Abstract
Environmental chemical exposure could be a contributor to the increasing obesity epidemic. Diazinon, an organophosphate insecticide, has been widely used in the agriculture, and exposure of the general population to diazinon has been reported. Diazinon has been known to induce neurotoxic effects mainly through the inhibition of acetylcholinesterase (AChE). However, its association with dysregulation of adipogenesis has been poorly investigated. The current study aimed to examine the mechanism of diazinon’s effect on adipogenesis using the 3T3-L1 preadipocytes combined with a single-cell-based high-content analysis. The results showed that diazinon induced lipid droplet accumulation in a dose-dependent manner. The dynamic changes of adipogenic regulatory proteins and genes were examined at the three stages of adipogenesis (induction, differentiation, and maturation) in 3T3-L1 cells treated with various doses of diazinon (0, 1, 10, 100 μM) using real-time quantitative RT-PCR and Western Blot respectively. Diazinon significantly induced protein expression of transcriptional factors CCAAT-enhancer-binding proteins α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ), their downstream proteins, fatty acid synthase (FASN), acetyl CoA carboxylase (ACC), fatty acid-binding protein 4 (FABP4), lipoprotein lipase (LPL), adiponectin and perilipin in dose and time-dependent manners. Similarly, the adipogenic genes were significantly induced in a dose and time-dependent manner compared to the relative controls. The current study demonstrates that diazinon promotes lipid accumulation and activates the adipogenic signaling pathway in the in vitro model.
Keywords: Diazinon, 3T3-L1, Adipogenesis, obesogen
1. Introduction
More than 36.5% of adults and 17% of children in the US have obesity or overweight [1], and obesity has become a growing health problem and closely associated with a far-ranging adverse effect on health, contributing to morbidity and mortality [2-7]. Obesity is a multifactorial disorder, and a susceptible genetic background indeed predisposes to obesity. However, the current rapid rise in the prevalence of obesity appears to be related to gene-environmental interaction [8, 9]. Accumulating evidence suggests that environmental chemicals, such as endocrine-disrupting chemicals (EDCs), could be contributing to the development of obesity and associated metabolic disorders [10-18]. EDCs are exogenous compounds that modulate the endogenous hormonal action through disrupting a variety of metabolic signaling pathways. Prenatal exposure to hexachlorobenzene (DDE, metabolites of pesticide DDT), and polychlorinated biphenyls (PCBs) were found to be associated with increased BMI and weight gain at an early age of children [19, 20]. Epidemiologic studies have shown that exposures to PCBs, dioxins, and phthalates were closely associated with the prevalence of diabetes [21, 22]. Occupational exposure to organochlorine and organophosphate (OP) insecticides have been shown to be associated with diabetes [23].
Diazinon is a widely applied OP insecticide in the US and the worldwide [24], approximately 13 million pounds of the active ingredient diazinon are used annually on agricultural sites [25]. The wide usage of diazinon in agriculture is likely to pose an adverse effect to human from exposure, through multiple pathways in both agricultural and non-agricultural communities. Although diazinon’s residential uses were banned in the US in 2004, diazinon residue has still been detected in the agricultural worker, general populations, and ecological risks, due to its wild usage in fruit and vegetable production [25, 26]. For example, diazinon residue has frequently been found in surface waters or drinking water [27], and transported from the site of application by precipitation, fog, and wind to other areas, and has the potential to migrate through the soil and into groundwater [28]. In addition, the metabolite of diazinon, 2-isopropyl-4-methyl-6-hydroxypyrimidine, has been detected in urine from 82% of American adults [29, 30]. Although the metabolites of diazinon in urine were below the limit of detection in adults, it was detected in 95% of children aged 6–11 years and the non-Hispanic community at 1.45 and 1.49 μg/L, respectively, in the NHANES 2001–2002 [31]. Diazinon can be transferred to the developing fetus if the pregnant women used OP containing products [32]. In addition, Occupational workers, farmworkers and their children had up to 10 fold higher of urinary metabolites than that of the NHANES survey during agricultural seasons [33]. Importantly, pesticides were found to be tracked into homes of farmworkers where children were highly exposed through take-home pesticide pathway [34]. Diazinon can be oxidatively degenerated to diaxozon, much more toxic than diazinon. Diazinon exposure can lead to various adverse health outcomes, not only limit to developmental neurotoxicity through acetylcholinesterase inhibition [35-37], but also induction of genotoxicity [38, 39] and DNA damage [40]. Recently, more studies have raised concerns regarding a multitude of toxic effects of OPs at the sub-toxic dose [41-43]. Interestingly, exposure to diazinon has been shown to cause the rise of incidence of acute pancreatitis [44-46], and reproductive disorder [47, 48], which is associated with endocrine and metabolic-related diseases [49, 50]. In addition, Ear-tags impregnated with diazinon have been widely used to effectively control ectoparasites on livestock. The cattle that wearing these ear tags gained significant weight, a net increase of 60% over 5 months as opposed to only a 28% weight gain in cattle without treating ear tags [51, 52]. An animal study also suggested that subclinical doses and repeated exposure of diazinon promoted weight gain predominately [53]. The further results showed that neonatal rats with low-dose of diazinon not only produced developmental neurotoxicity, but also have lasting effects on metabolism, and these metabolic defects were exacerbated when diazinon exposure occurred at a critical developmental stage [54-56]. This phenomenon was further reinforced by the impaired energy metabolism of the gut microbiome in mice treated with Diazinon [57].
To date, a few studies have investigated the mechanisms of insecticides on lipid and glucose metabolism, which ultimately contribute to weight gain, development of obesity, and related chronic metabolic diseases [58, 59]. Given the significance of adverse health effects and widespread use of diazinon, it is of great interest to understand the interplay between adipogenesis and diazinon at a subtoxic dose. In our previous study, we have established the impact of environmental exposure Benzyl butyl phthalate on adipogenesis using an in vitro model combined with high-content analysis. A cell-based high-content analysis emerges as a highly informative approach to analyze the regulation and dynamic changes at a single-cell level [60]. In the current study, we investigated whether diazinon regulates adipogenic signaling pathway and promotes adipogenesis using a multiparametric high-content analysis, suggesting an essential role of diazinon in adipogenesis.
2. Materials and Methods
2.1. Chemicals and reagents
Dulbecco’s modified Eagle’s medium (DMEM), antibiotics (penicillin and streptomycin), fetal bovine serum (FBS), and 0.25% trypsin were purchased from GE Healthcare Life Sciences (Logan, Utah). Insulin, dexamethasone (DEX),3-isobutyl-1- methylxanthine (IBMX), protease inhibitor cocktail, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). Diazinon (Cas: 33–41-5,) was purchased from Chem Service with a purity of 99.2% (West Chester, PA). The name of International Union of Pure and Applied Chemistry (IUPAC) is O,O-Diethyl O-[4-methyl-6-(propan-2-yl)pyrimidin-2-yl] phosphorothioate.
2.2. 3T3-L1 cell culture
3T3-L1 mouse preadipocytes were kindly gifted from Dr. Clifton Bailey’s laboratory at the University of Georgia. Cells were maintained in DMEM composed of high glucose, 10% FBS and 100 U/mL penicillin and streptomycin in a 37°C, 5% CO2 humidified environment. The cultured cells were maintained in a sub-confluent condition and change of media every 2–3 days.
2.3. 3T3-L1 differentiation and treatments
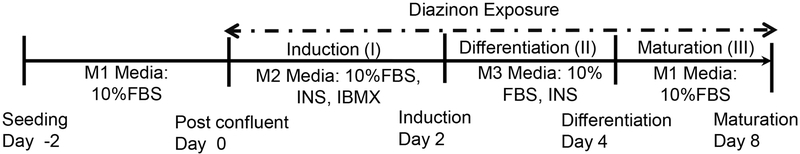
3T3-L1 cells were cultured to 100% confluence (M1 medium: DMEM containing 10% FBS) in a 12-well plate, 35 mm dish, or 96 well-plate, for RNA, protein, or high-content analysis, respectively. This time point is denoted as day 0. After post-confluence, cells were then incubated in adipogenic induction medium (M2 medium: DMEM containing 1 μM DEX, 0.5 mM IBMX, 167 nM insulin and 10% FBS) for two days, and then cultured in adipogenic differentiation medium (M3 medium: DMEM containing 167 nM insulin and 10% FBS) for another two days, followed by DMEM with 10% FBS (M1) for another four days. The cells cultured with DEX in the M2 medium were used as a positive control, in order to ensure the cell’s ability to differentiate into the adipocyte. To examine the effects of diazinon on adipogenesis of 3T3-L1 cells, diazinon was added to M1, M2 without DEX, and M3 media as indicated doses for a total 8 days. Cells in the vehicle (DMSO 0.05%) in M1, M2, and M3 media were set as the untreated control (CTL). Figure 1 illustrates the stages and treatment protocol.
