Abstract
BACKGROUND
Postoperative pulmonary complications (PPCs) are common after major abdominal surgery. The kinetics of plasma biomarkers could improve identification of patients developing PPCs, but the kinetics may depend on intraoperative ventilator settings.
OBJECTIVE
To test whether the kinetics of plasma biomarkers are capable of identifying patients who will develop PPCs, and whether the kinetics depend on the intraoperative level of positive end-expiratory pressure (PEEP).
DESIGN
A preplanned substudy of a randomised controlled trial.
SETTING
Operation room of five centres.
PATIENTS
Two hundred and forty-two adult patients scheduled for abdominal surgery at risk of developing PPCs.
INTERVENTIONS
High (12 cmH2O) versus low (≤2 cmH2O) levels of PEEP.
MAIN OUTCOME MEASURES
Individual PPCs were combined as a composite endpoint. Plasma samples were collected before surgery, directly after surgery and on the fifth postoperative day. The levels of the following were measured: tumour necrosis factor (TNF)-α, interleukin (IL)-6 and IL-8, the soluble form of the Receptor for Advanced Glycation End-products (sRAGE), Surfactant Protein (SP)-D, Clara Cell protein (CC)-16 and Krebs von den Lungen 6 (KL6).
RESULTS
Blood sampling was complete in 242 patients: 120 patients in the high PEEP group and 122 patients in the low PEEP group. Increases in plasma levels of TNF- IL-6, IL-8 and CC-16, and a decrease in plasma levels of SP-D were greater in patients who developed PPCs; however, the area under the receiver operating characteristic curve was low for all biomarkers. CC-16 was the only biomarker whose level increased more in patients who had received high levels of PEEP.
CONCLUSION
In patients undergoing abdominal surgery and at risk of developing PPCs, plasma levels of biomarkers for inflammation or lung injury showed distinct kinetics with development of PPCs, but none of the biomarkers showed sufficient prognostic value. The use of high levels of PEEP was associated with increased levels of CC-16, suggesting lung overdistension.
TRIAL REGISTRATION
The PROVHILO trial, including this substudy, was registered at clinicaltrials.gov ().
Introduction
Postoperative complications, in particular those involving the lungs, are common after major abdominal surgery.1 Postoperative pulmonary complications (PPCs) are associated with increased morbidity and mortality, as well as increased healthcare-related costs.1,2 Early recognition of patients likely to develop PPCs would allow measures to be taken in the perioperative phase to minimise the occurrence of such PPCs. Currently, the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) risk score seems to reliably predict the occurrence of PPCs.2,3
The plasma kinetics of biomarkers of inflammation and lung injury could help to identify those patients who will develop one or more PPCs. Potentially useful biomarkers include tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, the soluble form of the Receptor for Advanced Glycation End-products (sRAGE), Surfactant Protein (SP)-D, Clara Cell protein (CC)-16 and Krebs von den Lungen 6 (KL6).4–8 Perioperative kinetics ofthese biomarkers could also depend on intraoperative ventilator settings, including the level of positive end-expiratory pressure (PEEP).4 Table 1 summarises an overview of the main findings from previous trials suggesting a potential role in prediction of PPC for these biomarkers.9–14,25
Table 1.
Selection of biomarkers, rationale and relevance in the field of mechanical ventilation
| Biomarkers | Relevance | References |
|---|---|---|
| TNF-α | Higher plasma levels in patients developing pulmonary complications after oesophagus resection | 18-22 |
| Higher plasma levels in patients developing pulmonary complications after liver transplantation | ||
| Higher plasma levels in patients developing pulmonary complications after lung resection | ||
| IL-6 | Higher plasma levels in patients developing pulmonary complications after oesophagus resection | 18-20 |
| Higher plasma levels in patients developing pulmonary complications after liver transplantation | ||
| IL-8 | Plasma levels were lower with the use of protective ventilation in patients with uninjured lungs | 12,20 |
| sRAGE | Plasma levels decreased with the use of protective ventilation in patients without lung injury | 4,6,25 |
| Higher plasma levels were associated with an increased severity of lung injury | ||
| Plasma levels increased after mechanical ventilation during general anaesthesia for surgery | ||
| SP-D | Higher plasma levels correlated with clinical lung injury after coronary artery bypass surgery | 10 |
| CC-16 | Plasma levels correlated with compliance and were suggested to reflect lung overdistension | 9-11,14,25 |
| Plasma levels increased after mechanical ventilation during general anaesthesia for surgery | ||
| Plasma levels correlated with clinical lung injury after coronary artery bypass surgery | ||
| Higher plasma levels in patients with ARDS than patients with uninjured lungs | ||
| Plasma levels decreased with use of protective ventilation in patients with ARDS | ||
| Higher plasma levels were associated with worse outcome in patients with ARDS | ||
| KL6 | Higher plasma levels in patients with ARDS than patients with uninjured lungs | 9,13 |
| Plasma levels decreased with the use of protective ventilation in patients with ARDS. | ||
| Higher plasma levels were associated with worse outcome in patients with ARDS. | ||
ARDS, acute respiratory distress syndrome; CC-16, Clara cell protein; IL, interleukin; KL-6, Krebs von den Lungen-6; SP-D, surfactant protein D; sRAGE, soluble receptor for advanced glycation end-products; TNF, tumour necrosis factor.
