Abstract
OBJECTIVES:
To assess the multimorbidity burden and its association with clinical outcomes in adults with heart failure (HF) according to sex, age, and HF type.
DESIGN:
Retrospective cohort study.
SETTING and PARTICIPANTS:
114, 553 adults with HF from five healthcare delivery systems across the U.S.
MEASUREMENTS:
We characterized patients with respect to the presence of 26 chronic conditions categorized into quartiles based on overall burden of comorbidity (0–4, 5–6, 7–8, ≥9). Outcomes included all-cause death and hospitalizations for HF or any cause. Multivariable Cox regression was used to evaluate the adjusted association of categorized burden of multimorbidity burden with outcomes.
RESULTS:
Among 114,553 adults with HF, adjusted hazard ratios (HR) for all-cause death among those with 5–6, 7–8, or 9 or more morbidities (vs. 0–4) were HR 1.27 (95% CI, 1.24–1.31), HR 1.52 (95% CI, 1.48–1.57), and HR 1.92 (95% CI, 1.86–1.99), respectively. There was a graded, higher adjusted rate of any-cause hospitalization associated with 5–6, 7–8, or 9 or more morbidities (vs. 0–4): HR 1.28 (95% CI, 1.25–1.30), HR 1.47 (95% CI, 1.44–1.50), and HR 1.77 (95% CI, 1.73–1.82), respectively. Similar findings were observed for HF-specific hospitalization in those with 5–6, 7–8, or 9 or more morbidities (vs. 0–4): HR 1.22 (95% CI, 1.19–1.26), HR 1.39 (95% CI, 1.34–1.44), and HR 1.68 (95% CI, 1.61–1.74), respectively. Consistent findings were seen by sex, age group and HF type (preserved, reduced, borderline HF), with the relationship between categorical burden of multimorbidity and outcomes especially prominent among those <65 years.
CONCLUSION:
After adjustment higher levels of multimorbidity predict worse HF outcomes and may be an important consideration in strategies to improve clinical and patient-centered outcomes.
Keywords: multimorbidity, heart failure, comorbidity, multiple chronic conditions
Introduction
Heart failure (HF) develops frequently in the presence of multiple other concomitant chronic conditions which may complicate clinical management and treatment decision-making.1–10 Previous studies have shown that the majority of adults with HF have five or more chronic medical conditions.1–3
As a consequence, health care providers face the prospect of simultaneously managing HF and multiple chronic conditions.4–7;11,12 Therefore, it can be challenging to apply clinical practice guidelines which do not currently consider how multimorbidity contributes to undesirable outcomes such as adverse interactions among drugs and diseases.4 Furthermore, in selected studies, multimorbidity was associated with higher risks of hospitalization and death among patients with HF.3–6
As Rich et al have written, “… most randomized clinical trials have either explicitly excluded older adults or have enrolled only relatively healthy older patients. As a result, the generalizability of the results of most major clinical trials to older patients, especially those >75 years of age with multimorbidity, is uncertain.”13 Our rationale in pursuing this investigation was to fill gaps in knowledge highlighted by Rich and colleagues by better characterizing the association between multimorbidity and adverse outcomes in HF patients cared for in real-world clinical settings. Gaining additional insight into these relationships is crucial to advancing the care of the growing population of patients with HF.
In addition, over recent years, HF epidemiology has changed with an increase in the prevalence of HF with preserved ejection fraction (HFpEF), especially in older patients, as compared with reduced ejection fraction (HFrEF) or borderline ejection fraction (HFbEF).10,14 Through the Cardiovascular Research Network (CVRN),15 we sought to better define the association of burden of multimorbidity with clinical outcomes in adults with heart failure (HF) according to sex, age, and HF type.
