Abstract
In humans, increased red blood cell distribution width (RDW) values are associated with higher morbidity and mortality in a variety of pathological processes. The main objective of this study was to evaluate RDW in dogs with a diverse range of pathologies. Clinical data from 276 dogs were retrospectively evaluated. Significantly higher RDW values were found in dogs with primary immune-mediated hemolytic anemia (P < 0.0001), immune-mediated thrombocytopenia (P < 0.0004), hyperadrenocorticism (P < 0.0001), hypothyroidism (P = 0.0220), hepatic vascular anomaly (P < 0.0001), pneumonia (P < 0.0001), chronic kidney disease (P = 0.0005), multi-centric lymphoma (P = 0.0002), and myxomatous mitral valve degeneration (P = 0.0032). However, there was extensive overlap with the values from healthy dogs, limiting the diagnostic value of RDW in this setting. Although RDW may have a role as a potential prognostic indicator, further studies would be necessary to address this.
Résumé
Évaluation de l’indice de distribution des globules rouges chez des chiens avec différentes maladies. Chez les humains, une augmentation des valeurs de l’indice de distribution des globules rouges (RDW) est associée avec une plus grande morbidité et mortalité dans une variété de processus pathologiques. L’objectif principal de la présente étude était d’évaluer la RDW chez des chiens avec une variété de pathologies. Les données cliniques de 276 chiens ont été rétrospectivement évaluées. Des valeurs significativement plus élevées de RDW ont été trouvées chez des chiens avec une anémie hémolytique primaire à médiation immunitaire (P < 0,0001), une thrombocytopénie à médiation immunitaire (P < 0,0004), de l’hyperadrénocorticisme (P < 0,0001), de l’hypothyroïdisme (P < 0,0220), une anomalie vasculaire hépatique (P < 0,0001), une pneumonie (P < 0,0001), une maladie rénale chronique (P = 0,0005), un lymphome multicentrique (P = 0,0002), et une dégénérescence myxomateuse de la valvule mitrale (P = 0,0032). Toutefois, il y avait un chevauchement important avec les valeurs provenant de chiens en santé, limitant ainsi la valeur diagnostique de RDW dans ce contexte. Bien que le RDW peut avoir un rôle d’indicateur potentiel de pronostic, des études supplémentaires seraient nécessaires pour y répondre.
(Traduit par Dr Serge Messier)
Introduction
The red blood cell distribution width (RDW) is the coefficient of variation of the red blood cell (RBC) volume and as such is a measure of anisocytosis or the variability in size of circulating erythrocytes in a sample. This quantitative parameter is routinely calculated by many hematology analyzers and is easily incorporated into standard hematology results.
In human medicine, increased RDW values can result from a variety of physiological and pathological processes (1). Physiological causes include ageing (2,3), ethnicity (4), moderate physical exercise (5,6), and pregnancy (7). In pathological settings, RDW is predictably increased when RBC regeneration is present, most commonly in response to hemorrhagic or hemolytic anemia, but remains within the reference interval in many non-regenerative anemias. The interpretation of RDW in combination with mean corpuscular volume (MCV) may further aid the subclassification of anemias (6). For example, increased MCV with an RDW within the reference interval is associated with aplastic anemia and chronic liver disease while MCV within the reference interval and increased RDW is associated with early iron, cobalamin, or folate deficiency and chronic hepatobiliary disease. Many other pathological causes for increased RDW values with or without associated anemia have been reported including cardiorespiratory, vascular, neoplastic, endocrine, pancreatic, renal, hepatic, and bone marrow disorders, nutritional deficiencies, chronic or acute systemic inflammation, sepsis, and the use of certain drugs such as nebivolol and metoprolol (6,8–10). Indeed, an increased RDW provides important prognostic information and is a predictor of mortality in heart failure, community acquired pneumonia, hematological malignancies, acute pancreatitis, kidney disease, obstructive jaundice caused by malignancy and sepsis or septic shock (6). It is also a useful indicator of both clinical and subclinical hypothyroidism (11). The mechanisms involved are numerous and varied and include disturbances of erythropoiesis, chronic inflammation, and nutritional deficiencies, either alone or in various combinations (6).
In dogs, information on RDW is more limited. Microcytosis and increased RDW occur in health in certain breeds such as the Japanese shiba, Akita and Hokkaido, and macrocytosis and increased RDW occur in toy and miniature poodles (12,13). Increased RDW has been reported in Alaskan malamutes, Drentse partridge dog, and miniature schnauzers with hereditary symptomatic and asymptomatic stomatocytosis (14). The RDW is predictably increased in dogs with regenerative but not in non-regenerative anemias and/or with iron deficiency (13). The RDW can itself also assist in predicting a regenerative or non-regenerative response. In one study of 143 anemic dogs, a 1% increase in RDW increased the odds of having regenerative rather than non-regenerative anemia by a factor of 1.3 (15). In another study conducted in 4521 anemic dogs, the sole use of MCV and mean corpuscular hemoglobin concentration (MCHC) resulted in significant underdetection of a regenerative response. However, an increased RDW value (> 14%) improved the detection of regeneration compared with other erythrocyte indices (16).
