During the time that this remarkable drug is relieving pain a very curious psychical condition manifests itself; namely, that the diminution of the pain seems to be due to its fading away in the distance, so that the pain becomes less and less, just as the pain in a delicate ear would grow less and less as a beaten drum was carried farther and farther out of the range of hearing. This condition is probably associated with the other well-known symptom produced by the drug; namely, the prolongation of time.
—Hobart Amory Hare, M.D., 1887 (1)
The history of cannabinoids has been one of contending narratives and dueling interpretations. As analgesics, their use has been so common that William Osler—often referred to as the founder of modern medicine—endorsed cannabis as “the best treatment for migraine headaches” (2). Sir John Russell Reynolds, Queen Victoria’s personal physician, was said to prescribe hemp tincture to Her Majesty to relieve painful menstrual cramps (1). More surprisingly, even though the Koran (2:219) specifically prohibits intoxicants, Muslim physicians tried prescribing hashish in doses that would kill pain but not intoxicate, to comply with the Prophet’s wishes. There have also been skeptics along the way, including those who have claimed that “the road to Hades is lined with marihuana plants” (1).
Centuries later, the debate is alive and well. Should cannabis be legal? Does it have any legitimate medical use? If so, for what? As legislation and public perceptions rapidly shift, different states have allowed its use for a wide range of medical and psychiatric indications (e.g., cancer, glaucoma, human immunodeficiency virus, epilepsy, and posttraumatic stress disorder) and even recreationally. Perhaps most commonly, though, has been a return to the idea that cannabinoids may be especially well suited for treating pain.
Chronic pain has, at least indirectly, become one of the most pressing public health concerns in the United States. It is highly prevalent, affecting 10% of the U.S. population, and leads to considerable disability (3). More significantly, it is inextricably linked to the harrowing opioid epidemic. Each day, more than 90 Americans die from opioid overdoses, representing an enormous societal tragedy and economic burden (4). There is a desperate need for new treatments that can both address the underlying pathology and avert further problems caused by addiction. The idea that cannabis—still categorized by the U.S. Drug Enforcement Administration as a Schedule I substance (“no currently accepted medical use and a high potential for abuse”)—may play a beneficial role is both tantalizing and highly controversial (5).
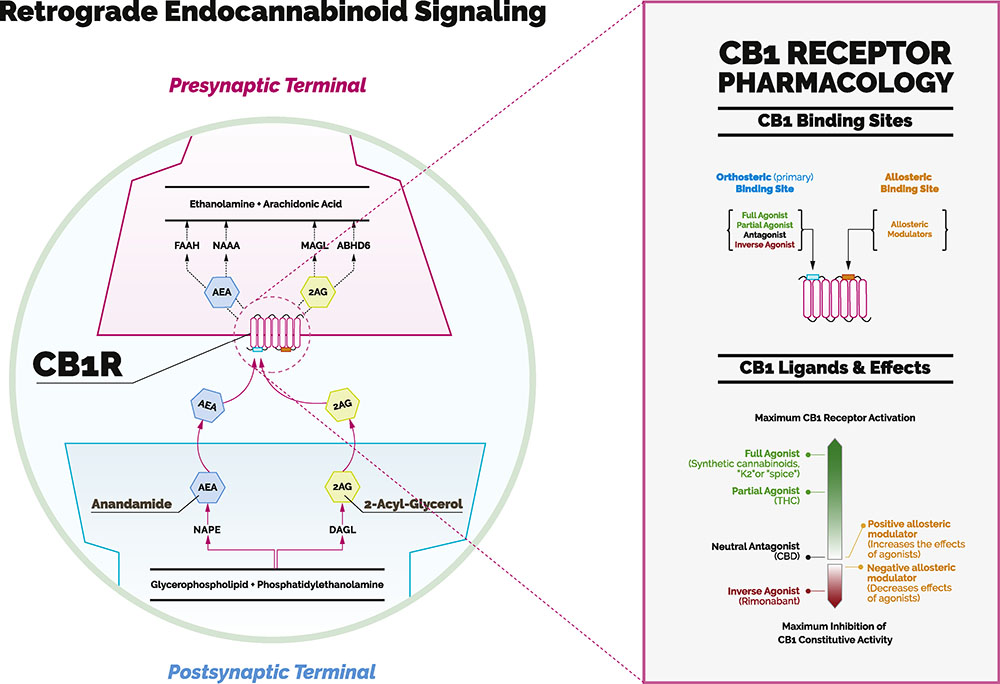
The question is complicated by the fact that our understanding of the endogenous cannabinoid system (ECS) is so new. It was not until the 1990s that the Israeli chemist Raphael Mechoulam and his collaborators described its key components: two G protein–coupled cannabinoid receptors (CB1 and CB2 receptors), the endogenous cannabinoid ligands (or endocannabinoids), and a set of synthetic and degradative enzymes (Figure 1) (6). Other researchers described a unique property of the ECS—that it acts primarily via retrograde signaling (i.e., from the postsynaptic to the presynaptic neuron). Subsequently, ECS components were identified extensively throughout nociceptive pathways and were found to play crucial roles in endogenous pain control—both by modulating the sensory development and resolution of pain, and also by regulating factors that can strongly influence it (e.g., via different cytokines that act at different steps throughout the inflammatory cascade) (6,7).
