Abstract
Early cancer detection, its monitoring, and therapeutical prediction are highly valuable, though extremely challenging targets in oncology. Significant progress has been made recently, resulting in a group of devices and techniques that are now capable of successfully detecting, interpreting, and monitoring cancer biomarkers in body fluids. Precise information about malignancies can be obtained from liquid biopsies by isolating and analyzing circulating tumor cells (CTCs) or nucleic acids, tumor-derived vesicles or proteins, and metabolites. The current work provides a general overview of the latest on-chip technological developments for cancer liquid biopsy. Current challenges for their translation and their application in various clinical settings are discussed. Microfluidic solutions for each set of biomarkers are compared, and a global overview of the major trends and ongoing research challenges is given. A detailed analysis of the microfluidic isolation of CTCs with recent efforts that aimed at increasing purity and capture efficiency is provided as well. Although CTCs have been the focus of a vast microfluidic research effort as the key element for obtaining relevant information, important clinical insights can also be achieved from alternative biomarkers, such as classical protein biomarkers, exosomes, or circulating-free nucleic acids. Finally, while most work has been devoted to the analysis of blood-based biomarkers, we highlight the less explored potential of urine as an ideal source of molecular cancer biomarkers for point-of-care lab-on-chip devices.
I. INTRODUCTION
Worldwide, cancer kills one person every 4 s.1 Its detection in the early stage can improve the prognosis and the efficacy of the surgical and chemotherapeutic protocols. Moreover, treating malignancies requires precise monitoring and prediction of the therapeutic responses. As a result, the discovery of reliable cancer indicators or biomarkers must be correlated with the development and implementation of new chip-based cancer diagnostic technologies.
One of the new approaches employed for this purpose is “liquid biopsy,” the collection of small amounts of body fluids for laboratory examination. Practically, liquid biopsy presents several advantages from the patient's and clinician's perspectives. The benefits to patients stem from the minimal invasiveness, significant reduction of the patient's discomfort, the possibility to be performed in patients in whom invasive sampling via typical tissue biopsies is contraindicated due to the high invasiveness of the tumor [e.g., non-small cell lung cancer (NSCLC), pancreatic cancer, prenatal diagnosis of cancer], or when inadequate quantities of tumor samples are available. Liquid biopsy also presents minimal risks for the medical professionals who collect the samples: the procedures are usually simple, easy to perform, or associated with strict standard protocols and personal protective equipment (PPE) that shield the specialists and prevent direct contact with the biofluid collected. Moreover, it is a fresh tumor-derived material, free of preservatives, that can be more quickly processed.2,3 The shorter turnaround time decreases the time between sampling and treatment administration, an interval considered crucial to avoid or minimize the alteration of the tumor's genetic composition.2,4 Clinically, liquid biopsy can provide the same genetic information as the tissue sample plus the global molecular “status” of the patient.5,6 This additional information can address the challenge imposed by the tissue heterogeneity. Since liquid biopsy is primarily used to analyze cell-free nucleic acids in peripheral blood and other bodily fluids, such as cerebrospinal fluid, urine, and ascites fluid, it is used for genetic analysis of tissues with high cell turnover. For instance, circulating-free tumor DNA (ctDNA) originates from all tumor lesions/cells and can be isolated from biofluids from different body compartments to contribute to tumor diagnostics: it shows the intra- and intertumor genetic heterogeneity associated with tumor staging, the multiple loci, metastasis, vascularization, and size. Moreover, cfDNA originates from normal regenerating tissues in various states, such as hematopoiesis, immune reactions, inflammatory processes, and hypoxia-related necrosis. Therefore, an important possible clinical implication for future personalized medicine is the fact that the samples contain many biocomponents that can provide vital information about the cancer's biomarkers.
Since liquid biopsy can be highly informative compared with tissue biopsy samples, the isolation and characterization of specific cancer biomarkers have real potential to effectively support the cancer diagnosis, treatment, and disease monitoring. Furthermore, follow-up during remission, prediction, and characterization of the treatment effects, accurate estimation of the acquired resistance, and detection of somatic and epigenetic alterations, stratification, and pharmacodynamics are also possible. The above arguments regarding the analysis of liquid biopsy components have recently led to major new findings,7 which may lead the way toward a new understanding of translational efforts. In this direction, microfluidic devices are ideal candidates to boost translation of biomarkers to the clinical level. The design and engineering of these devices need to consider variables defined mostly by the intended application (isolation or analysis), the type of biomarkers, and their source and intended clinical use. The present review discusses technologies, recent advances, and the potential for miniaturization taking into consideration all major variables of testing molecular cancer biomarkers: two major on-chip functions (isolation and analysis), five major classes of tumor-derived biomarkers, two main sources (blood and urine), two sources that are specific for a limited panel of cancers (feces/stool and saliva/sputum), and five main applications in the clinical setting. Each variable is discussed with an emphasis on recent advances and the potential for on-chip miniaturization.
II. CANCER LIQUID BIOPSY: BIOMARKERS FROM BIOFLUIDS
A. Biomarkers
Biocomponents that can be used as specific and relevant indicators for cancer research are present in liquid biopsy samples in various formats that can be grouped according to their size as (1) cellular aggregates (circulating tumor microemboli), (2) free cells (circulating tumor cells, circulating endothelial progenitor cells, and cancer stem cells), (3) platelets and cellular vesicles (exosomes), and (4) macro- and nanomolecules (nucleic acids and proteins).
“Circulating tumor clusters” are defined as clusters of circulating tumor cells (CTCls). Little is known about the biological properties, composition, or metastatic capability of these clusters comparative to those of single CTCs. They seem to have several unexpected features, such as a lack of either apoptotic cells indicating a survival advantage or of proliferating cells which may make them less susceptible to the effects of chemotherapy.8 Other authors have also highlighted the higher capacity for seeding distant metastases and considered that the clusters of CTCs may be related to a worse clinical outcome.4,9 Cima et al.10 discovered a distinct population of cell clusters that circulated in the blood of colorectal cancer patients: tumor-derived endothelial cells. These clusters expressed both epithelial and mesenchymal markers that are consistent with CTCs phenotyping.
“Circulating tumor cells (CTCs)” are tumor-derived cells that have separated from tumor tissue and entered the blood circulation. Biochemically, in the simplest definition, a cell is a CTC if it coexpresses the epithelial-cell-adhesion molecule (EpCAM) and cytokeratin(s) (CKs), as evidenced in metastatic stages of prostate, colon, and breast cancers,11 and misses the leukocyte common antigen CD45. CTCs, upon staining with 4′,6-diamidino-2-phenylindole (DAPI) are nucleated entities since the integrity of the nucleus is demonstrated by this procedure. However, to be defined as CTC cells have to express genomic alterations as well.
“Circulating endothelial progenitor cells (CEPCs)” are capable of inducing tumor vascularization, a major hallmark of cancer metastasis. The level of CEPCs, similar to the prognostic importance of CTCs, is correlated with poorer overall survival (OS), recurrence-free survival (RFS), and response duration (RD) but not associated with progression-free survival (PFS). However, the definition of CEPCs is not universally accepted; their detection is challenging and large-scale high-quality clinical studies should be conducted to clarify their correlation with the clinically important outcomes.12
“Cancer stem cells (CSCs)” are now considered critical to the metastatic process. Also, their tumorigenic capacity is significant compared with other malignant cells belonging to the same tumor. CSCs have autorenewal and differentiation capability. Therefore, they can generate non-CSC progenies and are capable of unlimited proliferation.13–15 Similar to CTCs, CSCs have specific biomarkers: the aldehyde dehydrogenase 1 (ALDH1), the EpCAM/CD44/CD47/MET, the CEA/CK/CD133 mRNA, and the CD44high/CD24low phenotype expressed.4,15
“Platelets” are considered another relevant liquid biopsy biocomponent with an important role in tumorigenesis. Recent literature consolidates the roles of platelets in wound healing and tissue remodeling, while introducing and highlighting new aspects: the role of platelets as conveyors of systemic signals, the selective release of their bioactive cargo, and their role in tumor metastasis and progression.16,17
“Exosomes” are small vesicles enveloped by membranes and comprise functional biomolecules (such as proteins, RNA, and DNA) most likely discharged by viable tumor cells.7,11 However, exosomes derive from multiple cell types, share several protein markers, or transport a small proportion of proteins further related to the physiological and pathophysiological conditions of the secretory cell.
“Circulating tumor nucleic acids (ctNAs)” comprise “cell-free DNA (cfDNA),” “microRNA (miRNA),” and “cell-free miRNAs (cfmiRNAs).” Cell-free DNA (cfDNA) is fragmented DNA of cancer cells. The release may be passive after apoptosis, phagocytosis or cellular necrosis, or active from living cells.18 Circulating tumor DNA (ctDNA) of direct clinical relevance for cancer study represents only a part of cfDNA. This proportion can be quantified and this value can also be one useful indicator of the patient's status.2 Siravegna et al.19 while focusing on ctDNA examined how different forms of liquid biopsy can be exploited to guide patient care and eventually integrated into clinical practice. MicroRNA (miRNA) refers to fragments of single-stranded noncoding RNA of 19–25 nucleotides, resulting from hairpin precursor molecules that comprise 70–120 nucleotides. Cell-free miRNAs (cfmiRNAs) have been shown to be present in blood, urine, and other biofluids and have excellent stability during storage and handling.
“Proteins”11 as circulating soluble protein tumor markers (CEA, PSA, CA125, the MUC-1 antigens CA15-3 and CA27.29, and CA19-9) are possible clinical indicators of different types of cancer therapeutic responses.
B. Biofluids
The analysis of these liquid biopsy components can reveal substantial information about the intrapatient tumor heterogeneity and supply a partial or complete genomic landscape of all cancerous lesions (primary and metastases). Additionally, recent technological advances have enabled researchers to obtain precious data about tumor-linked genetic alterations to identify genetic and epigenetic aberrations and track genomic evolution.2,4
This was possible because cancer biomarkers can be found in different body fluids secreted or excreted by the body, physiologically and pathologically. Therefore, the biofluids commonly used in investigations and in liquid biopsies include blood, urine, and saliva. The biomarkers secreted in biological fluids are either in free form, in exfoliated cells, or other membranes. The drawbacks of body fluid analysis include the scarcity and fast degradation of the biomarkers in the sample and also the lack of marker specificity.
“Blood” is an essential biofluid capable of providing information related to diverse physiological and pathological events. Peripheral blood (PB) is typically the liquid biopsy of choice because it is a well-defined material, it can easily be collected upon obtaining the informed consent from the patients, and it can be collected frequently based upon the testing requests.20 Therefore, CTCs and exosomes from blood are considered biological circulating transporters of signal molecules representing the cells of origin and have great potential as noninvasive diagnostic tools.
“Urine”-based tests could offer large-scale screening for biomarkers, as urine is one readily collectable relatively cell-free biofluid. To support this option, several proteomic studies have presented various methods to reveal the urinary extracellular vesicles and related proteins and to correlate them to unique malignancies and to a broad range of urogenital diseases.21,22 For instance, the ExoMir Kit uses a filter-based approach to capture exosomes from cell-free fluid samples. The captured particles are lysed to release their contents and to extract RNA for further identification of biomarkers for prostate cancer.23 Moreover, the NanoSight microarray was employed in conjunction with ultracentrifugation and PCR to identify specific urinary extracellular vesicles derived biomarkers for urinary bladder cancer.24 Several groups25,26 also reviewed new methods of exosome-based diagnostics and discussed the challenges and the opportunities of using urine for fluid biopsy analysis.
“Saliva” is also a readily accessible biofluid which provides important information about a wide range of diseases from viral and bacterial infections to cardio, autoimmune, and renal diseases and even cancer.27 It has the major advantage of easy collection in sufficient quantities, with minimal anxiety and discomfort for the patients. Saliva was already used as liquid biopsy for cancer detection using LOC (lab on a chip) devices. Sugimoto et al.28 used capillary electrophoresis time-of-flight mass spectrometry (CE-TOF-MS) to develop a metabolic profile of oral, breast, and pancreatic cancer patients, periodontal disease patients, as well as healthy control volunteers. The saliva samples were analyzed without pretreatment (except for centrifugation, to remove any solid particles). The study results suggested that “cancer-specific signatures are embedded in saliva metabolites.” Jokerst et al.29 also proposed a microfluidic platform (the Nano-bio-chip system) for the measurement of CEA, CA125, and Her-2/Neu (CerbB-2), as cancer markers isolated from serum and saliva samples. They used gold nanoparticles (NPs) as the detection element. Zilberman and Sonkusale30 proposed an optical microfluidic system to diagnose gastric cancer using saliva as a liquid biopsy. Their microfluidic system with embedded sensors quantified the body fluids by-products (NH3 and CO2) of a urea-processing enzyme secreted from Helicobacter pylori. However, the low levels of salivary biomarkers combined with the heterogeneity of the sample matrix and the high level of mucins and proteolytic enzymes31 make differentiation between background and target-specific signals difficult.29
“Blood vs urine”: indeed, urine emerges as an intriguing alternative source of cfDNA besides plasma or serum. Not only is it less invasive while easily available in much larger sample volumes than blood, but it also yields overall comparable results in terms of cfDNA yields and ctDNA biomarker detection.32,33 Interestingly, this trend appears to be consistent among different malignancies such as breast, prostate, urinary bladder cancer, and non-small cell lung cancer (NSCLC). The similar results are independent of the method used for nucleic acid isolation and type of biomarker, indicating a potential universal use of urine for cancer detection. Under certain circumstances, analyzing urine appears even more attractive than plasma analysis. For instance, there are cases that require large quantities of body fluids for PCR-based detection of Kras mutations, which suggest that the inhibitory factors in serum/plasma may be more limiting than those in urine.34 Table I presents various studies comparing the efficiency of plasma vs urine sampling. Because of the attractiveness of on-chip devices for point-of-care (POC) applications, the use of urine as the main fluid from which tumor-derived biomarkers such as ctDNA and vesicles can be analyzed is surprisingly under-represented in LOC studies (compared to studies dealing with blood-based cancer biomarkers). Since the majority of LOC-based reported studies employed peripheral blood samples, the present review will further develop this aspect and introduce relevant examples.
TABLE I.
Comparative study: plasma vs urine.
| Disease | Biomarker | Isolation method | Detection method | Result urine | Results plasma/serum | Comparison plasma vs urine | Reference |
|---|---|---|---|---|---|---|---|
| Bladder urothelial carcinoma | Total DNA | GFX™ kit (Amersham Pharmacia Biotech) (urine) | GeneQuant RNA/DNA Calculator—Amersham Pharm. Biotech | 34–107 ng/ml | 30–106 ng/ml | Comparable levels of total cfDNA | 32 |
| Breast cancer | Total DNA methylated promoters of RASSF1A andRAR?2 genes | Patented glass-filters (silica) | Methylation-specific PCR(MSP) | DNA 6–50 ng/ml, 90% concordance with plasma | N/A | Comparable methylation detection | 35 |
| Colorectal carcinoma or adenomatous polyps | Total DNA Frequency of detectable mutated Kras DNA | Resin binding (Wizard Plus Mini-Prep DNA Purification System) | Restriction enriched polymerase chain reaction | 23.7 ng/ml Sensitivity 95% | 7.4 ng/ml Sensitivity 40% | Concentration of DNA in plasma was significantly lower than in either serum or urine. The incidence of mutated Kras DNA detected in urine was significantly higher than in either plasma or serum. | 34 |
| NSCLC | EGFR mutations | QIAamp DNA Circulating Nucleic Acid Kit | Next-generation sequencing analysis of EGFR mutations | Specificity 72%–92% | Specificity 87%–100% | Comparable sensitivity of EGFR mutation detection between blood and urine | 33 |
| NSCLC | EGFR mutations | Qiagen's QIAamp Circulating Nuclei Acid kit | Droplet digital PCR | 6.1 ng/ml 88% concordance with tissue 98% concordance with plasma | 2.31 ng/ml | Urinary ctDNA yielded close correlation of EGFR mutation status when compared to baseline primary tissue. Virtually all samples detected via urine specimens were uncovered in plasma samples. | 36 |
| Prostate cancer | GSTP1 promoter hypermethylation | QIAamp RNA Viral Kit | Methylation-specific PCR | Sensitivity 72% | Sensitivity 77% | GSTP1 promoter hypermethylation was found in 90% of tumors, 72% of plasma or serum samples, 76% of urine from patients with prostate cancer. | 37 |
III. CTCS
A. Relevance of CTCs
CTCs can be isolated and identified morphologically and genetically.2,15 Their distinct phenotypic and genomic features were considered crucial for the local and distant tumors' dissemination (micro and macrometastasis).38 However, it is still unclear whether (1) the release of CTCs into the blood circulation is an accidental or a programmed course of biological events,7 (2) the self-seeding and metastasis destruction by the primary tumor itself are valid steps,7,14 or (3) all CTCs have indeed cancer-seeding capabilities.14
Nevertheless, the detection of CTCs is considered a consistent estimation of the metastatic risk and one possible early warning signal39,40 in esophageal adenocarcinoma41 or prostate cancer.42 CTCs detection has prognostic value in stages III and IV cutaneous malignant melanoma.52 In patients with Ewing,53 and gastro-oesophageal tumors,41 identifying CTCs was an interpreter of the disease development and a decisive evidence toward role of CTCs as a marker of the survival rate and disease progression. Furthermore, the CTCs count may detect the patients with an aggressive tumor and monitor the malignant progress.43,44 Clinical studies45 also evidenced the role CTCs play in patients' classification upon the disease prognostic.41 For instance, in hepatocellular carcinoma patients, CTCs identification and count were strongly correlated with the tumor aggressiveness and degree.46 The CTCs count can further predict: the progression-free survival (PFS) and overall survival (OS) in metastatic neuroendocrine tumors (NETs),47 in breast cancer,48 in colorectal cancer patients,49 the disease-free survival (DFS) and OS in breast cancer,50 the metastasis-free survival (MFS) in stage II and III breast cancer,51 the PFS in early breast cancer, in metastatic breast cancer, in lung cancer, gastric cancer, nonmetastatic colorectal cancer testicular germ cell tumors, esophageal cancer, and squamous cell carcinoma of the oral cavity,15 or the DFS in progressive head and neck squamous cell carcinoma (HNSCC).43 While the direct proportionality between the post-therapy CTCs count and OS was noteworthy in patients with metastatic pancreatic cancer,54 combining the CTCs enumeration with LDH levels was considered as the first indicative biomarker for the survival rate in castration-resistant prostate cancer (CRPC) patients.55 Other studies reported a significant association between the CTCs count, the progressive disease (PD) and the OS of patients with metastatic neuroendocrine neoplasms (NENs),59 esophageal adenocarcinoma (EAC),41 colorectal cancer (CRC),60 and hepatocellular carcinoma.46
Besides the diagnostic and staging value, the CTCs count can significantly contribute to the evaluation of therapeutic efficiency13 and to the cancer drugs screening.44 In this direction, Yap et al.56 discussed the key technical aspects of CTCs as a biomarker in pharmacodynamics (PD), and as a prognostic and predictive factor for breast, colorectal, and prostate cancers. Smith et al.57 compared Quantitative Polymerase Chain Reaction (QPCR) and Immunocytochemical Techniques (ICCTs) as CTCs detection methods in patients with progressive, locally advanced, and metastatic breast cancer (all stage IV) prior the start of systemic treatment. They reported that both methods reflected the results of systemic treatment and the clinical development (progression or regression) of the disease. It is important to mention that research groups developed CTC-based platforms for rapid evaluation of individualized susceptibility to drugs,44 and for real-time prediction of resistance to hormone therapy in patients with HoR+ MBC.58 Therefore, the CTCs count may be used to estimate of PFS and biological behavior of cancer, actively helping clinicians in diagnosis and pre- or post-therapy histological evaluation.
B. Challenges in CTCs isolation
Several reasons make the isolation/separation of CTCs from a biological sample and their subsequent studies delicate and difficult processes.61
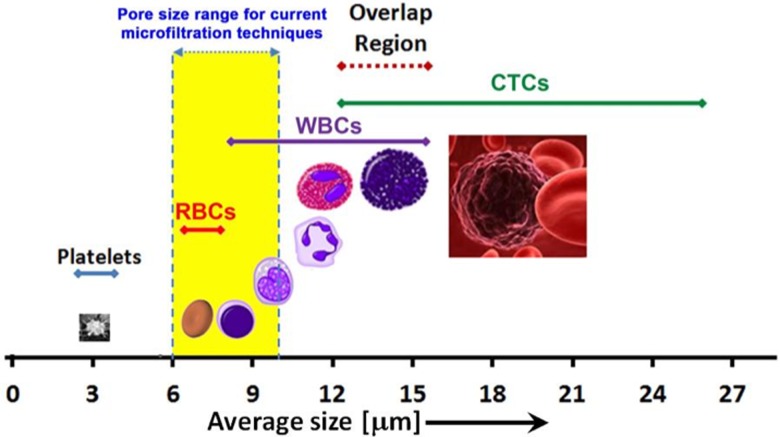
The large morphological heterogeneity of CTCs makes their separation (and definition) extremely difficult. The numbers, sizes, and densities of different types of cells present in the blood are associated with variations in the CTCs count from one type of cancer to another (Table II).9 Moreover, the CTCs count is even lower in the incipient stages of cancer and explains a statistical distribution associated with a high rate of false negative results.62 Cellular variations due to the presence of apoptotic and small CTCs,56 of unnucleated cells, necrotic cells, and even cellular fragments would also diminish significantly the efficiency of capturing all CTCs when the traditional identification methods, such as cytopathology or immunofluorescence staining using FISH was used (Fig. 1).63
TABLE II.
Comparison between the size and concentration of different cells types in human blood with those of CTCs.
| Cell type | Concentration cells/ml | Diameter (μm) | |
|---|---|---|---|
| Red blood cells (RBCs) | 4.6–6.2 × 109 (in males) | 6–8 (disk shape 2–2.5 μm-thick) |
|
| 4.2–5.4 × 109 (in females) | |||
| Platelets (thrombocytes) | 1.5–4 × 108 | 2–3 | |
| White blood cells (WBCs) | Neutrophils | 3–5.8 × 106 | 10–12 |
| Eosinophils | 5–25 × 104 | ||
| Basophils | 1.5–5 × 104 | 12–15 | |
| Lymphocytes | 1–3 × 106 | 7–8 (small lymphocytes) 12…15 (large lymphocytes) |
|
| Monocytes | 3–5 × 105 | 15…30 | |
| Total | 4.5–11 × 106 | - | |
| CTCs | 1–10 | 12–27 | |
FIG. 1.
Distribution of size of hematopoietic cells and comparison with the size range of CTCs. The overlap in size between WBCs and CTCs is also highlighted.
The lack of one or more universally usable tumor-specific markers is another challenge in the isolation of CTCs. The CTCs' surface marker antigens are shielded by a protective outer cloak of platelets (aggregated to the tissue factor proteins displayed on the cellular surfaces).13
In order to overcome such difficulties, possible solutions were analyzed: (1) extracting a volume of peripheral blood much larger than the typical 5–7.5 ml;61,64,65 (2) using only a slightly increased volume of blood (e.g., 10 or 15 ml), but collected with increased frequency; (3) developing highly sensitive and specific isolation devices; and (4) postseparation CTCs culturing.
C. CTCs capture: Parameters and main methods
Previous reviews highlighted, analyzed, and compared the essential features of the most relevant platforms and mini-/microsystems reported: miniaturized structure with reduced volume and weight, superior sensitivity, improved cell recovery, high purity, enhanced enrichment, shorter analysis time, less sample/reagent consumption, low cost, portability, ease of usage and disposability, capability to perform fast and cheap analysis even at the point-of-care.65,66 The separation of CTCs can be characterized and evaluated quantitatively by several factors. Smith et al.67 provided an overview of the “key performance parameters:” yield, purity, enrichment factor, throughput, viability, and WBC depletion. However, supplementary features may be considered when evaluating the functionality and the implementation of a CTCs capture system for clinical applications: reproducibility, time needed to completely process a sample of whole blood, robustness, ease of use (related to automation), the need of sample pretreatment protocols, cost, and test turnaround time.68 In terms of their usage frequency, the separation efficiency is the most often mentioned parameter, sometimes either purity or the enrichment factor can be given, and in a few cases, the throughput may be indicated as well. Therefore, the standardization-related issues slow down significantly the practical implementation in clinical settings. Table III summarizes the key features presented by the extensive body of research.
TABLE III.
Summary of the main topics covered in the reviews on CTCs.
| Reference | Key topics discussed in the corresponding review paper | Other topics/Remarks | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biological/properties of CTCs | Summary of CTC capture methods | Comparison table of CTC capture devices: I/WO = including commercial | 2 isolation methods | CTC characterization methods | Clinical applications/relevance of CTCs | CTC culture | EMT | Other tumor cell types (CTCls, CSCs, etc.) | Nanodevices and/or nanomaterials | |||||
| Label-free | Affinity-based | Functional based | In vivo | |||||||||||
| 4 | Y | I | Y | Y | Y | Y | ||||||||
| 18 | I | Y | Other circulating tumor materials (ctNAs, exosomes, etc.) | |||||||||||
| 90 | Y | Y | Y | Ia | Y | Y | ||||||||
| 89 | Y | Y | Y | Y | DTCs properties & isolation | |||||||||
| 93 | Y | Y | I | Y | Y | |||||||||
| 56 | Y | Y | I | Y | Y | |||||||||
| 65 | Y | Yb | I | Yb | Y | Y | Other circulating tumor materials (ctNAs, exosomes, etc.) | |||||||
| 111 | Y | I | Y | Roadmap for clinical validation and qualification of CTC assays | ||||||||||
| 66 | Y | Yb | Y | Y | High flow rates = high throughputs | |||||||||
| 67 | Yc | Yc | ||||||||||||
| 84 | Y | Y | WO | |||||||||||
| 99 | Y | Y | CS | |||||||||||
| 102 | Y | Y | WO | Y | Y | |||||||||
| 81 | Y | Yb | I | Y | Y | Y | Cell release for post-separ. Analysis | |||||||
| 82 | Y | Y | I | Y | ||||||||||
| 83 | Y | Y | WO DEP | Y | Y | Positive vs negative enrichment | ||||||||
| 85 | Y | Y | Y | I | Y | Y | Cell-release for post-separation and analysis | |||||||
| 86 | Y | Y | Y | Y | Y | Y | NMR material for CTC detection | |||||||
| 87 | Y | Y | I | Y | Y | Y | ||||||||
| 92 | Y | Y | Y | Y | ||||||||||
| 91 | Y | Y | Y | Y | Y | |||||||||
| 88 | Y | Y | Y | Y | Y | Y | ||||||||
| 94 | Y | Y | Y | I | Y | |||||||||
| 95 | Y | Yb | Y | Y | Y | Y | Y | |||||||
| 96 | Yd | Y | Y | Positive vs negative enrichment | ||||||||||
| 97 | Yd | |||||||||||||
| 98 | Y | Y | CS /CT by electric & magnetic methods Microsystems best solution for CTC isolation requirements | |||||||||||
| 100 | Y | WO | Y | EIS - possible characterization tool | ||||||||||
| 77, 103, 104 | Y DEP | DEP combine the high purity isolation with conservation of cell membrane DEP - potential of identifying cells with different phenotypes | ||||||||||||
| 105 | Y | Y | I | Y | Y | Y | ||||||||
| 118 | Y | Y | Y | Y | ||||||||||
| 119 | Y | Yb | WO | Y | Y | Y | ||||||||
| 107 | Y | Yb | I | Y | ||||||||||
| 110 | I | |||||||||||||
| 120 | Ye | Ye | ||||||||||||
Only immunological.
The usage of aptamers used for that specific purpose (affinity-based capture OR detection/characterization).
Fundamental mechanisms of cell adhesion and transport.
Only immunomagnetic-based.
Only using polymers for CTCs' isolation; CS/CT = Focus on “general” cell sorting (CS) OR cell trapping (CT) methods rather than specifically for CTC capture.
All microfluidic devices employ one or more of the following seven technologies for CTCs isolation/separation:
-
•
flow cytometry,69
-
•
hydrodynamic and biorheological methods,70,71
-
•
size-based filtration using mechanical sieving,72,73
-
•
affinity-based capture in conjunction with geometrical obstacles,74
-
•
affinity-based capture with immunomagnetic micro- and nanoparticles,75
-
•
electrokinetic, mainly employing dielectrophoresis (DEP), and76,77
-
•
acoustophoretic.78,79
Each method has its own characteristics, advantages, and drawbacks. The following metareview will highlight how these techniques, their characteristics, and other features of interest were highlighted and/or compared in various previous reviews.
Any of the above mentioned methods used to separate a desired bioparticle from a complex sample can be considered either a positive or a negative isolation method. Positive isolation describes the direct and exclusive extraction of the specific targeted cells (CTCs) from the biofluid. Negative isolation implies the complete exclusion of the initial cells and components from the sample, other than those targeted cells, which will be harvested. The positive isolation of CTCs, e.g., affinity-based capture, has the downside caused by significant false positives (due to the dual positive cells, CD45, and cytokeratins);66 hence, studied investigated methods for negative isolation of CTCs. They are mainly based on the removal of nontarget cells, especially the WBCs, whose sizes overlap with that of most CTCs. For this purpose, antibody targets to the CD45 leukocyte surface marker can be used,80,81 since the preliminary removal of RBCs and platelets is relatively easy. However, problems may still appear due to the CTCs heterogeneity and their dynamism. Additionally, the negative selection approach typically requires several successive steps to remove both the WBCs and RBCs. This aspect usually increases the complexity of the system, complicates its structure, and increases the number of processing steps. Therefore, the risk of losing CTCs and/or damaging them also increases. Nevertheless, the key advantage of negative selection resides in the ability to isolate all types of CTCs, including those that do not necessarily express certain specific markers.82 Moreover, it can easily provide intact and viable cells for valuable subsequent processing (e.g., genomic analyses).
D. A metareview on CTCs capture methods and related devices
Recent research highlighted the CTCs' properties, clinical relevance, isolation, and detection, as well as their characteristic properties. This section compiles the most relevant reviews summarizing the successful CTCs acquiring methods denominated as “enrichment,” “separation,” “trapping,” “capture,” or “isolation” (ideally without any other cells present at the output) as well as their postseparation analytical techniques for various purposes such as phenotypic and genomic characterizations denominated as “detection,” “identification,” or “characterization.”
In this direction, the work of Smith et al.57 is critical for the understanding of both affinity-based and label-free capture mechanisms and for the development of microfluidic devices based on such phenomena. It detailed the fundamental mechanism of adhesion and the transport mechanisms of rare cells capture in microfluidic devices, the factors that influence these mechanisms, the design strategies, and their practical applications in a transport context. The central role of microfluidics in LOCs for CTCs isolation and analysis was also emphasized in two reviews by Hyun and Jung.66,83 One review83 presented the two fundamental methodologies to isolate CTCs: affinity-based and label-free. The authors discussed a variety of late CTC developments such as the use of aptamers (single-stranded nucleic acids with specific binding capacity for proteins), the NanoVelcro family of devices, the cointegration of CTCs isolation with their subsequent on-chip culture, and the issue of using high flow rates to attain high throughputs. This review detailed label-free techniques such as dielectrophoresis (DEP) and hydrophoresis, with details of the essential characteristics of many DEP- and Field Flow Fractionation (FFF)-based devices. The other review66 focused on microfluidic approaches with an emphasis on positive vs negative enrichment methods and devices. The authors also proposed the use of LOC systems as ultimate technology for complete CTCs isolation and analysis. The combination of both isolation and on-chip single-cell analysis was presented as a possible solution for addressing some the CTCs' challenging characteristics, such as stem cell phenotype and heterogeneity. Performing separation of CTCs from a sample together with full analysis (including genetic/genomic one) of single CTCs in one chip/microsystem was advocated as one alternative that could solve the problems arising from the CTCs heterogeneity. Such a multipotent LOC platform could also shed light on many other related cells of similar (or even higher) future importance such as circulating endothelial cells (CECs), circulating cancer stem cells (CCSCs), circulating progenitor cells (CPCs), and nucleated red blood cells (nRBCs). Therefore, it could provide information which may lead to highly effective patient-tailored diagnosis and treatment.66
Moreover, notable claims on separation efficiency emerged from the review of Chen et al.,84 who summarized the hydrodynamic, DEP, immunochemical and magnetophoretic approaches for rare cells separation and their subsequent analysis. The review concluded that currently “a benchmarking standard is lacking to effectively compare the different methods based on efficiency.” It also highlighted other parameters such as purity, throughput, and operability with whole blood samples and considered the clinical applications of such a microsystem. In order to complete the understanding of the existing available CTCs capture and isolation systems, the recent review by Green et al.85 presented other significant issues, such as the release and recovery of captured CTCs, their usage for subsequent culturing and downstream molecular analysis, as well as the identification of CTC subpopulations and heterogeneity. It focused on the iChip, compared existing available CTCs capture systems, and summarized most of the methods used for CTCs isolation (affinity-, size- and dielectric-based). It also included a short section dedicated to in vivo CTCs analysis. Relevant discussions on CTCs analysis can also be found in the work of Li et al.,86 who presented the biophysical and biochemical properties of CTCs. They examined material-related aspects relevant for CTCs capture: rationally designed interfaces, nanostructured substrates for CTCs capture, and the usage of immunospecific magnetic nanoparticles in micronuclear magnetic resonance (μNMR). They reviewed various types of devices and methods: geometrically enhanced affinity-based isolation, immunomagnetic based on inertial forces, deformability, or various hydrodynamic effects. Furthermore, Sun et al.87 reviewed the methods typically used to separate CTCs, with reference to the most important commercially available assays, their operation principles, and basic performance features. However, only a small part of this review was devoted to miniaturized on-chip realizations. The strong points of this paper were the details on the two major methods (nucleic acid and cytometric) typically employed by clinicians to identify CTCs after their capture, and the summary of the techniques for molecular and genetic characterization of CTCs. The EMT of CTCs and the numerous clinical applications of CTCs detection were also evaluated. The paper confirmed again the predominant use of microfluidic technologies to isolate CTCs and the researchers' focus on their on-chip analysis, especially at a genomic level. In this direction, Alix-Panabières and Pantel88 discussed the epithelial–mesenchymal plasticity as a key challenge in positive, affinity-based CTCs isolation and recapitulated the methods for both enrichment of CTCs and their subsequent analysis for identification, immunological, or molecular characterization. The same authors dedicated other reviews to CTCs, isolation, detection, and clinical importance. The earliest of them presented the features of CTCs and DTCs isolation methods, including the functional assay EPISPOT (EPithelial ImmunoSPOT). The authors discussed other relevant issues as well, such as the search for potentially metastatic stem cells and the incompletely understood biology of tumor cell shedding.89 Another review dedicated to cancer metastases discussed the clinical and prognostic relevance of these cells and presented their detection methods, including traditional macroscopic methods such as gradient centrifugation or various modern microscopy techniques. The immunological approaches for detecting CTCs and DTCs (CellSearch® and the CTCs chip) and their main features were also summarized and compared. This paper is one of the very few which touched on cancer dormancy and metastatic stem cells, introduced a possible schematic model of tumor cell circulation and cancer dormancy, and discussed the factors that may regulate the onset of metastasis.90 A subsequent paper summarized the main strategies for CTCs enrichment (based on physical or biological properties) and detection (protein-based or mRNA-based strategies), the latter being necessary in order to identify only the CTCs from the captured cells which may also include a substantial fraction of WBCs. The review also presented novel alternatives explored for yield enhancement (e.g., the GILUPI® CellCollector) and briefly discussed the challenge posed by EMT for CTCs detection,91 a crucial factor contributing to the reported drawbacks of CellSearch®. Yet another review intended to provide a conceptual framework of CTC assays and to highlight the significant challenges of CTCs research. It reviewed the most important CTCs detection approaches, such as the protein expression-based technologies, including the impact of EMT and the corresponding possible measures to counteract its deleterious effect on the CTCs count, the physical property-based technologies, and the functional assays. The latter is particularly useful in discovering the “metastasis initiator cells” (MICs). The authors debated the suitability of CTCs for clinical applications: the early tumor detection, the molecular/genomic characterization, the characterization of primary tumors in order to identify particular distant organs as potential sites of metastatic relapse, the relevance of EMT and the overall evolution of CTCs after they are released in blood.92 The clinical relevance of CTCs as pharmacodynamics, prognostic, and intermediate endpoint biomarkers represented one strong element extensively discussed by Yap et al.56 The paper also compared the clinical relevance of both CTCs in general and of CellSearch® in metastatic breast cancer (MBC) and classified the CTCs assays by their respective underlying mechanisms. Therefore, it presented, in particular, the immunomagnetic separation method (by positive selection of EpCAM antibodies or by negative selection of CD45 antibodies), and the microfluidic ones upon either physical (size, deformability, density), or electrical characteristics (DEP-based capture). Furthermore, Lee et al.93 compared and summarized the underlying operational principles of the current commercial technologies for enrichment and detection of CTCs (immunomagnetic, microfluidic, physical filtration, or density gradient).