In contrast to human patients, RDW values did not significantly differ between healthy dogs and those with myxomatous mitral valve degeneration (MMVD), either compensated or with congestive heart failure (17). In dogs with pulmonary hypertension, increased RDW values were found, but considerable overlap existed between diseased and healthy animals (18). Increased RDW values in these dogs did not have prognostic significance and an association with survival was not established. In one other study, RDW was evaluated in 946 adult dogs with a variety of largely unspecified illnesses. Most dogs (93.5%) had reference interval RDW values. Only 4% had elevated values including 2 poodles with macrocytosis, 2 with portosystemic shunts and the remainder with unspecified hemorrhagic disorders associated with inflammation or neoplasia. Low values were found in 2.5% of dogs and were largely associated with trauma-induced inflammation such as fractures (13).
Given the paucity of information on RDW values in dogs with specific disease conditions, the aim of this study was to expand the current knowledge in RDW by evaluating this parameter in a more diverse range of pathologies, and to report the relationship with MCV. It was hypothesized that, as in humans, RDW values increase in association with specific categories of disease.
Materials and methods
Case selection
The case logs of 4 residents in small animal medicine in the University College Dublin Veterinary Hospital (UCDVH), within a 4-year time frame (2014–2018), were retrospectively searched for dogs diagnosed with conditions that have been associated with increased RDW in humans including: hypothyroidism, hyperadrenocorticism (HAC), diabetes mellitus (DM), meningitis of unknown origin (MUO), idiopathic epilepsy (IE), hepatic vascular anomaly (HVA), hepatitis, pancreatitis, multicentric lymphoma (MCL), MMVD, primary immune-mediated hemolytic anemia (IMHA), immune-mediated thrombocytopenia (IMTP), inflammatory bowel disease (IBD), chronic kidney disease (CKD), pneumonia, eosinophilic bronchopneumopathy (EBP), and chronic bronchitis (CB). Case logs were used as they provided detailed information on how a diagnosis was achieved and on follow-up of individual dogs.
Data examined from medical records included: signalment, history, physical examination findings at presentation, complete blood (cell) count (CBC), serum biochemistry, urinalysis at the time of diagnosis, and investigations required to achieve a definitive diagnosis. Complex cases with more than one prominent disease process were excluded to allow creation of mutually exclusive groups. Dogs that had received treatments known to influence RDW in humans (e.g., blood transfusions or chemotherapy) were also excluded.
Hypothyroidism was diagnosed based on supportive clinical and clinicopathological features and decreased total thyroxine (T4) with increased canine thyroid stimulating hormone (cTSH) concentrations. In cases with reference interval cTSH concentrations but clinical suspicion of hypothyroidism, decreased free T4 concentration measured by equilibrium dialysis was used for clarification, alone or in combination with positive thyroglobulin autoantibody results. A diagnosis of HAC was based on supportive clinical and clinicopathological features, ultrasonographic findings, and abnormal ACTH-stimulation test and low dose dexamethasone suppression test results. A diagnosis of DM was based on appropriate clinical signs and persistent fasting hyperglycemia with concomitant glycosuria. Meningitis of unknown origin was diagnosed based on neurological signs, increased cerebrospinal fluid (CSF) nucleated cell count, and in some cases consistent magnetic resonance imaging (MRI) findings. A diagnosis of IE was made based on clinicopathological findings, CSF analysis, and MRI findings allowing exclusion of reactive seizures and structural epilepsy. A diagnosis of HVA was made by means of abdominal ultrasonography, computed tomography with angiography, and histopathology when required. Hepatitis was diagnosed based on clinicopathological and histopathological findings demonstrating inflammatory changes consistent with chronic hepatitis or cholangitis. Pancreatitis was diagnosed based on the combination of presenting clinical signs, ultrasonographic findings, and serum pancreatic lipase immunoreactivity concentrations. Multicentric lymphoma was diagnosed based on peripheral lymphadenopathy and cytological or histopathological results of the affected nodes. A diagnosis of MMVD was established by means of echocardiographic examination and classified according to the American College of Veterinary Internal Medicine consensus statement (19). Only animals classified as having stage B2 (asymptomatic with radiographic or echocardiographic evidence of cardiac remodeling) or C (with past or current clinical signs of heart failure associated with structural heart disease), were included. A diagnosis of primary IMHA was made based on the presence of spherocytes, positive saline agglutination test, Coombs test, and the exclusion of underlying conditions. A presumptive diagnosis of IMTP was based on the presence of severe thrombocytopenia and the exclusion of other potential causes. Inflammatory bowel disease was diagnosed based on the presence of gastrointestinal signs, hypocobalaminemia and/or low folate concentration, and supportive histopathological findings. Chronic kidney disease was diagnosed based on persistent azotemia (> 3-month duration). The stage of the disease was classified according to the International Renal Interest Society (IRIS). Pneumonia, EBP, and CB were diagnosed based on duration of clinical signs, diagnostic imaging findings, and compatible bronchoalveolar cytological and microbiological findings.
The healthy group comprised dogs being screened for the first time for blood donation purposes during the same time period. They all had unremarkable histories, physical examination findings, and routine hematological and biochemical results.
Hematology
Samples were collected by jugular, cephalic, or saphenous venipuncture into 1.3 mL blood tubes containing K3-EDTA (Sarstedt AG & Co. KG, Nümbrecht, Germany) and all samples were transferred immediately to the UCDVH Diagnostic Laboratory for analysis. On arrival, samples were mixed gently on an automatic roller and a full hematology analysis, including MCV and RDW, was carried out using an ADVIA 2120 analyzer (Siemens Medical Solutions, Fernwald, Germany) within 2 h of collection. Prior to sample analysis, manufacturer-produced control samples were run daily to ensure all parameters were being appropriately measured. In addition, biweekly external quality control scheme (RIQAS) validation was performed.