The ‘PROtective Ventilation using HIgh versus LOw PEEP (PROVHILO)’ trial was a randomised controlled study of intraoperative ventilation, comparing high levels with low levels of PEEP, in patients under general anaesthesia for scheduled open abdominal surgery at intermediate or high risk of PPCs according to the ARISCAT score.15 There was a high incidence of PPCs up to postoperative day 5 in the PROVHILO trial but with no differences between the randomisation groups.16 In a preplanned substudy of this trial, blood samples were collected before surgery, directly after surgery and on postoperative day 5 to test whether the kinetics of the biomarkers predicted the development of PPCs. Second, we assessed whether the plasma levels of these biomarkers were correlated with the level of PEEP used during intraoperative ventilation. We hypothesised that the kinetics of biomarkers of inflammation and lung injury differ between patients who do and do not develop PPCs, and that they discriminate between those patients who develop one or more PPCs from patients who do not. We further hypothesised that the kinetics of the bio-markers are dependent on the intraoperative level of PEEP.
Materials and methods
Ethics
This was a preplanned substudy, whose aims, hypotheses and design were registered prior to start of the main study, the PROVHILO trial.15,16 Written informed consent was obtained from all participants before randomisation. The Institutional Review Board of all participating centres in this substudy approved the study protocol. The PROVHILO trial, including this substudy, was registered at clinicaltrials.gov(NCT01441791). The information on ethical approval at each centre is as follows.
-
(1)
Ethical approval for this study (Ethical Committee No. MEC 10/251) was provided by the Ethical Committee of the Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands on 31 January 2011.
-
(2)
Ethical approval for this study (Ethical Committee No. EK 130032011) was provided by the Ethical Committee of the Technische Universitat Dresden, Dresden, Germany on 20 April 2011.
-
(3)
Ethical approval for this study [Ethical Committee No. 837.284.11 (7826)] was provided by the Ethical Committee of der Johanned Guterberg-Universitat Mainz, Mainz, Germany on 29 June 2011.
-
(4)
Ethical approval for this study (Ethical Committee No. 11/204) was provided by the Ethical Committee of Hospital de la Santa Creu I Sant Pau, Barcalona, Spain on 01 December 2011.
-
(5)
Ethical approval for this study (Ethical Committee No. 12–000234) was provided by the Ethical Committee of Mayo Clinics, Rochester, Minnesota, USA on 03 August 2012.
-
(6)
Ethical approval for this study (Ethical Committee No. 2012-P-000062/1) was provided by the Ethical Committee of Massachusetts General Hospital, Boston, USA on 03 July 2012.
Study sites
The PROVHILO trial included 30 centres,9 but this substudy only ran in the Academic Medical Centre (Amsterdam, Netherlands), the Hospital Santa Creu I Sant Pau (Barcelona, Spain), the University Hospital Carl Gustav Carus (Dresden, Germany), the University Medical Center Mainz (Mainz, Germany), the Massachusetts General Hospital (Boston, Massachusetts, USA) and the Mayo Clinic, Rochester (Rochester, Minnesota, USA).
Study design
Briefly, in the PROVHILO study, patients aged 18 years or older, scheduled for open abdominal surgery under general anaesthesia, and with intermediate or high risk for PPCs according to the ARISCAT score,2 were eligible for participation. Patients were excluded if they had a low ARISCAT score,2 or had undergone a planned laparoscopic surgery, or were pregnant, or had a BMI more than 40 kg m−2, or severe cardiac or pulmonary comorbidities or another disorder that might have compromised well tolerated trial procedure.15 Finally, patients who gave consent for participation in the PROVHILO trial but who declined collection of blood were excluded from participation in this substudy.
Ventilation strategies
Patients were randomly allocated to receive intraoperative ventilation with either high levels of PEEP (12 cmH2O) along with lung recruitment manoeuvres (the ‘high PEEP group’) or low levels of PEEP (≤2cmH2O) only (the ‘low PEEP group’). In both arms of the trial, tidal volume was set at 8mlkg−1 predicted body weight (PBW) and the fraction of inspired oxygen (FIO2) was at least 0.40 to a target SpO2 of at least 92%. Respiratory rate was set to maintain end-tidal partial pressure of carbon dioxide (PETCO2) between 4.67kPammHg and 6.0 kPa, and with an inspiration to expiration ratio of 1 to 2.
Postoperative pulmonary complications
The definition of a PPC included the occurrence of unexpected hypoxaemia mandating supplementary oxygen, severe hypoxaemia, bronchospasm, suspected pulmonary infection, pulmonary infiltrate, aspiration pneumonitis, development of acute respiratory distress syndrome (ARDS), atelectasis, pleural effusion, pulmonary oedema caused by cardiac failure and pneumothorax (for definitions of these PPCs, see eTable 1, http://links.lww.com/EJA/A114). Until postoperative day 5, assessors who remained blinded to the intraoperative level of PEEP scored whether PPCs occurred.
Blood sampling
Blood was sampled via a venous puncture preoperatively, before induction of anaesthesia, directly after surgery and on postoperative day 5. Samples were centrifuged at 1500 to 2000 rpm for 15 min to obtain serum, and the supernatant was stored at –80°C (for more information see PROVHILO Standard of Procedure in the Appendix).
Measurements
Samples were shipped to and analysed batch-wise in the Academic Medical Centre (Amsterdam, Netherlands). Levels of TNF-α, IL-6, IL-8, sRAGE, SP-D, CC-16 and KL6 were measured in batches using customised Human Premixed Multi-Analyte Kit, Magnetic Luminex Screening Assay (R&D Systems, Minneapolis, Minnesota, USA), according to the protocol for each kit provided by the manufacturer.