Figure 1.
The endocannabinoid system consists of cannabinoid receptors 1 and 2 (CB1 and CB2); endogenous transmitters, known as endocannabinoids, including anandamide (AEA) and 2-arachidonoylglycerol (2AG); synthetic enzymes, including diacylglycerol lipase (DAGL), N-acylphosphatidylethanolamine (NAPE), and monoacylglycerol lipase (MAGL); and degradative enzymes, including fatty acid amide hydrolase (FAAH), N-acylethanolamine–hydrolyzing acid amidase (NAAA), and 2-arachidonoylglycerol (ABHD6). CB1 receptors are located primarily in the brain. CB2 receptors are located mainly in the periphery and are not shown in this figure. The endocannabinoids are retrograde messengers: they are released by a postsynaptic dendrite or cell body and travel “backward” across the synapse to the axon terminal of a presynaptic neuron. Inset: The CB1 receptor is one of the several receptors activated by cannabinoids. It contains an orthosteric (or traditional) site and an allosteric site. Different levels of G protein–coupled receptor activation can be produced by distinct ligands. Some cannabinoid ligands work by activating CB1 receptors (agonists) and others by blocking its activation (antagonists). As exemplified in this panel, the CB1 receptor is not like a light switch that can be turned on (e.g., by Δ9-tetrahydrocannabinol [THC], the main psychoactive constituent of cannabis) and off (e.g., by cannabidiol [CBD], a nonpsychoactive cannabinoid). It is more appropriate to think of it as a dimmer switch that can be adjusted to the intended level. “Pure” allosteric modulators either increase or lessen the effects of orthosteric cannabinoid ligands.
Given this intricate connection to the body’s pain control system, the ECS quickly became a compelling target for developing novel analgesic treatments. Initial strategies included the administration of plant-based or synthetic cannabinoid ligands. Similarly, mimicking work with other neurotransmitter systems, researchers also attempted to enhance endocannabinoid signaling by inhibiting their degradative enzymes (6). The thought with both strategies was that increasing activation of CB1 receptor (both in the periphery and in the central nervous system) could lead to pain relief.
Sadly, as alluring as this approach seemed at the time, extensive research has yielded relatively little success. Only nabilone, dronabinol, and a blend of Δ9-tetrahydrocannabinol and cannabidiol have been approved for treatment by various governments [for uses of spasticity, pain, and nausea (8)]. Recently, the initial enthusiasm for degradative enzyme inhibitors has been tempered by negative results in clinical trials of osteoarthritis-related pain, in addition to serious adverse events (6).
How can we make sense of these failures? The biggest reason appears to be the ubiquity and diversity of ECS components. They are found in a range of tissues, including not only different types of neurons but also peripheral immune cells and glial cells in the brain (6). Moreover, across these sites (and as might be expected), ligand actions are variable, with both excitatory and inhibitory effects at cannabinoid and noncannabinoid receptors. For example, anandamide, one of the most studied endocannabinoids, is an agonist at the capsaicin receptor, but has inhibitory actions at the CB1 and CB2 receptors (6). One other unique attribute is that endocannabinoids are synthesized and released on demand rather than being stored and transported in vesicles. This makes their actions specific to the microenvironment in which they are expressed. Taken together, we see that the activity of the ECS can induce a wide range of effects, including either antinociception or pronociception, depending on the specific region and its underlying physiological state (6). It follows that a broad “shotgun” approach to targeting this system is not feasible.
So what, then, is the way forward? Is it possible to leverage the peculiarities of the ECS to treat pain while minimizing harm?
Recent research in G protein–coupled receptor function may offer a solution. Historically, our tendency has been to think of drugs as activating receptors at the main binding site for their endogenous ligands (the orthosteric site). In recent years, however, we have come to appreciate that ligands can activate receptors in distinctive and nonbinary ways. One way they can do this is by binding at a different set of allosteric sites (from the Greek allos [ἄλλoς], “other,” and stereos [στερεὀς], “solid [object]”) (Figure 1) (9). Activation at these sites can modify the conformation of the G protein–coupled receptor and thereby augment or diminish the effect of the traditional ligand. Critically, while orthosteric sites tend to be highly conserved, the allosteric sites may have diverse subtypes with distinct properties across the body.