Methods
Source population
The source population included members from five participating healthcare delivery systems within the CVRN. Sites included Kaiser Permanente Northern California, Kaiser Permanente Colorado, Kaiser Permanente Northwest, Kaiser Permanente Southern California, and Fallon Health in Massachusetts. These healthcare systems provide care to an ethnically and socioeconomically diverse population across varying practice settings and geographically diverse areas. Each site also developed and maintained a Virtual Data Warehouse (VDW) which served as the primary data source for subject identification and characterization.15,16 The VDW is a distributed standardized data resource comprised of electronic datasets at each CVRN site, populated with linked demographic, administrative, ambulatory pharmacy, outpatient laboratory test results, and health care utilization (ambulatory visits and network and non-network hospitalizations with diagnoses and procedures) data for members receiving care at participating sites.15,16
Institutional review boards at participating sites approved the study and a waiver of consent was obtained due to the nature of this observational study.
Study Sample
We first identified all persons aged ≥21 years with diagnosed HF based on either having been hospitalized with a primary discharge diagnosis of HF and/or having ≥3 ambulatory visits coded for HF between January 1, 2005 and December 31, 2012. We used the following International Classification of Diseases, Ninth Edition (ICD-9) codes to identify patients with HF: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, and 428.9. Previous studies have shown a positive predictive value of >95% for admissions with a primary discharge diagnosis of HF based on these codes when compared against chart review using Framingham clinical criteria.15,16 For the outpatient HF definition, we required ≥3 ambulatory visits with associated HF diagnoses, with ≥1 of the visits being to a cardiologist to enhance specificity for having HF.
We ascertained information on quantitative and/or qualitative assessments of left ventricular systolic function from the results of echocardiograms, radionuclide scintigraphy, other nuclear imaging modalities, and left ventriculography test results available from site-specific databases complemented by manual chart review. We classified patients into categories of HFpEF, HFrEF, and HFbEF. We defined HFpEF as either a reported left ventricular ejection fraction ≥50% and/or based on a physician’s qualitative assessment of preserved or normal systolic function.17 HFrEF was defined either by a reported left ventricular ejection fraction ≤40% and/or based on a physician’s qualitative assessment of moderate, moderate to severe, or severe systolic dysfunction.17 HFbEF was defined as a reported left ventricular ejection fraction between 41% to <50% and/or based on a physician’s qualitative assessment of mildly reduced systolic function.17
Assessment of multimorbidity burden
We selected 26 conditions for analysis based on their high prevalence or previously reported association with worse outcomes among patients with HF. We included 14 of the 19 comorbidities recommended by the U.S. Department of Health and Human Services’ Strategic Framework on Multiple Chronic Conditions.18 The conditions included in this investigation also incorporated the 10 conditions recently specified by Drye and colleagues under a task order from the Centers for Medicare and Medicaid Services (CMS) to systematically narrow the list of chronic conditions identified by CMS’s Chronic Condition Data Warehouse to those most relevant to risk for hospitalization.19 The 26 chronic conditions included in our analysis were: acute myocardial infarction, unstable angina, coronary heart disease, ventricular tachycardia or fibrillation, mitral or aortic valve disease, atrial fibrillation, ischemic stroke or transient ischemic attack, peripheral arterial disease, diabetes mellitus, dyslipidemia, hypertension, chronic kidney disease, hospitalized bleeds, anemia, arthritis, chronic obstructive pulmonary disease, asthma, cancer, chronic liver disease, osteoporosis, thyroid disease, dementia, depression, hearing impairment, mobility impairment and visual impairment. We did not have systematically available information on mental disorders such as autism or schizophrenia, as well as HIV infection status. All conditions were identified using previously described methods20–23 using data from inpatient, emergency and ambulatory diagnoses and procedures, dispensed prescription medications, and laboratory test results found in the VDW (definitions and specific codes included in Appendix Table B).
