Abstract
In stem cell-based dental tissue engineering, the goal is to create tooth-like structures using scaffold materials to guide the dental stem cells. In this study, the effect of fiber alignment and hydroxyapatite content in biodegradable electrospun PLGA scaffolds have been investigated. Fiber orientation of the scaffolds was random or aligned in bundles. For scaffolds with prefabricated orientation, scaffolds were fabricated from PLGA polymer solution containing 0, 10 or 20 % nano-hydroxyapatite. The scaffolds were seeded with porcine cells isolated from tooth buds (dental mesenchymal, dental epithelial, and mixed dental mesenchymal/epithelial cells). Samples were collected at 1, 3 and 6 weeks. Analyses were performed for cell proliferation, ALP activity, and cell morphology. Fiber alignment showed an effect on cell orientation in the first week after cell seeding, but had no long-term effect on cell alignment or organized calcified matrix deposition once the cells reach confluency. Scaffold porosity was sufficient to allow migration of mesenchymal cells. Hydroxyapatite incorporation did not have a positive effect on cell proliferation, especially of epithelial cells, but seemed to promote differentiation. Concluding, scaffold architecture is important to mesenchymal cell morphology, but has no long-term effect on cell alignment or organized ECM deposition. nHA incorporation does have an effect on cell proliferation, differentiation and ECM production, and should be regarded as a bioactive component of dental bioengineered scaffolds.
Keywords: Dental tissue engineering, Tooth bud stem cells, Electrospinning, Hydroxyapatite, PLGA
Introduction
For decades, researchers in the field of tissue engineering have been investigating the possibility to create ‘biological teeth’ ex vivo. Such teeth constructs would not be associated with drawbacks related to artificial tooth replacement. For example, synthetic materials upon implantation can elicit undesired immune responses. Dental implants lack the resilience and pliability that natural teeth have. Furthermore, dental implants are less suitable for children, because they do not keep up with craniofacial growth [1, 2].
To create tooth (-like) material in vitro, first a biodegradable scaffold is necessary, which can be seeded with the cells that are capable of forming dental tissues. Such an approach is commonly referred to as scaffold-based tooth regeneration [3]. Since teeth originate from both ectodermal as ectomesenchymal germ layers, subsequently two types of stem cells will need to be added. First, cells of ectodermal origin to form the enamel. Second, cells from ectomesenchymal origin to form dentin, pulp, cementum, PDL and alveolar bone. The cell population present in immature tooth buds seems optimal for this purpose, as previous research has shown that cells isolated from tooth germs can be used to bioengineer small anatomically correct whole tooth crowns consisting of pulp tissue, dentin, and enamel [4]. Seeding of these epithelial and mesenchymal cells on biodegradable scaffolds potentially creates teeth of predetermined size and shape, supporting the presence of odontogenic stem cells in the tooth germ [5]. Still, no functional teeth of proper morphology including the root have been created yet from adult stem cells. Currently designed scaffolds lack the accurate three-dimensional organization to allow the spatial development of a completely natural tooth. Furthermore, the tooth bud stem cells respond to the physical as well as the chemical composition of the microenvironment. Thus, advanced scaffold production techniques will have to be investigated and employed to structure, as well as modulate the exact scaffold composition.
Electrospinning provides a promising method to manufacture scaffolds suitable for tissue engineering. With electrospinning, nanometric fibres are produced of the same size range as actual extracellular matrix (ECM). The simplicity of the technique makes it cost effective, whereas all involved parameters are easily modified [6]. A recent adaptation of the electrospinning approach, by collecting the fibers from a water bath, allows to obtain scaffolds of infinite thickness. Also this approach can be employed to develop highly organized scaffolds. The organization of the fibers could provide guidance to the adherent cells, resulting in alignment of these cells along the fiber axis [7, 8]. Thus, such development could later aid the creation of morphologically correct constructs for PDL, dentin and enamel.
To further optimize the chemical composition, especially the introduction of (nano)hydroxyapatite (nHA) particles should be regarded. The addition of bioactive material is proven to have an osteoinductive effect [9]. The effect of nHA on dental stem cells has not been studied yet in detail, especially with respect to stem cells from ectodermal origin.
