Abstract
Background:
Atherosclerosis is a major risk factor for morbidity and mortality. However, there is sparse epidemiologic data regarding risk factors for superior mesenteric artery calcification (SMAC), the association between SMAC and disease in other arterial beds, or the independent contribution of SMAC to risk of mortality.
Objectives:
To test the hypothesis that presence and extent of SMAC are associated with cardiovascular disease (CVD) risk factors, calcification in other arterial beds, and both cardiovascular and all-cause mortality, independent of classic risk factors and calcification in other arterial beds.
Methods:
Arterial calcification in the SMA, celiac trunk, coronaries, thoracic aorta, abdominal aorta and iliac arteries was evaluated by computed tomography in adults with no known CVD. Multiple logistic regression models examined risk factor associations for SMAC, and SMAC as a risk factor for calcification in other arterial beds. Cox models were used to examine the association between SMAC and mortality.
Results:
The average age of subjects was 56 years; 43.7% (1877 of 4300) were women, and 6.7% (290) had SMAC. Age (OR: 1.09, 95%CI: 1.06–1.11), male sex (1.79, 1.08–3.03), dyslipidemia (1.38, 1.01–1.88) and any smoking (1.60, 1.20–2.14) were associated with SMAC presence. Notably, neither body mass index, body fat percentage, hypertension, diabetes nor family history of coronary heart disease were significant risk factors for the presence of SMAC.SMAC presence was associated with calcification in all five other arterial beds (6.02, 3.76–9.66). Over a median follow-up time of 9.4 years, there were 234 deaths, 76 of which were CVD-related. SMAC extent, represented as per-unit increase in log[SMAC score+1] (1.31, 1.01–1.71), was significantly associated with CVD mortality after full adjustment for risk factors and calcification in other arterial beds. SMAC presence (1.52, 1.10–2.12) and extent (1.25, 1.06–1.48) were also both significantly associated with all-cause mortality after full adjustment.
Conclusions:
SMAC is associated with specific CVD risk factors as well as calcification in all other arterial beds. SMAC extent was significantly associated with incident cardiovascular mortality, while both SMAC presence and extent were significantly associated with all-cause mortality, even after adjustment for risk factors and calcification in other arterial beds. Further studies are needed to determine if SMAC is simply a marker for advanced and systemic disease, or if it confers increased mortality risk through an independent mechanism.
Introduction
Subclinical atherosclerosis can manifest as calcification in several arterial beds, even in individuals who are not traditionally defined as high-risk1. While calcification in vessels such as the coronary and renal arteries have been shown to have unique risk factors and additional contribution to mortality2–3, little of such data is available for the superior mesenteric artery (SMA).
The SMA arises from the abdominal aorta just distal to the celiac trunk and supplies the small intestine, part of the large intestine, and the pancreas. Presumably, SMA calcification represents progression of the same disease process that leads to atherosclerosis and calcified disease in other systemic arterial beds. Indeed, hyperlipidemia, diabetes, and smoking, all of which are well-established risk factors for atherosclerosis, are also risk factors for chronic mesenteric ischemia4. In this regard, specific radiographic qualitative and quantitative studies would further strengthen these associations or perhaps reveal additional prognostic value of the finding of superior mesenteric artery calcification (SMAC) for incident cardiovascular events and mortality.
This study tested the hypothesis that SMAC presence and extent are positively associated with cardiovascular disease (CVD) risk factors, calcified atherosclerosis in other arterial beds, and both cardiovascular and all-cause mortality, even after adjusting for risk factors and presence of systemic atherosclerotic disease.
Methods
Subjects
From March 1, 2000 to July 3, 2003, 5,156 healthy individuals without known CVD presented for preventive medicine services at a university-affiliated disease prevention center in San Diego, California. Of these, 4,300 had complete survey and vascular calcification data and were included in our analysis. Subjects were self- or physician-referred to obtain preventive health information and testing. Subjects completed a health history questionnaire that included information on history of hypertension, diabetes, high cholesterol, smoking (ever or never smoker), medications, and family history of coronary heart disease (CHD). The protocol for this study was approved by the Human Research Protection Program at the University of California, San Diego and all subjects gave informed consent.