Outcomes
Follow-up occurred from index date of meeting eligibility criteria through December 31, 2013. Patients were censored if they disenrolled from the health plan or reached the end of the study follow-up. Hospitalizations were identified from each site’s VDW, and admissions for HF were based on a primary discharge diagnosis for HF using the same ICD-9 inclusion codes. Deaths were identified from hospital and billing claims databases, administrative health plan databases, state death certificate registries, and Social Security Administration files as available at each site. These approaches have yielded >97% vital status information in prior studies.20–22
Data Analysis
All analyses were performed using SAS software, version 9.3 (Cary, NC). The 26 chronic conditions included in this study were summed for each study subject to create a multimorbidity count. We categorized our study population into four multimorbidity burden groups based on the sample distribution (0–4, 5–6, 7–8 and ≥9 chronic conditions). We compared differences in baseline patient characteristics and medication use patterns across the comparison multimorbidity burden groups using chi square tests for categorical variables and t-tests for continuous variables. Cox proportional hazards regression models were used to examine the associations between burden of multimorbidity and each outcome of interest after adjustment for potential confounding variables. Covariates included study site; demographic characteristics (age, sex, race/ethnicity); selected laboratory and physiologic variables (systolic blood pressure, body mass index, estimated glomerular filtration rate, hemoglobin, serum potassium); and receipt of relevant cardiac medications within 120 days before the index date based on dispensed outpatient prescriptions (ACE Inhibitors [ACEi],Angiotensin II receptor blockers ([ARB], calcium channel blockers, beta-blockers, warfarin, digoxin, statins, and antiplatelet agents). Consistent with the objectives of our study, we also performed separate analyses stratified by patient gender, age group (<55, 55–64, 65–74, 75–84, 85–94, and ≥95 years), and HF type (HFpEF, HFrEF and HFbEF).
Results
Study Population
We identified a total of 114,553 eligible adults with HF in whom left ventricular systolic function data were available. Mean age was 75.0 (12.8) years, 45.9% were women, and 73.5% were white (Table 1). Patients had a mean of 6.0 chronic conditions, with 26.4% of patients having 0–4, 29.3 % having 5–6, 26.9% having 7–8, and 17.4% having 9 or more of the 26 chronic conditions examined at the time of study entry. With regard to type of HF, 51.3% had HFpEF, 34.3% had HFrEF, and 14.4% had HFbEF. Frequency distribution of chronic conditions overall and stratified by multimorbidity burden are summarized in Table 2. Use of all classes of cardiovascular medications was higher with greater burden of multimorbidity at entry (Appendix Table A).
Table 1:
Patients’ characteristics for adults with heart failure, overall and stratified by multimorbidity burden.
| Variables | Overall n=114,553 (%) | 0–4 morbidities n=30,282 (%) | 5–6 morbidities n=33,594 (%) | 7–8 morbidities n=30,764 (%) | ≥9 morbidities n=19,913 (%) |
|---|---|---|---|---|---|
| Median (interquartile range) age, years | 75.0 (65.0–83.0) | 66.0 (56.0–77.0) | 75.0 (66.0–83.0) | 78.0 (70.0–84.0) | 80.0 (73.0–85.0)** |
| Age category, years | ** | ||||
| 55–64 | 15.1 | 24.3 | 15.5 | 10.7 | 7.0 |
| 65–74 | 24.6 | 24.2 | 26.6 | 24.5 | 22.1 |
| 75–84 | 32.0 | 19.8 | 32.5 | 37.4 | 41.2 |
| 85–94 | 18.0 | 9.1 | 17.2 | 22.4 | 26.3 |
| 95 and older | 1.7 | 1.2 | 1.8 | 1.9 | 1.8 |
| Women | 45.9 | 36.1 | 44.2 | 50.3 | 56.8** |
| White/European race | 73.5 | 67.6 | 73.2 | 75.8 | 79.1** |
| Heart failure type | |||||
| Preserved ejection fraction | 31.8 | 22.1 | 31.2 | 36.4 | 40.4 |
| Reduced ejection fraction | 21.3 | 29.6 | 20.8 | 17.4 | 15.5 |
| Borderline reduced ejection fraction | 8.9 | 9.2 | 9.0 | 8.9 | 8.3 |
| Current or former smoker | 39.8 | 35.9 | 38.4 | 41.2 | 45.8** |
Linear trend p<0.001
Table 2:
Frequency of chronic conditions for adults with heart failure, overall and stratified by multimorbidity burden.