Figure 1. Illustration of the treatment schedule and maturation stages of 3T3-L1 preadipocytes.
3T3-L1 cells were cultured to 100% confluence in 10% FBS DMEM (M1 medium), denoted as day 0. To differentiate the pre-adipocyte 3T3-L1 cells as a positive control (Pos), cells were incubated with 1 μM DEX in adipogenic induction medium (M2 medium: DMEM containing, 0.5 mM IBMX, 167 nM insulin and 10% FBS) for 2 days (day 2). To examine the effect of a compound on the adipogenesis, diazinon was added to the M2 medium for two days, M3 medium for two days, and M1 medium for another four days. Cells cultured in the vehicle (DMSO 0.1%) in all medium was set as the untreated control (CTL). The cellular differentiation was processed into three stages: induction (day 0–2), differentiation (day 2–4) and maturation (day 4–8).
2.4. Oil Red O staining and quantification of lipid droplets and cellular triglyceride levels in cell lysates
3T3-L1 cells were seeded in a 12-well plate and treated with diazinon of various concentrations or a vehicle control from day 0 to day 8. On day 8, cells were fixed in fresh 4% paraformaldehyde, and the intracellular lipid droplets were stained with a filtered solution of 60% Oil Red O in 100% 2-isopropanol. Stained cells were observed with an Olympus IX71 (TH4–100) imaging system with 20X phase contrast objectives. Oil Red O was extracted from cells with isopropanol, and optical density (OD) was then measured at a wavelength of 510 nm (Gen5, BioTek). The cells were also stained with Nile Red using AdipoRed Assay according to the manufacturer’s instructions (Lonza, MD), the fluorescence intensity was measured at 572 nm (Gen5, BioTek). In order to determine triglyceride levels more specifically, the triglyceride levels from the cell lysates were quantified using Infinity Triglycerides Reagent (Thermo Scientific, MA) and Triglyceride Standard (Pointe Scientific, Canton, MI). The 3T3-L1 cells were cultured in the 6-well plate, and treated with various doses of Diazinon, DEX as a positive control (pos), and vehicle (DMSO 0.05%) as the negative control (Ctl). After treatment for 10 days, the cells were washed with cold PBS twice, and the cell lysates were re-suspended and homogenized in 5% NP-40 solution. The cell lysates were then harvested, and heated for 5 mins in the 95ºC water bath, and vortexed for 30 seconds. This step was repeated twice, and then cool samples to room temperature. The Triglyceride levels, then were assayed with Infinity Triglyceride Reagent according to the manufacturer’s instructions. The protein content of the cell lysates was also measured by the Bradford protein assay following the manufacturer’s instruction (BioRad, Hercules, CA). The final triglyceride level was normalized with the protein content. The experiment was performed with three technical replicates, repeated three times.
2.5. A single-cell based high-content analysis (HCA)
3T3-L1 cells were seeded in a black frame 96-well plate (Corning, NY), and treated with diazinon as indicated concentrations from day 0 to day 8. Cells were then fixed with 4% paraformaldehyde and then washed with PBS twice. A staining solution containing Hoechst 33342 (1 μM) for nuclear staining, and LipidTOX for neutral lipid staining (Life Technologies, NY) was added to each well and incubated at room temperature for 30 min. Image acquisition and image-based quantification were performed using ArrayScan® VTI HCS Reader with HCS Studio™ 2.0 Cell Analysis Software (Thermo Scientific, MA). Images were acquired with high resolution (1024 × 1024), and 49 fields for each well were extracted. After feature extraction, the cell-based data were exported to JMP (SAS) or GraphPad Prism (San Diego, CA) for further statistical analyses. Lipid droplets were detected with the SpotDetector® algorithm of BioApplication. The method was based on a two-channel acquisition assay, which uses a 20 × objective (NA 0.5), a Hamamatsu ORCA-ER digital camera in combination with a 0.63 × coupler, and Carl Zeiss microscope optics for automatic image acquisition. Channel one applies BGRFR 386–23 for Hoechst 33342 (nuclei), and the objects were identified. This nuclear identification was used as a measure of cell number, and the nuclear area was determined by the staining Hoechst 33342. The spots (lipid droplets) were detected in channel two (BGRFR 549–15 filter). The average fluorescence intensity of spot, spot totally intensity per spot count, spot average area and average intensity of FABP4 were reported. The setting of the SpotDetector algorithm (version 4.1) was optimized for LipidTox analysis in channel two, and the thresholds were set to ensure that only lipid droplets of certain size and intensity were selected for analysis. Lipid counts, the intensity of the spot and intensity of FABP4 was normalized to the number of the nucleus. The experiment was performed with 8 technical replicates and repeated twice.
2.6. RNA isolation and quantitative real-time RT-PCR (qRT-PCR)
3T3-L1 cells were seeded in a 12-well plate and treated with diazinon of various concentrations or vehicle control media from day 0 to day 8. Total RNAs were isolated at the end of each stage as indicated in Figure 1. The quality and quantity of total RNA were measured on a Nanodrop (Thermo Scientific, MA). cDNA was reverse transcribed from 2 μg total RNA using iScript Reverse Transcription (BioRad, Hercules, CA). Using an aliquot of the synthesized cDNA, qRT-PCR was conducted with SsoAdvanced Universal SYBR Green Supermix (BioRad, CA). The amplification conditions were initially denatured at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 30s, annealing at 55–57°C for 30s and elongation at 72°C for 30s. Melt-curve analysis was performed to confirm that the signal was not of possible primer-dimers but of the expected amplification product. Oligonucleotide primers were designed using Primer 3.0 software and UCSC Genome Bioinformatics (https://genome.ucsc.edu/) or according to the published sequences [61]. The level of glyceraldehydes-3-phosphate dehydrogenase (GAPDH) transcript was used to normalize each sample. Results are expressed as relative fold change of treatment over control. Two independent experiments were performed, and each test condition was conducted in triplicates.
2.7. Protein isolation and western blot analysis
3T3-L1 cells were seeded in 35 mm dishes and treated with diazinon of various concentrations or control media from day 0 to day 8. At the end of each stage, cells were harvested and lysed with ice-cold cell lysis buffer (Cell Signaling, Boston, MA). The cell lysates were sonicated on ice, and the soluble materials were collected from the supernatants after centrifugation at 13,000 rpm for 15 min at 4°C. The protein concentration was determined according to the manufacturer’s instructions (BioRad, CA). 10 μg of total protein were resolved by 4–12% Bis-Tris polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, MA). The specific protein expression was detected with monoclonal rabbit antibodies against Peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT/enhancer binding protein α (C/EBPα), fatty acid synthase (FASN), Acetyl CoA (ACC), adiponectin, perilipin, and mouse antibody against housekeeping proteins β-actin for overnight at 4 °C. The blots were washed five times with Tris-buffered saline containing 0.05% Tween 20 (TBS-T), and then incubated with a horseradish peroxidase-conjugated secondary anti-rabbit or anti-mouse IgG antibodies (Jackson Immuno Research, PA) for 1.5 h at room temperature. Immunoreactivity bands were visualized by enhanced chemiluminescence (BioRad, CA). The intensity of individual bands was quantified using Image J densitometry software (NIH, 1.49), and results are expressed as relative fold change of treatment over control after normalization with housekeeping protein.
2.8. Statistical analysis
The data are shown as the mean ± standard deviation from multiple experiments. Statistical significance was determined using one-way analysis of variances, and Tukey's multiple comparison test, with statistically significant at the cutoff level of p < 0.05 or p<0.01 (GraphPad, Prism5, CA).