Laboratory measurements
Total, high-density, and low-density cholesterol and glucose measurements were obtained by fingerstick using the Cholestech LDX system (Cholestech, Hayward, Calif). Individuals with a total/HDL cholesterol ratio greater than 5, or reported using a medication to treat high cholesterol, were classified as dyslipidemic. Diabetes was defined by use of prescribed diabetes medications at the time or a random glucose level greater than 200 mg/dL. While seated, blood pressure was measured in the right arm by automated oscillometry after 5 minutes at rest. Hypertension was defined as a systolic pressure or diastolic pressure greater than 140 or 90 mm Hg, respectively, or a self-reported history of physician-diagnosed hypertension and current use of an antihypertensive medication.
Imaging
Non-contrast computed tomography imaging was conducted using an Imatron C-150 scanner (General Electric, San Francisco, California). Images for each arterial bed were obtained from the base of the skull to the pubic symphysis. Each bed was obtained by a distinct scan of the segment in question by using slice thicknesses of 3 mm for the coronary bed, 5 mm for the thorax, and 6 mm through the neck, abdomen, and pelvis. Imaging of the heart, thorax, and abdomen was conducted during separate breath holds at 50% maximal inspiration. These images were subsequently analyzed for the presence and extent of vascular calcification in the coronary arteries, thoracic aorta, abdominal aorta, superior mesenteric artery, celiac trunk, and iliac arteries. Quantitative calcium scores for each arterial bed were determined according to the method described by Agatston et al5. Data on a subgroup of these individuals have been previously reported3.
The superior mesenteric artery was identified at its anterior takeoff from the aorta, and was tracked caudally until no longer discernible. In cases where calcification extended from the aorta into the ostia of the SMA, a vertical plane simulating the wall of the aorta traversing the ostium was drawn to approximate the lumen of the aorta. Any calcification medial to the plane was excluded from the calculation as SMAC.
Mortality data
All-cause mortality was determined up to December 31st, 2010 using Social Security Death Index searches and the National Death Index. Date and cause of death were extracted from death certificates and/or by the National Death Index search. The cause of mortality was ascertained by physician adjudicators (MA, MC).
Statistical analysis
Baseline characteristics stratified by SMAC absence vs. presence were examined using Chi-squared analysis or Welch’s unequal variances T-test as appropriate. Proportions of calcification in other arterial beds were compared between those with and without SMAC using chi-squared analysis. Age- and sex-adjusted Spearman rank correlation was used to determine partial coefficient correlations of the extent of calcification (represented as log10[score+1]) between the SMA and the non-SMA arterial beds included in the analysis (the thoracic aorta, abdominal aorta, coronary arteries, iliac arteries and celiac trunk).
As the prevalence of SMAC did not exceed 10% in our study sample, a multiple logistic regression model was used to determine risk factor associations for SMAC presence. The risk factors included were age, sex, body mass index (BMI), body fat percentage (BF%), dyslipidemia, any smoking, hypertension, diabetes, and a family history of CHD. The model was then further adjusted for calcification in five other arterial beds (abdominal aorta, thoracic aorta, coronary arteries, iliac arteries and celiac trunk). A similar analysis was performed using multiple linear regression, with SMAC extent (represented as log10[SMAC score + 1]) as the outcome variable. In a separate logistic regression model, SMAC presence was used as a predictor for systemic calcified atherosclerosis, defined as having calcification in all five of the aforementioned non-SMA arterial beds. The model was first adjusted for age and sex, and then for all risk factors. A similar analysis using SMAC extent rather than presence as a predictor was performed.
Survival analysis was performed using Kaplan-Meier curves and Cox regression models. SMAC presence or SMAC extent were used in separate models as the exposure variable. Each model was adjusted for age and sex, then CVD risk factors, then calcification in other arterial beds (to account for collinearity, this was represented as the number of other arterial beds with calcium score > 0, ranging from 0 to 5).