| Variables | Overall n=114,553 (%) | 0–4 morbidities n=30,282 (%) | 5–6 morbidities n=33,594 (%) | 7–8 morbidities n=30,764 (%) | ≥9 morbidities n=19,913 (%) |
|---|---|---|---|---|---|
| Medical history | |||||
| Acute myocardial infarction | 11.0 | 5.7 | 8.7 | 13.0 | 19.9** |
| Unstable angina pectoris | 4.3 | 1.5 | 3.3 | 5.3 | 8.9** |
| Coronary heart disease | 18.5 | 8.4 | 14.5 | 22.3 | 34.9** |
| Ventricular tachycardia or fibrillation | 2.6 | 1.5 | 2.0 | 2.8 | 5.0** |
| Mitral or aortic valve disease | 31.4 | 16.5 | 25.7 | 37.1 | 54.6** |
| Atrial fibrillation | 33.1 | 18.3 | 29.5 | 38.7 | 52.6** |
| Ischemic stroke or transient ischemic | 5.1 | 1.2 | 3.0 | 6.0 | 13.2** |
| attack | |||||
| Peripheral arterial disease | 5.5 | 0.9 | 2.9 | 6.8 | 15.2** |
| Diabetes mellitus | 40.8 | 16.3 | 40.2 | 52.0 | 61.6** |
| Dyslipidemia | 76.7 | 51.8 | 79.1 | 87.6 | 93.7** |
| Hypertension | 77.6 | 46.9 | 81.8 | 91.4 | 95.6** |
| Chronic kidney disease | 50.7 | 19.8 | 47.7 | 66.7 | 78.0** |
| Hospitalized bleeds | 4.7 | 1.0 | 2.4 | 5.3 | 13.1** |
| Anemia | 36.0 | 11.4 | 29.3 | 48.0 | 66.0** |
| Arthritis | 38.7 | 14.9 | 33.6 | 49.5 | 66.7** |
| Chronic obstructive pulmonary disease | 25.8 | 11.2 | 20.5 | 31.2 | 48.7** |
| Asthma | 15.1 | 6.9 | 11.9 | 17.5 | 29.6** |
| Cancer, systemic | 5.4 | 1.9 | 3.8 | 6.5 | 11.4** |
| Chronic liver disease | 3.0 | 1.6 | 2.6 | 3.3 | 5.3** |
| Osteoporosis | 12.8 | 3.0 | 8.8 | 16.0 | 29.5** |
| Thyroid disease | 16.2 | 4.8 | 11.8 | 20.1 | 35.1** |
| Dementia | 4.8 | 1.3 | 3.1 | 5.7 | 11.8** |
| Depression | 14.6 | 4.7 | 10.1 | 17.7 | 32.3** |
| Hearing impairment | 17.6 | 5.6 | 13.6 | 22.6 | 35.0** |
| Mobility impairment | 4.0 | 0.6 | 2.2 | 5.0 | 10.9** |
| Visual impairment | 70.8 | 39.0 | 72.9 | 85.4 | 92.9** |
Linear trend p<0.001
Association between burden of multimorbidity and the principal study outcomes
Compared to patients with the lowest comorbidity burden (0–4 comorbidities), higher comorbidity burden was associated with higher all-cause mortality in crude analyses: unadjusted hazard ratios (HRs) were 1.59 (95% CI: 1.55–1.63) for 5–6 comorbidities, 2.20 (95% CI: 2.15–2.26) for 7–8 comorbidities, and 3.17 (95% CI: 3.09–3.25) comorbidities. After adjustment for demographic characteristics, laboratory and physiologic variables, medications, and study site, there remained a graded increased risk of death with greater morbidity burden: adjusted HR 1.27 (95% CI: 1.24–1.31) for 5–6 comorbidities, HR 1.52 (95% CI: 1.48–1.57) for 7–8 comorbidities, and HR 1.92 (95% CI: 1.86–1.99) for ≥9 comorbidities (Figure 1A).