Analysis plan
The primary aim was to describe the kinetics of the plasma biomarkers and to determine their predictive value for the development of PPCs. For the latter, we restricted the analysis to samples obtained before and directly after surgery, as levels on postoperative day 5 were too late to be of prognostic value for PPCs that had already developed on that day.
The secondary aim was to determine whether the kinetics of biomarkers were dependent on the intraoperative ventilation strategy. For this purpose, all samples were used, including those obtained on postoperative day 5.
Two post-hoc analyses were performed: one in which we repeated the first analyses but restricted the outcome to severe PPCs, that is excluding ‘unexpected need for supplementary oxygen’ from the composite endpoint; and one in which we stratified the patients into four groups according to the level of PEEP and development of PPC (high PEEP and PPC versus high PEEP and no PPC versus low PEEP and PPC versus low PEEP and no PPC).
Statistical analysis
Qualitative data were expressed as numbers or percentages, and quantitative data as means ± standard deviation or medians (interquartile range), and 10 to 90% range where applicable. Baseline characteristics between groups were assessed with Mann-Whitney tests for quantitative parameters and Chi-square test or Fisher’s exact test for categorical variables.
To evaluate differences in plasma levels of biomarkers over time with respect to outcome (i.e. one or more PPCs versus no PPC) and randomisation groups (i.e. high PEEP versus low PEEP), repeated measures analysis of variance using Bonferroni correction with repeated contrast was used. The interaction between outcome and randomisation group was assessed including the two variables as fixed-effect in the model. In all analyses, plasma levels of biomarkers were analysed after logarithmic (log10) transformation to achieve homoscedasticity and normal distribution. Change of biomarker levels was expressed in relative changes in samples obtained directly after surgery (level directly after surgery divided by level before surgery) and in samples obtained on postoperative day 5 (level at postoperative day 5 divided by level before surgery). We controlled the false discovery rate using the Benjamini-Hochberg procedure using a false discovery rate of 0.2.
The prognostic capability of biomarkers that showed a difference in the postoperative levels between the outcome groups was determined using the area under the curve of the receiver operating characteristic curve (ROC-AUC). For this, we looked at the absolute levels of each biomarker, and its relative change. We used the following cut-offs for classifying the predictive value of each single, or a combination of biomarkers: 0.90 to 1.00 = excellent; 0.80 to 0.90 = good; 0.70 to 0.80 = fair; 0.60 to 0.70 = poor; and 0.50 to 0.60 = very poor. Where possible, the optimal cut-off was determined using a Youden index for the selection of the highest sum of sensitivity and specificity.
All analyses were conducted with SPSS v20 (IBM SPSS Statistics for Windows, Version 20.0; IBM Corp., Armonk, New York, USA), and R v.2.12.0. For all analyses, two-sided P values less than 0.05 were considered significant.
Results
Patients
Blood sampling was complete in 242 patients; 120 patients received high PEEP and 122 patients low PEEP for intraoperative ventilation (Fig. 1). Baseline characteristics, intraoperative ventilation characteristics and outcomes are presented in eTable 2, http://links.lww.com/EJA/A114, eTable 3 and eTable 4, http://links.lww.com/EJA/A114. Baseline characteristics, intraoperative ventilation characteristics and outcomes in patients included in this substudy were not different from patients included in the main analysis of the PROVHILO trial.15
Fig. 1.
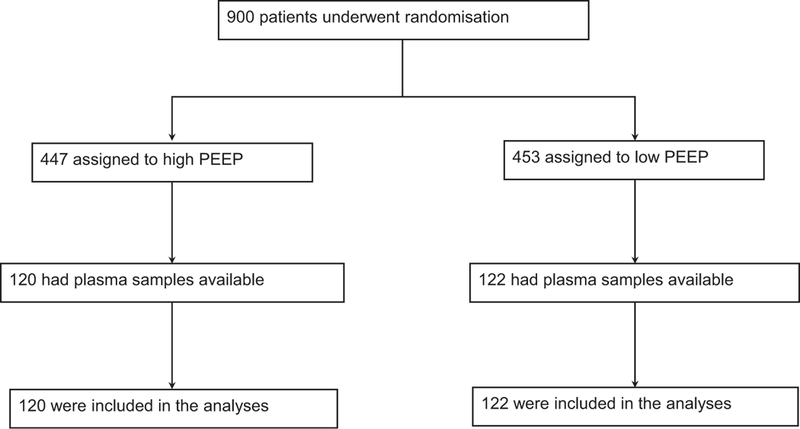
Flow chart.
Changes in plasma levels of biomarkers in the perioperative phase
Plasma levels of each biomarker before and after surgery are presented in Table 2. Median plasma levels of TNF-α, IL-6, IL-8 and CC-16 increased, median plasma levels of SP-D and KL6 decreased, while median plasma level of sRAGE did not change after surgery. Changes in plasma levels of IL-6 [3.3 (2.1 to 5.6) pgml−1 to 117.2 (48.1 to 245.0) pgml−1; P< 0.001] and IL-8 [7.0 (4.5 to 10.2) pgm1−1 to 18.4 (9.5 to 38.3) pgml−1; P < 0.001] in samples obtained directly after surgery were much larger, and seen in almost all patients. Plasma levels of IL-6 and IL-8 decreased towards preoperative levels on postoperative day 5, though were still significantly higher than before surgery. Increase in plasma levels of CC—16 was found only in samples obtained directly after surgery. Plasma levels of SP-D and KL6 increased slightly from samples obtained directly after surgery to samples obtained on postoperative day 5.