The level of significance used in this study was p < 0.05. All statistical analysis was performed using IBM SPSS Statistics Version 22 (IBM corp., Armonk, New York).
Results
Baseline characteristics
The average age was 56 years, 43.7% (1877 of 4300) were women, and 6.7% (290 of 4300) had SMAC. Among those with SMAC, the median Agatston score was 51.3 (interquartile range 22.0 to 112.3). Those with SMAC were likely to be older (69.7 vs. 55.1 years, P<.001) and male (73.1% vs. 55.1%, P<.001). They also tended to have hypertension (38.6% vs. 21.3%, P<.001), diabetes (10.0% vs. 3.1%), a history of smoking (65.2% vs. 42.5%), and a higher body fat percentage (31.4% vs 29.7%, P<.001) (Table I). Those with SMAC had, on average, 3.9 other arterial beds with calcification present, compared to 1.9 other beds in those without SMAC. Moreover, 21.4% of those with SMAC had calcification in all five other beds, compared to 1.1% of those without SMAC (Table I). Only two subjects had SMAC without calcification in any other bed. Among the entire sample, calcification in the coronaries was most common (55.2%), followed by the abdominal aorta (54.6%) then the iliacs (49.4%) (Table II). Those with SMAC had a higher prevalence of calcification in each arterial bed compared to those without SMAC. The partial correlation coefficients between log-transformed calcium scores in different arterial beds are shown in Table III. The extent of SMA calcification correlated most with that of the celiac trunk (correlation coefficient = 0.306, P<.001) and thoracic aorta (0.260, P<.001), and least with that of the coronary arteries (0.170, P<.001).
Table I.
Unadjusted Baseline Characteristics According to Absence or Presence of SMAC
| Characteristic | SMAC present (n = 290) | SMAC absent (n = 4010) | p-value |
|---|---|---|---|
| Agea | 69.7 +/− 8.6 | 55.1 +/− 10.7 | <.001 |
| Female sex (Y/N)b | 78 (26.9) | 1799 (44.9) | <.001 |
| Body Mass Indexa | 26.9 +/− 3.8 | 27.0 +/− 4.5 | .678 |
| Body Fat %a | 31.4 +/− 6.9 | 29.7 +/− 7.6 | <.001 |
| Dyslipidemia (Y/N)b | 95 (32.8) | 1108 (27.6) | .060 |
| Ever smoked (Y/N)b | 189 (65.2) | 1704 (42.5) | <.001 |
| Hypertension (Y/N)b | 112 (38.6) | 853 (21.3) | <.001 |
| Diabetes (Y/N)b | 29 (10.0) | 126 (3.1) | <.001 |
| Family History of CHD (Y/N)b | 139 (47.9) | 2021 (50.4) | .422 |
| Deceased (any cause, Y/N)b | 67 (23.1) | 167 (4.2) | <.001 |
| Deceased (cardiovascular cause, Y/N)b | 30 (10.3) | 46 (1.1) | <.001 |
| All other beds have calcification (Y/N)b | 62 (21.4) | 43 (1.1) | <.001 |
| Number of other beds with calcificationa | 3.9 +/− 0.8 | 1.9 +/− 1.5 | <.001 |
Welch’s Unequal Variances T-Test, values are mean +/− SD.
Pearson Chi-Squared Test, values are n (%).
Table II.
Prevalence of Calcification In Other Arterial Beds According to Absence or Presence of SMAC
| Total n=4300 n (%) |
SMAC present n = 290 n (%) |
SMAC absent n (%) |
p-valuea | |
|---|---|---|---|---|
| Thoracic aorta | 1632 (38.0) | 267 (92.1) | 1365 (34.0) | <.001 |
| Abdominal aorta | 2349 (54.6) | 285 (98.3) | 2064 (51.5) | <.001 |
| Coronary arteries | 2372 (55.2) | 260 (89.7) | 2112 (52.7) | <.001 |
| Iliac arteries | 2125 (49.4) | 276 (95.2) | 1849 (46.1) | <.001 |
| Celiac trunk | 120 (2.8) | 67 (23.1) | 53 (1.3) | <.001 |
Pearson Chi-Squared, values are n (%).
