Abstract
Key discoveries in aging research have been made possible with the use of model organisms. Caenorhabditis elegans is a short-lived nematode that has become a well-established system to study aging. The practicality and powerful genetic manipulations associated with this metazoan have revolutionized our ability to understand how organisms age. 25 years after the publication of the discovery of the daf-2 gene as a genetic modifier of lifespan, C. elegans remains as relevant as ever in the quest to understand the process of aging. Nematode aging research has proven useful in identifying transcriptional regulators, small molecule signals, cellular mechanisms, epigenetic modifications associated with stress resistance and longevity, and lifespan-extending compounds. Here, we review recent discoveries and selected topics that have emerged in aging research using this incredible little worm.
Keywords: C. elegans, Aging, Proteostasis, Epigenetic, Transcription factors
1. Longevity-associated transcriptional regulators
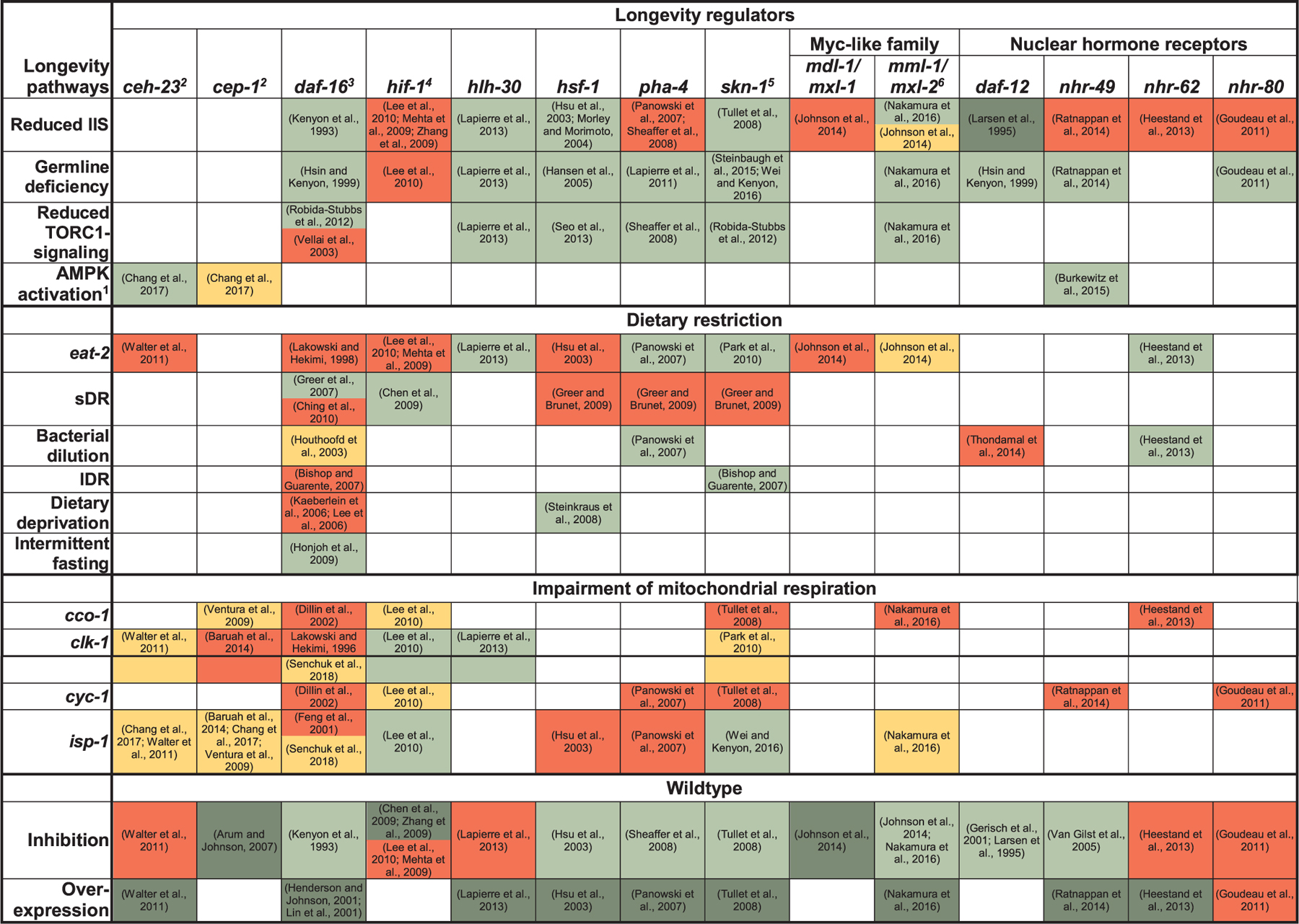
The model organism C. elegans was fundamental in establishing that aging is regulated by cellular signaling pathways that sense environmental or internal stress (Kenyon, 2010). Examples for such stresses or perturbations that affect C. elegans lifespan include reduced insulin/IGF-1 like signaling (IIS), germline ablation, dietary restriction (DR, i.e. reduced food intake without starvation), reduced TOR-activity, and inhibition of the mitochondrial electron transport chain (ETC) (Kenyon, 2010; Riera et al., 2016). Yet, it is increasingly becoming clear that different upstream stimuli employ partially overlapping sets of downstream mediators and processes that ultimately produce lifespan extension. Examples for such mediators include the widely conserved transcription factors DAF-16 (FOXO), HLH-30 (TFEB), PHA-4 (FOXA), HIF-1 (HIF1A), HSF-1 (HSF1) SKN-1 (NRF2), as well as nuclear hormone receptors (Table 1). Notably, maintaining coordinated expression of genes in various stress resistance pathways and avoiding transcriptional drift allows animals to live longer (Rangaraju et al., 2015). Currently, the regulation and integration of the activity of these transcription factors with environmental and metabolic stimuli is not completely understood. Recent studies have focused on characterizing modifications and interactions between these transcription factors and new regulators with roles in lifespan modulation.
Table 1.
Major longevity pathways and longevity-associated transcription factors in C. elegans. Other classes of regulators such as micro RNAs and transcriptional coregulators were omitted for simplicity. Green shading indicates that a factor is required for a particular lifespan-extending treatment (RNAi or loss/reduction of function mutation, or dietary restriction regimen) to extend lifespan or to maintain normal lifespan in otherwise wildtype animals. Yellow shading indicates a partial requirement, red shading no requirement, dark green further extension, and white not explicitly tested. sDR: solid DR, lDR: liquid DR. Cf. (Greer and Brunet, 2009) for a more detailed description of these dietary restriction regimens. Note that (Greer and Brunet, 2009) list additional DR-methods not included in this table (Chen et al., 2009; Dillin et al., 2002; Feng et al., 2001; Gerisch et al., 2001; Hansen et al., 2005; Honjoh et al., 2009; Houthoofd et al., 2003; Hsin and Kenyon, 1999; Hsu et al., 2003; Kaeberlein et al., 2006; Kenyon et al., 1993; Lakowski and Hekimi, 1998; Larsen et al., 1995; Lee et al., 2006; Mehta et al., 2009; Morley and Morimoto, 2004; Park et al., 2010; Robida-Stubbs et al., 2012; Senchuk et al., 2018; Seo et al., 2013; Steinbaugh et al., 2015; Steinkraus et al., 2008; Vellai et al., 2003; Zhang et al., 2009; Lee et al., 2010; Lapierre et al., 2013; Panowski et al., 2007; Sheaffer et al., 2008; Tullet et al., 2008; Johnson et al., 2014; Nakamura et al., 2016; Ratnappan et al., 2014; Heestand et al., 2013; Goudeau et al., 2011; Wei and Kenyon, 2016; Lapierre et al., 2011; Van Gilst et al., 2005a; Henderson and Johnson, 2001; Lin et al., 2001; Arum and Johnson, 2007; Walter et al., 2011; Baruah et al., 2014; Chang et al., 2017a; Ventura et al., 2009; Thondamal et al., 2014; Bishop and Guarente, 2007; Greer and Brunet, 2009; Greer et al., 2007; Ching et al., 2010; Burkewitz et al., 2015).
|
Notes.
AMPK activation achieved by transgenic overexpression of a constitutively active aak-2 (AMPKα) construct; note that aak-2 is also required for longevity upon sDR and mutation of the TORC1 substrate rsks-1 (Chen et al., 2013; Greer and Brunet, 2009; Greer et al., 2007), partially upon bacterial dilution and daf-2, isp-1 or clk-1 mutation (Apfeld et al., 2004; Chen et al., 2013; Curtis et al., 2006; Greer and Brunet, 2009), but not upon eat-2 mutation or germline deficiency (Curtis et al., 2006; Greer and Brunet, 2009); aak-2 mutants are shorter-lived than wildtype (Apfeld et al., 2004).
ceh-23 and cep-1: these two transcription factors act in a common pathway to modulate lifespan of ETC-compromised worms (Chang et al., 2017a).
daf-16: sDR-regimens used by (Greer et al., 2007) and (Ching et al., 2010) differed in terms of plate preparation and were initiated at different times of life (day 4 of adulthood vs day 1 of adulthood); isoforms used in overexpression studies in wildtype were daf-16a1 (Lin et al., 2001) and a2 (Henderson and Johnson, 2001).
hif-1: Differences in the observed effects of hif-1 null mutations on wildtype lifespan may in part be due to different temperature regimens used in the respective studies (Lee et al., 2010)
skn-1: Effect of overexpression of skn-1 on lifespan was examined using a transgene coding for the SKN-1B/C isoforms (Tullet et al., 2008).
mml-1/mxl-2: Although (Johnson et al., 2014) and (Nakamura et al., 2016) both used the same mutants [mml-1(ok8499), mxl-2(tm1516)], culture conditions differed in terms of the bacterial food source (HT115 vs OP50) and the use of FUDR (400 μM vs no FUDR).
1.1. DAF-16 (FOXO)
The sole C. elegans member of the evolutionarily conserved forkhead box O (FOXO) family of transcription factors is encoded by the gene daf-16, which plays key roles in maintaining homeostasis under stress and in extending lifespan in response to various stimuli (reviewed in (Eijkelenboom and Burgering, 2013; Kenyon, 2010)). How do cells modulate DAF-16 activity? Subcellular localization, transcriptional activity and stability of DAF-16 are tightly regulated by posttranslational modifications (Calnan and Brunet, 2008). When activity of the insulin/IGF1-like receptor is reduced, phosphorylation of DAF-16 by AKT-1/2 and binding of 14–3-3 proteins ceases and DAF-16 can accumulate in the nucleus (Kenyon, 2010; Manning and Toker, 2017). The nuclear import of DAF-16 can also be modulated by reactive oxygen species (ROS) via disulfide bond formation with transportin-1 (IMB-2) (Putker et al., 2013). Several factors have been identified that regulate DAF-16 by targeting its upstream kinase AKT-1, such as the SCF ubiquitin ligase complex and daf-12 (cf. below) via micro-RNAs mir-84 and mir-243 (Chaudhari and Kipreos, 2017; Shen et al., 2012). Post-translational modifications of DAF-16 include phosphorylation by AAK-2 (AMPK) (Greer et al., 2007), acetylation by CBP-1 (p300/CBP) (Chiang et al., 2012b), methylation by PRMT-1 (PRMT1) (Takahashi et al., 2011), ubiquitylation by RLE-1 (RC3H1/Roquin-1) (Li et al., 2007) and deubiquitylation by MATH-33 (USP7/HAUSP) (Heimbucher et al., 2015).
MBK-1, the C. elegans ortholog of the mammalian FOXO1 kinase DYRK1A also modulates DAF-16, but a kinase-substrate relationship has not formally been established (Mack et al., 2017). Nuclear factors also modulate DAF-16 function and they include histone deacetylase SIR-2.1 (Berdichevsky et al., 2006), transcriptional regulator HCF-1 (HCF1) (Li et al., 2008), nuclear mRNA exporter HEL-1 (Seo et al., 2015), the SWI/SNF-complex (Riedel et al., 2013) and, potentially, ZFP-1 (AF10) (Riedel et al., 2013; Singh et al., 2016). In addition, the plasma membrane-associated protein EAK-7 (TLDC1) (Alam et al., 2010), the PP4 regulatory subunit SMK-1 (PPP4R3A) (Wolff et al., 2006), the neuronal micro-RNA mir-71 (Boulias and Horvitz, 2012), the cytoskeletal adapter protein KRI-1 (KRIT1/CCM1) (Berman and Kenyon, 2006), the transcription elongation factor TCER-1 (TCERG1) (Ghazi et al., 2009), the RNA-binding protein PHI-62 (RNASEK) (McCormick et al., 2012) and the C-type lectin domain containing protein IRG-7 (Yunger et al., 2017) can modulate DAF-16 function, but their mechanism of action is not fully understood. Altogether, these modifications modulate DAF-16-mediated stress resistance and longevity.
1.2. HLH-30 (TFEB)
A key longevity mechanism is the autophagy process (see cellular mechanisms of longevity below) and it is in part modulated by transcription (reviewed in (Lapierre et al., 2015)). A major regulator of autophagy and lysosomal gene expression is the Transcription Factor EB (TFEB), an autophagy enhancer found in C. elegans as HLH-30. HLH-30/TFEB is required for the autophagic response to starvation (O’Rourke and Ruvkun, 2013; Settembre et al., 2013), for innate immunity (Visvikis et al., 2014) and for lifespan extension in different long-lived nematode mutants (Lapierre et al., 2013). The nuclear localization of HLH-30/TFEB is modulated via phosphorylation by mTOR (Lapierre et al., 2013; Roczniak-Ferguson et al., 2012; Settembre et al., 2011). In the nucleus, HLH-30/TFEB function is regulated via competition with MXL-3/MAX (O’Rourke and Ruvkun, 2013) and by interaction with proteins of the Mondo-complex (Nakamura et al., 2016). Interestingly, HLH-30/TFEB and DAF-16/FOXO are both required for longevity associated with reduced lipid secretion (Seah et al., 2016), suggesting potential nuclear interactions between these transcription factors. The nuclear export of HLH-30/TFEB is regulated by nuclear export protein XPO-1/XPO1 and selective inhibitors of nuclear export enhance HLH-30/TFEB activity (Silvestrini et al., 2018). Consequently, enhancing lysosomal function pharmacologically via HLH-30/TFEB activation leads to lifespan extension in C. elegans (Silvestrini et al., 2018; Wang et al., 2017). Therefore, modulation of HLH-30/TFEB nuclear localization may be an exploitable strategy to stimulate the autophagy/lysosomal pathway and improve somatic maintenance.
1.3. PHA-4 (FOXA)
Another member of the forkhead box family of transcription factors, PHA-4(FOXA), was originally identified as a central factor in foregut development (reviewed in (Mango, 2009)) and later found to also be a key transcription factor in lifespan extension upon dietary restriction (Panowski et al., 2007). PHA-4 was also found to be important in the long lifespan of germline-less animals (Lapierre et al., 2011; O’Rourke et al., 2013). The expression of the transcription factors pha-4 and skn-1 (see below) can be modulated by micro RNAs miR-71 and miR-228 (Smith-Vikos et al., 2014). In line with its negative role on lifespan, TOR signaling impairs the function of PHA-4 (Lapierre et al., 2011; Sheaffer et al., 2008). During development, PHA-4 binds promoters of multiple genes (Zhong et al., 2010) and affects chromatin dynamics and RNA polymerase function (Fakhouri et al., 2010; Hsu et al., 2015). PHA-4′s role also includes the modulation of the expression of superoxide dismutases and autophagy genes associated with lifespan extension (Lapierre et al., 2011; Panowski et al., 2007).
1.4. HIF-1 (HIF1)
The hypoxia-inducible factor-1 (HIF-1) has been linked to lifespan extension in various longevity models (reviewed in (Hwang and Lee, 2011)). For instance, in C. elegans, reduction of the conserved acyl-CoA binding protein MMA-1/ACBP-1 (Shamalnasab et al., 2017) or the inhibition of the E3 ligase elongin (Hwang et al., 2015) require HIF-1 activation for lifespan extension. Moreover, in the mitochondrial mutant isp-1, where ketoacids levels are elevated (Butler et al., 2013), HIF-1 activity is increased and contributes to the lifespan extension (Mishur et al., 2016). Interestingly, supplementing animals with ketoacid α-ketoglutarate is sufficient to extend lifespan in C. elegans by reducing the ability of mitochondria to produce ATP, thereby activating autophagy (Chin et al., 2014). Iron starvation by frataxin suppression also stimulates mitochondrial autophagy (mitophagy) in part via HIF-1 activation (Schiavi et al., 2015). Recent work has uncovered that neuronal HIF-1 modulates serotonin signaling to the intestine, where a xenobiotic response is elicited via HLH-30-regulated expression of flavin-containing monooxygenase 2 (Leiser et al., 2015). Altogether, the requirement of HIF-1 on lifespan extension appears to be context-dependent (Table 1).
1.5. HSF-1 (HSF1)
The heat shock transcription factor HSF-1 (HSF1) increases the expression of chaperones in response to various proteotoxic stressors, including but not limited to heat (reviewed in (Li et al., 2017)). More recently, maintenance of cytoskeletal integrity was identified as another mechanism through which HSF-1 increases thermotolerance (Akerfelt et al., 2010; Baird et al., 2014). Under non-stress conditions, HSF-1 regulates developmental and metabolic genes as well as genes involved in collagen biogenesis (Akerfelt et al., 2010; Brunquell et al., 2016). As in other organisms, activation of C. elegans HSF-1 upon heat shock involves oligomerization and apparently, changes in posttranslational modifications, including phosphorylation (Anckar and Sistonen, 2011; Chiang et al., 2012a). Reduction of insulin/IGF-1 signaling, but not heat, activates HSF-1 by promoting phosphorylation of DDL-1 (CCDC53) by an unidentified kinase, which leads to destabilization of the DDL-1/DDL-2 (WASH2)/HSB-1 (HSBP1)-complex that inhibits HSF-1. Upon heat shock, at least in larvae, but apparently not in adult worms (Berber et al., 2016), the protein kinase HPK-1 indirectly activates HSF-1 by interfering with inhibitory HSF-1 sumoylation (Das et al., 2017). HSF-1 is also subjected to complex regulation during thermal stress and DR by the integrin-linked kinase PAT-4 (ILK) and the deacetylase SIR-2.1 (SIRT1) (Kumsta et al., 2014; Raynes et al., 2012). While persistent heat stress is unequivocally detrimental to nematode survival, it is interesting to note that intermittent heat shock can extend lifespan via HSF-1 activation (Kumsta et al., 2017).