Statistical analysis
Data were examined for normality using the Shapiro-Wilk test. Non-parametric data were reported as median (range) and parametric data were reported as mean ± standard deviation (SD). In the control group, the association between age, body weight, and RDW, and MCV and RDW were evaluated using Spearman’s correlation test because of non-parametric data distribution in at least one of each group. Comparison among different gender groups [male entire (ME)/neutered (MN), female entire (FE)/neutered (FN)] and the RDW results among all groups were evaluated by means of the Kruskal-Wallis 1-way analysis of variance (ANOVA). To determine which specific subgroups were significantly different from the healthy control group, a Dunn’s multiple comparison post-hoc test was also conducted. The reference interval for RDW was established using the 5% to 95% of values from the healthy group. All statistical analyses were performed using SPSS (IBM Corporation, Armonk, New York, USA) software. Statistical differences were considered significant at P < 0.05. Graphical data were presented using GraphPad Prism software (La Jolla, California, USA).
Results
Study population
The healthy control group included 79 animals and comprised various breeds including crossbreed (n = 23), Labrador retriever (n = 11), golden retriever (n = 9), boxer (n = 4), greyhound (n = 4), German shepherd (n = 4), Siberian husky (n = 3), and other breeds (n = 21) in smaller numbers. Of these dogs, 48 were male (25 ME and 23 MN), and 31 were female (16 FE and 15 FN). The median age was 3 y (range: 1 to 7 y). The mean body weight was 34.4 kg (± 9.12 kg). The mean RDW was 12.8% (± 0.71%). There was no significant correlation between RDW and body weight or age [rs = 0.189, 95% confidence interval (CI): −0.069 to 0.409, P = 0.123; rs = −0.83, 95% CI: −0.340 to 0.156, P = 0.466, respectively]. The reference interval was calculated as 11.7% to 14.3%.
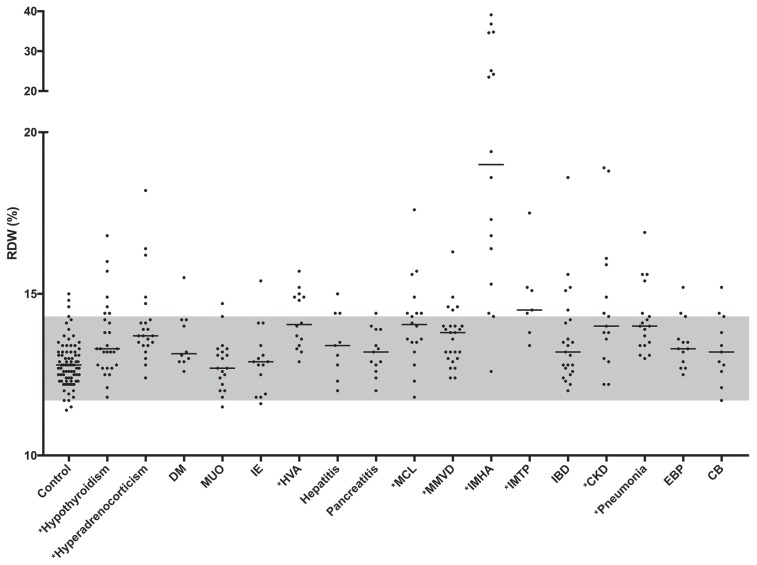
The study group included 276 dogs of different breeds (n = 52) with no one breed representing more than 15% of the total, except for the MMVD group in which Cavalier King Charles spaniels comprised almost 1/3 of the dogs. In total, 145 were male (54 ME and 91 MN) and 131 were female (27 FE and 104 FN). The median age was 7 y (range: 0.3 to 17 y). The animals were grouped as follows: hypothyroidism (n = 28), MMVD (n = 25), IBD (n = 23), HAC (n = 22), MUO (n = 19), pneumonia (n = 18), MCL (n = 18), IMHA (n = 16), CKD (n = 15), IE (n = 15), HVA (n = 14), EBP (n = 13), pancreatitis (n = 13), CB (n = 11), DM (n = 10), hepatitis (n = 9), and IMTP (n = 7). There was a statistically significant (P < 0.0001) difference in RDW values among all groups. When compared to the control group, significantly higher RDW values were found in dogs in the following groups: IMHA, IMTP, HAC, hypothyroidism, HVA, pneumonia, CKD, MCL, and MMVD, but there was considerable overlap among all groups (Table 1, Figure 1).
Table 1.