Table III.
Age- and Sex- Adjusted Spearman Rank Correlation Between Log-Transformeda Calcium Scores In Arterial Beds
| Arterial bed | Superior mesenteric artery | Thoracic Aorta | Abdominal Aorta | Coronary Arteries | Iliac Arteries | Celiac Trunk |
|---|---|---|---|---|---|---|
| Superior mesenteric artery | 1.000 | .260 | .180 | .170 | .204 | .306 |
| Thoracic Aorta | .260 | 1.000 | .432 | .283 | .411 | .173 |
| Abdominal Aorta | .180 | .432 | 1.000 | .363 | .613 | .107 |
| Coronary Arteries | .170 | .283 | .363 | 1.000 | .348 | .121 |
| Iliac Arteries | .204 | .411 | .613 | .348 | 1.000 | .124 |
| Celiac Trunk | .306 | .173 | .107 | .121 | .124 | 1.000 |
Values are partial correlation coefficients; p-value <.001 for all values.
Represented as log10(score+1)
Risk Factors
In a fully adjusted multiple logistic regression model (Table IV), age per 1 year increment (OR 1.09; 95%CI: 1.06–1.11), dyslipidemia (1.38; 1.01–1.88) and any smoking (1.60; 1.20–2.14) were significantly associated with the presence of SMAC, while female sex was inversely associated (0.56; 0.33–0.93). BMI, BF%, HTN, diabetes and family history of CHD were not significantly associated with SMAC presence. Using a multiple linear regression model, the risk factors significantly associated with an increase in SMAC extent, expressed as % change (using the relation % change = 100*[ebeta-coefficient−1]), were age (% change 0.80%, 95%CI 0.60%−0.90%), male sex (4.97%, 0.30%−9.43%), any smoking (4.08%, 1.41%−6.72%), and diabetes (7.14%, 0.20%−14.57%) (Table V); BMI, BF%, dyslipidemia, HTN nor family history were significantly associated with SMAC extent.
Table IV.
Logistic regression model of risk factors for the presence of superior mesenteric artery calcification
| Sex- and age-adjusted | Risk adjusteda | Fully adjustedb | ||||
|---|---|---|---|---|---|---|
| Variable | OR | 95%CI | OR | 95%CI | OR | 95%CI |
| Age | 1.15 | 1.13 to 1.17 | 1.15 | 1.13 to 1.17 | 1.09 | 1.06 to 1.11 |
| Female Sex | 0.35 | 0.26 to 0.47 | 0.42 | 0.26 to 0.69 | 0.56 | 0.33 to 0.93 |
| Body mass index | - | - | 0.99 | 0.94 to 1.05 | 0.99 | 0.94 to 1.05 |
| Body fat percentage | - | - | 0.99 | 0.96 to 1.03 | 0.99 | 0.95 to 1.03 |
| Dyslipidemia | - | - | 1.40 | 1.04 to 1.88 | 1.37 | 1.01 to 1.88 |
| Any Smoking | - | - | 2.09 | 1.58 to 2.76 | 1.60 | 1.20 to 2.14 |
| Hypertension | - | - | 1.40 | 1.05 to 1.87 | 1.21 | 0.90 to 1.62 |
| Diabetes | - | - | 1.65 | 1.01 to 2.69 | 1.16 | 0.70 to 1.93 |
| Family History of CHD | - | - | 1.08 | 0.83 to 1.42 | 0.95 | 0.72 to 1.26 |
| Abdominal Aorta calcification | - | - | - | - | 4.27 | 1.68 to 10.86 |
| Thoracic Aorta Calcification | - | - | - | - | 3.52 | 2.19 to 5.67 |
| Coronary artery calcification | - | - | - | - | 1.74 | 1.14 to 2.67 |
| Iliac artery calcification | - | - | - | - | 2.77 | 1.54 to 5.00 |
| Celiac trunk calcification | - | - | - | - | 4.29 | 2.79 to 6.60 |
Additionally adjusted for body mass index, body fat percentage, dyslipidemia, any smoking, hypertension, diabetes, and family history of chronary heart disease.
