_ie-1_fwd | TACAGACGACCGCCGATTTA | 156 |
| 22137–22156 | prCrpeNPV_ie-1_rev | ATGCAACTGTAACGAGCAGC | ||
| lef-8 | 32325–32344 | prCrpeNPV_lef-8_fwd | GTAAGGATCCTCAGCCGTCA | 154 |
| 32459–32478 | prCrpeNPV_lef-8_rev | TTTGTCCAGCAGTGAAACCG | ||
| dnapol | 54688–54707 | prCrpeNPV_dnapol_fwd | TGCACCGAAGACGCTTTAAG | 168 |
| 54862–54855 | prCrpeNPV_dnapol_rev | ACGGTACAAATTATCGCGGC | ||
| vp39 | 64790–64809 | prCrpeNPV_vp39_fwd | CTGCCAACGGTAAACCTTCC | 158 |
| 64928–64947 | prCrpeNPV_vp39_rev | AATGGTTTTGCCCATACCCG | ||
| f-protein | 105755–105774 | prCrpeNPV_fusion_fwd | TCAAATCGAACTTGGCCACG | 162 |
| 105897–105961 | prCrpeNPV_fusion _rev | AGCGCATCAATGAACTTGCC | ||
| actin | actin_Cp_f | TGGGACAGAAGGACTCGTAC | 223 | |
| actin_Cp_r | TGGGTCATCTTTTCTCTGTTG | |||
Supplementary Material
ACKNOWLEDGMENTS
We thank Birgit Ruoff for the excellent support in bioassay studies and cell culture experiments as well as data analysis, Karin Undorf-Spahn and Eva Fritsch for their experimental advice concerning the bioassay studies, and Robin Nikolei for carefully reading the manuscript, as well as Doris El Mazouar and Eckhard Gabrys for the technical assistance.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.00795-19.
REFERENCES
- 1.Asser-Kaiser S, Radtke P, El-Salamouny S, Winstanley D, Jehle JA. 2011. Baculovirus resistance in codling moth (Cydia pomonella L.) caused by early block of virus replication. Virology 410:360–367. doi: 10.1016/j.virol.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Huber J. 1998. Western Europe, p 201–215. In Hunter-Fujita FR, Entwistle PF, Evans HF, Crook NE (ed), Insect viruses and pest management. Wiley, New York, NY. [Google Scholar]
- 3.Lacey LA, Thomson D, Vincent C, Arthurs SP. 2008. Codling moth granulovirus: a comprehensive review. Biocontrol Sci Technol 18:639–663. doi: 10.1080/09583150802267046. [DOI] [Google Scholar]
- 4.ICTV. 2016. Virus taxonomy: 2016 release. International Committee on Taxonomy of Viruses, London, United Kingdom: http://www.ictvonline.org/virustaxonomy.asp. [Google Scholar]
- 5.Lange M, Jehle JA. 2003. The genome of the Cryptophlebia leucotreta granulovirus. Virology 317:220–236. doi: 10.1016/S0042-6822(03)00515-4. [DOI] [PubMed] [Google Scholar]
- 6.Huber J. 1986. Use of baculoviruses in pest management programs, p 181–202. In Granados RR, Federici BA (ed), The biology of baculoviruses. Volume II Practical application for insect control. CRC Press, Boca Raton, Florida. [Google Scholar]
- 7.Herniou EA, Arif BM, Becnel JJ, Blissard GW, Bonning BC, Harrison RL, Jehle JA, Theilmann DA, Vlak JM. 2011. Baculoviridae, p 163–174. In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ (ed), Virus taxonomy. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 8.Gebhardt MM, Eberle KE, Radtke P, Jehle JA. 2014. Baculovirus resistance in codling moth is virus isolate-dependent and the consequence of a mutation in viral gene pe38. Proc Natl Acad Sci U S A 111:15711–15716. doi: 10.1073/pnas.1411089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wennmann J, Radtke P, Eberle K, Gueli Alletti G, Jehle J. 2017. Deciphering single nucleotide polymorphisms and evolutionary trends in isolates of the Cydia pomonella granulovirus. Viruses 9:227. doi: 10.3390/v9080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanada Y. 1964. A granulosis virus of the codling moth, Carpocapsa pomonella (Linnaeus) (Olethreutidae, Lepidoptera). J Invertebr Pathol 6:378–380. [DOI] [PubMed] [Google Scholar]
- 11.Jehle JA, Schulze-Bopp S, Undorf-Spahn K, Fritsch E. 2017. Evidence for a second type of resistance against Cydia pomonella granulovirus in field populations of codling moths. Appl Environ Microbiol 83:e02330-16. doi: 10.1128/AEM.02330-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asser-Kaiser S, Fritsch E, Undorf-Spahn K, Kienzle J, Eberle KE, Gund NA, Reineke A, Zebitz CP, Heckel DG, Huber J, Jehle JA. 2007. Rapid emergence of baculovirus resistance in codling moth due to dominant, sex-linked inheritance. Science 317:1916–1918. doi: 10.1126/science.1146542. [DOI] [PubMed] [Google Scholar]