Dunn’s multiple comparisons of red blood cell distribution width (RDW) between healthy dogs (n = 79) and those with various diseases (n = 276). All results are expressed as median and range. Significant P-values (< 0.05) are in bold.
| Group | Number of animals | RDW | P-value |
|---|---|---|---|
| Hypothyroidism | 28 | 13.3 (11.8 to 16.8) | 0.0220 |
| Hyperadrenocorticism | 22 | 13.7 (12.4 to 18.2) | < 0.0001 |
| Diabetes mellitus | 10 | 13.1 (12.6 to 15.5) | 0.5334 |
| Meningitis of unknown origin | 19 | 12.7 (11.5 to 14.7) | > 0.9999 |
| Idiopathic epilepsy | 15 | 12.9 (11.6 to 15.4) | > 0.9999 |
| Hepatic vascular anomaly | 14 | 14 (12.9 to 15.7) | < 0.0001 |
| Hepatitis | 9 | 13.4 (12 to 15) | > 0.9999 |
| Pancreatitis | 13 | 13.2 (12 to 14.4) | > 0.9999 |
| Multi-centric lymphoma | 18 | 14 (11.8 to 17.6) | 0.0002 |
| Myxomatous mitral valve degeneration | 25 | 13.8 (12.4 to 16.3) | 0.0032 |
| Immune-mediated hemolytic anemia | 16 | 19 (16 to 39.1) | < 0.0001 |
| Immune-mediated thrombocytopenia | 7 | 14.5 (13.4 to 17.5) | 0.0004 |
| Inflammatory bowel disease | 23 | 13.2 (12 to 18.6) | 0.2629 |
| Chronic kidney disease | 15 | 14 (12.2 to 18.9) | 0.0005 |
| Pneumonia | 18 | 14 (13 to 16.9) | < 0.0001 |
| Eosinophilic bronchoneumopathy | 13 | 13.3 (12.5 to 15.2) | 0.3585 |
| Chronic bronchitis | 11 | 13.2 (11.7 to 15.2) | > 0.9999 |
Figure 1.
Red blood cell distribution width (RDW) in all groups. The median value for each group is represented by a solid line. The reference interval established from the healthy control group is represented in gray. Asterisks (*) indicate the groups with significantly increased RDW values. DM — diabetes mellitus; MUO — meningitis of unknown origin; IE — idiopathic epilepsy; HVA — hepatic vascular anomaly; MCL — multicentric lymphona; MMVD — myxomatous mitral valve degeneration; IMHA — immune-mediated hemolytic anemia; IMTP — immune-mediated thrombocytopenia; IBD — inflammatory bowel disease; CKD — chronic kidney disease; EBP — eosinophilic bronchopneumopathy; CB — chronic bronchitis.
The median RDW was above the reference interval in 2 groups of dogs, those diagnosed with IMHA and those diagnosed with IMTP. In the former group, 7 dogs had markedly increased RDW values (range: 23.5% to 39.1%). In all other groups where RDW was significantly increased, the median value remained within the reference interval. In the group with HAC, 5 dogs had mildly to moderately increased RDW (range: 14.7% to 18.2%). Notably, none of them had evidence of anemia, and increased MCV was only observed in 1 animal. In the group with hypothyroidism, increased RDW values were observed in 7 dogs (range: 14.4% to 16.8%), and of these, only 2 dogs had mild non-regenerative, microcytic, normochromic and moderate, normocytic, hyperchromic anemia, respectively. The third dog had increased RDW without evidence of anemia. In the group with HVA, 6 dogs had RDW values above the reference interval (range: 14.8% to 15.7%), and of these, only 2 dogs had evidence of mild non-regenerative anemia, 1 of them being normocytic, normochromic, and the other microcytic and normochromic. In the group with pneumonia, 5 dogs had increased RDW (range: 14.4% to 16.9%); however, anemia was only noted in 2 of them, which was classified as normocytic, normochromic in both cases. In the group with CKD, RDW was increased in 6 dogs (range: 14.4% to 18.9%) and anemia was noted in all of them. All these dogs were classified as having IRIS stage 3 or 4 CKD. In the remaining 9 dogs with reference interval RDW, 1, 2, 5 and 1 dogs were classified as IRIS stage 1, 2, 3, and 4 CKD, respectively. The type of anemia was classified as follows: 4 normocytic, normochromic, 2 normocytic, hyperchromic, and 1 microcytic, normochromic. In the group with MCL, 7 dogs had increased RDW (range: 14.4% to 17.6%), with a mild normocytic, normochromic anemia identified in 1 of them. In the group with MMVD, 4 dogs with MMVD B2 and 1 dog with MMVD C had increased RDW (range: 14.5% to 16.3%), with mild anemia (normocytic, normochromic) identified in only one of them. Notably, this animal had no additional comorbidities at the time of diagnosis.