Additionally adjusted for presence of calcification in the coronary arteries, thoracic aorta, abdominal aorta, iliac arteries, and celiac trunk.
Table V.
Linear regression models of risk factors for the extent of superior mesenteric artery calcification
| Sex- and age-adjusted | Risk adjusteda | Fully adjustedb | ||||
|---|---|---|---|---|---|---|
| Variable | % change in SMAC score+1c | 95%CI | % change in SMAC score+1 | 95%CI | % change in SMAC score+1 | 95%CI |
| Age | 1.41% | 1.21% to 1.51% | 1.31% | 1.11% to 1.41% | 0.80% | 0.60% to 0.90% |
| Female Sex | −8.61% | −11.00% to − 6.20% | −9.06% | −13.41% to − 4.40% | −4.97% | −9.43% to −0.30% |
| Body mass index | - | - | −0.50% | −1.00% to 0.00% | −0.30% | −0.80% to − 0.20% |
| Body fat percentage | - | - | 0.10% | −0.30% to 0.50% | −0.10% | −0.40% to 0.30% |
| Dyslipidemia | - | - | 2.12% | −0.90% to 5.23% | 2.43% | −0.50% to 5.44% |
| Any Smoking | - | - | 5.55% | 2.84% to 8.33% | 4.08% | 1.41% to 6.72% |
| Hypertension | - | - | 4.08% | 0.80% to 7.47% | 2.94% | −0.20% to 6.08% |
| Diabetes | - | - | 13.88% | 6.08% to 22.14% | 7.14% | 0.20% to 14.57% |
| Family History of CHD | - | - | 1.11% | −1.49% to 3.77% | −0.20% | −2.57% to 2.33% |
| Abdominal Aorta calcification | - | - | - | - | −2.57% | −5.82% to 0.90% |
| Thoracic Aorta Calcification | - | - | - | - | 10.30% | 6.72% to 14.00% |
| Coronary artery calcification | - | - | - | - | 1.41% | −1.39% to 4.39% |
| Iliac artery calcification | - | - | - | - | 3.87% | 0.50% to 7.36% |
| Celiac trunk calcification | - | - | - | - | 117.49% | 101.58% to 134.90% |
Additionally adjusted for body mass index, body fat percentage, dyslipidemia, any smoking, hypertension, diabetes, and family history of coronary heart disease in a single model.
Additionally adjusted for presence of calcification in the coronary arteries, thoracic aorta, abdominal aorta, iliac arteries, and celiac trunk in a single model.
% change in SMAC score + 1 calculated using the following equation: 100*(eB-coefficient −1).
Systemic calcified atherosclerosis
In the study sample, 1058 subjects did not have calcification in any of the five other arterial beds. Of these, only 2 (0.2%) had SMAC. Conversely, 105 subjects had calcification in all five other arterial beds, and, of these, 62 (59.0%) also had SMAC. To further investigate the relationship between the extent of calcified atherosclerosis and the presence of SMAC, “advanced atherosclerosis” was defined as having calcification in all five other arterial beds. Separate logistic regression models adjusted for risk factors showed that SMAC presence conferred a 6.02 (3.76–9.66) higher odds for advanced atherosclerosis, while a per unit increase in log[SMAC score+1] was associated with a 2.54 (95%CI 2.009–3.201) higher odds.
In a linear regression model using number of arterial beds with calcification as a continuous dependent variable, and SMAC presence as the independent variable, SMAC presence was associated with 2.13 more calcified beds (95%CI: 1.95–2.30). When adjusted for all risk factors, effect size was attenuated to 0.65 more calcified beds (0.51–0.79).