As of yet, it remains unknown if scaffold organization or hydroxyapatite content affect the behavior of tooth bud stem cells. Thus, the goal of this study is to determine the effect of both fiber orientation and nHA incorporation on epithelial and mesenchymal dental stem cells, which will provide insight in the mechanisms involved in tooth development. We especially hypothesize that (1) tooth bud stem cells will recognize and align along the fiber axis of oriented electrospun scaffolds via contact guidance; and (2) that nHA content of the scaffold will promote odontogenic differentiation.
Materials and methods
Polymer preparation
PLGA(50:50) was purchased from PURAC biochem bv (Gorinchem, the Netherlands). It consists of 52 % D,L-lactide and 48 % glycolide, with a molecular weight (Mw) of 153,000. Nano-hydroaxyapatite (nHA) was a product from Budenheim (Budenheim, Germany), kindly provided by Dr. Marc Bohner, RMS foundation, Bettlach, Switzerland. Acetone and N,N-dimethylformamide (DMF) were both in HPLC grade and purchased from Lab-Scan analytical sciences (Gliwice, Poland) and VWR International Ltd (England, UK), respectively.
Scaffold preparation
To prepare electrospinning dope, PLGA was dissolved in Acetone/DMF (v/v = 6:4) at a concentration of 0.2 g/ml. For the dope-containing nHA, a defined amount of nHA was suspended in the same solvent by ultrasonification and vigorous stirring before adding the polymers. The polymer:nHA weight ratio was 1:1 and 2:1, respectively.
Before electrospinning, the prepared dope was fed into a plastic syringe, which was controlled by a syringe pump (KD Scientific Inc. Holliston, MA) at a feeding rate of 1.5–3.0 ml/h, depending on the stability of the fibres. A Teflon tube was used to connect the syringe and a blunt-end nozzle with an inner diameter of 0.8 mm, which was set up vertically.
To prepare cross aligned scaffolds, a grounded water bath was placed under the nozzle and the distance between the water surface and the nozzle was adjusted to 18 cm. Next to the water bath, a motorized take-up mandrel of 9.6 mm in diameter had been placed, of which the rotary and axial lineal speed were set at 35 rpm and 300 mm/min, respectively. During electrospinning, a high voltage of 21 kV was applied on the nozzle to generate a stable polymer jet. The resulting PLGA fibers were deposited on the water surface and then drawn to a rotating take-up mandrel, to become a wrapped bundle of fibers. To prepare the conventional randomly oriented electrospun scaffold with randomly oriented fibers, a rotating aluminum drum instead of the water bath was placed in the path of the polymer jet.
After spinning the scaffold materials were heat treated at 65 °C for 30 min for stress annealing and to prevent fibers from intensive shrinking during cell culture. Then the scaffolds were cut off from the mandrel and freeze dried (Table 1).
Table 1.
Scaffold compositions
| Hydroxyapatite content | |||
|---|---|---|---|
| Fiber orientation | 0 % | 10 % | 20 % |
| Random | ✓ | ||
| Aligned | ✓ | ✓ | ✓ |
Cell culture
For cell culture the scaffolds were cut to 6 mm diameter with a biopunch (Acuderm, Fort Lauderdale, FL, USA). Before cell seeding the scaffolds were treated with 75 % ethanol overnight, subsequently washed with PBS buffer (GIBCO, Invitrogen, Carlsbad, CA, USA), and stored in medium until use.
Six-month-old Yucatan minipig second molar tooth buds were carefully removed and tooth progenitor cells were harvested as previously described [10], with the following modifications. Enamel and pulp organs were surgically separated, minced into small tissue pieces (<1 mm3) in sterile HBSS containing penicillin/streptomycin/amphotericin B, digested in HBSS containing collagenase (0.4 mg/ mL) and dispase (0.2 mg/mL) at 37 °C for 1 h, followed by mechanical trituration. Single cell suspensions were generated by filtration through a 40-μm cell sieve, and repeatedly washed in cell culture medium. Cells were conserved with DMSO and stored in liquid nitrogen until use.