- 13.Alletti G, Sauer AJ, Weihrauch B, Fritsch E, Undorf-Spahn K, Wennmann JT, Jehle JA. 2017. Using next generation sequencing to identify and quantify the genetic composition of resistance-breaking commercial isolates of Cydia pomonella granulovirus. Viruses 9:250. doi: 10.3390/v9090250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berling M, Blachere-Lopez C, Soubabere O, Lery X, Bonhomme A, Sauphanor B, Lopez-Ferber M. 2009. Cydia pomonella granulovirus genotypes overcome virus resistance in the codling moth and improve virus efficiency by selection against resistant hosts. Appl Environ Microbiol 75:925–930. doi: 10.1128/AEM.01998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberle KE, Asser-Kaiser S, Sayed SM, Nguyen HT, Jehle JA. 2008. Overcoming the resistance of codling moth against conventional Cydia pomonella granulovirus (CpGV-M) by a new isolate CpGV-I12. J Invertebr Pathol 98:293–298. doi: 10.1016/j.jip.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Graillot B, Bayle S, Blachere-Lopez C, Besse S, Siegwart M, Lopez-Ferber M. 2016. Biological characteristics of experimental genotype mixtures of Cydia pomonella granulovirus (CpGV): ability to control susceptible and resistant pest populations. Viruses 8:147. doi: 10.3390/v8050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kienzle J, Zimmer J, Volk F, Zebitz CP. 2008. Field tests with Madex Plus against CpGV-resistant codling moth populations in organic orchards in 2006. Ecofruit. Proceedings of the 13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing. Fördergemeinschaft Ökol Obstbau EV FOKO, Weinsberg, Germany. [Google Scholar]
- 18.Sauer AJ, Fritsch E, Undorf-Spahn K, Nguyen P, Marec F, Heckel DG, Jehle JA. 2017. Novel resistance to Cydia pomonella granulovirus (CpGV) in codling moth shows autosomal and dominant inheritance and confers cross-resistance to different CpGV genome groups. PLoS One 12:e0179157. doi: 10.1371/journal.pone.0179157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauer AJ, Schulze-Bopp S, Fritsch E, Undorf-Spahn K, Jehle JA. 2017. A third type of resistance to Cydia pomonella granulovirus in codling moths shows a mixed Z-linked and autosomal inheritance pattern. Appl Environ Microbiol 83:e01036-17. doi: 10.1128/AEM.01036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsberg T, Jukes MD, Krejmer-Rabalska M, Rabalski L, Knox CM, Moore SD, Hill MP, Szewczyk B. 2018. Morphological, genetic and biological characterisation of a novel alphabaculovirus isolated from Cryptophlebia peltastica (Lepidoptera: Tortricidae). J Invertebr Pathol 157:90–99. doi: 10.1016/j.jip.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Rohrmann GF, Erlandson MA, Theilmann DA. 30 January 2014, posting date Genome sequence of an alphabaculovirus isolated from Choristoneura murinana. Genome Announc 2:e01135-13. doi: 10.1128/genomeA.01135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen Q, Qi YM, Wu Y, Chan LCL, Nielsen LK, Reid S. 2011. In vitro production of Helicoverpa baculovirus biopesticides—automated selection of insect cell clones for manufacturing and systems biology studies. J Virol Methods 175:197–205. doi: 10.1016/j.jviromet.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Naser WL, Harvey JP, Huger AM, Huber J. 1987. Choristoneura murinana nuclear polyhedrosis virus: comparative biochemical and biological examination of replication in vivo and in vitro. J Gen Virol 68:1251–1260. doi: 10.1099/0022-1317-68-5-1251. [DOI] [Google Scholar]
- 24.Winstanley D, Crook NE. 1993. Replication of Cydia pomonella granulosis virus in cell cultures. J Gen Virol 74:1599–1609. doi: 10.1099/0022-1317-74-8-1599. [DOI] [PubMed] [Google Scholar]