Mortality
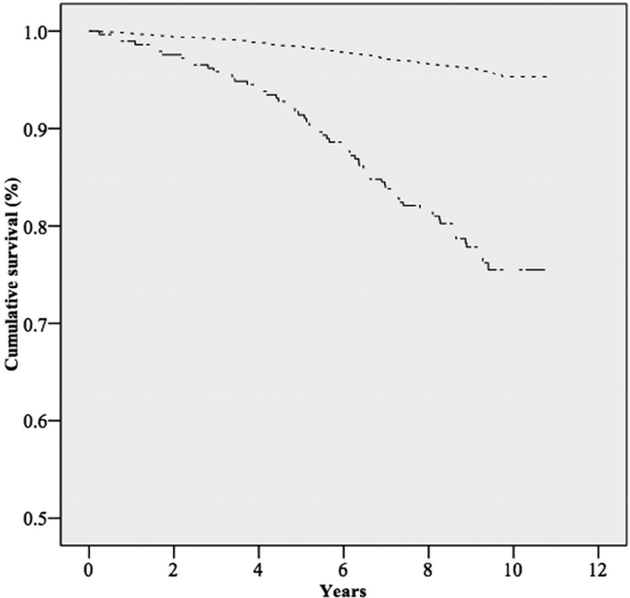
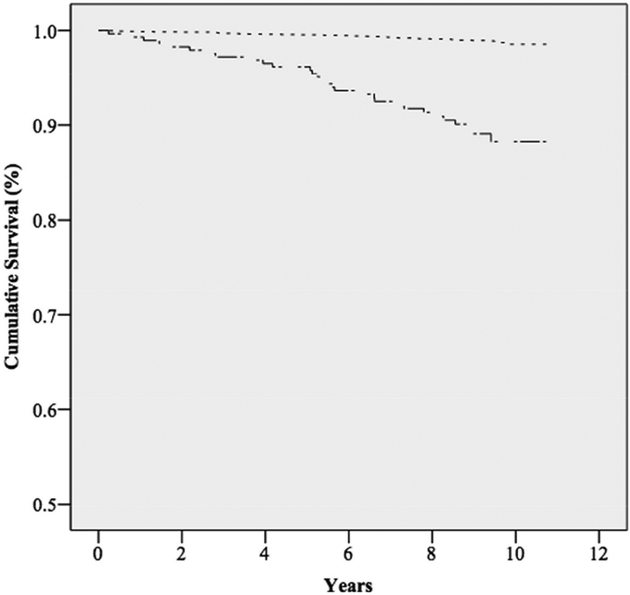
Over a median follow-up time of 9.4 years, there were a total of 234 deaths, of which, 76 were determined to be of a cardiovascular cause (Table I). Kaplan-Meier curves for overall survival (Figure 1) and cardiovascular survival (Figure 2) demonstrated significant differences (p-value <.001). Mean overall survival for those without SMAC was 10.6 years (95%CI: 10.53–10.61), compared to 9.6 years (9.26–9.84) for those with SMAC. Similarly, the mean survival from cardiovascular mortality for those without SMAC was 10.7 years (10.71–10.75), compared to 10.2 years (9.94–10.39) for those with SMAC.
Figure I. Kaplan-Meier curve for overall survival among those with or without superior mesenteric artery calcification.
There is a significant difference in cumulative overall survival between those with and without SMAC (log rank test P<.001). Note that the Y-axis begins at 0.5.
 Without SMAC
Without SMAC
 With SMAC
With SMAC
Figure II. Kaplan-Meier curve for cardiovascular survival among those with or without superior mesenteric artery calcification.
There is a significant difference in cumulative cardiovascular survival between those with and without SMAC (log rank test P<.001). Note that the Y-axis begins at 0.5.