The pig dental epithelial (DEP) tooth bud cells were recovered from cryopreservation and seeded in a T175 cm2 flask (Corning Inc., Corning, NY, USA), and expanded using epithelial medium [LHC-8, (#12678–017, GIBCO), 10 % FBS, 1 % PSA, 0.5 ug/mL Epinephrine]. The dental mesenchymal (DME) tooth bud cells were expanded for 7 days in mesenchymal medium [Advanced DMEMF12 (#12634, GIBCO), 10 % FBS, 25 ug/mL Ascorbic Acid, 1 % PSA, 1 % Glutamax].
After confluency, cells were trypsinized, centrifuged at 1500 rpm for 5 min and resuspended in media to a concentration of 400 cells/μL. Scaffolds were seeded with 10 μL cell suspension and incubated at 37 °C for 1 h before adding media. For further culture, osteogenic factors (100 nM dexamethasone, 10 mM β-glycerolphosphate and 50 μg/ml ascorbic acid) were added to the media. For mixed epithelial and mesenchymal tooth bud cells (DEP–ME), culture epithelial and mesenchymal media were mixed 1:1. Medium was changed every 2–3 days. Control samples received identical treatment, without seeded cells. Samples and controls were collected after 1, 3 and 6 weeks.
Microscopical analysis
Samples were washed twice with PBS, and fixed with 3.7 % formalin for 1 h, washed twice with PBS, and stored in PBS. For analysis the samples were dehydrated in a graded series of ethanol (50, 70, 80, 90 and 100 %), xylene, embedded in paraffin and sectioned.
H&E staining
Samples were washed twice with xylene and subsequently rehydrated in a graded series of ethanol for 2 min (100, 95, 70, 50 and 0 %). Samples were stained with Hematoxylin for 1 minute, and washed with tap water. The samples were briefly submerged in diluted hydrochloric acid and subsequently ammonia water. After washing with water the samples were stained with eosin for 20 s. Samples then were dehydrated again with ethanol (95, 100 %) and xylene, and mounted with Permount (Fisher Chemicals, Pittsburg, PA, USA). Samples were analyzed using Zeiss Axiophot microscopes and photos were taken by digital Zeiss Axiocam camera.
DAPI staining
Samples were washed twice with xylene, rehydrated in a graded series of ethanol, and mounted with mounting medium with DAPI (Vectashield, Vector Labs, Burlingame, CA, USA). Samples were analyzed using Zeiss Axiophot microscopes under fluorescent filter and photos were taken by digital Zeiss Axiocam camera.
SEM analysis
Samples were rinsed two times with PBS, fixed in 2.5 % glutaraldehyde for 2 h at 4 °C, and washed three times with 0.1 M sodium-cacodylate buffer for 15 min at 21 °C. The samples were post-fixed with 1 % Osmium Tetroxide for 1 h on ice. After fixation, samples were washed three times again with 0.1 M sodium-cacodylate buffer. Thereafter, samples were dehydrated in a graded series of ethanol (30, 50, 70, 80, 90 and 100 %), and stored in 100 % ethanol. Before SEM analysis the samples were critical point dried. Non-cell culture treated samples received similar treatment before SEM analysis. All specimens were sputtered with gold and examined and recorded using SEM at an accelerating voltage of 10 kV.
ALP assay
At the respective sample collection days, the media from each well was collected and stored in separate tubes at −80 °C until further analysis. Before use the samples were defrosted completely at room temperature. To 80 μL sample 100 μL buffer was added, and directly before reading 20 μL of substrate solution (Alkaline phosphatase Liquicolor, no. 2900, Stanbio, Boerne, TX, USA). Change in absorption at 405 nm was monitored for 10 min by kinetic reading function of a microplate reader (Spectramax M2, Molecular devices, Sunnyvale, CA, USA).
Alamar blue viability assay
After collection of the medium for ALP analysis 400 μL new media, premixed with 40 μL Alamar blue reagent (Invitrogen, Carlsbad, CA, USA), was added to each well. After 1 h incubation at 37 °C the media was collected and stored in tubes with 200 μL 3 % SDS at −80 °C until further analysis.
For analysis samples were defrosted, 100 μL of each sample was pipetted in a black 96-well plate with a clear bottom and fluorescence was measured at 590 nm, after excitation at 544 nm on the microplate reader.