Table 2.
Plasma level of biomarkers of inflammation and lung injury according to groups
| TNF-α (pg ml−1) | IL-6 (pg ml−1) | IL-8 (pg ml−1) | sRAGE (μg ml−1) | SP-D (μg ml−1) | CC-16 (μg ml−1) | KL6 (units ml−1) | |
|---|---|---|---|---|---|---|---|
| Overall cohort | |||||||
| Preoperative | 8.5 (4.2 to 11.2) | 3.3 (2.1 to 5.6) | 7.0 (4.5 to 10.2) | 1.9 (1.5 to 2.4) | 9.2 (5.0 to 1.6) | 15.7 (10.1 to 22.7) | 18.3 (11.1 to 27.0) |
| Postoperative | 8.8 (4.0 to 12.1) | 11 7.2 (48.1 to 245.0) | 18.4 (9.5 to 38.3) | 1.9 (1.5 to 2.5) | 5.5 (3.3 to 10.0) | 17.8 (10.9 to 22.7) | 13.4 (7.8 to 19.8) |
| Postoperative day 5 | 9.0 (4.8 to 1 2.2) | 13.7 (7.4 to 32.8) | 10.0 (6.5 to 14.5) | 2.0 (1.4 to 2.6) | 6.2 (3.7 to 10.7) | 11.9 (7.6 to 19.6) | 12.8 (7.1 to 18.5) |
| P* | 0.003 | <0.001 | <0.001 | 0.783 | <0.001 | <0.001 | <0.001 |
| PPCs | |||||||
| Preoperative | 9.0 (5.2 to 11.5) | 3.7 (2.4 to 6.1) | 7.2 (5.0 to 10.2) | 2.1 (1.7 to 2.5) | 9.6 (5.5 to 1 6.8) | 15.6 (11.2 to 23.3) | 18.1 (12.0 to 26.2) |
| Postoperative | 9.5 (5.3 to 13.4) | 153.7 (80.8 to 344.3) | 22.3 (12.5 to 46.1) | 2.1 (1.5 to 2.6) | 5.6 (3.3 to 10.3) | 18.5 (12.6 to 29.4) | 13.2 (8.5 to 18.4) |
| Postoperative day 5 | 10.2 (5.6 to 12.9) | 18.3 (10.3 to 50.7) | 10.5 (7.7 to 17.4) | 2.1 (1.5 to 2.7) | 6.5 (4.3 to 1 2.9) | 13.9 (8.3 to 21.7) | 12.9 (7.2 to 17.4) |
| P* | 0.001 | <0.001 | <0.001 | 0.406 | <0.001 | <0.001 | <0.001 |
| No PPC | |||||||
| Preoperative | 7.5 (3.5 to 11.1) | 2.9 (1.7 to 4.6) | 6.2 (3.6 to 10.2) | 1.8 (1.4 to 2.3) | 8.1 (4.4 to 1.4) | 14.2 (7.5 to 22.6) | 18.1 (9.3 to 28.2) |
| Postoperative | 7.5 (3.4 to 11.5) | 62.7 (32.1 to 143.8) | 13.7 (7.4 to 25.0) | 1.7 (1.4 to 2.3) | 5.1 (3.0 to 9.4) | 1 6.3 (9.8 to 26.3) | 13.6 (7.3 to 22.7) |
| Postoperative day 5 | 6.5 (4.2 to 11.1) | 9.8 (5.6 to 1 6.5) | 8.0 (4.2 to 12.4) | 1.8 (1.4 to 2.3) | 5.6 (3.2 to 8.6) | 10.3 (6.6 to 1 7.0) | 12.8 (6.1 to 21.7) |
| P* | 0.683 | <0.001 | <0.001 | 0.523 | <0.001 | <0.001 | <0.001 |
| P ** | 0.150 | 0.024 | 0.188 | 0.271 | 0.014 | 0.014 | 0.246 |
| High PEEP | |||||||
| Preoperative | 8.2 (4.1 to 11.1) | 3.2 (2.1 to 4.9) | 7.1 (4.5 to 10.0) | 2.0 (1.6 to 2.3) | 9.6 (5.1 to 1.9) | 16.1 (9.0 to 22.0) | 17.8 (11.1 to 26.4) |
| Postoperative | 9.0 (4.2 to 1 2.3) | 125.5 (51.3 to 290.6) | 19.2 (10.4 to 43.1) | 2.1 (1.5 to 2.6) | 5.4 (3.4 to 10.0) | 18.0 (11.0 to 30.1) | 13.0 (8.7 to 18.8) |
| Postoperative day 5 | 9.5 (4.8 to 11.8) | 13.7 (8.7 to 18.8) | 10.2 (6.6 to 16.7) | 2.1 (1.4 to 2.6) | 6.5 (3.9 to 11.4) | 11.5 (7.9 to 20.1) | 12.7 (6.8 to 17.8) |
| P* | 0.019 | <0.001 | <0.001 | 0.443 | <0.001 | <0.001 | <0.001 |
| Low PEEP | |||||||
| Preoperative | 9.0 (4.3 to 11.6) | 3.6 (2.0 to 6.2) | 6.9 (4.3 to 10.4) | 1.8 (1.5 to 2.4) | 8.9 (5.0 to 14.5) | 15.6 (10.4 to 23.1) | 19.9 (11.1 to 27.7) |
| Postoperative | 8.5 (3.9 to 1 2.2) | 115.5 (44.8 to 215.8) | 16.1 (8.1 to 35.3) | 1.8 (1.4 to 2.4) | 5.6 (3.1 to 10.2) | 17.8 (10.7 to 25.5) | 14.1 (7.4 to 22.6) |
| Postoperative day 5 | 8.9 (4.7 to 12.6) | 13.3 (7.3 to 32.4) | 9.2 (6.4 to 13.8) | 1.8 (1.4 to 2.3) | 5.9 (3.7 to 10.1) | 11.9 (7.2 to 1 8.5) | 13.1 (7.2 to 19.0) |
| P* | 0.201 | <0.001 | <0.001 | 0.900 | <0.001 | <0.001 | <0.001 |
| P ** | 0.542 | 0.533 | 0.854 | 0.478 | 0.482 | 0.231 | 0.944 |
Values are medians (interquartile range). CC-16, Clara cell protein; IL, interleukin; KL-6, Krebs von den Lungen-6; PEEP, positive end expiratory pressure; PPCs, postoperative pulmonary complications; SP-D, surfactant protein D; sRAGE, soluble receptor for advanced glycation end-products; TNF, tumour necrosis factor.