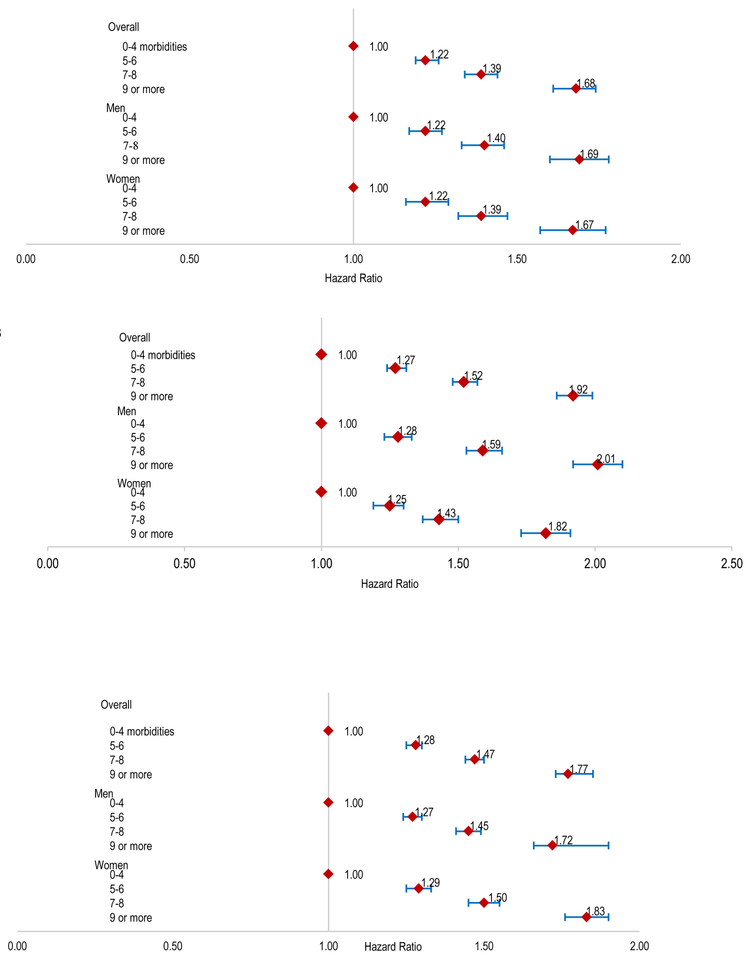
Figure 1.
Multivariable association between burden of multimorbidity and risk of death from any cause (A); risk of heart failure-related hospitalization (B) or risk of hospitalization for any cause(C) in adults with heart failure, overall and stratified by gender.
Compared to patients with 0–4 comorbidities, unadjusted HRs for HF-specific hospitalization were 1.06 (95% CI, 1.03–1.09) for 5–6 comorbidities, 1.18 (95% CI, 1.15–1.22) for 7–8 comorbidities, and 1.42 (95% CI, 1.38–1.47) for ≥9 comorbidities. In multivariable analyses, compared to persons with 0–4 comorbidities, there were higher adjusted risks with greater multimorbidity: HR 1.22 (95% CI: 1.19–1.26) for 5–6 comorbidities, HR 1.39 (95% CI: 1.34–1.44) for 7–8 comorbidities, and HR 1.68 (95% CI, 1.61–1.74) for ≥9 comorbidities Figure 1B).