Statistical analysis
Results of the cell viability assay and ALP activity assay were analyzed by ANOVA and post hoc Tukey test.
Results
General observation
All samples exhibited uneventful culturing, all samples could be harvested, and no infections were recorded. By visual inspection, nHA-containing scaffolds seemed to have lost consistency at the 6-week time period, whereas for the control samples no difference in morphological appearance could be noted over the time of the experiment.
Light microscopical analysis
The H&E staining demonstrated that most cells had proliferated on the seeding side on top of the scaffold. Still, in all four scaffold types cellular growth and penetration throughout the scaffold were occasionally observed as well. Noticeably, a more elongated orientation in cellular shape along the fiber axis of the scaffolds was observed with pre-orientated fibers. In contrast, cells appeared more compact in shape in the randomly oriented scaffolds (Fig. 1a, b). The DAPI staining and fluorescent microscopy corroborated the presence of the cells throughout the sample (Fig. 1c, d).
Fig. 1.

H&E and DAPI staining, all after 3 weeks of cell culture. Scale bar 50 μm. a DEP–ME on an aligned, 0 % nHA scaffold. b DME on a random, 0 % nHA scaffold. c DEP–ME cells on an aligned, 0 % nHA scaffold. d DME on a random, 0 % nHA scaffold
SEM analysis
When observing the starting materials, the randomly oriented scaffold exhibited a consistent fiber diameter (Fig. 2a) with a relatively smooth surface of the individual fibers (Fig. 2b). In the aligned scaffold there was a clear angle approximating 30° between the fiber axes (Fig. 2c). The orientation of the individual fibers minimally deviated from the principal fiber axis. Surface texture of the individual fibers was similar to the random scaffold (Fig. 2d). The scaffold with 10 % nHA again demonstrated a clear angle between the fiber axes (Fig. 2e). However, fiber diameter was less consistent, and the individual fibers were oriented along the principal fiber axes to a lesser extent. Examination of the surface texture demonstrated regular distribution of nHA particles throughout the fibers (Fig. 2f). The three-dimensional structure of the scaffold was more distinct in the scaffold with 20 % nHA due to a more variable fiber diameter (Fig. 2g). Again the individual fibers were lesser oriented along the principal fiber axes. The nHA particles were distributed evenly through the fibers, although the surface seemed more densely covered with hydroxyapatite when compared to the 10 % scaffold (Fig. 2h).
Fig. 2.

SEM image of scaffolds before further treatment. Top images ×500, scale bar 50 μm, lower images ×10,000, scale bar 3 μm
After cell seeding, only few cells were visible on the week 1 specimens. Alignment along the individual fibers was most apparently observed for the DME cells (Fig. 3). At the later stages cellular alignment was no longer recognizable as cells could no longer be distinguished individually (Fig. 4).
Fig. 3.
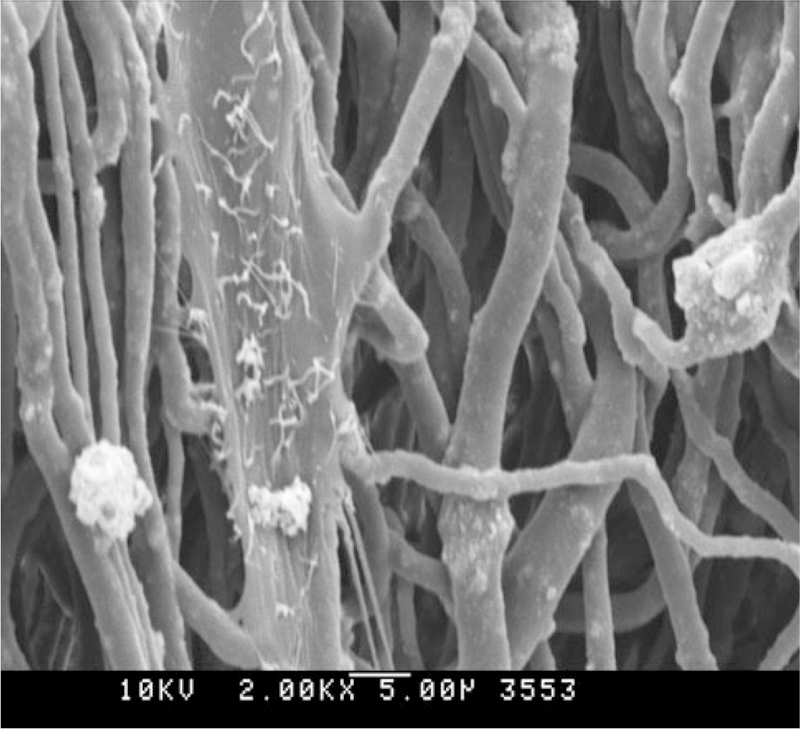
SEM image of DME cell after 1 week; note the apparent cellular alignment with the scaffold architecture. ×2000, scale bar 5 μm
Fig. 4.