Value is for comparison over time [postoperative versus preoperative and postoperative day 5 versus postoperative (repeated contrast)].
Comparison between groups overtime (PPC versus No PPC and High PEEP versus Low PEEP).
Associations between plasma levels of biomarkers and occurrence of postoperative pulmonary complications
Plasma levels of biomarkers in patients with and without PPCs are presented in Table 2. Relative changes of plasma levels are shown in Fig. 2. The degree of increase of TNF-α, IL-6, IL-8 and decrease of SP-D differed between patients who did and who did not develop PPCs. The ROC curves for the absolute levels of individual biomarkers in samples obtained directly after surgery, and for their relative change, are shown in Fig. 3. None of the biomarkers had an AUC-ROC higher than 0.7 (poor prognostic capacity for PPCs, eTable 5, http://links.lww.com/EJA/A114).
Fig. 2.
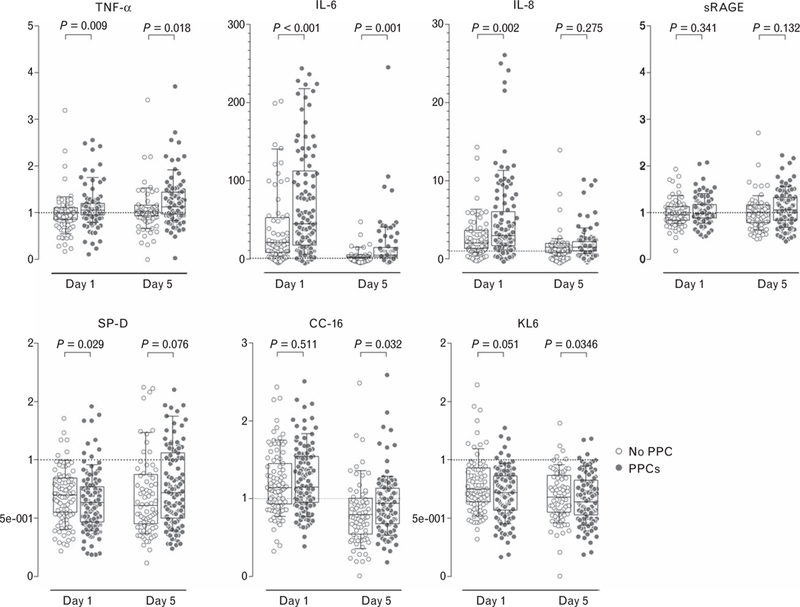
Relative changes in plasma levels of biomarkers of inflammation (TNF-α, IL-6, IL-8, sRAGE) or lung injury (SP-D, CC-16, KL6) in patients who developed (closed symbols) or did not develop (open symbols) one or more postoperative pulmonary complications. Box plots show medians (horizontal line within the box), interquartile ranges (lower and upper edges of the box) and the 10th to 90th percentile range (ends of whiskers). P values indicate the statistical significance. CC-16, Clara cell protein; IL, interleukin; KL6, Krebs von den Lungen-6; SP-D, surfactant protein D; sRAGE, soluble receptor for advanced glycation end-products; TNF, tumour necrosis factor.
Fig. 3.
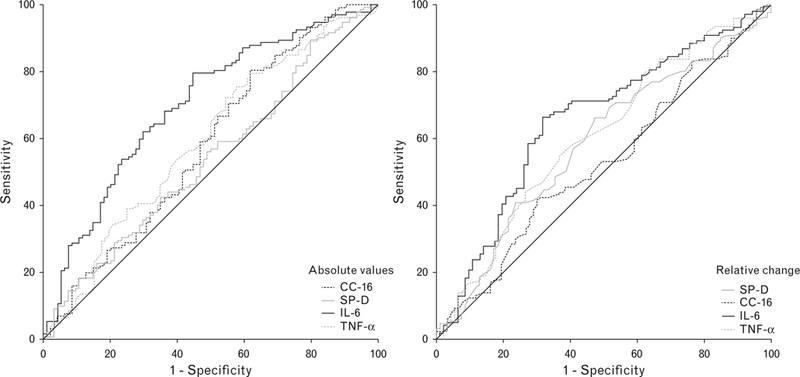
Receiver operating characteristic curves for the development of one or more postoperative pulmonary complications for biomarkers showing different kinetics after surgery. Absolute levels in the postoperative sample (left); relative changes after surgery (right). CC-16, Clara cell protein; IL, interleukin; SP-D, surfactant protein D; TNF, tumour necrosis factor.