1.6. SKN-1 (NRF2)
Beyond its function in inducing phase II detoxification genes upon oxidative stress, SKN-1 (NRF-2) has been implicated in the response to other stressors such as ER stress and starvation, and in various homeostatic processes even in the absence of stress, such as proteostasis and lipid metabolism (reviewed in (Blackwell et al., 2015)). Interestingly, in the context of reduced IIS, skn-1 is only required for longevity under conditions that do not induce dauer-like traits (Ewald et al., 2015; Tullet et al., 2008). Under basal conditions, SKN-1 is inhibited by phosphorylation by AKT-1/2, SGK-1 (Tullet et al., 2008) and GSK-3 (An et al., 2005) while upon oxidative stress, SKN-1 is activated by PMK-1/p38 MAP-kinase dependent phosphorylation (Inoue et al., 2005). Apparently downstream of PMK-1, GSK-3 and IIS-signaling, the WD40-repeat protein WDR-23 and the CUL-4/DDB-1 E3 ligase complex modulate SKN-1 activity (Choe et al., 2009). Importantly, a similar WDR23-DDB1-CUL4 axis appears to regulate NRF2 in mammalian cells independently to the previously established KEAP1-CUL3 axis (Lo et al., 2017). SKR-1/2 (orthologues of the mammalian SCF-ubiquitin ligase complex member SKP1) also promote SKN-1 target gene expression upon oxidative stress (Wu et al., 2016a) and were reported to be required for longevity of daf-2 mutant C. elegans (Ghazi et al., 2007). Evidence suggested that DAF-16 is a target of SKR-1/2, although SKN-1 was not examined in this context (Ghazi et al., 2007). Interestingly, a recent study suggested that skn-1 can be transcriptionally regulated by daf-16 and that skn-1 mediated stress resistance may not be necessary for longevity (Tullet et al., 2017). However, whether these regulatory connections are limited to artificial settings such daf-16 overexpression remains unclear.
1.7. Nuclear hormone receptors
Signaling via nuclear hormone receptors affects metabolism, xenobiotic responses, stress resistance and longevity (reviewed in (Hoffmann and Partridge, 2015)). For instance, the nuclear hormone receptor DAF-12 and bile acid like steroids called dafachronic acids (DA) (Antebi, 2013) are required for germline-longevity and metabolomics analyses identified specific DAs as endogenous ligands for DAF-12 (Mahanti et al., 2014; Motola et al., 2006). DA biosynthesis appears to be distributed across several tissues and may include contributions from the somatic gonad, consistent with the notion that DAs contribute to the longevity-promoting signal from this tissue (Antebi, 2013). While exogenous DA can increase the lifespan of somatic gonad-deficient, but not of somatic gonad-competent, germline-less animals (Yamawaki et al., 2010), DA’s ability to extend wildtype lifespan is controversial (Gerisch et al., 2007; Yamawaki et al., 2010), and this requirement may be more robust at 25 °C (Li et al., 2015). Moreover, there are conflicting reports on elevated DA-levels in germline-deficient glp-1 animals (Li et al., 2015; Shen et al., 2012). Recently, DA has also been implicated in DR-induced longevity, but in this context, DA signals through the nuclear hormone receptor NHR-8, rather than DAF-12, to repress let-363 (mTOR) mRNA-levels (Antebi, 2013; Thondamal et al., 2014). Lifespan extension by DR was also linked to NHR-62 (HNF4a)-mediated gene regulation (Heestand et al., 2013).
NHR-80 is another nuclear hormone receptor whose elevated expression is required for the longevity of germline-less animals (Goudeau et al., 2011). This NHR-80 upregulation is only partially dependent on daf-12 and daf-16, (Goudeau et al., 2011). Moreover, NHR-80 has been reported to physically interact with NHR-49 (HNF4/PPARa) (Pathare et al., 2012) and NHR-49 is also upregulated in germline-less glp-1 animals, however, dependent on daf-16 (Ratnappan et al., 2014). In addition to its role in modulating expression of β-oxidation genes (Van Gilst et al., 2005a,b), NHR-49 has recently been shown to mediate a transcriptional response to fasting and oxidative stress (Goh et al., 2018). Endogenous ligands for NHR-80 and NHR-49 are currently unknown, although the monounsaturated fatty acid oleic acid (OA) is a candidate NHR-80 ligand (Goudeau et al., 2011).
2. Longevity-regulating signals
Small molecules and endocrine signals have been linked to longevity and mediate changes in different signaling pathways with effects on downstream transcription factors and effector mechanisms. Recent studies in longevity regulation have focused on cell-autonomous and cell-non-autonomous signals to modulate organismal lifespan.
2.1. Neuroendocrine signals
Observations such as lifespan modulatory effects of sensory perception through olfactory and gustatory neurons (Alcedo and Kenyon, 2004; Apfeld and Kenyon, 1999) or inhibition of the DR-induced longevity response of peripheral tissues by diffusible compounds from the bacterial food source indicated a role for (neuro)endocrine signals in lifespan regulation (Bishop and Guarente, 2007; Smith et al., 2008). A recent study suggested that upon DR, DAF-7 (TGFβ) secreted by ASI neurons constitutes a pro-longevity signal that contributes to intestinal DAF-16 activation (Fletcher and Kim, 2017). Moreover, an age-associated decrease in DAF-7 levels may explain why C. elegans’ sensitivity to the longevity-promoting effects of DR decreases over time (Fletcher and Kim, 2017). In contrast, in fed animals, lifespan is extended when DAF-7 signaling is suppressed by branched chain amino acids (BCAAs) from the periphery that activate let-363 in ASI neurons (Mansfeld et al., 2015). Thus, although global inhibition of TOR extends lifespan, activating TOR can also exert this effect, when occurring in specific neurons (Mansfeld et al., 2015). Supplementation with the BCAT-1 (branched-chanin-amino-acid aminotransferase) substrate L-leucine or RNAi knockdown of bcat-1 or hlh-15 (NHLH1), which regulates bcat-1 transcription, is sufficient to extend C. elegans lifespan dependent on daf-16 and hsf-1 (Mansfeld et al., 2015). An independent study also reported that daf-7’s role in lifespan regulation is dependent on feeding state and suggested that combinatorial expression of daf-7 and the serotonin biosynthetic enzyme tph-1 (tryptophan hydroxylase) encodes food availability in vivo (Entchev et al., 2015). On the other hand, the ASI and ASJ-derived insulin like peptide INS-6 apparently mediates a bacterial food-derived anti-longevity signal that is sufficient to block DAF-16 nuclear accumulation in peripheral tissues and, partially, longevity in otherwise food-restricted C. elegans (Artan et al., 2016).
2.2. Reactive oxygen species
ROS have been implicated in aging because of their potential to cause macromolecular damage, (Finkel, 2011). Yet, treatment with low doses of ROS-generators such as paraquat and jugulone can lead to lifespan extension dependent on hif-1 and aak-2 (AMPKα) or on daf-16 and sir-2.1, respectively (Heidler et al., 2010; Hwang et al., 2014; Yang and Hekimi, 2010). Within the cell, ROS are generated as a by-product during mitochondrial electron transport and certain enzymatic reactions, but also as a primary product from professional ROS generating enzymes such as NADPH-oxidases (Finkel, 2011). Apart from dose, the localization of ROS generation within the cell and the precise ROS species may be important factors that determine the cellular and organismal outcome of ROS presence (Heidler et al., 2010; Lee et al., 2010; Yang and Hekimi, 2010). Of note, superoxide anions, a ROS species that cannot cross biological membranes (Krause, 2007), appears to be particularly important in at least some C. elegans longevity paradigms, such as daf-2, the mitochondrial mutants nuo-6 and isp-1 (Yang and Hekimi, 2010) and germline-less worms (Wei and Kenyon, 2016). Thus, the localization of professional superoxide generators such as NADPH-oxidases and, as proposed recently, globins, and eventually, their interplay with superoxide dismutases, allow to spatially control redox signaling (De Henau et al., 2015; Krause, 2007; Schaar et al., 2015). ROS originating from mitochondria or from the ER through the NADPH-oxidase BLI-3 (DUOX1/2) cause inhibitory sulfenylation of the ER-stress sensing kinase inositol requiring enzyme-1 (IRE-1) (Hourihan et al., 2016), consequently inhibiting the UPRER (see below) and inducing a p38/SKN-1 mediated antioxidant response. bli-3, ROS and skn-1 also mediate lifespan extension in response to loss of memo-1 (ortholog of mammalian mediator of ErbB2 driven cell motility) (Ewald et al., 2017) and enhanced pathogen resistance upon elevated proline catabolism (Liang et al., 2013; Tang and Pang, 2016). Moreover, a transient ROS-signal generated by enhanced proline catabolism in daf-2 worms contributes to their longevity (Zarse et al., 2012). Therefore, the impact of ROS production on redox balance and signaling in different compartments of the cell remains to be elucidated. In summary, depending on the context, ROS are not only damaging agents that promote aging, but are also emerging as important signaling molecules that can promote longevity.
2.3. Hydrogen sulfide (H2S)
Increased endogenous H2S production has been reported to be critical for various DR-induced benefits in diverse organisms, including longevity in eat-2 mutant C. elegans (Hine et al., 2015). Moreover, H2S has been implicated in the longevity of glp-1 worms (Wei and Kenyon, 2016) and exogenous H2S extends worm lifespan (Miller and Roth, 2007). H2S is generated during sulfur amino acid metabolism and acts as a gaseous messenger molecule that modulates cellular signaling through protein sulfhydration and other mechanisms (Kabil et al., 2014; Paul and Snyder, 2012). The ability of worms to tolerate low levels of H2S depends on skn-1 and hif-1 and indeed, in germline-deficient worms, H2S, rather than ROS, appears to activate skn-1 (Budde and Roth, 2010; Miller and Roth, 2007; Topalidou and Miller, 2017; Wei and Kenyon, 2016). Interestingly, hif-1 is not required for the H2S-mediated longevity of eat-2 and glp-1 worms (Table 1). Recently, the sulfide-quinone oxidoreductase SQRD-1, which mediates H2S benefits in cultured cells (Hine et al., 2015) has also been implicated in maintaining proteostasis in H2S-exposed worms (Horsman and Miller, 2016). Whether increased levels of H2S are a broad mechanism for longevity remains to be determined.
2.4. Nutrient and energy sensors
The best-established links between metabolism and aging stem from the discovery that the major amino acid sensor and growth regulator, the mechanistic Target Of Rapamycin (mTOR) as well as the energy sensor AMP-activated protein kinase (AMPK) modulate lifespan across phyla (Burkewitz et al., 2014; Hansen and Kapahi, 2010; Lapierre and Hansen, 2012; Laplante and Sabatini, 2012). Lifespan extension upon deficiency in the ribosomal protein S6 kinase, a key TOR-complex 1 substrate (Kapahi et al., 2010) was recently reported to require the arginine kinase ARGK-1 (ortholog of creatine kinase) (McQuary et al., 2016). argk-1 is dispensable for daf-2 and eat-2 longevity and appears to function together with aak-2/AMPK (McQuary et al., 2016). Yet, the precise regulatory mechanisms that link ARGK-1 activation to RSKS-1 (S6K) inactivation and AAK-2 activation remain to be determined. AAK- and its substrate, the CREB-regulated transcriptional co-activator CRTC-1 (Mair et al., 2011) were also implicated in longevity of ETC-compromised by activating the homeobox transcription factor CHE-23 (EMX1/2) and CEP-1/p53 (Chang et al., 2017a). How AAK-2 modulates CEP-1 activity has not been elucidated but it is interesting to note that mammalian p53 may be a substrate of AMPK (Jones et al., 2005). Altogether, energy levels and nutrient status are key molecular cues for cells to initiate stress resistance and survival mechanisms that affect lifespan.
3. Cellular processes mediating longevity
Longevity-associated transcription factors modulate genes that drive the activity and efficiency of complex processes in the cell, which translates into improved somatic maintenance. Major proteostatic pathways have been linked to lifespan extension and include protein degradation pathways such as the autophagy/lysosomal pathway and the ubiquitin proteasome system as well as protein metabolism in the endoplasmic reticulum and the mitochondria. Aging animals are characterized by proteostatic decline (Ben-Zvi et al., 2009), altered protein turnover (Dhondt et al., 2017) and the accumulation of insoluble proteins (Reis-Rodrigues et al., 2012). A cell’s response to the global loss of protein stability and solubility during aging includes enhanced autophagic degradation (Chang et al., 2017b; Chapin et al., 2015; David et al., 2010), disaggregation (Nillegoda et al., 2015), but also, intriguingly, packaging of aggregating proteins into chaperone-enriched aggregates (Moll et al., 2016; Walther et al., 2015). Here, we describe new findings in cellular processes with benefits on proteostasis, stress resistance and lifespan.
3.1. Autophagy
mTOR and AMPK modulate the process of autophagy, a recycling mechanism that results in the sequestration and lysosome-mediated breakdown of damaged macromolecules and organelles into basic components that become substrates for various biogenic pathways. This cellular “rejuvenation” pathway has emerged as a central mechanism in the ability of cells to maintain proteostasis, signaling and transcriptional signatures associated with survival. The ability of cells to engage and maintain autophagic flux is in part governed by transcription factors such as HLH-30/TFEB, PHA-4/FOXA and DAF-16/FOXO that translocate to the nucleus to enhance autophagy and lysosomal gene expression (Lapierre et al., 2015). More recently, selective autophagy of particular cellular cargo has been linked to longevity. Breakdown of compromised mitochondria by mitophagy has been shown to be important for prolonged lifespan in the worm (Palikaras et al., 2015). Autophagy stimulation can be recapitulated using pharmacological agents against upstream negative regulators (Galluzzi et al., 2017a, b). For instance, inhibitors of mTOR can activate autophagy and lysosomal biogenesis in part via HLH-30/TFEB activation (Roczniak-Ferguson et al., 2012; Settembre et al., 2011). Specifically, targeting the activity of TFEB has emerged as a viable option to stimulate autophagy. However, pharmacological targeting of TFEB has been particularly challenging since several drugs improving the nuclear localization of TFEB and lysosomal biogenesis have lysosomotropic properties that inhibit mTOR and impair lysosome function in cells (Lu et al., 2017). Nonetheless, new small molecule activators of autophagy via TFEB activation are emerging (Wang et al., 2017; Silvestrini et al., 2018). Other transcription factors, such as HSF-1, have been shown to modulate autophagy gene expression in the context of heat shock (Kumsta et al., 2017), suggesting that autophagy induction is a converging process cells use to maintain the soma under various extrinsic stresses. Lysosome biogenesis via expression of lysosomal proteins and degradation enzymes is increased in long-lived animals (Florez-McClure et al., 2007; Lapierre et al., 2013; Li et al., 2016; McColl et al., 2008, 2010). Lysosomal pH in the intestine can be modulated by DAF-16/FOXO-mediated transcriptional upregulation of proton v-ATPase genes (Baxi et al., 2017). A recent study highlighted that induction of the lysosomal proton V-ATPase subunit VHA-13 during fertilization is sufficient to efficiently clear damaged proteins in oocytes (Bohnert and Kenyon, 2017), demonstrating that lysosomal enhancement can restore proteostasis. Proper autophagosome assembly is crucial in the response to stress and in longevity. Longevity of eat-2, glp-1, rsks-1 and daf-2 mutant worms is dependent on the autophagy machinery (Lapierre et al., 2015). Specifically, autophagy in chemosensory neurons mediates signaling to the intestine (Minnerly et al., 2017) and autophagy in intestinal cells is essential for the integrity of the worm gut (Gelino et al., 2016). These data in the worm link the new molecular understanding of the autophagy machinery with animal physiology and longevity.
The relationship between lipid metabolism, autophagy and lifespan is emerging as a key interaction in longevity (Hansen et al., 2013; Lapierre et al., 2012). Autophagy is required for the accumulation of neutral lipids in the intestine of nematodes (Lapierre et al., 2013). Lipid composition in membranes correlates with longevity (Hulbert et al., 2007) and biogenesis of particular lipids correlates with long lifespan in C. elegans (Shmookler Reis et al., 2011). Aging markedly changes overall lipid composition and leads to accumulation of very long chain fatty acids (Gao et al., 2017). Recent evidence points to a potential role for oleic acid in longevity (Han et al., 2017), although supplementation experiments have not robustly shown lifespan extension (Goudeau et al., 2011). Regulated lipid turnover has been linked to long-term survival (Narbonne and Roy, 2009). In particular, enhanced lysosomal lipolysis has been shown to extend lifespan (Lapierre et al., 2011; Wang et al., 2008) and to mediate lipid signals driving nuclear hormone receptor (NHR) signaling (Folick et al., 2015; Seah et al., 2016). Indeed, NHR signaling is a central longevity mechanism in different long-lived models (Goudeau et al., 2011; Heestand et al., 2013; Ratnappan et al., 2014) (Table 1). Fatty acids such as oleylethanolamine, derived from lysosomal lipolysis and transported by lipid binding proteins such as LBP-8, have been linked to NHR signaling longevity (Folick et al., 2015). However, lipid signals have not been systematically addressed in the context of aging. Larger polyunsaturated lipids, such as omega-3 and −6 fatty acids have been linked to NHR signaling, autophagy activation and germline signaling (Lynn et al., 2015; O’Rourke et al., 2013; Qi et al., 2017). In addition, cholesterol can drive DAF-16/FOXO activity via lipid-binding protein NSBP-1 (Cheong et al., 2013; Ihara et al., 2017). These studies warrant further understanding of the integration of various fatty acids and sterols with signaling and proteostatic pathways during the process of aging.