SEM image of aligned 0 % nHA scaffolds after 6 weeks of cell culture. a DEP. b DME. c DEP–ME. ×500, scale bar 40 μm
Of course, it was also impossible to distinguish DME from DPE in the combined culture. Still, comparing to the individually seeded DME and DEP over the different time periods, the mixed DEP–ME appeared more proliferative. This was especially apparent on the aligned scaffold without nHA, although such assessment remains based on visual inspection only.
Moreover, there was an effect of cell type on the ECM production. In all scaffold types ECM formation could be distinguished since the first week, and seemed more pronounced at the third week. DME cells began to form a continuous layer already at week 3. Underneath this cell layer there was extensive ECM deposition, becoming thicker and more abundant toward the latter time point (Fig. 5a), and showing a highly organized structure. DEP cells also started to form a continuous cell layer from week 3; however, less extensively than the DME cells and only on the scaffolds without nHA. Also, the small amount of ECM remained on the cell membrane, and did not disperse on the scaffold. However, DEP–ME cells together seemed most efficient, and covered the scaffolds without nHA completely with ECM already at week 3. After 6 weeks, there was extensive ECM deposition present in the majority of the samples. DME excreted collagen-like fibrils (Fig. 5). These fibrils were highly organized, even without the guidance of an aligned scaffold (Fig. 5a). DEP produced calcified nodules (Fig. 4a). Combined DEP–ME cells displayed the most evident matrix deposition and formation of calcified nodules (Fig. 4c). When comparing scaffold types, the nHA scaffolds appeared to show lesser matrix deposition for both DEP and DME (Fig. 5c, d).
Fig. 5.

SEM image of DME after 6 weeks of cell culture. a Random 0 % nHA. b Aligned 0 % nHA. c Aligned 10 % nHA. d Aligned 20 % nHA. ×2000, scale bar 5 μm
Alamar blue viability assay
DME showed proliferation between the first and the third week on the scaffolds without nHA (Fig. 6). After this, the cell number reached a plateau. In contrast, the nHA-containing materials gave rise to significantly lower cell numbers at week 3, but exceeded proliferation compared to the other scaffolds at week 6, without showing any sign of reaching a plateau value.
Fig. 6.

Cell viability (*p<0.05, **p<0.01)
For the DEP cells, markedly lower values were observed as compared to DME. Still, again the scaffolds without nHA resulted in high initial proliferation, while on the nHA scaffolds the proliferation rate was extremely low. After 6 weeks, the higher (20 %) nHA content gave significantly lesser cell growth compared to the 10 %. Again, only the cells on the scaffolds without nHA seemed to reach a plateau phase.
The combined DEP–ME cell cultures did not grow well on the randomly oriented scaffolds, as after both 3 and 6 weeks the cell proliferation was significantly lower than on any other scaffold. In contrast, on the aligned scaffold cell proliferation was significantly enhanced. On nHA-containing scaffolds, DEP–ME demonstrated a very different development compared to the separate cultures, i.e., high initial cell proliferation, while at 3 weeks a plateau value was reached.
Alkaline phosphatase activity
DME showed an increasing ALP activity from week 1 to 3 (Fig. 7). After 6 weeks this activity had diminished to about 50 % of the maximal value. On the nHA-containing scaffolds, ALP activity continued to increase in a linear fashion. However, maximal ALP activity never exceeded the maximal ALP activity on the non-nHA scaffolds.