Associations between plasma levels of biomarkers and the intraoperative ventilation strategy
Plasma levels of biomarkers in the randomisation groups are presented in Table 2, and relative changes are depicted in Fig. 4. Only the relative change in plasma levels of CC-16 in samples obtained directly after surgery were statistically significant, with larger values in patients receiving high PEEP during surgery. The association remained significant after adjustments for multiple comparisons (eTable 6, http://links.lww.com/EJA/A114).
Fig. 4.
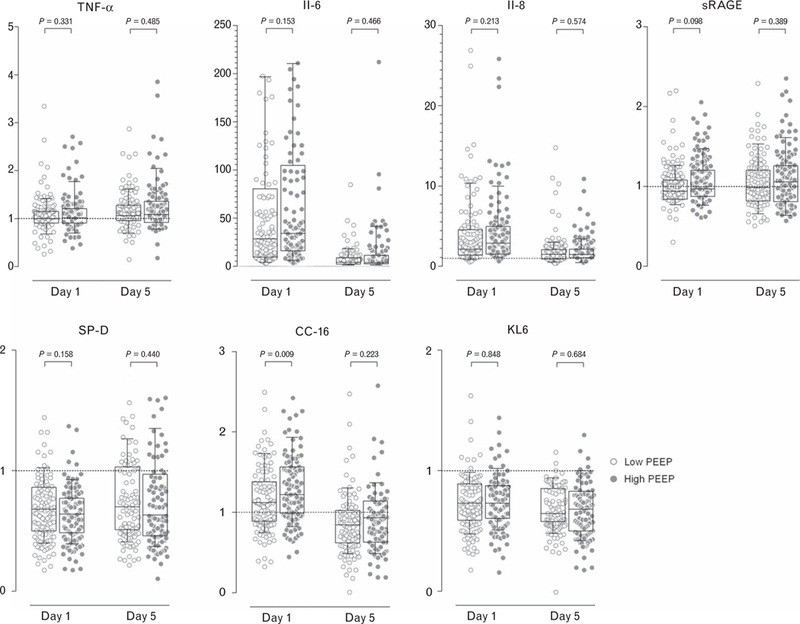
For patients who developed one or more postoperative pulmonary complications, the relative changes in plasma levels of biomarkers of inflammation (TNF-α, IL-6, IL-8, sRAGE) or lung injury (SP-D, CC-16, KL6) in patients ventilated with high (closed symbols) versus low (open symbols) PEEP. Box plots show medians (horizontal line within the box), interquartile ranges (lower and upper edges of the box) and the 10th to 90th percentile range (ends of whiskers). P values indicate the statistical significance. CC-16, Clara cell protein; IL, interleukin; KL6, Krebs von den Lungen-6; SP-D, surfactant protein D; sRAGE, soluble receptor for advanced glycation end-products; TNF, tumour necrosis factor.
Post-hoc analysis focusing on severe postoperative pulmonary complications
When ‘unexpected need for supplementary oxygen’ was excluded from the composite outcome, only the kinetics of IL-6 was different between patients who did and who did not develop one or more PPCs (eFigure 2, http://links.lww.com/EJA/A114, and eTable 7, http://links.lww.com/EJA/A114).
The results according to the four stratification groups are shown in eTables 8, http://links.lww.com/EJA/A114, 9 and 10, http://links.lww.com/EJA/A114. The degree of increase in plasma levels of TNF- IL-6 and IL-8 in the postoperative sample and of IL-6 and CC-16 in the sample taken on postoperative day 5 differed between the four groups analysed (eTable 9, http://links.lww.com/EJA/A114). The association remained significant after adjustments for multiple comparisons (eTable 10, http://links.lww.com/EJA/A114).
Discussion
This is the first study to assess the prognostic accuracy of several biomarkers for predicting PPCs and to describe their behaviour according to the level of intraoperative PEEP during low tidal ventilation in adult patients undergoing planned major open abdominal surgery.
The results of this preplanned substudy of the PROV-HILO trial can be summarized as follows: plasma levels of TNF-α, IL-6, IL-8, SP-D, CC-16 and KL6 changed following major abdominal surgery and the changes were higher for IL-6 and IL-8. Patients who developed PPCs showed a larger perioperative change in plasma levels of TNF-α, IL-6, IL-8 and SP-D, but none of the biomarkers had sufficient prognostic accuracy to predict PPCs. Patients who developed severe PPCs showed a larger perioperative change in plasma levels of IL-6 only. Patients ventilated with high PEEP who developed PPC showed a larger perioperative change in plasma levels of TNF-α, IL-6, IL-8 and CC-16, only. CC-16 was the only biomarker to present different plasma concentrations according to intraoperative level of PEEP, with higher plasma levels directly after surgery in patients receiving high PEEP.
The prospective design of the present study, the completeness of follow-up and the fact that occurrence of PPCs was scored by assessors who remained blind to the intraoperative ventilation strategy prevented some bias. In addition, PPCs were defined a priori, the number of participants was comparatively large and patients’ characteristics, as well as type of surgery were relatively homogeneous. Also, in this substudy, we determined the effect of two different levels of PEEP during intraoperative ventilation on kinetics of various biomarkers. Importantly, all patients were ventilated with low tidal volumes; thus, the sole effect of PEEP and recruitment manoeuvres on kinetics of the biomarkers could be studied.