3. Results
3.1. Effect of Diazinon on cell number and nuclear area in 3T3-L1 cells
In order to select a sub-toxic dose of diazinon for a subsequent adipogenesis study in 3T3-L1 cells, the cell number was determined using an Arrayscan VTI HCS reader with HCS Studio 2.0 Target Activation BioApplication module (Thermo Scientific, MA). The nuclei were stained with Hoechst 33342, and fluorescence intensities of the nuclei were examined and compared in 3T3-L1 treated with Diazinon at concentrations of 1, 10, 25, 50 and 100 μM. It was found that diazinon treatments decreased the cell number slightly at 25 and 50 μM, but significantly decreased at 100 μM as illustrated in Figure 2. Therefore, the concentrations below 100 μM of diazinon were selected in the following experiments to examine the effect of diazinon on the adipogenesis without obvious cytotoxicity. To clarify whether nuclear condensation that occurs alongside the differentiation, we also found that the nucleus size was reduced by approximately 10% during adipocyte differentiation after diazinon treatment, which likely is associated with alteration of the chromosome [62].
Figure 2. Effect of diazinon treatment on cell count and nuclear area.
The cell counts (A: the treated versus control) and nuclear area (B) were determined by Cellomics reader with TargetActivation Application using High Content Analysis (ArrayScan, Thermo Scientific). 3T3-L1 cells were treated with diazinon for whole stages of differentiation at indicated doses. On day 8 of maturation, the nuclei were stained with Hoechst 33342 (Thermo Scientific, MA), and the fluorescence intensities and area of the nuclei were measured. The data show the means ± SD (N=4). The data were represented as three independent experiments. Statistical analysis was conducted by one-way ANOVA followed by Tukey's multiple comparison tests. Asterisks * and ** represent statistical significance at p < 0.05, or p < 0.01, respectively, versus the control.
3.2. Diazinon increases lipid droplet accumulation and triglyceride levels in cell lysates of 3T3-L1 cells
In order to visualize diazinon’s adipogenic characteristics, Oil Red O staining was used to observe lipid accumulation in 3T3-L1 cells. DEX treatment, a positive control, shows approximately 90% Oil Red O staining cells in each field, indicating a high degree of lipid droplet accumulation (Figure 3). Diazinon treatment significantly increases lipid droplet accumulation in a dose-dependent manner as compared with the untreated control. During the differentiation process for 8 days, the 3T3-L1 preadipocytes underwent morphological changes from spindle-like to round, and accumulated intracellular lipids in both a positive control and diazinon treated conditions (Figure 3). Quantitative measurement of intracellular lipid droplets shows diazinon treatments above 50 μM significantly increase lipid staining as compared to control (p < 0.01) (Figure 3F). The triglyceride was stained with another dye-based Nile Red (AdipoRed), the content of triglyceride was increased starting at 10 μM of diazinon (Figure 3G). In order to distinguish stored triglyceride inside of the cells from the accumulated fat-soluble diazinon, the triglyceride levels in cell lysates were further quantified based on lipase enzyme-based assay using Infinity Triglycerides Reagent (Thermo Scientific, MA). The cellular triglyceride levels were determined by comparison to a triglyceride standard curve. Diazinon treatment increased the cellular triglycerides in a dose-dependent manner, and significantly increased cellular triglycerides at 50 μM (Figure 4). This result further confirmed that diazinon promotes the cellular triglycerides, not fat-soluble compound diazinon itself in the cells.
Figure 3. Effects of diazinon on the lipid droplets accumulation using Oil Red O staining in 3T3-L1 cells.
3T3-L1 preadipocytes were exposed to diazinon in the M2 medium for two days, M3 medium for two days and M1 medium for maturation for four days. On day 8 of maturation, the cells were stained with Oil Red O. Each experiment was repeated twice with two replicates. A: vehicle control, B: 1 μM, C: 10 μM, D: 100 μM, E: positive control (Pos, treated with DEX). F: Quantification of Oil Red O staining cells. Oil Red O was extracted from cells with isopropanol, and optical density (OD) of Oil Red O in the extract was measured at a wavelength of 510 nm. G: The triglyceride content was quantified using AdipoRed Assay Reagent. Data are expressed as a relative fold change from the treatment versus control. Asterisks ** represent statistical significance at p < 0.01, respectively, versus the control. Scale bar: 50 μm.
Figure 4. Quantification of Triglyceride levels in cell lysates.
The 3T3-L1 cells were cultured in the 6-well plate, and treated with various doses of Diazinon, Dex as a positive control, and vehicle (DMSO, 0.05%) as the negative control (Ctl). After the treatment 10 days, the cell were re-suspended and homogenized in 5% NP-40 solution. The triglyceride levels were quantified and expressed relative to total protein levels. Asterisks * represents statistical significance at p < 0.05, and ** represents statistical significance at p < 0.01,
3.3. Multiparametric high-content analysis (HCA) of concentration-dependent effects of diazinon
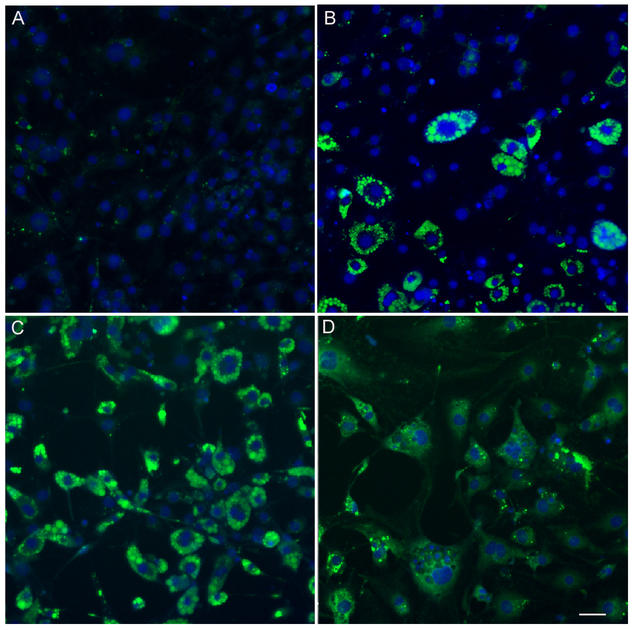
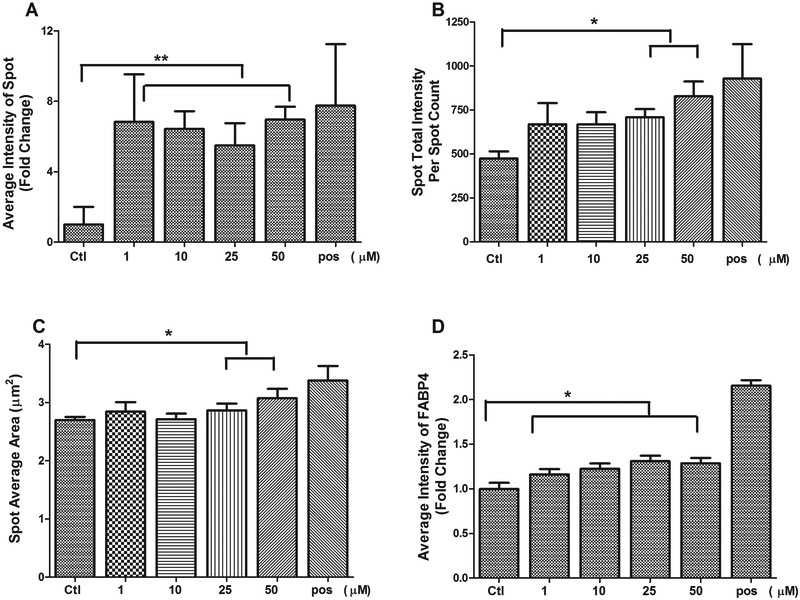
To further quantification of adipocyte differentiation at a single-cell level, a single-cell-based HCA was used to characterize multiparametric features in 3T3-L1 cell treated with diazinon. Lipid accumulation has been used as the primary indicator for the differentiation of preadipocytes into mature adipocytes. We applied a neutral lipid staining, LipidTOX, to investigate the adipogenic effects of diazinon treatment in a high-throughput and high-content format. We evaluated the effects of diazinon on the lipid droplet accumulation in a wide range of concentrations (1, 10, 25 50 and 100 μM). A single-cell-based HCA for the nuclear, lipid droplets and lipid-associated fatty acid binding protein 4 (FABP4) were quantified. Figure 5A (LipidTOX) and 5B (FABP4) show representative fluorescence images of the lipid droplets or FABP4 staining, respectively, after the treatments. As shown in Figure 4A, the cells in control show evenly distributed nuclei with barely detectable green fluorescent lipid. In the treated conditions, we observed a dose-dependent increase of LipiTOX (Figure 5A) or FABP4 fluorescence (Figure 5B), suggesting diazinon-induced adipogenic effect in 3T3-L1 cells as compared to relative control. Similar morphological changes were observed with increasing lipid accumulation with increasing dose of diazinon, while nuclei count did not decrease. Quantification of lipid droplet accumulation, as measured by the Spot Detector Algorithm, increases significantly, whereas the average intensity of FABP4 shows some parallel increases at the end of differentiation, but less extent (Figure 6D). Lipid droplets are the lipid storage organelles, and the size of lipid droplets is associated with the capacity of fat storage. The parameters for lipid droplet size, including Spot Total Intensity Per Spot Count and Spot Average Area, were determined (Figure 6C). As shown in Figure 5, the droplet size in 3T3-L1 cells treated with 1 (B), 25 (C), and 50 μM (D) significantly increased as compared with the relative controls. Many other parameters derived from the lipid droplet images also increased in a similar manner, including Spot Total Intensity Per Spot Count and Average Intensity of Spot. Overall, these data demonstrated that Diazinon induced the lipid droplets and its associated protein FABP4 (Figure E-H) with multiple adipogenesis specific endpoints.