Fig. 7.

ALP activity (*p<0.05, **p<0.01)
In DEP cultures, ALP activity remained below the measuring threshold. The combined DEP–ME exhibited a similar trend to DME on the aligned scaffold without nHA, although the curve runs at about 50 % of the value of DME. This is consistent with the cell seeding (2000 mesenchymal cells and 2000 epithelial cells for DEP–ME, versus 4000 mesenchymal cells for the separate group).
Discussion
This investigation aimed to systematically study cell behavior of mesenchymal and epithelial cells from dental tooth buds, on scaffolds with different topological and chemical cues, to assess the requirements for scaffolds to support organized dental tissue formation. Four scaffold types were prepared, with a random or aligned fiber orientation, and 0, 10 or 20 % nHA content. In general, the scaffold topography influenced cell morphology, the effect of the chemical component, however, was not as evident.
Regarding our study set-up, the materials used in this study proved easy to produce and handle. However, collection of the 20 % nHA fibers was more challenging than the other materials, since the high hydroxyapatite content made the fibers more fragile and susceptible to breaking. Adding a surfactant to the polymer solution could secure a more uniform nHA distribution, thus improving the mechanical properties of the scaffold [11]. Addition of hydroxyapatite also increased fiber diameter distribution and surface roughness, and possibly influenced pore size as well. Samples with nHA became very fragile and difficult to handle after 6 weeks of cell culture, especially with the higher nHA concentration. Controls without cells did not display an obvious decrease in strength. The fragility of the scaffolds could be due to the customized electrospinning method used, since previous research indicates an increased scaffold strength upon nHA incorporation in conventional electrospinning methods [12, 13]. Possibly these effects will be of lesser importance when going to actual, larger sized scaffolds. In the current investigation scaffold thickness was very limited, since the aim of this research was mainly to investigate surface characteristics. However, increasing scaffold thickness would be very easy to achieve, especially for the aligned materials, by increasing the processing time.
Other technical remarks should address the cell culture procedure. In the current study, little distinction could be made between the cell types on basis of morphological visual inspection alone. Thus, future work should, prior to seeding, employ separate fluorescent labels for both cell types. Such a set up would allow for individual and quantitative measurements to be made, even in the mixed cultures. When scaffold architecture and cell seeding are optimized in this fashion, immunohistochemistry could unravel the effect of cell type on production of the major ECM components (e.g., collagen, fibronectin, laminin). Further, the matrix mineralization process should be studied in close detail with the aid of transmission electron microscopy.
As dental epithelial stem cells are relatively hard to come by, there has been little research on the effect of different scaffold compositions on this cell type. It seemed that the seeding density, proliferation capacity, and/or scaffold properties were less than optimal, causing a relatively low cell count for all cell types. Increasing scaffold dimensions, porosity, or surface roughness could possibly promote cell in growth, thereby providing a niche for cell proliferation and increasing total cell count [14].
Regarding the results, DME cells initially aligned along the individual fibers, due to the cells’ intrinsic capability to migrate and morphologically adapt to their surroundings. In the first week, DME exhibited the characteristic spindle shaped morphology with an elongated cell body along the individual fibers, which is consistent with previous research [7]. When the scaffold became more densely covered with cells, the fiber alignment no longer affected cellular morphology since intercellular contact guidance was more dominant than the bioengineered microenvironment. In contrast, the DEP cells had a lesser tendency to adapt to topological organization or to migrate, which was evident at visual inspection of scaffold surface and penetration of the scaffolds with DEP. Consequently, fiber orientation did not show to have any effect on calcified matrix deposition. Nevertheless, the incapability to guide ECM deposition does not necessarily mean that specific scaffold architecture is redundant, since previous research indicated specific roughness and topography could promote differentiation of cells from both mesoderm as ectodermal origin [15].