The important aspects of the technology and methodology of CTCs separation were reviewed by Hong and Zu.65 They compared many types of CTCs separation microdevices from the perspectives of physical dimension and assay performance characteristics, including clinical sample volume, preparation, processing, and total turnaround times (with CellSearch® as reference). They evaluated other circulating tumor materials, such as CTCls, cell fragments, and circulating DNA as new separation and possible characterization targets, and they assessed the emerging approaches for CTCs characterization, including telomerase-based and aptamer-based assays and functional analysis.
Similarly, the review of Krebs et al.4 presented a large range of commercially available technologies for CTCs separation and compared their methods of CTCs enrichment and of CTCs detection/characterization. Apart from the discussion on the biology of CTCs, EMT, CTCls, and other types of cells and their clinical relevance, the paper mentioned a more recent automated technology, the DEPArray. Such a technology, which employs a closed system of mobile electrostatic cages to block and move cells has been employed to image and isolate either single or cluster CTCs.
Barradas and Terstappen94 reiterated the relevant commercial technologies for CTCs capture and compared them from the underlying isolation principle point of view (physical and/or biological properties that define CTCs). Filtration-based technologies were briefly examined. The paper evidenced itself due to the details on the biological properties of CTCs, the CTCs in vitro culture (one previously proposed microfluidic setup that allowed cancer cells to be transferred from the microchip device on which they were captured, to a cell culture dish), and in vivo models.
The review of Gold et al.18 also summarized a large range of commercially available CTCs capture methods and specified the antibodies against which protein markers were employed for each method. Importantly, it detailed the clinical application of CTCs and introduced the clinical value of circulating cell-free and tumor-cell DNA. Subsequent sections were dedicated to the tumor-specific gene mutations, the epigenetic alterations, cfRNA, circulating miRNA, exosomes, and circulating microvesicles.
An extensive paper by Esmaeilsabzali et al.95 introduced both the features and importance of CTCs as well as the enumeration of the methods and devices for their isolation. The paper included information about chips based on various principles: size-based filtration, size-dependent hydrodynamic separation, electrokinetic separation, antibody-based isolation onto a solid matrix (magnetic beads or a microfluidic channel with various obstacles), and affinity-based capture with aptamers. Immunochemistry and nucleic acid-based methods for CTCs detection and characterization were also presented. Qian et al.81 introduced additional information regarding the microfluidic technologies indented to capture and characterize CTCs (immunoaffinity, filtration, hydrodynamic, DEP-based). Notably, this is one of the very few works which reviewed the simultaneous application of multiple CTCs isolation methods for enhanced performance. It also discussed cell-releasing technologies for subsequent downstream usage of the captured CTCs (e.g., for culture and/or genomic analyses). It recapitulated as well a large range of CTCs separation methods and devices, and quantitatively compared different CTCs isolation methods in terms of recovery rate, specificity, viability, throughput, and simplicity of downstream analysis. To be mentioned here is the fact that the paper included sections on capture of CTCs using aptamers and nanostructured surfaces or nanomaterials, both relatively new but increasingly popular methods. The clinical utility of CTCs as a prognostic factor, measure of the therapeutic effectiveness, molecular profiling tool, and potential metastasis suppression indicator was also examined.
In contrast to the broad analysis of Qian's review, Zborowski and Chalmers96 focused exclusively on separation by magnetic sorting. After briefly reviewing the potential of rare circulating cells as cancer biomarker and the traditional CTCs enumeration and analysis by optical means, the authors discussed the magnetic cell separation technologies. The distinction between positive and negative separation for enrichment of CTCs as well as of other rare cells was included. Similarly, the work of Chen et al.97 focused exclusively on immunomagnetic enrichment techniques and, unlike Zborowski and Chalmers, it detailed integrated realizations for miniaturized approaches. After succinctly presenting macroscopic magnetic-based separation systems such as MagSweeper and CellSearch®, the paper detailed the physical principles of immunomagnetic separation and reviewed the microchip assays based on such principles. Lastly, approaches based on micromagnets were also introduced and discussed. Furthermore, the paper of Bocchi et al.98 summarized the essential physical principles of cell manipulation using electric or magnetic forces. The review presented the microsystems that meet to the best extent the ideal requirements of rare cell isolation and handling system: high throughput and automation, high sensitivity and selectivity of the separation methods, and suitable mechanical and fluidic interfaces to the macroscopic world.
Since label-free methods were introduced as alternatives to affinity-based isolation, recent work focused on elucidating their technical advantages and further applications. A recent work by Murlidhar et al.82 compared affinity-based and label-free isolation techniques. They evaluated the advantages and disadvantages of some devices illustrative for each method and for their practical utility. However, they reviewed only size-based label-free methods, even if they employed various principles and devices (filtration, DEP, hydrodynamic, and acoustic). As expected, in all cases, the heterogeneity of CTCs is the key reason behind their relatively limited applicability. Several nanomaterial-based devices (using CNTs or TiO2 nanofibers) were also mentioned in the affinity-based section. A similar approach was used by Shields et al.,99 who classified the cell separation methods in two categories: (1) active, which use external fields exerted upon the cells to reach the desired separation and (2) passive, based on the intrinsic differences in size and physical properties of various cells. Three succeeding sections illustrated a few large families of cell sorting (and the subfamilies of interest for each): fluorescent label-based, bead-based, and label-free. The key operational principles were mentioned and graphically presented: electrokinetic-based, acoustophoresis, magnetophoresis, and optical manipulations. However, the paper itself did not focus on the specific separation of CTCs alone, but rather on introducing the large range of methods used for cell sorting in general.
Other reviews focused exclusively on label-free approaches for isolation and manipulation of CTCs. Cima et al.100 and Iliescu et al.101 described in detail two large categories of separation methods: based on mechanical or on electrical properties, respectively. Jin et al.102 presented current CTCs separation based on biochemical and biophysical methods. It described the CellSearch® system and indicated some reviews which focused on both generic cell separation and specific detection and characterization of CTCs. It described the biophysical and biomechanical characteristics of CTCs (including a historical perspective) exploited by various devices. The review introduced one aspect rarely discussed: the appropriateness of using cancer cell lines for CTCs modeling. A few label-free separation methods and devices were presented: filtration, hydrodynamic chromatography, and DEP, with a comparison of the performance metrics of some most indicative devices for each method. Methods for analysis of CTCs after separation (including identification, enumeration, and characterization) were scrutinized and evaluated comparatively.102 Furthermore, Xing et al.103 enumerated single and combined DEP methods for isolation of various cancer cells and presented their specific features. A general review on DEP characterization and separation of cancer cells was recently provided by Chan et al.76 Similarly, the comprehensive review of Iliescu et al.77,104 detailed the label-free methods for isolation, detection, and characterization of CTCs using only DEP. This review underlined the unique feature of CTCs isolation using DEP that consists in combining the specificity of the isolation method (a feature of affinity-based procedure) with the preservation of the cellular membrane surface (viability-related aspect particular to label-free methods). Additionally, DEP has the capability of recognizing cells based on their specific phenotypes.102 The difference in the capacitances between the cellular membranes of CTCs and the other blood cells can be the key for improving the capture efficiency, the purity, and the cells' viability, for a final downstream analysis. Likewise, DEP-based enrichment methods were presented by Arya et al.105 who also listed CTCs enrichment methods based on magnetic or geometrically enhanced affinity, size, density, and deformability. An entire section focused on a large range of CTCs detection/identification strategies summarizing the essential features of various techniques and realizations, as well as the various previous clinical studies involving CTCs. Yu et al.106 also discussed CTCs detection technologies, although in their paper “detection” encompassed not only isolation but also identification techniques. They presented the isolation techniques based on physical properties, affinity immunocapture, inclusive geometrically enhanced ones, as well as a few miniaturized platforms. The identification techniques described were nucleic acid-based. It is worth mentioning the approach to the molecular profiling of CTCs and their clinical applicability as prognostic markers. Identification and postprocessing of CTCs was the focus of Yu et al. who specifically presented the LOC microsystems for CTCs separation and the difficulties raised by the traditional systems of CTCs isolation and detection. Analytical miniaturization in LOCs in parallel with continuous development toward co-integration with nanotechnologies was highlighted as possible solutions to overcome the difficulties and to offer new opportunities with significant impact on biomedical research. Materials and architectures for on-chip CTCs capture and investigation were detailed, followed by the reviewed main CTCs isolation techniques: size-, DEP-, and affinity-based. The CTCs isolation techniques geometrically enhanced by microposts, pillars, or aptamers were also mentioned. One section on chip detection summarized the reported realizations that mainly employed various optical identification methods such as immunofluorescence, absorbance, scattering, interference spectroscopy, Raman spectroscopy. Other important techniques were also detailed: electrical (Electrochemical Impedance Spectroscopy—EIS), Surface Acoustic Wave (SAW)- and NMR-based. A unique feature distinguishing this paper is a special section dedicated to nanotechnology-facilitated postisolation CTCs culture and analysis. A similar focus, but on a larger scale and in greater detail, was shared by the review of Yoon et al.107 This work is entirely devoted to the emerging role of nanomaterials and their applications: the design of recent devices in order to overcome current limitations of existing CTCs capture and analysis methods/tools (CellSearch®, size-based filtration, and microfluidic devices). The paper enumerated and briefly presented the features, the performance, and the fabrication methods of devices incorporating or using various nanobased solutions: the techniques based on magnetic nanoparticles (MNPs) and on other nanomaterials: Au nanoparticles (NPs), carbon nanotubes (CNTs), nanowires (NWs), nanopillars, nanofibers, nanoroughened surfaces, and graphene oxide. The alternative role of aptamers (both DNA and RNA-based) as a capture moiety was also presented. Besides nanoparticles, polymers were observed. Myung et al.108 concentrated only on the role of polymers in the isolation of CTCs: physical-based separation, separation in solution with injectable polymer-coated inorganic NPs, and surface capture using biopolymers, hybrids, dendrimers, and silane chemistries. However, all proposed solutions need attentive evaluation from the commercial perspective.
E. Commercially available systems
Previous research analyzed the commercialization of various devices designed to isolate, capture, and analyze the CTCs and the related difficulties. Green et al.85 compared some of the currently used CTCs capture and analysis systems and highlighted the current high-end tools and approaches in CTCs enumeration and characterization. One more summary of existing commercially available solutions with clinical applications to prostate cancer was tabulated by Miyamoto et al.109 Another comparison of recent platforms devised for CTCs capture can be found in the report of Kling.110 The report briefly discussed the increasing importance of the isolated CTCs for extracting vital information in subsequent postseparation analyses. It also highlighted the failings of EpCAM-based separation and mentioned the various technical solutions, inclusive a possible significantly improved CellSearch® version.
The review of Parkinson et al.111 analyzed the lack of standardization of clinical validation and qualification of CTCs enumeration and/or characterization assays as the main cause for the current lack of universal acceptance of CTCs in clinical work. After a detailed comparison of numerous commercially available CTCs assays and technologies, it debated the biomarker development process and the criteria to be considered for the best clinical framework. The paper discussed the clinical trial design, the qualification study strategy, and the process which outlines a possible roadmap for clinical validation and clinical qualification in CTCs case. Table III summarizes the key features presented by the extensive body of research.
The features of CellSearch® EpCAM-based capture of CTCs is the basis of the commercially available and FDA-approved CellSearch® system (Veridex). It is an affinity-based immunomagnetic isolation platform, which uses ferrofluid nanoparticles coated with anti-EpCAM antibodies to attach onto CTCs. These complexes are subsequently retained when passing through a magnetic field, and analyzed with fluorescence microscopy.112 It is important to mention that the shelf life is at least 72 h.81 There are two different kits: the CellSearch Epithelial Cell Kit (CEK) and the CellSearch Profile Kit (CPK). In CEK, captured CTCs are made permeable and then labeled with DAPI and specific antibodies for CD45 and a few CKs (namely, 8, 18 and 19), and are then analyzed by semiautomated counting. Contrary to CEK, which includes cell labeling or enumeration, the CPK method employs only the anti-EpCAM-based positive immunomagnetic selection for isolation of CTCs and subsequent molecular characterization. Only the CEK procedure is FDA-approved for clinical usage. Indeed, the great majority of reports indicating the usage of CellSearch® have actually employed the CEK.113 The recent paper of Swennenhuis et al.114 gives another detailed presentation of the CellSearch® kits, of their features, of their numerous applications, and of their usage flexibility for various tests, some not necessarily clinically cleared (e.g., the usage for other body fluids besides blood, or changing the antibodies and/or the assay conditions).
Tests performed by Allard et al.45 with 7.5 ml of blood using CEK indicated a linear enumeration of the spiked tumor cells over the scale of 5 to 1142 cells, and a mean recovery higher than 85% at each spike level. Unfortunately, the CEK's capture yield is more than 20 times lower than that of the CPK method (when using actual samples containing CTCs, not spiking blood with cells from cancer lines), and low purity (0.01%–0.1% compared to 60%–70% for CPK) due to a high degree of leukocyte contamination. Few large clinical trials of MBC patients specifically reported a median number of 5 isolated CTCs per 7.5 ml of blood. This number was considered sufficient to provide insights regarding the clinical prognostic. Nevertheless, CEK's low capture yield and poor purity drastically limits the ability to perform detailed postseparation molecular analyses. The review of Masuda et al.15 indicated that CellSearch® introduced false-positive results related to the patients with benign colorectal conditions. Riethdorf et al.115 assessed the analytical power of the CEK system for clinical applications to MBC patients and reported:
-
•
intra- and inter-assay precision above 95%,
-
•
recovery rate of spiked samples between 80% and 82%, and
-
•
CTCs in just 70% of the actual samples from MBC patients, and
-
•
more recent findings indicated efficiency values of ∼82% in MBC patients.87
Other authors also showed that CellSearch® suffers from low sensitivity. For head and neck squamous cell carcinoma (HNSCC) patients, the sensitivity between ∼16% and 29%, was lower than that of RT-PCR and much lower than that of immunocytochemistry.43 It provided at least two CTCs in 7.5 ml of blood, although the HD-CTC assay provided an average number of CTCs 100× larger. Coumans et al.116 revealed that the immunomagnetic enrichment and staining procedure used by the CellSearch® resulted in a 3.3-fold loss of EpCAM+ CTCs and is thus a main cause of this method's reduced sensitivity. Using flow cytometry could achieve a potential threefold recovery improvement, even though this result is still insufficient to find CTCs in just 7.5 ml of blood in all patients. Likewise, the samples from newly diagnosed nonmetastatic breast cancer patients, after treating the WBCs with the MAINTRAC process, exhibited extraordinarily high concentrations of epithelial CTCs, similar to the number of monocytes. The CTC counts were 10 times higher than the previously reported when using CellSearch®.X.20,48,117
The sensitivity of CellSearch® is also limited in melanoma, pancreatic, and ovarian cancer, regardless of the stage of the disease,121 and in localized prostate and breast cancer.122 In lung cancer patients no noteworthy variation in the CellSearch® CTCs count was observed after therapy, in contrast with the data provided by the in vivo GILUPI CellCollector which reflected reductions for complete or partial responders and increases for patients with progressive disease.9 An interesting comparison between three different CTCs isolation systems was performed by Kuske et al.123 They studied CTCs separation in nonmetastatic prostate cancer patients by combining three independent CTCs assays: CellSearch®, the in vivo GILUPI CellCollector, and the EPISPOT (an EpCAM-independent assay that enriches CTCs while depleting the leukocytes and identifies viable prostate cancer cells upon the secretion of PSA). CTCs were found in 37%, 54.9%, and 58.7% of patients using CellSearch®, the GILUPI CellCollector and EPISPOT, respectively. Only CTCs isolation by EPISPOT before radical prostatectomy significantly correlated with PSA serum values and clinical tumor stage. On the other hand, matched pair analysis of samples before and after surgery indicated a significant decrease of CTCs captured after treatment only when using the in vivo GILUPI CellCollector.
Besides the limited CTCs capture efficiency suggested by these reports, CellSearch's® CEK has other significant drawbacks as well: it is costly and time-consuming, and requires extensive labor owing to its multiple immunofluorescent staining,124 its clinical utility is constrained by its relatively high blood consumption (min. 7.5 ml) and its limited dynamic range (the ratio of the highest to the lowest number of enumerated CTCs).125 The narrow dynamic range of CellSearch® in mainly all tumor types might disqualify it as a regular pharmacodynamics biomarker or “surrogate end point.”4 Moreover, CellSearch® is also beset by low purity.118,119 Specifically, Sieuwerts et al.126 found that the CellSearch® system cannot capture or identify mesenchymal transitioned breast cancer cell lines with low or no expression of EpCAM. The previously mentioned tests of Allard et al.45 showed that CellSearch® detected >2 CTCs in only 57% of prostate cancers, 37% of breast cancers, 37% of ovarian cancers, 30% of colorectal cancers, 20% of lung cancers, and 26% of other cancers. It is thus very difficult, and often impossible, to find CTCs by CellSearch® in some cancers, such as pancreatic and non-small cell lung cancer. Similarly, strong homogeneous, membranous EpCAM expression was observed in ileal and pancreatic neuroendocrine tumors, whereas variable EpCAM expression was observed in bronchopulmonary NETs.59 All these results are understandable in the light of Went et al.127 findings: analysis of 134 different histological tumor types and subtypes revealed EpCAM expression in only 98 of 131 tumor categories. The tumors with the strongest EpCAM+ expression were adenocarcinomas of the colon (81%) and pancreas (78%), followed by hormone-refractory adenocarcinomas of the prostate (71%). Endometrioid carcinomas of the uterus more frequently had stronger EpCAM expression (81%) than did serous carcinomas (32%). In contrast, most soft-tissue tumors and all lymphomas were EpCAM– (EpCAM-negative). Additionally, both the EpCAM expression and that of CKs vary among various CTCs subpopulations.128
It can be concluded that another key reason for the drawbacks of CellSearch® is the incapacity of EpCAM as an ideal marker due also to its downregulation in malignant epithelial cells undergoing EMT, and its lack of expression in nonepithelial solid malignancies.66,103 Since the large CTCs' heterogeneity, the efficacy of EpCAM-based antigen isolation might be low. This can be explained by the expression level of antigens that depends on cell type, cellular transformation stage, and of tumor type.103 For example, CTCs were not present in 36% of 292 metastatic cancer patients examined using the CellSearch® technique and 29% expressed at least one of the EMT markers, suggesting a drastic underestimation of CTCs by CellSearch® due partly to CTCs undergoing EMT.129 Likewise, Punnoose et al.44 highlighted that many patients, even with late-stage cancers do not have any detectable CTCs by the CellSearch® platform, mainly due to its EpCAM-based enrichment protocol which makes it insensitive to CTCs with down-regulated EpCAM after EMT. Other authors also reported failure to identify subtypes of CTCs resembling normal type breast cancer using CellSearch®. In contrast, significantly higher numbers of CTCs could be isolated using an EpCAM-independent separation method compared to EpCAM-based enrichment (69.2% vs 42.3%) in breast cancer patients, suggesting that a mixture of EpCAM+ and EpCAM− tumor cells circulate in the blood.130 It was also hypothesized that the metastatic progression of esophageal cancer may be supported by both EpCAM+ and EpCAM− cancer cells in a context-dependent manner, and that this different phenotype may also be histotype-specific. This, again, would limit the usage of CellSearch® for such patients.41 The number of CTCs isolated from patients with advanced non-small cell lung cancer (NSCLC) is very low when using the CellSearch® system due to their missing EpCAM expression.131 The CellSearch® system is also not indicated for detection of CTCs in hepatocellular carcinoma (HCC) patients because, although HCC cells are epithelial cells, EpCAM is expressed in only about 35% of HCC cases. Instead, a distinctive magnetic cell separation system that performed enumeration, immunomorphologic identification, and genetic analysis of CTCs from peripheral blood samples of HCC patients has been realized and validated. This platform uses the interaction of the asialoglycoprotein receptor (AGPR) exclusively expressed on hepatocytes with its ligand, biotinylated asialofetuin. Since normal hepatocytes do not enter the circulation unless they become tumorous, any cells found in peripheral blood by the system are circulating HCC cells.46
It is clear that, despite its wide usage and some proven clinical relevance as a prognostic test for breast, colon/colorectal and prostate cancers, CellSearch® is also marked by important disadvantages. Its deficiencies lowered the clinical reliability and technical standardization of CTCs studies which employed this tool, and the potential of becoming a “gold standard” clinical test, next to the CT scan and the protein biomarkers. Furthermore, many clinical issues cannot be addressed by EpCAM+ CTCs counting alone. Early detection, predicting, and monitoring responses to therapy may be achievable using technologies that are fundamentally different from CellSearch®. Therefore, identifying specific clinical needs is a good approach to better guide the design of microsystems that could use alternative biomarkers such as EpCAM− CTCs, CTCs clusters, live CTCs, or completely different classes of cancer biomarkers.
Non-EpCAM-based CTCs isolation strategies were investigated. A recent study showed that reversible shifts between EMT and CTCs phases were associated with each therapeutic cycle and disease progression, and it also indicated the important role played by EMTs in the blood-borne metastasis of human breast cancer. It also highlighted that EMT could be both a highly relevant clinical biomarker indicative of therapeutic resistance and a potential drug target.119 After the EMT transition, the cells change from an epithelial to a mesenchymal phenotype, dedifferentiate, increase mobility, and drop cell adhesion. Apparently, these changes contribute to a higher cellular metastatic capacity, survival, and drug resistance.49 Indeed, it was suggested that EpCAM− CTCs are very invasive and aggressive, with increased metastatic capabilities in the brain and lungs. Hence, the loss of EpCAM− CTCs due to the incapability of capturing them may impact considerably the current and future clinical significance of CTCs for personalized medicine, for accurate diagnosis, and prognosis.66,132 The review of Masuda et al.15 listed other specific markers useful only for the isolation of CTCs with metastatic potential, such as (1) HER-2, critically involved in the progression of some aggressive breast cancer or gastric cancer, (2) the Estrogen Receptor (ER), expressed in ca. 70% of patients with breast cancer, (3) the programmed death ligand 1 (PD-L1), which promotes tumor progression by preventing autoimmunity, (4) Plastin3 (PLS3), a recently identified CTCs marker present in CTCs with an EpCAM+ epithelial phenotype and in EpCAM mesenchymal cells, and (5) the EpCAM, CD44, CD47, and MET expressed by the metastasis-initiating cells (MICs) among CTCs, which could be biomarkers for metastasis or relapse.
Therefore, the key disadvantages of EpCAM-based CTCs detection can be minimized by using instead a mix of more antibodies to target multiple antigens, such as Anti-CK combined with anti-EpCAM antibodies,133 A triple marker set of P- and E-selectin, and anti-EpCAM,120 A four-marker set consisting of EpCAM, HER-2, EGFR, and MUC-1 determining a 400% increase in separation sensitivity using a point-of-care micronuclear magnetic resonance (μNMR) system.134 A rich mixture of up to 12 antibodies or more,128 Monoclonal antibodies against HEA, ErbB2, and EGFR for circulating breast cancer cells,135 Nuclear androgen-receptor splice variant 7 (AR-V7) protein for prostate cancer,63 HER-2,136 and cell surface associated Mucin 1 (MUC-1) can be used for breast cancer.137 Moreover, in EAC, a wider-ranging “epithelial” phenotype was isolated by capturing three CTCs classes: EpCAM+/CKs+, EpCAM−/CKs+ and EpCAM+/CKs− CTCs, permitting the identification of EpCAM− cells.41
Other references mentioned combining the CellSearch® enrichment step with CD146 to improve detection rates of breast cancer cell lines, adding anti-CD49f to the immunostaining cocktail,94 as well as using antibodies directed against several epithelial (EpCAM, HER2, MUC1, epidermal growth factor receptor, folate-binding protein receptor, TROP-2) or mesenchymal/stem cell antigens (c-MET, N-cadherin, CD318, and mesenchymal stem cell antigen).61 When compared to a surface functionalized with anti-EpCAM only, the biomimetic surfaces on epoxy-covered glass slides functionalized with P- and E-selectin and anti-EpCAM induced distinct reactions in cell models (HL-60 and MCF-7 as models of leukocytes and CTCs, respectively), and significantly improved separation and capture (more than threefold enhancement). These results were believed to be due to mimicking both the biological processes of combined stationary binding and dynamic cell rolling.120 Functionalized anti-EpCAM with seventh-generation (G7) PAMAM dendrimers immobilized more anti-EpCAM molecules than the PEGylated surfaces and also induced substantially more cells to be bound than the PEGylated surfaces for all 3 cell lines that were tested (MDA-MB-361, MCF-7, and MDA-MB-231 cells as CTC models), namely, the capture efficiency was enhanced 1.7–3.7-fold.138 Also, a study by Liu et al.139 found that chemokine receptors (CRs), such as CXCR4, CCR6, CCR7, and CCR9 were present on CTCs of patients with solid tumors. Although the tests were performed on blood samples only from metastatic carcinoma or melanoma patients, their findings suggested that CRs could be involved in CTCs proliferation and migration indicating CTCs as the potential CR-antagonist therapeutic target.
Other commercial solutions added to the previously mentioned list of the antigens leverages on a plurality of selection methods that may be better than a simple single-antibody-based CTCs capture.140 One example is the AdnaTest™ (AdnaGen) which isolates CTCs via magnetic beads conjugated with both epithelial and tumor-specific antibodies (available on colon, breast, and prostate cancer) followed by epithelial mRNAs detection.87 However, in contrast to CellSearch®, the AdnaTest™ system seems to have low/no prognostic value in metastatic breast cancer (MBC) patients.50 Another option is to avoid using CellSearch® and employ some other method instead. For instance, a FACS-based method, which isolated and captured EpCAM + CD45− cells was proposed and used to isolate average 100-fold more putative CTCs than CellSearch®: >50 events/sample in 58% vs 10% of patients, or >10 events/sample in 88% vs 32%, respectively.63
A nested reverse transcription (RT)-PCR assay for uroplakins (UPs) II was integrated to detect CTCs in the peripheral blood of patients with urothelial cancer. Regarding its use in other cancers, it was observed that positive detection rates increased with tumor extension, but UP-II-positive cells were not detected anymore in two patients who responded positively to the applied systemic chemotherapy.141 Also, a methodology has been developed for using a gastrointestinal-specific anti-CK20 antibody to isolate CTCs in colorectal cancer (CRC) patients' blood.142
Other platforms besides CellSearch® and AdnaTest™ are now commercially available, such as (1) IsoFlux (Fluxion Biosciences) and the magnetic-activated cell sorting MACS™ (Miltenyi Biotec) are immunomagnetic cell sorting systems, (2) Ficoll–Hypaque (GE Healthcare) and Oncoquick (Greiner Bio-One) employ density gradient separation to separate CTCs and mononuclear cells from other blood cells, (3) Oncoquick employs a porous membrane to exclude cross-contamination of distinct layers, and (4) RosetteSep™ (Stem Cell Technologies) improves the specificity of standard density gradient selection procedure via a negative CTCs enrichment that removes undesired cells (RBCs and WBCs) from the sample in a subsequent density gradient centrifugation step.20
A comparison of MACS™ with Ficoll–Hypaque, Oncoquick, and RosetteSep™ revealed that the former showed a better CTCs recovery rate with higher reproducibility, sensitivity, and accuracy than the other three assays and could be used to screen real samples.18,26 The literature also provides another comparison between CellSearch®, Oncoquick, MACS™, and ISET (Isolation by Size of Epithelial Tumor Cells, an enrichment technique based on size).143,144 ISET seems to be the only one specifically capable to separate not only CTCs but circulating tumor clusters/CTCls as well.
Other filtration-based products now available on the market are ScreenCell®, ClearCell (Clearbridge BioMedics), and RareCell.125 ScreenCell® is a recent device that also employs polycarbonate filters with randomly distributed pores. When used with H2030 cells, it provided an average efficiency of 91.2% and 74% with five and two spiked cells, respectively.105,145 Size- and geometry-controlled microcavity arrays with 10 000 apertures have also been designed to capture spiked tumor cells based on the differences in size and deformability of cells. The process ran with unprocessed human whole blood. Such filtering microcavity arrays, which do not longer rely on EpCAM expression, have been successfully demonstrated for the entrapment of CTCs from the blood of non-small cell lung cancer (NSCLC). It is known that these tumor cells are very difficult to be detected using CellSearch® due to their drastically down-regulated EpCAM expression.131,146 Probably, the independence of the antigen expression on the CTCs surface would be a feature which characterizes the filtration-based commercial products. However, they also exhibit the serious disadvantages of the size-based filtering devices: such as the insufficient purity of the isolated CTCs, the noncapture of small CTCs, and the difficulty of subsequently retrieving and using the captured cells for downstream genetic analyses.
1. Trends in CTCs separation microdevices
The downsides of the CellSearch® system have encouraged many researchers to develop new systems, some of which surpass its performance.48,147
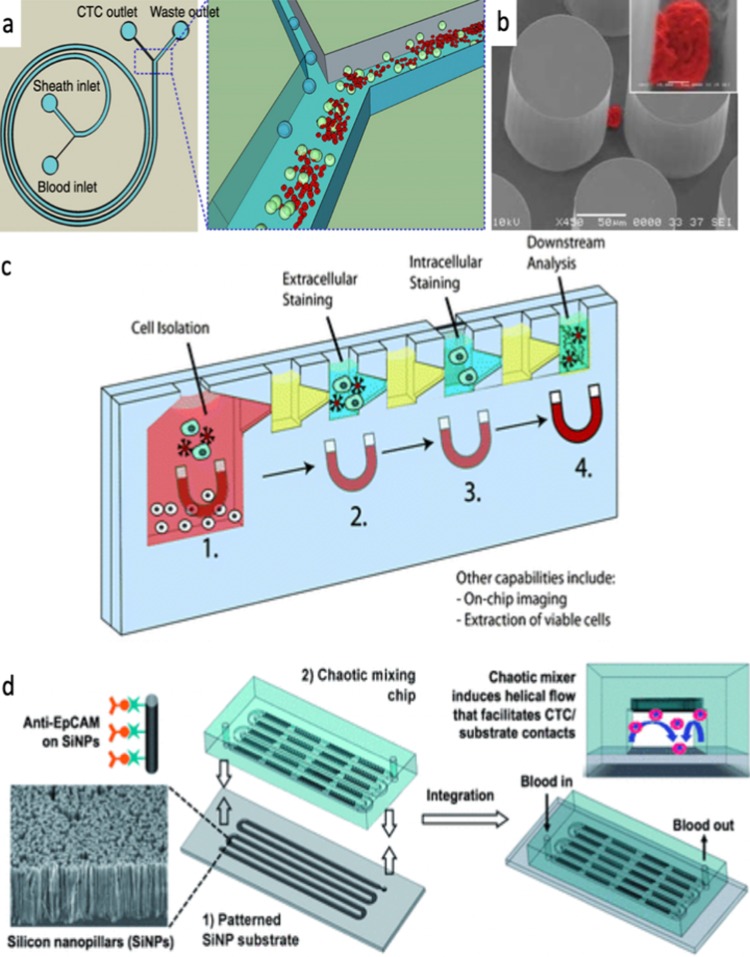
A highly performant device has been reported by Warkiani et al.148 The chip consists of a very long spiral microfluidic channel. It uses the combined inertial and hydrodynamic Dean forces present in curvilinear microchannels to perform label-less size- and deformity-based isolation of viable CTCs [Fig. 2(a)]. Despite its simplicity, the device achieved ≥85% recovery of spiked cells from various cancer cell lines and 99.99% reduction of WBCs in whole blood at high throughput. When used on blood samples taken from patients with advanced-stage metastatic breast and lung cancers, it exhibited 100% collection of CTCs with very high purity (∼4 log depletion of WBCs), so that the CTCs could be used for subsequent processing. However, the additional preparatory steps to lyse the RBCs in the blood sample and then remove them by centrifugation complicated the procedure and reduced its attractiveness.48,148 Nevertheless, the concept has been successfully implemented and the chip denoted as CTCchip® FR1 (Clearbridge BioMedics) is the key element of the commercially available ClearCell® FX1 system.149,150
FIG. 2.