Long-lived animals coordinate their lipid stores with lysosomal lipolysis by reducing the expression of large lipid transporters called vitellogenins (DePina et al., 2011; Dong et al., 2007; Murphy et al., 2003; Seah et al., 2016). In turn, lipids bound for yolk protein biogenesis are re-routed to storage, remodeling, and signaling associated with autophagy and somatic maintenance (Seah et al., 2016). Lipid redistribution is accompanied by improvements in lysosome function and nuclear hormone receptor signaling. While enhanced vitellogenesis is not detrimental in C. elegans (Seah et al., 2016), rearrangement of lipid stores by reduced vitellogenesis is essential for the ability of animals to survive starvation (Harvald et al., 2017). Some, but not all long-lived animals have enhanced lipogenesis that leads to increased lipid storage (Amrit et al., 2016; Perez and Van Gilst, 2008). Animals unable to concomitantly increase lipogenesis or redistribute lipids have decreased lipid stores when autophagy and lysosomal lipolysis are enhanced (Schiavi et al., 2013; Wang et al., 2011). Interestingly, lipid droplet biogenesis has recently been linked to longevity via modulation of the intake of fatty acid to mitochondria (Nguyen et al., 2017). These findings point to an intra-organelle integration involving lipid droplet biogenesis and mitochondrial function that can be modulated by the autophagy/lysosomal pathways and nuclear hormone signaling.
3.2. Unfolded protein response of the endoplasmic reticulum
The endoplasmic reticulum manages biochemical changes in its lumen via the unfolded protein response (UPRER). This multibranch pathway has a number of ER luminal sensors that transmit the information resulting in gene expression changes that reset ER homeostasis. The sensor proteins are IRE-1, PERK, and ATF-6. The ER transmembrane stress sensor IRE-1 (Chen and Brandizzi, 2013) modulates the UPR-related transcription factor XBP-1 through splicing of its mRNA to permit synthesis of the functional transcription factor. Together with its role in the antioxidant defense (Hourihan et al., 2016), as discussed above, these combined functions place IRE-1 into the center of cellular homeostasis and stress response. It is particularly interesting that IRE-1 can receive distinct inputs that result in different downstream consequences. Of note, IRE-1 signaling to SKN-1 or via the UPR both encode a stress signal and the respective responses have been linked to longevity. Interestingly, a recent study likewise linked the stress response via skn-1 and ire-1 with enhanced fitness (Mark et al., 2016). Vitamin D promotes protein homeostasis and longevity by triggering skn-1 and ire-1 UPR branch pathways. These data further support the concept of ER hormesis and show that a certain tone in UPRER signaling can be a mechanism for enhanced fitness and longevity. Hormesis is an adaptive response to a low level of detrimental stress that triggers an adaptation which subsequently leads to stress resistance and robustness. Conceptually, this is akin to mitohormesis, the process by which low doses of ROS have beneficial effects on mitochondrial function (Schulz et al., 2007). ER stress signaling can thus be a trigger for an adaptive response that mediates longevity in the worm. Upon stress, PERK phosphorylates eIF2α, which reduces initiation of mRNA translation and leads to expression of ATF4 that participates in nuclear gene expression changes enhancing ER protein folding capacity. ATF-6 is likewise an ER luminal sensor that becomes processed in the Golgi apparatus upon stress to directly activate expression of gene that mitigate ER stress.
While the role of the UPRER in stress adaptation is intriguing, it remains elusive if ER signaling pathways might also be involved in reversing aging. A recent study showed that larval starvation in the worm results in a number of age-associated phenotypes, which are reversed upon return of the animals to food (Roux et al., 2016). Excitingly, this “correction” of age-associated phenotypes, with the exception of protein aggregates, was dependent on IRE-1. This points to two possible roles of IRE-1 in longevity. For instance, a signal of ER stress and UPRER might be required for normal homeostasis. Alternatively, during development IRE-1 might have functions that are entirely distinct from ER sensing and downstream signaling. Certainly, future work will address the question of whether age-associated phenotypes will also be reversible in the adult worm, and whether IRE-1 might be involved in this process.
The FOXO transcription factor DAF-16 also promotes ER homeostasis. Specifically, DAF-16 releases ER stress by enhancing autophagy-mediated degradation independently of IRE-1 UPR-pathway activated genes, such as ERAD genes (Safra et al., 2014). While ER stress does not directly trigger DAF-16, its activity promotes ER homeostasis. In addition, DAF-16 interacts with the UPRER-activated transcription factor XBP-1 (Henis-Korenblit et al., 2010). This orchestrated function of DAF-16 clearly demonstrates the critical role of the ER in longevity. Several additional observations support a link between UPRER signaling and longevity. Mutant toxic proteins themselves initiate an UPR (Fardghassemi et al., 2017; Singh and Aballay, 2017). However, is a reduction of protein misfolding sufficient to extend lifespan? Forward genetic approaches were used to directly identify factors that simultaneously enhance stress resistance and extend lifespan. Heat, which leads to protein folding stress, can be a proxy for protein aggregation stress. A screen for resistance to heat stress identified novel alleles in many longevity genes, including the daf-2 gene (Munoz and Riddle, 2003). Importantly, protein aggregates accumulate with age in C. elegans (David et al., 2010), and human disease-associated toxic proteins aggregate in aging transgenic worms (Morley et al., 2002). A screen for resistance to tunicamycin, which triggers ER stress through inhibition of N-glycosylation, yielded a large number of resistant and long-lived mutant strains (Denzel et al., 2014). Of note, activation of the metabolic hexosamine pathway, which provides substrates for N-glycosylation, extended lifespan through engagement of autophagy, ERAD, and mild upregulation of proteasome activity. This suggested that degradation of proteins can suffice to extend lifespan in the absence of disease linked aggregation prone proteins. Moreover, it was found that compounds that directly bind to amyloid protein aggregates can extend worm lifespan (Alavez et al., 2011). This effect was hsf-1 and skn-1 dependent and thus it is unclear if it results from direct action on protein aggregates, or from altered stress signaling.
In addition to supporting the formation of autophagosomes, the ER is the site of de novo lipid droplet biogenesis, which is an essential process in the worm (Choudhary et al., 2015). Consistently, the ER is also a site of lipid and membrane composition sensing. Lipid dis-equilibrium is per se sufficient to trigger the UPR in the absence of disrupted protein folding (Hou et al., 2014). Moreover, IRE-1 acts as a direct sensor for ER membrane composition (Promlek et al., 2011; Volmer et al., 2013). Thus, IRE-1 is positioned at a very interesting cross road of protein and lipid homeostasis. How downstream signaling integrates and differentiates between the two processes will be exciting field of future research.
The UPRER was traditionally considered a cell-autonomous mechanism maintaining cellular protein homeostasis. Recent data, however, have expanded this view by demonstrating cell-non-autonomous regulation of the UPRER. Ectopic expression of spliced xbp-1 in the worm’s nervous system triggered peripheral expression of the UPR target gene hsp-4 and extended lifespan (Taylor and Dillin, 2013). Interestingly, this effect was ire-1 dependent, demonstrating that the peripheral response requires the entire arm of the UPRER, including the stress sensor. This work suggests the presence of yet unidentified neuroendocrine signaling molecules that mediate the cell-nonautonomous effect on proteostasis (Taylor et al., 2014).
3.3. The ubiquitin-proteasome system (UPS)
Cellular protein turnover is mediated in part by the ubiquitin-proteasome system, in which polyubiquitylation factors identify and mark aberrant proteins for degradation. Does enhancing proteasome function prevent organismal aging? Evidence of lifespan extension through induction of proteasome subunit expression, assembly or activity suggest that this is indeed the case (Chondrogianni et al., 2015; Vilchez et al., 2012). In addition, treatment with the proteasome activating compound 18α-Glycyrrhetinic Acid was shown to extend lifespan in the worm (Papaevgeniou et al., 2016). In line with this, loss of proteasome activity explains the lifespan reduction in glucose-fed animals (Fitzenberger et al., 2013). In addition, protein aggregates related to neurodegeneration were shown to block proteasome activity (Ayyadevara et al., 2015) and proteasome inhibition elicited a stress response via SKN-1 and autophagy (Keith et al., 2016; Lehrbach and Ruvkun, 2016). The UPS pathway was linked to the longevity-related insulin signaling longevity pathway. Surprisingly, the ubiquitin ligase CHIP regulates the insulin receptor DAF-2 directly by monoubiquitination and subsequent endocytic-lysosomal degradation. CHIP activity thus maintains low DAF-2 cell surface abundance, low insulin signaling tone, consequently affecting longevity (Tawo et al., 2017). With increased demand on the UPS with aging, CHIP activity towards DAF-2 is reduced, resulting in enhanced DAF-2 expression with age. This work suggests a cross talk between protein aggregates and DAF-2 expression that results in a self-accelerating cycle between protein aggregates that eliminates the protective low insulin signaling tone. Unexpectedly, recent studies of long-lived daf-2 animals have demonstrated that enhancement of proteasomal function is not necessarily a common mechanism for longevity. Lower proteasome activity was observed in daf-2 animals as well as reduced protein turnover (Stout et al., 2013). In addition, the half-life of proteins in daf-2 animals is extended (Depuydt et al., 2016; Dhondt et al., 2016; Visscher et al., 2016), which suggests that long lifespan may be achieved by globally enhancing protein stability thereby reducing the global requirement for rapid turnover and synthesis.
3.4. The mitochondrial unfolded protein response (UPRmt)
Perturbations in mitochondrial protein homeostasis triggers the mitochondrial unfolded protein response that induces nuclear gene expression changes to cope with the stress. This results in expression of mitochondria-associated protective genes to restore mitochondrial function (Qureshi et al., 2017). Although first described in mammalian cells, key components of the UPRmt-pathway have been identified in C. elegans (Qureshi et al., 2017), including the mitochondrial quality control protease CLPP-1 (CLPP), the peptide transporter HAF-1 (ABCB10), the transcription factors ATFS-1 (ATF4/5) and DVE-1 (SATB1/2), and the ubiquitin-like protein UBL-5 (UBL5), (Pellegrino et al., 2013; Qureshi et al., 2017). Complementary to ATFS-1 mediated changes in transcription, the eIF2α kinase GCN-2 lowers cytosolic protein translation when activated by increased ROS from dysfunctional mitochondria (Qureshi et al., 2017). Initial studies implicating the UPRmt (Durieux et al., 2011), or more broadly, mitonuclear imbalance (Houtkooper et al., 2013), into longevity of worms with compromised mitochondrial function were subsequently challenged (Bennett and Kaeberlein, 2014; Bennett et al., 2014). Indeed, several conditions have been identified in which mitochondrial perturbation shortens lifespan in the presence of an active UPRmt (Bennett and Kaeberlein, 2014). In some cases, induction of the UPRmt apparently even confers a disadvantage, for example in a short-lived heteroplasmic strain (Liau et al., 2007), where a constitutively active UPRmt contributes to maintenance and propagation of mutated mitochondrial genomes (Gitschlag et al., 2016; Lin et al., 2016).
Recent work identified additional regulators of the UPRmt and of longevity-associated factors upon mitochondrial impairment in C. elegans. Mitochondrial stress induces chromatin changes dependent on the apparently nematode-specific protein LIN-65 and the H3K9me2-forming methyltransferase MET-2 (SETDB1) (Tian et al., 2016) (Table 2). Moreover, the H3K27me2/3 demethylases JMJD-1.2 (PHF8) and JMJD-3.1 (JMJD3) strongly contribute to longevity of ETC-compromised, but not of eat-2 animals (Merkwirth et al., 2016). Interestingly, only jmjd-3.1 was required for glp-1 (Labbadia and Morimoto, 2015) and (partially) daf-2 longevity (Merkwirth et al., 2016). Of note, positive correlations between PHF8/JMJD3 and UPRmt signaling mediators/targets are also observed in murine tissues (Merkwirth et al., 2016). On the other hand, the transaldolase TALD-1 and other pentose phosphate pathway enzymes, whose knockdown extends C. elegans lifespan, were identified as suppressors of the UPRmt (Bennett et al., 2017). Another recent study (Munkacsy et al., 2016) described a novel pathway that is activated upon disruption of mitochondrial function that contributes to the extended lifespan of ETC defective animals and comprises the kinases DLK-1 (MAP3K12), SEK-3 (MAP2K4) and PMK-3 (MAPK14) and the reporter gene Ptbb-6::GFP. ETC-knockdown in the nervous system increases lifespan and induces the UPRmt in a distant issue, the intestine, suggesting an endocrine signal (“mitokine”) to coordinate mitochondrial stress signaling and eventually lifespan across tissues (Durieux et al., 2011). A recent study from the same group expanded this cell non-autonomous activation of the UPRmt to neuronal stress upon polyQ-expression (Berendzen et al., 2016). Among other factors, UPRmt induction in this context was dependent on the neuro-transmitter serotonin. Serotonin was also required to transmit a peripheral UPRmt activating signal upon other forms of neuronal stress (Berendzen et al., 2016), but whether it also transmits the lifespan-modulatory signal when the neuronal ETC is impaired has not been explicitly tested. Of note, serotonin also mediates a cell-nonautonomous signal from neurons to the intestine which stabilizes HIF-1 (Leiser et al., 2015).
Table 2.
Methyl marks and their regulators implicated in C. elegans lifespan modulation. Mammalian orthologs of regulators are given in parentheses. Effect of the methyl mark on chromatin: A/activating, R/repressive; Change with age (globally): ≈/unchanged, ↓/decreased, ↑/increased; Enzymatic activity: +/methyltransferase forming the respective mark, −/demethylase removing the respective mark; lifespan effect (of knockdown/depletion of the regulator in wildtype worms): ≈/unchanged, ↓/decreased, ↑/increased, tg/transgenerational effect (Greer et al., 2010, 2014, 2016; McColl et al., 2008; Maures et al., 2011; Ni et al., 2012; Towbin et al., 2012; Tian et al., 2016; Merkwirth et al., 2016; Wang et al., 2018; Hamilton et al., 2005; Tian et al., 2016; Maures et al., 2011; Labbadia and Morimoto, 2015; Pu et al., 2015; Jin et al., 2011).
| Mark | Effect | Change with age | Regulator (ortholog) | Enzymatic activity | Lifespan effect | Germline dependent1 | Ref |
|---|---|---|---|---|---|---|---|
| H3K4 me1/2 | A | SET-17 (PRDM7,−11) | + | tg ≈ | (Greer et al., 2016; Greer et al., 2014) | ||
| SET-30 (SMYD1–3) | tg ↑ | ||||||
| LSD-12 (LSD1/KDM1A) | − | ↑ | (Maures et al., 2011; McColl et al., 2008) | ||||
| SPR-5 (LSD1/KDM1A) | tg ↑ | No | (Greer et al., 2016) | ||||
| H3K4 me3 | A | ≈3 | SET-2 (SETD1A,B/KMT2F,G) | + | ↑ | Yes | (Greer et al., 2010) |
| RBR-24 (JARID1A,B/KDM5A,B) | − | ↓/↑5 | Yes/no5 | (Greer et al., 2010; Ni et al., 2012) | |||
| H3K9 me2 | R | MET-2 (SETDB1/KMT1E) | + | ↓ | (Tian et al., 2016; Towbin et al., 2012) | ||
| JMJD-1.26 (PHF8) | − | ≈ | (Merkwirth et al., 2016) | ||||
| H3K9 me3 | R | ↓3 | SET-26 (SETD5, KMT2E)7 | + | ↑/↑5 | no5 | (Greer et al., 2010; Hamilton et al., 2005; Ni et al., 2012; Wang et al., 2018) |
| SET-25 (EHMT2/KMT1C) | + | ≈ | (Tian et al., 2016; Towbin et al., 2012) | ||||
| JMJD-28 (JMJD2A-D/KDM4A-D) | − | ↑5/ tg ≈ | Yes5 | (Greer et al., 2016; Greer et al., 2014; Ni et al., 2012) | |||
| H3K27 me2 | R | JMJD-1.26 (PHF8) | − | ≈ | (Merkwirth et al., 2016) | ||
| H3K27 me3 | R | ↓3 | MES-2 (EZH2/KMT6)9 | + | ↑5 | No5 | (Ni et al., 2012) |
| UTX-1 (UTX/KDM6A) | − | ↑/↑5 | No/no5 | (Jin et al., 2011; Maures et al., 2011; Ni et al., 2012) | |||
| JMJD-3.16 (JMJD3/KDM6B) | − | ≈ | (Labbadia and Morimoto, 2015; Merkwirth et al., 2016) | ||||
| H3K36 me3 | A10 | ≈3, 11, 12 | MET-1 (SETD2/KMT3A) | + | ↓ | No | (Pu et al., 2015) |
Notes.
Germline dependence assessed by measuring lifespan of sterile glp-1(e2144ts) worms; germline dependency means that deficiency/knockdown of the regulator is not able to modulate lifespan in germline-deficient glp-1(e2144ts) worms.
Catalytic activity as H3K4me1/2-generating methyltransferase not firmly established (reviewed in (Greer and Shi, 2012)).
Experiments to asses global levels of H3K4me3, H3K9me3, H3K27me3 and H3K36me3 in young compared to aged worms were conducted in glp-1(e2144ts) worms (Ni et al., 2012); the effect of age on H3K27me3 in glp-1 animals was confirmed in (Maures et al., 2011).
rbr-2 also displays H3K4me2-demethylase activity, at least in vitro (Christensen et al., 2007).
Lifespan experiments conducted in the presence of FUDR in (Hamilton et al., 2005; Ni et al., 2012) and in some experiments in (Jin et al., 2011).
Reported as H3K9/27me2 (JMJD-1.2) and H3K27me3 (JMJD-3.1) demethylases in C. elegans,(Agger et al., 2007; Kleine-Kohlbrecher et al., 2010) but, as discussed in (Merkwirth et al., 2016), the mammalian ortholgs PHF8 and JMJD3 display broader substrate-specificity.
The highly similar set-26 paralog set-9 was identified as a lifespan regulator in an RNAi-study (Ni et al., 2012), but a recent study using mutants indicated that only set-26 can modulate lifespan (Wang et al., 2018). SET-9/26 were predicted to be catalytically inactive (Ni et al., 2012) and one study providing in vitro evidence that SET-26 mediates H3K9me3, but not methylation of other H3-lysine residues (Greer et al., 2014) is opposed by another study that found no decrease in H3K9me3 upon set-9/26 inactivation in vivo, but suggested that SET-9/26 bind to H3K4me3 (Wang et al., 2018).