Figure5. A single cell-based high-content analysis (HCA) of concentration-dependent effects of diazinon on the neutral lipid droplets accumulation (A-D) and FABP4 expression (E-H).
The 3T3-L1 were inoculated in a 96-well plate and treated with diazinon at the doses as indicated. On maturation stage of 8 days, the cells were stained with LipidTOX for neutral lipid (green) and Hoechst 33327 for nuclei (blue). The images were acquired with a 20 × objective and representative image from 49 fields/well were shown. A: vehicle control, B: 1 μM, C: 10 μM, D: 25 μM. Cells were also probed with a specific primary antibody of FABP4 (Cell Signaling), followed by DyLight 650 conjugated secondary antibodies(red). Nuclei were stained with Hoechst 33327 (blue). E: vehicle control, F: 1 μM, G: 10 μM, H: 25 μM. Scale bar is 50 μm
Figure 6. Quantification of lipid droplets including the average intensity of spot (A), Spot total intensity per spot count (B), Spot average area (C) and the average intensity of FABP4 (D) in 3T3-L1 cells treated with diazinon.
The pixel intensity of the target was evaluated using TargetActivation BioApplication with HCS Studio™ 2.0 Cell Analysis Software (Thermo Scientific, MA). These intensity levels were normalized to the number of the nucleus. Each bar represents the mean ± SE (n = 4 replicates), and the experiments were repeated for three times. Statistical analysis was conducted by one-way ANOVA followed by Tukey's multiple comparison tests. Asterisks *and ** represent statistical significance at p < 0.05 or p < 0.01, respectively, versus the relative control.
3.4. Diazinon increased adipogenic protein expression.
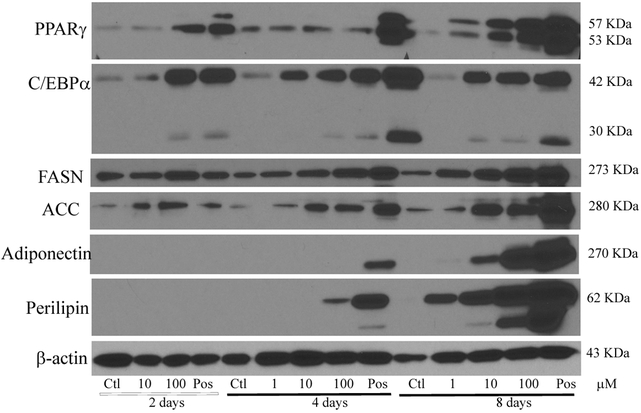
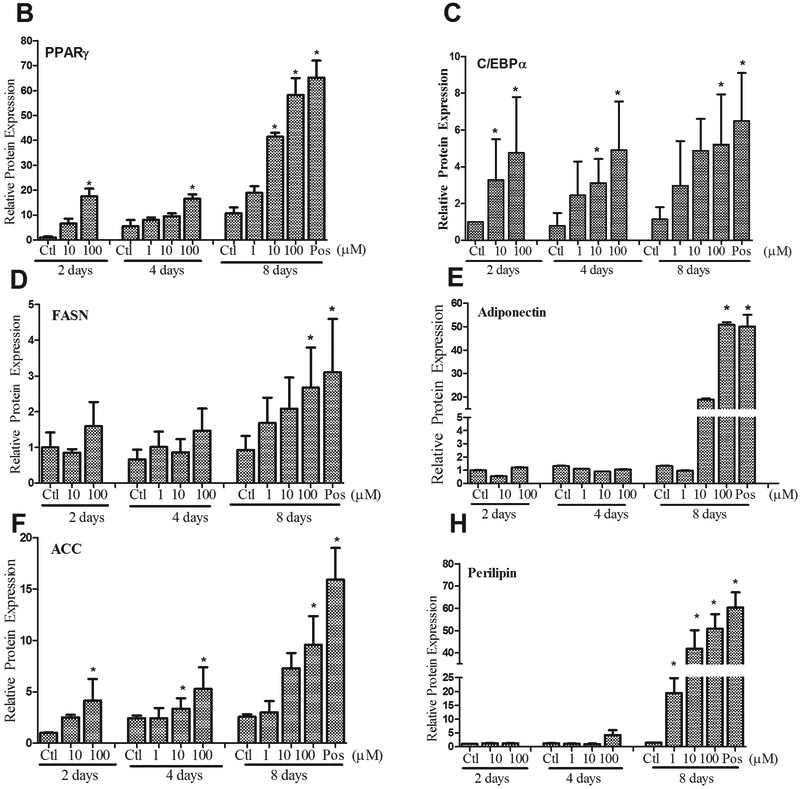
In order to further examine diazinon’s adipogenic efficacy during the differentiation process, adipogenic protein expression was examined in 3T3-L1 cells treated with diazinon (1 μM, 10 μM, 100 μM). The adipogenesis associated proteins, including Fatty acid synthase (FASN), PPARγ, C/EBPα, acetyl-CoA carboxylase (ACC), perilipin and adiponectin, were investigated at the end of each stage of differentiation process (Figure 1, including induction, differentiation, and maturation). As shown in Figure 7A, all the positive controls of each stage show adipogenic proteins, except Perilipin and adiponectin, and exceptionally high abundance of expression levels at the end of the stage (Day 8, maturation). Adipogenic proteins significantly increased with increasing dose and exposure time in 3T3-L1 cells treated with diazinon (Figure 7B-H). Specifically, the protein expression of PPARγ and C/EBPα, two major transcriptional factors for adipogenensis, were increased with diazinon at a low dose of 10 μM starting from the second stage (day 4). As indicated in Figure 7B, PPARγ expression was significantly induced as early as the first stage at 100 μM treatment, and at the lowest dose of 1 μM at the third stage. C/EBPα protein expression was significantly increased in all stages after treatment with 10 μM and 100 μM of diazinon. All examined proteins had a statistically significant increase in expression of the third stage with treatment 10 and 100 μM diazinon.
Figure 7. Diazinon induced adipogenic protein expressions in 3T3-L1 cells using Western blot analysis.
A: Cells were treated with diazinon for 8 days and 1 mM DEX treatment in the M1 media was served as the positive control. Cells were harvested at each stage of differentiation process on day 2, day 4, and day 8, respectively. The proteins were extracted and measured with Western blot analysis using the primary antibodies including PPAR-γ, C/EBP-α, acetyl-CoA Carboxylase (ACC), adiponectin, FABP4, fatty acid synthase (FASN), and perilipin. β-actin was used as an internal standard for the protein loading (A). The protein expression including PPAR-γ (B), C/EBP-α (C), fatty acid synthase (FASN, D), adiponectin (E), acetyl-CoA Carboxylase (ACC, F) and perilipin (G) were quantified using densitometric program Image J ( NIH, V1.49), and quantitative data are expressed as relative protein expression compared with the control after normalization with β-actin (Figure 7B). The data are shown as the mean ± SE of replicates (N=3). Pos indicates positive control. Statistical analysis was conducted by one-way ANOVA followed by Tukey's multiple comparison tests. Asterisks *and ** represent statistical significance at p < 0.05 or p < 0.01, respectively, versus the relative control.