Schematic representations of various types of CTC isolation microdevices reported in the literature: (a) Label-free CTCs isolation from a blood sample using inertial microfluidics. Reproduced with permission from Warkiani et al., Nat. Protoc. 11(1), 134 (2016). Copyright 2016 Nature Publishing Group. (b) SEM photo of the micropillars in the device of Nagrath et al. with a captured NCI-H1650 lung cancer cell. Reproduced with permission from Nagrath et al., Nature 450(7173), 1235 (2007). Copyright 2007 Nature Publishing Group. (c) The VerIFAST device for cell isolation, cellular staining, and downstream analysis. Reproduced with permission from Casavant et al., Lab Chip 13(3), 391–396 (2013). Copyright 2013 The Royal Society of Chemistry. (d) Schematic illustrations of how the CTCs captured in the NanoVELCRO device may also be released for subsequent processing, respectively. Reproduced with permission from Wang et al., Angew. Chem. 123(13), 3140–3144 (2011). Copyright 2011 John Wiley and Sons.
The simplest way to improve efficiency of the affinity-based cell capture principle is to increase the total area of the functionalized surface, which can be achieved even as the overall size of the device decreases. This is the basic idea behind using arrays of functionalized micropillars in biochips, as in the device of Chang et al.,151 although one of the first and probably one of the best known microchip in this category is that of Nagrath et al.152 The latter contained 78 000 cylindrical microposts functionalized with anti-EpCAM antibodies [see Fig. 2(b)], and had a yield 10–100 times higher than that of CellSearch® (e.g., 79 cells per ml of blood collected from breast cancer patients). The same principle was included when designing the GEDI (Geometrically Enhanced Differential Immunocapture) microdevice (Cornell University). Like Nagrath's device, it also employed microposts (80 μm in diameter), functionalized with antibodies. The posts were arranged in a regular array with 100 μm gaps but each subsequent row was offset from the one before by 7 μm. This caused the higher frequency of larger cancer cells -walls collision and wall adherence , compared with other cells in the blood that flow smoothly.153
“MagSweeper” (Stanford University) incorporated a magnetic rod to stir a sample of blood prelabeled with anti-EpCAM antibody-coated magnetic beads. It enriched CTCs to offer a higher CTCs isolation purity than CellSearch® and the ability to isolate live cells.4,154
“VerIFAST” [University of Wisconsin; see Fig. 2(c)] employed the Immiscible Filtration Assisted by Surface Tension (IFAST) to separate the cells of interest labeled with paramagnetic particles (PMPs) from the nontarget cells and unnecessary particles. The system allowed undesirable particles to move away from the path designed for the target cells, laterally along the walls rather than transversely along the floor surface. The cells and reagents were then directed inside two wells separated by a membrane, and towards the sieve chamber, where excess unbound PMPs were also removed by filtering through the dividing microporous membrane. The device enabled several complicated washing procedures to be conducted in the absence of centrifugation or transfer steps, as well as extra- and intracellular staining followed by downstream analysis. The device offered “good” capture efficiency (>80%) and purity (>70%). The viable cells isolated with it can also be extracted for subsequent processing. The device was successfully used to isolate rare non-small cell lung cancer cells for diagnostic and pharmacodynamic analysis.155,156
“NanoVelcro” [Fig. 2(d)]153,155,157–164 integrated two key elements into the same microdevice. The first component was a 3D-nanostructured substrate coated with agents that can capture cancer cells, and it considerably enhanced the effectiveness of this process, due to intensified local interactions between the nanostructured surface and the cellular membrane components. The second component, the top part of the device comprised a PDMS blender with herringbone features embedded that generated a vertical flow to increase the cell-substrate contact along a 22 cm microchannel connected with the mixer.59,60 When the nanosurface was functionalized with anti-EpCAM antibody-based capture agent, tests with breast (MCF7), prostate (PC3), and bladder (T24) cell lines provided efficiencies >95% for flows ≤2 ml/h. Using only 1 ml blood samples the device detected CTCs in 17 out of 26 prostate cancer patients and in larger numbers (after normalization to a 7.5 ml volume) than those provided by CellSearch®.161,165 When anti-EpCAM were replaced by DNA-aptamers to functionalize the nanosurface, the device's capturing of non-small cell lung cancer (NSCLC) CTCs from peripheral blood, and recovering the immobilized NSCLC CTCs after an enzymatic treatment significantly improved.60
“The HBCTC chip and CTC-iChip” (Massachusetts General Hospital, employing negative depletion to extract the desired CTCs).85,166,167 The CTC-iChip, is a platform that combined hydrodynamic separation,168 and immunomagnetic purification as the last step to achieve better results: increased capture and purity.81,169 It first eliminated the RBCs and platelets from the sample by using hydrodynamic sorting based on deterministic lateral displacement in a chamber with an range of pillars. The rest of the sample containing the large nucleated cells was aligned within a microfluidic channel by inertial focusing and mixed with antibody-tagged magnetic particles that may attach to either WBCs or CTCs, for subsequent positive or negative depletion. The actual separation was achieved in an ultimate chamber, under an externally induced magnetic field, which deflected CTCs and WBCs to different outlets. In the former case (magnetic beads attaching to WBCs), a full label-free negative separation of CTCs was achieved, although the purity was lower than the one obtained with positive selection methods due to contamination with WBCs. Tests done with cancer cells spiked in blood on the device in the negative depletion mode showed capture efficiencies of ∼97% even for cancer cells with no EpCAM expression, and a purity of 2.5 log. The positive depletion mode provided purity of 3.5 log, and capture efficiency of only 10% for cancer cells with no EpCAM expression compared to ∼78% for those who expressed EpCAM. It also clearly displayed the capacity of processing substantial volumes of whole blood (8 ml/h), at high throughput (107 cells/s) and high efficiency, with greater sensitivity than CellSearch® at low CTCs numbers (<30 CTCs/7.5 ml). The authors considered the negative depletion mode capable to detect all cancers that express vascular invasion, unlike current technologies which showed major practical limitations in this respect.85,167 Consequently, the iChip was advertised as a performant marker-free CTCs isolation system.85
The “Ephesia system” (CNRS and Institute Curie, France) combined hydrodynamic and immunomagnetic sorting.170,171 The first segment of this system used water-based ferrofluid dots (“magnetic ink”) patterned in a hexagonal array onto glass, at the bottom of a microfluidic channel. Antibody-functionalized superparamagnetic microbeads introduced into this channel under an external magnetic field, self-organized in tall columns. The large number of beads in a column allowed the use of several types of beads with a large number of desired antibodies to ensure the capture of CTCs with a large range of cellular expression. After removing the magnetic field, the columns maintained their cohesion and remained attached to the magnetic dots despite the flow through the channel at modest velocities (<20 μm/s).170 The second component of the system was a microfluidic structure that was first developed and optimized to maximize the flow throughput and interaction with the columns through high flow rate and homogeneity along the capture areas, and to minimize the footprint and shear stress.172 The two elements were combined upon adding extra PDMS microposts to filter debris that could interfere with the self-organized columns or cause clogging. Using anti-EpCAM functionalized magnetic beads obtained an average capture efficiency of ∼90%, although the value decreased for very low concentrations. The highly specific capture of the CTCs was demonstrated by the 0.26% of nonepithelial cells that were captured at concentrations greater than 107 cells/ml. The device was comparable or better than CellSearch® in 10 out of 13 cases studied, and its capture efficiency in patients with metastatic cancers was 75% for prostate cancer and 80% for breast cancer.171 However, using Ephesia system implied a pretreatment of all whole blood samples prior their processing in the chip: incubation with tetrameric antibody complexes and centrifugation for removal of RBCs and nearly all of the CD45+ cells were used for samples larger than 1 ml, and three times dilution without centrifugation were applied to samples smaller than 1 ml.171
“ApoStream™” based on DEP-FFF was pioneered by Gupta et al.173–175 It achieved a high throughput label-free isolation (independent of antigen expression levels, including EpCAM), and a high recovery of viable CTCs despite an efficiency of only 75%.174 Similar principles were then used by the same group in other DEP-based devices reported subsequently.176–179
The “GILUPI® CellCollector” is unique and performs in vivo direct CTCs isolation when directly applied in the peripheral arm venous flow. It consisted of a gold-coated medical wire with an anti-EpCAM functionalized hydrogel layer at its tip. This wire was carefully inserted in a catheter that has first been placed into the patient's median cubital vein. The device appears to have better performance than CellSearch®: the isolated CTCs are in sufficient number for treatment monitoring and could be subsequently used in downstream molecular analyses.9 Furthermore, the number of CTCs captured by it in PCa patients correlated well with OS when considering a cut off of ≥5 CTCs (namely the mortality risk was 7 times greater than that for patients with fewer than 5 CTCs), and the captured CTCs were also molecularly characterized.180
Other devices for direct clinical applications employed functionalization of nanostructured surfaces or polymeric coatings and greatly improved the capture of CTCs in the blood samples in the presence or even absence of affinity capture probes.161,181,182 Yoon et al.58 designed a micropost-free chip to capture CTCs. It contained graphene oxide (GO) absorbed onto a surface-patterned gold microarray to facilitate a noncovalent immobilization of antibodies. A rapid microfluidic cell sorter (μFCS) device was also designed for size-based separation of CTCs from unprocessed whole blood. It is important to mention that the device allowed in situ analysis for comprehensive molecular profiling, and on-chip culture for genetic analyses and drug screening.183 Recently, the Cluster-Chip emerged as label-free microfluidic chip developed to capture CTC clusters from unprocessed blood, based upon their physical properties, and free of biomarkers. The Cluster-Chip identified CTCs clusters in more than 30% of the patients with metastatic cancers.184 Other recent technologies also demonstrated the possible integration of several magnetic sensing chips into one microfluidic system, for improved throughput. Consequently, the μHall platform showed capability of detecting separate cells from a sample comprising rich in blood cells.185 The results of a clinical trial showed higher sensitivity than CellSearch®.186
Another approach is to employ aptamers to capture CTCs with a large selection of techniques and devices.81 One example with promising results is a simple PDMS-on-glass microfluidic device employing cell-affinity chromatography upon selective cell capture of immobilized DNA-aptamers. It provided a 135 times better enrichment of the CTCs in one complete procedure, at a 96% purity.187 Similarly, dual-aptamer functionalization of the surface of an amorphous carbon electrode enabled the successful ultrasensitive electrochemical detection of a single cell in 109 whole blood cells,188 while DNA aptamer-functionalized barcode particles provided a CTCs capture efficiency of 90%. Moreover, the captured cells could also be released subsequently (using exonuclease I) with an efficiency of 86%, and the activity of the released CTCs is 97%.189
Some of the devices listed above are very complex and advanced, but all attempt to provide improved speed and/or efficiency over CellSearch® and/or to provide viable CTCs at the output for postcapture analyses, a vital feature that CellSearch® lacks.125,169
Consequently, the development of the next generation of instruments capable to deliver similar or better performance than the existing commercial CellSearch®-like systems has clear specific targets: a detailed molecular profiling of the isolated CTCs, an increased miniaturization, a much more simplified operation and less time-consuming preliminary sample preparations. Ultimately, all newly proposed tools aim at becoming the new and uncontested “gold standard” in clinical use.
F. Isolation of other tumor-associated cancer cells and clusters
While higher sensitivities may be obtained with other methods, their clinical implementation has to be demonstrated. Recent research has characterized the presence of tumor-derived stromal cells in the blood of early cancer patients with nearly complete separation of cases and controls. These cells, while being tumor-derived and formally CTCs, are not classical epithelial tumor cells bearing metastatic potential but rather accessory cells present in the tumor. This indicates that CTCs chips may be employed in early cancer detection by targeting other different populations than classical CTCs. For instance, using a filtration method allowed Circulating Cancer-Associated Macrophage-Like Cells (CAML) to be identified in 83% of stage I/II disease patients but not in healthy individuals.190 These findings were reproduced in a follow-up prospective study.191 Mehran et al.192 reported the presence of tumor-derived endothelial cells characterized by the expression of CD276. Tumor-derived endothelial cells (CTECs) were detected by virtue of their specific CD276 marker when using flow cytometry. Compared to normal endothelial cells, the number of CTECs could distinguish healthy volunteers from esophageal and lung cancer patients. In another study, Cima et al.10 used a silicon-based microfiltration device to isolate CD31 positive endothelial cell clusters from CRC patients. These cell clusters were shown to be tumor-derived and were present in 86% of stage I/IIa colorectal cancer patients but only in 2.2% healthy individuals. Separating and analyzing the number of tumor-derived stromal cells instead of classical EpCAM+ CTCs may be a valid alternative for the application of the CTCs count at earlier disease stages.
G. Current trends: Combining two or more isolation methods
One of the greatest challenges in diagnosing cancer at early stages is the engineering of tests with nearly 100% specificity (confirming the true negatives and avoiding false-positive test results). Because a common cancer disease may be present in 0.1% of the general population, a diagnostic test with 100% sensitivity (all cancers detected) and 99% specificity (only 1% false positives), while experimentally sound in the lab by using equal numbers of cases and controls, could result in catastrophic outcomes if implemented clinically: for each cancer patient diagnosed correctly, it would diagnose nine healthy individuals as having the disease. Combining two or more independent methods,100 e.g., a gold standard test with proven high sensitivity together with other tests to rule out false positive findings, are current strategies that may significantly improve performance for early cancer detection . Certain realizations combined two (or more) methods in a single system and optimized them for the desired very high purity, yield and viability4,100
For instance, Lin et al.193 realized a PDMS device comprising a hydrodynamic concentrator and a microfluidic ratchet mechanism, shown in Fig. 3(a). The first stage removed WBCs based on their sizes, while the second separated CTCs in funnel shaped constrictions using both size and deformability. Tests performed with samples comprising WBC suspensions spiked with cancer cells at a concentration of 103:1 led to a 97% yield, 75% purity, and a 3000-fold enrichment of CTCs relative to the concentration of WBCs due to removing of 99.7% of the WBCs. However, the device was not tested using whole blood, which have ratios of WBCs:CTCs of >106:1 and may lead to a poorer performance of the device.
FIG. 3.
Different LOC solutions that combine two or more methods for efficient CTCs isolation: (a) Schematic of the concentrator mechanism and of the ratchet cell sorter (Lin et al.). Reproduced with permission from Lin et al., Biomicrofluidics 7(3), 034114 (2013). Copyright 2013 AIP Publishing LLC. (b) A Multi-Obstacle Architecture (MOA) for filtration of CTCs. Reproduced with permission from Kim et al., Lab Chip 12(16), 2874–2880 (2012). Copyright 2012 The Royal Society of Chemistry. (c) Cross-sectional view of the microaperture chip system of Chang et al. (CTCs immunomagnetic isolation together with subsequent size-based filtration). Reproduced with permission from Chang et al., IEEE Sensors J. 14(9), 3008–3013 (2014). Copyright 2014 IEEE. (d) Cross-sectional image and 3D representation of the geometrically activated surface interaction (GASI) chip for negative enrichment of CTCs. The asymmetric herringbone structure creates a helical fluid flow that enhances significantly the interaction between the cells and the antibody-coated channel surface. Depletion of WBCs can be achieved by functionalizing the chip surfaces with CD45 antibodies, thus allowing CTCs to flow freely to the outlet. Reproduced with permission from Hyun et al., Anal. Chem. 85(9), 4439–4445 (2013). Copyright 2013 American Chemical Society.
A combination of two or three different techniques improved the performance of the previously mentioned VerIFAST, NanoVelcro, and Ephesia, and CTC-iChip, respectively.
Using a similar approach to the CTC-iChip, Kim et al.194 devised the platform and combined “selective size amplification” (SSA) and a multi-obstacle architecture (MOA) filter. In SSA, anti-EpCAM functionalized magnetic microbeads attached onto the CTCs and artificially increased the CTCs diameter. This removed any size overlap issue with leukocytes and allowed to overcome the typical compromise between recovery rate and purity attribute of stand-alone label-free size-based filtration. The MOA filter, shown in Fig. 3(b), consisted of micropillars arranged in a zig-zag fashion with gaps decreasing gradually from 30 μm to 8 μm in order to achieve an increasing density of filtering obstacles. The entire platform provided a maximal capture rate of 99% for an optimal diameter of 3 μm for the magnetic microbeads, at a purity of 272 peripheral blood leukocytes per ml (PBL/ml). However, the chip was not tested with actual clinical samples but by “spiking” 100 cells (from breast carcinoma cell lines, mainly MCF-7) with microbeads in 1 ml of whole blood injected at a flow rate of 20 μl/min. Additionally, the MOA filter was fabricated using an original silicon-on-glass process.195 Zhang et al.196 used the related Size Amplified Immune Magnetic Microbeads (SAIMM) strategy which combined membrane filtration with immunomagnetic separation employing anti-EpCAM functionalized microbeads of same size as in the previous report (3 μm). First, membrane filtration (pore diameter: 8 μm) removed most of the WBCs and the unattached magnetic microbeads, then magnetic separation of the CTCs followed. Isolation of MCF-7 cells spiked in human blood indicated capture efficiencies higher than 90% for a number of spiked cells between 50 and 200, but reduced to ∼79% for only 5 cells per 1 ml of blood, with a reported 133% for the detection limit of 2 cells/ml. In all cases, purity was >98%. For optimal results, the sample required a long incubation time (1 h) with the magnetic microbeads.
A similar approach, but using the separation methods in reverse order (immunomagnetic isolation, followed by size-based filtration), was used by Chang et al.197 [Fig. 3(c)]. In this case, the size of the CTCs was artificially increased by the attached beads. This was done in order to address the previously mentioned problem of poor selectivity for size-based separation due to the similar dimensions of CTCs and other cells, especially WBCs. Antibody-functionalized magnetic microbeads (1 μm in diameter) initially attached to the CTCs present in the sample were then input into a chip comprising a membrane with circular 8 μm-diameter microapertures. The chip was positioned in a fluidic arrangement at a certain distance from a permanent external magnet which created a magnetic field normal onto the fluid flow. The bead-bound CTCs were attracted towards the magnet, but because their size was larger than that of the apertures they were blocked by the membrane and captured onto it. In contrast, RBCs easily passed through and thus were filtered out. The system operated even at large flows rates of up to ∼4 ml/min. By removing the magnet the trapped cells could be easily released for subsequent processing or analysis, without the need of adding another biochemical agent in the fluidic chamber. Yields of up to 90% were achieved at 1 ml/min, if an adequate incubation time or a sufficient amount of beads were used. If anti-EGFR conjugated beads were used, the yield remained quasiconstant for flow rates up to 4 ml/min for MCF-7 and A549 cancer cell types, but decreased dramatically to as low as 20% for anti-EpCAM conjugated beads and A549 cancer cells.197 The system required few preliminary sample preprocessing steps: the RBCs were lysed by a buffer and the sample that remained was resuspended in a PBS solution, spiked with CTCs for testing, and then incubated with anti-EpCAM beads. However, the processing time increased. Like any other size-based filtration membrane mentioned earlier, this system was able to trap WBCs. The two methods used in this system for CTCs isolation were employed simultaneously while the sample was analyzed, and only the CTCs release step followed the sample analysis stage.
The method proposed by Chung et al.198 enriched the blood sample by immunomagnetic isolation with anti-EpCAM-coated beads then size filtered the larger cell-bead complexes with a commercially available membrane. The sample underwent additional magnetic concentrations, followed by immunochemical trapping on electrodes to detect/identify only the CTCs by EIS (Electrical Impedance Spectroscopy). Therefore, it avoided nonspecific binding of other cells. The first cell enrichment stage was realized in a syringe setup, with efficiencies above 70% for as few as 10 spiked MCF7 cells per 0.5 ml blood. It increased above 85% for larger numbers of spiked cells. The second stage was integrated in a microfluidics chip with a magnetic trapping chamber, and - an array of electrodes of matched circular and ring-shaped electrodes for EIS. The operation of the system required careful sequencing of steps (accurate application and removal of the external magnetic fields in the different stages), had limited efficiency, unknown purity, and did not operate with whole blood. The EIS electrode array provided only an estimate number of trapped CTCs.
Other approaches which employed two methods have also been demonstrated. For instance, in negative isolation of CTCs, RBCs were removed by lysis in a 1st stage. A 2nd stage magnetically removed WBCs from the sample by using specific markers (such as CD45 or CD61).199,200 This combined approach was no longer biased by any particular CTCs marker (i.e., it is independent of the CTCs' phenotype, thus enabling isolation of CTCs without a classical epithelial phenotype). Furthermore, the CTCs were unaffected and easily available at the outlet for subsequent analysis. However, the capture efficiency and purity were not very high: 77% with an average nucleated cell log depletion of 2.56, in the paper of Wu et al., and an average recovery of ∼83% with a 2.59 log enrichment for the optimal protocol in the report of Yang et al.200 Additionally, all these systems were hardly miniaturized.
A smaller two-stage realization combined microfluidic magnetic-activated cell sorting (μMACS) with a “geometrically activated surface interaction” (GASI) chip.201 The latter structure, detailed in Fig. 3(d), employed asymmetrical herringbone structures to enhance interactions of the CTCs with the inner surfaces of the microfluidic channel. The former chip was realized in polymethylmethacrylate (PMMA), the latter in PDMS and it had been initially developed and tested separately for negative enrichment of CTCs by capturing WBCs on the CD45 antibody-coated channel surfaces. However, the full system employed the μMACS stage for removal of WBCs and the GASI stage for positive enrichment of the CTCs. The μMACS stage had higher than 99% depletion efficiency for healthy blood, but a 90.97% recovery at the ideal inlet flow rate of 400 μl/min when both leukocytes and cancer cells were loaded at a constant concentration of 106 cells/ml into the inlet, with CTCs enrichment 763.14 times higher. The GASI chip provided capture efficiencies between 67% and 99% for different cell lines. The entire set-up comprised two stages and was tested by separating 1000 cancer cells from cell lines that had been spiked into 5 ml blood samples. It also classified, with purities from 10.19% to 22.91%, heterogeneous CTCs based on their features, such as their EpCAM or HER2 surface protein expression. However, no capture efficiency was given for the entire system.202
Sajay et al.68 proposed several platforms that combined the same two distinct steps. In an initial version, a μMACS removal of WBCs was first performed with magnetic particles conjugated with anti-CD45 antibodies. Then, the CTCs were captured on a microfabricated slit membrane; the RBCs were filtered out with the flow of the remaining sample. The special shape of each slit permitted deformable or flat/elongated cells to easily pass through the slits (nearly 100% depletion of RBCs), and still some fluid to flow. The system had an average CTCs isolation efficiency of 94% from 2 ml of spiked whole blood diluted in 2 ml of buffer, a 2.25 log enrichment for multiple cell lines, and a WBC depletion efficiency above 90%. However, this platform required dilution of the initial blood sample. The subsequent version included the microslit membrane in a chip made from PMMA, combined with the setting used to immunomagnetically separate WBC. This hybrid arrangement demonstrated a WBC depletion of ∼97% and a capture efficiency above 92% for MCF-7 and NCI-H1975 cancer cell lines. Additionally, the system was also tested using 15 clinical samples that included 12 Non-Small-Cell Lung Carcinoma (NSCLC) and 3 colorectal (CRC) cancer cases. CTCs were successfully detected in all 15 samples (the CTCs counts per 2 ml of sample ranged from as low as 1 to as high as 51, with a mean value of ∼12.67), with WBC log depletion values ranging from 2.01 to 2.79, with an overall mean value of ∼2.378.203A third version maintained the integrated method, but included both stages into a single microfluidic LOC platform. The first stage was a μMACS module of a microfluidic chamber realized in a PMMA chip and an array of externally placed magnets. The second module comprised the microslit membrane, also within the same PMMA chip. The immunomagnetic separation module alone achieved an excellent value of ∼3.19 for the log WBC depletion.204 Its combination with the microslit filter in the integrated on-chip realization functioned with 2 ml of undiluted whole blood. It ensured the exclusion of more than 99.9% of WBCs (log WBC depletion of ∼3.94) and an average recovery of spiked tumor cells of different types larger than 80% (ranged from 77% to ∼95%), with a total time to process the assay of 50 min.205 However, the system was not tested on clinical samples.
Mohamadi et al.206 combined immunomagnetic separation with filtration on physical obstacles in a microfluidic chip. First, CTCs were labeled with EpCAM-targeted NPs, at various extents depending on their phenotype. Second, X-shaped physical obstacles were used in four zones of the chip to generate limited regions of reduced flow velocity (i.e., velocity valleys, VVs). There, the external magnetic force overcame the lowered drag force, and the tagged cells were captured. This resulted in spatial sorting of various CTCs in different bins, depending on their magnetic moment, i.e., on their phenotypes (from high to low, with a 100-fold difference in EpCAM expression). As anticipated, the capture efficiency fell with increasing flow rate. Hence, tuning the flow rate by modifying the channel cross section ensured the capture of a wide range of cell types. For instance, even cells with significantly reduced EpCAM expression levels were trapped at higher than 90% efficiency at very low flow rates (0.5 ml/h). When tested with VCaP cells spiked into whole blood, the device captured less than 0.01% of the WBCs introduced, and the specificity of the recovered cancer cells was 10 000-fold and higher.207 A modified version of the device also performed mRNA profiling of the captured CTCs. For this purpose, the chip included electrodes in the trapping chambers for subsequent lysis of trapped cells, and two additional chambers with electrochemical sensors for mRNA profiling (nanostructured microelectrodes—NMEs—on which peptide nucleic acid-PNA probes specific to complementary mRNA targets were immobilized). Once mRNA was released from the captured cells, the lysate flowed to the chambers with electrochemical sensors, where the released mRNA hybridized to PNA probes immobilized on the surface of NMEs and determined an increase in current after hybridization as measured by differential pulse voltammetry. The capture efficiency was 97% compared to only ∼60% obtained with CellSearch®, while the level of WBC capture was reported to be ∼100 times lower than typical levels of nonspecific cells captured by CellSearch® and much lower than for other CTC capture methods previously reported. The chip was tested with low numbers (5−500) of VCaP cells spiked into 2 ml of blood, and the electrochemical sensor provided statistically significant signal changes even for as few as 2 cells in 1 ml of blood.207 This is one of the very few notable cases in which CTCs post-analysis was also carried out on the same chip or in the same system. Other examples are that of Pratt et al.208 and of Han et al.209 The former used the previously mentioned GEDI microdevice as a platform for a one-step controllable chemical extraction of unbroken nuclei from captured PCa cells, and performed a genetic copy number variation analysis. In the latter, Han et al.209 used a complex microsystem comprising silicon- and glass-based chips to both separate and characterize suspended breast cancer cells in peripheral blood. RBCs were first removed continuously with a magnetophoretic microseparator. Second, a Si chip was used downstream to capture and analyze the remaining cells using EIS and probably to remove the undesired cells. This chip easily distinguished between human breast cancer cell lines at various stages of their pathology (MCF-7, MDA-MB-231, and DA-MB-435) with different electrophysiological properties compared to normal human breast cell line MCF-10A. However, although the EIS analysis showed clear differences between cancer cells and normal ones, it was not clear how exactly the other healthy or undesired cells would be removed from the sample, particularly the WBCs, and with what separation efficiency.
A rather different example implemented twice an immunomagnetic negative selection process by using a multistage magnetic gradient (generated externally by a four-stage concentric circular permanent magnet to trap labeled cells in double trapping areas for increased efficiency and purity. The device was built in a disk-based rotating microfluidic structure for easy fluid handling and processing multiple samples simultaneously. A complex operation cycle employed the centrifugal force and capillary forces to actuate the sample through the fluidic device and to enable the trapping and enumeration of the desired cells. The structure was tested only on a mix of two cell lines (Jurkat and MCF7) and mononuclear cells (MNCs) purified from unprocessed blood. A depletion rate of 99.96% was achieved between the Jurkat and MCF7 cells, while the depletion rate for CD45+-Jurkat cells was predicted to be 99.99%. The average isolation yield for MCF7 cells was ∼65% in a mixture of MCF7 and Jurkat cells, and ∼59% in the MCF7 & MNCs mixture.210
Another microfluidic chip combined multi-orifice flow fractionation (MOFF, a hydrodynamic size-based label-free separation method in its own right, which had been successfully used previously for separation of cells, including CTCs)210,211 as a first enrichment stage to remove excess blood cells using DEP. Although the device successfully demonstrated label-free high-speed continuous flow-through separation of CTCs at a 126 ml/min flow rate, it was not tested on whole blood. Instead, RBCs, WBCs and MCF-7 breast carcinoma cells were first separated by centrifugation from whole blood or harvested from culture, then resuspended and mixed in a working buffer. The MOFFF stage showed a RBC separation efficiency of ∼84% for an optimal flow at a Reynold's number value Re = 70. The DEP part exhibited separation efficiencies of 99.24% and 94.23% for RBCs and WBCs, respectively, and a separation efficiency of 75.18% for MCF-7cells, for which the final enrichment factor was 162.118
A distinct class of devices merged an electrokinetic (typically DEP) with a fluidic-based method as complete label-free CTCs separation. For instance, DEP and immunocapture when combined proved to work synergistically for high purity CTCs isolation. In this case, a Hele-Shaw flow cell included interdigitated electrodes, and its glass surface was functionalized with monoclonal antibody J591 with high specificity to prostate cancer. At low shear rates, changing the DEP parameters (frequency, voltage) allowed increased control flexibility by generating either positive- or negative-DEP (pDEP or nDEP). It either enhanced 2 times or decreased almost 3 times the density of cells captured onto the functionalized surfaces. In a system that combined DEP and immunocapture, nDEP could increase the CTCs adhesion, while nDEP could prevent the leukocytes' specific attachment onto the capture surfaces, and decrease the nonspecific adhesion of WBCs.212 Despite the better results predicted, no updates are available regarding the values for the capture efficacy and purity. Furthermore, Dielectrophoretic–Magnetic-Activated Cell Sorter (iDMACS) emerged as a two-input, multiple-output device that integrated DEP with magnetic separation.213
To conclude, the combination of more types of isolation methods attempted at bringing together the advantages of each method in order to enhance the overall performance. Label-free methods with their simple design and fabrication, may require less (or even no) preparation of the sample and may provide greater throughput than affinity-based methods. Unfortunately, the label-free methods may damage the cells or clog easily and exhibit poor selectivity as they also may isolate noncancerous cells. The results are a low purity and, consequently, false positives.100 Importantly, the affinity-based approaches exhibited larger specificity and sensitivity, hence, exhibited improved capture efficiency and purity. However, once CTCs are trapped with affinity-based methods, it may be very difficult to release them for subsequent analyses. Additionally, the specificity of affinity-based methods may be insufficient or too narrowly defined, as clearly illustrated previously when the unsuitability of EpCAM-based isolation of CTCs was demonstrated. Furthermore, multimarker functionalization is a viable solution for this problem, although it may entail a significant complication of the device and/or a more complex preparation protocol prior to using the LOC microsystem. Moreover, it may also be a highly efficient and promising solution which could deliver the future “gold standard” for CTCs isolation.
H. CTCs culturing
CTCs culturing is an extremely promising technology which may compensate the greatest drawback of CTCs isolation. It can thus provide sufficient material for other subsequent (e.g., genomic) analyses that might reveal vital information about the cancer, even when the initial separation process may provide a very small CTCs count at its output.
CTCs can proliferate as tumor spheroids in serum-free media supplemented with EGF and basic fibroblast growth factor in hypoxia (4% O2), but CTCs culturing has also been recently achieved using an EpCAM-based approach.214 Interesting to highlight that, within 3 months most of the CTCs lines obtained from cultures would generate tumors with histological and immunohistochemical features similar to the primary tumor. However, the success of this method appears to depend on the given therapy and was successful only with CTCs collected from patients who were off therapy or who progressed on treatment.8 Nevertheless, culturing CTCs from patients might lead to the establishment of new approaches for predicting response to therapy and may enable a much easier genotyping to reveal treatment-induced mutation which had previously been absent in the respective primary tumors.8 Culturing CTCs from clinical specimens was formally demonstrated for the first time using negative isolation.215 from peripheral blood of patients with advanced breast cancer.216 Living CTCs could give rise to clones with peculiar resistance and sensitivity to chemotherapeutic drugs119 especially in advanced breast, prostate, and small lung cancer patients.217
On-chip CTCs isolation and culture may be applied to generate patient-derived samples data about drug response and seems to be achieved using negative selection methods instead of positive label-free or marker-based approaches. It is still to be determined if this approach can isolate clones that reflect the metastatic disease or if this method can be superior to standard biopsies, which are increasingly performed at metastatic sites for providing fresh material for DNA sequencing but also for in vitro biologic tests.218 The nucleic acids of CTCs, as well as the circulating nucleic acids or the exosomes may uncover crucial information about the primary tumor and the secondary metastases and could also indicate which targeted drugs may be most efficient for each specific patient, all vital data which cannot be obtained by counting CTCs only.
I. Genetic and molecular analysis of CTCs
The previously mentioned AdnaTest™ was clinically validated in a cohort of PCa patients to detect the seventh variant form of the androgen receptor (Arv7), a biomarker related to resistance to enzatulamide and abiraterone. In this landmark study, patients with Arv7-positive CTCs were associated with resistance to both drugs. Interestingly, the purity of the samples was about 0.1%.219 This demonstrates that low purity, high efficiency may be tolerated for on-chip detection of specific mRNA variants in CTCs.