JMJD-2 also demethylates H3K36me3/2/1 in vitro (Greer et al., 2014).
EZH2, as part of the Polycomb repressive complex 2 (PRC2), has been reported to regulate all forms of H3K27 methylation (Cao et al., 2002; Ferrari and Pasini, 2013). The study that found a role for C. elegans MES-2 regulating H3K27me2/3 levels did not examine H3K27 monomethylation (Bender et al., 2004).
Also suppresses cryptic transcription, which is increased in aged (FUDR-treated) worms (Sen et al., 2015).
Genome-wide, H3K36me3 patterns do not dramatically change during aging, but gain/loss of H3K36me3 is observed at a subset of genes (Pu et al., 2015).
Experiment conducted by (Pu et al., 2015) in germline-deficient glp-1(e2144ts) worms.
3.5. Heat-shock response
A major player in the proteostasis machinery is the heat shock response. Orchestrated by the key regulator HSF-1, the heat shock response is critical for maintaining homeostasis during aging. Impressive studies have shown how the heat shock response declines precipitously at early adult stages in the worm (Ben-Zvi et al., 2009; Labbadia and Morimoto, 2015), positioning a decline in proteostasis as a very early event in aging in the worm. Recently, they were able to identify suppressors of this phenotype through forward genetic screens and demonstrated that a reduction in mitochondrial ETC activity maintains the heat shock response (Labbadia et al., 2017). This work sheds light on an interesting interplay between mitochondrial activity and cytosolic protein homeostasis. While reduced ETC function has long been associated with longevity, it had not been known that this involves a downstream function of HSF-1, thus linking two major longevity pathways.
In further support of this concept, depletion of a major UPRmt transcriptional target, the mitochondrial chaperone hsp-6, triggers a stress response in the cytosol (MCSR: mitochondrial to cytosolic stress response) dependent on multiple UPRmt-mediators and on the key transcriptional regulator of the cytosolic heat shock response, hsf-1 (Kim et al., 2016). Moreover, hsp-6 depletion triggered the dve-1 and hsf-1 dependent expression of lipid metabolic genes, which are not induced under conditions that activate only dve-1 or hsf-1. MSCR induction improved cytosolic protein homeostasis not just in C. elegans but also in a human cell culture model (Kim et al., 2016). Of note, although hsp-6 depletion/MSCR induction apparently has beneficial effect on proteostasis in polyQ-challenged animals, lifespan of wildtype worms is shortened by hsp-6 RNAi (Kimura et al., 2007)
3.6. Protein synthesis
Reduced protein synthesis is a consequence of a number of longevity interventions, including genetic models of longevity in the worm such as the eat-2 DR model, or the inhibition of TOR (Hansen et al., 2007). However, reduced protein synthesis appears to be per se sufficient for lifespan extension. A first indication of this came from initial RNAi longevity screens (Hamilton et al., 2005; Hansen et al., 2007; Lee et al., 2003) that found that knockdown of a number of ribosomal and translation genes resulted in lifespan extension. Reducing translation improves all-over robustness, for example under conditions of ER stress (Howard et al., 2016), and is a characteristic of long-lived daf-2 animals (Depuydt et al., 2013). Further reducing translation in daf-2 animals leads to extreme longevity (Chen et al., 2013). Protein synthesis reduction via RNA polymerase PolII inhibition can also mediate lifespan extension (Filer et al., 2017). Moreover, genetic and pharmacological inhibition of mRNA translation extends worm lifespan (Cattie et al., 2016; Syntichaki et al., 2007; Takauji et al., 2016). Interestingly, proteome stability is also sensitive to nascent peptide-ribosome interactions (Kirstein-Miles et al., 2013) as well as to ribosomal dynamics governed by codon translation optimization (Nedialkova and Leidel, 2015).
Why does reduced protein synthesis extend lifespan? One explanation is the reduced demand on the protein folding machinery. Age-dependent changes in protein abundance contribute to protein aggregation as abundant proteins strongly contribute to protein aggregates (Walther et al., 2015). Globally reducing protein synthesis might thus prevent such catastrophic shift in solubility. Reducing load on the protein homeostasis system via reducing protein synthesis, might thus delay protein misfolding by improving translation fidelity and chaperone availability (Hansen et al., 2007; Pan et al., 2007; Syntichaki et al., 2007). This is consistent with the disposable soma theory of aging: fast growth in early life is beneficial and protein misfolding is readily suppressed in young animals due to efficient and responsive proteostatic mechanisms (Kirkwood, 2005). Thus, there is no trade-off in young animals. As animals age, however, protein folding capacity shrinks while the proteome composition shifts significantly, and proteins form insoluble aggregates. With reduced protein synthesis, this effect might be delayed.
In addition, there is a signaling response to reduced protein synthesis. During genetic inhibition of mRNA translation, there is a specific response of the SKN-1 transcription factor (Li et al., 2011) that results in the expression of cytoprotective genes, including atf-5 and haf-7. This suggests that reducing protein synthesis is not only per se protective but also triggers a signaling response via SKN-1 that boosts robustness. Similarly, while eIF2α phosphorylation inhibits protein synthesis, it also triggers the ATF-5-dependent transcriptional response. ATF-5 is thus the transcriptional output of the PERK arm of the UPRER. The mammalian homologue ATF4 initiates expression of genes involved in oxidative stress and amino acid metabolism, as well as apoptosis, and the yeast homologue GCN4 is involved in caloric restriction and amino acid starvation. Worm ATF-5 target genes have not been specifically addressed.
ER stress triggers the phosphorylation of eIF2α by the ER kinase PERK. eIF2α is the master regulator of the integrated stress response, which, in the worm, also receives input by general control non-derepressible 2 (GCN-2) kinase that signals amino acid shortage and mitochondrial stress. eIF2α is a critical component of cap-dependent mRNA translation machinery and its phosphorylation leads to reduced levels of protein synthesis (Pakos-Zebrucka et al., 2016). In the mammalian system, upstream open reading frame (uORF) regulated transcripts become expressed under these conditions, most importantly the bZIP transcription factor ATF4, which is a homolog of the yeast GCN4 (Vattem and Wek, 2004).
Another aspect of protein synthesis that has emerged recently relates to the roles of splicing factors in the specific and global modulation of proteomes (Heintz et al., 2017; Tabrez et al., 2017) as well as RNA quality control pathways (Son et al., 2017) and nucleoli formation (Tiku et al., 2016). How protein synthesis rates and overall proteostasis are modulated at the RNA level to provide cellular conditions conducive for longevity remains an important area of research and is bound to continue to yield interesting clues on the rate of aging.
4. Epigenetic modifications associated with lifespan
Epigenetic changes, i.e. changes in histone post-translational modification patterns, DNA methylation and chromatin remodeling have been proposed as a hallmark of aging (Lopez-Otin et al., 2013). Studies in C. elegans identified several chromatin modifiers that influence lifespan, in some cases even in subsequent generations. As many epigenetic regulators are conserved, these insights from C. elegans may be broadly applicable.
4.1. Histone expression and modifications, and nucleosome positioning
Beyond sirtuins, a family of NAD +-dependent histone deacetylases whose longevity-promoting function in C. elegans has been challenged (although evidence for beneficial effects on mammalian lifespan and healthspan is substantial), other modifiers of histone methylation have been implicated in C. elegans lifespan regulation (Giblin et al., 2014; Imai and Guarente, 2016) (Table 2). While marked changes in global levels of euchromatin (active) methyl marks have not been observed, heterochromatin (repressed) marks appear to decrease as C. elegans ages (Benayoun et al., 2015). Although these and other findings in C. elegans are consistent with the notion that loss of heterochromatin and redistribution of euchromatin is detrimental to a long lifespan (Benayoun et al., 2015), the picture is not entirely uniform. For example, decreasing levels of the H3K27me3 demethylase UTX-1 extends worm lifespan (Benayoun et al., 2015; Jin et al., 2011; Maures et al., 2011; Ni et al., 2012), while decreasing levels of the apparent H3K27me3-forming methyltransferase MES-2 was reported to not shorten, but rather, to extend worm lifespan (Benayoun et al., 2015; Ni et al., 2012). The same pattern is observed for depletion of regulators of another repressive mark, H3K9me3, with the caveats that the demethylase JMJD-2 also appears to deplete the activating H3K36 mark and that the function of SET-9/26 as H3K9me3 generating methyl-transferases is not firmly established (Greer et al., 2014; Greer and Shi, 2012; Ni et al., 2012). Integrating different studies is further complicated by different experimental conditions in the respective studies, such as the use of FUDR or of the sterile glp-1(e2144ts) strain. Modifiers of the activating H3K4me3 influence C. elegans lifespan through lipid metabolism, characterized by increased accumulation of lipids, particularly lipids containing monounsaturated fatty acids (Han et al., 2017). On the other hand, the activating H3K36me3 mark has been suggested to promote longevity by restricting gene expression changes and suppressing cryptic transcription (Pu et al., 2015; Sen et al., 2015). Of note, methyltransferases and demethylases frequently possess a broad substrate specificity and many studies do not formally rule out the possibility that the identified regulators modulate lifespan, at least in part, through targets other than histone proteins (Greer and Shi, 2012).
Some regulators of histone methylation have been reported to interact with well-established longevity pathways. For example, utx-1 knockdown extends lifespan of eat-2 and of wildtype animals in a daf-16 dependent manner but does not increase daf-2 longevity (Jin et al., 2011; Maures et al., 2011; Ni et al., 2012). Moreover, the daf-2 gene appears to be a direct UTX-1 methylation target in both worms and mammalian cells (Jin et al., 2011; Maures et al., 2011). On the other hand, lifespan extension by set-9/26 or ash-2 knockdown was at best partially dependent on DAF-16 (Greer et al., 2010; Ni et al., 2012). Furthermore, multiple methyltransferases and demethylases (Table 2) have been examined for their lifespan-regulatory effect in germline-deficient glp-1 worms. Ability or inability of particular knockdowns to extend glp-1 lifespan has been taken as evidence that these factors modulate lifespan by acting in the soma/germline (Greer et al., 2010; Hamilton et al., 2005; Jin et al., 2011; Maures et al., 2011; Ni et al., 2012). However, these findings are further consistent with the notion that these knockdowns trigger lifespan-extending mechanisms that are not yet, or already, active in long-lived glp-1 worms.
Reduced core histone expression during aging has been observed in multiple species, including C. elegans (Benayoun et al., 2015) and has been proposed to contribute to aging by precluding proper maintenance of chromatin structure, thus broadly dysregulating transcription as found in yeast (Feser et al., 2010). Although levels of endogenous H3 protein were decreased in aged compared to young adult glp-1 worms (Ni et al., 2012) a recent study provided evidence that at least a particular H3-variant, HIS-71 increases during aging (Narayan et al., 2016). Of note, changes in the relative levels of individual histone variants have been reported previously to occur during cellular senescence and mammalian aging (Benayoun et al., 2015).
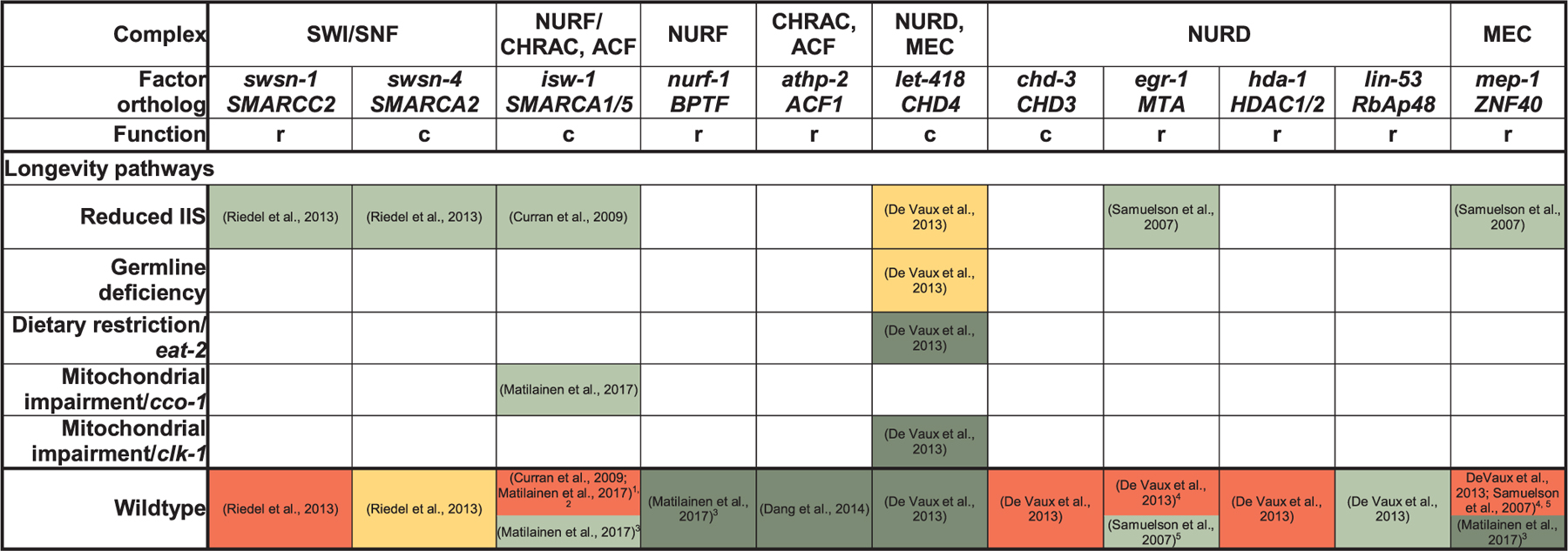
The transcriptional landscape can further be changed by ATP-dependent chromatin remodelers (Clapier et al., 2017) (Table 3). Members of the SWI/SNF complex do at best mildly shorten C. elegans wildtype lifespan when inactivated, but are required for DAF-16 dependent processes, such as daf-2 longevity and dauer formation (Riedel et al., 2013). Conversely, regulation of transcription of daf-16d/f by SWI/SNF may contribute to longevity (Bansal et al., 2014), although the particular importance of daf-16d/f for lifespan extension in daf-2 worms (Kwon et al., 2010) has been challenged (Chen et al., 2015). Depletion of isw-1 (orthologous to the ATPases hSNF2L [NURF-complex] and hSNF2H [CHRAC- and ACF-complexes] (Clapier and Cairns, 2009)), in daf-2 (Curran et al., 2009) and cco-1 RNAi animals (Matilainen et al., 2017) shortens their extended lifespan, while isw-1 overexpression extends wildtype lifespan (Matilainen et al., 2017). On the other hand, loss of let-418 (Mi2β/CHD4, ATPase of the NuRD complex) also shortens daf-2 and glp-1 longevity, while further extending wildtype, eat-2 and clk-1 RNAi lifespan (De Vaux et al., 2013). However, effects on wildtype lifespan upon depletion of isw-1 or mep-1 (ZNF40), a component of the LET-418 containing MEC-complex, varied depending on the RNAi regimen (Table 3) (Curran et al., 2009; De Vaux et al., 2013; Matilainen et al., 2017; Passannante et al., 2010). Moreover, upon depletion of regulatory subunits of the NURF (Matilainen et al., 2017), CRAC/ACF (Dang et al., 2014), NURD and MEC-complexes (De Vaux et al., 2013), different effects than for depletion of isw-1/let-418 (Curran et al., 2009; Matilainen et al., 2017; De Vaux et al., 2013) have been reported. Thus, it is possible that these ATPases regulate lifespan through several of their complexes (De Vaux et al., 2013) and that some complexes may play different roles during development and adulthood (Matilainen et al., 2017)
Table 3.
Role of ATP-dependent chromatin remodelers in C. elegans lifespan regulation. Green shading indicates that a factor is required for a particular lifespan-extending treatment (RNAi or loss/reduction of function mutation or Dietary restriction regimen) to extend lifespan or to maintain normal lifespan in otherwise wildtype animals. Yellow shading indicates a partial requirement, red shading no requirement, dark green further extension, and white not explicitly tested. Function refers to the function of a particular factor within the ATP-dependent chromatin-remodeling complex (c: catalytic, or r: regulatory subunit) (Riedel et al., 2013; Curran et al., 2009; De Vaux et al., 2013; Matilainen et al., 2017; Samuelson et al., 2007; Dang et al., 2014).
|
Notes.
RNAi was performed only during adulthood by (Curran et al., 2009).
The (Matilainen et al., 2017) study used different RNAi regimens and in some cases, also examined mutants; in this case, RNAi was performed from L1.
Cf. previous note; different RNAi-regimens were applied in the (Matilainen et al., 2017) study; in this case, RNAi was initiated already in the parental generation starting in L1-L3 and the experimental F1 was kept on RNAi-plates.
The (De Vaux et al., 2013) study examined genetic mutations for all genes of interest, with the exception of egr-1 and hda-1, which were knocked down by RNAi starting in L4. Other experimental conditions (lifespans measured at 25 °C, use of FUDR) were the same than in the (Samuelson et al., 2007) study.
The (Samuelson et al., 2007) study used RNAi from L4.
4.2. DNA methylation
Directed DNA methylation, at least in mammals, occurs most prominently at the 5-carbon of cytosine (5-methylcytosine, 5-mC) residues in CpG dinucleotides and leads to transcriptional repression (Benayoun et al., 2015; O’Brown and Greer, 2016; Sen et al., 2016). CpG methylation patterns change as humans age and have been proposed as a reliable biomarker of aging (Horvath, 2013). 5-mC is thought to be absent in C. elegans, but recent studies detected the presence of 6-methyladenine (6-mA) in worms (Greer et al., 2015) and also in fruit flies (O’Brown and Greer, 2016; Zhang et al., 2015). Subsequent studies provided new evidence for the presence of 6-mA even in mammals and evolutionary conservation of 6-mA regulating methyltransferases and demethylases further supports the concept that 6-mA exerts regulatory functions in multicellular eukaryotes (O’Brown and Greer, 2016). While 6-mA in bacteria serves to distinguish self and foreign DNA (O’Brown and Greer, 2016), it has been implicated into transposon repression and developmental processes in D. melanogaster (Zhang et al., 2015) and into the transgenerational regulation of fertility and longevity by the H3K4me2 demethylase SPR-5 (cf. below) in C. elegans.