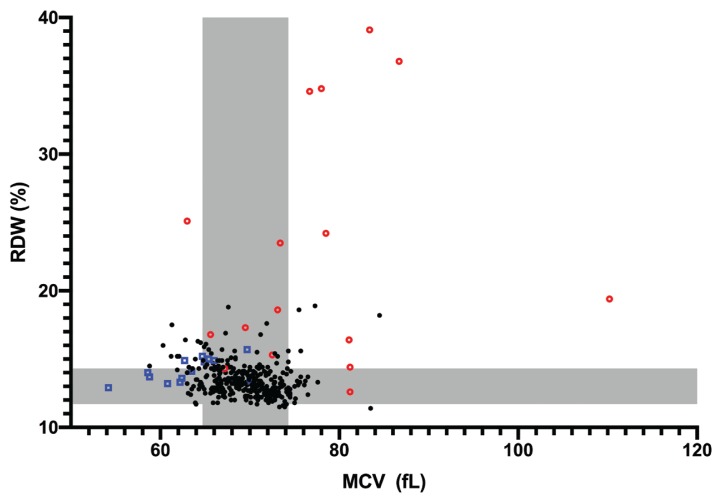
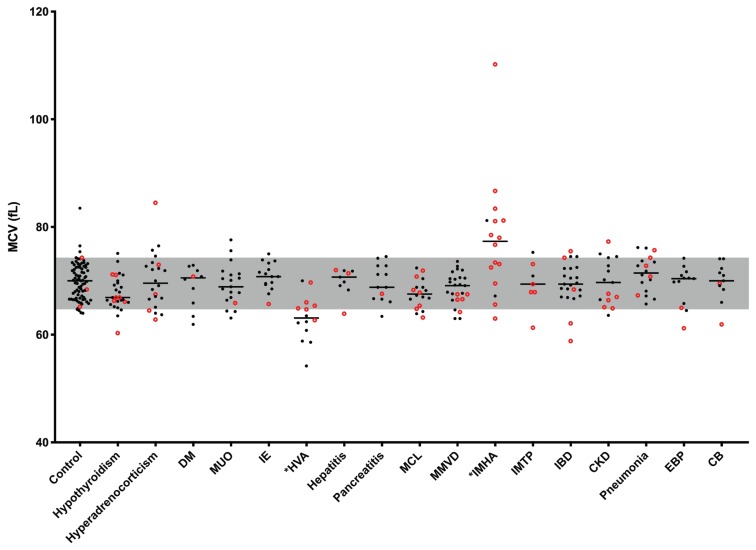
There was a poor but significant negative correlation (rs = −0.183, 95% CI: −0.284 to −0.07, P = 0.0005) between RDW and MCV (Figure 2). The majority of MCV values were within the reference interval in most subgroups despite significantly increased RDW values in several of these groups (Figure 3). Dogs with HVA had significantly (P < 0.0001) lower, MCV values and those with IMHA had significantly (P = 0.0423) higher MCV values. These 2 groups also had median MCV values outside the reference interval. In the HVA group, all the dogs with an MCV below the reference interval had portosystemic shunts and none were anemic. In the IMHA group, severe regenerative anemia with macrocytosis was observed in nearly all dogs, accounting for the increased MCV values. There was no significant correlation between the reticulocyte count and RDW in dogs with IMHA or IMTP (rs = −0.131, 95% CI: −0.524 to 0.310, P = 0.553).
Figure 2.
Correlation between red blood cell distribution width (RDW) and mean corpuscular volume (MCV) values. The reference intervals established from the control group are represented in gray. Blue squares represent dogs with HVA. Red circles represent dogs with IMHA. Abbreviations listed in Figure 1.
Figure 3.
Mean corpuscular volume (MCV) in all groups. The median value for each group is represented by a solid line. The MCV values of dogs with an increased RDW are represented by red circles. The reference interval established from the control group is represented in gray. Asterisks (*) indicate the groups with significantly different values. Abbreviations listed in Figure 1.
Discussion
The RDW has been studied extensively in humans as a readily available quantitative measure of erythrocyte anisocytosis. It is used to augment diagnostic information in anemic patients but can also provide prognostic information in patients with a wide variety of disease conditions. In view of the relative paucity of data on canine RDW, the main aim of this paper was to establish the variation of RDW in cohorts of dogs with various diseases and to compare these results to a healthy control population. Significantly increased RDW values were observed in dogs with cardiorespiratory, renal, neoplastic, hepatic, endocrine, and immune-mediated disease. However, within these groups, there was a broad spread of individual values with the median RDW falling within the reference interval for all except 2 conditions. Dogs with IMHA or IMTP had higher RDW values than those observed in healthy dogs. However, despite this, there was marked individual variation with some dogs having values within the reference interval.
The RDW is derived from the mean and standard deviation of red cell volume. In this study there was a poor but significant correlation between MCV and RDW. This significance likely represents the relatively large sample size and should not be considered of practical importance in light of the low correlation coefficient. Notably, a significant increase in RDW was detected in several groups despite a lack of significant difference in MCV compared with the control group. The poor correlation between MCV and RDW was clearly apparent on visual inspection of the data and was likely due to the dominant influence of heterogeneity in size distribution of erythrocytes on the RDW.
In dogs, congenital hepatic shunts may produce microcytosis with or without anemia, thought to be due to altered iron metabolism resulting from liver dysfunction (20). In agreement with this, dogs in the current study with HVA group had a significantly lower MCV compared with the control group. In theory, this decrease in MCV could lead to an increase in RDW. However, although 6 dogs in the HVA group had increased RDW, MCV was within the reference interval in 5 of these, with only 1 being microcytic. In addition, 7 of the 8 dogs with microcytosis had RDW values within the reference interval. Therefore, the increased RDW observed in these dogs is likely to reflect the degree of anisocytosis rather than the presence of microcytosis. Five of the six dogs with increased RDW were diagnosed with extra- or intra-hepatic portosystemic shunts and none of them had evidence of anemia.
A significant difference between the MCV of cases and controls was also identified in the IMHA group. In contrast to the HVA group, this group had a significantly increased MCV; however, again it appeared that the RDW reflected more than just the increased MCV, with 5 of the 13 dogs with increased values having an MCV within the reference interval. The markedly increased RDW in this group predominantly reflects the presence of macrocytic anemia with a strong regenerative response. However, the possibility of an additional contribution from microcytic poikilocytosis in some cases cannot be excluded. Although spherocytes were present in many of these cases, they were not, in the main, contributing to the increased RDW as the volume of these cells is not typically decreased. Although these cells appear smaller on a blood smear, this apparent reduction in size reflects a decrease in their surface area to volume ratio rather than their volume.