Stathopoulou et al.220 showed that reverse transcription-polymerase chain reaction (RT-PCR)-based identification of CK19 mRNA positive cells in the peripheral blood of stage I and II breast cancer patients prior the start of an adjuvant therapy is a marker of unsatisfactory clinical outcome. For example, detection of peripheral-blood CK19+ cells before adjuvant therapy was correlated with decreased disease-free interval and OS, while an multivariate analysis confirmed that such an event “was an independent prognostic factor for disease relapse and death.” Similarly, semi-quantitative RT-PCR in CRC patients showed significantly elevated levels of CK19 mRNA, probably originating from circulating malignant cells. Moreover, the increased level of CK19 mRNA associated directly with advancing Dukes' stage and with the serum CEA level, hence the authors concluded that measuring the CK19 mRNA levels may be beneficial for cancer staging and disease monitoring.221
Chip-based digital PCR (dPCR) mutational analyses of CTCs (captured in lung cancer patients with the GILUPI CellCollector) revealed mutations in the KRAS and EGFR genes relevant to treatment decisions, and were confirmed in the primary tumors of CRC patients.9 Chromosome 17 aneusomy observed in 90% of CRC patients with CK20+ CTCs matched with those from the primary tumors.142 Examination of the CRC patients' circulating venous blood by RT-PCR revealed that the cancer-induced marker CK20 mRNA was present in 42.1% of the patients and that it was also significantly correlated with lymph node metastasis. Additionally, the 5-year survival rate for CK20-positive patients was 62.5% in contrast to 87.5% for the CK20-negative patients. The authors concluded that CK20 is strongly related to lymph node metastasis and prognosis, suggesting its usefulness for the diagnosis of CRC recurrence. However, CK20 did not predict liver metastasis.222
Molecular characterization of CTCs in metastatic CRC compared the derived and the original molecular signatures of the metastatic tumor and the primary carcinoma, and recognized genes which facilitate malignant cells' ability to evade apoptosis, invade, and colonize distant tissues during metastatic process.49 The latest metastatic progression models indicated it depends both on pre-determined traits as well as postdissemination genetic instability resulting in clonal evolution that enables the DTCs to continue to evolve independently of the primary tumor of origin.223 Once DTC infiltrated the new microenvironment, they progress, with or without a latent period towards overt metastasis. The collective capability of CTCs “to infiltrate, survive in latency and colonize distant organs” are functional expressions of specific genetic or epigenetic alterations “that provide metastasis initiation, progression and virulence functions.”224 Moreover, CTCs and DTCs have been found to share similar characteristics of genotype and phenotype with certain breast cancer stem cells (CSCs).225 Phenotyping and genotyping of CTCs could provide direct and immediate insight regarding the current disease status, the possible pathways involved in tumor progression and those underlying development of clinical cancer dormancy.116 As such, the postseparation analysis of CTCs may guide treatment decisions and thus enable doctors to follow a much flexible and adaptive treatment regimen.
For instance, CTCs captured using epithelial-based immunomagnetic enrichment were subsequently used for molecular detection of the genes CK-19, mammaglobin, γ-aminobutyric acid type A receptor π subunit (GABA Aπ), B305D-C, and B726P in order to achieve an efficient early detection of breast cancer.226 Another study employed the quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and compared the expression levels of 35 CTC-specific genes encoding the estrogen receptor (ER), including ESR1, in metastatic breast cancer (MBC) and the matching primary tumors. The divergent genetic profiles of CTCs and primary tumor suggested no prognostic consequences, contrary to the alterations in ER-status between primary tumors and CTCs that might have prognostic consequences. For instance, a combination of ER-negative primary tumor and ER-positive CTCs detected in patients prognosticated a 8.5 months median time-to-treatment switch (i.e., the interval between start of first-line and second-line treatment or death, whichever comes first) compared to a 2.1 months in patients with a simultaneous ER-negative tumor and CTCs.227 Moreover, recent results showed special beneficial responses of epithelial CTCs to inhibitors of DAN replication in some newly diagnosed breast cancer patients, while previous studies explained the almost certain existence of drug-resistant subclones prior the therapeutic approach. These rare, drug-refractory subclones or tumor stem cells proliferate predominantly after the treatment exactly as a result of the selective pressure of drug exposure which increased their fitness. Therefore, it is recommended to perform the genomic profile of the primary tumor and of the increasing tumoral population in order to better assess such a possibility and to explore any sensitivities to targeted therapy.20 Additionally, the molecular characterization of CTCs can be a powerful tool in establishing the characterization of the effects of drugs on cell membrane antigens on CTCs, the identification as well the assessment of genetically distinct subpopulations of CTCs and/or their selective reduction as a result of antitumor treatment. For these purposes, a range of protein-based assays, including HER-2, γ-H2AX, epidermal growth factor receptor (EGFR), and insulin-like growth factor-I receptor (IGF-IR) expression, as well as androgen receptor (AR) signaling on CTCs, have been explored as promising PD biomarkers in clinical trials.56 The review of Masuda et al.15 indicates that cultured CTCs from MBC patients exhibit DNA copy number increases in the FGFR1, Myc, CCND1, HER-2, TOP2A, and ZNF217 genes using fluorescence in situ hybridization (FISH) analysis and subsequent phenotype investigation of these cells revealed signatures of recurrent gains in CTCs. Findings of other recent genomic examinations, e.g., research on the premetastatic niche features, are also presented in Masuda's review. HER-2 gene amplification and TP53 gene deletion were observed in circulating hepatocellular carcinoma (HCC) cells detected in the PB of HCC patients.46
Another valuable, noninvasive tool for routine tumor profiling is FISH analysis of CTCs. Its usage in 77 men with metastatic CRPC indicated that patients with CTCs counts >10 exhibited high-level chromosomal amplification of the androgen receptor (AR) gene in 38% of the samples and relative gain of the MYC gene in 56% of samples. 24 out of 42 patients with MYC and 8p FISH results registered MYC/8p ratios larger or equal to 1.5. Also, 12 out of the 25 patients with results for AR, MYC, and 8p, registered AR amplification and gained of MYC relative to 8p copy number. The sample showed substantially increased rate of AR amplification in CTCs compared with those from nontreated confided prostate cancer and from locally recurrent castration-resistant tumor. This suggested the acquisition of the amplification phonotype during progression to the castration-resistant state. High-level amplification of AR was expressed in 35% of the studied patients, and in 50% of the patients with CTCs counts larger than 10 cells per 7.5 ml blood. The results also showed that 73% (29 out of 40) of the abnormal samples expressed cells with FISH patterns consistent with tetraploidy, and that 38% (11 out of the 29) of samples contained various abnormal cells. Notably, the high CTCs count was correlated with polyploidy and with advanced cancer, including metastatic stage. However, an incontestable bidirectional correspondence between the CTC counts and various irregularities cannot be claimed since cells with atypical morphology and abnormal FISH patterns could be detected in many patients even in the absence of obvious CTCs. The authors considered the increased nonspecific abnormal FISH patterns in samples with low CTCs counts as sampling bias.228 Other studies also showed that AR amplification, although not common in primary/diagnostic tumor specimens, had been detected in >50% of castration-resistant lesions, and the AR genomic amplification and copy number gain had frequencies similar to those reported for late-stage tumors. Scher et al.63 indicated that AR amplify-cation and copy gain occurring under the selective pressure of androgen deprivation therapy may represent a marker of sensitivity to a second-generation AR antagonist, and that cells that have initiated true AR-amplification events also developed increased chromosomal instability, demonstrated by additional abnormalities.
Quantitative detection of phosphorylated H2AX (γH2AX) as nuclear DNA damage marker in CTCs was considered to be more sensitive than total CTCs counts alone for assessing drug effects in patients with a variety of advanced malignancies. Cancer cell lines treated with increasing therapeutically relevant doses of topotecan and then spiked into whole blood exhibited a fraction of γH2AX-positive cells that increased in a concentration-dependent manner (though in a strongly nonlinear fashion for some cell types). Another potential application mentioned by the authors for the monitoring of γH2AX signal is to determine the half-life of CTCs after shedding from the tumor, which was considered that could have important both theoretical and diagnostic significance.229
IV. CELL-FREE AND CIRCULATING TUMOR NUCLEIC ACIDS
Cell-free nucleic acids (cfNAs), including cell-free DNA (cfDNA), mRNA, and miRNA, have drawn great attention in the past decade as a valuable blood biomarker in cancer patients.230,231 The presence of cfDNA in body fluids was first time reported seventy years ago by Mandel and Metais.232 Circulating tumor nucleic acids (ctNAs) can arise from apoptosis and necrosis of cancer cells, secretion being also a source of ctNAs,233 and therefore represent the most clinically relevant part of cfNAs. Collection and analysis of ctNAs from liquid biopsies allow precious tumor related genetic information to be obtained and can thus be used for diagnosis and suggest the most efficient patient-tailored therapy, as was briefly highlighted earlier and as will be detailed in depth in the subsequent sections. Additionally, ctDNA can actually be taken up by host cells, and the biology of these cells is affected by this uptake, possibly influencing the overall metastasis evolution.7
The review of Lim et al.49 indicates the important advantages cfDNA and cfRNA exhibit over CTCs.
-
•
They reflect the circulating tumor burden.
-
•
They can be more readily detected even in early disease (when the CTC count is very low, making CTCs detection difficult or the interpretation of the CTCs count problematic), although this may require using sensitive molecular techniques such as qRT-PCR.
-
•
They are reliable biomarkers insensitive to the EMT which significantly impacts many CTCs detection methods.
-
•
They have a clinical utility complementary to that of CTCs.
Thus, while cfNAs presents the highest effectiveness in early disease as a screening and monitoring tool, CTCs have well-known prognostic value. Still, cfRNA may also exhibit prognostic and predictive utility, e.g., correlation with relapse, role in postoperative surveillance or assessing the effect of radiotherapy and their corresponding impact on PFS and OS. Just as CTCs, cfNAs may also be used in addition to standard surveillance methods for enhanced prognosis although they seem to have less utility in treatment response monitoring.49
A. cfDNA
Genetic and epigenetic alterations that are meaningful for the development, progression, and therapeutic resistance can be found in cfDNA. Some of the alterations include change of DNA content,234,235 tumor-specific loss of heterozygosis (LOH),234,236,237 tumor suppressor genes (TSG) mutations,238,239 oncogenes mutations,240,241 as well as epigenetic changes.242
The work of Schwarzenbach et al.243 established a relationship between the occurrence of CTCs and cfDNA in the blood of PCa patients. Both the plasma cfDNA levels and the detection of CTCs significantly correlated with the diagnosis subgroups of localized (stage M0) and metastasized (stage M1) PCa and with the tumor stage of these patients. Thus, CTCs were found in 71% and 92% of the M0 and M1 patients, while mean cfDNA concentrations were 451 ng/ml and 957 ng/ml, respectively. The CTCs count also correlated with tumor stage (mean values of 3.5 and 10 for stage M0 and M1, respectively) and increasing Gleason scores. Furthermore, significant associations of the CTC count with the frequency of allelic imbalances (detected using PCR) at certain markers were observed. Medeiros et al.244 identified a nitric oxide related genetic factor associated with micrometastazation of prostate cancer and hypothesized that genotypes with the a allele of the endothelial nitric oxide synthase gene (ecNOS4a/b) polymorphism may facilitate the survival of CTCs in cancer patients' blood. In another study145 CTCs (captured employing the CPK, not the FDA-approved CEK of CellSearch® typically used by most of the authors) and cfDNA (analyzed using plasma genotyping by droplet digital PCR for EGFR19del, L858R, and T790M) were evaluated in EGFR-mutant NSCLC patients treated with erlotinib until progression. The study found that high levels of cfDNA but not high levels of CTCs correlated with PFS: the median PFS for patients with high baseline cfDNA was 9.3 months vs 14 months for patients with a low baseline level of cfDNA. It was concluded that cfDNA and CTCs are complementary tools, but serial cfDNA monitoring may offer greater clinical utility and be a potentially more effective prognostic biomarker than the CTCs count at the time of disease diagnosis.
Huang and Hoon 52 showed that ctDNA mutations—especially of the B-Raf proto-oncogene—can be used as a prognostic factor for therapeutic response in melanoma patients. Their results confirmed that the absence of ctDNA mutations at baseline may be a prognostic marker for better disease outcome. Additionally, ctDNA mutations may also be used to monitor the response to therapy in stage IV melanoma patients. Furthermore, analyzed ctDNA in melanoma patients with a novel single-molecule digital sequencing assay identified rare genomic alterations at significantly high sensitivity and specificity. It also provided a valuable comprehensive detection of clinically applicable mutations in melanoma patients. Microsatellite instability and loss of heterozygosity detected in ctDNA correlate with clinical outcomes, such as a decrease in survival rate and correlation with systemic treatment response. Similarly, the presence of a methylated gene was significantly associated with lower OS.
Changes in cfDNA quantity are able to elucidate information related to pathology of cancer as well as cell cycle.245,246 Liu et al.247 analyzed cfDNA content in serum samples with a microfluidic chip, employing “microfluidic cylindrical illumination confocal spectroscopy and fluorescence burst size analysis” to determine the size of the cfDNA in the chip. Their assay was performed on lung cancer patients' serum samples using a simple DNA intercalating dye, that did not require DNA isolation or amplification procedures. They demonstrated the feasibility of using microfluidic single-molecule spectroscopy as an easy-to-use alternative for RT-PCR to analyze cfDNA content.
Regions of DNA instability or loss of heterozygosity have been found in many types of cancers and can be used as a biomarker in liquid biopsy for tumor screening.248,249 Erikson et al.250 developed a microfluidic device composed of PDMS and glass that used an electrokinetic-based approach. The device is capable of detecting single-nucleotide polymorphism (SNP) which could lead to LOH. By applying external electric potentials, the device was able to achieve precise control of coupled thermal, shear, and electrical energies. Consequently, pumping, flow regulators, heating elements and temperature sensors were not needed. Magnetic beads can also be used to immobilize the DNA probe. Wang et al.251 combined electrophoretic driving together with magnetic beads-based sandwich hybridization to detect SNP in oral cancer. The target DNA is hybridized with a capture probe together with a signal probe, forming a “sandwich” structure. The magnetic beads can then capture the resulting complex and produce a fluorescence signal which can be detected by an LIF detector. Their method could achieve sensitive and reliable detection of SNP without using any complex sample labeling procedures.
Tumor suppressor genes (TSGs) have high frequency of mutations in many cancer types and contributed to progression of tumor.238 One example is the BRCA1 gene, a TSG commonly found mutated in breast and ovarian cancer patients.252 Xu et al.253 designed a PMMA microfluidic chip embedded with a planar waveguide to detect mutations in BRCA1. The surface of the chip was chemically activated and covalently attached with DNA probe. A noncontact microspotter was used to print the probes to the microfluidic channels. Hybridization of targets with the probes was detected with an imaging microscope with high resolution and large field-of-view. With this approach, they could detect cfDNA polymorphism present in less than 1% of the total DNA content.
Microfluidic devices have been developed for detection of oncogene mutations as well. Das et al.254 reported the first electrochemical approach for direct detection of mutated cfNAs from serum samples. They fabricated an electrochemical clamp assay chip with molecules specific for different nucleic acid sequences and clamp molecules for elimination of cross reactivity [Fig. 4(a)]. They proved the detection of cancer-related mutation in cfNAs without enzymatically amplifying the sequences. Their chip enables specific, rapid and sensitive detection of sequences from 5 fg of isolated RNA by utilizing a peptide nucleic acid (PNA)-modified microsensor. The chip accurately detected mutated sequences in serum samples of lung cancer and melanoma patients. This chip-based method brings several advantages over existing methods: capability to utilize unpurified serum to achieve the same accuracy as PCR, significantly shorter sample volume and analysis time, and lower cost per test. Another approach employed droplet-based devices for detection of oncogene mutations. Pekin et al.255 developed a microfluidic chip for microdroplet-based digital PCR on the chip. They compartmentalized genomic DNA (gDNA) together with Taqman®-probes specific for mutant and wild-type genes in droplets. Green and red fluorescence signals enabled quantitative determination of mutated KRAS oncogene in the gDNA. The system was sensitive enough for detecting cfDNA in blood, stool, lymph samples and showed great specificity in detection of oncogene mutation. Miotke et al.256 proposed a microfluidic platform for highly sensitive detection and quantification of DNA copy number and variation in single nucleotides. It used droplet digital polymerase chain reaction (ddPCR), and its effectiveness was further enhanced by using EvaGreen to better differentiate between target and reference DNA. The target and reference products were independently quantified by manipulating the length of their respective amplicons. In this way, the cost and need for optimization was significantly reduced from the commonly used two-color fluorescent-oligonucleotide probe (TaqMan) design. The system demonstrated the detection of copy number changes in proto-oncogene FLT3 and point mutation in oncogene BRAF.
FIG. 4.
Microfluidic approaches for cfNA isolation: (a) The sensor-based clamp-chip for electrochemical analysis of cfNA mutations developed by Das et al. The microsensor is modified by a PNA probe and only targets mutant nucleic acids. Nontarget mutants and wild-type sequences are washed away. Electrochemical readout is generated by an electrocatalytic reporter system. cfNA hybridization is observed by differential pulse voltammetry signal changes. Reproduced with permission from Das et al., Nat. Chem. 7(7), 569 (2015). Copyright 2015 Nature Publishing Group. (b) On-chip surface acoustic wave lysis and ion-exchange nanomembrane detection of exosomal RNA for pancreatic cancer study and diagnosis developed by Taller et al. Reproduced with permission from Taller et al., Lab Chip 15(7), 1656–1666 (2015). Copyright 2015 The Royal Society of Chemistry.
In the past decade, next-generation sequencing (NGS) has been extensively employed to study DNA and RNA mutations using quantifiable and digital data.257 NGS reveals genetic alterations in cancer by comparing tumor related sequence to the wild-type somatic sequences. NGS could be especially useful in studying heterogeneous tumors, for example head and neck squamous cell carcinoma (HNSCC),258 PCa259 and CRC.260 Russo et al.260 first reported the usage of single molecule, real time, circular consensus sequencing (SMRT-CCS) to detect mutations in stool DNA samples of CRC patients. Mutations at 0.5% frequency could be detected using this noninvasive and sensitive assay. The detection was achieved with commercially available PacBio RS technology. Apart from the PacBio RS technology, nanopore sequencing is another technology developed for sequencing a single molecule of DNA without the need of amplification. It relies on the detection of different electrical or optical signals during the transit of DNA through a nanopore.261 The movement of the DNA strands loaded onto a lipid bilayer can be driven by an externally applied voltage. In this manner, the DNA strand does not need to be modified for sequencing. Clarke et al.262 used this technology to detect monophosphates and to distinguish methylated cytosines in a single DNA molecule. This is useful for detecting single base pair mutation as well as DNA methylation commonly found in cancer.
In addition to DNA mutation, epigenetic alterations also strongly influence the development and progression of cancer. Methylated DNA has been found to be present in liquid biopsy of various types of cancer.263–268 Since detecting cfDNA methylation is becoming a promising approach in cancer prognosis, microfluidic technologies have been actively developed for analyzing DNA methylation.269 Wang et al.270 developed a microfluidic system that could detect the DNA methylation present in ovarian cancer. They integrated the DNA methylation assay onto a chip, together with the stages for DNA isolation, HpaII/MspI endonuclease digestion, and nucleic acid amplification. Compared with standard detection assays using bisulfite treatment and endonuclease digestion, their automated system presented a rapid way of diagnosing ovarian cancer using liquid biopsy samples. Furthermore, a comparison of the sensitivity and specificity of detecting tumor-specific mutations in circulating tumor ctDNA was necessary. Recently, von Bubnoff et al.271 provided information on the various technologies and compared ELISA, amplicon seq/dPCR, CellSearch, hybrid-capture seq, Cobas. BEAMing, BEAMing, AS_qPCR for various breast, non-small cell lung, colorectal cancers, gastrointestinal stromal tumors, and melanoma.
B. cfRNA, mRNA, and miRNA
In spite of the increased RNAse content in the blood stream, cfRNA is possible to be detected in the blood using reverse transcription real-time PCR (RT-PCR) since they are packaged into exosomes and are stable.272,273 Compared with PCR and sequencing assays, LOC methods can be designed for automated sample analysis in a cost-effective way.274
Molecular methods that use RT-PCR to amplify target messenger RNA (mRNA) can easily distinguish CTCs from WBCs, one of the key problems faced by many CTC separation methods, and the usage of multimarker assays also provides increased sensitivity. However, there also are serious concerns related to the extraction, storage, and processing of the relatively unstable mRNA that may lead to loss of sensitivity and misidentification of tumor-specific markers, resulting in false-positives due to low-level of marker expression in noncancerous or benign cells, or in free nucleic acids that can be present in blood.225 The report of Madhavan et al.275 documented the capability of circulating miRNAs to discriminate metastatic breast cancer (MBC) patients depending on their CTCs status by establishing a panel of circulating miRNAs that proved capable of differentiating MBC cases from healthy controls. Among these, miR-200b emerged as the best parameter for differentiating CTC-positive from CTC-negative MBC patients, and it was also “the most accurate miRNA individually for predicting PFS and OS, and its prediction accuracy increased by a small margin when used in combination with CTCs.” The report also highlighted the potential of circulating miRNAs as prognostic markers which was considered to be similar or even better than that of CTCs. Additionally, their stability and the relatively cheap methods used for their isolation and detection make circulating miRNA an increasingly attractive and useful biomarker for prognostication and prediction in MBC either alone or in combination with CTCs and which could thus be used as an early detection marker of metastasis. Huang and Hoon52 used a modified qRT-PCR directly-in-plasma assay to identify circulating miRNAs in a low volume of plasma (10 ml) of cutaneous malignant melanoma patients and assess its clinical utility. The results revealed that the cmiR-210 level was much higher in the disease-free patients than in normal, healthy donors, and found significant correlation between poor prognosis and increased levels of cmiR-210 prior the systemic recurrence specific to stage III patients.
A number of LOC devices have been designed to detect and analyze cfRNA, as summarized in Table IV. One example of a microfluidic chips for isolation of miRNA in liquid biopsy from pancreatic cancer patients is presented in Fig. 4(b).276 Imaging techniques can also be used to identify miRNA of interest. Roy et al.277 reported a microarray device to detect miRNA by differential interference contrast (DIC) imaging.
TABLE IV.
Summary of previously reported microfluidics devices for isolation and characterization of cfNA.
| Disease | Biomarker | Detection technique | Reference |
|---|---|---|---|
| Lung cancer | Change in DNA content | Microfluidic single molecule spectroscopy | 247 |
| Several cancer types | Single-nucleotide polymorphism—SNP | Electrokinetically driven microfluidic control | 250 |
| Oral cancer | Single-nucleotide polymorphism—SNP | Magnetic beads-based “sandwich” hybridization | 251 |
| Breast cancer | TSG mutation | Integrated waveguide for microarray | 253 |
| Lung cancer and melanoma | Oncogene mutation | Electrochemical clamp assay | 254 |
| Several cancer types | Oncogene mutation | Droplet-based digital PCR Single Color Droplet Digital PCR | 255 |
| Ovarian cancer | DNA methylation | Digestion-based PCR assay | 270 |
| Colon cancer | DNA methylation | Microfluidic-based surface plasmon resonance (SPR) detection | 280 |
| Pancreatic cancer | miRNA in exosome | SAW lysis and ion-exchange nanomembrane detection | 276 |
| Glioblastoma | miRNA in exosome | Immunoaffinity, antibody-binding | 281 |
| Melanoma | miRNA in exosome | Electrophoresis-driven microfluidic filtration | 282 |
| Glioblastoma | miRNA | Differential interference contrast (DIC) imaging technique | 277 |
| Breast cancer | miRNA | Photonic crystals fabricated from silicon, and resonance coupling laser scanning | 278 |
By utilizing DIC to image individual reporting gold nanoparticles (NPs) tagged to capture the desired miRNA sequence, as many as 300 copies of target miRNAs in a sample volume of 1 μl could be detected. The resolution of this microarray technique was higher than that of fluorescence scanning, and the need to amplify the target miRNA sequence present in liquid biopsy samples was also avoided. Another method for optical detection of cell-free miRNA was developed by George et al.278 It employed a silicon-based photonic crystal (PC) sensor for detection of RNA and other biomarkers. This was the first silicon-based PC-enhanced fluorescence (PCEF) detection platform for detection of cancer biomarker with sensitivity at pg/ml level. In order to successfully detect such minute quantities of miRNA, the PCEF was fabricated on a SiO2 and TiO2 substrate having very low autofluorescence. The incident light was coupled to a PC resonant mode to further enhance detection of weak fluorescent signal from the fluorescent microarray. A breast cancer miRNA biomarker miR-21 was detectable at a concentration of 0.6pM. In recent years, FDA-approved several platforms and assays to facilitate nucleic acid-based viral and cancer diagnostics. Egatz-Gomez et al.279 presented both groups: the FDA-approved platforms for genomic and messenger viral RNA as well as the miRNA-based tests aiming at a better guiding therapeutic management. The direct clinical application of the tests based on the differential expression of miRNA biomarkers is: the classification of cancer of unknown primary origin, lung cancer type classification, thyroid cancer stratification, kidney cancer type and breast cancer metastasis and recurrence analysis. However, despite these promising preliminary data, more evidence is required to prove its usefulness in clinical applications.
V. EXOSOMES
Currently, exosomes are regarded as distinct cellular entities specifically capable of carrying various cargos to be shared between cells.283–289 It is generally admitted that exosomes are small, lipid bilayer membrane nanovesicles of endocytic origin with a highly selective and variable content, secreted both by nearly all cells in vitro and in vivo290,291 and involved in intercellular communication either in physiologic or pathological conditions.292 Exosomes have several morphological characteristics in common: size, density, shape, general lipid or protein composition and subcellular origin.285,286,288,293 Exosomes also transport diverse cellular constituents from their parent cells, including proteins,294 mRNA and miRNA,295,296 DNA,297 and have been shown to play several roles in modulating the tumor microenvironment.298 Generally, malignancies are associated with high levels of exosomes released into blood circulation, fact which increases the diagnostic value of exosomes.294,299
As exosomes represent an enriched source of biomolecules, such as proteins and nucleic acids, it is hypothesized that they provide a peripheral exosomal-derived type of biomarker for malignancies, neurodegenerative, cardiovascular, or other diseases that would be more reliable than cerebrospinal fluid (CSF), blood, or urine samples analysis.300 The relevance of exosomes is highlighted in the review of Alberter et al.,301 who indicated that in pancreatic ductal adenocarcinoma the amount of exosomes in the blood correlated with disease stage, and even early stages of disease were reliably detected with 100% sensitivity and specificity. Recently, Barile and Vassalli302 reviewed the current understanding of physiological and pathophysiological roles of the exosomes, their potential as disease biomarkers, and drug delivery systems as well as their prognostic relevance. They presented the ongoing clinical studies of diagnostic exosomes and EV markers.
However, the major clinical application of exosomes for diagnostic purposes raised unique challenges, originating primarily in their morphological heterogeneity and their secretion. Generally, the protocols for isolation and analysis of microvesicles from blood are laborious281,303 and the conventional assays typically involved time-consuming, low recovery and purity ultracentrifugation to concentrate large volumes of samples and extensive processing for detection (Western blot, enzyme-linked immunosorbent assays—ELISA).290
Since the current methods are difficult to be implemented in clinical settings, particularly in studies that implicate a large amount of samples or rare molecular targets, new technologies emerged as a series of miniaturized systems, microfluidic technology has been adapted to address both isolation and analysis. The currently used microfluidics-based exosome separation methods are classified into three categories:
-
•
size based,
-
•
dynamic, and
-
•
immune-affinity-based.
“Size-based” exosome separation devices include nanofilters, nanoporous membranes, or nanoarrays to trap the vesicles when fluids flow through the channel. For instance, one sandwich-like device comprised one detachable membrane filter (with 0.1 μm pores), two permanent ring magnets to size-selectively enrich exosomes from large sample volumes304 and one capillary layer, introduced beneath the membrane to guide the purified exosomes to the collection channel. The design305 enabled filter units to be easily replaced during processing of large samples. Recently, an acoustic nanofilter system separated exosomes in a contact-free continuous flow. Ultrasound standing waves exerted differential acoustic force on the vesicles which were separated by size and density. The procedure registered high-resolution separation, high yield, as well as a capacity for fast and effective exosome isolation from small samples.306 Recently, the method employing the nano-DLD arrays built within a microchannel quantification of the exosomes and other demonstrated the high-resolution separation of particles of diameters from 20 to 110 nm, inclusive of exosomes. Therefore, it showed potential for on-chip sorting.
“Dynamic” methods were developed to integrate external forces, such as ultrasound or flow field-flow fractionation (FIFFF) into new microfluidics technologies for faster and easier separation of exosomes. For instance, Davies et al.282 avoided usage of antibody selection and developed a microfluidic filtration system to isolate exosomes and derive mRNA from whole blood samples. Their method avoided laborious centrifugation and antibody-based affinity purification. By integrating a porous polymer monolithic membrane (PPM) into a PMMA chip, vesicles could be separated from cells and debris according to their sizes. By adjusting the size of the membrane pores, they were able to selectively isolate exosomes and reject cellular components. Moreover, another acoustic nanofilter system separated EVs by density and size with 80% recovery rate for exosomes.306 However, the data on the FIFF device concluded the noncomplicated nanostructure and not the purity and recovery rate of the separated subcellular species in the studied samples.307
“Immunoaffinity-based microfluidics” devices achieved specific separation since it relied on specific biomarkers on the exosomes' membranes. These devices comprised modified microchannels with antibodies or magnetic beads with adapted affinity. One example, as shown in Fig. 5(a), is the ExoChip, a microfluidic device fabricated from PDMS and functionalized with antibodies against CD63, an antigen commonly overexpressed in exosomes. This device enabled profiling and quantifying of exosomes when subsequent specific staining with a fluorescent carbocyanine dye (DiO), immuno-electron-microscopy and Western blotting of the recovered exosomes with intact RNA were applied. Similar solutions have been developed to facilitate rapid, accurate, highly sensitive screening and highly specificity diagnostic tests. The microfluidic devices which use microscale channels to operate small liquid samples and carries reactions in parallel were coupled with the emerging multidimensional nanostructures. The application of multidimensional nanostructures, including nanoparticles,299,308 nanopillars, nanowires,309 nanoporous layers, and graphene-based materials58,310,311 integrated with microfluidic channels facilitates biomolecular and cellular separation, and these examples provide an indication of how microfluidic technologies integrating nanostructures have many potential biomedical and biological end-uses.299,304,306,312–318 Liu et al.311 developed a ciliated nanowire-on-micropillar structure to isolate exosomes using only conventional microfabrication techniques. The microscaled fluid channels allowed efficient processing of a small sample (∼100 μl) at low risk of clogging. Another example is depicted by Fig. 5(b). The newly emerged devices were designed upon methods to collect intact exosomes directly from biological samples and replace ultracentrifugation or other precipitation methods. ExoSearch [Fig. 5(c)] also developed as a robust design for in situ detection and analysis.319
FIG. 5.
Different LOC solutions for exosome isolation: (a) The ExoChip (top) and its operation procedure (bottom) used for exosomes isolation and analysis. Reproduced with permission from Kanwar et al., Lab Chip 14(11), 1891–1900 (2014). Copyright 2014 The Royal Society of Chemistry. (b) ExoSearch: a robust, continuous-flow design provides enriched preparation of blood plasma exosomes for in situ, multiplexed detection using immunomagnetic beads for quantitative isolation and release of blood plasma exosomes in a wide range of preparation volumes. Reproduced with permission from Zhao et al., Lab Chip 16(3), 489–496 (2016). Copyright 2016 The Royal Society of Chemistry. (c) Nano-IMEX: (A) Schematic of a single-channel PDMS/glass device, with the exploded-view highlighting the coated PDMS chip containing an array of Y-shaped microposts. (B) Surface of the channel and microposts coated with graphene oxide and polydopamine as a nanostructured interface for the sandwich ELISA of exosomes with enzymatic fluorescence signal amplification. Reproduced with permission from Zhang et al., Lab Chip 16(16), 3033–3042 (2016). Copyright 2016 The Royal Society of Chemistry.
While different chromatographic methods were developed for isolation of biomolecule, proteins, enzymes, or antibodies, size exclusion chromatography (SEC) was used in combination with ultrafiltration by Benedikter et al.320 for exosomes purification. In their experiment, the sample underwent centrifugation and ultrafiltration before SEC for size extrusion of the exosomes. The main advantage of the method relied on the use of buffer with controllable osmolarity and viscosity, which prevented the loss of biological function. However, there was a risk of sample contamination with lipoprotein and protein aggregation.321 An quantitative evaluation and optimization of the previous method was presented in Ref. 322 The method was tested by spiking healthy plasma with cancer cell-derived exosomes. The study showed that cancer extracellular vesicles-associated proteins were detectable by nano-LC-MS/MS.
Nuclear magnetic resonance implemented on a microfluidic platform (μNMR) was also used for magnetic detection and analysis of exosomes. It was designed to enrich the exosomes previously labeled magnetically and to detect exosomal protein markers. Petersen et al.323 demonstrated the efficacy of field flow fractionation methods in separating exosome subpopulations without immune-affinity or other labeling steps.323 Moreover, the nanoplasmonic exosome detector, in which SPR is used for label-free exosome sensing, added to the existing miniaturized systems for sample preparation (microfluidics) and for protein analyses (analytical tools).324 The i-MER platform was designed as one small device to support exosome enrichment, RNA extraction, reverse transcription, and analysis in real-time of distinct RNA targets. The procedure included the capture of cancer exosomes present in serum onto magnetic microbeads with affinity ligands (i.e., anti-CD63 and anti-EGFR). Once the exosomal population was immuno-enriched it was lysed and the lysate flew through a glass bead filter. The packed glass beads efficiently adsorbed RNA onto their surface. The subsequent steps consisted of RNA extraction followed by the reverse-transcription for real-time amplification and quantification.318 Recently, one surface-enhanced Raman scattering (SERS)-based procedure for tumor-derived exosomes was described for qualitative and quantitative detection. When target exosomes are present in the sample to analyze, they are captured by the SERS nanoprobes and magnetic nanobeads in a form of sandwich-type immunocomplexes. These can be precipitated with a magnet, allowing the SERS signals to be detected in the newly formed precipitates.36 In comparison, Chen et al.281 presented another easy and rapid method to isolate exosomes using immunoaffinity. Binding antibodies onto the chip surface was now used to capture exosomes. This was a faster and simpler method of exosome isolation compared with current protocols involving high-speed centrifugation and filtration.
The most commonly used techniques to purify exosomes and their related clinical potential are briefly presented in Table V. The recent work addressed the clinical potential of the conventional and of the newly emerged microfluidic-based devices.314,325,326 In contrast with the detection deficiencies of CellSearch®, exosomes have been instantly detectable in early- and late-stage pancreatic cancer, melanoma, glioblastoma, and other cancers for which CTCs isolation is currently difficult or even impossible.327–329 Despite the advances of new technologies,330 there is a stringent need of standardized performance of the existing models towards a higher reproducibility of the exosomes' recovery despite the variations within samples.
TABLE V.