4.3. Transgenerational epigenetic inheritance of longevity
Evidence for transgenerational inheritance of longevity was first provided by a study in C. elegans which reported that deficiency in H3K4me3 (COMPASS)-complex components (ASH-2/ASH2L, WDR-5/WDR5 or SET-2/SETD1A) extended lifespan not just in mutant animals but also in genetically wildtype progeny from crosses with wildtype worms (Greer et al., 2011). Subsequently, the COMPASS complex was implicated in transgenerational inheritance of increased adult stress resistance when parents, but not progeny, experienced various forms of environmental stress during development (Kishimoto et al., 2017). Similarly, starvation induces transgenerational effects on multiple phenotypes including growth, reproduction and stress resistance (Jobson et al., 2015) and the COMPASS complex, as well as AMPK, ensure reproductive fitness in progeny of starved parents (Demoinet et al., 2017). More recently, another paradigm of transgenerational lifespan regulation was described in C. elegans deficient for the H3K4me2 demethylase SPR-5 (Greer et al., 2016). The spe-5 paradigm differs from the COMPASS paradigm (Greer et al., 2011) in several aspects and appears to transgenerationally regulate a different set of genes (Greer et al., 2016). Of note, transgenerational longevity effects are not observed for wildtype descendants from parents deficient in other chromatin modifiers or established longevity genes such as utx-1, set-9, set-15 and daf-2 (Greer et al., 2011).
5. Pharmacologic lifespan extension
Apart from enabling fundamental insights into the biology of aging through genetic studies, C. elegans has been proposed to aid in the search for compounds that may promote healthy aging in more complex organism (Lucanic et al., 2017). The most efficient regimen to extend lifespan in model organisms is dietary restriction (Kapahi et al., 2017) and the particular DR-variant of caloric restriction already has been shown to improve health in non-human primates (Mattison et al., 2017). Accordingly, compounds that mimic the effect of DR appear particularly promising in extending healthspan in humans (Calvert et al., 2016; Lucanic et al., 2016). Recent bioinformatics and high throughput experimental screening approaches lead to the identification of candidate CR/DR mimetics in C. elegans that now require investigation in higher organisms (Calvert et al., 2016; Lucanic et al., 2016). Additional compounds that recently were shown to increase wildtype C. elegans lifespan in candidate testing or small scale screening approaches include small molecules and metabolites such as dimethyl sulfide (Guan et al., 2017), α-ketoacids (Mishur et al., 2016), fructose (Zheng et al., 2017), the d-fructose epimer d-allulose (Shintani et al., 2017), the ω−3 polyunsaturated fatty acid alpha-linolenic acid (ALA) and ALA-derived oxylipin-metabolites (Qi et al., 2017), the proteasome activator 18α-Glycyrrhetinic Acid, a triterpenoid from licorice (Papaevgeniou et al., 2016) and FDA-approved drugs such as rifampicin for tuberculosis (Golegaonkar et al., 2015) and the angiotensin-converting enzyme inhibitor captopril (Kumar et al., 2016) and hydralazine, which are both used to treat hypertension (Dehghan et al., 2017). Many of these compounds apparently act, at least in part, by activating or stabilizing lifespan-regulatory key transcription factors (Table 1), such as daf-16 (18α-Glycyrrhetinic Acid, rifampicin, captopril), hlh-30 (selective inhibitors of nuclear export), hif-1 (α-ketoacids), nhr-49 (ALA) and skn-1 (ALA-metabolites, 18 α-Glycyrrhetinic, hydralazine). Moreover, several recent studies described molecular mechanisms of action for C. elegans lifespan-extending drugs identified earlier. For example the serotonine and noradrenaline receptor antagonist Mianserin, an antidepressant, has been shown to act by modulating synaptic transmission and cell-non-autonomously inducing oxidative stress response genes in peripheral tissues (Petrascheck et al., 2007; Rangaraju et al., 2015). The nonsteroidal anti-inflammatory drug Aspirin extends C. elegans lifespan through mechanisms that overlap with daf-16-, eat-2-induced DR- and germline signaling (Ayyadevara et al., 2013; Huang et al., 2017; Wan et al., 2013) while the major lipid in bee royal jelly, 10-Hydroxy-2-decenoic acid, acts through the eat-2- and TORC1-pathways (Honda et al., 2015, 2011). Intermediate doses of the green tea polyphenol epigallocatechine gallate engage AMPK, sir-2.1 and daf-16 for C. elegans lifespan extension (Abbas and Wink, 2009; Xiong et al., 2018). Vitamin D exerts beneficial effects on longevity and protein homeostasis via skn-1, ire-1 and xbp-1 (Mark et al., 2016; Messing et al., 2013). For the antidiabetic drug Metformin, for which first trials have been designed to test their health-promoting effects in humans (Barzilai et al., 2016), multiple mechanisms for C. elegans lifespan extension have been reported, including disruption of the folate and methionine cycles in the bacterial food source (Cabreiro et al., 2013), and, in the worm itself, impairment of mitochondrial complex I, TORC1 inhibition and activation of AMPK and SKN-1 (Chen et al., 2017; De Haes et al., 2014; Onken and Driscoll, 2010; Wu et al., 2016b). Importantly, some of these molecular mechanisms appear to be conserved between worms and humans (Chen et al., 2017; Wu et al., 2016b). In summary, these recent reports support the view that C. elegans is not just exceptionally useful for uncovering genetic pathways, but also for designing pharmacologic strategies to modulate aging.
6. Future perspective
A central question in aging research remains whether extended longevity equates a long and healthy lifespan (Hansen and Kennedy, 2016). Indeed, how genetic and metabolic changes correlate with healthspan has been recently debated. While lifespan extension represents a temporal scaling (Stroustrup et al., 2016), early indications suggested that long-lived daf-2 animals unexpectedly have lower activity later in life (Bansal et al., 2015; Zhang et al., 2016). However, further studies on healthspan have determined that aging daf-2 animals are not necessarily unhealthy (Hahm et al., 2015; Podshivalova et al., 2017). One of the important goals in aging research will remain to carefully determine whether lifespan extending interventions maintain a satisfying level of health in the later stages of life.
Multiple studies highlight that reducing the load of aggregating toxic proteins improves fitness and contributes to longevity downstream of many, if not all, longevity pathways. It remains less clear if the stress signaling pathways responsible for clearing aggregates also have broader beneficial effects, including perhaps metabolic changes or alterations in protein synthesis. In addition, organelle remodeling is emerging as a component of cyto-protective mechanisms in cells. For instance, mitochondrial dynamics has recently been linked to longevity (Chaudhari and Kipreos, 2017; Weir et al., 2017). Moving forward, characterizing interactions and identifying biochemical and genetic mechanisms for coordination between tissues and organelles will be key to better understand how cells respond to nutrient signaling and stress to protect the soma.
Acknowledgments
Funding source and acknowledgements
We would like to thank Dr. Shi Quan Wong for comments. M.S.D. was funded by grants from the European Research Council (ERC-2014StG-640254) and the German Federal Ministry of Education and Research (01GQ1423A). L.R.L. was funded by grants from the NIH/NIA (R00 AG042494 and R01 AG051810), a Glenn Foundation for Medical Research Award for Research in Biological Mechanisms of Aging and a Junior Faculty Grant from the American Federation for Aging Research. H.I.D.M. was funded by grants from the Aktion Daniel Swarovski and the Tyrolean Science Fund (Tiroler Wissenschaftsfonds).
References
- Abbas S, Wink M, 2009. Epigallocatechin gallate from green tea (Camellia sinensis) increases lifespan and stress resistance in Caenorhabditis elegans. Planta Med 75, 216–221. [DOI] [PubMed] [Google Scholar]
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, Issaeva I, Canaani E, Salcini AE, Helin K, 2007. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731–734. [DOI] [PubMed] [Google Scholar]
- Akerfelt M, Morimoto RI, Sistonen L, 2010. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol 11, 545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam H, Williams TW, Dumas KJ, Guo C, Yoshina S, Mitani S, Hu PJ, 2010. EAK-7 controls development and life span by regulating nuclear DAF-16/FoxO activity. Cell Metab 12, 30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavez S, Vantipalli MC, Zucker DJ, Klang IM, Lithgow GJ, 2011. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature 472, 226–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Kenyon C, 2004. Regulation of C. elegans longevity by specific gustatory and olfactory neurons. Neuron 41, 45–55. [DOI] [PubMed] [Google Scholar]
- Amrit FR, Steenkiste EM, Ratnappan R, Chen SW, McClendon TB, Kostka D, Yanowitz J, Olsen CP, Ghazi A, 2016. DAF-16 and TCER-1 facilitate adaptation to germline loss by restoring lipid homeostasis and repressing reproductive physiology in C. elegans. PLoS Genet 12, e1005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK, 2005. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. U. S. A 102, 16275–16280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anckar J, Sistonen L, 2011. Regulation of HSF1 function in the heat stress response: implications in aging and disease. Annu. Rev. Biochem 80, 1089–1115. [DOI] [PubMed] [Google Scholar]
- Antebi A, 2013. Regulation of longevity by the reproductive system. Exp. Gerontol 48, 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, Kenyon C, 1999. Regulation of lifespan by sensory perception in Caenorhabditis elegans. Nature 402, 804–809. [DOI] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R, 2004. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev 18, 3004–3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artan M, Jeong DE, Lee D, Kim YI, Son HG, Husain Z, Kim J, Altintas O, Kim K, Alcedo J, et al. , 2016. Food-derived sensory cues modulate longevity via distinct neuroendocrine insulin-like peptides. Genes Dev 30, 1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arum O, Johnson TE, 2007. Reduced expression of the Caenorhabditis elegans p53 ortholog cep-1 results in increased longevity. J. Gerontol. A Biol. Sci. Med. Sci 62, 951–959. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Bharill P, Dandapat A, Hu C, Khaidakov M, Mitra S, Reis R.J. Shmookler, Mehta JL, 2013. Aspirin inhibits oxidant stress, reduces age-associated functional declines, and extends lifespan of Caenorhabditis elegans. Antioxid. Redox Signal 18, 481–490. [DOI] [PubMed] [Google Scholar]
- Ayyadevara S, Balasubramaniam M, Gao Y, Yu LR, Alla R, Reis R. Shmookler, 2015. Proteins in aggregates functionally impact multiple neurodegenerative disease models by forming proteasome-blocking complexes. Aging Cell 14, 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR, Manning G 3rd, Dillin A, 2014. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science 346, 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Kwon ES, Conte D, Liu H Jr., Gilchrist MJ, MacNeil LT, Tissenbaum HA, 2014. Transcriptional regulation of Caenorhabditis elegans FOXO/DAF-16 modulates lifespan. Longev. Healthspan 3, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Zhu LJ, Yen K, Tissenbaum HA, 2015. Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proc. Natl. Acad. Sci. U. S. A 112, E277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruah A, Chang H, Hall M, Yuan J, Gordon S, Johnson E, Shtessel LL, Yee C, Hekimi S, Derry WB, et al. , 2014. CEP-1, the Caenorhabditis elegans p53 homolog, mediates opposing longevity outcomes in mitochondrial electron transport chain mutants. PLoS Genet 10, e1004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA, 2016. Metformin as a tool to target aging. Cell Metab 23, 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxi K, Ghavidel A, Waddell B, Harkness TA, de Carvalho CE, 2017. Regulation of lysosomal function by the DAF-16 forkhead transcription factor couples reproduction to aging in Caenorhabditis elegans. Genetics 207, 83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun BA, Pollina EA, Brunet A, 2015. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol 16, 593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender LB, Cao R, Zhang Y, Strome S, 2004. The MES-2/MES-3/MES-6 complex and regulation of histone H3 methylation in C. elegans. Curr. Biol 14, 1639–1643. [DOI] [PubMed] [Google Scholar]
- Bennett CF, Kaeberlein M, 2014. The mitochondrial unfolded protein response and increased longevity: cause, consequence, or correlation? Exp. Gerontol 56, 142–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CF, Wende H. Vander, Simko M, Klum S, Barfield S, Choi H, Pineda VV, Kaeberlein M, 2014. Activation of the mitochondrial unfolded protein response does not predict longevity in Caenorhabditis elegans. Nat. Commun 5, 3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett CF, Kwon JJ, Chen C, Russell J, Acosta K, Burnaevskiy N, Crane MM, Bitto A, Wende H. Vander, Simko M, et al. , 2017. Transaldolase inhibition impairs mitochondrial respiration and induces a starvation-like longevity response in Caenorhabditis elegans. PLoS Genet 13, e1006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Miller EA, Morimoto RI, 2009. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences of the United States of America 106, 14914–14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berber S, Wood M, Llamosas E, Thaivalappil P, Lee K, Liao BM, Chew YL, Rhodes A, Yucel D, Crossley M, et al. , 2016. Homeodomain-Interacting Protein Kinase (HPK-1) regulates stress responses and ageing in C. elegans. Sci. Rep 6, 19582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky A, Viswanathan M, Horvitz HR, Guarente L, 2006. C. elegans SIR-2.1 interacts with 14–3-3 proteins to activate DAF-16 and extend life span. Cell 125, 1165–1177. [DOI] [PubMed] [Google Scholar]
- Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S, Liu Y, Dillin A, 2016. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell 166, 1553–1563 e1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman JR, Kenyon C, 2006. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell 124, 1055–1068. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L, 2007. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549. [DOI] [PubMed] [Google Scholar]
- Blackwell TK, Steinbaugh MJ, Hourihan JM, Ewald CY, Isik M, 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med 88, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert KA, Kenyon C, 2017. A lysosomal switch triggers proteostasis renewal in the immortal C. elegans germ lineage. Nature 551, 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulias K, Horvitz HR, 2012. The C. elegans MicroRNA mir-71 acts in neurons to promote germline-mediated longevity through regulation of DAF-16/FOXO. Cell Metab 15, 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunquell J, Morris S, Lu Y, Cheng F, Westerheide SD, 2016. The genome-wide role of HSF-1 in the regulation of gene expression in Caenorhabditis elegans. BMC Genom 17, 559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde MW, Roth MB, 2010. Hydrogen sulfide increases hypoxia-inducible factor-1 activity independently of von Hippel-Lindau tumor suppressor-1 in C. elegans. Mol. Biol. Cell 21, 212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkewitz K, Zhang Y, Mair WB, 2014. AMPK at the Nexus of energetics and aging. Cell Metab [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkewitz K, Morantte I, Weir HJM, Yeo R, Zhang Y, Huynh FK, Ilkayeva OR, Hirschey MD, Grant AR, Mair WB, 2015. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell 160, 842–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JA, Mishur RJ, Bhaskaran S, Rea SL, 2013. A metabolic signature for long life in the Caenorhabditis elegans Mit mutants. Aging Cell 12, 130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabreiro F, Au C, Leung KY, Vergara-Irigaray N, Cocheme HM, Noori T, Weinkove D, Schuster E, Greene ND, Gems D, 2013. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell 153, 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan DR, Brunet A, 2008. The FoxO code. Oncogene 27, 2276–2288. [DOI] [PubMed] [Google Scholar]
- Calvert S, Tacutu R, Sharifi S, Teixeira R, Ghosh P, de Magalhaes JP, 2016. A network pharmacology approach reveals new candidate caloric restriction mimetics in C. elegans. Aging Cell 15, 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y, 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298, 1039–1043. [DOI] [PubMed] [Google Scholar]
- Cattie DJ, Richardson CE, Reddy KC, Ness-Cohn EM, Droste R, Thompson MK, Gilbert WV, Kim DH, 2016. Mutations in nonessential eIF3k and eIF3l genes confer lifespan extension and enhanced resistance to ER stress in Caenorhabditis elegans. PLoS Genet 12, e1006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HW, Pisano S, Chaturbedi A, Chen J, Gordon S, Baruah A, Lee SS, 2017a. Transcription factors CEP-1/p53 and CEH-23 collaborate with AAK-2/AMPK to modulate longevity in Caenorhabditis elegans. Aging Cell 16, 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang JT, Kumsta C, Hellman AB, Adams LM, Hansen M, 2017b. Spatiotemporal regulation of autophagy during Caenorhabditis elegans aging. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin HC, Okada M, Merz AJ, Miller DL, 2015. Tissue-specific autophagy responses to aging and stress in C. elegans. Aging (Albany NY) 7, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari SN, Kipreos ET, 2017. Increased mitochondrial fusion allows the survival of older animals in diverse C. elegans longevity pathways. Nat. Commun 8, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Brandizzi F, 2013. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol 23, 547–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Thomas EL, Kapahi P, 2009. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5, e1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Li PW, Goldstein BA, Cai W, Thomas EL, Chen F, Hubbard AE, Melov S, Kapahi P, 2013. Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep 5, 1600–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AT, Guo C, Itani OA, Budaitis BG, Williams TW, Hopkins CE, McEachin RC, Pande M, Grant AR, Yoshina S, et al. , 2015. Longevity genes revealed by integrative analysis of isoform-specific daf-16/FoxO mutants of Caenorhabditis elegans. Genetics 201, 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ou Y, Li Y, Hu S, Shao LW, Liu Y, 2017. Metformin extends C. elegans lifespan through lysosomal pathway. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong MC, Lee HJ, Na K, Joo HJ, Avery L, You YJ, Paik YK, 2013. NSBP-1 mediates the effects of cholesterol on insulin/IGF-1 signaling in Caenorhabditis elegans. Cell. Mol. Life Sci 70, 1623–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL, 2012a. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 148, 322–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang WC, Tishkoff DX, Yang B, Wilson-Grady J, Yu X, Mazer T, Eckersdorff M, Gygi SP, Lombard DB, Hsu AL, 2012b. C. elegans SIRT6/7 homolog SIR-2.4 promotes DAF-16 relocalization and function during stress. PLoS Genet 8, e1002948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin RM, Fu X, Pai MY, Vergnes L, Hwang H, Deng G, Diep S, Lomenick B, Meli VS, Monsalve GC, et al. , 2014. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 510, 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching TT, Paal AB, Mehta A, Zhong L, Hsu AL, 2010. drr-2 encodes an eIF4H that acts downstream of TOR in diet-restriction-induced longevity of C. elegans. Aging Cell 9, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe KP, Przybysz AJ, Strange K, 2009. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol 29, 2704–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, Gonos ES, 2015. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J 29, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary V, Ojha N, Golden A, Prinz WA, 2015. A conserved family of proteins facilitates nascent lipid droplet budding from the ER. J. Cell Biol 211, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K, 2007. RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128, 1063–1076. [DOI] [PubMed] [Google Scholar]