In the group with MCL, almost half of the dogs showed increased RDW; however, only 1 of them had anemia. This increased RDW may be related to the presence of inflammation or malnutrition caused by cancer progression (21). In humans, a higher RDW at diagnosis has been associated with more aggressive and advanced disease and low response rate in Hodgkin lymphoma (22). In a recent study conducted in patients with diffuse large B-cell lymphoma, an increased RDW was established as an independent prognostic marker of poor outcome (23).
In the group with MMVD, RDW values were significantly higher than those in healthy dogs. This contrasts with the single study that had previously evaluated this association, in which no difference was identified (17). In the present study, an increased RDW was observed in 5 dogs with MMVD. Although an association between RDW and disease severity was not calculated because of small sample size, it is interesting to note that more animals with MMVD B2 compared with C1 had increased RDW values. In humans with cardiovascular disorders, RDW is used to predict the onset of heart failure and as a prognostic indicator (24). Proposed underlying mechanisms for the abnormal RDW include microvascular disorders, anemia, presence of inflammatory cytokines, oxidative stress, increased concentration of free cholesterol, thrombosis, nutritional deficiencies, concomitant renal dysfunction, and activation of the neuro-humoral and adrenergic systems secondary to decreased erythropoietin concentration (17,25).
The RDW values were significantly higher in the dogs with CKD. In humans with CKD, RDW is significantly associated with the stage of the disease (26). In CKD, the gradual decline of renal erythropoietin synthesis and hyporesponsiveness are thought to be responsible for the reduced production of RBCs and the formation of erythrocytes of different sizes, ultimately impacting on the RDW (27).
The RDW was evaluated in 3 respiratory disorders, including bacterial pneumonia, CB, and EBP. However, the RDW was only significantly increased in the group with pneumonia. In a recent human study, patients with community-acquired pneumonia presenting with increased RDW were more likely to have complicated admissions, hospitalizations, and increased 30- and 90-day mortalities (6). Although the exact mechanism of association between increased RDW and pulmonary disease has not been identified, inflammation and oxidative stress have been proposed as possible causes (28).
The RDW was significantly higher in dogs with hypothyroidism and HAC. In the former subgroup, anemia was identified in almost half of the dogs. Anemia is known to develop in approximately 40% to 50% of hypothyroid dogs, presumably resulting from decreased production of erythropoietin and lack of a direct stimulatory effect of thyroid hormones on bone marrow (29–31). Thyroid hormones play an important physiological role in erythropoiesis by increasing the secretion of erythropoietin (32). In both humans and dogs, thyroid hormone deficiency can substantially influence the size variability of circulating RBCs, and thus RDW (29,30,32). To the authors’ knowledge, there are no current publications reviewing the relationship between RDW and HAC.
There was no significant difference in RDW between the healthy group and the dogs diagnosed with DM, MUO, IE, hepatitis, pancreatitis, and IBD, in contrast to the corresponding human diseases. Evaluation of RDW in human patients with DM revealed that elevated RDW values were associated with higher and lower prevalence of disease in different studies (33–36). No studies evaluating the relationship between RDW and MUO were found. In human patients with IE, an increased RDW is considered valuable as it can be used as both a surrogate marker of long-term memory of hypoxemia and as an independent marker of seizure status (37). In human liver disease, RDW is an independent prognostic parameter of 3-month mortality. Moreover, it has been reported to be significantly increased in patients with non-alcoholic fatty liver disease and hepatic malignancy, and is also strongly correlated with the severity of hepatic fibrosis (6). Proposed mechanisms to explain the increased RDW in hepatic disease include down-regulation of erythropoietin receptor expression, chronic inflammation, nutritional deficiencies, and increased red cell destruction (38). In pancreatic disorders, 1 study reported that RDW was significantly higher in human patients with acute pancreatitis during hospital admission (8). The RDW has been also reported to be a sensitive predictor of mortality in these patients (6).
In the healthy control group presented here, no association was found between RDW and age, body weight, or gender. These results are in agreement with a recent study in healthy dogs and in dogs with MMVD (17). However, it should be noted that only large breed dogs were assessed in the healthy group, thus limiting the likelihood of identifying a possible correlation between RDW and body weight.
Hematological tests in this study were performed using the ADVIA 2120 (Siemens Medical Solutions). Considerable variation among different analyzers has been reported for multiple hematological parameters, including RDW. In a recent human study, RDW values varied between different hematology analyzers with bias always exceeding the desirable quality specifications (e.g., ± 1.7%) (39). This limits the comparability of this parameter among studies and hinders the use of identical reference ranges and decisional thresholds across different clinical investigations. To date, a validated reference range for canine RDW has not been published for the ADVIA 2120. An excellent to fair agreement for multiple hematological parameters was observed when the ADVIA 120 and 2120 models were compared but RDW was not evaluated (40). Thus, the current reference interval derived for RDW in healthy dogs cannot be extrapolated to results from other hematology analyzers.
This study had several limitations. Firstly, the study was not longitudinal, and all variables, including RDW, were assessed with a single measurement, which might have been influenced by biological variability or measurement error. Secondly, the small sample size among some of the subgroups might have precluded the identification of a difference resulting in a type II error. Thirdly, although none of the breeds known to have abnormal RDW were included, both groups included multiple breeds and, therefore, the possibility of breed-related RDW variation cannot be excluded. Lastly, RDW values are largely dependent on the methodology and measurement technique employed and this is an important limitation for comparability of RDW values between different studies.