Summary of exosomes' isolation techniques and applications.
| Isolation technique | Applications | Reference |
|---|---|---|
| Differential ultracentrifugation—conventional technique | The most common technique in research with limited clinical: Annexin V, the tetraspannin CD63, the heat shock protein Hsp70 and TSG101 Microfluidic platforms: porous microstructure/size filtration (not validated with clinical samples, and no analysis of cargo protein or RNA) |
44, 282, 293, 299, 303, and 331–337 |
| Density gradient ultracentrifugation | Commercial kit-OptiPrep | 303, 331, 338, and 339 |
| Micro-/ultrafiltration and other size exclusion methods | Isolation of exosomes from small (0.5–10 ml) samples Microfluidic platforms: porous microstructure/Size filtration (no clinical validation) |
44, 282, 299, 332, 335, 337, and 340–343 |
| Solvent precipitation | ExoQuick™ (System Bioscience) | 344 and 345 |
| Total Exosome Isolation™ (Life Technologies) | ||
| Exospin (Cell Guidance System) commercial kit | ||
| Immunoaffinity capture (IAC) | Liquid biopsy | 11, 281, 296, 303, 312, 314, 318, 319, 324, and 345–350 |
Microfluidic platforms:
|
||
| Nanoshearing chip, electrohydrodynamic flow assisted IC: high sensitivity, inline detection | ||
| Electric Field Induced Release and Measurement (EFIRM) technique: RNA and protein molecules | ||
| Field flow fractionation (FFF) | High consistency and specificity | 307, 323, 351, and 352 |
In vivo—mouse melanoma B16-F10 cell line
|
||
| Nanoparticle tracking analysis | EC-EXs and EPC-EXs:CD105/CD144, CD34/ KDR | 353–356 |
| Size exclusion chromatography (SEC) and mini-SEC | Mini-SEC: solid tumor (head and neck squamous cell carcinoma) and acute myeloid leukemia (AML): CD9, TSG101; TGF-b1-associated pro-peptide and latency-associated protein (LAP), PD-1, PD-L1, COX-2, FasL, or CD39/CD73 ecto-enzymes; leukemia blast-relevant proteins: CD34, CD44, CD96, CD123 and CLL-1; TEX alter functions of activated NK cells or T cells | 333 and 357–360 |
Once isolated, exosomes which usually exist in large amounts in biological fluids have to be analyzed from banked and frozen biological samples361 for specific markers which characterize the primary tumor, the pathological process and the tumoral microenvironment.294,299 The morphological and the functional aspects were considered for the analysis of exosomes. A plethora of methods were employed, such as Western Blot (WB), nanoplasmonic colorimetric assay, Atomic Force Microscopy (AFM), scanning Helium Ion Microscopy (HIM), qNano, transmission electron microscopy (TEM) immunofluorescence (IF) assay, reverse transcription real-time PCR (RT-PCR). Each of these methods can contribute with information regarding the particle size and counts, molecular profiles of the transmembrane proteins and of the cargo and the functional aspects of the exosomes. WB results, for instance indicated the exosomes' ability to modify functions of immune cells (NK cells or T cells). The phenotyping of exosomes identified disease-specific protein targets for solid tumors such as
- •
-
•
epidermal growth factor receptor (EGFR) for brain cancers;294,299
-
•
human epidermal growth factor receptor 2 receptor (HER2/neu) for breast cancer;365
-
•
stereotypic markers TSG101, Alix, CD63, metastatic factors, transduction molecules, lipid raft, and lipid raft-associated components for CRC;362 and
-
•
proteoglycan glypican-1 (GPC) for pancreatic cancer with absolute sensitivity and specificity.
Kadota et al.366 summarized the exosomal proteins as possible biomarkers for lung cancer and discussed the previous studies which identified the differential protein profiles of exosomes in various body fluids of patients with lung cancer. Sandfeld-Paulsen et al.367 presented exosomal proteins as individual diagnostic biomarkers and discussed multimarkers models in lung cancer diagnosis. Recently, Minciacchi et al.368 reviewed the advantages and limitations of EV-based liquid biopsy approaches and discussed the studies on protein, DNA and RNA while focusing on the strong promises of interrogation of circulating EV in vivo for prostate cancer. They also presented the workflow for EV detection and analysis from PCa patients' biofluids and the potential clinical use of various commercial miniaturized assays for standardization of EV purification and analysis. The exosomal proteomic profile also revealed, in addition to targeted proteins, nucleic acids: RNAs295,296 and DNAs.297 Subsequent studies evidenced the potential of exosomal DNAs for tumor molecular analysis, therefore as reflective tumor marker.297,369,370 Moreover, the various types of RNAs can also be used as reflective disease markers, since their levels or patterns showed correlation with various types of cancers:
-
•
Exosomal miRNA in lung adenocarcinoma,371–373 pancreatic cancer,276 glioblastoma,281 primary colorectal cancer,374 melanoma,282 ovarian cancer,375 postliver transplantation hepatocellular carcinoma,376 and aggressive urinary bladder cancer.377
-
•
mRNAmutant (EGFRvIIImRNA) and miRNAs in glioblastoma295 and ovarian,375 prostate,23,367,378 and gastric cancers.379
It has been noticed that the alterations in mRNA expression levels clearly had functional consequences of diagnosis importance, therefore the exosome shuttle RNA (esRNA) comprising mRNA and miRNA is foreseen as a biomarker for cancer early diagnosis and surveillance.296 In this direction, plasma exosomal miRNA showed promising prognostic biomarkers for castration-resistant prostate380 and lung cancer381 patients with adequate prospective validation for further evaluation. Moreover, the stability of the exosomal cfRNA makes their detection in the blood possible.272,273 The paper of Siravegna et al.19 compares the clinical applications of liquid biopsy collected ctDNA, CTCs, and exosomes for capturing the molecular heterogeneity of metastatic cancers.
Since the coding and noncoding exosomal RNA profiles proved to be of diagnostic importance, the analytical methods play their crucial roles in this process. Paolini et al.382 recently approached the current available separation and analytical methods and evaluated the impact of their combinations upon the purity and further biophysical and biochemical analysis of the samples, including the impact of the residual matrix upon the biological activity of the exosomes isolated through purification. They presented the biochemical characterization of exosome preparations from 1 ml Multiple Myeloma (MM) pool with four different protocols for exosome preparation: differential centrifugation steps (P3), purification with a precipitation kit (Exo PK), and obtained with discontinuous iodixanol gradient. The samples were electrophoresed and analyzed by Western Blot (WB) for the presence of typical vesicular markers to analyze the influence of separation methods on analysis and biomarker-based diagnosis.
Another example is the nano-interfaced microfluidic exosome (nano-IMEX) chip383 based on a new graphene oxide/polydopamine (GO/PDA) nanocoating technology. It greatly enhanced the detection sensitivity, the dynamic range, and it allowed direct quantitative evaluation of circulating exosomes from smallest samples (2 μl) of unprocessed plasma. This way, it addressed one of the key challenges in the clinical development of exosomes as biomarkers. It also successfully distinguished ovarian cancer cases from healthy controls and quantified the expression of exosomal markers in a patient in response to cancer treatment.11 The development of platforms which employed quantum dots (QDs) and fluorescence nanoparticle tracking analysis (NTA) also allowed the phenotyping of the circulating epithelial tumor-derived exosomes.353,375
Furthermore, several groups focused on point-of-care diagnostic and monitoring platforms which incorporate isolation coupled with a downstream analysis technique. In this direction, Lee et al.306 showed label-free size-based purification of exosomes using an acoustic-based microfluidic device, while Taller et al.276 designed a microfluidic chip for the analysis of exosomal miRNA in pancreatic cancer. They utilized two chips in conjunction [Fig. 4(c)]: one using surface acoustic waves (SAW) for exosome lysis and another with ion-exchange nanomembrane for RNA sensing. A transducer generated SAW, which refracted in the liquid sample and set it in motion; at the same time, electromechanical coupling generates an electric wave. By targeting microRNA (miRNA), they extracted exosomal RNA from pancreatic cancer cell line and achieved a 38% lysis rate as well as a detection limit of 2 pM.
Chen et al.281 presented an immunoaffinity-based microfluidic method for fast and specific isolation of exosomes in serum samples. They obtained microvesicles from small volumes of blood serum and cell culture condition medium to extract high-quality RNA. Furthermore, the Taylor group repurposed MACS to isolate exosomes from serum samples of early stage ovarian cancer.375 Vaidyanathan et al.346 also developed an immunoaffinity-based high specific device to isolate exosomes from an immunocapture site using nanoshearing, a tunable alternating current-based electrohydrodynamic (AC EHD) method. The exosomes captured on this chip were subsequently incubated with antifluorescein HRP antibody and tetramethylbenzidine (TMB) to induce a colorimetric readout for a macroscopic identification. Dudani et al.384 demonstrated the clinical potential of the combination of isolation of exosomes via inertial lift force in microfluidic channels and their quantification via immunocapture. μNMR was used to outline the glioblastoma multiforme (GBM)-derived exosomes. An initial clinical test used the platform to evaluate the exosomes in the blood samples of GBM patients, as well as healthy controls. The results showed that the exosomal protein mapping could contribute effectively to an accurate disease diagnosis and treatment monitoring.299 Davies et al.282 developed a microfluidic filtration system to isolate exosomes and derive mRNA from whole blood samples. They harnessed the electrophoretic mobility difference between soluble proteins and exosomes to increase the RNA extraction yield per unit of protein.
The microfluidic technology significantly increased the limit of detection to ∼106 vesicles/ml, considerably reduced sample depletion or waste, and analysis time, while enabling the investigation of certain biological events previously inaccessible.385 We believe that the constant progress of microfluidic technology for exosome isolation and molecular analysis with focus on immediate clinical applications will ultimately conduct the development of a possible “gold standard” device and method which could be used to isolate, purify and use exosomes as a valuable tool for analysis of liquid biopsies.
VI. PROTEINS
Protein-based biomarkers have been used for screening cancer for decades. For instance, the prostate-specific antigen (PSA) is a protein secreted by prostate cells which has been used as tool for PCa screening since 1990s.240 However, it is still debatable whether PSA testing is accurate for cancer screening.
Alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), cancer antigen 125 (CA125), and carbohydrate antigen 153 (CA153) are the most commonly used cancer biomarkers to validate microfluidic LOC devices.246,386,387 Protein-based biomarkers include peptides, globular proteins, fibrous proteins, and membrane proteins.388 These biomarkers are obtained from various biological samples, including amniotic fluid, blood, saliva, tissue/cells, urine, and more.388,389 The current available, protein-based cancer biomarkers are listed in Table VI. The key limitation of the traditional circulating tumor markers in clinical practice is their lack of specificity, as the plasma serum levels of one specific biomarker are elevated in multiple types of cancer. For instance, a high level of AFP in the serum is present in germ cell tumors, HCC, gastric, pancreatic, colonic and bronchogenic carcinomas. Similarly, CEA used to monitor medullary thyroid cancer is also elevated in gastric, pancreatic, liver, breast, and ovarian cancers. The clinical utility and limitations of other serum biomarkers are detailed in the work of Dakubo.390 To increase the accuracy, some authors proposed to use cocktails of antibodies or a panel of protein-based biomarkers.112
TABLE VI.
Previously reported protein-based biomarkers and their applications.
| Biomarker | Cancer type | Reference |
|---|---|---|
| α-Fetoprotein (AFP) | Testicular, germ cell tumors, HCC | 387, 392, 397, and 398 |
| Aldehyde dehydrogenase (ALDH) | Glioma (GLM), colorectal cancer (CRC) | 399 |
| α-Methylacyl-CoA racemase (AMACR) | Prostate | 388 |
| Androgen-receptor splice variant 7 (AR-V7) | Prostate | 63 |
| Bladder tumor-associated antigen (BTA) | Bladder | 392 and 397 |
| B2M | Multiple Myeloma | 390 |
| Calcitonin | Medullary thyroid | 390 |
| Carcinoma antigen 15-3 (CA15-3) | Breast | 392, 393, 397, 398, and 400 |
| Carcinoma antigen 19-9 (CA19-9) | Colon, pancreatic | 392, 397, 398, and 400 |
| Carcinoma antigen 27-29 (CA27-29) | Breast | 388, 392, 397, and 400 |
| Carcinoma antigen 125 (CA125) | Ovarian | 392, 397, and 398 |
| Carcinoma antigen 242 (CA242) | Colon | 400 |
| Carcinoembryonic antigen (CEA) | Bladder, breast, colon, lung, medullary thyroid | 388, 392, 397, 398, and 400 |
| Carboxypeptidase A4 (CPA4) | Pancreatic | 401 |
| Cytokeroatins (CKs) | Breast, lung | 388, 392, 402, and 403 |
| CYFRA21 | NSCLC | 390 |
| Epidermal growth factor receptor (EGFR) | Colon, lung | 388, 392, and 397 |
| Fibrin degradation protein (FDP) | Bladder | 392, 397, and 402 |
| Human chorionic gonadotropin-β (hCGβ) | Testicular | 392, 397, and 400 |
| Human epidermal growth factor receptor 2 (HER-2) | Breast | 388, 392, 397, 398, 400, and 402–405 |
| KIT | Gastrointestinal stromal (GIST) | 392 and 397 |
| Mucin 1, cell surface associated (MUC-1) | Bladder, breast | 392 and 397 |
| Nuclear matrix protein 22 (NMP22) | Bladder | 392 and 397 |
| Neuron-specific enolase (NSE) | Lung, endocrine, SCLC | 388 |
| Oestrogen and progesterone receptor OPR | Breast | 397 |
| Plasminogen activator type 1 inhibitor (PAI-1) | Breast | 388 |
| Prostate-specific antigen (PSA) | Prostate | 392, 393, 397, and 398 |
| ProGRP | Lung, SCLC | 390 |
| SCCA | Lung | 390 |
| Trefoil factor 3 (TFF3) | Colon | 388 |
| Thyroglobulin | Thyroid | 392, 397, 398, 402, and 405 |
| TIMP metallopeptidase inhibitor 1 (TIMP1) | Colon | 400 |
| Tissue polypeptide antigen (TPA) | Breast | 388 and 400 |
| Urokinase-type plasminogen activator (uPA) | Breast | 388 |
To detect and quantify protein-based biomarkers, researchers use several methods mainly based on immunoaffinity binding, such as ELISA, electrochemical immunoassays, immunohistochemical (IHC) assays and immunomagnetic assays.71,199,388,391–393 Electrophoresis has also been employed to isolate and identify proteins in microfluidic systems.388 Likewise, SAW-and field effect transistor (FET)-based biosensor systems were used for label-free and real-time detection of multiple protein-based biomarkers.388,394 Nanotechnology has also been applied in this field, and both NPs and QDs are commonly used, e.g., for biological labeling and biosensing.395,396
To the general advantages offered by LOC devices such as automatization, high sensitivity, low cost, multifunctionality, and portability, some more specific ones add: microfluidics for cancer diagnostics using protein-based biomarkers are related to the small volume of sample required, sample preparation and detection on the same chip. However, despite these major advantages such microsystems also have a few disadvantages:
-
•
The number of available biomarkers is limited. New biomarkers are needed, especially for efficient early detection of cancer.
-
•
User-friendly tools and methods are required, as current methods require highly qualified staff to employ them correctly.
-
•
Minimally invasive sampling for analysis is also important for patient comfort. Current methods still need to be improved significantly in this respect.
VII. METABOLITES
The complexity of cancer metabolism is widely acknowledged. A number of physiological responses, such as hypoxia,406 low pH,407 and the Warburg Effect,408 have been observed and associated with cancer. Naturally, researchers study the metabolism of these physiological effects in an attempt to discover a “metabolic biomarker” that can be used to diagnose cancer.409 More often than so, and unsurprisingly, a group of metabolic biomarkers, not just one particular metabolic biomarker, have been found to be linked to certain cancers.410 Hypoxia inducible factors (HIFs) have been known to be potential biomarkers of the hypoxic condition of cancer tumor. In turn, the metabolic changes, which include changes to 4-hydroxyproline, fructose, and aspartate, caused by these HIFs have been found to be reflective of metabolic differences between cancer and normal cells.411 The role of phosphoinositide 3-kinase (P3IK) in tumor biology412 has also prompted researchers to look at P3IK-related metabolic biomarkers. Using mass spectrometry (MS) as the detection method, plasma concentrations of metabolites such as amino acids, acylcarnitines, and phosphatidylcholines can be easily determined. The effectiveness of this approach has led to the evaluation of the efficacy of pictilisib, which is a class I P3IK inhibitor, in treating cancer.413 This metabolomics approach has been typically used to screen for metabolic biomarkers reflective of various types of cancers.414,415 Thus, CRC patients can be differentiated from healthy controls by using GC-TOFMS and UPLC-QTOFMS mass spectrometric methods to analyze a panel of metabolites, the results of which are subject to statistical analyses. Not surprisingly, metabolic differences were found in many metabolic processes, including the Krebs and urea cycles.416
The PSA has long been known an unreliable indicator of prostate cancer, but it continues to be a mainstay in prostate cancer diagnosis.417 Progressively, blood plasma and serum samples can potentially serve to differentiate between prostate cancer patients and healthy patients, by using a combination of various detection methods (magnetic resonance spectroscopy, gas chromatography, and mass spectrometry) and univariate/multivariate statistical analyses to provide a better prediction of prostate cancer.418 In a comprehensive study of breast cancer metabolites, 368 out of 468 metabolites were found to be significantly different between cancerous and normal tissues.
Among these, the cytidine-5-monophosphate/penta-decanoic acid ratio was observed to be able to discriminate between cancerous and normal tissues with a detection sensitivity and specificity of 94.8% and 93.9% respectively.419 Blood serum samples of patients with esophageal cancer have also been assessed by metabolomics with great promise, using ultrahigh performance liquid chromatography as the separation method and nuclear magnetic resonance as the detection method.132 Liquid chromatography-mass spectrometry has been utilized to differentiate between the hypermethylated BRCA1 functionality of breast cancer patients from nonmethylated BRCA1 functionality of nonpatients.420 Gas chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry have been used to identify nine metabolites that can distinguish between pancreatic cancer and chronic pancreatitis, which is clearly an important advance in the fight against the deadly pancreatic cancer.421 Fast ultrahigh performance liquid chromatography-tandem mass spectrometry (FPLC-MS) was able to identify glycocholic acid, taurocholic acid, chenodeoxycholic acid, among others, as being significantly different in prostate cancer patients over normal patients.422 UPLC-MS has also identified spermine and isovalerate as biomarkers of endometrial cancer.423 Similarly, both creatine riboside and N-acetylneuraminic acid, which have long been associated with lung cancer, metabolomically display a positive correlation with tumor size.424 Kahlert et al.399 compiled relevant information on the cancer stem cells (CSC) markers and their metabolic signatures and presented the association with distinct metabolites or metabolic genes while discussing the importance of the CSCs' metabolic status in oncogenesis and therapy of glioblastoma (GBM) and colorectal cancer (CRC).
As we can see, metabolomics is an extremely promising method to assess the status of many cancer-related physiological conditions and cancers. However, the method necessarily involves some chromatographic separation of the metabolites and their mass spectrometric (MS) detection. Chromatographic separation is necessary due to the large number of metabolites to assess, and mass spectrometric detection is ideal as it can easily detect a large number of metabolites simultaneously and with very high sensitivity. Given the importance of these two essential steps, they need to be incorporated into a chip in order to realize high-performance metabolomics-based LOC devices for cancer diagnosis.64 To achieve on-chip chromatographic separation, microsolid phase extraction425–428 and liquid chromatography columns429,430 were incorporated into a chip to achieve desalting of the biological sample and separation of the metabolites in the biological sample, respectively. It has also become possible to incorporate electrospray nozzle emitters into a chip that can directly transfer the chromatographed metabolites into the electrospray ionization chamber for MS detection.351,426,431–433 By using a multinozzle emitter array, the metabolomic analyses of biological samples could be carried out with high throughput, thus greatly increasing the turnover frequency of sample analyses.434 Clearly, metabolomic analysis of metabolites using LOC microsystems may soon be realized once on-chip integration of both chromatographic separation and ESI-MS detection becomes possible.
VIII. MATERIALS USED FOR FABRICATION OF LOC DEVICES
The materials most often used for the fabrication of microfluidic systems are PDMS and glass. PDMS is processed using soft lithography.435 which is easy to use, fast and enables low cost proof-of-concept prototyping.436 Although PDMS has very useful properties for biological and microfluidic applications the successfully translation of PDMS-based fabrication technology to mass production is limited mainly due to inherent batch-to-batch variations and slow processing.437,438 Other limitations are related to its porosity and hydrophobicity, thus requiring careful a priori be considerations for the materials to be chosen for each specific application (CTCs enrichment, cfNA, exososmes, and metabolites, etc.). A positive aspect is that PDMS or glass substrates' surfaces can be functionalized via self-assembled silane monolayers, which can then be further modified chemically.
Glass-silicon technology439 has the advantage of robustness. Micropatterning of the silicon surface can be achieved with high accuracy, while chemical functionalization (e.g., silane chemistry) can be also easily performed. The relative high cost of fabricating a device in this technology is compensated by the reusability of the device using on-site cleaning. The main disadvantages remain the expertise required for chip processing and the relative longer processing time. Additionally, despite its high cost, silicon is the only substrate to offer the capability of embedding “intelligence” by incorporating signal conditioning and data processing integrated circuits into the same chip, thus realizing a “smart” chip or microsystem. This is extremely advantageous for highly automated platforms and/or for increased flexibility and ease of use.
Amorphous thermoplastic polymers including PMMA, polycarbonate, and cyclic olefin (co)polymers are other attractive materials for microfluidic devices, e.g., for on-chip liquid biopsy.201,205,253,440 The capability of easily scaling up the fabrication process of devices realized in these materials using injection molding (low fabrication cost) represents their main attraction, while laser cutting or micromilling are other possible alternatives for fast prototyping. Overall, the selection of the materials in which microfluidic devices are to be fabricated is strongly related to the particular method of analysis and/or of detection, as is illustrated next for a few key applications. A microfluidic filter was reported by Mohamed et al.441 consisting of rectangular pillars and fluidic channels and chamber etched in silicon (using deep RIE), and which were sealed using a glass wafer. In contrast, a microfilter device for CTCs enrichment with a series of pool and dam structures fully fabricated in glass was also reported.442 Membrane–based filtration can employ a large range of micropatternable materials such as polycarbonate,443 parylene C,444,445 nickel,146 or silicon.10,73 Microsieves fabrication using microtechnology ensures both fast processing and a very good control of the pore size and of its dispersion (especially for silicon due to the very well controlled deep RIE process).
Microfluidic separation of the CTCs based on inertial forces mainly employed PDMS/glass technology, aspect somehow justified by the simplicity of the device structure. Sun et al.446 report a PDMS-based double-spiral device for separation of cancer cells spiked into the blood. A detailed protocol of CTCs enrichment using hydrodynamic forces in curvilinear PDMS microchannels is presented by Warkiani et al.112 A PDMS/glass device is also used by Tanaka et al.447,448 for inertial separation of cancer cells from RBCs in straight microfluidic channels.
Microfluidics devices for CTCs enrichment based on cell deformability are also based on soft lithography, e.g., the PDMS device realized by Tan et al.449 using an array of traps, each trap consisting of three pillars (3–4 μm in diameter) closely located next to one another along an arc with a radius of 20 μm.
In contrast, CTCs isolation based on electrical properties of the cells (mainly using DEP) involved a wide range of materials/technologies. For example, a PDMS/silicon DEP device with 3D electrodes for label-free counting of human colorectal carcinoma cells from blood was realized by Xing et al.103 The actual microfluidic structure of the device was performed on a SOI wafer, while PDMS was used only for sealing. The cancer cell recovery was 82%, but—compared with other similar DEP-based CTC isolation devices—the main advantage of this structure is a low joule thermal dissipation due to the 3D electrodes and the high thermal conductivity of silicon.450 Most of the DEP devices used to separate cancer cells used thin film electrodes having different shapes and patterned onto a glass substrate, on top of which the microfluidic structure was built in PDMS using soft lithography.
For exosomes isolation a 100 nm porous PMMA membrane was successfully used for their filtration.282 Another implementation for exosomes isolation employed an acoustic nanofilter.306 in a PDMS microfluidic channel bonded on a piezoelectric substrate (LiNO3) on which a pair of interdigitated transducer electrodes were patterned. The μNMR filter previously mentioned299 is another PDMS/glass structure while the ExoChip is a fully PDMS-based microfluidic device.314
A recent development is the application of nanostructures or nanostructured surfaces for cell migration, proliferation, or differentiation. The nanostructured surface (obtained by using nanofibers, nanogroves, or nanotips) enhances cell adhesion due to its large surface area. This principle was applied using different types of nanostructures for cancer cell isolation.451 Wang et al.162 used a silicon nanopillar array (SiNPA) coated with anti-EpCAM to increase significantly the efficiency of capture of CTCs by intensifying local interactions between the nanopillar structure and the nanoscale features of the cellular surface, such as microvili and filipodia. This SiNPA was integrated together with a microfluidic chaotic mixer in a PDMS/silicon microfluidic structure.161 Lee et al.309 used a surface functionalized quartz nanowire array (QNWA) integrated with laser scanning cytometry for identification of CTCs. Nanostructured PDMS functionalized with anti-EGFR aptamers was used for CTCs isolation, as the nanotopography of the PDMS substrate increased the efficiency of trapping by augmenting the aptamer immobilization area.452 Nevertheless, coating the surface with functionalized nanoparticles can also be used as an easier alternative for CTCs enrichment.453
A wide range of materials were used for the exosome isolation. Rho et al.304 al used classical soft lithography to define the microfluidic circuit ingeniously integrated with Polycarbonate membrane filters (400 nm pore diameter; Nuclepore, Whatman) using magnetic clamping. Meanwhile, vesicles could be separated from cells and debris filtration process . Davies et al.282 integrated a porous polymer monolithic membrane into a PMMA chip. PDMS/glass material are mainly used for the isolation of exosomes using immunoaffinity-based microfluidics devices. The advantage these materials offered was the easy surface modification of glass surface.314 Nucleic acid detection was performed either using PDMS/glass chips (Erikson et al.250) or thermoplastic polymers such as PMMA (Xu et al.253).
IX. CONCLUSIONS
CellSearch® is currently the de facto standard widely used by the majority of clinicians for CTCs isolation, mainly because it is the only FDA-approved CTCs isolation method in breast, colon, and prostate cancer, and also because it allows an easy comparison with the already vast number of results previously published in the literature. However, although they have some advantages and are popular, EpCAM-based CTCs isolation in general, and CellSearch®, in particular, are afflicted by serious deficiencies. Consequently, many novel CTCs isolation devices have appeared lately, all aiming to replace CellSearch® by providing enhanced performance and/or the possibility to extract the isolated CTCs and subsequently analyze them in order to extract vital information, e.g., genomic, about the cancer. Yet, despite many recent efforts to develop and test numerous novel devices/methods for isolation of CTCs, an ideal technology optimized for this purpose that could serve as a new “golden standard” is still unavailable. Nevertheless, the isolation and analysis of CTCs and their usage as biomarkers for cancer diagnosis has benefited from the development of specific LOC devices and their corresponding analytical methods, as was underlined by the large number of recent publications and commercialized devices. A few years ago, we suggested100 that the combination of two isolation methods “can be not only an interesting approach but a direction to be followed” (giving the correct credit to Moon et al.118 and Han et al.209). The body of research reviewed in this paper highlighted that this has indeed been the approach followed to obtain high-performance separation of CTCs, many of which claim to be better than CellSearch®. Therefore, the future “golden standard” will most probably combine two (or maybe several?) separation methods. Furthermore, such an equipment should most probably be miniaturized, and may also comprise postseparation analysis and embedded intelligence (both in hardware and software) for signal conditioning as well as data processing and interpretation. This is in line with the current trend and demand for realizing highly sophisticated “smart” systems with increased functionality and ease of use at the point of care even by nonspecialists. Furthermore, the realization and standardized adoption and usage of such advanced equipment could be the first step toward personalized healthcare. It may also bring closer the more distant goal of realizing a machine for holistic separation and analysis of all liquid biopsy components. Such a tool is more than necessary in order to ultimately provide highly efficient predictive cancer medicine for truly personalized diagnosis and treatment. In any case, although engineering challenges do exist, the main hurdles will not be only technical. The complexity and difficulty of clinical trials and validation will also need to be overcome in order to pave the way to the wide acceptance of such an equipment by medical practitioners worldwide.
Besides the detection and characterization of CTCs, their culturing and/or their postprocessing are also becoming increasingly important. Particularly, the genomic analyses can reveal a wealth of crucial information about the patient's disease. However, for CTCs, the main challenge for cancer detection in body fluids other than PB remains the relative reduced concentration of tumor specific cfNAs, proteins, exosomes, or metabolites in a liquid biopsy sample. Moreover, this relative low number is fluctuating as a result of biological variations. In order to address this and other shortcomings, microfluidic devices can offer a multitude of approaches which were described in this work.
The usage of exosomes as elements of intercellular communication and active contributors to malignant processes makes them highly attractive targets for detection and analysis of cancer. As a number of studies demonstrated,281,282,312,314,318,319,324 the specific proteins and nucleic acids in the exosomes' cargo—if enriched prior to the analytical readout—could be an important and valuable source of biomarkers for cancer diagnosis and therapeutic monitoring. This conclusion was supported by various optimistic features of the exosomes' extracted from fresh or frozen biofluids. The high quality of the coding and noncoding RNA was considered an avenue towards the development of specific assays to detect mutations, and changes in expressions of RNA from both living and apoptotic cells. Moreover, the RNA analysis of the exosomes from liquid biopsy samples could complement the information coming from classical tumor DNA analysis and clarify specific cancer processes and eventually lead towards personalized medicine. The support given to cancer biomarker focused research stemmed from the unique features of malignant diseases known as highly dynamic processes with turning points upon internal or external inputs including the therapeutics themselves. Considering such aspects, liquid biopsy may become not only a noninvasive diagnostic method using microfluidic devices but may also turn out to be a key strategy/method for developing a personalized targeted therapy and its subsequent patient-tailored monitoring. New approaches were discussed to identify the potential fields for implementation of liquid biopsies in melanoma, knowing that this particular type of skin cancer is highly metastasizing one with no easily accessible mutational analysis and adequate predictive analysis that direct therapy timely decision. The modalities discussed included the use of
-
•
CTCs and the role of CellSearch platform in enumeration of the immune-magnetically captured and fluorescently labeled CS146 and HMW-MAA+ cells from whole blood,
-
•
ctDNA and its relevance for preradiological detection of disease progression and the mechanism of resistance,
-
•
circulating mRNA and exosomal mRNA as melanoma biomarkers in rapport with applied chemotherapy, and
-
•
miRNA and its potential to detect disease recurrence and progression in order to clarify the consistency.454
The emerging technology for captures and characterization, including the deep sequencing technology, of liquid biopsy components is the pathway to biomarkers and genomic profiles discovery, validation, and standardization for clinical translation. Consequently, in 2016, the detection of EGFR in ctDNA by the Cobas EGFR Mutation Test v2 achieved FDA approvals a prerequisite for treatment with erlotinib.455 At present, the routine clinical use of ctDNA analysis is limited to commercially available cobasR (Roche Molecular Systems, Pleasanton, CA, USA), therascreeenR EGFR (Qiagen, Hilden Germany), or validated FDA and EMA-approved noncommercial tests for NSCLS. However, significant progress is still required to further address the perfect match between the type of the biofluid collected from cancer patients and the desired lab application in order to maximize the potential of the extracted biomarkers. For instance, CTCs could be beneficial for drug resistance monitoring, while cfDNA would be more sensitive when mutations are diagnosed. Moreover, early detection of malignancies could be facilitated by the isolation and analysis of the stromal cells. The common traditional serum biomarkers in clinical practice such as AFP, B2M, Calcitonin, CA015.3, CA-19.9, CA0125, CEA, CYFRA21, NSE, ProGRP, PSA, SCCA, despite their clinical utility in diagnosis and treatment monitoring displayed a lack of specificity with low potential as screening tests.390 Furthermore, despite the ability to easily quantify the proteins on chip, their specificity as biomarkers is still low. Consequently, the various microfluidic platforms that emerged try to increase the sensitivity of the isolation process and to facilitate further the in situ analysis. Large clinical trials are ongoing to prove clinical utility: (1) early cancer detection and (2) to predict relapse and steer therapy.456
Future development of all the reviewed instruments and methods targets essentially their ability to isolate biomarkers from very small samples of biofluids, from samples which comprise very small amounts of biomarkers or from samples collected from cancer patients who express rare mutations. Furthermore, the ultimate clinical challenge is to perfect the technology, to increase the dynamic range of the platforms and produce cost-effective specific and sensitive devices for personalized medicine.
Note: This paper is part of the special issue on Microfluidics, Circulating Biomarkers and Cancer.
Contributor Information
Igor Cima, Email: .
Ciprian Iliescu, Email: .
REFERENCES
- 1.World Health Organization, International Agency for Research on Cancer (2018).