3.5. Diazinon induced dynamic changes in adipogenic regulatory genes
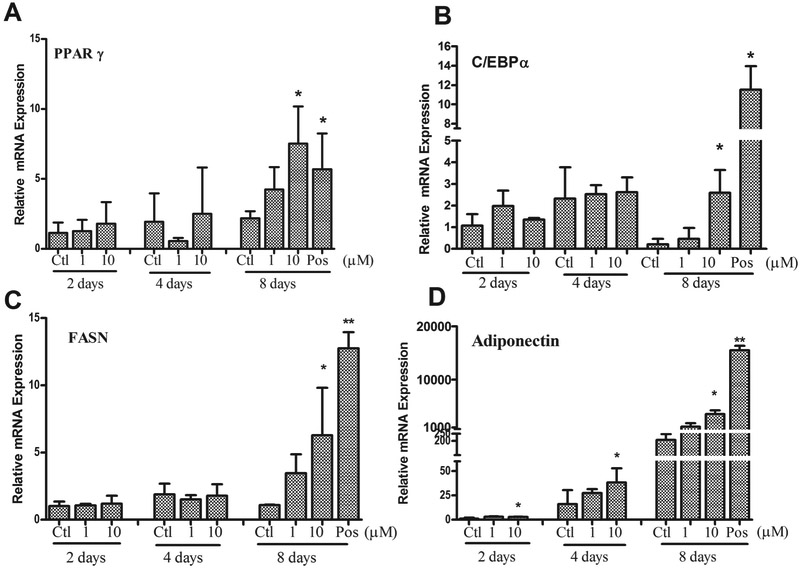
In order to examine the adipogenic signaling pathway, we measured the adipogentic regulatory genes at the end of each stage of adipogenesis: induction (day 2), differentiation (day 4) and maturation (day 8). As shown in Figure 8, all tested gene expression in the positive control were significantly increased with dramatic 10 to 1000-fold increases in a time-dependent manner (only show the third stage). Diazinon treatment significantly induced all tested gene expression at 10 μM in the third stage (Day 8) as compared with the control. Adiponectin, adipocyte differentiation marker, was significantly induced at the early stage (day 2), with dramatic induction at both the differentiation (30 fold) and maturation stage (1000 fold). The enzyme that regulates adipogenesis, fatty acid synthase (FASN), was also significantly induced at 10 μM of diazinon during the maturation (day 8).
Figure 8. Diazinon induced adipogenic regulatory gene expressions in 3T3-L1 cells using quantitative real-time RT-PCR (QRT-PCR).
The 3T3-L1 preadipocytes were treated with diazinon. Total RNA was isolated during induction on the day 2, differentiation on the day 4 and maturation on the day 8. The vehicle control at day 2 was served as controls (CTL). Changes in mRNA expression including PPAR-γ (A), C/EBP-α (B), fatty acid synthase (FASN, C), adiponectin (D), were evaluated using qRT-PCR and results were expressed as fold change of gene expression compared with the control after normalization with glyceraldehydes-3-phosphate dehydrogenase (GAPDH). Data are shown as the mean ± SE of triplicate (n = 3) from three independent experiments. Statistical analysis was conducted by one-way ANOVA followed by Tukey's multiple comparison tests. PC indicates positive control. Asterisks * represents statistical significance at p < 0.05, versus the control.
4. Discussion
The cellular basis of obesity is increased lipid droplets within the adipocyte. As cytoplasmic lipid droplet accumulation is a hallmark of adipogenesis, a compound capable of inducing lipid accumulation is considered as a potential obesogen. In the present study, we have applied a neutral lipid staining combined with a high-content analysis, and have shown that this novel approach provided much sensitive and robust measurement as compared with the conventional approach. Besides, the current study demonstrated that diazinon significantly promotes adipogenesis through the up-regulation of adipogenic transcription factors, C/EBPα and PPARγ, and multiple adipogenic specific genes and proteins in a dose-dependent and stage-specific manner. These results may provide valuable implication in understanding adipogenesis induced by organophosphate pesticide and other toxicants.
Diazinon has been widely used in agriculture in the USA and worldwide, in spite of the cancellation of residential use products by USEPA in 2004. Due to the pesticides’ mitigation and take-home exposure, general populations and ecological risks are still above the levels of health concerns [25]. Regulatory standards for human exposure of OPs are determined based on their inhibitory effects on cholinesterase activity. However, recent studies have raised more concerns regarding a multitude of toxic mechanisms of OPs at the sub-toxic dose [41-43]. High-content analysis has been shown to be highly sensitive and specific to examine subtle changes of toxicity within the cell using sub-lethal doses [63]. A single-cell-based HCA with LipidTOX staining showed significant induction of morphological change of lipid droplets at the lowest dose of 1 μM, and elevation of the size of lipid droplet at 25 μM. These results are approximately 10 times more sensitive than Oil Red O staining. Moreover, HCA provided multiple cellular parameters, including the number of the nucleus, the area of the nucleus, the average intensity of lipid droplet, size of lipid droplet, and associated protein expression FABP4, where the conventional toxicity assays showed none. The dose for adipogenic effects of diazinon was far less than those doses causing cytotoxic effects. Interestingly, the low dose of diazinon (1 μM) could significantly affect PPARγ, one of the main transcriptional factors for adipogenesis, while the higher dose of diazinon may have adverse health effects on other organ systems, such as the neurological system. Also, previous findings suggest that the size and number of the lipid droplets within adipocytes could affect the secretion of free fatty acids, which is associated with the development of insulin resistance [64]. In the current study, we have found that diazinon significantly induced greater in size of lipid droplet, suggesting another aspect of the mechanism involved in diazinon-induced adipogenesis. Therefore, it is apparent advantages using HCA as a high-throughput screen platform for environmental obesogen screening.
It has been reported that urine levels of diazinon ranged from 1.45 and 1.49 μg/L (metabolites), in the National Health and Nutrition Examination Survey (NHANES) 2001–2002 [31]. The NHANES is a program of studies designed to evaluate the health and nutritional status of the population in the United States. Besides, diazinon has been detected in rain and groundwater at the concentration up to 76 ug/L, and freshwater fish with LD50 values ranging from 90 to 7800 μg/L [65], which estimated to be 0.3–25 μM. Since organophosphate insecticides are highly lipophilic and are known to bioaccumulate in the body fat tissue, fish and milk. Human exposure to organophosphates may occur either during manufacture or use in agriculture, gardening, or even food-chain ([66-69]. According to EPA [70], the no-observed-adverse-effect-level (NOAEL) of acute dietary is 0.25 mg/kg/day, the lowest-observed-adverse-effect level (LOAEL) of acute dietary is 2.5 mg/kg/day, and the NOAEL of chronic dietary is 0.02 mg/kg/day based on the endpoint of plasma cholinesterase inhibition. To date, most animal studies that have examined the toxic effects of diazinon have used doses in the range of 0.06–100 mg/kg, and the dose range for most in vitro studies is 50–600 μM [70-74]. The National Institute for Occupational Safety and Health (NIOSH) recommended exposure limit (REL) is 0.1 mg/m3 [75]. The concentrations of diazinon used in this study (1, 10, 25, 50 and 100 μM) fall within the range of concentrations discussed above and have biological or ecotoxicology relevance.
3T3-L1 cells have been extensively used as an in vitro model for assessing adipogenesis and applied to screen environmental obesogens as an alternative in vitro model [76-78]. Differentiation of adipocyte involves multiple steps and several signaling pathways, a dynamic examination of the process of differentiation will be very crucial to accurately decipher the molecular mechanisms for the identification of potential obesogen. Combing with HCA platform, our previous and the current study have provided a more in-depth assessment by simultaneously imaging and correlating multiple parameters [61, 79].