- Clapier CR, Cairns BR, 2009. The biology of chromatin remodeling complexes. Annu. Rev. Biochem 78, 273–304. [DOI] [PubMed] [Google Scholar]
- Clapier CR, Iwasa J, Cairns BR, Peterson CL, 2017. Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes. Nat. Rev. Mol. Cell Biol 18, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran SP, Wu X, Riedel CG, Ruvkun G, 2009. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature 459, 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis R, O’Connor G, DiStefano PS, 2006. Aging networks in Caenorhabditis elegans: AMP-activated protein kinase (aak-2) links multiple aging and metabolism pathways. Aging Cell 5, 119–126. [DOI] [PubMed] [Google Scholar]
- Dang W, Sutphin GL, Dorsey JA, Otte GL, Cao K, Perry RM, Wanat JJ, Saviolaki D, Murakami CJ, Tsuchiyama S, et al. , 2014. Inactivation of yeast Isw2 chromatin remodeling enzyme mimics longevity effect of calorie restriction via induction of genotoxic stress response. Cell Metab 19, 952–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R, Melo JA, Thondamal M, Morton EA, Cornwell AB, Crick B, Kim JH, Swartz EW, Lamitina T, Douglas PM, et al. , 2017. The homeodomain-interacting protein kinase HPK-1 preserves protein homeostasis and longevity through master regulatory control of the HSF-1 chaperone network and TORC1-restricted autophagy in Caenorhabditis elegans. PLoS Genet 13, e1007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C, 2010. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8, e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haes W, Frooninckx L, Van Assche R, Smolders A, Depuydt G, Billen J, Braeckman BP, Schoofs L, Temmerman L, 2014. Metformin promotes lifespan through mitohormesis via the peroxiredoxin PRDX-2. Proc. Natl. Acad. Sci. U. S. A 111, E2501–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Henau S, Tilleman L, Vangheel M, Luyckx E, Trashin S, Pauwels M, Germani F, Vlaeminck C, Vanfleteren JR, Bert W, et al. , 2015. A redox signalling globin is essential for reproduction in Caenorhabditis elegans. Nat. Commun 6, 8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vaux V, Pfefferli C, Passannante M, Belhaj K, von Essen A, Sprecher SG, Muller F, Wicky C, 2013. The Caenorhabditis elegans LET-418/Mi2 plays a conserved role in lifespan regulation. Aging Cell 12, 1012–1020. [DOI] [PubMed] [Google Scholar]
- Dehghan E, Zhang Y, Saremi B, Yadavali S, Hakimi A, Dehghani M, Goodarzi M, Tu X, Robertson S, Lin R, et al. , 2017. Hydralazine induces stress resistance and extends C. elegans lifespan by activating the NRF2/SKN-1 signalling pathway. Nat. Commun 8, 2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demoinet E, Li S, Roy R, 2017. AMPK blocks starvation-inducible transgenerational defects in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 114, E2689–E2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzel MS, Storm NJ, Gutschmidt A, Baddi R, Hinze Y, Jarosch E, Sommer T, Hoppe T, Antebi A, 2014. Hexosamine pathway metabolites enhance protein quality control and prolong life. Cell 156, 1167–1178. [DOI] [PubMed] [Google Scholar]
- DePina AS, Iser WB, Park SS, Maudsley S, Wilson MA, Wolkow CA, 2011. Regulation of Caenorhabditis elegans vitellogenesis by DAF-2/IIS through separable transcriptional and posttranscriptional mechanisms. BMC Physiol 11, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt G, Xie F, Petyuk VA, Shanmugam N, Smolders A, Dhondt I, Brewer HM, Camp DG, Smith RD 2nd, Braeckman BP, 2013. Reduced insulin/insulin-like growth factor-1 signaling and dietary restriction inhibit translation but preserve muscle mass in Caenorhabditis elegans. Mol. Cell. Proteom.: MCP 12, 3624–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depuydt G, Shanmugam N, Rasulova M, Dhondt I, Braeckman BP, 2016. Increased protein stability and decreased protein turnover in the Caenorhabditis elegans Ins/IGF-1 daf-2 mutant. The journals of gerontology. Ser. A: Biol. Sci. Med. Sci 71, 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt I, Petyuk VA, Cai H, Vandemeulebroucke L, Vierstraete A, Smith RD, Depuydt G, Braeckman BP, 2016. FOXO/DAF-16 activation slows down turnover of the majority of proteins in C. elegans. Cell Rep 3028–3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhondt I, Petyuk VA, Bauer S, Brewer HM, Smith RD, Depuydt G, Braeckman BP, 2017. Changes of protein turnover in aging Caenorhabditis elegans. Mol. Cell Proteomics 16, 1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C, 2002. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Venable JD, Au N, Xu T, Park SK, Cociorva D, Johnson JR, Dillin A, Yates JR 3rd, 2007. Quantitative mass spectrometry identifies insulin signaling targets in C. elegans. Science 317, 660–663. [DOI] [PubMed] [Google Scholar]
- Durieux J, Wolff S, Dillin A, 2011. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelenboom A, Burgering BM, 2013. FOXOs: signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol 14, 83–97. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Patel DS, Zhan M, Steele AJ, Lu H, Ch’ng Q, 2015. A gene-expression-based neural code for food abundance that modulates lifespan. eLife 4, e06259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald CY, Landis JN, Abate J. Porter, Murphy CT, Blackwell TK, 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519, 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald CY, Hourihan JM, Bland MS, Obieglo C, Katic I, Mazzeo L.E. Moronetti, Alcedo J, Blackwell TK, Hynes NE, 2017. NADPH oxidase-mediated redox signaling promotes oxidative stress resistance and longevity through memo-1 in C. elegans. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri TH, Stevenson J, Chisholm AD, Mango SE, 2010. Dynamic chromatin organization during foregut development mediated by the organ selector gene PHA-4/FoxA. PLoS Genet 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardghassemi Y, Tauffenberger A, Gosselin S, Parker JA, 2017. Rescue of ATXN3 neuronal toxicity in Caenorhabditiselegans by chemical modification of endoplasmic reticulum stress. Dis. Model. Mech 10, 1465–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Bussiere F, Hekimi S, 2001. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644. [DOI] [PubMed] [Google Scholar]
- Ferrari KJ, Pasini D, 2013. Regulation and function of DNA and histone methylations. Curr. Pharm. Des 19, 719–733. [PubMed] [Google Scholar]
- Feser J, Truong D, Das C, Carson JJ, Kieft J, Harkness T, Tyler JK, 2010. Elevated histone expression promotes life span extension. Mol. Cell 39, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D, Thompson MA, Takhaveev V, Dobson AJ, Kotronaki I, Green JWM, Heinemann M, Tullet JMA, Alic N, 2017. RNA polymerase III limits longevity downstream of TORC1. Nature 552, 263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, 2011. Signal transduction by reactive oxygen species. J. Cell Biol 194, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzenberger E, Boll M, Wenzel U, 2013. Impairment of the proteasome is crucial for glucose-induced lifespan reduction in the mev-1 mutant of Caenorhabditis elegans. Biochim. Biophys. Acta 1832, 565–573. [DOI] [PubMed] [Google Scholar]
- Fletcher M, Kim DH, 2017. Age-dependent neuroendocrine signaling from sensory neurons modulates the effect of dietary restriction on longevity of Caenorhabditis elegans. PLoS Genet 13, e1006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-McClure ML, Hohsfield LA, Fonte G, Bealor MT, Link CD, 2007. Decreased insulin-receptor signaling promotes the autophagic degradation of beta-amyloid peptide in C. elegans . Autophagy 3, 569–580. [DOI] [PubMed] [Google Scholar]
- Folick A, Oakley HD, Yu Y, Armstrong EH, Kumari M, Sanor L, Moore DD, Ortlund EA, Zechner R, Wang MC, 2015. Aging. Lysosomal signaling molecules regulate longevity in Caenorhabditis elegans. Science 347, 83–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Baehrecke EH, Ballabio A, Boya P, Bravo-San Pedro JM, Cecconi F, Choi AM, Chu CT, Codogno P, Colombo MI, et al. , 2017a. Molecular definitions of autophagy and related processes. EMBO J 36, 1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Bravo-San Pedro JM, Levine B, Green DR, Kroemer G, 2017b. Pharmacological modulation of autophagy: therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov 16, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao AW, Chatzispyrou IA, Kamble R, Liu YJ, Herzog K, Smith RL, van Lenthe H, Vervaart MAT, van Cruchten A, Luyf AC, et al. , 2017. A sensitive mass spectrometry platform identifies metabolic changes of life history traits in C. elegans. Sci. Rep 7, 2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelino S, Chang JT, Kumsta C, She X, Davis A, Nguyen C, Panowski S, Hansen M, 2016. Intestinal autophagy improves healthspan and longevity in C. elegans during dietary restriction. PLoS Genet 12, e1006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A, 2001. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev. Cell 1, 841–851. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Rottiers V, Li D, Motola DL, Cummins CL, Lehrach H, Mangelsdorf DJ, Antebi A, 2007. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc. Natl. Acad. Sci. U. S. A 104, 5014–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C, 2007. Regulation of Caenorhabditis elegans lifespan by a proteasomal E3 ligase complex. Proc. Natl. Acad. Sci. U. S. A 104, 5947–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazi A, Henis-Korenblit S, Kenyon C, 2009. A transcription elongation factor that links signals from the reproductive system to lifespan extension in Caenorhabditis elegans. PLoS Genet 5, e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin W, Skinner ME, Lombard DB, 2014. Sirtuins: guardians of mammalian healthspan. Trends Genet 30, 271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitschlag BL, Kirby CS, Samuels DC, Gangula RD, Mallal SA, Patel MR, 2016. Homeostatic responses regulate selfish mitochondrial genome dynamics in C. elegans. Cell Metab 24, 91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh GYS, Winter JJ, Bhanshali F, Doering KRS, Lai R, Lee K, Veal EA, Taubert S, 2018. NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golegaonkar S, Tabrez SS, Pandit A, Sethurathinam S, Jagadeeshaprasad MG, Bansode S, Sampathkumar SG, Kulkarni MJ, Mukhopadhyay A, 2015. Rifampicin reduces advanced glycation end products and activates DAF-16 to increase lifespan in Caenorhabditis elegans. Aging Cell 14, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudeau J, Bellemin S, Toselli-Mollereau E, Shamalnasab M, Chen Y, Aguilaniu H, 2011. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol 9, e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Brunet A, 2009. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell 8, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Shi Y, 2012. Histone methylation: a dynamic mark in health, disease and inheritance. Nat. Rev. Genet 13, 343–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A, 2007. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol 17, 1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Hauswirth AG, Green EM, Leeman DS, Maro GS, Han S, Banko MR, Gozani O, Brunet A, 2010. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 466, 383–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A, 2011. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 479, 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Beese-Sims SE, Brookes E, Spadafora R, Zhu Y, Rothbart SB, Aristizabal-Corrales D, Chen S, Badeaux AI, Jin Q, et al. , 2014. A histone methylation network regulates transgenerational epigenetic memory in C. elegans. Cell Rep 7, 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Blanco MA, Gu L, Sendinc E, Liu J, Aristizabal-Corrales D, Hsu CH, Aravind L, He C, Shi Y, 2015. DNA methylation on N6-Adenine in C. elegans. Cell 161, 868–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Becker B, Latza C, Antebi A, Shi Y, 2016. Mutation of C. elegans demethylase spr-5 extends transgenerational longevity. Cell Res 26, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XL, Wu PF, Wang S, Zhang JJ, Shen ZC, Luo H, Chen H, Long LH, Chen JG, Wang F, 2017. Dimethyl sulfide protects against oxidative stress and extends lifespan via a methionine sulfoxide reductase A-dependent catalytic mechanism. Aging Cell 16, 226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, Nam HG, 2015. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat. Commun 6, 8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton B, Dong Y, Shindo M, Liu W, Odell I, Ruvkun G, Lee SS, 2005. A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19, 1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Schroeder EA, Silva-Garcia CG, Hebestreit K, Mair WB, Brunet A, 2017. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 544, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kapahi P, 2010. In: Hall MN, Tamanoi F (Eds.), TOR Signaling and Aging. In The Enzymes Academic Press, Burlington, pp. 279–299. [Google Scholar]
- Hansen M, Kennedy BK, 2016. Does Longer Lifespan Mean Longer Healthspan? Trends Cell Biol 26, 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Hsu AL, Dillin A, Kenyon C, 2005. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C, 2007. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6, 95–110. [DOI] [PubMed] [Google Scholar]
- Hansen M, Flatt T, Aguilaniu H, 2013. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab 17, 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvald EB, Sprenger RR, Dall KB, Ejsing CS, Nielsen R, Mandrup S, Murillo AB, Larance M, Gartner A, Lamond AI, et al. , 2017. Multi-omics analyses of starvation responses reveal a central role for lipoprotein metabolism in acute starvation survival in C. elegans. Cell Syst 5, 38–52 e34. [DOI] [PubMed] [Google Scholar]
- Heestand BN, Shen Y, Liu W, Magner DB, Storm N, Meharg C, Habermann B, Antebi A, 2013. Dietary restriction induced longevity is mediated by nuclear receptor NHR-62 in Caenorhabditis elegans. PLoS Genet 9, e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler T, Hartwig K, Daniel H, Wenzel U, 2010. Caenorhabditis elegans lifespan extension caused by treatment with an orally active ROS-generator is dependent on DAF-16 and SIR-2.1. Biogerontology 11, 183–195. [DOI] [PubMed] [Google Scholar]
- Heimbucher T, Liu Z, Bossard C, McCloskey R, Carrano AC, Riedel CG, Tanasa B, Klammt C, Fonslow BR, Riera CE, et al. , 2015. The deubiquitylase MATH-33 controls DAF-16 stability and function in metabolism and longevity. Cell Metab 22, 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz C, Doktor TK, Lanjuin A, Escoubas C, Zhang Y, Weir HJ, Dutta S, Silva-Garcia CG, Bruun GH, Morantte I, et al. , 2017. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nature 541, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE, 2001. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol 11, 1975–1980. [DOI] [PubMed] [Google Scholar]
- Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee SJ, Cary M, Kenyon C, 2010. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc. Natl. Acad. Sci. U. S. A 107, 9730–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, et al. , 2015. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JM, Partridge L, 2015. Nuclear hormone receptors: roles of xenobiotic detoxification and sterol homeostasis in healthy aging. Crit. Rev. Biochem. Mol. Biol 50, 380–392. [DOI] [PubMed] [Google Scholar]
- Honda Y, Fujita Y, Maruyama H, Araki Y, Ichihara K, Sato A, Kojima T, Tanaka M, Nozawa Y, Ito M, et al. , 2011. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS ONE 6, e23527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Araki Y, Hata T, Ichihara K, Ito M, Tanaka M, Honda S, 2015. 10-hydroxy-2-decenoic acid, the major lipid component of royal jelly, extends the lifespan of Caenorhabditis elegans through dietary restriction and target of rapamycin signaling. J. Aging Res 425261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjoh S, Yamamoto T, Uno M, Nishida E, 2009. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457, 726–730. [DOI] [PubMed] [Google Scholar]
- Horsman JW, Miller DL, 2016. Mitochondrial sulfide quinone oxidoreductase prevents activation of the unfolded protein response in hydrogen sulfide. J. Biol. Chem 291, 5320–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, 2013. DNA methylation age of human tissues and cell types. Genome Biol 14, R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou NS, Gutschmidt A, Choi DY, Pather K, Shi X, Watts JL, Hoppe T, Taubert S, 2014. Activation of the endoplasmic reticulum unfolded protein response by lipid disequilibrium without disturbed proteostasis in vivo. Proc. Natl. Acad. Sci. U. S. A 111, E2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan JM, Mazzeo L.E. Moronetti, Fernandez-Cardenas LP, Blackwell TK, 2016. Cysteine sulfenylation directs IRE-1 to activate the SKN-1/Nrf2 antioxidant response. Mol. Cell 63, 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR, 2003. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp. Gerontol 38, 947–954. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J, 2013. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard AC, Rollins J, Snow S, Castor S, Rogers AN, 2016. Reducing translation through eIF4G/IFG-1 improves survival under ER stress that depends on heat shock factor HSF-1 in Caenorhabditis elegans. Aging Cell [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsin H, Kenyon C, 1999. Signals from the reproductive system regulate the lifespan of C. elegans. Nature 399, 362–366. [DOI] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C, 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 300, 1142–1145. [DOI] [PubMed] [Google Scholar]
- Hsu HT, Chen HM, Yang Z, Wang J, Lee NK, Burger A, Zaret K, Liu T, Levine E, Mango SE, 2015. TRANSCRIPTION. Recruitment of RNA polymerase II by the pioneer transcription factor PHA-4. Science 348, 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XB, Mu XH, Wan QL, He XM, Wu GS, Luo HR, 2017. Aspirin increases metabolism through germline signalling to extend the lifespan of Caenorhabditis elegans. PLoS ONE 12, e0184027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA, 2007. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev 87, 1175–1213. [DOI] [PubMed] [Google Scholar]
- Hwang AB, Lee SJ, 2011. Regulation of life span by mitochondrial respiration: the HIF-1 and ROS connection. Aging (Albany NY) 3, 304–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang AB, Ryu EA, Artan M, Chang HW, Kabir MH, Nam HJ, Lee D, Yang JS, Kim S, Mair WB, et al. , 2014. Feedback regulation via AMPK and HIF-1 mediates ROS-dependent longevity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 111, E4458–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W, Artan M, Seo M, Lee D, Nam HG, Lee SJ, 2015. Inhibition of elongin C promotes longevity and protein homeostasis via HIF-1 in C. elegans. Aging Cell 14, 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara A, Uno M, Miyatake K, Honjoh S, Nishida E, 2017. Cholesterol regulates DAF-16 nuclear localization and fasting-induced longevity in C. elegans. Exp. Gerontol 87, 40–47. [DOI] [PubMed] [Google Scholar]