In conclusion, RDW is a simple, routinely calculated hematological parameter that is associated with several disease processes. In this study, RDW values were significantly higher in dogs with IMHA, IMTP, HAC, hypothyroidism, HVA, pneumonia, CKD, MCL, and MMVD; however, there was a broad overlap with control values for individual dogs. The role and prognostic significance of RDW in these and other pathologies remain to be determined. Increased RDW values were associated with a more advanced stage of CKD and with the presence of pulmonary bacterial infection in dogs with respiratory disease. Extremely increased RDW values support the diagnosis of IMHA, reflecting the presence of macrocytic anemia. Additional studies are necessary to establish reference intervals in dogs with further consideration of age, breed and body weight, and to further evaluate the diagnostic value and prognostic significance of RDW in the specific diseases mentioned. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. 1991;9:71–74. doi: 10.1016/0736-4679(91)90592-4. [DOI] [PubMed] [Google Scholar]
- 2.Cheng CK, Chan J, Cembrowski GS, Assendelft OW. Complete blood count reference interval diagrams derived from NHANES III: Stratification by age, sex, and race. Lab Hematol. 2004;10:42–53. [PubMed] [Google Scholar]
- 3.Patel KV, Semba RD, Ferrucci L, et al. Red cell distribution width and mortality in older adults: A meta-analysis. J Gerontol A Biol Sci Med Sci. 2010;65:258–265. doi: 10.1093/gerona/glp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saxena S, Wong ET. Heterogeneity of common hematologic parameters among racial, ethnic, and gender subgroups. Arch Pathol Lab Med. 1990;114:715–719. [PubMed] [Google Scholar]
- 5.Lippi G, Cervellin G, Favaloro EJ, Plebani M. In Vitro and In Vivo Hemolysis: An Unresolved Dispute in Laboratory medicine. Belin, Boston: De Gruyter; 2012. Red blood cell distribution width; p. 4. [Google Scholar]
- 6.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 7.Shehata HA, Ali MM, Evans-Jones JC, Upton GJ, Manyonda IT. Red cell distribution width (RDW) changes in pregnancy. Int J Gynaecol Obstet. 1998;62:43–46. doi: 10.1016/s0020-7292(98)00069-1. [DOI] [PubMed] [Google Scholar]
- 8.Karabulut KU, Narci H, Uçar Y, Uyar M. Association between red blood cell distribution width and acute pancreatitis. Med Sci Monit. 2014;27:2448–2452. doi: 10.12659/MSM.891075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balta S, Demirkol S, Cakar M, Aydogan M, Akhan M. The red cell distribution width may be affected by many factors in the clinical practice. J Clin Diagn Res. 2013;7:1830. doi: 10.7860/JCDR/2013/6007.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fici F, Celik T, Balta S, et al. Comparative effects of nebivolol and metoprolol on red cell distribution width and neutrophil/lymphocyte ratio in patients with newly diagnosed essential hypertension. J Cardiovasc Pharmacol. 2013;62:388–393. doi: 10.1097/FJC.0b013e31829f716a. [DOI] [PubMed] [Google Scholar]
- 11.Yu HM, Park KS, Lee JM. The value of red blood cell distribution width in subclinical hypothyroidism. Arq Bras Endocrinol Metabol. 2014;58:30–36. doi: 10.1590/0004-2730000002836. [DOI] [PubMed] [Google Scholar]
- 12.Aniolek O, Barc A, Jarosinka A, Gajewski Z. Evaluation of frequency and intensity of asymptomatic anisocytosis in the Japanese dog breeds Shiba, Akita, and Hokkaido. Acta Vet Brno. 2017;86:385–391. [Google Scholar]
- 13.Perret D, Trumel C, Diquélou A, Dossin O, Guelfi JF. Líndice de distribution des globules rouges (IDR) chez le chien. Analyse de 1400 cas [Red blood cell distribution width (RDW) in dogs. Analysis of 1400 cases] Revue Méd Vét. 2001;152:549–554. [Google Scholar]
- 14.Bonfanti U, Comazzi S, Paltrinieri S, Bertazzolo W. Stomatocytosis in 7 related standard schnauzers. Vet Clin Pathol. 2004;33:234–239. doi: 10.1111/j.1939-165x.2004.tb00379.x. [DOI] [PubMed] [Google Scholar]
- 15.Neiger R, Hadley J, Pfeiffer DU. Differentiation of dogs with regenerative and non-regenerative anaemia on the basis of their red cell distribution width and mean corpuscular volume. Vet Rec. 2002;150:431–434. doi: 10.1136/vr.150.14.431. [DOI] [PubMed] [Google Scholar]
- 16.Hodges J, Christopher MM. Diagnostic accuracy of using erythrocyte indices and polychromasia to identify regenerative anemia in dogs. J Am Vet Med Assoc. 2011;238:1452–1458. doi: 10.2460/javma.238.11.1452. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmini C, Poser H, Pria AD, et al. Red blood cell distribution width in dogs with chronic degenerative valvular disease. J Am Vet Med Assoc. 2013;243:1806–1815. doi: 10.2460/javma.243.6.858. [DOI] [PubMed] [Google Scholar]
- 18.Swann JW, Sudunagunta S, Covey HL, English K, Hendricks A, Connolly DJ. Evaluation of red cell distribution width in dogs with pulmonary hypertension. J Vet Cardiol. 2014;16:227–235. doi: 10.1016/j.jvc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med. 2009;23:1142–1150. doi: 10.1111/j.1939-1676.2009.0392.x. [DOI] [PubMed] [Google Scholar]