Two major transcriptional factors PPARγ and C/EBPα are found to be involved in the regulation adipogenesis, and both these transcriptional factors induce the expression of genes that are involved in insulin sensitivity, lipogenesis, and lipolysis, including lipogenic gene: fatty acid synthase (FASN), the adipocyte-derived factor adiponectin, lipoprotein lipase (LPL), and the lipid droplet-associated protein, perilipin [80-83]. Diazinon, in the current study, induced differential dynamic changes of these two transcriptional factors, with a significantly higher expression of both C/EBPα and PPARγ. Our finding strongly supports that diazinon treatment increased lipid droplet in adipocyte due to increasing of both adiponectin and perilipin at both mRNA and protein expression levels. Perilipin was found exclusively on the outer surface of lipid droplets, but not for any other subcellular compartment of cells [84]. Adiponectin is exclusively expressed in the differentiated adipocyte [85], and an increase in adiponectin expression is one of the main criteria for lipid accumulation in the adipocyte. Adiponectin modulates some metabolic processes, such as glucose regulation and fatty acid oxidation[86]. Transgenic mice with increased adiponectin show impaired adipocyte differentiation and increased energy expenditure associated with protein uncoupling [87]. Similarly, p,p′-dichlorodiphenyldichloroethylene (DDE), a lipophilic insecticide, has found to promote adipogenesis and increase adiponectin expression in the in vitro using 3T3-L1 [88]. Interestedly, although adiponectin is secreted from adipose tissue, many studies have found that circulating adiponectin is inversely correlated with body mass index in obese individuals, in particular, those with visceral obesity [89, 90]. Adiponectin expression is regulated at the transcriptional, translational, and post-translational levels, and its post-translational modification by glycosylation and hydroxylation can result in a low molecular weight trimer, a middle molecular weight hexamer, and a high molecular weight multimer (38). The observed increase in adiponectin expression following Diazinon treatment in vitro might be due to an increase of high molecular weight isoform. The in vivo animal study will be needed to examine the effect of Diazinon treatment on the adiponectin level.
So far, a few studies investigated the interplay between pesticide exposure and adipogenesis [79, 91]. Rats chronically exposed to chlorpyrifos, another organophosphate, at a sub-toxic dose (5 mg/kg/day) was found to gain weight and fat accumulation [92]. Organophosphate insecticides are known as inhibitors of AChE, the enzyme responsible for the degradation of acetylcholine. Thus, exposure to sufficient levels of organophosphate insecticides would be expected to increase the accumulation of acetylcholine, potentially leading to overstimulation and consequent downregulation of its receptors. The involvement of acetylcholine binding to muscarinic receptors in pancreatic cells ultimately leads to impaired homeostasis of glucose and insulin resistance [23, 93]. This group of pesticides has shown to share similar molecular mechanism to induce adipogenesis, and varying degrees of response may be dependent on exposure level duration and chemical structure. Another category of insecticides, organochlorines, such as DDT and DDE, have been shown to induce adipogenesis, and disrupt lipid metabolism [94]. Although these in vitro data offer valuable information that correlated with in vivo, the integrated approach, such as innovative high-content analysis, provides qualitative and quantitative measurement, which might attempt to extrapolate to the in vivo. Our findings indicate the classification of diazinon as an obesogen using 3T3-L1 in vitro model. However, more studies are needed to fully understand the molecular pathway and in vivo assessment.
In summary, this study has found that diazinon induced lipid droplet accumulation and altered adipogenesis signaling pathway through changes of multiple adipogenic regulated genes and proteins. To better understand an escalating global epidemic of obesity, the current study may help identify the fundamental underlying interplay between insecticide exposure and the development of obesity. However, further in vivo study of as well as human population study, are necessary to reveal the role of environmental obesogen in the development of obesity.
Highlights.
Diazinon is an organophosphate insecticide and widely used in the agriculture.
Effects and mechanisms of diazinon on adipogenesis was examined in the 3T3-L1.
Diazinon induced lipid droplet accumulation in a dose-dependent manner.
The dynamic changes of adipogenic regulatory proteins and genes were observed.
Diazinon promotes lipid accumulation and activates the adipogenic pathway.
5. Acknowledgments
This work was supported by the Centers for Disease Control and Prevention, The National Institute for Occupational Safety and Health (NIOSH) under award R21 OH 010473; National Institute of Environmental Health Sciences of the National Institutes of Health under award R43ES027374; Alternatives Research & Development Foundation (ARDF).
References
- [1].C. NCHS Data Brief, Prevalence of Obesity Among Adults and Youth: United States, 2011–2014, (2015). [PubMed]
- [2].Hossain P, Kawar B, El Nahas M, Obesity and diabetes in the developing world--a growing challenge, The New England journal of medicine, 356 (2007) 213–215. [DOI] [PubMed] [Google Scholar]
- [3].Allender S, Rayner M, The burden of overweight and obesity-related ill health in the UK, Obesity reviews : an official journal of the International Association for the Study of Obesity, 8 (2007) 467–473. [DOI] [PubMed] [Google Scholar]
- [4].Hursting SD, Dunlap SM, Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue, Annals of the New York Academy of Sciences, 1271 (2012) 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].McTigue KM, Chang YF, Eaton C, Garcia L, Johnson KC, Lewis CE, Liu S, Mackey RH, Robinson J, Rosal MC, Snetselaar L, Valoski A, Kuller LH, Severe obesity, heart disease, and death among white, African American, and Hispanic postmenopausal women, Obesity, 22 (2014) 801–810. [DOI] [PubMed] [Google Scholar]
- [6].Buckley JP, Herring AH, Wolff MS, Calafat AM, Engel SM, Prenatal exposure to environmental phenols and childhood fat mass in the Mount Sinai Children's Environmental Health Study, Environ Int, 91 (2016) 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Buckley JP, Engel SM, Mendez MA, Richardson DB, Daniels JL, Calafat AM, Wolff MS, Herring AH, Prenatal Phthalate Exposures and Childhood Fat Mass in a New York City Cohort, Environ Health Perspect, 124 (2016) 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stanner SA, Yudkin JS, Fetal programming and the Leningrad Siege study, Twin research : the official journal of the International Society for Twin Studies, 4 (2001) 287–292. [DOI] [PubMed] [Google Scholar]
- [9].Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL, The global obesity pandemic: shaped by global drivers and local environments, Lancet, 378 (2011) 804–814. [DOI] [PubMed] [Google Scholar]
- [10].Janesick A, Blumberg B, Endocrine disrupting chemicals and the developmental programming of adipogenesis and obesity, Birth defects research. Part C, Embryo today : reviews, 93 (2011) 34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Newbold RR, Padilla-Banks E, Jefferson WN, Environmental estrogens and obesity, Molecular and cellular endocrinology, 304 (2009) 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baillie-Hamilton PF, Chemical toxins: a hypothesis to explain the global obesity epidemic, Journal of alternative and complementary medicine, 8 (2002) 185–192. [DOI] [PubMed] [Google Scholar]
- [13].Newbold RR, Impact of environmental endocrine disrupting chemicals on the development of obesity, Hormones, 9 (2010) 206–217. [DOI] [PubMed] [Google Scholar]
- [14].Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N, Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes, Environ Health Perspect, 116 (2008) 1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ, Effects of endocrine disruptors on obesity, International journal of andrology, 31 (2008) 201–208. [DOI] [PubMed] [Google Scholar]
- [16].Migliarini B, Piccinetti CC, Martella A, Maradonna F, Gioacchini G, Carnevali O, Perspectives on endocrine disruptor effects on metabolic sensors, General and comparative endocrinology, 170 (2011) 416–423. [DOI] [PubMed] [Google Scholar]
- [17].Heindel JJ, Newbold R, Schug TT, Endocrine disruptors and obesity, Nat Rev Endocrinol, 11 (2015) 653–661. [DOI] [PubMed] [Google Scholar]
- [18].Wei Y, Zhu J, Nguyen A, Urinary concentrations of dichlorophenol pesticides and obesity among adult participants in the U.S. National Health and Nutrition Examination Survey (NHANES) 2005–2008, Int J Hyg Environ Health, 217 (2014) 294–299. [DOI] [PubMed] [Google Scholar]
- [19].Verhulst SL, Nelen V, Hond ED, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K, Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life, Environ Health Perspect, 117 (2009) 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO, Diabetes an relation to serum levels of polychlorinated biphenyls and chlorinated pesticides an adult native Americans, Environ Health Persp, 115 (2007) 1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Mainous AG, Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey, Environ Res, 103 (2007) 413–418. [DOI] [PubMed] [Google Scholar]
- [22].Deierlein AL, Wolff MS, Pajak A, Pinney SM, Windham GC, Galvez MP, Silva MJ, Calafat AM, Kushi LH, Biro FM, Teitelbaum SL, and the Breast C, P. Environment Research, Longitudinal associations of phthalate exposures during childhood and body size measurements in young girls, Epidemiology, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Montgomery MP, Kamel F, Saldana TM, Alavanja MC, Sandler DP, Incident diabetes and pesticide exposure among licensed pesticide applicators: Agricultural Health Study, 1993–2003, American journal of epidemiology, 167 (2008) 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].USEPA, Reregistration Eligibility Decision for Diazinon, US Environmental Protection Agency Office of Pesticide Programs; (2006). [Google Scholar]
- [25].EPA, Diazinon IRED Facts, in: U.S.E.P. Agency; (Ed.) Pesticides: Reregistration, 2016. [Google Scholar]
- [26].ATSDR, Public Health Statement: Diazinon CAS # 333–41-5, in: P.H.S. Department of Health and Human Services (Ed.), Center for Disease Control and Prevention, 2008. [Google Scholar]
- [27].Aggarwal V, Deng X, Tuli A, Goh KS, Diazinon-chemistry and environmental fate: a California perspective, Rev Environ Contam Toxicol, 223 (2013) 107–140. [DOI] [PubMed] [Google Scholar]
- [28].EPA, TOXICOLOGICAL PROFILE FOR DIAZINON, Agency for Toxic Substances and Disease Registry, (2008). [PubMed] [Google Scholar]
- [29].Hill RH Jr., Head SL, Baker S, Gregg M, Shealy DB, Bailey SL, Williams CC, Sampson EJ, Needham LL, Pesticide residues in urine of adults living in the United States: reference range concentrations, Environ Res, 71 (1995) 99–108. [DOI] [PubMed] [Google Scholar]
- [30].MacIntosh DL, Kabiru C, Echols SL, Ryan PB, Dietary exposure to chlorpyrifos and levels of 3,5,6-trichloro-2-pyridinol in urine, Journal of exposure analysis and environmental epidemiology, 11 (2001) 279–285. [DOI] [PubMed] [Google Scholar]
- [31].CDC, Centers for Disease Control and Prevention (CDC). , Fourth National Report on Human Exposure to Environmental Chemicals., 2009, pp. 1/24/13.