- Imai SI, Guarente L, 2016. It takes two to tango: NAD(+) and sirtuins in aging/longevity control. NPJ Aging Mech. Dis 2, 16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K, 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev 19, 2278–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Li J, Green CD, Yu X, Tang X, Han D, Xian B, Wang D, Huang X, Cao X, et al. , 2011. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab 14, 161–172. [DOI] [PubMed] [Google Scholar]
- Jobson MA, Jordan JM, Sandrof MA, Hibshman JD, Lennox AL, Baugh LR, 2015. Transgenerational effects of early life starvation on growth, reproduction, and stress resistance in Caenorhabditis elegans. Genetics 201, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Llop JR, Farrell SF, Yuan J, Stolzenburg LR, Samuelson AV, 2014. The Caenorhabditis elegans Myc-Mondo/Mad complexes integrate diverse longevity signals. PLoS Genet 10, e1004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB, 2005. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283–293. [DOI] [PubMed] [Google Scholar]
- Kabil O, Motl N, Banerjee R, 2014. H2S and its role in redox signaling. Biochim. Biophys. Acta 1844, 1355–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein TL, Smith ED, Tsuchiya M, Welton KL, Thomas JH, Fields S, Kennedy BK, Kaeberlein M, 2006. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L, 2010. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 11, 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P, Kaeberlein M, Hansen M, 2017. Dietary restriction and lifespan: lessons from invertebrate models. Ageing Res. Rev 39, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith SA, Maddux SK, Zhong Y, Chinchankar MN, Ferguson AA, Ghazi A, Fisher AL, 2016. Graded proteasome dysfunction in Caenorhabditis elegans activates an adaptive response involving the conserved SKN-1 and ELT-2 transcription factors and the autophagy-lysosome pathway. PLoS Genet 12, e1005823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ, 2010. The genetics of ageing. Nature 464, 504–512. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R, 1993. A C. elegans mutant that lives twice as long as wild type [see comments]. Nature 366, 461–464. [DOI] [PubMed] [Google Scholar]
- Kim HE, Grant AR, Simic MS, Kohnz RA, Nomura DK, Durieux J, Riera CE, Sanchez M, Kapernick E, Wolff S, et al. , 2016. Lipid biosynthesis coordinates a mitochondrial-to-Cytosolic stress response. Cell 166, 1539–1552 e1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Tanaka N, Nakamura N, Takano S, Ohkuma S, 2007. Knockdown of mitochondrial heat shock protein 70 promotes progeria-like phenotypes in caenorhabditis elegans. J. Biol. Chem 282, 5910–5918. [DOI] [PubMed] [Google Scholar]
- Kirkwood TB, 2005. Understanding the odd science of aging. Cell 120, 437–447. [DOI] [PubMed] [Google Scholar]
- Kirstein-Miles J, Scior A, Deuerling E, Morimoto RI, 2013. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J 32, 1451–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto S, Uno M, Okabe E, Nono M, Nishida E, 2017. Environmental stresses induce transgenerationally inheritable survival advantages via germline-to-soma communication in Caenorhabditis elegans. Nat. Commun 8, 14031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Kohlbrecher D, Christensen J, Vandamme J, Abarrategui I, Bak M, Tommerup N, Shi X, Gozani O, Rappsilber J, Salcini AE, et al. , 2010. A functional link between the histone demethylase PHF8 and the transcription factor ZNF711 in X-linked mental retardation. Mol. Cell 38, 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause KH, 2007. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol 42, 256–262. [DOI] [PubMed] [Google Scholar]
- Kumar S, Dietrich N, Kornfeld K, 2016. Angiotensin converting enzyme (ACE) inhibitor extends Caenorhabditis elegans life span. PLoS Genet 12, e1005866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta C, Ching TT, Nishimura M, Davis AE, Gelino S, Catan HH, Yu X, Chu CC, Ong B, Panowski SH, et al. , 2014. Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging Cell 13, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta C, Chang JT, Schmalz J, Hansen M, 2017. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun 8, 14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ES, Narasimhan SD, Yen K, Tissenbaum HA, 2010. A new DAF-16 isoform regulates longevity. Nature 466, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI, 2015. Repression of the heat shock response is a programmed event at the onset of reproduction. Mol. Cell 59, 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Brielmann RM, Neto MF, Lin YF, Haynes CM, Morimoto RI, 2017. Mitochondrial stress restores the heat shock response and prevents proteostasis collapse during aging. Cell Rep 21, 1481–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S, 1998. The genetics of caloric restriction in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 95, 13091–13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Hansen M, 2012. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol. Metab 23, 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Gelino S, Melendez A, Hansen M, 2011. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr. Biol 21, 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Melendez A, Hansen M, 2012. Autophagy links lipid metabolism to longevity in C. elegans. Autophagy 8, 144–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, De Magalhaes Filho CD, McQuary PR, Chu CC, Visvikis O, Chang JT, Gelino S, Ong B, Davis AE, Irazoqui JE, et al. , 2013. The TFEB orthologue HLH-30 regulates autophagy and modulates longevity in Caenorhabditis elegans. Nat. Commun 4, 2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre LR, Kumsta C, Sandri M, Ballabio A, Hansen M, 2015. Transcriptional and epigenetic regulation of autophagy in aging. Autophagy 11, 867–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM, 2012. mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PL, Albert PS, Riddle DL, 1995. Genes that regulate both development and longevity in Caenorhabditis elegans. Genetics 139, 1567–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Lee RY, Fraser AG, Kamath RS, Ahringer J, Ruvkun G, 2003. A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat. Genet 33, 40–48. [DOI] [PubMed] [Google Scholar]
- Lee GD, Wilson MA, Zhu M, Wolkow CA, de Cabo R, Ingram DK, Zou S, 2006. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 5, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hwang AB, Kenyon C, 2010. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr. Biol 20, 2131–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrbach NJ, Ruvkun G, 2016. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiser SF, Miller H, Rossner R, Fletcher M, Leonard A, Primitivo M, Rintala N, Ramos FJ, Miller DL, Kaeberlein M, 2015. Cell nonautonomous activation of flavin-containing monooxygenase promotes longevity and health span. Science 350, 1375–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao B, Lee SM, Bennett K, Fang D, 2007. RLE-1, an E3 ubiquitin ligase, regulates C. elegans aging by catalyzing DAF-16 polyubiquitination. Dev. Cell 12, 235–246. [DOI] [PubMed] [Google Scholar]
- Li J, Ebata A, Dong Y, Rizki G, Iwata T, Lee SS, 2008. Caenorhabditis elegans HCF-1 functions in longevity maintenance as a DAF-16 regulator. PLoS Biol 6, e233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Matilainen O, Jin C, Glover-Cutter KM, Holmberg CI, Blackwell TK, 2011. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet 7, e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TM, Liu W, Lu S, Zhang YP, Jia LM, Chen J, Li X, Lei X, Dong MQ, 2015. No Significant Increase in the Delta4- and Delta7-Dafachronic Acid Concentration in the Long-Lived glp-1 Mutant, nor in the Mutants Defective in Dauer Formation. G3 5, 1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen B, Zou W, Wang X, Wu Y, Zhao D, Sun Y, Liu Y, Chen L, Miao L, et al. , 2016. The lysosomal membrane protein SCAV-3 maintains lysosome integrity and adult longevity. J. Cell Biol 215, 167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Labbadia J, Morimoto RI, 2017. Rethinking HSF1 in stress, development, and organismal health. Trends Cell Biol 27, 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhang L, Natarajan SK, Becker DF, 2013. Proline mechanisms of stress survival. Antioxid. Redox Signal 19, 998–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau WS, Gonzalez-Serricchio AS, Deshommes C, Chin K, LaMunyon CW, 2007. A persistent mitochondrial deletion reduces fitness and sperm performance in heteroplasmic populations of C. elegans. BMC Genet 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C, 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet 28, 139–145. [DOI] [PubMed] [Google Scholar]
- Lin YF, Schulz AM, Pellegrino MW, Lu Y, Shaham S, Haynes CM, 2016. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature 533, 416–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JY, Spatola BN, Curran SP, 2017. WDR23 regulates NRF2 independently of KEAP1. PLoS Genet 13, e1006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Sung T, Lin N, Abraham RT, Jessen BA, 2017. Lysosomal adaptation: how cells respond to lysosomotropic compounds. PLoS ONE 12, e0173771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M, Garrett T, Yu I, Calahorro F, Asadi Shahmirzadi A, Miller A, Gill MS, Hughes RE, Holden-Dye L, Lithgow GJ, 2016. Chemical activation of a food deprivation signal extends lifespan. Aging Cell 15, 832–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucanic M, Plummer WT, Chen E, Harke J, Foulger AC, Onken B, Coleman-Hulbert AL, Dumas KJ, Guo S, Johnson E, et al. , 2017. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat. Commun 8, 14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DA, Dalton HM, Sowa JN, Wang MC, Soukas AA, Curran SP, 2015. Omega-3 and −6 fatty acids allocate somatic and germline lipids to ensure fitness during nutrient and oxidative stress in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 112, 15378–15383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack HID, Zhang P, Fonslow BR, Yates JR, 2017. The protein kinase MBK-1 contributes to lifespan extension in daf-2 mutant and germline-deficient Caenorhabditis elegans. Aging 9, 1414–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanti P, Bose N, Bethke A, Judkins JC, Wollam J, Dumas KJ, Zimmerman AM, Campbell SL, Hu PJ, Antebi A, et al. , 2014. Comparative metabolomics reveals endogenous ligands of DAF-12, a nuclear hormone receptor, regulating C. elegans development and lifespan. Cell Metab 19, 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A, 2011. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature 470, 404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mango SE, 2009. The molecular basis of organ formation: insights from the C. elegans foregut. Annu. Rev. Cell Dev. Biol 25, 597–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Toker A, 2017. AKT/PKB signaling: navigating the network. Cell 169, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfeld J, Urban N, Priebe S, Groth M, Frahm C, Hartmann N, Gebauer J, Ravichandran M, Dommaschk A, Schmeisser S, et al. , 2015. Branched-chain amino acid catabolism is a conserved regulator of physiological ageing. Nat. Commun 6, 10043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KA, Dumas KJ, Bhaumik D, Schilling B, Davis S, Oron TR, Sorensen DJ, Lucanic M, Brem RB, Melov S, et al. , 2016. Vitamin d promotes protein homeostasis and longevity via the stress response pathway genes skn-1, ire-1, and xbp-1. Cell Rep 17, 1227–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilainen O, Sleiman MSB, Quiros PM, Garcia S, Auwerx J, 2017. The chromatin remodeling factor ISW-1 integrates organismal responses against nuclear and mitochondrial stress. Nat. Commun 8, 1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM, 2017. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun 8, 14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maures TJ, Greer EL, Hauswirth AG, Brunet A, 2011. The H3K27 demethylase UTX-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell 10, 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Killilea DW, Hubbard AE, Vantipalli MC, Melov S, Lithgow GJ, 2008. Pharmacogenetic analysis of lithium-induced delayed aging in Caenorhabditis elegans. J. Biol. Chem 283, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl G, Rogers AN, Alavez S, Hubbard AE, Melov S, Link CD, Bush AI, Kapahi P, Lithgow GJ, 2010. Insulin-like signaling determines survival during stress via posttranscriptional mechanisms in C. elegans. Cell Metab 12, 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick M, Chen K, Ramaswamy P, Kenyon C, 2012. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell 11, 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuary PR, Liao CY, Chang JT, Kumsta C, She X, Davis A, Chu CC, Gelino S, Gomez-Amaro RL, Petrascheck M, et al. , 2016. C. elegans S6K mutants require a creatine-kinase-like effector for lifespan extension. Cell Rep 14, 2059–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R, Steinkraus KA, Sutphin GL, Ramos FJ, Shamieh LS, Huh A, Davis C, Chandler-Brown D, Kaeberlein M, 2009. Proteasomal regulation of the hypoxic response modulates aging in C. elegans. Science 324, 1196–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C, Jovaisaite V, Durieux J, Matilainen O, Jordan SD, Quiros PM, Steffen KK, Williams EG, Mouchiroud L, Tronnes SU, et al. , 2016. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell 165, 1209–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing JA, Heuberger R, Schisa JA, 2013. Effect of vitamin D3 on lifespan in Caenorhabditis elegans. Curr. Aging Sci 6, 220–224. [DOI] [PubMed] [Google Scholar]
- Miller DL, Roth MB, 2007. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 104, 20618–20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnerly J, Zhang J, Parker T, Kaul T, Jia K, 2017. The cell non-autonomous function of ATG-18 is essential for neuroendocrine regulation of Caenorhabditis elegans lifespan. PLoS Genet 13, e1006764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishur RJ, Khan M, Munkacsy E, Sharma L, Bokov A, Beam H, Radetskaya O, Borror M, Lane R, Bai Y, et al. , 2016. Mitochondrial metabolites extend lifespan. Aging Cell 15, 336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll L, Ben-Gedalya T, Reuveni H, Cohen E, 2016. The inhibition of IGF-1 signaling promotes proteostasis by enhancing protein aggregation and deposition. Faseb J 30, 1656–1669. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI, 2004. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol. Biol. Cell 15, 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JF, Brignull HR, Weyers JJ, Morimoto RI, 2002. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 99, 10417–10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. , 2006. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 124, 1209–1223. [DOI] [PubMed] [Google Scholar]
- Munkacsy E, Khan MH, Lane RK, Borror MB, Park JH, Bokov AF, Fisher AL, Link CD, Rea SL, 2016. DLK-1, SEK-3 and PMK-3 are required for the life extension induced by mitochondrial bioenergetic disruption in C. elegans. PLoS Genet 12, e1006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz MJ, Riddle DL, 2003. Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 163, 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C, 2003. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424, 277–283. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Karalay O, Jager PS, Horikawa M, Klein C, Nakamura K, Latza C, Templer SE, Dieterich C, Antebi A, 2016. Mondo complexes regulate TFEB via TOR inhibition to promote longevity in response to gonadal signals. Nat. Commun 7, 10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan V, Ly T, Pourkarimi E, Murillo AB, Gartner A, Lamond AI, Kenyon C, 2016. Deep proteome analysis identifies age-related processes in C. elegans. Cell Syst 3, 144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbonne P, Roy R, 2009. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457, 210–214. [DOI] [PubMed] [Google Scholar]
- Nedialkova DD, Leidel SA, 2015. Optimization of codon translation rates via tRNA modifications maintains proteome integrity. Cell 161, 1606–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TB, Louie SM, Daniele JR, Tran Q, Dillin A, Zoncu R, Nomura DK, Olzmann JA, 2017. DGAT1-dependent lipid droplet biogenesis protects mitochondrial function during starvation-induced autophagy. Dev. Cell 42, 9–21 e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Ebata A, Alipanahiramandi E, Lee SS, 2012. Two SET domain containing genes link epigenetic changes and aging in Caenorhabditis elegans. Aging Cell 11, 315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillegoda NB, Kirstein J, Szlachcic A, Berynskyy M, Stank A, Stengel F, Arnsburg K, Gao X, Scior A, Aebersold R, et al. , 2015. Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature 524, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brown ZK, Greer EL, 2016. N6-methyladenine: a conserved and dynamic DNA mark. Adv. Exp. Med. Biol 945, 213–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke EJ, Ruvkun G, 2013. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol 15, 668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G, 2013. omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev 27, 429–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onken B, Driscoll M, 2010. Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-PLoS ONE 5, e8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM, 2016. The integrated stress response. EMBO Rep 17, 1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K, Lionaki E, Tavernarakis N, 2015. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521, 525–528. [DOI] [PubMed] [Google Scholar]
- Pan KZ, Palter JE, Rogers AN, Olsen A, Chen D, Lithgow GJ, Kapahi P, 2007. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A, 2007. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447, 550–555. [DOI] [PubMed] [Google Scholar]
- Papaevgeniou N, Sakellari M, Jha S, Tavernarakis N, Holmberg CI, Gonos ES, Chondrogianni N, 2016. 18alpha-glycyrrhetinic acid proteasome activator decelerates aging and alzheimer’s disease progression in Caenorhabditis elegans and neuronal cultures. Antioxid. Redox Signal 25, 855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SK, Link CD, Johnson TE, 2010. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J 24, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passannante M, Marti CO, Pfefferli C, Moroni PS, Kaeser-Pebernard S, Puoti A, Hunziker P, Wicky C, Muller F, 2010. Different Mi-2 complexes for various developmental functions in Caenorhabditis elegans. PLoS ONE 5, e13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathare PP, Lin A, Bornfeldt KE, Taubert S, Van Gilst MR, 2012. Coordinate regulation of lipid metabolism by novel nuclear receptor partnerships. PLoS Genet 8, e1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul BD, Snyder SH, 2012. H(2)S signalling through protein sulfhydration and beyond. Nat. Rev. Mol. Cell Biol 13, 499–507. [DOI] [PubMed] [Google Scholar]