 Without SMAC
Without SMAC
 With SMAC
With SMAC
In Cox regression models fully adjusted for age, sex, risk factors and calcification in other arterial beds (Table VI), SMAC presence (HR: 1.52, 95%CI 1.10–2.12) and each log-increase in SMAC+1 (1.25, 1.06–1.48) were both significantly associated with an increased risk of total mortality. For cardiovascular mortality, SMAC presence was associated with a hazard ratio of 1.61 (0.94–2.74) after full adjustment; each log-increase in SMAC+1 was associated with a hazard ratio of 1.31 (1.01–1.71) after full adjustment.
Table VI.
Hazard of all-cause or cardiovascular mortality given superior mesenteric artery calcification presence or extent.
| Age- and sex-adjusted HR (95%CI) | Risk adjusteda HR (95%CI) | Fully adjustedb HR (95%CI) | |
|---|---|---|---|
| All-cause mortality (234 deaths) | |||
| SMAC presence (yes/no) | 1.82 (1.32 to 2.49) | 1.67 (1.21 to 2.31) | 1.52 (1.10 to 2.12) |
| SMAC extent (log[SMAC score+1]) | 1.35 (1.15 to 1.58) | 1.30 (1.11 to 1.53) | 1.25 (1.06 to 1.48) |
| Cardiovascular mortality (76 deaths) | |||
| SMAC presence | 2.15 (1.29 to 3.61) | 1.86 (1.10 to 3.15) | 1.61 (0.94 to 2.74) |
| SMAC extent | 1.47 (1.15 to 1.89) | 1.40 (1.08 to 1.80) | 1.31 (1.01 to 1.71) |
SMAC = superior mesenteric artery calcification
Additionally adjusted for body mass index, body fat percentage, dyslipidemia, any smoking, hypertension, diabetes, and family history of coronary heart disease in a single model.
Additionally adjusted for presence of calcification in the coronary arteries, thoracic aorta, abdominal aorta, iliac arteries, and celiac trunk in a single model.
Discussion
In this study of a relatively large community-based sample, we tested the hypotheses that superior mesenteric artery calcification (SMAC) is associated with cardiovascular disease (CVD) risk factors, atherosclerosis in other arterial beds, and an increase in both cardiovascular and all-cause mortality. The findings indicate that those with SMAC have significantly higher levels of calcified atherosclerosis in other arterial beds and a significantly higher risk for incident CVD and total mortality compared to those without SMAC.
Of the risk factors included in this analysis, age and history of any smoking were associated with both the presence and extent of SMAC. Dyslipidemia was associated with presence of SMAC, but not extent. On the other hand, while diabetes conferred the highest increase in risk for SMAC extent, the adjusted association with SMAC presence was not significant. The other classic risk factors included in the analysis, notably hypertension, were not significantly associated with either SMAC presence nor extent. This is contrary to prior findings where hypertension was significantly associated with calcification in the other mesenteric arteries (i.e. abdominal aorta and renal arteries)3,6. That SMAC is associated with a unique set of risk factors compared to calcification in coronary arteries7 and nearby arterial beds may be suggestive of a separate pathophysiologic process, and may further inform the risk of other forms of vascular disease.
Our findings suggest the SMAC presence is associated with increased risk of calcification of any of the other arterial beds. Both SMAC presence and extent also confer a significantly higher risk of calcified atherosclerosis in all five of these beds. More specifically, the overall prevalence of SMAC in our study sample was 6.7%, and only 2 subjects had SMAC without also having any calcification in any other bed, while more than half of the subjects with calcification in all five other beds had SMAC. Taken together, these findings suggest that SMAC occurs late in subclinical atherosclerotic disease. It would be interesting to see if SMAC is of prognostic value, or can predict the onset of symptomatic disease, such as chronic mesenteric ischemia or acute coronary syndromes. It has been shown, though, that significant disease of mesenteric vessels, as determined by sonography, does not necessarily indicate symptomatic mesenteric ischemia.8
Similar to earlier findings for renal artery calcium in this cohort9, SMAC was associated with increased total and CVD mortality. It is possible that SMAC is a marker for advanced and systemic atherosclerotic disease, and our findings affirm that presence and extent of disease in the SMA correlates with that in other arterial beds. However, given that SMAC remained significant after adjusting for risk factors and calcification in other arterial beds, alternative explanations should be entertained. For example, that there is residual confounding for the association of SMAC with mortality, or that SMAC may be a proxy for pathophysiologic processes separate from those leading to atherosclerotic disease, as was posited in our previous discussion regarding the unique risk factors for SMAC. Further studies would be needed to examine these associations in greater detail.