- [32].Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP, Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns, Environ Health Perspect, 111 (2003) 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thompson B, Griffith WC, Barr DB, Coronado GD, Vigoren EM, Faustman EM, Variability in the take-home pathway: farmworkers and non-farmworkers and their children, J Expo Sci Environ Epidemiol, 24 (2014) 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Coronado GD, Vigoren EM, Thompson B, Griffith WC, Faustman EM, Organophosphate pesticide exposure and work in pome fruit: evidence for the take-home pesticide pathway, Environ Health Perspect, 114 (2006) 999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Flaskos J, The developmental neurotoxicity of organophosphorus insecticides: a direct role for the oxon metabolites, Toxicol Lett, 209 (2012) 86–93. [DOI] [PubMed] [Google Scholar]
- [36].Munoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, Iglesias V, Alvarado S, Concha C, Rojas E, Vega C, Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: a systematic review, Neurotoxicology, 39 (2013) 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yen J, Donerly S, Levin ED, Linney EA, Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish, Neurotoxicol Teratol, 33 (2011) 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boussabbeh M, Ben Salem I, Hamdi M, Ben Fradj S, Abid-Essefi S, Bacha H, Diazinon, an organophosphate pesticide, induces oxidative stress and genotoxicity in cells deriving from large intestine, Environ Sci Pollut Res Int, 23 (2016) 2882–2889. [DOI] [PubMed] [Google Scholar]
- [39].Jones RR, Barone-Adesi F, Koutros S, Lerro CC, Blair A, Lubin J, Heltshe SL, Hoppin JA, Alavanja MC, Beane Freeman LE, Incidence of solid tumours among pesticide applicators exposed to the organophosphate insecticide diazinon in the Agricultural Health Study: an updated analysis, Occup Environ Med, 72 (2015) 496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kashanian S, Gholivand MB, Ahmadi F, Ravan H, Interaction of diazinon with DNA and the protective role of selenium in DNA damage, DNA Cell Biol, 27 (2008) 325–332. [DOI] [PubMed] [Google Scholar]
- [41].Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA, Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade, Toxicology and applied pharmacology, 145 (1997) 158–174. [DOI] [PubMed] [Google Scholar]
- [42].Crumpton TL, Seidler FJ, Slotkin TA, Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos?, Brain research. Developmental brain research, 121 (2000) 189–195. [DOI] [PubMed] [Google Scholar]
- [43].Crumpton TL, Seidler FJ, Slotkin TA, Developmental neurotoxicity of chlorpyrifos in vivo and in vitro: effects on nuclear transcription factors involved in cell replication and differentiation., Brain research, 857 (2000) 87–98. [DOI] [PubMed] [Google Scholar]
- [44].Roeyen G, Chapelle T, Jorens P, de Beeck BO, Ysebaert D, Necrotizing pancreatitis due to poisoning with organophosphate pesticides, Acta gastro-enterologica Belgica, 71 (2008) 27–29. [PubMed] [Google Scholar]
- [45].Harputluoglu MM, Kantarceken B, Karincaoglu M, Aladag M, Yildiz R, Ates M, Yildirim B, Hilmioglu F, Acute pancreatitis: an obscure complication of organophosphate intoxication, Human & experimental toxicology, 22 (2003) 341–343. [DOI] [PubMed] [Google Scholar]
- [46].Hsiao CT, Yang CC, Deng JF, Bullard MJ, Liaw SJ, Acute pancreatitis following organophosphate intoxication, J Toxicol Clin Toxicol, 34 (1996) 343–347. [DOI] [PubMed] [Google Scholar]
- [47].Adamkovicova M, Toman R, Cabaj M, Massanyi P, Martiniakova M, Omelka R, Krajcovicova V, Duranova H, Effects of subchronic exposure to cadmium and diazinon on testis and epididymis in rats, ScientificWorldJournal, 2014 (2014) 632581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chiu YH, Afeiche MC, Gaskins AJ, Williams PL, Petrozza JC, Tanrikut C, Hauser R, Chavarro JE, Fruit and vegetable intake and their pesticide residues in relation to semen quality among men from a fertility clinic, Hum Reprod, 30 (2015) 1342–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sadr-Azodi O, Orsini N, Andren-Sandberg A, Wolk A, Abdominal and total adiposity and the risk of acute pancreatitis: a population-based prospective cohort study, The American journal of gastroenterology, 108 (2013) 133–139. [DOI] [PubMed] [Google Scholar]
- [50].Lowenfels AB, Maisonneuve P, Sullivan T, The changing character of acute pancreatitis: epidemiology, etiology, and prognosis, Current gastroenterology reports, 11 (2009) 97–103. [DOI] [PubMed] [Google Scholar]
- [51].Spradbery JP, Tozer RS, The efficacy of diazinon impregnated ear tags against buffalo fly and resulting weight gains and diazinon residues in meat and milk, Aust Vet J, 73 (1996) 6–10. [DOI] [PubMed] [Google Scholar]
- [52].Maciel W, Lopes WD, Cruz B, Teixeira W, Felippelli G, Sakamoto CA, Favero FC, Buzzulini C, Soares V, Gomes LV, Bichuette M, da Costa AJ, Effects of Haematobia irritans infestation on weight gain of Nelore calves assessed with different antiparasitic treatment schemes, Prev Vet Med, 118 (2015) 182–186. [DOI] [PubMed] [Google Scholar]
- [53].Baconi DL, Barca M, Manda G, Ciobanu AM, Balalau C, Investigation of the toxicity of some organophosphorus pesticides in a repeated dose study in rats, Rom J Morphol Embryol, 54 (2013) 349–356. [PubMed] [Google Scholar]
- [54].Adigun AA, Wrench N, Seidler FJ, Slotkin TA, Neonatal dexamethasone treatment leads to alterations in cell signaling cascades controlling hepatic and cardiac function in adulthood, Neurotoxicol Teratol, 32 (2010) 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Adigun AA, Wrench N, Seidler FJ, Slotkin TA, Neonatal organophosphorus pesticide exposure alters the developmental trajectory of cell-signaling cascades controlling metabolism: differential effects of diazinon and parathion, Environ Health Perspect, 118 (2010) 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Slotkin TA, Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity?, Reprod Toxicol, 31 (2011) 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gao B, Bian X, Mahbub R, Lu K, Sex-Specific Effects of Organophosphate Diazinon on the Gut Microbiome and Its Metabolic Functions, Environ Health Perspect, 125 (2017) 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kim J, Park Y, Yoon KS, Clark JM, Park Y, Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes, J Biochem Mol Toxicol, 28 (2014) 418–424. [DOI] [PubMed] [Google Scholar]
- [59].Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M, Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study, Environ Health Perspect, 120 (2012) 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yin L, Yu KS, Lu K, Yu X, Benzyl butyl phthalate promotes adipogenesis in 3T3-L1 preadipocytes: A High Content Cellomics and metabolomic analysis, Toxicol In Vitro, 32 (2016) 297–309. [DOI] [PubMed] [Google Scholar]
- [61].Yin L, Yu KS, Lu K, Yu X, Benzyl butyl phthalate promotes adipogenesis in 3T3-L1 preadipocytes: A High Content Cellomics and metabolomic analysis, Toxicology in Vitro, 32 (2016) 297–309. [DOI] [PubMed] [Google Scholar]
- [62].Kuroda M, Tanabe H, Yoshida K, Oikawa K, Saito A, Kiyuna T, Mizusawa H, Mukai K, Alteration of chromosome positioning during adipocyte differentiation, J Cell Sci, 117 (2004) 5897–5903. [DOI] [PubMed] [Google Scholar]
- [63].Clarke R, Connolly L, Frizzell C, Elliott CT, High content analysis: a sensitive tool to detect and quantify the cytotoxic, synergistic and antagonistic effects of chemical contaminants in foods, Toxicol Lett, 233 (2015) 278–286. [DOI] [PubMed] [Google Scholar]
- [64].Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. , PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance, Mol Cell, 4 (1999) 597–609. [DOI] [PubMed] [Google Scholar]
- [65].NPIC, Diazinon, Technical Fact Sheet. http://npic.orst.edu/factsheets/archive/diazinontech.html#references, (2011).