- 2.Crowley E., Di Nicolantonio F., Loupakis F., and Bardelli A., Nat. Rev. Clin. Oncol. 10(8), 472 (2013). 10.1038/nrclinonc.2013.110 [DOI] [PubMed] [Google Scholar]
- 3.Vaidyanathan R., Soon R. H., Zhang P., Jiang K., and Lim C. T., Lab Chip 19(1), 11–34 (2019). 10.1039/C8LC00684A [DOI] [PubMed] [Google Scholar]
- 4.Krebs M. G., Metcalf R. L., Carter L., Brady G., Blackhall F. H., and Dive C., Nat. Rev. Clin. Oncol. 11(3), 129–144 (2014). 10.1038/nrclinonc.2013.253 [DOI] [PubMed] [Google Scholar]
- 5.Ilié M. and Hofman P., Trans. Lung Cancer Res. 5(4), 420 (2016). 10.21037/tlcr.2016.08.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlinger M., Rowan A. J., Horswell S., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., and Tarpey P., N. Eng. J. Med. 2012(366), 883–892 (2012). 10.1056/NEJMoa1113205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alix-Panabières C. and Pantel K., Cancer Discov. 6(5), 479–491 (2016). 10.1158/2159-8290.CD-15-1483 [DOI] [PubMed] [Google Scholar]
- 8.Pantel K. and Speicher M., Oncogene 35(10), 1216–1224 (2016). 10.1038/onc.2015.192 [DOI] [PubMed] [Google Scholar]
- 9.Gorges T. M., Penkalla N., Schalk T., Joosse S., Riethdorf S., Tucholski J., Lucke K., Wikman H., Jackson S. M., Brychter N. et al. , Clin. Cancer Res. 22, 2197 (2016). 10.1158/1078-0432.CCR-15-1416 [DOI] [PubMed] [Google Scholar]
- 10.Cima I., Kong S. L., Sengupta D., Tan I. B., Phyo W. M., Lee D., Hu M., Iliescu C., Alexander I., Goh W. L., Rahmani M., Suhaimi N.-A. M., Vo J. H., Tai J. A., Tan J. H., Chua C., Ten R., Lim W. J., Chew M. H., Hauser C. A., van Dam R. M., Lim W.-Y., Prabhakar S., Lim B., Koh P. K., Robson P., Ying J. Y., Hillmer A. M., and Tan M.-H., Sci. Trans. Med. 8(345), 345ra389 (2016). 10.1126/scitranslmed.aad7369 [DOI] [PubMed] [Google Scholar]
- 11.Zhang C., Guan Y., Sun Y., Ai D., and Guo Q., Cancer Lett. 374(2), 216–223 (2016). 10.1016/j.canlet.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 12.Ge Y.-Z., Wu R., Lu T.-Z., Xin H., Yu P., Zhao Y., Liu H., Xu Z., Xu L.-W., Shen J.-W. et al. , Med. Oncol. 32(1), 332 (2015). 10.1007/s12032-014-0332-x [DOI] [PubMed] [Google Scholar]
- 13.Chaffer C. L. and Weinberg R. A., Science 331(6024), 1559–1564 (2011). 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 14.Comen E., Norton L., and Massague J., Nat. Rev. Clin. Oncol. 8(6), 369–377 (2011). 10.1038/nrclinonc.2011.64 [DOI] [PubMed] [Google Scholar]
- 15.Masuda T., Hayashi N., Iguchi T., Ito S., Eguchi H., and Mimori K., Mol. Oncol. 10(3), 408–417 (2016). 10.1016/j.molonc.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAllister S. S. and Weinberg R. A., Nat. Cell Biol. 16(8), 717–727 (2014). 10.1038/ncb3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantel K. and Alix-Panabières C., Nat. Rev. Gastroenterol. Hepatol. 14(2), 73–74 (2017). 10.1038/nrgastro.2016.198 [DOI] [PubMed] [Google Scholar]
- 18.Gold B., Cankovic M., Furtado L. V., Meier F., and Gocke C. D., J. Mol. Diagn. 17(3), 209–224 (2015). 10.1016/j.jmoldx.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siravegna G., Marsoni S., Siena S., and Bardelli A., Nat. Rev. Clin. Oncol. 14(9), 531 (2017). 10.1038/nrclinonc.2017.14 [DOI] [PubMed] [Google Scholar]
- 20.Pachmann K., Camara O., Kavallaris A., Krauspe S., Malarski N., Gajda M., Kroll T., Jorke C., Hammer U., and Altendorf-Hofmann A., J. Clin. Oncol. 26(8), 1208–1215 (2008). 10.1200/JCO.2007.13.6523 [DOI] [PubMed] [Google Scholar]
- 21.Drake R. R. and Kislinger T., Expert Rev. Proteomics 11(2), 167–177 (2014). 10.1586/14789450.2014.890894 [DOI] [PubMed] [Google Scholar]
- 22.Liu T., Mendes D. E., and Berkman C. E., Int. J. Oncol. 44(3), 918–922 (2014). 10.3892/ijo.2014.2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant R., Pawlowski T., Catto J., Marsden G., Vessella R., Rhees B., Kuslich C., Visakorpi T., and Hamdy F., Br. J. Cancer 106(4), 768 (2012). 10.1038/bjc.2011.595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez A., Loizaga A., Arceo R., Lacasa I., Rabade A., Zorroza K., Mosen-Ansorena D., Gonzalez E., Aransay A. M., and Falcon-Perez J. M., Cancers 6(1), 179–192 (2014). 10.3390/cancers6010179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuhrmann G., Herrmann I. K., and Stevens M. M., Nano Today 10(3), 397–409 (2015). 10.1016/j.nantod.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagni P., Cretich M., Benussi L., Tonoli E., Ciani M., Ghidoni R., Santini B., Galbiati E., Prosperi D., and Chiari M., Anal. Chim. Acta 902, 160–167 (2016). 10.1016/j.aca.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 27.Malamud D., J. Am. Dent. Assoc. 137(3), 286 (2006). 10.14219/jada.archive.2006.0158 [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto M., Wong D. T., Hirayama A., Soga T., and Tomita M., Metabolomics 6(1), 78–95 (2010). 10.1007/s11306-009-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jokerst J. V., Raamanathan A., Christodoulides N., Floriano P. N., Pollard A. A., Simmons G. W., Wong J., Gage C., Furmaga W. B., and Redding S. W., Biosens. Bioelectron. 24(12), 3622–3629 (2009). 10.1016/j.bios.2009.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zilberman Y. and Sonkusale S. R., Biosens. Bioelectron. 67, 465–471 (2015). 10.1016/j.bios.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 31.Streckfus C. F., Bigler L. R., Zwick M., J. Oral Pathol. Med. 35(5), 292–300 (2006). 10.1111/j.1600-0714.2006.00427.xo [DOI] [PubMed] [Google Scholar]
- 32.de Almeida E. F. P., Abdalla T. E., Arrym T. P., de Oliveira Delgado P., Wroclawski M. L., da Costa Aguair Alves B., de S Gehrke F., Azzalis L. A., Alves S., and Tobias-Machado M., Clin. Biochem. 49(16–17), 1274–1277 (2016). 10.1016/j.clinbiochem.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 33.Reckamp K. L., Melnikova V. O., Karlovich C., Sequist L. V., Camidge D. R., Wakelee H., Perol M., Oxnard G. R., Kosco K., and Croucher P., J. Thoracic Oncol. 11(10), 1690–1700 (2016). 10.1016/j.jtho.2016.05.035 [DOI] [PubMed] [Google Scholar]
- 34.Su Y. H., Wang M., Brenner D. E., Norton P. A., and Block T. M., Ann. N. Y. Acad. Sci. 1137(1), 197–206 (2008). 10.1196/annals.1448.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bryzgunova O. E., Skvortsova T. E., Kolesnikova E. V., Starikov A. V., Rykova E. Y., Vlassov V. V., and Laktionov P. P., Ann. N. Y. Acad. Sci. 1075(1), 334–340 (2006). 10.1196/annals.1368.045 [DOI] [PubMed] [Google Scholar]
- 36.Zong S., Wang L., Chen C., Lu J., Zhu D., Zhang Y., Wang Z., and Cui Y., Anal. Methods 8(25), 5001–5008 (2016). 10.1039/C6AY00406G [DOI] [Google Scholar]
- 37.Goessl C., Muller M., Heicappell R., Krause H., and Miller K., Ann. N. Y. Acad. Sci. 945(1), 51–58 (2001). 10.1111/j.1749-6632.2001.tb03863.x [DOI] [PubMed] [Google Scholar]
- 38.Hüsemann Y., Geigl J. B., Schubert F., Musiani P., Meyer M., Burghart E., Forni G., Eils R., Fehm T., and Riethmüller G., Cancer Cell 13(1), 58–68 (2008). 10.1016/j.ccr.2007.12.003 [DOI] [PubMed] [Google Scholar]
- 39.Racila E., Euhus D., Weiss A. J., Rao C., McConnell J., Terstappen L. W., and Uhr J. W., Proc. Natl. Acad. Sci. U.S.A. 95(8), 4589–4594 (1998). 10.1073/pnas.95.8.4589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tkaczuk K. H. R., Goloubeva O., Tait N. S., Feldman F., Tan M., Lum Z.-P., Lesko S. A., Van Echo D. A., and Ts’o P. O., Breast Cancer Res. Treat. 111(2), 355–364 (2008). 10.1007/s10549-007-9771-9 [DOI] [PubMed] [Google Scholar]
- 41.Gallerani G. and Fabbri F., Int. J. Mol. Sci. 17(8), 1266 (2016). 10.3390/ijms17081266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghossein R. A., Scher H. I., Gerald W. L., Kelly W., Curley T., Amsterdam A., Zhang Z.-F., and Rosai J., J. Clin. Oncol. 13(5), 1195–1200 (1995). 10.1200/JCO.1995.13.5.1195 [DOI] [PubMed] [Google Scholar]
- 43.Wu X.-L., Tu Q., Faure G., Gallet P., Kohler C., and Bittencourt M. D. C., Sci. Rep. 6, 20210 (2016). 10.1038/srep20210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punnoose E. A., Atwal S., Liu W., Raja R., Fine B. M., Hughes B. G., Hicks R. J., Hampton G. M., Amler L. C., and Pirzkall A., Clin. Cancer Res. 18(18), 2391–2401 (2012). 10.1158/1078-0432.CCR-11-3148 [DOI] [PubMed] [Google Scholar]
- 45.Allard W. J., Matera J., Miller M. C., Repollet M., Connelly M. C., Rao C., Tibbe A. G., Uhr J. W., and Terstappen L., Clin. Cancer Res. 10(20), 6897–6904 (2004). 10.1158/1078-0432.CCR-04-0378 [DOI] [PubMed] [Google Scholar]
- 46.Xu W., Cao L., Chen L., Li J., Zhang X.-F., Qian H.-H., Kang X.-Y., Zhang Y., Liao J., Shi L.-H. et al. , Clin. Cancer Res. 17, 3783 (2011). 10.1158/1078-0432.CCR-10-0498 [DOI] [PubMed] [Google Scholar]
- 47.Antonuzzo L., Meoni G., and Di Costanzo F., J. Clin. Oncol. 31(19), 2518–2518 (2013). 10.1200/JCO.2013.49.2132 [DOI] [PubMed] [Google Scholar]
- 48.Cristofanilli M., Budd G. T., Ellis M. J., Stopeck A., Matera J., Miller M. C., Reuben J. M., Doyle G. V., Allard W. J., and Terstappen L. W., N. Eng. J. Med. 351(8), 781–791 (2004). 10.1056/NEJMoa040766 [DOI] [PubMed] [Google Scholar]
- 49.Lim S. H., Becker T. M., Chua W., Caixeiro N. J., Ng W., Kienzle N., Tognela A., Lumba S., Rasko J. E., and de Souza P., Cancer Lett. 346(1), 24–33 (2014). 10.1016/j.canlet.2013.12.019 [DOI] [PubMed] [Google Scholar]
- 50.Bidard F.-C., Proudhon C., and Pierga J.-Y., Mol. Oncol. 10(3), 418–430 (2016). 10.1016/j.molonc.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banys-Paluchowski M., Schneck H., Blassl C., Schultz S., Meier-Stiegen F., Niederacher D., Krawczyk N., Ruckhaeberle E., Fehm T., and Neubauer H., Geburtshilfe Frauenheilkd. 75(03), 232–237 (2015). 10.1055/s-0035-1545788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang S. K. and Hoon D. S., Mol. Oncol. 10(3), 450–463 (2016). 10.1016/j.molonc.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schleiermacher G., Peter M., Oberlin O., Philip T., Rubie H., Mechinaud F., Sommelet-Olive D., Landman-Parker J., Bours D., and Michon J., J. Clin. Oncol. 21(1), 85–91 (2003). 10.1200/JCO.2003.03.006 [DOI] [PubMed] [Google Scholar]
- 54.De Bono J. S., Scher H. I., Montgomery R. B., Parker C., Miller M. C., Tissing H., Doyle G. V., Terstappen L. W., Pienta K. J., and Raghavan D., Clin. Cancer Res. 14(19), 6302–6309 (2008). 10.1158/1078-0432.CCR-08-0872 [DOI] [PubMed] [Google Scholar]
- 55.Danila D. C., Fleisher M., and Scher H. I., Clin. Cancer Res. 17(12), 3903–3912 (2011). 10.1158/1078-0432.CCR-10-2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yap T. A., Lorente D., Omlin A., Olmos D., and de Bono J. S., Clin. Cancer Res. 20(10), 2553–2568 (2014). 10.1158/1078-0432.CCR-13-2664 [DOI] [PubMed] [Google Scholar]
- 57.Smith B. M., Slade M. J., English J., Graham H., Lüchtenborg M., Sinnett H. D., Cross N. C., and Coombes R. C., J. Clin. Oncol. 18(7), 1432–1439 (2000). 10.1200/JCO.2000.18.7.1432 [DOI] [PubMed] [Google Scholar]
- 58.Yoon H. J., Kim T. H., Zhang Z., Azizi E., Pham T. M., Paoletti C., Lin J., Ramnath N., Wicha M. S., and Hayes D. F., Nat. Nanotechnol. 8(10), 735 (2013). 10.1038/nnano.2013.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khan M. S., Tsigani T., Rashid M., Rabouhans J. S., Yu D., Luong T. V., Caplin M., and Meyer T., Clin. Cancer Res. 17(2), 337–345 (2011). 10.1158/1078-0432.CCR-10-1776 [DOI] [PubMed] [Google Scholar]
- 60.Huang X., Gao P., Song Y., Sun J., Chen X., Zhao J., Liu J., Xu H., and Wang Z., BMC Cancer 14(1), 976 (2014). 10.1186/1471-2407-14-976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stoecklein N. H., Fischer J. C., Niederacher D., and Terstappen L. W., Expert Rev. Mol. Diagn. 16(2), 147–164 (2016). 10.1586/14737159.2016.1123095 [DOI] [PubMed] [Google Scholar]
- 62.Pierga J.-Y., Bidard F.-C., Mathiot C., Brain E., Delaloge S., Giachetti S., de Cremoux P., Salmon R., Vincent-Salomon A., and Marty M., Clin. Cancer Res. 14(21), 7004–7010 (2008). 10.1158/1078-0432.CCR-08-0030 [DOI] [PubMed] [Google Scholar]
- 63.Scher H. I., Lu D., Schreiber N. A., Louw J., Graf R. P., Vargas H. A., Johnson A., Jendrisak A., Bambury R., and Danila D., JAMA Oncol. 2(11), 1441–1449 (2016). 10.1001/jamaoncol.2016.1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin L. and Lin J.-M., J. Pharm. Anal. 5(6), 337–347 (2015). 10.1016/j.jpha.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong B. and Zu Y., Theranostics 3(6), 377–394 (2013). 10.7150/thno.5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hyun K.-A. and Jung H.-I., Lab Chip 14(1), 45–56 (2014). 10.1039/C3LC50582K [DOI] [PubMed] [Google Scholar]
- 67.Smith J. P., Barbati A. C., Santana S. M., Gleghorn J. P., and Kirby B. J., Electrophoresis 33(21), 3133–3142 (2012). 10.1002/elps.201200263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sajay B. N. G., Chang C.-P., Ahmad H., Chung W. C., Puiu P. D., and Rahman A. R. A., Biomed. Microdevices 15(4), 699–709 (2013). 10.1007/s10544-013-9757-9 [DOI] [PubMed] [Google Scholar]
- 69.Ohnaga T., Shimada Y., Takata K., Obata T., Okumura T., Nagata T., Kishi H., Muraguchi A., and Tsukada K., Mol. Clin. Oncol. 4(4), 599–602 (2016). 10.3892/mco.2016.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aghaamoo M., Zhang Z., Chen X., and Xu J., Biomicrofluidics 9(3), 034106 (2015). 10.1063/1.4922081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z., Chen X., and Xu J., Biomicrofluidics 9(2), 024108 (2015). 10.1063/1.4916645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ao Z., Moradi K., Cote R. J., and Datar R. H., Circulating Tumor Cells (Springer, 2016), pp. 29–45. [Google Scholar]
- 73.Lim L. S., Hu M., Huang M. C., Cheong W. C., Gan A. T. L., Looi X. L., Leong S. M., Koay E. S. C., and Li M. H., Lab Chip 12(21), 4388–4396 (2012). 10.1039/c2lc20750h [DOI] [PubMed] [Google Scholar]
- 74.Son Y. J., Kang J., Kim H. S., and Yoo H. S., Biomacromolecules 17(3), 1067–1074 (2016). 10.1021/acs.biomac.5b01689 [DOI] [PubMed] [Google Scholar]
- 75.Yildiz I., Nanotechnol. Rev. 5(3), 331–340 (2016). 10.1515/ntrev-2015-0012 [DOI] [Google Scholar]
- 76.Chan J. Y., Ahmad Kayani A. B., Md Ali M. A., Kok C. K., Yeop Majlis B., Hoe S. L. L., Marzuki M., Khoo A. S. B., Ostrikov K., Ataur Rahman M., and Sriram S., Biomicrofluidics 12(1), 011503 (2018). 10.1063/1.5010158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iliescu F. S. and Iliescu C., Ann. Acad. Rom. Sci. 9(2), 27–42 (2016). [Google Scholar]
- 78.Karthick S., Pradeep P. N., Kanchana P., and Sen A. K., Lab Chip 18(24), 3802–3813 (2018). 10.1039/C8LC00921J [DOI] [PubMed] [Google Scholar]
- 79.Augustsson P., Magnusson C., Lilja H., and Laurell T., “Acoustophoresis in tumor cell enrichment,” in Circulating Tumor Cells: Isolation and Analysis (Wiley, London, 2016), pp. 227–248. 10.1002/9781119244554.ch10 [DOI] [Google Scholar]
- 80.Casavant B. P., Mosher R., Warrick J. W., Maccoux L. J., Berry S. M., Becker J. T., Chen V., Lang J. M., McNeel D. G., and Beebe D. J., Methods 64(2), 137–143 (2013). 10.1016/j.ymeth.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qian W., Zhang Y., and Chen W., Small 11(32), 3850–3872 (2015). 10.1002/smll.201403658 [DOI] [PubMed] [Google Scholar]
- 82.Murlidhar V., Rivera-Báez L., and Nagrath S., Small 12(33), 4450–4463 (2016). 10.1002/smll.201601394 [DOI] [PubMed] [Google Scholar]
- 83.Hyun K. A. and Jung H. I., Electrophoresis 34(7), 1028–1041 (2013). 10.1002/elps.201200417 [DOI] [PubMed] [Google Scholar]
- 84.Chen Y., Li P., Huang P.-H., Xie Y., Mai J. D., Wang L., Nguyen N.-T., and Huang T. J., Lab Chip 14(4), 626–645 (2014). 10.1039/c3lc90136j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green B. J., Saberi Safaei T., Mepham A., Labib M., Mohamadi R. M., and Kelley S. O., Angew. Chem. Int. Ed. 55(4), 1252–1265 (2016). 10.1002/anie.201505100 [DOI] [PubMed] [Google Scholar]
- 86.Li Y. Q., Chandran B. K., Lim C. T., and Chen X., Adv. Sci. 2(11), 1500118 (2015). 10.1002/advs.201500118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sun Y.-F., Yang X.-R., Zhou J., Qiu S.-J., Fan J., and Xu Y., J. Cancer Res. Clin. Oncol. 137(8), 1151–1173 (2011). 10.1007/s00432-011-0988-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alix-Panabières C. and Pantel K., Lab Chip 14(1), 57–62 (2014). 10.1039/C3LC50644D [DOI] [PubMed] [Google Scholar]
- 89.Alix-Panabières C., Riethdorf S., and Pantel K., Clin. Cancer Res. 14(16), 5013–5021 (2008). 10.1158/1078-0432.CCR-07-5125 [DOI] [PubMed] [Google Scholar]
- 90.Pantel K., Alix-Panabières C., and Riethdorf S., Nat. Rev. Clin. Oncol. 6(6), 339–351 (2009). 10.1038/nrclinonc.2009.44 [DOI] [PubMed] [Google Scholar]
- 91.Alix-Panabières C. and Pantel K., Clin. Chem. 59(1), 110–118 (2013). 10.1373/clinchem.2012.194258 [DOI] [PubMed] [Google Scholar]
- 92.Alix-Panabières C. and Pantel K., Nat. Rev. Cancer 14(9), 623–631 (2014). 10.1038/nrc3820 [DOI] [PubMed] [Google Scholar]
- 93.Lee J. S., Magbanua M. J. M., and Park J. W., Breast Cancer Res. Treat. 160(3), 411–424 (2016). 10.1007/s10549-016-4014-6 [DOI] [PubMed] [Google Scholar]
- 94.Barradas A. and Terstappen L. W., Cancers 5(4), 1619–1642 (2013). 10.3390/cancers5041619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esmaeilsabzali H., Beischlag T. V., Cox M. E., Parameswaran A. M., and Park E. J., Biotech. Adv. 31(7), 1063–1084 (2013). 10.1016/j.biotechadv.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 96.Zborowski M. and Chalmers J., Anal. Chem. 83(21), 8050–8056 (2011). 10.1021/ac200550d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen P., Huang Y.-Y., Hoshino K., and Zhang X., Lab Chip 14(3), 446–458 (2014). 10.1039/C3LC51107C [DOI] [PubMed] [Google Scholar]
- 98.Bocchi M., Scarselli E. F., and Guerrieri R., IEEE Trans. Electron. Devices 57(1), 244–255 (2010). 10.1109/TED.2009.2035026 [DOI] [Google Scholar]
- 99.Shields IV C. W., Reyes C. D., and López G. P., Lab Chip 15(5), 1230–1249 (2015). 10.1039/C4LC01246A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cima I., Wen Yee C., Iliescu F. S., Min Phyo W., Lim K. H., Iliescu C., and Han Tan M., Biomicrofluidics 7(1), 011810 (2013). 10.1063/1.4780062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iliescu F. S., Cima I., Dumitrescu-Ionescu D., and Iliescu C., in Circulating Tumor Cells (CTCs): Detection Methods, Health Impact and Emerging Clinical Challenges, edited by Ray P. C. (Nova Science Publishers, Inc., 2016), pp. 89–117. [Google Scholar]
- 102.Jin C., McFaul S. M., Duffy S. P., Deng X., Tavassoli P., Black P. C., and Ma H., Lab Chip 14(1), 32–44 (2014). 10.1039/C3LC50625H [DOI] [PubMed] [Google Scholar]
- 103.Xing X., Poon R. Y., Wong C. S., and Yobas L., Biosens. Bioelectron. 61, 434–442 (2014). 10.1016/j.bios.2014.05.054 [DOI] [PubMed] [Google Scholar]
- 104.Iliescu F. S., Sim W. J., Heidari H., Poenar D. P., Miao J., Taylor H. K., and Iliescu C., Electrophoresis 40(10), 1457–1477 (2019). 10.1002/elps.201800446 [DOI] [PubMed] [Google Scholar]
- 105.Arya S. K., Lim B., and Rahman A. R. A., Lab Chip 13(11), 1995–2027 (2013). 10.1039/c3lc00009e [DOI] [PubMed] [Google Scholar]
- 106.Yu L., Ng S. R., Xu Y., Dong H., Wang Y. J., and Li C. M., Lab Chip 13(16), 3163–3182 (2013). 10.1039/c3lc00052d [DOI] [PubMed] [Google Scholar]
- 107.Yoon H. J., Kozminsky M., and Nagrath S., ACS Nano 8(3), 1995–2017 (2014). 10.1021/nn5004277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Myung J. H., Gajjar K. A., Han Y. E., and Hong S., Polym. Chem. 3(9), 2336–2341 (2012). 10.1039/c2py20420g [DOI] [Google Scholar]
- 109.Miyamoto D. T., Sequist L. V., and Lee R. J., Nat. Rev. Clin. Oncol. 11(7), 401 (2014). 10.1038/nrclinonc.2014.82 [DOI] [PubMed] [Google Scholar]
- 110.Kling J., Nat. Biotechnol. 30(7), 578–580 (2012). 10.1038/nbt.2295 [DOI] [PubMed] [Google Scholar]
- 111.Parkinson D. R., Dracopoli N., Petty B. G., Compton C., Cristofanilli M., Deisseroth A., Hayes D. F., Kapke G., Kumar P., and Lee J. S., J. Trans. Med. 10(1), 138 (2012). 10.1186/1479-5876-10-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warkiani M. E., Khoo B. L., Wu L., Tay A. K. P., Bhagat A. A. S., Han J., and Lim C. T., Nat. Protoc. 11(1), 134 (2016). 10.1038/nprot.2016.003 [DOI] [PubMed] [Google Scholar]
- 113.Flores L., Kindelberger D., Ligon A., Capelletti M., Fiorentino M., Loda M., Cibas E., Jänne P., and Krop I., Br. J. Cancer 102(10), 1495–1502 (2010). 10.1038/sj.bjc.6605676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swennenhuis J., van Dalum G., Zeune L., and Terstappen L., Expert Rev. Mol. Diagn. 16(12), 1291–1305 (2016). 10.1080/14737159.2016.1255144 [DOI] [PubMed] [Google Scholar]
- 115.Riethdorf S., Fritsche H., Müller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., and Jänicke F., Clin. Cancer Res. 13(3), 920–928 (2007). 10.1158/1078-0432.CCR-06-1695 [DOI] [PubMed] [Google Scholar]
- 116.Coumans F. A., Ligthart S. T., Uhr J. W., and Terstappen L. W., Clin. Cancer Res. 18(20), 5711–5718 (2012). 10.1158/1078-0432.CCR-12-1585 [DOI] [PubMed] [Google Scholar]
- 117.Cristofanilli M., Hayes D. F., Budd G. T., Ellis M. J., Stopeck A., Reuben J. M., Doyle G. V., Matera J., Allard W. J., and Miller M. C., J. Clin. Oncol. 23(7), 1420–1430 (2005). 10.1200/JCO.2005.08.140 [DOI] [PubMed] [Google Scholar]
- 118.Moon H.-S., Kwon K., Kim S.-I., Han H., Sohn J., Lee S., and Jung H.-I., Lab Chip 11(6), 1118–1125 (2011). 10.1039/c0lc00345j [DOI] [PubMed] [Google Scholar]
- 119.Yu M., Bardia A., Wittner B. S., Stott S. L., Smas M. E., Ting D. T., Isakoff S. J., Ciciliano J. C., Wells M. N., and Shah A. M., Science 339(6119), 580–584 (2013). 10.1126/science.1228522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Myung J. H., Launiere C. A., Eddington D. T., and Hong S., Langmuir 26(11), 8589 (2010). 10.1021/la904678p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Allard B., Pommey S., Smyth M. J., and Stagg J., Clin. Cancer Res. 19(20), 5626–5635 (2013). 10.1158/1078-0432.CCR-13-0545 [DOI] [PubMed] [Google Scholar]
- 122.Ignatiadis M., Rothé F., Chaboteaux C., Durbecq V., Rouas G., Criscitiello C., Metallo J., Kheddoumi N., Singhal S. K., Michiels S., Veys I., Rossari J., Larsimont D., Carly B., Pestrin M., Bessi S., Buxant F., Liebens F., Piccart M., and Sotiriou C., PLoS One 6(1), e15624 (2011). 10.1371/journal.pone.0015624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuske A., Gorges T. M., Tennstedt P., Tiebel A.-K., Pompe R., Preißer F., Prues S., Mazel M., Markou A., and Lianidou E., Sci. Rep. 6, 39736 (2016). 10.1038/srep39736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gu Y., Ju C., Li Y., Shang Z., Wu Y., Jia Y., and Niu Y., Biosens. Bioelectron. 66, 24–31 (2015). 10.1016/j.bios.2014.10.070 [DOI] [PubMed] [Google Scholar]
- 125.Lin M., Chen J.-F., Lu Y.-T., Zhang Y., Song J., Hou S., Ke Z., and Tseng H.-R., Acc. Chem. Res. 47(10), 2941–2950 (2014). 10.1021/ar5001617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sieuwerts A. M., Kraan J., Bolt J., van der Spoel P., Elstrodt F., Schutte M., Martens J. W., Gratama J.-W., Sleijfer S., and Foekens J. A., J. Natl. Cancer Inst. 101(1), 61–66 (2009). 10.1093/jnci/djn419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Went P. T., Lugli A., Meier S., Bundi M., Mirlacher M., Sauter G., and Dirnhofer S., Hum. Pathol. 35(1), 122–128 (2004). 10.1016/j.humpath.2003.08.026 [DOI] [PubMed] [Google Scholar]
- 128.Mikolajczyk S. D., Millar L. S., Tsinberg P., Coutts S. M., Zomorrodi M., Pham T., Bischoff F. Z., and Pircher T., J. Oncol. 2011, 252361 (2011). 10.1155/2011/252361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kasimir-Bauer S., Hoffmann O., Wallwiener D., Kimmig R., and Fehm T., Breast Cancer Res. 14(1), R15 (2012). 10.1186/bcr3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gorges T. M., Tinhofer I., Drosch M., Röse L., Zollner T. M., Krahn T., and von Ahsen O., BMC Cancer 12(1), 178 (2012). 10.1186/1471-2407-12-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hosokawa M., Kenmotsu H., Koh Y., Yoshino T., Yoshikawa T., Naito T., Takahashi T., Murakami H., Nakamura Y., and Tsuya A., PLoS One 8(6), e67466 (2013). 10.1371/journal.pone.0067466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang X., Xu L., Shen J., Cao B., Cheng T., Zhao T., Liu X., and Zhang H., Biochimica Biophys. Acta Mol. Basis Dis. 1832(8), 1207–1216 (2013). 10.1016/j.bbadis.2013.03.009 [DOI] [PubMed] [Google Scholar]
- 133.Deng G., Herrler M., Burgess D., Manna E., Krag D., and Burke J. F., Breast Cancer Res. 10(4), R69 (2008). 10.1186/bcr2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ghazani A. A., Castro C. M., Gorbatov R., Lee H., and Weissleder R., Neoplasia 14(5), 388–395 (2012). 10.1596/neo.12696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hager G., Tong D. C.-C., Schiebel I., Rezniczek G. A., Watrowski R., Speiser P., and Zeillinger R., Ginecol. Oncol. 98(2), 211–216 (2005). 10.1016/j.ygyno.2005.04.042 [DOI] [PubMed] [Google Scholar]
- 136.Riethdorf S., Müller V., Zhang L., Rau T., Loibl S., Komor M., Roller M., Huober J., Fehm T., and Schrader I., Clin. Cancer Res. 16(9), 2634–2645 (2010). 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 137.Lacroix M., Endocr. Relat. Cancer 13(4), 1033–1067 (2006). 10.1677/ERC-06-0001 [DOI] [PubMed] [Google Scholar]
- 138.Myung J. H., Gajjar K. A., Saric J., Eddington D. T., and Hong S., Angew. Chem. Int. Ed. 50(49), 11769–11772 (2011). 10.1002/anie.201105508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu Z., Fusi A., Klopocki E., Schmittel A., Tinhofer I., Nonnenmacher A., and Keilholz U., J. Trans. Med. 9(1), 70 (2011). 10.1186/1479-5876-9-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zieglschmid V., Hollmann C., Gutierrez B., Albert W., Strothoff D., Gross E., and Böcher O., Anticancer Res. 25(3A), 1803–1810 (2005). [PubMed] [Google Scholar]
- 141.Lu J.-J., Kakehi Y., Takahashi T., Wu X.-X., Yuasa T., Yoshiki T., Okada Y., Terachi T., and Ogawa O., Clin. Cancer Res. 6(8), 3166–3171 (2000). [PubMed] [Google Scholar]
- 142.Wong S. C. C., Chan C. M. L., Ma B. B. Y., Hui E. P., Ng S. S. M., San Lai P. B., Cheung M. T., Lo E. S. F., Chan A. K. C., and Lam M. Y. Y., Clin. Cancer Res. 15(3), 1005–1012 (2009). 10.1158/1078-0432.CCR-08-1515 [DOI] [PubMed] [Google Scholar]
- 143.Paterlini-Brechot P. and Benali N. L., Cancer Lett. 253(2), 180–204 (2007). 10.1016/j.canlet.2006.12.014 [DOI] [PubMed] [Google Scholar]
- 144.Zhe X., Cher M. L., and Bonfil R. D., Am. J. Cancer Res. 1(6), 740 (2011). [PMC free article] [PubMed] [Google Scholar]
- 145.Desitter I., Guerrouahen B. S., Benali-Furet N., Wechsler J., Jänne P. A., Kuang Y., Yanagita M., Wang L., Berkowitz J. A., and Distel R. J., Anticancer Res. 31(2), 427–441 (2011). [PubMed] [Google Scholar]
- 146.Hosokawa M., Hayata T., Fukuda Y., Arakaki A., Yoshino T., Tanaka T., and Matsunaga T., Anal. Chem. 82(15), 6629–6635 (2010). 10.1021/ac101222x [DOI] [PubMed] [Google Scholar]
- 147.Dharmasiri U., Njoroge S. K., Witek M. A., Adebiyi M. G., Kamande J. W., Hupert M. L., Barany F., and Soper S. A., Anal. Chem. 83(6), 2301–2309 (2011). 10.1021/ac103172y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Warkiani M. E., Khoo B. L., Tan D. S.-W., Bhagat A. A. S., Lim W.-T., Yap Y. S., Lee S. C., Soo R. A., Han J., and Lim C. T., Analyst 139(13), 3245–3255 (2014). 10.1039/C4AN00355A [DOI] [PubMed] [Google Scholar]
- 149.Chudasama D. Y., Freydina D. V., Freidin M. B., Leung M., Fernandez A. M., Rice A., Nicholson A. G., Karteris E., Anikin V., and Lim E., Ann. Trans. Med. 4(24), 480 (2016). 10.21037/atm.2016.12.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.De Giorgi V., Pinzani P., Salvianti F., Panelos J., Paglierani M., Janowska A., Grazzini M., Wechsler J., Orlando C., and Santucci M., J. Invest. Dermatol. 130(10), 2440–2447 (2010). 10.1038/jid.2010.141 [DOI] [PubMed] [Google Scholar]
- 151.Chang W. C., Lee L. P., and Liepmann D., Lab Chip 5(1), 64–73 (2005). 10.1039/b400455h [DOI] [PubMed] [Google Scholar]