A similar pattern between multimorbidity burden and hospitalizations from any cause was observed. Unadjusted hazard ratios for any-cause hospitalization were 1.12 (95% CI: 1.10–1.14) for patients with 5–6 comorbidities, 1.31 (95% CI: 1.29–1.33) for 7–8 comorbidities, and 1.65 (95% CI, 1.62–1.69) for ≥9 comorbidities, as compared to persons with 0–4 comorbidities. After adjustment for potential confounders, compared to persons with 0–4 comorbidities, greater multimorbidity remained associated with a graded higher risk of hospitalization: HR 1.28 (95% CI: 1.25–1.30) for 5–6 comorbidities, HR 1.47 (95% CI: 1.44–1.50) for 7–8 comorbidities, and HR 1.77 (95% CI: 1.73–1.82) for ≥9 comorbidities (Figure 1C).
Association between burden of multimorbidity and outcomes by gender
In men, compared to those with 0–4 comorbidities, multivariable adjusted hazard ratios for all-cause mortality for persons with 5–6, 7–8, or 9 or more comorbidities were 1.28 (95% CI:1.23–1.33), 1.59 (95% CI:1.53–1.66), and 2.01 (95% CI:1.92–2.10), respectively (Figure 1A). In women, compared to persons with 0–4 comorbidities, adjusted hazard ratios were 1.25 (95% CI: 1.19–1.30) for 5–6 comorbidities, 1.43 (95% CI: 1.37–1.50) for 7–8 comorbidities, and 1.82 (95% CI: 1.73–1.91) for ≥9 comorbidities, respectively (Figure 1A).
Similar trends were found in the multivariable adjusted associations of greater multimorbidity with HF-specific and any-cause hospitalization among both men and women (Figure 1B and 1C).
Association between burden of multimorbidity and outcomes by age
Across a wide age range, there was a consistent association between higher burden of multimorbidity and worse clinical outcomes. However, the relative impact of multimorbidity burden on outcomes was greater in younger vs. older patients (Figures 2A, 2B and 2C). For example, among individuals aged 55–64 years, compared to patients with 0–4 comorbidities, greater morbidity ( 5–6, 7–8, or 9 or more morbidities) was associated with notably higher risk of dying from any cause (adjusted HRs 1.49 [95% CI:1.37–1.62], 2.01 [95% CI:1.83–2.21] and 2.53 [95% CI:2.26–2.83], respectively), while the association was less prominent in the oldest age group of ≥95 years (adjusted HRs 1.11 [95% CI:0.95–1.30], 1.23 [95% CI:1.04–1.45] and 1.47 [95% CI:1.22–1.77], respectively (Figure 2A).
Figure 2.
Multivariable association between burden of multimorbidity and risk of death from any cause (A); heart failure related-hospitalization (B) and hospitalization for any cause (C) in adults with heart failure stratified by age.
Similar trends were found in the multivariable associations of greater morbidity burden with HF-specific and any cause hospitalizations, in which the strength of association was less prominent with older age (Figure 2B and 2C).
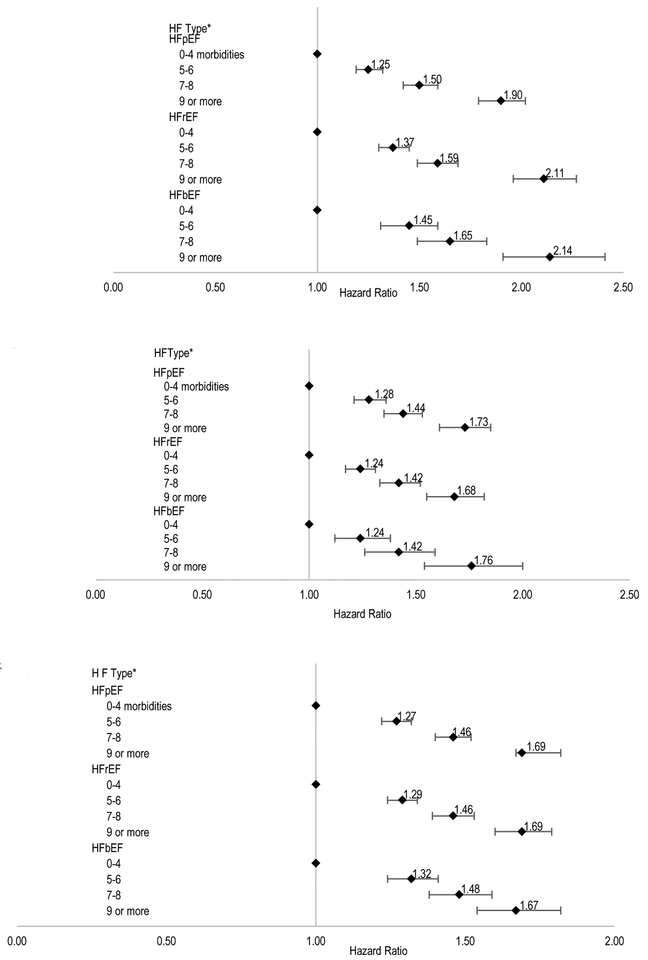
Association between burden of multimorbidity and outcomes by type of HF