- [66].Kazemi AMTM, Valizadeh R, Naserian AA, and Soni A, Organophosphate pesticides: A general review, http://www.resjournals.com/ARJ, 2(9) (2012) 512– 522. [Google Scholar]
- [67].Melgar MJ, Santaeufemia M, Garcia MA, Organophosphorus pesticide residues in raw milk and infant formulas from Spanish northwest, J Environ Sci Health B, 45 (2010) 595–600. [DOI] [PubMed] [Google Scholar]
- [68].Salas JH, Gonzalez MM, Noa M, Perez NA, Diaz G, Gutierrez R, Zazueta H, Osuna I, Organophosphorus pesticide residues in Mexican commercial pasteurized milk, J Agric Food Chem, 51 (2003) 4468–4471. [DOI] [PubMed] [Google Scholar]
- [69].Sanghi R, Pillai MK, Jayalekshmi TR, Nair A, Organochlorine and organophosphorus pesticide residues in breast milk from Bhopal, Madhya Pradesh, India, Human & experimental toxicology, 22 (2003) 73–76. [DOI] [PubMed] [Google Scholar]
- [70].EPA, Reregistration Eligibility Decision for Diazinon, (2004).
- [71].Bagherpour Shamloo H, Golkari S, Faghfoori Z, Movassaghpour A, Lotfi H, Barzegari A, Yari Khosroushahi A, Lactobacillus Casei Decreases Organophosphorus Pesticide Diazinon Cytotoxicity in Human HUVEC Cell Line, Adv Pharm Bull, 6 (2016) 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Ogasawara N, Matsushima M, Kawamura N, Atsumi K, Yamaguchi T, Ochi H, Kusatsugu Y, Oyabu S, Hashimoto N, Hasegawa Y, Ueyama J, Kawabe T, Modulation of immunological activity on macrophages induced by diazinon, Toxicology, 379 (2017) 22–30. [DOI] [PubMed] [Google Scholar]
- [73].Gokalp O, Buyukvanli B, Cicek E, Ozer MK, Koyu A, Altuntas I, Koylu H, The effects of diazinon on pancreatic damage and ameliorating role of vitamin E and vitamin C, Pestic Biochem Phys, 81 (2005) 123–128. [Google Scholar]
- [74].ATSDR, TOXICOLOGICAL PROFILE FOR DIAZINON (2008). [PubMed]
- [75].CDC, NIOSH Pocket Guide to Chemical Hazards: Diazinon, (2005).
- [76].Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, Vanparys C, Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect, PloS one, 8 (2013) e77481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pereira-Fernandes A, Vanparys C, Hectors TL, Vergauwen L, Knapen D, Jorens PG, Blust R, Unraveling the mode of action of an obesogen: mechanistic analysis of the model obesogen tributyltin in the 3T3-L1 cell line, Molecular and cellular endocrinology, 370 (2013) 52–64. [DOI] [PubMed] [Google Scholar]
- [78].Pereira-Fernandes A, Vanparys C, Vergauwen L, Knapen D, Jorens PG, Blust R, Toxicogenomics in the 3T3-L1 Cell Line, a New Approach for Screening of Obesogenic Compounds, Toxicological Sciences, 140 (2014) 352–363. [DOI] [PubMed] [Google Scholar]
- [79].Mangum LH, Howell GE, Chambers JE, Exposure to p,p′-DDE enhances differentiation of 3T3-L1 preadipocytes in a model of sub-optimal differentiation, Toxicology Letters, 238 (2015) 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Madsen MS, Siersbaek R, Boergesen M, Nielsen R, Mandrup S, Peroxisome proliferator-activated receptor gamma and C/EBPalpha synergistically activate key metabolic adipocyte genes by assisted loading, Molecular and cellular biology, 34 (2014) 939–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ntambi JM, Young-Cheul K, Adipocyte differentiation and gene expression, The Journal of nutrition, 130 (2000) 3122S–3126S. [DOI] [PubMed] [Google Scholar]
- [82].Rosen ED, The transcriptional basis of adipocyte development, Prostaglandins, leukotrienes, and essential fatty acids, 73 (2005) 31–34. [DOI] [PubMed] [Google Scholar]
- [83].Cai H, Dong LQ, Liu F, Recent Advances in Adipose mTOR Signaling and Function: Therapeutic Prospects, Trends Pharmacol Sci, 37 (2016) 303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Garcia A, Sekowski A, Subramanian V, Brasaemle DL, The central domain is required to target and anchor perilipin A to lipid droplets, J Biol Chem, 278 (2003) 625–635. [DOI] [PubMed] [Google Scholar]
- [85].Berg AH, Combs TP, Du X, Brownlee M, Scherer PE, The adipocyte-secreted protein Acrp30 enhances hepatic insulin action, Nat Med, 7 (2001) 947–953. [DOI] [PubMed] [Google Scholar]
- [86].Diez JJ, Iglesias P, The role of the novel adipocyte-derived hormone adiponectin in human disease, Eur J Endocrinol, 148 (2003) 293–300. [DOI] [PubMed] [Google Scholar]
- [87].Bauche IB, El Mkadem SA, Pottier AM, Senou M, Many MC, Rezsohazy R, Penicaud L, Maeda N, Funahashi T, Brichard SM, Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation, Endocrinology, 148 (2007) 1539–1549. [DOI] [PubMed] [Google Scholar]
- [88].Howell G 3rd, Mangum L, Exposure to bioaccumulative organochlorine compounds alters adipogenesis, fatty acid uptake, and adipokine production in NIH3T3-L1 cells, Toxicol In Vitro, 25 (2011) 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA, Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia, J Clin Endocrinol Metab, 86 (2001) 1930–1935. [DOI] [PubMed] [Google Scholar]
- [90].Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE, Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex, Diabetologia, 46 (2003) 459–469. [DOI] [PubMed] [Google Scholar]
- [91].Kim J, Sun Q, Yue Y, Yoon KS, Whang K-Y, Marshall Clark J, Park Y, 4,4′-Dichlorodiphenyltrichloroethane (DDT) and 4,4′-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture, Pesticide Biochemistry & Physiology, 131 (2016) 40–45. [DOI] [PubMed] [Google Scholar]
- [92].Meggs WJ, Brewer KL, Weight Gain Associated with Chronic Exposure to Chlorpyrifos in Rats, Journal of Medical Toxicology, 3 (2007) 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Androutsopoulos VP, Hernandez AF, Liesivuori J, Tsatsakis AM, A mechanistic overview of health associated effects of low levels of organochlorine and organophosphorous pesticides, Toxicology, 307 (2013) 89–94. [DOI] [PubMed] [Google Scholar]
- [94].Kim J, Sun Q, Yue Y, Yoon KS, Whang KY, Marshall Clark J, Park Y, 4,4'-Dichlorodiphenyltrichloroethane (DDT) and 4,4'-dichlorodiphenyldichloroethylene (DDE) promote adipogenesis in 3T3-L1 adipocyte cell culture, Pestic Biochem Physiol, 131 (2016) 40–45. [DOI] [PubMed] [Google Scholar]