It also was apparent that DME generally exhibited higher cell proliferation and more ECM production than DEP. At visual inspection, combination of the two cell types (DEP–ME) seemed to promote proliferation and ECM production of the DEP cells. However, ALP proved to be invalid as a marker for differentiation in DEP, so further research should investigate the effect of combining DME and DEP on DEP differentiation. All cell groups showed formation of calcified nodules from the third week onwards, although DME demonstrated a significantly higher density of nodules, both in the third and sixth week. However, the predictive value of mineralization in vitro will always need to be confirmed in vivo calcified tissue formation models, as previous research shows [16].
The scaffolds without hydroxyapatite showed high early cell proliferation and ALP activity, and SEM images illustrated prominent ECM production. The diminished proliferation toward the sixth week might be caused by differentiation of the cells, or depletion of the cells from nutrition due to the extensive presence of ECM and thus induced apoptotic cell death. Curiously, DEP–ME showed a very low proliferation and ALP activity assay data on the random scaffold, which was contradictive to the SEM image of these samples. Imaging showed total coverage of the scaffold with cells, and extensive production of calcified nodules. Possibly, such calcification influenced the readout from the cell proliferation and ALP assay, by inhibiting diffusion of the reagents to the cells.
Since the evident success of the bioactive HA in bone tissue engineering, the study design mainly focused on qualitatively assessing whether a similar trend would hold true for dental tissue engineering. Obviously, the addition of nHA to the scaffolds had some very distinct effects on cell proliferation and differentiation. The most pronounced and notable effect was the limited proliferation DEP manifested on the nHA-infused scaffolds. Second, SEM images demonstrated only little calcified matrix formation on all scaffolds with nHA. Previous studies emphasize that although there are some indications that nHA has an adverse effect, gene expression is altered in many ways because of the mineral presence, and this is only a small facet of the protein-level cellular response [12]. Besides its bioactive properties, nHA has alkaline properties which theoretically could reduce acidity of degraded products of PLGA, thereby promoting proliferation [17]. However, visual inspection displayed inhibited cell proliferation on the scaffolds with nHA. Hypothetically the proliferation, and thus the overall mineralization, could have been less pronounced because nHA had promoted a more rapid differentiation process. However, at the last time point most ECM was present on the scaffolds without nHA. It is clear that the presence of nHA has effect on proliferation, alters gene expression, and affects composition and production rate of ECM, but additional research should be done to investigate exactly which intra-cellular mechanisms are up- or downregulated.
We hypothesized that tooth bud cells would recognize and align along the fiber axis of oriented electrospun scaffolds via contact guidance. This statement seems to have only restricted validity, since DEP are not influenced by scaffold architecture. DME cells indeed do respond to the fiber alignment. Concerning the promotion of odontogenic differentiation by nHA content of the scaffold, it is evident that nHA has an effect on proliferation, differentiation and ECM production of both mesenchymal and epithelial cells. However, to assess the exact intracellular mechanism additional research should be performed.
Concluding, scaffold architecture is important to mesenchymal cell morphology, but has no long-term effect on cell alignment or organized ECM deposition. nHA incorporation does have an effect on cell proliferation, differentiation and ECM production, and should be regarded as a bioactive component of dental bioengineered scaffolds.
Acknowledgments
We would like to acknowledge the support received from the Radboud University (EHCVM), and NIH/NIDCR grant DE016132–06 (PCY).
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Elisabeth H. C. van Manen, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands
Weibo Zhang, Division of Tissue Engineering, Department of Oral and Maxillofacial Pathology, Tufts University School of Dental Medicine, Boston, MA, USA.
X. Frank Walboomers, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands.
Betsy Vazquez, Division of Tissue Engineering, Department of Oral and Maxillofacial Pathology, Tufts University School of Dental Medicine, Boston, MA, USA.
Fang Yang, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands.
Wei Ji, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands.
Na Yu, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands.