For the outcome of all-cause death, among patients with confirmed HFpEF, compared to individuals with 0–4 comorbidities, adjusted hazard ratios for 5–6, 7–8, or ≥9 comorbidities were 1.25 (95% CI:1.19–1.32), 1.50 (95% CI:1.42–1.59) and 1.90 (95% CI:1.79–2.02), respectively. The direction and strength of association between higher multimorbidity burden and the risk of dying were similar for patients with HFrEF or HFbEF (Figure 3A).
Figure 3.
Multivariable association between burden of multimorbidity and risk of death (A); risk of heart failure-related hospitalization (B) or risk of hospitalization for any cause(C) in adults with heart failure stratified by type of heart failure.
* HFpEF: HF preserved ejection fraction; HFrEF: HF reduced ejection fraction; HFbEF: HF borderline ejection fraction
Similar trends were observed for greater multimorbidity and the outcomes of HF-specific and any-cause hospitalization according to type of HF (Figure 3B and 3C).
Discussion
In this large, population-based study of adults with HF, after adjustment we found a graded, increasing risk of adverse clinical outcomes with greater multimorbidity burden. Similar trends were found in men and women, across a broad age spectrum, and among those with preserved, reduced, and borderline ejection fraction findings. Of special note, worse prognoses associated with greater multimorbidity burden were especially prominent among persons younger than 65 years of age, an important finding that highlights the fact that the clinical implications of multimorbidity for both the health of populations and individuals are not limited just to older adults with HF.24
Several previous studies have examined the association between multimorbidity burden and clinical outcomes in patients with HF.5,25,26 The HF Pilot Survey of the EURObservational Research Program of the European Society of Cardiology examined the association between multimorbidity burden and adverse outcomes in 3,226 patients with HF.25 Compared to patients with none of the 10 comorbidities examined, those with ≥3 comorbidities were approximately ninefold more likely to die during a one-year follow-up, and fivefold more likely to be hospitalized for HF during the period of follow-up.25 Similarly, in a study of 18,300 fee-for-service Medicare beneficiaries with HF, those with ≥5 chronic conditions had a twofold higher 1-year risk of dying compared to those with ≤2 conditions.5 Finally, findings from an outpatient cohort of veterans with HF (2,843 HFpEF, 6,599 HFrEF) also revealed that HF patients presenting with ≥8 non-cardiac-related comorbidities had a 60% higher relative risk of being hospitalized for any cause compared to those with no comorbidities.26
Our work builds on these prior studies within the context of a larger and more demographically diverse population of adults with confirmed HFpEF, HFrEF, and HFbEF, as well as examining an expanded set of comorbidities and outcomes. Of note, while we found that the associations of greater multimorbidity with death and hospitalization were strongest in individuals aged ≤65 years, the impact on a population-level is largest among older patients with HF since the prevalence of morbidity burden is highest in the oldest patients.