One important finding was that plasma levels of three biomarkers of inflammation, TNF-α, IL-6 and IL-8, increased after surgery. TNF-α and IL-6 levels remained high during the first five postoperative days, suggesting abdominal surgery induces a strong systemic inflammatory response that lasts for several days. IL-8 levels decreased after the first postoperative day, as IL-8 is a key mediator associated with inflammation and it plays a key role in neutrophil recruitment and degranulation, a higher increase earlier in the postoperative phase is to be expected.5,17 Our data confirm results from previous investigations in patients undergoing abdominal surgery,4,18,21 and cardiac or lung surgery.5,17,22 However, the plasma levels of all three biomarkers were modest. Previous studies showed that plasma levels of biomarkers were not affected by surgery in patients with healthy lungs.5,11,12,23,25 In all of these studies, the change in plasma levels of biomarkers was independent of intraoperative ventilation settings. The present study also shows distinct kinetics of TNF-α, IL-6, IL-8 between patients who do and do not develop PPCs, which is partly in accordance with what has been reported in other studies investigating patients undergoing lung resection,22 oesophagectomy18,19,21 or liver transplantation.20 All three biomarkers had poor prognostic accuracy and cannot be used to predict PPCs with any reliability.
Plasma levels of SP-D and KL6 decreased after surgery. SP-D is responsible for modulation of host defence functions and maintenance of phospholipid homeostasis in the lung, and usually increases in inflammation associated with epithelial and peripheral airways damage.5,17 Similarly, KL6 is increased in association with injury to alveolar type II cells and airway glandular epithelium.9,24 There are only a few investigations into the kinetics of these two biomarkers in patients undergoing mechanical ventilation for surgery. One study showed no change in plasma levels of SP-D after general surgery,25 whereas, in patients undergoing cardiac surgery, an increase in plasma levels of SP-D was observed.10 A tempting suggestion could be that mechanical ventilation during surgery affects the epithelial integrity of the lungs increasing the leakage of these two biomarkers into the circulation. However, higher plasma levels of SP-D and KL6 during ventilation have been found in patients with ARDS,9,13 and a rise in levels of this biomarker was attenuated with lung-protective ventilation.9,24 Patients in the PROVHILO trial also received intraoperative ventilation with low tidal volumes. Of note, a positive fluid balance after surgery could somewhat dilute the concentration of a biomarker, resulting in the modest decrease in plasma levels of SP-D.26 Further, we did not observe any difference in SP-D and KL6 between patients with and without PPCs. Overall, our data suggest that epithelial and peripheral airways damage is minor after surgery whether patients develop PPCs or not. The increase in CC-16 suggested a process of Clara cell injury, or increased epithelial permeability consistent with lung tissue injury.
The difference in postoperative relative changes in plasma levels of CC-16 between patients ventilated with high versus low PEEP, suggests that high PEEP may cause lung injury during surgery. CC-16 is a lung epithelium-specific small protein found in small airways only,14 and a recent study suggested that plasma CC-16 shows a positive correlation with lung compliance in surgical patients, and raised levels may indicate lung overdistension.11 However, this remains speculative, as we did not assess the extent of overdistension, and cannot say whether or not overdistension was associated with the increases in plasma levels of CC-16. Notably, in patients with ARDS, higher levels of CC-16 were found to be associated with an increased risk of death, fewer ventilator-free days and increased frequency of nonpulmonary multiple organ failure.14
The stress response induced by surgery may have served as a trigger for the increase in the levels of some of the biomarkers measured in this investigation. As discussed above, it has been shown before that systemic levels of markers of inflammation, endothelial activation and coagulation increase after surgery, especially major surgery, even though those increases were much less than those seen with, for example sepsis. Of note, IL-6 and IL-8 have been shown to be useful in classifying patients with ARDS.27,28 Also, in ARDS, changes in systemic levels of IL-6 have been shown to be associated with outcome and the levels of IL-6 depend on the ventilation parameters.29,30 Taken together, the changes found in the present study suggest that after surgery patients, and mainly those developing PPCs, might present an increased inflammatory activity, with prolonged chemotaxis, reduced activation of the surfactant system as well as alveolar epithelial and peripheral airway damage, at least in the early postoperative phase.
Although the results of this study suggest that none of the biomarkers were helpful in predicting the development of PPCs, several reasons for their poor prognostic capacity in this study should be considered. First, we sampled blood only directly after surgery and on postoperative day 5. Measurements performed more closely to the time of occurrence of PPC could have led to a better prognostic performance. Second, the analysis was limited to measurement of seven biomarkers and to those that could be measured in combination employing two customized Luminex kits. Although all seven biomarkers are accredited as important biomarkers for inflammation or lung injury, we cannot rule out that other biomarkers would have performed better. Third, the PROVHILO trial included only patients at an intermediate or a high risk of PPCs, according to the ARISCAT score2,3 and only patients scheduled for open abdominal surgery. Biomarkers tested in this study might behave differently in other perioperative settings, for example in patients with a lower risk of PPCs and patients undergoing other types of surgery. Finally, levels of biomarkers were measured in plasma, while measurements performed in even more interesting.
Conclusion
The development of PPCs was associated with larger changes in plasma levels of biomarkers of inflammation or lung injury in patients undergoing major abdominal surgery. None of these biomarkers had sufficient prognostic capacity. The use of high compared with low PEEP during intraoperative ventilation was associated with higher plasma levels of a marker of epithelial lung injury, namely CC-16, directly after surgery.