- 152.Nagrath S., Sequist L. V., Maheswaran S., Bell D. W., Irimia D., Ulkus L., Smith M. R., Kwak E. L., Digumarthy S., and Muzikansky A., Nature 450(7173), 1235 (2007). 10.1038/nature06385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gleghorn J. P., Pratt E. D., Denning D., Liu H., Bander N. H., Tagawa S. T., Nanus D. M., Giannakakou P. A., and Kirby B. J., Lab Chip 10(1), 27–29 (2010). 10.1039/B917959C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Talasaz A. H., Powell A. A., Huber D. E., Berbee J. G., Roh K.-H., Yu W., Xiao W., Davis M. M., Pease R. F., and Mindrinos M. N., Proc. Natl. Acad. Sci. U.S.A. 106(10), 3970–3975 (2009). 10.1073/pnas.0813188106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Casavant B. P., Guckenberger D. J., Berry S. M., Tokar J. T., Lang J. M., and Beebe D. J., Lab Chip 13(3), 391–396 (2013). 10.1039/C2LC41136A [DOI] [PubMed] [Google Scholar]
- 156.Casavant B. P., Strotman L. N., Tokar J. J., Thiede S. M., Traynor A. M., Ferguson J. S., Lang J. M., and Beebe D. J., Lab Chip 14(1), 99–105 (2014). 10.1039/C3LC50912E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hou S., Zhao H., Zhao L., Shen Q., Wei K. S., Suh D. Y., Nakao A., Garcia M. A., Song M., and Lee T., Adv. Mater. 25(11), 1547–1551 (2013). 10.1002/adma.201203185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Y.-T., Zhao L., Shen Q., Garcia M. A., Wu D., Hou S., Song M., Xu X., Ouyang W.-H., and Ouyang W. W.-L., Methods 64(2), 144–152 (2013). 10.1016/j.ymeth.2013.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sekine J., Luo S. C., Wang S., Zhu B., Tseng H. R., and Yu H. H., Adv. Mater. 23(41), 4788–4792 (2011). 10.1002/adma.201102151 [DOI] [PubMed] [Google Scholar]
- 160.Shen Q., Xu L., Zhao L., Wu D., Fan Y., Zhou Y., Ouyang W. H., Xu X., Zhang Z., and Song M., Adv. Mater. 25(16), 2368–2373 (2013). 10.1002/adma.201300082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang S., Liu K., Liu J., Yu Z. T. F., Xu X., Zhao L., Lee T., Lee E. K., Reiss J., and Lee Y. K., Angew. Chem. 123(13), 3140–3144 (2011). 10.1002/ange.201005853 [DOI] [Google Scholar]
- 162.Wang S., Wang H., Jiao J., Chen K. J., Owens G. E., Kamei K. I., Sun J., Sherman D. J., Behrenbruch C. P., and Wu H., Angew. Chem. 121(47), 9132–9135 (2009). 10.1002/ange.200901668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang N., Deng Y., Tai Q., Cheng B., Zhao L., Shen Q., He R., Hong L., Liu W., and Guo S., Adv. Mater. 24(20), 2756–2760 (2012). 10.1002/adma.201200155 [DOI] [PubMed] [Google Scholar]
- 164.Zhao L., Lu Y. T., Li F., Wu K., Hou S., Yu J., Shen Q., Wu D., Song M., and Ouyang W. H., Adv. Mater. 25(21), 2897–2902 (2013). 10.1002/adma.201205237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chen J. F., Ho H., Lichterman J., Lu Y. T., Zhang Y., Garcia M. A., Chen S. F., Liang A. J., Hodara E., Zhau H. E. et al. , Cancer 121(18), 3240–3251 (2015). 10.1002/cncr.29455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karabacak N. M., Spuhler P. S., Fachin F., Lim E. J., Pai V., Ozkumur E., Martel J. M., Kojic N., Smith K., and Chen P.-I. J. N. P., Nat. Protoc. 9(3), 694 (2014). 10.1038/nprot.2014.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ozkumur E., Shah A. M., Ciciliano J. C., Emmink B. L., Miyamoto D. T., Brachtel E., Yu M., Chen P.-I., Morgan B., and Trautwein J., Sci. Trans. Med. 5(179), 179ra147 (2013). 10.1126/scitranslmed.3005616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Huang L. R., Cox E. C., Austin R. H., and Sturm J. C., Science 304(5673), 987–990 (2004). 10.1126/science.1094567 [DOI] [PubMed] [Google Scholar]
- 169.Sollier E., Go D. E., Che J., Gossett D. R., O’Byrne S., Weaver W. M., Kummer N., Rettig M., Goldman J., and Nickols N., Lab Chip 14(1), 63–77 (2014). 10.1039/C3LC50689D [DOI] [PubMed] [Google Scholar]
- 170.Saliba A.-E., Saias L., Psychari E., Minc N., Simon D., Bidard F.-C., Mathiot C., Pierga J.-Y., Fraisier V., and Salamero J., Proc. Natl. Acad. Sci. U.S.A. 107(33), 14524–14529 (2010). 10.1073/pnas.1001515107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Autebert J., Coudert B., Champ J., Saias L., Guneri E. T., Lebofsky R., Bidard F.-C., Pierga J.-Y., Farace F., and Descroix S., Lab Chip 15(9), 2090–2101 (2015). 10.1039/C5LC00104H [DOI] [PubMed] [Google Scholar]
- 172.Saias L., Autebert J., Malaquin L., and Viovy J.-L., Lab Chip 11(5), 822–832 (2011). 10.1039/c0lc00304b [DOI] [PubMed] [Google Scholar]
- 173.Gascoyne P. R., Noshari J., Anderson T. J., and Becker F. F., Electrophoresis 30(8), 1388–1398 (2009). 10.1002/elps.200800373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gupta V., Jafferji I., Garza M., Melnikova V. O., Hasegawa D. K., Pethig R., and Davis D. W., Biomicrofluidics 6(2), 024133 (2012). 10.1063/1.4731647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vykoukal J., Vykoukal D. M., Freyberg S., Alt E. U., and Gascoyne P. R., Lab Chip 8(8), 1386–1393 (2008). 10.1039/b717043b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alshareef M., Metrakos N., Juarez Perez E., Azer F., Yang F., Yang X., and Wang G., Biomicrofluidics 7(1), 011803 (2013). 10.1063/1.4774312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shim S., Stemke-Hale K., Noshari J., Becker F. F., and Gascoyne P. R., Biomicrofluidics 7(1), 011808 (2013). 10.1063/1.4774307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shim S., Stemke-Hale K., Tsimberidou A. M., Noshari J., Anderson T. E., and Gascoyne P. R. C., Biomicrofluidics 7(1), 011807 (2013). 10.1063/1.4774304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sonnenberg A., Marciniak J. Y., Skowronski E. A., Manouchehri S., Rassenti L., Ghia E. M., Widhopf G. F., Kipps T. J., and Heller M., Electrophoresis 35(12–13), 1828–1836 (2014). 10.1002/elps.201400016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Theil G., Fischer K., Weber E., Medek R., Hoda R., Lücke K., and Fornara P., PLoS One 11(8), e0158354 (2016). 10.1371/journal.pone.0158354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li W., Reátegui E., Park M.-H., Castleberry S., Deng J. Z., Hsu B., Mayner S., Jensen A. E., Sequist L. V., and Maheswaran S., Biomaterials 65, 93–102 (2015). 10.1016/j.biomaterials.2015.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen W., Weng S., Zhang F., Allen S., Li X., Bao L., Lam R. H., Macoska J. A., Merajver S. D., and Fu J., ACS Nano 7(1), 566–575 (2012). 10.1021/nn304719q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chung J., Shao H., Reiner T., Issadore D., Weissleder R., and Lee H., Adv. Healthc. Mater. 1(4), 432–436 (2012). 10.1002/adhm.201200046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sarioglu A. F., Aceto N., Kojic N., Donaldson M. C., Zeinali M., Hamza B., Engstrom A., Zhu H., Sundaresan T. K., and Miyamoto D. T., Nat. Methods 12(7), 685 (2015). 10.1038/nmeth.3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Muluneh M. and Issadore D., Lab Chip 14(23), 4552–4558 (2014). 10.1039/C4LC00869C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Issadore D., Chung J., Shao H., Liong M., Ghazani A. A., Castro C. M., Weissleder R., and Lee H., Sci. Trans. Med. 4(141), 141ra192 (2012). 10.1126/scitranslmed.3003747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xu Y., Phillips J. A., Yan J., Li Q., Fan Z. H., and Tan W., Anal. Chem. 81(17), 7436–7442 (2009). 10.1021/ac9012072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qu L., Xu J., Tan X., Liu Z., Xu L., and Peng R., ACS Appl. Mater. Interfaces 6(10), 7309–7315 (2014). 10.1021/am5006783 [DOI] [PubMed] [Google Scholar]
- 189.Zheng F., Cheng Y., Wang J., Lu J., Zhang B., Zhao Y., and Gu Z., Adv. Mater. 26(43), 7333–7338 (2014). 10.1002/adma.201403530 [DOI] [PubMed] [Google Scholar]
- 190.Adams D. L., Martin S. S., Alpaugh R. K., Charpentier M., Tsai S., Bergan R. C., Ogden I. M., Catalona W., Chumsri S., and Tang C.-M., Proc. Nat. Acad. Sci. U.S.A. 111(9), 3514–3519 (2014). 10.1073/pnas.1320198111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Adams D. L., Adams D. K., Alpaugh R. K., Cristofanilli M., Martin S. S., Chumsri S., Tang C.-M., and Marks J. R., Cancer Epidemiol. 25(7), 1037–1042 (2016). 10.1158/1055-9965.EPI-15-1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mehran R., Nilsson M., Khajavi M., Du Z., Cascone T., Wu H. K., Cortes A., Xu L., Zurita A., Schier R. et al. , Cancer Res. 74(10), 2731–2741 (2014). 10.1158/0008-5472.CAN-13-2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lin B. K., McFaul S. M., Jin C., Black P. C., and Ma H., Biomicrofluidics 7(3), 034114 (2013). 10.1063/1.4812688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kim M. S., Sim T. S., Kim Y. J., Kim S. S., Jeong H., Park J.-M., Moon H.-S., Kim S. I., Gurel O., Lee S. S. et al. , Lab Chip 12(16), 2874–2880 (2012). 10.1039/c2lc40065k [DOI] [PubMed] [Google Scholar]
- 195.Park J. M., Lee J. Y., Lee J. G., Jeong H., Oh J. M., Kim Y. J., Park D., Kim M. S., Lee H. J., Oh J. H., Lee S. S. et al. , Anal. Chem. 84(17), 7400–7407 (2012). 10.1021/ac3011704 [DOI] [PubMed] [Google Scholar]
- 196.Zhang H., Wang Y., Li Q., Zhang F., and Tang B., Chem. Commun. 50(53), 7024–7027 (2014). 10.1039/C4CC02342K [DOI] [PubMed] [Google Scholar]
- 197.Chang C.-L., Jalal S. I., Huang W., Mahmood A., Matei D. E., and Savran C. A., IEEE Sensors J. 14(9), 3008–3013 (2014). 10.1109/JSEN.2014.2321167 [DOI] [Google Scholar]
- 198.Chung Y.-K., Reboud J., Lee K. C., Lim H. M., Lim P. Y., Wang K. Y., Tang K. C., Ji H., and Chen Y., Biosens. Bioelectron. 26(5), 2520–2526 (2011). 10.1016/j.bios.2010.10.048 [DOI] [PubMed] [Google Scholar]
- 199.Wu Y., Deighan C. J., Miller B. L., Balasubramanian P., Lustberg M. B., Zborowski M., and Chalmers J. J., Methods 64(2), 169–182 (2013). 10.1016/j.ymeth.2013.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yang L., Lang J. C., Balasubramanian P., Jatana K. R., Schuller D., Agrawal A., Zborowski M., and Chalmers J., Biotechnol. Bioeng. 102(2), 521–534 (2009). 10.1002/bit.22066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hyun K.-A., Lee T. Y., and Jung H.-I., Anal. Chem. 85(9), 4439–4445 (2013). 10.1021/ac3037766 [DOI] [PubMed] [Google Scholar]
- 202.Hyun K.-A., Lee T. Y., Lee S. H., and Jung H.-I., Biosens. Bioelectron. 67, 86–92 (2015). 10.1016/j.bios.2014.07.019 [DOI] [PubMed] [Google Scholar]
- 203.Sajay B. N. G., Chang C.-P., Ahmad H., Khuntontong P., Wong C. C., Wang Z., Puiu P. D., Soo R., and Rahman A. R. A., Biomed. Microdevices 16(4), 537–548 (2014). 10.1007/s10544-014-9856-2 [DOI] [PubMed] [Google Scholar]
- 204.Gourikutty S. B. N., Chang C.-P., and Puiu P. D., J. Chromatogr. B 1011, 77–88 (2016). 10.1016/j.jchromb.2015.12.016 [DOI] [PubMed] [Google Scholar]
- 205.Gourikutty S. B. N., Chang C.-P., and Poenar D. P., J. Chromatogr. B 1028, 153–164 (2016). 10.1016/j.jchromb.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 206.Mohamadi R. M., Besant J. D., Mepham A., Green B., Mahmoudian L., Gibbs T., Ivanov I., Malvea A., Stojcic J., and Allan A. L., Angew. Chem. Int. Ed. 54(1), 139–143 (2015). 10.1002/anie.201409376 [DOI] [PubMed] [Google Scholar]
- 207.Mohamadi R. M., Ivanov I., Stojcic J., Nam R. K., Sargent E. H., and Kelley S. O., Anal. Chem. 87(12), 6258–6264 (2015). 10.1021/acs.analchem.5b01019 [DOI] [PubMed] [Google Scholar]
- 208.Pratt E. D., Stepansky A., Hicks J., and Kirby B. J., Anal. Chem. 86(22), 11013–11017 (2014). 10.1021/ac503453v [DOI] [PubMed] [Google Scholar]
- 209.Han K.-H., Han A., and Frazier A. B., Biosens. Bioelectron. 21(10), 1907–1914 (2006). 10.1016/j.bios.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 210.Chen C.-L., Chen K.-C., Pan Y.-C., Lee T.-P., Hsiung L.-C., Lin C.-M., Chen C.-Y., Lin C.-H., Chiang B.-L., and Wo A. M., Lab Chip 11(3), 474–483 (2011). 10.1039/C0LC00332H [DOI] [PubMed] [Google Scholar]
- 211.Moon H.-S., Kwon K., Hyun K.-A., Seok Sim T., Chan Park J., Lee J.-G., and Jung H.-I., Biomicrofluidics 7(1), 014105 (2013). 10.1063/1.4788914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Huang C., Santana S. M., Liu H., Bander N. H., Hawkins B. G., and Kirby B. J., Electrophoresis 34(20–21), 2970–2979 (2013). 10.1002/elps.201370191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim U. and Soh H. T., Lab Chip 9(16), 2313–2318 (2009). 10.1039/b903950c [DOI] [PubMed] [Google Scholar]
- 214.Kang J. H., Krause S., Tobin H., Mammoto A., Kanapathipillai M., and Ingber D. E., Lab Chip 12(12), 2175–2181 (2012). 10.1039/c2lc40072c [DOI] [PubMed] [Google Scholar]
- 215.Baccelli I., Schneeweiss A., Riethdorf S., Stenzinger A., Schillert A., Vogel V., Klein C., Saini M., Bäuerle T., and Wallwiener M., Nat. Biotechnol. 31(6), 539 (2013). 10.1038/nbt.2576 [DOI] [PubMed] [Google Scholar]
- 216.Yu M., Bardia A., Aceto N., Bersani F., Madden M. W., Donaldson M. C., Desai R., Zhu H., Comaills V., and Zheng Z., Science 345(6193), 216–220 (2014). 10.1126/science.1253533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hodgkinson C. L., Morrow C. J., Li Y., Metcalf R. L., Rothwell D. G., Trapani F., Polanski R., Burt D. J., Simpson K. L., and Morris K., Nat. Med. 20(8), 897 (2014). 10.1038/nm.3600 [DOI] [PubMed] [Google Scholar]
- 218.Robinson D., Van Allen E. M., Wu Y.-M., Schultz N., Lonigro R. J., Mosquera J.-M., Montgomery B., Taplin M.-E., Pritchard C. C., and Attard G., Cell 161(5), 1215–1228 (2015). 10.1016/j.cell.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Antonarakis E. S., Lu C., Wang H., Luber B., Nakazawa M., Roeser J. C., Chen Y., Mohammad T. A., Chen Y., and Fedor H. L., N. Eng. J. Med. 371(11), 1028–1038 (2014). 10.1056/NEJMoa1315815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Stathopoulou A., Vlachonikolis I., Mavroudis D., Perraki M., Kouroussis C., Apostolaki S., Malamos N., Kakolyris S., Kotsakis A., and Xenidis N., J. Clin. Oncol. 20(16), 3404–3412 (2002). 10.1200/JCO.2002.08.135 [DOI] [PubMed] [Google Scholar]
- 221.Wong I. H., Yeo W., Chan A. T., and Johnson P. J., Cancer Lett. 162(1), 65–73 (2001). 10.1016/S0304-3835(00)00630-3 [DOI] [PubMed] [Google Scholar]
- 222.Katsumata K., Sumi T., Mori Y., Hisada M., Tsuchida A., and Aoki T., Int. J. Clin. Oncol. 11(5), 385–389 (2006). 10.1007/s10147-006-0590-5 [DOI] [PubMed] [Google Scholar]
- 223.Nguyen D. X. and Massagué J., Nat. Rev. Genet. 8(5), 341 (2007). 10.1038/nrg2101 [DOI] [PubMed] [Google Scholar]
- 224.Nguyen D. X., Bos P. D., and Massagué J., Nat. Rev. Cancer 9(4), 274 (2009). 10.1038/nrc2622 [DOI] [PubMed] [Google Scholar]
- 225.Ross J. S. and Slodkowska E. A., Am. J. Clin. Pathol. 132(2), 237–245 (2009). 10.1309/AJCPJI7DEOLKCS6F [DOI] [PubMed] [Google Scholar]
- 226.Reinholz M. M., Nibbe A., Jonart L. M., Kitzmann K., Suman V. J., Ingle J. N., Houghton R., Zehentner B., Roche P. C., and Lingle W. L., Clin. Cancer Res. 11(10), 3722–3732 (2005). 10.1158/1078-0432.CCR-04-1483 [DOI] [PubMed] [Google Scholar]
- 227.Onstenk W., Sieuwerts A. M., Weekhout M., Mostert B., Reijm E. A., van Deurzen C. H., Bolt-de Vries J. B., Peeters D. J., Hamberg P., and Seynaeve C., Cancer Lett. 362(1), 36–44 (2015). 10.1016/j.canlet.2015.03.020 [DOI] [PubMed] [Google Scholar]
- 228.Leversha M. A., Han J., Asgari Z., Danila D. C., Lin O., Gonzalez-Espinoza R., Anand A., Lilja H., Heller G., and Fleisher M., Clin. Cancer Res. 15(6), 2091–2097 (2009). 10.1158/1078-0432.CCR-08-2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang L. H., Pfister T. D., Parchment R. E., Kummar S., Rubinstein L., Evrard Y. A., Gutierrez M. E., Murgo A. J., Tomaszewski J. E., and Doroshow J. H., Clin. Cancer Res. 16(3), 1073–1084 (2010). 10.1158/1078-0432.CCR-09-2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Fernandez-Mercado M., Manterola L., Larrea E., Goicoechea I., Arestin M., Armesto M., Otaegui D., and Lawrie C. H., J. Cell. Mol. Med. 19(10), 2307–2323 (2015). 10.1111/jcmm.12625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schwarzenbach H., Hoon D. S., and Pantel K., Nat. Rev. Cancer 11(6), 426 (2011). 10.1038/nrc3066 [DOI] [PubMed] [Google Scholar]
- 232.Mandel P. and Metais P., C. R. Acad. Sci. Paris 142, 241–243 (1948). [Google Scholar]
- 233.Choi J. J., C. F. Reich, III, and Pisetsky D. S., Immunology 115(1), 55–62 (2005). 10.1111/j.1365-2567.2005.02130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kamat A. A., Baldwin M., Urbauer D., Dang D., Han L. Y., Godwin A., Karlan B. Y., Simpson J. L., Gershenson D. M., and Coleman R. L., Cancer 116(8), 1918–1925 (2010). 10.1002/cncr.24997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wimberger P., Roth C., Pantel K., Kasimir-Bauer S., Kimmig R., and Schwarzenbach H., Int. J. Cancer 128(11), 2572–2580 (2011). 10.1002/ijc.25602 [DOI] [PubMed] [Google Scholar]
- 236.Schwarzenbach H., Pantel K., Kemper B., Beeger C., Otterbach F., Kimmig R., and Kasimir-Bauer S., Breast Cancer Res. 11(5), R71 (2009). 10.1186/bcr2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Sunami E., Shinozaki M., Higano C. S., Wollman R., Dorff T. B., Tucker S. J., Martinez S. R., Singer F. R., and Hoon D. S., Clin. Chem. 55(3), 559–567 (2009). 10.1373/clinchem.2008.108498 [DOI] [PubMed] [Google Scholar]
- 238.De Roock W., Biesmans B., De Schutter J., and Tejpar S., Mol. Diagn. Ther. 13(2), 103–114 (2009). 10.1007/BF03256319 [DOI] [PubMed] [Google Scholar]
- 239.Levine A. J. and Oren M., Nat. Rev. Cancer 9(10), 749 (2009). 10.1038/nrc2723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Castells A., Puig P., Móra J., Boadas J., Boix L., Urgell E., Solé M., Capellà G., Lluís F., and Fernández-Cruz L., J. Clin. Oncol. 17(2), 578–578 (1999). 10.1200/JCO.1999.17.2.578 [DOI] [PubMed] [Google Scholar]
- 241.Downward J., Nat. Rev. Cancer 3(1), 11 (2003). 10.1038/nrc969 [DOI] [PubMed] [Google Scholar]
- 242.Klose R. J. and Bird A. P., Trends Biochem. Sci. 31(2), 89–97 (2006). 10.1016/j.tibs.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 243.Schwarzenbach H., Alix-Panabières C., Müller I., Letang N., Vendrell J.-P., Rebillard X., and Pantel K., Clin. Cancer Res. 15(3), 1032–1038 (2009). 10.1158/1078-0432.CCR-08-1910 [DOI] [PubMed] [Google Scholar]
- 244.Medeiros R., Morais A., Vasconcelos A., Costa S., Carrilho S., Oliveira J., and Lopes C., Cancer Lett. 189(1), 85–90 (2003). 10.1016/S0304-3835(02)00118-0 [DOI] [PubMed] [Google Scholar]
- 245.Gormally E., Caboux E., Vineis P., and Hainaut P., Mutat. Res. Rev. Mutat. 635(2–3), 105–117 (2007). 10.1016/j.mrrev.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 246.Guan G., Wu L., Bhagat A. A., Li Z., Chen P. C., Chao S., Ong C. J., and Han J., Sci. Rep. 3, 1475 (2013). 10.1038/srep01475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu K. J., Brock M. V., Shih I.-M., and Wang T.-H., J. Am. Chem. Soc. 132(16), 5793–5798 (2010). 10.1021/ja100342q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.El-Naggar A. K., Mao L., Staerkel G., Coombes M. M., Tucker S. L., Luna M. A., Clayman G. L., Lippman S., and Goepfert H., J. Mol. Diagn. 3(4), 164–170 (2001). 10.1016/S1525-1578(10)60668-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lindblad-Toh K., Tanenbaum D. M., Daly M. J., Winchester E., Lui W.-O., Villapakkam A., Stanton S. E., Larsson C., Hudson T. J., and Johnson B. E., Nat. Biotechnol. 18(9), 1001 (2000). 10.1038/79269 [DOI] [PubMed] [Google Scholar]
- 250.Erickson D., Liu X., Venditti R., Li D., and Krull U. J., Anal. Chem. 77(13), 4000–4007 (2005). 10.1021/ac050236r [DOI] [PubMed] [Google Scholar]
- 251.Wang Z., Fan Y., Chen J., Guo Y., Wu W., He Y., Xu L., and Fu F., Electrophoresis 34(15), 2177–2184 (2013). 10.1002/elps.201300131 [DOI] [PubMed] [Google Scholar]
- 252.Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., and Ding W., Science 266(5182), 66–71 (1994). 10.1126/science.7545954 [DOI] [PubMed] [Google Scholar]
- 253.Xu F., Datta P., Wang H., Gurung S., Hashimoto M., Wei S., Goettert J., McCarley R. L., and Soper S. A., Anal. Chem. 79(23), 9007–9013 (2007). 10.1021/ac7016597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Das J., Ivanov I., Montermini L., Rak J., Sargent E. H., and Kelley S. O., Nat. Chem. 7(7), 569 (2015). 10.1038/nchem.2270 [DOI] [PubMed] [Google Scholar]
- 255.Pekin D., Skhiri Y., Baret J.-C., Le Corre D., Mazutis L., Salem C. B., Millot F., El Harrak A., Hutchison J. B., and Larson J. W., Lab Chip 11(13), 2156–2166 (2011). 10.1039/c1lc20128j [DOI] [PubMed] [Google Scholar]
- 256.Miotke L., Lau B. T., Rumma R. T., and Ji H. P., Anal. Chem. 86(5), 2618–2624 (2014). 10.1021/ac403843j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Shendure J. and Ji H., Nat. Biotechnol. 26(10), 1135 (2008). 10.1038/nbt1486 [DOI] [PubMed] [Google Scholar]
- 258.Jessri M. and Farah C. S., Oral Oncol. 50(4), 247–253 (2014). 10.1016/j.oraloncology.2013.12.017 [DOI] [PubMed] [Google Scholar]
- 259.Valdes-Mora F. and Clark S., Oncogene 34(13), 1609 (2015). 10.1038/onc.2014.111 [DOI] [PubMed] [Google Scholar]
- 260.Russo G., Patrignani A., Poveda L., Hoehn F., Scholtka B., Schlapbach R., and Garvin A. M., Appl. Transl. Genom. 7, 32–39 (2015). 10.1016/j.atg.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Morey M., Fernandez-Marmiesse A., Castineiras D., Fraga J. M., Couce M. L., and Cocho J. A., Mol. Genet. Metab. 110(1–2), 3–24 (2013). 10.1016/j.ymgme.2013.04.024 [DOI] [PubMed] [Google Scholar]
- 262.Clarke J., Wu H.-C., Jayasinghe L., Patel A., Reid S., and Bayley H. J. N. N., Nat. Nanotechnol. 4(4), 265 (2009). 10.1038/nnano.2009.12 [DOI] [PubMed] [Google Scholar]
- 263.Abou-Hassan A., Neveu S., Dupuis V., and Cabuil V. J. R. A., RSC Adv. 2(30), 11263–11266 (2012). 10.1039/c2ra21799f [DOI] [Google Scholar]
- 264.Carmona F. J., Azuara D., Berenguer-Llergo A., Fernández A. F., Biondo S., de Oca J., Rodriguez-Moranta F., Salazar R., Villanueva A., Fraga M. F. et al. , Cancer Prev. Res. 6(7), 656 (2013). 10.1158/1940-6207.CAPR-12-0501 [DOI] [PubMed] [Google Scholar]
- 265.Delpu Y., Cordelier P., Cho W., and Torrisani J., Int. J. Mol. Sci. 14(7), 15029–15058 (2013). 10.3390/ijms140715029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dietrich D., Kneip C., Raji O., Liloglou T., Seegebarth A., Schlegel T., Flemming N., Rausch S., Distler J., Fleischhacker M. et al. , Int. J. Oncol. 40(3), 825–832 (2012). 10.3892/ijo.2011.1264 [DOI] [PubMed] [Google Scholar]
- 267.Jones P. A., Nat. Rev. Genet. 13(7), 484 (2012). 10.1038/nrg3230 [DOI] [PubMed] [Google Scholar]
- 268.Masood S., El-Gabry E., Zhang C., and Wang Z., Diagn. Cytopathol. 44(8), 670–675 (2016). 10.1002/dc.23505 [DOI] [PubMed] [Google Scholar]
- 269.Kurita R. and Niwa O., Lab Chip 16(19), 3631–3644 (2016). 10.1039/C6LC00829A [DOI] [PubMed] [Google Scholar]
- 270.Wang C.-H., Lai H.-C., Liou T.-M., Hsu K.-F., Chou C.-Y., and Lee G.-B., Microfluid. Nanofluidics 15(5), 575–585 (2013). 10.1007/s10404-013-1179-8 [DOI] [Google Scholar]
- 271.von Bubnoff N., Oncol. Res. Treat. 40(7–8), 409–416 (2017). 10.1159/000478864 [DOI] [PubMed] [Google Scholar]
- 272.Halait H., DeMartin K., Shah S., Soviero S., Langland R., Cheng S., Hillman G., Wu L., and Lawrence H. J., Diagn. Mol. Pathol. 21(1), 1–8 (2012). 10.1097/PDM.0b013e31823b216f [DOI] [PubMed] [Google Scholar]
- 273.O’Driscoll L., Kenny E., Mehta J. P., Doolan P., Joyice H., Gammell P., Hill A., O’Daly B., O’Gorman D., and Clynes M., Cancer Genomics Proteomics 5(2), 95–104 (2008). [PubMed] [Google Scholar]
- 274.Kelley S. O., Mirkin C. A., Walt D. R., Ismagilov R. F., Toner M., and Sargent E. H., Nat. Nanotechnol. 9(12), 969 (2014). 10.1038/nnano.2014.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Madhavan D., Zucknick M., Wallwiener M., Cuk K., Modugno C., Scharpff M., Schott S., Heil J., Turchinovich A., Yang R., Benner A., Riethdorf S., Trumpp A., Sohn C., Pantel K., Schneeweiss A., and Burwinkel B., Clin. Cancer Res. 18(21), 5972–5982 (2012). 10.1158/1078-0432.CCR-12-1407 [DOI] [PubMed] [Google Scholar]
- 276.Taller D., Richards K., Slouka Z., Senapati S., Hill R., Go D. B., and Chang H.-C., Lab Chip 15(7), 1656–1666 (2015). 10.1039/C5LC00036J [DOI] [PubMed] [Google Scholar]
- 277.Roy S., Soh J. H., and Gao Z., Lab Chip 11(11), 1886–1894 (2011). 10.1039/c0lc00638f [DOI] [PubMed] [Google Scholar]
- 278.George S., Chaudhery V., Lu M., Takagi M., Amro N., Pokhriyal A., Tan Y., Ferreira P., and Cunningham B., Lab Chip 13(20), 4053–4064 (2013). 10.1039/c3lc50579k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Egatz-Gomez A., Wang C., Klacsmann F., Pan Z., Marczak S., Wang Y., Sun G., Senapati S., and Chang H.-C., Biomicrofluidics 10(3), 032902 (2016). 10.1063/1.4948525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kurita R., Yanagisawa H., Yoshioka K., and Niwa O., Anal. Chem. 87(22), 11581–11586 (2015). 10.1021/acs.analchem.5b03520 [DOI] [PubMed] [Google Scholar]
- 281.Chen C., Skog J., Hsu C.-H., Lessard R. T., Balaj L., Wurdinger T., Carter B. S., Breakefield X. O., Toner M., and Irimia D., Lab Chip 10(4), 505–511 (2010). 10.1039/B916199F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Davies R. T., Kim J., Jang S. C., Choi E.-J., Gho Y. S., and Park J., Lab Chip 12(24), 5202–5210 (2012). 10.1039/c2lc41006k [DOI] [PubMed] [Google Scholar]
- 283.Andaloussi S. E., Mäger I., Breakefield X. O., and Wood M. J., Nat. Rev. Drug Discov. 12(5), 347 (2013). 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- 284.Jia S., Zocco D., Samuels M. L., Chou M. F., Chammas R., Skog J., Zarovni N., Momen-Heravi F., and Kuo W. P., Expert Rev. Mol. Diagn. 14(3), 307–321 (2014). 10.1586/14737159.2014.893828 [DOI] [PubMed] [Google Scholar]
- 285.Johnstone R. M., Adam M., Hammond J., Orr L., and Turbide C., J. Biol. Chem. 262(19), 9412–9420 (1987). [PubMed] [Google Scholar]
- 286.Johnstone R., Mathew A., Mason A., and Teng K., J. Cell. Physiol. 147(1), 27–36 (1991). 10.1002/jcp.1041470105 [DOI] [PubMed] [Google Scholar]
- 287.Pan B.-T. and Johnstone R. M., Cell 33(3), 967–978 (1983). 10.1016/0092-8674(83)90040-5 [DOI] [PubMed] [Google Scholar]
- 288.Trams E. G., Lauter C. J., Salem J. N., and Heine U., Biochim. Biophys. Acta Biomembr. 645(1), 63–70 (1981). 10.1016/0005-2736(81)90512-5 [DOI] [PubMed] [Google Scholar]
- 289.Vlassov A. V., Magdaleno S., Setterquist R., and Conrad R., Biochim. Biophys. Acta Gen. Subj. 1820(7), 940–948 (2012). 10.1016/j.bbagen.2012.03.017 [DOI] [PubMed] [Google Scholar]
- 290.Simpson R. J., Lim J. W., Moritz R. L., and Mathivanan S., Expert Rev. Proteomics 6(3), 267–283 (2009). 10.1586/epr.09.17 [DOI] [PubMed] [Google Scholar]
- 291.Théry C., Ostrowski M., and Segura E., Nat. Rev. Immunol. 9(8), 581 (2009). 10.1038/nri2567 [DOI] [PubMed] [Google Scholar]
- 292.Azmi A. S., Bao B., and Sarkar F. H., Cancer Metastasis Rev. 32(3–4), 623–642 (2013). 10.1007/s10555-013-9441-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Van der Pol E., Böing A. N., Harrison P., Sturk A., and Nieuwland R., Pharmacol. Rev. 64(3), 676–705 (2012). 10.1124/pr.112.005983 [DOI] [PubMed] [Google Scholar]
- 294.Graner M. W., Alzate O., Dechkovskaia A. M., Keene J. D., Sampson J. H., Mitchell D. A., and Bigner D. D., FASEB J. 23(5), 1541–1557 (2009). 10.1096/fj.08-122184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Skog J., Würdinger T., Van Rijn S., Meijer D. H., Gainche L., Curry Jr W. T., Carter B. S., Krichevsky A. M., and Breakefield X. O., Nat. Cell Biol. 10(12), 1470 (2008). 10.1038/ncb1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., and Lötvall J. O., Nat. Cell Biol. 9(6), 654 (2007). 10.1038/ncb1596 [DOI] [PubMed] [Google Scholar]
- 297.Balaj L., Lessard R., Dai L., Cho Y.-J., Pomeroy S. L., Breakefield X. O., and Skog J., Nat. Commun. 2, 180 (2011). 10.1038/ncomms1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.D’Asti E., Garnier D., Lee T. H., Montermini L., Meehan B., and Rak J., Front. Physiol. 3, 294 (2012). 10.3389/fphys.2012.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shao H., Chung J., Balaj L., Charest A., Bigner D. D., Carter B. S., Hochberg F. H., Breakefield X. O., Weissleder R., and Lee H., Nat. Med. 18(12), 1835 (2012). 10.1038/nm.2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Cheng L., Sharples R. A., Scicluna B. J., and Hill A. F., J. Extracell. Vesicles 3(1), 23743 (2014). 10.3402/jev.v3.23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Alberter B., Klein C. A., and Polzer B., Expert Rev. Mol. Diagn. 16(1), 25–38 (2016). 10.1586/14737159.2016.1121099 [DOI] [PubMed] [Google Scholar]