Since we present the results of an observation study and there is sparse data on the epidemiology of SMAC, we recommend neither elective whole-body screening CT scans nor specific imaging to assess for SMAC at this time. However, the incidental finding of SMAC on a non-contrast CT scan obtained for another indication could be used to inform risk factor management.
Strengths of this study include a large sample size and availability of imaging and data pertaining to other arterial beds. Also, the mortality outcomes were collected systematically and adjudicated by a physician panel. Limitations to our study include the large proportion of self-referred individuals, likely of higher socioeconomic status on average, which may limit the generalizability of our results. Additional history such as abdominal or gastrointestinal complaints were not collected. For our analysis, the definition of hypertension, dyslipidemia and diabetes included ongoing pharmacologic treatments (to include statins, beta-blockers and ACE inhibitors). However, specific medications or dosages were not collected for each participant.
Conclusion
SMAC presence is associated with a unique set of traditional risk factors for cardiovascular disease, raising the possibility of differing pathophysiologic processes contributing to the development and/or progression of SMAC. The presence of any SMAC was associated with greatly increased odds for calcification in all other arterial beds and suggests that calcification of the superior mesenteric artery occurs with subclinical, yet widely-manifest, systemic atherosclerotic disease. Finally, SMAC was significantly associated with increased total and cardiovascular mortality even after adjustment for risk factors and calcification in other arterial beds. Further research may help to clarify whether SMAC itself may be an independent risk factor for increased mortality, or if SMAC serves as a proxy for other risk factors that independently contribute to mortality.
Abbreviations List
- 95%CI
95% Confidence Interval
- BMI
Body mass index
- BF%
Body fat percentage
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- HR
Hazard ratio
- OR
Odds ratio
- SMA
Superior mesenteric artery
- SMAC
Superior mesenteric artery calcification
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fernández-friera L, Peñalvo JL, Fernández-ortiz A, Ibañez B, López-Melgar B, Laclaustra M et al. Prevalence, Vascular Distribution, and Multiterritorial Extent of Subclinical Atherosclerosis in a Middle-Aged Cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation. 2015;131(24):2104–13. [DOI] [PubMed] [Google Scholar]
- 2.Odink AE, van der Lugt A, Hofman A, Hunink MG, Breteler MM, Krestin GP, et al. Risk factors for coronary, aortic arch and carotid calcification; The Rotterdam Study. J Hum Hypertens 2010;24:86–92. [DOI] [PubMed] [Google Scholar]
- 3.Allison MA, Lillie EO, DiTomasso D, Wright CM, Criqui MH. Renal artery calcium is independently associated with hypertension. J Am Coll Cardiol 2007;50:1578–83. [DOI] [PubMed] [Google Scholar]
- 4.Sreenarasimhaiah J Chronic mesenteric ischemia. Best Pract Res Clin Gastroenterol. 2005;19(2):283–95. [DOI] [PubMed] [Google Scholar]
- 5.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R, et al. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–32. [DOI] [PubMed] [Google Scholar]
- 6.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24(2):331–6. [DOI] [PubMed] [Google Scholar]
- 7.Kronmal RA, Mcclelland RL, Detrano R, Shea S, Lima JA, Cushman M et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115(21):2722–30. [DOI] [PubMed] [Google Scholar]
- 8.Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: predictive value of Doppler sonography. AJR Am J Roentgenol. 1993;161(5):985–8. [DOI] [PubMed] [Google Scholar]
- 9.Rifkin DE, Ix JH, Wassel CL, Criqui MH, Allison MA. Renal artery calcification and mortality among clinically asymptomatic adults. J Am Coll Cardiol. 2012;60(12):1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]