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: the European Society of Anaesthesiology and the Academic Medical Centre (Amsterdam, the Netherlands) financially supported and endorsed the original trial. They had no role in design, data collection, data analysis, data interpretation or writing of the report of neither the original trial nor this substudy.
Footnotes
Conflicts of interest: none.
Presentation: none.
The PROVE Network: the PROtective VEntilation Network (http://www.provenet.eu).
References
- 1.Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med 2014; 2: 1007–1015. [DOI] [PubMed] [Google Scholar]
- 2.Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010; 113:1338–1350. [DOI] [PubMed] [Google Scholar]
- 3.Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014; 121:219–231. [DOI] [PubMed] [Google Scholar]
- 4.Jabaudon M, Futier E, Roszyk L, et al. Association between intraoperative ventilator settings and plasma levels of soluble receptor for advanced glycation end-products in patients without preexisting lung injury. Respirology 2015; 20:1131–1138. [DOI] [PubMed] [Google Scholar]
- 5.Wrigge H, Uhlig U, Zinserling J, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg 2004; 98:775–781. [DOI] [PubMed] [Google Scholar]
- 6.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 2008; 63:1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shirasawa M, Fujiwara N, Hirabayashi S, et al. Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 2004; 9:165–174. [DOI] [PubMed] [Google Scholar]
- 8.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 2006; 173:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Determann RM, Royakkers AA, Haitsma JJ, et al. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med 2010; 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels GE, Gu YJ, van Oeveren W, et al. The utility of lung epithelium specific biomarkers in cardiac surgery: a comparison of biomarker profiles in on- and off-pump coronary bypass surgery. J Cardiothorac Surg 2013; 8:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez-Bustamante A, Klawitter J, Repine JE, et al. Early effect of tidal volume on lung injury biomarkers in surgical patients with healthy lungs. Anesthesiology 2014; 121:469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 2008; 108:46–54. [DOI] [PubMed] [Google Scholar]
- 13.Kondo T, Hattori N, Ishikawa N, et al. KL-6 concentration in pulmonary epithelial lining fluid is a useful prognostic indicator in patients with acute respiratory distress syndrome. Respir Res 2011; 12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesur O, Langevin S, Berthiaume Y, et al. Outcome value of Clara cell protein in serum of patients with acute respiratory distress syndrome. Intensive Care Med 2006; 32:1167–1174. [DOI] [PubMed] [Google Scholar]
- 15.Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014; 384:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmes SN, Severgnini P, Jaber S, et al. Rationale and study design of PROVHILO: a worldwide multicenter randomized controlled trial on protective ventilation during general anesthesia for open abdominal surgery. Trials 2011; 12:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weingarten TN, Whalen FX, Warner DO, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth 2010; 104:16–22. [DOI] [PubMed] [Google Scholar]
- 18.D’Journo XB, Michelet P, Marin V, et al. An early inflammatory response to oesophagectomy predicts the occurrence of pulmonary complications. Eur J Cardiothorac Surg 2010; 37:1144–1151. [DOI] [PubMed] [Google Scholar]
- 19.Katsuta T, Saito T, Shigemitsu Y, et al. Relation between tumour necrosis factor alpha and interleukin 1beta producing capacity of peripheral monocytes and pulmonary complications following oesophagectomy. Br J Surg 1998; 85:548–553. [DOI] [PubMed] [Google Scholar]
- 20.Wen XH, Kong HY, Zhu SM, et al. Plasma levels of tumor necrotic factor-alpha and interleukin-6, −8 during orthotopic liver transplantation and their relations to postoperative pulmonary complications. Hepatobiliary Pancreat Dis Int 2004; 3:38–41. [PubMed] [Google Scholar]
- 21.Lu SL, Hsu FM, Tsai CL, et al. Serum transforming growth factor-β1 change after neoadjuvant chemoradiation therapy is associated with postoperative pulmonary complications in esophageal cancer patients undergoing combined modality therapy. Int J Radiat Oncol Biol Phys 2015; 93:1023–1031. [DOI] [PubMed] [Google Scholar]
- 22.de la Gala F1, Piñeiro P, Garutti I, et al. Systemic and alveolar inflammatory response in the dependent and nondependent lung in patients undergoing lung resection surgery: a prospective observational study. Eur J Anaesthesiol 2015; 32:872–880. [DOI] [PubMed] [Google Scholar]
- 23.Wrigge H, Uhlig U, Baumgarten G, et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Med 2005; 31:1379–1387. [DOI] [PubMed] [Google Scholar]
- 24.Eisner MD, Parsons P, Matthay MA, et al. Acute Respiratory Distress Syndrome Network. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax 2003; 58:983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Determann RM, Wolthuis EK, Choi G, et al. Lung epithelial injury markers are not influenced by use of lower tidal volumes during elective surgery in patients without preexisting lung injury. Am J Physiol Lung Cell Mol Physiol 2008; 294:L344–L350. [DOI] [PubMed] [Google Scholar]
- 26.Prowle JR, Leitch A, Kirwan CJ, Forni LG. Positive fluid balance and AKI diagnosis: assessing the extent and duration of ‘creatinine dilution’. Intensive Care Med 2015; 41:160–161. [DOI] [PubMed] [Google Scholar]
- 27.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014; 2:611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Famous KR, Delucchi K, Ware LB, et al. ARDS subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 2016; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999; 282:54–61. [DOI] [PubMed] [Google Scholar]
- 30.Ranieri VM, Giunta F, Suter PM, Slutsky AS. Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 2000; 284:43–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