- 302.Barile L. and Vassalli G., Pharmacol. Ther. 174, 63–78 (2017). 10.1016/j.pharmthera.2017.02.020 [DOI] [PubMed] [Google Scholar]
- 303.Théry C., Amigorena S., Raposo G., and Clayton A., Curr. Protoc. Cell Biol. 30(1), 3.22.21–3.22.29 (2006). 10.1002/0471143030.cb0322s30 [DOI] [PubMed] [Google Scholar]
- 304.Rho J., Chung J., Im H., Liong M., Shao H., Castro C. M., Weissleder R., and Lee H., ACS Nano 7(12), 11227–11233 (2013). 10.1021/nn405016y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Wunsch B. H., Smith J. T., Gifford S. M., Wang C., Brink M., Bruce R. L., Austin R. H., Stolovitzky G., and Astier Y., Nat. Nanotechnol. 11(11), 936 (2016). 10.1038/nnano.2016.134 [DOI] [PubMed] [Google Scholar]
- 306.Lee K., Shao H., Weissleder R., and Lee H., ACS Nano 9(3), 2321–2327 (2015). 10.1021/nn506538f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kang D., Oh S., Reschiglian P., and Moon M. H., Analyst 133(4), 505–515 (2008). 10.1039/b716851a [DOI] [PubMed] [Google Scholar]
- 308.Chung H. J., Castro C. M., Im H., Lee H., and Weissleder R., Nat. Nanotechnol. 8(5), 369 (2013). 10.1038/nnano.2013.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lee S.-K., Kim G.-S., Wu Y., Kim D.-J., Lu Y., Kwak M., Han L., Hyung J.-H., Seol J.-K., and Sander C., Nano Lett. 12(6), 2697–2704 (2012). 10.1021/nl2041707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Hu W., He G., Zhang H., Wu X., Li J., Zhao Z., Qiao Y., Lu Z., Liu Y., and Li C. M., Anal. Chem. 86(9), 4488–4493 (2014). 10.1021/ac5003905 [DOI] [PubMed] [Google Scholar]
- 311.Liu Y., Dong X., and Chen P., Chem. Soc. Rev. 41(6), 2283–2307 (2012). 10.1039/C1CS15270J [DOI] [PubMed] [Google Scholar]
- 312.He M., Crow J., Roth M., Zeng Y., and Godwin A. K., Lab Chip 14(19), 3773–3780 (2014). 10.1039/C4LC00662C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Im H., Shao H., Park Y. I., Peterson V. M., Castro C. M., Weissleder R., and Lee H., Nat. Biotechnol. 32(5), 490 (2014). 10.1038/nbt.2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Kanwar S. S., Dunlay C. J., Simeone D. M., and Nagrath S., Lab Chip 14(11), 1891–1900 (2014). 10.1039/C4LC00136B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ko J., Carpenter E., and Issadore D., Analyst 141(2), 450–460 (2016). 10.1039/C5AN01610J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ng E., Chen K., Hang A., Syed A., and Zhang J. X., Ann. Biomed. Eng. 44(4), 847–862 (2016). 10.1007/s10439-015-1521-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Santana S. M., Antonyak M. A., Cerione R. A., and Kirby B. J., Biomed. Microdevices 16(6), 869–877 (2014). 10.1007/s10544-014-9891-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Shao H., Chung J., Lee K., Balaj L., Min C., Carter B. S., Hochberg F. H., Breakefield X. O., Lee H., and Weissleder R., Nat. Commun. 6, 6999 (2015). 10.1038/ncomms7999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhao Z., Yang Y., Zeng Y., and He M., Lab Chip 16(3), 489–496 (2016). 10.1039/C5LC01117E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Benedikter B. J., Bouwman F. G., Vajen T., Heinzmann A. C. A., Grauls G., Mariman E. C., Wouters E. F. M., Savelkoul P. H., Lopez-Iglesias C., Koenen R. R., Rohde G. G. U., and Stassen F. R. M., Sci. Rep. 7(1), 15297 (2017). 10.1038/s41598-017-15717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Böing A. N., van der Pol E., Grootemaat A. E., Coumans F. A. W., Sturk A., and Nieuwland R., J. Extracell. Vesicles 3(1), 23430 (2014). 10.3402/jev.v3.23430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Lane R. E., Korbie D., Trau M., and Hill M. M., Proteomics 19(8), 1800156 (2019). 10.1002/pmic.201800156 [DOI] [PubMed] [Google Scholar]
- 323.Petersen K. E., Manangon E., Hood J. L., Wickline S. A., Fernandez D. P., Johnson W. P., and Gale B. K., Anal. Bioanal. Chem. 406(30), 7855–7866 (2014). 10.1007/s00216-014-8040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Im H., Shao H., Weissleder R., Castro C. M., and Lee H., Expert Rev. Mol. Diagn. 15(6), 725–733 (2015). 10.1586/14737159.2015.1041378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Liga A., Vliegenthart A., Oosthuyzen W., Dear J., and Kersaudy-Kerhoas M., Lab Chip 15(11), 2388–2394 (2015). 10.1039/C5LC00240K [DOI] [PubMed] [Google Scholar]
- 326.Yang F., Liao X., Tian Y., and Li G., Biotechnol. J. 12(4), 1600699 (2017). 10.1002/biot.201600699 [DOI] [PubMed] [Google Scholar]
- 327.Costa-Silva B., Aiello N. M., Ocean A. J., Singh S., Zhang H., Thakur B. K., Becker A., Hoshino A., Mark M. T., and Molina H., Nat. Cell Biol. 17(6), 816 (2015). 10.1038/ncb3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Melo S. A., Luecke L. B., Kahlert C., Fernandez A. F., Gammon S. T., Kaye J., LeBleu V. S., Mittendorf E. A., Weitz J., and Rahbari N., Nature 523(7559), 177 (2015). 10.1038/nature14581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Peinado H., Alečković M., Lavotshkin S., Matei I., Costa-Silva B., Moreno-Bueno G., Hergueta-Redondo M., Williams C., García-Santos G., and Ghajar C. M., Nat. Med. 18(6), 883 (2012). 10.1038/nm.2753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Witwer K. W., Buzas E. I., Bemis L. T., Bora A., Lässer C., Lötvall J., Nolte-‘t Hoen E. N., Piper M. G., Sivaraman S., and Skog J., J. Extracell. Vesicles 2(1), 20360 (2013). 10.3402/jev.v2i0.20360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Dignat-George F., Freyssinet J.-M., and Key N. S., Platelets 20(3), 225–226 (2009). 10.1080/09537100902795500 [DOI] [PubMed] [Google Scholar]
- 332.György B., Módos K., Pállinger É, Pálóczi K., Pásztói M., Misják P., Deli M. A., Sipos Á, Szalai A., and Voszka I., Blood 117(4), e39–e48 (2011). 10.1182/blood-2010-09-307595 [DOI] [PubMed] [Google Scholar]
- 333.Hong C.-S., Funk S., Muller L., Boyiadzis M., and Whiteside T. L., J. Extracell. Vesicles 5(1), 29289 (2016). 10.3402/jev.v5.29289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Momen-Heravi F., Balaj L., Alian S., Mantel P.-Y., Halleck A. E., Trachtenberg A. J., Soria C. E., Oquin S., Bonebreak C. M., and Saracoglu E., Biol. Chem. 394(10), 1253–1262 (2013). 10.1515/hsz-2013-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Lawrie A., Albanyan A., Cardigan R., Mackie I., and Harrison P., Vox Sang. 96(3), 206–212 (2009). 10.1111/j.1423-0410.2008.01151.x [DOI] [PubMed] [Google Scholar]
- 336.Lamparski H. G., Metha-Damani A., Yao J.-Y., Patel S., Hsu D.-H., Ruegg C., and Le Pecq J.-B., J. Immunol. Methods 270(2), 211–226 (2002). 10.1016/S0022-1759(02)00330-7 [DOI] [PubMed] [Google Scholar]
- 337.Wang Z., Wu H.-J., Fine D., Schmulen J., Hu Y., Godin B., Zhang J. X., and Liu X., Lab Chip 13(15), 2879–2882 (2013). 10.1039/c3lc41343h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., and Hendrix A., J. Extracell. Vesicles 3(1), 24858 (2014). 10.3402/jev.v3.24858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Ingebrigtsen L. and Brandl M., AAPS PharmSciTech 3(2), 9–15 (2002). 10.1208/pt030207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Kang D., Oh S., Ahn S.-M., Lee B.-H., and Moon M. H., J. Proteome Res. 7(8), 3475–3480 (2008). 10.1021/pr800225z [DOI] [PubMed] [Google Scholar]
- 341.Lai R. C., Arslan F., Lee M. M., Sze N. S. K., Choo A., Chen T. S., Salto-Tellez M., Timmers L., Lee C. N., and El Oakley R. M., Stem Cell Res. 4(3), 214–222 (2010). 10.1016/j.scr.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 342.Merchant M. L., Powell D. W., Wilkey D. W., Cummins T. D., Deegens J. K., Rood I. M., McAfee K. J., Fleischer C., Klein E., and Klein J. B., Proteom. Clin. Appl. 4(1), 84–96 (2010). 10.1002/prca.200800093 [DOI] [PubMed] [Google Scholar]
- 343.Oh S., Kang D., Ahn S. M., Simpson R. J., Lee B. H., and Moon M. H., J. Sep. Sci. 30(7), 1082–1087 (2007). 10.1002/jssc.200600394 [DOI] [PubMed] [Google Scholar]
- 344.Yamada T., Inoshima Y., Matsuda T., and Ishiguro N., J. Vet. Med. Sci. 74(11), 1523–1525 (2012). 10.1292/jvms.12-0032 [DOI] [PubMed] [Google Scholar]
- 345.Taylor D. D., Zacharias W., and Gercel-Taylor C., Serum/Plasma Proteomics (Springer, 2011), pp. 235–246. [DOI] [PubMed] [Google Scholar]
- 346.Vaidyanathan R., Naghibosadat M., Rauf S., Korbie D., Carrascosa L. G., Shiddiky M. J., and Trau M., Anal. Chem. 86(22), 11125–11132 (2014). 10.1021/ac502082b [DOI] [PubMed] [Google Scholar]
- 347.Tauro B. J., Greening D. W., Mathias R. A., Ji H., Mathivanan S., Scott A. M., and Simpson R. J., Methods 56(2), 293–304 (2012). 10.1016/j.ymeth.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 348.Lässer C., Eldh M., and Lötvall J., J. Vis. Exp. (59), e3037 (2012). 10.3791/3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Bettazzi F., Hamid-Asl E., Esposito C. L., Quintavalle C., Formisano N., Laschi S., Catuogno S., Iaboni M., Marrazza G., and Mascini M., Anal. Bioanal. Chem. 405(2–3), 1025–1034 (2013). 10.1007/s00216-012-6476-7 [DOI] [PubMed] [Google Scholar]
- 350.Bulut D., Maier K., Bulut-Streich N., Börgel J., Hanefeld C., and Mügge A., J. Card. Fail. 14(4), 336–340 (2008). 10.1016/j.cardfail.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 351.Wu J., Wang S., Chen Q., Jiang H., Liang S., and Lin J. M., Anal. Chim. Acta 892, 132–139 (2015). 10.1016/j.aca.2015.08.020 [DOI] [PubMed] [Google Scholar]
- 352.Roda B., Zattoni A., Reschiglian P., Moon M. H., Mirasoli M., Michelini E., and Roda A., Anal. Chim. Acta 635(2), 132–143 (2009). 10.1016/j.aca.2009.01.015 [DOI] [PubMed] [Google Scholar]
- 353.Dragovic R., Collett G., Hole P., Ferguson D., Redman C., Sargent I., and Tannetta D., Methods 87, 64–74 (2015). 10.1016/j.ymeth.2015.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Gercel-Taylor C., Atay S., Tullis R. H., Kesimer M., and Taylor D. D., Anal. Biochem. 428(1), 44–53 (2012). 10.1016/j.ab.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 355.Horgan K., Shaw S., and Boirivant M., Curr. Protoc. Immunol. 85(1), 7.4.1–7.4.9 (2009). 10.1002/0471142735.im0704s85 [DOI] [PubMed] [Google Scholar]
- 356.Slipicevic A., Somasundaram R., Sproesser K., and Herlyn M., Molecular Diagnostics for Melanoma (Springer, 2014), pp. 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Taylor D. D. and Shah S., Methods 87, 3–10 (2015). 10.1016/j.ymeth.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 358.Welton J. L., Webber J. P., Botos L.-A., Jones M., and Clayton A., J. Extracell. Vesicles 4(1), 27269 (2015). 10.3402/jev.v4.27269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Lozano-Ramos I., Bancu I., Oliveira-Tercero A., Armengol M. P., Menezes-Neto A., Portillo H. A. D., Lauzurica-Valdemoros R., and Borràs F. E., J. Extracell. Vesicles 4(1), 27369 (2015). 10.3402/jev.v4.27369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.de Menezes-Neto A., Sáez M. J. F., Lozano-Ramos I., Segui-Barber J., Martin-Jaular L., Ullate J. M. E., Fernandez-Becerra C., Borrás F. E., and del Portillo H. A., J. Extracell. Vesicles 4(1), 27378 (2015). 10.3402/jev.v4.27378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Muller L., Hong C.-S., Stolz D. B., Watkins S. C., and Whiteside T. L., J. Immunol. Methods 411, 55–65 (2014). 10.1016/j.jim.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ji H., Greening D. W., Barnes T. W., Lim J. W., Tauro B. J., Rai A., Xu R., Adda C., Mathivanan S., Zhao W., Xue Y., Xu T., Zhu H. J., and Simpson R. J., Proteomics 13(10–11), 1672–1686 (2013). 10.1002/pmic.201200562 [DOI] [PubMed] [Google Scholar]
- 363.Mathivanan S., Lim J. W., Tauro B. J., Ji H., Moritz R. L., and Simpson R., Mol. Cell. Proteomics 9(2), 197–208 (2010). 10.1074/mcp.M900152-MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Runz S., Keller S., Rupp C., Stoeck A., Issa Y., Koensgen D., Mustea A., Sehouli J., Kristiansen G., and Altevogt P., Gynecol. Oncol. 107(3), 563–571 (2007). 10.1016/j.ygyno.2007.08.064 [DOI] [PubMed] [Google Scholar]
- 365.Amorim M., Fernandes G., Oliveira P., Martins-de-Souza D., Dias-Neto E., and Nunes D., Proteomics 14(12), 1472–1479 (2014). 10.1002/pmic.201300485 [DOI] [PubMed] [Google Scholar]
- 366.Kadota T., Yoshioka Y., Fujita Y., Kuwano K. and Ochiya T., Semin. Cell Dev. Biol. 67(3), 39–47 (2017). 10.1016/j.semcdb.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 367.Sandfeld-Paulsen B., Jakobsen K. R., Bæk R., Folkersen B. H., Rasmussen T. R., Meldgaard P., Varming K., Jørgensen M. M., and Sorensen B. S., J. Thoracic Oncol. 11(10), 1701–1710 (2016). 10.1016/j.jtho.2016.05.034 [DOI] [PubMed] [Google Scholar]
- 368.Minciacchi V., Zijlstra A., Rubin M. A., and Di Vizio D., Prostate Cancer 20(3), 251 (2017). 10.1038/pcan.2017.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Kahlert C., Melo S. A., Protopopov A., Tang J., Seth S., Koch M., Zhang J., Weitz J., Chin L., and Futreal A., J. Biol. Chem. 289(7), 3869–3875 (2014). 10.1074/jbc.C113.532267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Thakur B. K., Zhang H., Becker A., Matei I., Huang Y., Costa-Silva B., Zheng Y., Hoshino A., Brazier H., and Xiang J., Cell Res. 24(6), 766 (2014). 10.1038/cr.2014.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Aushev V. N., Zborovskaya I. B., Laktionov K. K., Girard N., Cros M.-P., Herceg Z., and Krutovskikh V., PLoS One 8(10), e78649 (2013). 10.1371/journal.pone.0078649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Rabinowits G., Gerçel-Taylor C., Day J. M., Taylor D. D., and Kloecker G. H., Clin. Lung Cancer 10(1), 42–46 (2009). 10.3816/CLC.2009.n.006 [DOI] [PubMed] [Google Scholar]
- 373.Silva J., García V., Zaballos A., Provencio M., Lombardía L., Almonacid L., García J. M., Domínguez G., Peña C., Diaz R. et al. , Eur. Respir. J. 37, 617–623 (2011). 10.1183/09031936.00029610 [DOI] [PubMed] [Google Scholar]
- 374.Ogata-Kawata H., Izumiya M., Kurioka D., Honma Y., Yamada Y., Furuta K., Gunji T., Ohta H., Okamoto H., and Sonoda H., PLoS One 9(4), e92921 (2014). 10.1371/journal.pone.0092921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Taylor D. D. and Gercel-Taylor C., Gynecol. Oncol. 110(1), 13–21 (2008). 10.1016/j.ygyno.2008.04.033 [DOI] [PubMed] [Google Scholar]
- 376.Sugimachi K., Matsumura T., Hirata H., Uchi R., Ueda M., Ueo H., Shinden Y., Iguchi T., Eguchi H., and Shirabe K., Br. J. Cancer 112(3), 532 (2015). 10.1038/bjc.2014.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Baumgart S., Heinzelmann J., Krause E., Stöckle M., Ostenfeld M. S., and Junker K., Eur. Urol. Suppl. 16(3), e897 (2017). 10.1016/S1569-9056(17)30577-8 [DOI] [Google Scholar]
- 378.Hessvik N. P., Phuyal S., Brech A., Sandvig K., and Llorente A., Biochim. Biophys. Acta 1819(11), 1154–1163 (2012). 10.1016/j.bbagrm.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 379.Ohshima K., Inoue K., Fujiwara A., Hatakeyama K., Kanto K., Watanabe Y., Muramatsu K., Fukuda Y., Ogura S.-I., and Yamaguchi K., PLoS One 5(10), e13247 (2010). 10.1371/journal.pone.0013247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Huang X., Yuan T., Liang M., Du M., Xia S., Dittmar R., Wang D., See W., Costello B. A., and Quevedo F., Eur. Urol. 67(1), 33–41 (2015). 10.1016/j.eururo.2014.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cazzoli R., Buttitta F., Di Nicola M., Malatesta S., Marchetti A., Rom W. N., and Pass H. I., J. Thoracic Oncol. 8(9), 1156–1162 (2013). 10.1097/JTO.0b013e318299ac32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Paolini L., Zendrini A., Di Noto G., Busatto S., Lottini E., Radeghieri A., Dossi A., Caneschi A., Ricotta D., and Bergese P., Sci. Rep. 6, 23550 (2016). 10.1038/srep23550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Zhang P., He M., and Zeng Y., Lab Chip 16(16), 3033–3042 (2016). 10.1039/C6LC00279J [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Dudani J. S., Gossett D. R., Tse H. T., Lamm R. J., Kulkarni R. P., and Carlo D. D., Biomicrofluidics 9(1), 014112 (2015). 10.1063/1.4907807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Iliescu F. S., Vrtačnik D., Neuzil P., and Iliescu C., Micromachines 10(6), 392 (2019). 10.3390/mi10060392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Wu Y., Xue P., Hui K. M., and Kang Y., Biosens. Bioelectron. 52, 180–187 (2014). 10.1016/j.bios.2013.08.039 [DOI] [PubMed] [Google Scholar]
- 387.Zang D., Ge L., Yan M., Song X., and Yu J., Chem. Commun. 48(39), 4683–4685 (2012). 10.1039/c2cc16958d [DOI] [PubMed] [Google Scholar]
- 388.Nahavandi S., Baratchi S., Soffe R., Tang S.-Y., Nahavandi S., Mitchell A., and Khoshmanesh K., Lab Chip 14(9), 1496–1514 (2014). 10.1039/C3LC51124C [DOI] [PubMed] [Google Scholar]
- 389.Ziober B. L., Mauk M. G., Falls E. M., Chen Z., Ziober A. F., and Bau H. H., Head Neck J. Sci. Spec. 30(1), 111–121 (2008). 10.1002/hed.20680 [DOI] [PubMed] [Google Scholar]
- 390.Dakubo G. D., Cancer Biomarkers in Body Fluids (Springer, 2016), pp. 75–102. [Google Scholar]
- 391.Hoshino K., Huang Y.-Y., Lane N., Huebschman M., Uhr J. W., Frenkel E. P., and Zhang X., Lab Chip 11(20), 3449–3457 (2011). 10.1039/c1lc20270g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Ludwig J. A. and Weinstein J. N., Nat. Rev. Cancer 5(11), 845 (2005). 10.1038/nrc1739 [DOI] [PubMed] [Google Scholar]
- 393.Rasooly A. and Jacobson J., Biosens. Bioelectron. 21(10), 1851–1858 (2006). 10.1016/j.bios.2006.01.003 [DOI] [PubMed] [Google Scholar]
- 394.Cheng S., Hideshima S., Kuroiwa S., Nakanishi T., and Osaka T. J. S., Sens. Actuators B Chem. 212, 329–334 (2015). 10.1016/j.snb.2015.02.038 [DOI] [Google Scholar]
- 395.Chan W. C. and Nie S., Science 281(5385), 2016–2018 (1998). 10.1126/science.281.5385.2016 [DOI] [PubMed] [Google Scholar]
- 396.Ni M. and Zhuo S., Ann. Phys. 527(7–8), 471–489 (2015). 10.1002/andp.201500119 [DOI] [Google Scholar]
- 397.Dixit C. K., Kadimisetty K., Otieno B. A., Tang C., Malla S., Krause C. E., and Rusling J. F., Analyst 141(2), 536–547 (2016). 10.1039/C5AN01829C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Polanski M. and Anderson N. L., Biomark. Insights 1, 1–48 (2006). 10.1177/117727190600100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kahlert U., Mooney S., Natsumeda M., Steiger H. J., and Maciaczyk J., Int. J. Cancer 140(1), 10–22 (2017). 10.1002/ijc.30259 [DOI] [PubMed] [Google Scholar]
- 400.Sturgeon C. M., Duffy M. J., Stenman U.-H., Lilja H., Brünner N., Chan D. W., Babaian R., Bast R. C., Dowell B., Esteva F. et al. , Clin. Chem. 54(12), e11–e79 (2008). 10.1373/clinchem.2008.105601 [DOI] [PubMed] [Google Scholar]
- 401.Sun L., Burnett J., Guo C., Xie Y., Pan J., Yang Z., Ran Y., and Sun D., Am. J. Cancer Res. 6(1), 91 (2016). [PMC free article] [PubMed] [Google Scholar]
- 402.Ludwig J. A., Szakács G., Martin S. E., Chu B. F., Cardarelli C., Sauna Z. E., Caplen N. J., Fales H. M., Ambudkar S. V., Weinstein J. N., and Gottesman M. M., Cancer Res. 66(9), 4808–4815 (2006). 10.1158/0008-5472.CAN-05-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Joensson H. N., Samuels M. L., Brouzes E. R., Medkova M., Uhlén M., Link D. R., and Andersson‐Svahn H., Angew. Chem., Int. Ed. 48(14), 2518–2521 (2009). 10.1002/anie.200804326 [DOI] [PubMed] [Google Scholar]
- 404.Sturgeon C. M., Clin. Chem. 48(8), 1151–1159 (2002). [PubMed] [Google Scholar]
- 405.Zhang Z., Bast R. C., Yu Y., Li J., Sokoll L. J., Rai A. J., Rosenzweig J. M., Cameron B., Wang Y. Y., Meng X. Y., Berchuck A. et al. , Cancer Res. 64(16), 5882–5890 (2004). 10.1158/0008-5472.CAN-04-0746 [DOI] [PubMed] [Google Scholar]
- 406.Vaupel P. and Mayer A., Cancer Metastasis Rev. 26(2), 225–239 (2007). 10.1007/s10555-007-9055-1 [DOI] [PubMed] [Google Scholar]
- 407.Damaghi M., Wojtkowiak J. W., and Gillies R. J., Front. Physiol. 4, 370 (2013). 10.3389/fphys.2013.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Vander Heiden M. G., Cantley L. C., and Thompson C. B., Science 324(5930), 1029–1033 (2009). 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Griffin J. L. and Shockcor J. P., Nat. Rev. Cancer 4(7), 551 (2004). 10.1038/nrc1390 [DOI] [PubMed] [Google Scholar]
- 410.Beger R., Metabolites 3(3), 552–574 (2013). 10.3390/metabo3030552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Armitage E. G., Kotze H. L., Allwood J. W., Dunn W. B., Goodacre R., and Williams K., Sci. Rep. 5, 15649 (2015). 10.1038/srep15649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Fruman D. A. and Rommel C., Nat. Rev. Drug Discov. 13(2), 140 (2014). 10.1038/nrd4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Ang J. E., Pandher R., Ang J. C., Asad Y. J., Henley A. T., Valenti M., Box G., de Haven Brandon A., Baird R. D., Friedman L. et al. , Mol. Cancer Ther. 15(6), 1412–1424 (2016). 10.1158/1535-7163.MCT-15-0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Hwang V. J. and Weiss R. H., Expert Opin. Drug Metab. Toxicol. 12(11), 1263–1265 (2016). 10.1080/17425255.2016.1238460 [DOI] [PubMed] [Google Scholar]
- 415.Wang X., Chen S., and Jia W., Curr. Pharmacol. Rep. 2(6), 293–298 (2016). 10.1007/s40495-016-0074-x [DOI] [Google Scholar]
- 416.Tan B., Qiu Y., Zou X., Chen T., Xie G., Cheng Y., Dong T., Zhao L., Feng B., and Hu X., J. Proteome Res. 12(6), 3000–3009 (2013). 10.1021/pr400337b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Harvey P., Basuita A., Endersby D., Curtis B., Iacovidou A., and Walker M., BMC Urol. 9(1), 14 (2009). 10.1186/1471-2490-9-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Giskeødegård G. F., Hansen A. F., Bertilsson H., Gonzalez S. V., Kristiansen K. A., Bruheim P., Mjøs S. A., Angelsen A., Bathen T. F., and Tessem M.-B., Br. J. Cancer 113(12), 1712 (2015). 10.1038/bjc.2015.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Budczies J., Denkert C., Müller B. M., Brockmöller S. F., Klauschen F., Györffy B., Dietel M., Richter-Ehrenstein C., Marten U., and Salek R. M., BMC Genomics 13(1), 334 (2012). 10.1186/1471-2164-13-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Roig B., Rodríguez-Balada M., Samino S., Lam E. W. F., Guaita-Esteruelas S., Gomes A. R., Correig X., Borràs J., Yanes O., and Gumà J., Sci. Rep. 7(1), 17831 (2017). 10.1038/s41598-017-17897-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Mayerle J., Kalthoff H., Reszka R., Kamlage B., Peter E., Schniewind B., Maldonado S. G., Pilarsky C., Heidecke C. D., Schatz P., Distler M., Scheiber J. A., Mahajan U. M., Weiss F. U., Grützmann R., and Lerch M. M., Gut 67(1), 128–137 (2018). 10.1136/gutjnl-2016-312432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liang Q., Liu H., Xie L. X., Li X., and Zhang A. H., RSC Adv. 7(5), 2587–2593 (2017). 10.1039/C6RA25007F [DOI] [Google Scholar]
- 423.Audet-Delage Y., Villeneuve L., Grégoire J., Plante M., and Guillemette C., Front. Endocrinol. 9, 87 (2018). 10.3389/fendo.2018.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Haznadar M., Cai Q., Krausz K. W., Bowman E. D., Margono E., Noro R., Thompson M. D., Mathé E. A., Munro H. M., and Steinwandel M. D., Cancer Epidemiol. Biomark. Prev. 25(6), 978–986 (2016). 10.1158/1055-9965.EPI-15-1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Chen Q., Wu J., Zhang Y., and Lin J. M., Anal. Chem. 84(3), 1695–1701 (2012). 10.1021/ac300003k [DOI] [PubMed] [Google Scholar]
- 426.Gao D., Li H., Wang N., and Lin J. M., Anal. Chem. 84(21), 9230–9237 (2012). 10.1021/ac301966c [DOI] [PubMed] [Google Scholar]
- 427.Mao S., Gao D., Liu W., Wei H., and Lin J. M., Lab Chip 12(1), 219–226 (2012). 10.1039/C1LC20678H [DOI] [PubMed] [Google Scholar]
- 428.Mao S., Zhang J., Li H., and Lin J.-M., Anal. Chem. 85(2), 868–876 (2012). 10.1021/ac303164b [DOI] [PubMed] [Google Scholar]
- 429.Lin S. L., Bai H. Y., Lin T. Y., and Fuh M. R., Electrophoresis 33(4), 635–643 (2012). 10.1002/elps.201100380 [DOI] [PubMed] [Google Scholar]
- 430.Sainiemi L., Nissilä T., Kostiainen R., Franssila S., and Ketola R. A., Lab Chip 12(2), 325–332 (2012). 10.1039/C1LC20874H [DOI] [PubMed] [Google Scholar]
- 431.Benetton S., Kameoka J., Tan A., Wachs T., Craighead H., and Henion J. D., Anal. Chem. 75(23), 6430–6436 (2003). 10.1021/ac030249+ [DOI] [PubMed] [Google Scholar]
- 432.Hoffmann P., Häusig U., Schulze P., and Belder D., Angew. Chem. Int. Ed. 46(26), 4913–4916 (2007). 10.1002/anie.200605152 [DOI] [PubMed] [Google Scholar]
- 433.Sun X., Kelly R. T., Tang K., and Smith R. D., Anal. Chem. 83(14), 5797–5803 (2011). 10.1021/ac200960h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Liu W., Chen Q., Lin X., and Lin J. M., Analyst 140(5), 1551–1554 (2015). 10.1039/C4AN02370F [DOI] [PubMed] [Google Scholar]
- 435.Xia Y., Gates B., Yin Y., and Lu Y., Adv. Mater. 12(10), 693–713 (2000). [DOI] [Google Scholar]
- 436.El-Ali J., Sorger P. K., and Jensen K. F., Nature 442(7101), 403–411 (2006). 10.1038/nature05063 [DOI] [PubMed] [Google Scholar]
- 437.Becker H., Lab Chip 10(3), 271–273 (2010). 10.1039/B925993G [DOI] [PubMed] [Google Scholar]
- 438.Myung J. H. and Hong S., Lab Chip 15(24), 4500–4511 (2015). 10.1039/C5LC00947B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Iliescu C., Taylor H., Avram M., Miao J., and Franssila S., Biomicrofluidics 6(1), 016505 (2012). 10.1063/1.3689939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Wang S., Akbas R. and Demirci U., in Mobile Health Technologies: Methods and Protocols (Humana Press, New York, 2015), pp. 111–121. [Google Scholar]
- 441.Mohamed H., McCurdy L. D., Szarowski D. H., Duva S., Turner J. N., and Caggana M., IEEE Trans. Nanobiosci. 3(4), 251–256 (2004). 10.1109/TNB.2004.837903 [DOI] [PubMed] [Google Scholar]
- 442.Chen Z., Zhang S., Tang Z., Xiao P., Guo X., and Lu Z., Surf. Interface Anal. 38(6), 996–1003 (2006). 10.1002/sia.2344 [DOI] [Google Scholar]
- 443.Pinzani P., Salvadori B., Simi L., Bianchi S., Distante V., Cataliotti L., Pazzagli M., and Orlando C., Hum. Pathol. 37(6), 711–718 (2006). 10.1016/j.humpath.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 444.Lin H. K., Zheng S., Williams A. J., Balic M., Groshen S., Scher H. I., Fleisher M., Stadler W., Datar R. H., Tai Y. C., and Cote R. J., Clin. Cancer Res. 16(20), 5011–5018 (2010). 10.1158/1078-0432.CCR-10-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zheng S., Lin H., Liu J. Q., Balic M., Datar R., Cote R. J., and Tai Y. C., J. Chromatogr. A 1162(2), 154–161 (2007). 10.1016/j.chroma.2007.05.064 [DOI] [PubMed] [Google Scholar]
- 446.Sun J., Li M., Liu C., Zhang Y., Liu D., Liu W., Hu G., and Jiang X., Lab Chip 12(20), 3952–3960 (2012). 10.1039/c2lc40679a [DOI] [PubMed] [Google Scholar]
- 447.Tanaka T., Ishikawa T., Numayama-Tsuruta K., Imai Y., Ueno H., Matsuki N., and Yamaguchi T., Lab Chip 12(21), 4336–4343 (2012). 10.1039/c2lc40354d [DOI] [PubMed] [Google Scholar]
- 448.Tanaka T., Ishikawa T., Numayama-Tsuruta K., Imai Y., Ueno H., Yoshimoto T., Matsuki N., and Yamaguchi T., Biomed. Microdevices 14(1), 25–33 (2012). 10.1007/s10544-011-9582-y [DOI] [PubMed] [Google Scholar]
- 449.Tan S. J., Lakshmi R. L., Chen P., Lim W. T., Yobas L., and Lim C. T., Biosens. Bioelectron. 26(4), 1701–1705 (2010). 10.1016/j.bios.2010.07.054 [DOI] [PubMed] [Google Scholar]
- 450.Tay F. E. H., Yu L., Pang A. J., and Iliescu C., Electrochim. Acta 52(8), 2862–2868 (2007). 10.1016/j.electacta.2006.09.022 [DOI] [Google Scholar]
- 451.Wang L., Asghar W., Demirci U., and Wan Y., Nano Today 8(4), 374–387 (2013). 10.1016/j.nantod.2013.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Wan Y., Mahmood M. A. I., Li N., Allen P. B., Kim Y. T., Bachoo R., Ellington A. D., and Iqbal S. M., Cancer 118(4), 1145–1154 (2012). 10.1002/cncr.26349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Han W., Allio B. A., Foster D. G., and King M. R., ACS Nano 4(1), 174–180 (2010). 10.1021/nn900442c [DOI] [PubMed] [Google Scholar]
- 454.Buder-Bakhaya K., Machiraju D., and Hassel J. C., Oncol. Res. Treat. 40(7–8), 430–434 (2017). 10.1159/000478893 [DOI] [PubMed] [Google Scholar]
- 455.Webb S., Nat. Biotechnol. 34(11), 1090–1094 (2016). 10.1038/nbt.3717 [DOI] [PubMed] [Google Scholar]
- 456.Alix-Panabières C. and Pantel K., Nat. Biomed. Eng. 1(4), 0065 (2017). 10.1038/s41551-017-0065 [DOI] [Google Scholar]