Daisy J. Spear, Division of Tissue Engineering, Department of Oral and Maxillofacial Pathology, Tufts University School of Dental Medicine, Boston, MA, USA
John A. Jansen, Department of Biomaterials, Radboud University Nijmegen Medical Centre 309 PB, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands
Pamela C. Yelick, Division of Tissue Engineering, Department of Oral and Maxillofacial Pathology, Tufts University School of Dental Medicine, Boston, MA, USA
References
- 1.Sicilia A, Cuesta S, Coma G, Arregui I, Guisasola C, Ruiz E, Maestro A. Titanium allergy in dental implant patients: a clinical study on 1500 consecutive patients. Clin Oral Implants Res 2008;19(8):823–35. [DOI] [PubMed] [Google Scholar]
- 2.Mankani N, Chowdhary R, Patil B, Madalli P. Dental implants in children and adolescents: a literature review. J Oral Implantol 2012. doi: 10.1563/AAIL-JOI-D-11-00186. [DOI] [PubMed] [Google Scholar]
- 3.Zivkovic P, Petrovic V, Naiman S, Stefanovic V. Stem cell-based dental tissue engineering. Sci World J 2010;10:901–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Abukawa H, Troulis MJ, Kaban LB, Vacanti JP, Yelick PC. Tissue engineered hybrid tooth-bone constructs. Methods 2009;47(2):122–8. [DOI] [PubMed] [Google Scholar]
- 5.Young CS, Terada S, Vacanti JP, Honda M, Bartlett JD, Yelick PC. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 2002;81(10):695–700. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Lim CT, Ramakrishna S, Huang ZM. Recent development of polymer nanofibers for biomedical and biotechnological applications. J Mater Sci Mater Med 2005;16(10):933–46. [DOI] [PubMed] [Google Scholar]
- 7.Shang S, Yang F, Cheng X, Walboomers XF, Jansen JA. The effect of electrospun fibre alignment on the behaviour of rat periodontal ligament cells. Eur Cell Mater 2010;19:180–92. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton DW, Oakley C, Jaeger NA, Brunette DM. Directional change produced by perpendicularly-oriented microgrooves is microtubule-dependent for fibroblasts and epithelium. Cell Motil Cytoskelet 2009;66(5):260–71. [DOI] [PubMed] [Google Scholar]
- 9.Götz W, Lenz S, Reichert C, et al. A preliminary study in osteoinduction by a nano-crystalline hydroxyapatite in the mini pig. Folia Histochem Cytobiol 2010;48(4):589–96. [DOI] [PubMed] [Google Scholar]
- 10.Abukawa H, Zhang W, Young CS, Asrican R, Vacanti JP, Kaban LB, Troulis MJ, Yelick PC. Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg 2009;67(2):335–47. [DOI] [PubMed] [Google Scholar]
- 11.Kim HW. Biomedical nanocomposites of hydroxyapatite/polycaprolactone obtained by surfactant mediation. J Biomed Mater Res A 2007;83(1):169–77. [DOI] [PubMed] [Google Scholar]
- 12.Dormer NH, Qiu Y, Lydick AM, Allen ND, Mohan N, Berkland CJ, Detamore MS. Osteogenic differentiation of human bone marrow stromal cells in hydroxyapatite-loaded microsphere-based scaffolds. Tissue Eng Part A 2012;18(7–8):757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas V, Jagani S, Johnson K, Jose MV, Dean DR, Vohra YK, Nyairo E. Electrospun bioactive nanocomposite scaffolds of polycaprolactone and nanohydroxyapatite for bone tissue engineering. J Nanosci Nanotechnol 2006;6(2):487–93. [DOI] [PubMed] [Google Scholar]
- 14.Zheng L, Yang F, Shen H, Hu X, Mochizuki C, Sato M, Wang S, Zhang Y. The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials 2011;32(29):7053–9. [DOI] [PubMed] [Google Scholar]
- 15.Massumi M, Abasi M, Babaloo H, Terraf P, Safi M, Saeed M, Barzin J, Zandi M, Soleimani M. The effect of topography on differentiation fates of matrigel-coated mouse embryonic stem cells cultured on PLGA nanofibrous scaffolds. Tissue Eng Part A 2012;18(5–6):609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang W, Ahluwalia IP, Literman R, Kaplan DL, Yelick PC. Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol based silk scaffolds. J Biomed Mater Res A 2011;97(4):414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F, Cui WJ, Xiong Z, Liu L, Bei JZ, Wang SG. Poly(L-lactide-co-glycolide)/tricalcium phosphate composite scaffold and its various changes during degradation in vitro. Polym Degrad Stabil 2006;91(12):3065–73. [Google Scholar]