- 25.Hilton S, Winstanley D. 2008. Biological characterization of an English granulovirus from the summer fruit tortrix moth, Adoxophyes orana. J Invertebr Pathol 97:298–305. doi: 10.1016/j.jip.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 26.Hilton S, Winstanley D. 2008. Genomic sequence and biological characterization of a nucleopolyhedrovirus isolated from the summer fruit tortrix, Adoxophyes orana. J Gen Virol 89:2898–2908. doi: 10.1099/vir.0.2008/002881-0. [DOI] [PubMed] [Google Scholar]
- 27.Wennmann JT, Köhler T, Gueli Alletti G, Jehle JA. 2015. Mortality of cutworm larvae is not enhanced by Agrotis segetum granulovirus and Agrotis segetum nucleopolyhedrovirus B coinfection relative to single infection by either virus. Appl Environ Microbiol 81:2893–2899. doi: 10.1128/AEM.03726-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kool M, Voncken JW, Van Lier FLJ, Tramper J, Vlak JM. 1991. Detection and analysis of Autographa californica nuclear polyhedrosis virus mutants with defective interfering properties. Virology 183:739–746. doi: 10.1016/0042-6822(91)91003-Y. [DOI] [PubMed] [Google Scholar]
- 29.Krell PJ. 1996. Passage effect of virus infection in insect cells. Cytotechnology 20:125–137. doi: 10.1007/BF00350393. [DOI] [PubMed] [Google Scholar]
- 30.Pijlman GP, Dortmans J, Vermeesch AMG, Yang K, Martens DE, Goldbach RW, Vlak JM. 2002. Pivotal role of the non-hr origin of DNA replication in the genesis of defective interfering baculoviruses. J Virol 76:5605–5611. doi: 10.1128/jvi.76.11.5605-5611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison RL. 2013. Concentration- and time-response characteristics of plaque isolates of Agrotis ipsilon multiple nucleopolyhedrovirus derived from a field isolate. J Invertebr Pathol 112:159–161. doi: 10.1016/j.jip.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Harrison RL, Puttler B, Popham H. 2008. Genomic sequence analysis of a fast-killing isolate of Spodoptera frugiperda multiple nucleopolyhedrovirus. J Gen Virol 89:775–790. doi: 10.1099/vir.0.83566-0. [DOI] [PubMed] [Google Scholar]
- 33.Hilton S, Kemp E, Keane G, Winstanley D. 2008. A bacmid approach to the genetic manipulation of granuloviruses. J Virol Methods 152:56–62. doi: 10.1016/j.jviromet.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Beperet I, Irons SL, Simon O, King LA, Williams T, Possee RD, Lopez-Ferber M, Caballero P. 2014. Superinfection exclusion in alphabaculovirus infections is concomitant with actin reorganization. J Virol 88:3548–3556. doi: 10.1128/JVI.02974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Undorf-Spahn K, Fritsch E, Huber J, Kienzle J, Zebitz CPW, Jehle JA. 2012. High stability and no fitness costs of the resistance of codling moth to Cydia pomonella granulovirus (CpGV-M). J Invertebr Pathol 111:136–142. doi: 10.1016/j.jip.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Crook NE, Spencer RA, Payne CC, Leisy DJ. 1985. Variation in Cydia pomonella granulosis virus isolates and physical maps of the DNA from three variants. J Gen Virol 66:2423–2430. doi: 10.1099/0022-1317-66-11-2423. [DOI] [Google Scholar]
- 37.Eberle KE, Wennmann JT, Kleespies RG, Jehle JA. 2012. Chapter II: basic techniques in insect virology, p 15–74. In Lacey LA. (ed), Manual of techniques in invertebrate pathology, 2nd ed Academic Press, San Diego, CA. [Google Scholar]
- 38.Ivaldi-Sender C. 1974. Techniques simples pour elevage permanent de la tordeuse orientale, Grapholita molesta (Lep., Tortricidae), sur milieu artificiel. Ann Zool Ecol Anim 2:337–343. [Google Scholar]
- 39.Abbott WS. 1925. A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 40.Therneau TM. 2015. A package for survival analysis in S version 2:38. https://CRAN.R-project.org/package=survival.
- 41.Kassambara A, Kosinski M. 2008. Survminer: drawing survival curves using “ggplot2”. R package version 0.4.3. https://cloud.r-project.org/web/packages/survminer/index.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.