The application of this work to the endocannabinoid system becomes immediately clear (9). One reason that previous treatment studies (e.g., with Δ9-tetrahydrocannabinol) have failed is because they activate CB1 receptors across the entire body and brain, with no specificity, causing unwanted psychoactive effects (8). In principle, the use of allosteric modulators would have the potential to act selectively—in the periphery, in the dorsal horn of the spinal cord, or in the supraspinal pain regulatory regions of the brain (6,9). Some groups have already investigated potential structural–activity relationships and region-specific mechanisms of new compounds (9).
Another key advantage of allosteric modulators is with respect to the temporal aspects of receptor activation. While orthosteric ligands immediately activate the corresponding receptor, a “pure” allosteric modulator will have an effect only when an endogenous ligand is already present—thus having the potential to modulate endogenous signaling pathways while still maintaining the temporal and spatial characteristics of normal signaling (9).
This issue of Biological Psychiatry includes an encouraging study by Slivicki et al. (10) that perfectly illustrates this strategy. Using the positive cannabinoid receptor allosteric modulator GAT211 the authors have shown a reduction in neuropathic and inflammatory pain in animals, without abuse liability (10). Such work offers a new direction for the development of pain therapeutics, holding the potential to develop subtype- and pathway-specific medications.
Exciting lines of inquiry include the development of multifunctional compounds that can regulate the ECS while simultaneously engaging with more traditional targets—thus leading to a superior therapeutic profile and minimizing unwanted side effects. For instance, one might imagine using cannabinoid allosteric modulators in conjunction with cyclooxygenase-2 inhibition or capsaicin receptor antagonism. In particular, patients with opioid-induced hyperalgesia and allodynia will greatly benefit from new analgesic approaches to peripheral targets (6,9,10). These are also reasons to believe that it may be possible to successfully leverage ECS targets in the central nervous system to help modulate the perception and experience of pain—including its affective and cognitive aspects (e.g., by decreasing anxiety or stress) (6).
As the ancient Muslim physicians were well aware, developing cannabis as a medicine is a challenging feat. Though it may be effective for pain, its psychiatric consequences may render it unusable on its own. Fittingly, the word they used for intoxicants, khamr, comes from the verb khamara (“to cover”). The innovative new class of cannabinoid allosteric modulators may finally have the potential to walk this narrow tightrope— relieving pain without “covering” the mind.
Acknowledgments and Disclosures
Clinical Commentaries are produced in collaboration with the National Neuroscience Curriculum Initiative (NNCI). David A. Ross, in his dual roles as co-chair of the NNCI and Education Editor of Biological Psychiatry, manages the development of these commentaries but plays no role in the decision to publish each commentary. The NNCI is supported by the National Institutes of Health Grant Nos. R25 MH10107602S1 and R25 MH08646607S1.
We thank Amanda Wang for her role in developing the figure.
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Booth M (2015): Cannabis: A History. New York: Thomas Dunne Books. [Google Scholar]
- 2.Boes CJ (2015): Osler on migraine. Can J Neurol Sci 42:144–147. [DOI] [PubMed] [Google Scholar]
- 3.Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN (2015): Rates of opioid misuse, abuse, and addiction in chronic pain: A systematic review and data synthesis. Pain 156:569–576. [DOI] [PubMed] [Google Scholar]
- 4.Rudd RA (2016): Increases in drug and opioid-involved overdose deaths - United States, 2010–2015. MMWR Morb Mortal Wkly Rep 66:35. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen S, Sabioni P, Trigo JM, Ware MA, Betz-Stablein BD, Murnion B, et al. (2017): Opioid-sparing effect of cannabinoids: A systematic review and meta-analysis. Neuropsychopharmacology 42:1752–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V (2017): The cannabinoid system and pain. Neuropharmacology 124:105–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zádor F, Wollemann M (2015): Receptome: Interactions between three pain-related receptors or the “triumvirate” of cannabinoid, opioid and TRPV1 receptors. Pharmacol Res 102:254–263. [DOI] [PubMed] [Google Scholar]
- 8.De Aquino JP, Sherif M, Radhakrishnan R, Cahill JD, Ranganathan M, D’Souza DC (2018): The psychiatric consequences of cannabinoids. Clin Ther 40:1448–1456. [DOI] [PubMed] [Google Scholar]
- 9.Khurana L, Mackie K, Piomelli D, Kendall DA (2017): Modulation of CB1 cannabinoid receptor by allosteric ligands: Pharmacology and therapeutic opportunities. Neuropharmacology 124:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slivicki RA, Xu Z, Kulkarni PM, Pertwee RG, Mackie K, Thakur GA, Hohmann AG (2018): Positive allosteric modulation of cannabinoid receptor type 1 suppresses pathological pain without producing tolerance or dependence. Biol Psychiatry 84:722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]