- Pellegrino MW, Nargund AM, Haynes CM, 2013. Signaling the mitochondrial unfolded protein response. Biochim. Biophys. Acta 1833, 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez CL, Van Gilst MR, 2008. A 13C isotope labeling strategy reveals the influence of insulin signaling on lipogenesis in C. elegans. Cell Metab 8, 266–274. [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB, 2007. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450, 553–556. [DOI] [PubMed] [Google Scholar]
- Podshivalova K, Kerr RA, Kenyon C, 2017. How a mutation that slows aging can also disproportionately extend end-of-Life decrepitude. Cell Rep 19, 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promlek T, Ishiwata-Kimata Y, Shido M, Sakuramoto M, Kohno K, Kimata Y, 2011. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol. Biol. Cell 22, 3520–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu M, Ni Z, Wang M, Wang X, Wood JG, Helfand SL, Yu H, Lee SS, 2015. Trimethylation of Lys36 on H3 restricts gene expression change during aging and impacts life span. Genes Dev 29, 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putker M, Madl T, Vos HR, de Ruiter H, Visscher M, van den Berg MC, Kaplan M, Korswagen HC, Boelens R, Vermeulen M, et al. , 2013. Redox-dependent control of FOXO/DAF-16 by transportin-1. Mol. Cell 49, 730–742. [DOI] [PubMed] [Google Scholar]
- Qi W, Gutierrez GE, Gao X, Dixon H, McDonough JA, Marini AM, Fisher AL, 2017. The omega-3 fatty acid alpha-linolenic acid extends Caenorhabditis elegans lifespan via NHR-49/PPARalpha and oxidation to oxylipins. Aging Cell 16, 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi MA, Haynes CM, Pellegrino MW, 2017. The mitochondrial unfolded protein response: signaling from the powerhouse. J. Biol. Chem 292, 13500–13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangaraju S, Solis GM, Thompson RC, Gomez-Amaro RL, Kurian L, Encalada SE, Niculescu AB, Salomon DR 3rd, Petrascheck M, 2015. Suppression of transcriptional drift extends C. elegans lifespan by postponing the onset of mortality. eLife 4, e08833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnappan R, Amrit FR, Chen SW, Gill H, Holden K, Ward J, Yamamoto KR, Olsen CP, Ghazi A, 2014. Germline signals deploy NHR-49 to modulate fatty-acid beta-oxidation and desaturation in somatic tissues of C. elegans. PLoS Genet 10, e1004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynes R, Leckey BD, Nguyen K Jr, Westerheide SD, 2012. Heat shock and caloric restriction have a synergistic effect on the heat shock response in a sir2.1-dependent manner in Caenorhabditis elegans. J. Biol. Chem 287, 29045–29053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, et al. , 2012. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell 11, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel CG, Dowen RH, Lourenco GF, Kirienko NV, Heimbucher T, West JA, Bowman SK, Kingston RE, Dillin A, Asara JM, et al. , 2013. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat. Cell Biol 15, 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riera CE, Merkwirth C, De Magalhaes Filho CD, Dillin A, 2016. Signaling networks determining life span. Annu. Rev. Biochem 85, 35–64. [DOI] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, Mizunuma M, Narasimhan SD, Neumann-Haefelin E, Sabatini DM, Blackwell TK, 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab 15, 713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roczniak-Ferguson A, Petit CS, Froehlich F, Qian S, Ky J, Angarola B, Walther TC, Ferguson SM, 2012. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal 5, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux AE, Langhans K, Huynh W, Kenyon C, 2016. Reversible age-related phenotypes induced during larval quiescence in C. elegans . Cell Metab 23, 1113–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safra M, Fickentscher R, Levi-Ferber M, Danino YM, Haviv-Chesner A, Hansen M, Juven-Gershon T, Weiss M, Henis-Korenblit S, 2014. The FOXO transcription factor DAF-16 bypasses ire-1 requirement to promote endoplasmic reticulum homeostasis. Cell Metab 20, 870–881. [DOI] [PubMed] [Google Scholar]
- Samuelson AV, Carr CE, Ruvkun G, 2007. Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev 21, 2976–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar CE, Dues DJ, Spielbauer KK, Machiela E, Cooper JF, Senchuk M, Hekimi S, Van Raamsdonk JM, 2015. Mitochondrial and cytoplasmic ROS have opposing effects on lifespan. PLoS Genet 11, e1004972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi A, Torgovnick A, Kell A, Megalou E, Castelein N, Guccini I, Marzocchella L, Gelino S, Hansen M, Malisan F, et al. , 2013. Autophagy induction extends lifespan and reduces lipid content in response to frataxin silencing in C. elegans. Exp. Gerontol 48, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavi A, Maglioni S, Palikaras K, Shaik A, Strappazzon F, Brinkmann V, Torgovnick A, Castelein N, De Henau S, Braeckman BP, et al. , 2015. Iron starvation-Induced mitophagy mediates lifespan extension upon mitochondrial stress in C. elegans. Curr. Biol 25, 1810–1822. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M, 2007. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6, 280–293. [DOI] [PubMed] [Google Scholar]
- Seah NE, de Magalhaes Filho CD, Petrashen AP, Henderson HR, Laguer J, Gonzalez J, Dillin A, Hansen M, Lapierre LR, 2016. Autophagy-mediated longevity is modulated by lipoprotein biogenesis. Autophagy 12, 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen P, Dang W, Donahue G, Dai J, Dorsey J, Cao X, Liu W, Cao K, Perry R, Lee JY, et al. , 2015. H3K36 methylation promotes longevity by enhancing transcriptional fidelity. Genes Dev 29, 1362–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen P, Shah PP, Nativio R, Berger SL, 2016. Epigenetic mechanisms of longevity and aging. Cell 166, 822–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senchuk MM, Dues DJ, Schaar CE, Johnson BK, Madaj ZB, Bowman MJ, Winn ME, Van Raamsdonk JM, 2018. Activation of DAF-16/FOXO by reactive oxygen species contributes to longevity in long-lived mitochondrial mutants in Caenorhabditis elegans. PLoS Genet 14, e1007268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo K, Choi E, Lee D, Jeong DE, Jang SK, Lee SJ, 2013. Heat shock factor 1 mediates the longevity conferred by inhibition of TOR and insulin/IGF-1 signaling pathways in C. elegans. Aging Cell 12, 1073–1081. [DOI] [PubMed] [Google Scholar]
- Seo M, Seo K, Hwang W, Koo HJ, Hahm JH, Yang JS, Han SK, Hwang D, Kim S, Jang SK, et al. , 2015. RNA helicase HEL-1 promotes longevity by specifically activating DAF-16/FOXO transcription factor signaling in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 112, E4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S, Erdin SU, Huynh T, Medina D, Colella P, et al. , 2011. TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre C, De Cegli R, Mansueto G, Saha PK, Vetrini F, Visvikis O, Huynh T, Carissimo A, Palmer D, Jurgen Klisch T, et al. , 2013. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol 15, 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamalnasab M, Dhaoui M, Thondamal M, Harvald EB, Faergeman NJ, Aguilaniu H, Fabrizio P, 2017. HIF-1-dependent regulation of lifespan in Caenorhabditis elegans by the acyl-CoA-binding protein MAA-1. Aging (Albany NY) 9, 1745–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaffer KL, Updike DL, Mango SE, 2008. The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr. Biol 18, 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Wollam J, Magner D, Karalay O, Antebi A, 2012. A steroid receptor-microRNA switch regulates life span in response to signals from the gonad. Science 338, 1472–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Sakoguchi H, Yoshihara A, Izumori K, Sato M, 2017. d-Allulose, a stereoisomer of d-fructose, extends Caenorhabditis elegans lifespan through a dietary restriction mechanism: a new candidate dietary restriction mimetic. Biochem. Biophys. Res. Commun 493, 1528–1533. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis RJ, Xu L, Lee H, Chae M, Thaden JJ, Bharill P, Tazearslan C, Siegel E, Alla R, Zimniak P, et al. , 2011. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging (Albany NY) 3, 125–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini MJ, Johnson JR, Kumar AV, Thakurta TG, Blais K, Neill ZA, Marion SW, St Amand V, Reenan RA, Lapierre LR, 2018. Nuclear export inhibition enhances HLH-30/TFEB activity, autophagy, and lifespan. Cell Rep 23, 1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Aballay A, 2017. Endoplasmic reticulum stress caused by lipoprotein accumulation suppresses immunity against bacterial pathogens and contributes to immunosenescence. mBio 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A, Kumar N, Matai L, Jain V, Garg A, Mukhopadhyay A, 2016. A chromatin modifier integrates insulin/IGF-1 signalling and dietary restriction to regulate longevity. Aging Cell 15, 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ED, Kaeberlein TL, Lydum BT, Sager J, Welton KL, Kennedy BK, Kaeberlein M, 2008. Age- and calorie-independent life span extension from dietary restriction by bacterial deprivation in Caenorhabditis elegans. BMC Dev. Biol 8, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Vikos T, de Lencastre A, Inukai S, Shlomchik M, Holtrup B, Slack FJ, 2014. MicroRNAs mediate dietary-restriction-induced longevity through PHA-4/FOXA and SKN-1/Nrf transcription factors. Curr. Biol 24, 2238–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son HG, Seo M, Ham S, Hwang W, Lee D, An SW, Artan M, Seo K, Kaletsky R, Arey RN, et al. , 2017. RNA surveillance via nonsense-mediated mRNA decay is crucial for longevity in daf-2/insulin/IGF-1 mutant C. elegans. Nat. Commun 8, 14749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh MJ, Narasimhan SD, Robida-Stubbs S, Moronetti Mazzeo LE, Dreyfuss JM, Hourihan JM, Raghavan P, Operana TN, Esmaillie R, Blackwell TK, 2015. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M, 2008. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell 7, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout GJ, Stigter EC, Essers PB, Mulder KW, Kolkman A, Snijders DS, van den Broek NJ, Betist MC, Korswagen HC, Macinnes AW, et al. , 2013. Insulin/IGF-1-mediated longevity is marked by reduced protein metabolism. Mol. Syst. Biol 9, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup N, Anthony WE, Nash ZM, Gowda V, Gomez A, Lopez-Moyado IF, Apfeld J, Fontana W, 2016. The temporal scaling of Caenorhabditis elegans ageing. Nature 530, 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syntichaki P, Troulinaki K, Tavernarakis N, 2007. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 445, 922–926. [DOI] [PubMed] [Google Scholar]
- Tabrez SS, Sharma RD, Jain V, Siddiqui AA, Mukhopadhyay A, 2017. Differential alternative splicing coupled to nonsense-mediated decay of mRNA ensures dietary restriction-induced longevity. Nat. Commun 8, 306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Daitoku H, Hirota K, Tamiya H, Yokoyama A, Kako K, Nagashima Y, Nakamura A, Shimada T, Watanabe S, et al. , 2011. Asymmetric arginine dimethylation determines life span in C. elegans by regulating forkhead transcription factor DAF-16. Cell Metab 13, 505–516. [DOI] [PubMed] [Google Scholar]
- Takauji Y, Wada T, Takeda A, Kudo I, Miki K, Fujii M, Ayusawa D, 2016. Restriction of protein synthesis abolishes senescence features at cellular and organismal levels. Sci. Rep 6, 18722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Pang S, 2016. Proline catabolism modulates innate immunity in Caenorhabditis elegans. Cell Rep 17, 2837–2844. [DOI] [PubMed] [Google Scholar]
- Tawo R, Pokrzywa W, Kevei E, Akyuz ME, Balaji V, Adrian S, Hohfeld J, Hoppe T, 2017. The ubiquitin ligase CHIP integrates proteostasis and aging by regulation of insulin receptor turnover. Cell 169, 470–482 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Dillin A, 2013. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Berendzen KM, Dillin A, 2014. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat. Rev. Mol. Cell Biol 15, 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thondamal M, Witting M, Schmitt-Kopplin P, Aguilaniu H, 2014. Steroid hormone signalling links reproduction to lifespan in dietary-restricted Caenorhabditis elegans. Nat. Commun 5, 4879. [DOI] [PubMed] [Google Scholar]
- Tian Y, Garcia G, Bian Q, Steffen KK, Joe L, Wolff S, Meyer BJ, Dillin A, 2016. Mitochondrial stress induces chromatin reorganization to promote longevity and UPR (mt). Cell 165, 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiku V, Jain C, Raz Y, Nakamura S, Heestand B, Liu W, Spath M, Suchiman HED, Muller RU, Slagboom PE, et al. , 2016. Small nucleoli are a cellular hallmark of longevity. Nat. Commun 8, 16083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalidou I, Miller DL, 2017. Caenorhabditis elegans HIF-1 is broadly required for survival in hydrogen sulfide. G3 7, 3699–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin BD, Gonzalez-Aguilera C, Sack R, Gaidatzis D, Kalck V, Meister P, Askjaer P, Gasser SM, 2012. Step-wise methylation of histone H3K9 positions heterochromatin at the nuclear periphery. Cell 150, 934–947. [DOI] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK, 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132, 1025–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullet JMA, Green JW, Au C, Benedetto A, Thompson MA, Clark E, Gilliat AF, Young A, Schmeisser K, Gems D, 2017. The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell 16, 1191–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Jolly A, Yamamoto KR, 2005a. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 3, e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gilst MR, Hadjivassiliou H, Yamamoto KR, 2005b. A Caenorhabditis elegans nutrient response system partially dependent on nuclear receptor NHR-49. Proc. Natl. Acad. Sci. U. S. A 102, 13496–13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC, 2004. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. U. S. A 101, 11269–11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F, 2003. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620. [DOI] [PubMed] [Google Scholar]
- Ventura N, Rea SL, Schiavi A, Torgovnick A, Testi R, Johnson TE, 2009. p53/CEP-1 increases or decreases lifespan, depending on level of mitochondrial bioenergetic stress. Aging Cell 8, 380–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues AP, Manning G, Dillin A, 2012. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature 489, 263–268. [DOI] [PubMed] [Google Scholar]
- Visscher M, De Henau S, Wildschut MHE, van Es RM, Dhondt I, Michels H, Kemmeren P, Nollen EA, Braeckman BP, Burgering BMT, et al. , 2016. Proteome-wide changes in protein turnover rates in C. elegans models of longevity and age-related disease. Cell Rep 16, 3041–3051. [DOI] [PubMed] [Google Scholar]
- Visvikis O, Ihuegbu N, Labed SA, Luhachack LG, Alves AM, Wollenberg AC, Stuart LM, Stormo GD, Irazoqui JE, 2014. Innate host defense requires TFEB-Mediated transcription of cytoprotective and antimicrobial genes. Immunity [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmer R, van der Ploeg K, Ron D, 2013. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. U. S. A 110, 4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter L, Baruah A, Chang HW, Pace HM, Lee SS, 2011. The homeobox protein CEH-23 mediates prolonged longevity in response to impaired mitochondrial electron transport chain in C. elegans. PLoS Biol 9, e1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DM, Kasturi P, Zheng M, Pinkert S, Vecchi G, Ciryam P, Morimoto RI, Dobson CM, Vendruscolo M, Mann M, et al. , 2015. Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell 161, 919–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan QL, Zheng SQ, Wu GS, Luo HR, 2013. Aspirin extends the lifespan of Caenorhabditis elegans via AMPK and DAF-16/FOXO in dietary restriction pathway. Exp. Gerontol 48, 499–506. [DOI] [PubMed] [Google Scholar]
- Wang MC, O’Rourke EJ, Ruvkun G, 2008. Fat metabolism links germline stem cells and longevity in C. elegans. Science 322, 957–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Min W, Freudiger CW, Ruvkun G, Xie XS, 2011. RNAi screening for fat regulatory genes with SRS microscopy. Nat. Methods 8, 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Niederstrasser H, Douglas PM, Lin R, Jaramillo J, Li Y, Olswald NW, Zhou A, McMillan EA, Mendiratta S, et al. , 2017. Small-molecule TFEB pathway agonists that ameliorate metabolic syndrome in mice and extend C. elegans lifespan. Nat. Commun 8, 2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Chaturbedi A, Wang M, An S, Velayudhan S. Santhi, Lee SS, 2018. SET-9 and SET-26 are H3K4me3 readers and play critical roles in germline development and longevity. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Kenyon C, 2016. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A 113, E2832–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HJ, Yao P, Huynh FK, Escoubas CC, Goncalves RL, Burkewitz K, Laboy R, Hirschey MD, Mair WB, 2017. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab 26, 884–896 e885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S, Ma H, Burch D, Maciel GA, Hunter T, Dillin A, 2006. SMK-1, an essential regulator of DAF-16-mediated longevity. Cell 124, 1039–1053. [DOI] [PubMed] [Google Scholar]
- Wu CW, Deonarine A, Przybysz A, Strange K, Choe KP, 2016a. The Skp1 homologs SKR-1/2 are required for the Caenorhabditis elegans SKN-1 Antioxidant/Detoxification response independently of p38 MAPK. PLoS Genet 12, e1006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, Mou F, Kacergis MC, Talkowski ME, Carr CE, et al. , 2016b. An ancient, unified mechanism for metformin growth inhibition in C. elegans and Cancer. Cell 167, 1705–1718 e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong LG, Chen YJ, Tong JW, Gong YS, Huang JA, Liu ZH, 2018. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol 14, 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki TM, Berman JR, Suchanek-Kavipurapu M, McCormick M, Gaglia MM, Lee SJ, Kenyon C, 2010. The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Hekimi S, 2010. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol 8, e1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunger E, Safra M, Levi-Ferber M, Haviv-Chesner A, Henis-Korenblit S, 2017. Innate immunity mediated longevity and longevity induced by germ cell removal converge on the C-type lectin domain protein IRG-7. PLoS Genet 13, e1006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M, 2012. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab 15, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA, 2009. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLoS ONE 4, e6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Huang H, Liu D, Cheng Y, Liu X, Zhang W, Yin R, Zhang D, Zhang P, Liu J, et al. , 2015. N6-methyladenine DNA modification in Drosophila. Cell 161, 893–906. [DOI] [PubMed] [Google Scholar]
- Zhang WB, Sinha DB, Pittman WE, Hvatum E, Stroustrup N, Pincus Z, 2016. Extended twilight among isogenic C. elegans causes a disproportionate scaling between lifespan and health. Cell Syst 3, 333–345 e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Gao C, Wang M, Tran P, Mai N, Finley JW, Heymsfield SB, Greenway FL, Li Z, Heber D, et al. , 2017. Lower doses of fructose extend lifespan in Caenorhabditis elegans. J. Diet. Suppl 14, 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong M, Niu W, Lu ZJ, Sarov M, Murray JI, Janette J, Raha D, Sheaffer KL, Lam HY, Preston E, et al. , 2010. Genome-wide identification of binding sites defines distinct functions for Caenorhabditis elegans PHA-4/FOXA in development and environmental response. PLoS Genet 6, e1000848. [DOI] [PMC free article] [PubMed] [Google Scholar]