- 20.Laflamme DP, Mahaffey EA, Allen SW, Twedt DC, Prasse KW, Huber TL. Microcytosis and iron status in dogs with surgically induced portosystemic shunts. J Vet Intern Med. 1994;8:212–216. doi: 10.1111/j.1939-1676.1994.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S, Fang F, Chen H, et al. Prognostic significance of the red blood cell distribution width in diffuse large B-cell lymphoma patients. Oncotarget. 2017;8:40724–40731. doi: 10.18632/oncotarget.16560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez Andrade B, Robredo B, Sartori F, et al. Red cell distribution width (RDW) at diagnosis is associated to advanced stage, worse response and poor prognosis in Hodgkin lymphoma. Blood. 2016;128:5373. [Google Scholar]
- 23.Perisa V, Zibar L, Sincic-Petricevic J, Knezovic A, Perisa I, Barbic J. Red blood cell distribution width as a simple negative prognostic factor in patients with diffuse large B-cell lymphoma: A retrospective study. Croat Med J. 2015;56:334–343. doi: 10.3325/cmj.2015.56.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippi G, Cervellin G. Risk assessment of post-infarction heart failure. Systematic review on the role of emerging biomarkers. Crit Rev Clin Lab Sci. 2014;51:13–29. doi: 10.3109/10408363.2013.863267. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Zhoy H, Tang Q. Red blood cell distribution width: A novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers. 2017;2017 doi: 10.1155/2017/7089493. 7089493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solak Y, Yilmaz MI, Saglam M, et al. Red cell distribution width is independently related to endothelial dysfunction in patients with chronic kidney disease. Am J Med Sci. 2014;347:118–124. doi: 10.1097/MAJ.0b013e3182996a96. [DOI] [PubMed] [Google Scholar]
- 27.Kario K, Matsuo T, Nakao K, Yamaguchi N. The correlation between red cell distribution width and serum erythropoietin titres. Clin Lab Haematol. 1991;13:222–223. doi: 10.1111/j.1365-2257.1991.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Lee JH, Kim K, et al. The clinical significance of changes in red blood cell distribution width in patients with community-acquired pneumonia. Clin Exp Emerg Med. 2016;3(3):139–147. doi: 10.15441/ceem.15.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mooney CT. Canine hypothyroidism: A review of aetiology and diagnosis. N Z Vet J. 2011;59:105–114. doi: 10.1080/00480169.2011.563729. [DOI] [PubMed] [Google Scholar]
- 30.Panciera D. Conditions associated with canine hypothyroidism. Vet Clin N Am Small Anim Pract. 2001;31:935–950. [PubMed] [Google Scholar]
- 31.Dixon RM, 1, Reid SW, Mooney CT. Epidemiological, clinical, haematological and biochemical characteristics of canine hypothyroidism. Vet Rec. 1999;145:481–487. doi: 10.1136/vr.145.17.481. [DOI] [PubMed] [Google Scholar]
- 32.Geetha JP, Srikrishna R. Role of red blood cell distribution width in thyroid dysfunction. Int J Biol Med Res. 2012;3:1476–1478. [Google Scholar]
- 33.Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arbel Y, Weitzman D, Raz R, et al. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality. A population-based study. Thromb Haemost. 2014;111:300–307. doi: 10.1160/TH13-07-0567. [DOI] [PubMed] [Google Scholar]
- 35.Engstrom G, Smith JG, Persson M, Nilsson PM, Melander O, Hedlad B. Red cell distribution width, haemoglobin A1C and incidence of diabetes mellitus. J Intern Med. 2014;276:174–183. doi: 10.1111/joim.12188. [DOI] [PubMed] [Google Scholar]
- 36.Chen PC, Sung FC, Chien KL, Hsu HC, Lee YT. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. Am J Epidemiol. 2010;171:214–220. doi: 10.1093/aje/kwp360. [DOI] [PubMed] [Google Scholar]
- 37.Eroglu T, Turkoglu AS, Bolac ES, Yildiz S, Yildiz N. Hemogram parameters in epilepsy may be indicators of chronic inflammation and hypoxemia. J Neurol Clin Neurosci. 2017;1:17–20. [Google Scholar]
- 38.Cengiz M, Candir BA, Yilmaz G, Akyol G, Ozenirler S. Is increased red cell distribution width an indicating marker of nonalcoholic steatohepatitis and fibrotic stage? World J Gastroenterol. 2013;19:7412–7418. doi: 10.3748/wjg.v19.i42.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lippi G, Pavesi F, Bardi M, Pipitone S. Lack of harmonization of red blood cell distribution width (RDW). Evaluation of four hematological analyzers. Clin Biochem. 2014;47:1100–1103. doi: 10.1016/j.clinbiochem.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Bauer N, Moritz A. Evaluation of three methods for measurement of hemoglobin and calculated hemoglobin parameters with the ADVIA 2120 and ADVIA 120 in dogs, cats, and horses. Vet Clin Pathol. 2008;37:173–179. doi: 10.1111/j.1939-165X.2008.00039.x. [DOI] [PubMed] [Google Scholar]