Our study had several strengths and limitations that should be considered in interpreting the study findings. While we did not individually adjudicate each patient’s records to confirm the presence of clinical HF, prior studies in our population have shown a high positive predictive value for our algorithms to identify patients with HF.27,28 Our study uniquely included a large sample of patients with confirmed HFbEF. Few studies have included this interesting group of HF patients, that some have asserted have different clinical characteristics and outcomes compared to those with HFpEF and HFrEF.29 Of note, across the range of left ventricular systolic function, we observed similar findings in relation to multimorbidity burden. We also relied on automated clinical databases for ascertainment of the presence of comorbidities and use of medications, which may have led to some misclassification. Our study was conducted among an insured population living in five geographic areas of the US. We believe, however, that our findings are generalizable to other healthcare settings and uninsured persons in the U.S. within “real-world” practice settings, given the diverse nature and large size of the studied population. Another study limitation was that detailed information about the duration, severity, or extent of the chronic conditions studied was unavailable. However, our approach to characterizing multimorbidity—namely summing the number of chronic conditions—addressed the limitation of considerable heterogeneity and complexity with existing approaches to examining the burden of multimorbidity. Some examples of the most commonly used approaches include the Charlson and Elixhauser indices, which while often employed in observational research studies of prognosis, may be less practical and applicable for use by health care providers delivering face-to-face care to “real” patients in “real-world” clinical settings in real-time.30 Our straightforward approach to characterizing the burden of multimorbidity in HF patients may be readily applied as health care systems continue to implement novel informatics approaches to assist in clinical decision-making at point-of-care.31
Our findings underscore the importance of the association between multimorbidity burden and adverse outcomes in patients with varying types of HF, middle-aged and older persons, and in men as well as women. Multimorbidity in patients with HF substantially increases the complexity of their care, as practitioners face the challenge of managing multiple conditions simultaneously that may increase therapy-associated side effects.4,28,32–35 With the aging of the US population, the numbers of patients affected by HF will increase dramatically over the coming decades,1,2 especially those with a high burden of multimorbidity, which will create greater demands on health care providers and the systems in which they work. A clearer understanding of the impact of multimorbidity burden on important outcomes in patients with HF will help health care providers, patients, and their caregivers in discussions around the prognosis of this complex condition. Clinical trials of treatments for HF have usually excluded older adults with a high burden of comorbidity, which has led to evidence gaps in how to optimally treat this complex, vulnerable population.32–34
In conclusion, our study highlights the uniformly negative prognosis of greater morbidity burden across multiple clinical outcomes. Our findings also suggest that large observational studies can help to identify important high risk HF subgroups and to inform the design of future randomized trials focused on those patients at highest risk for adverse outcomes.
Supplementary Material
Appendix A: Medication use for adults with heart failure, overall and stratified by multimorbidity burden
Appendix B: Chronic Conditions Codes
Table 3:
Medication use for adults with heart failure, overall and stratified by multimorbidity burden
| Medication | Overall n=114,553 (%) | 0–4 morbidities n=30,282 (%) | 5–6 morbidities n=33,594 (%) | 7–8 morbidities n=30,764 (%) | ≥9 morbidities n=19,913 (%) |
|---|---|---|---|---|---|
| Angiotensin converting enzyme | 64.7 | 55.1 | 67.2 | 69.1 | 68.1** |
| inhibitor or angiotensin II receptor | |||||
| blocker | |||||
| βeta-blocker | 65.7 | 54.3 | 67.2 | 71.2 | 71.9** |
| Aldosterone receptor antagonist | 7.5 | 8.0 | 7.5 | 7.0 | 7.4** |
| Digoxin | 16.0 | 14.2 | 15.9 | 16.6 | 18.1** |
| Calcium channel blocker | 31.7 | 16.9 | 31.7 | 39.4 | 42.5** |
| Nitrates | 21.9 | 12.7 | 20.2 | 26.4 | 31.7** |
| Antiarrhythmic drugs | 7.2 | 4.6 | 6.4 | 8.2 | 10.9** |
| Anticoagulant | 24.6 | 17.1 | 24.1 | 27.5 | 32.4** |
| Non-aspirin antiplatelet agent | 11.3 | 5.8 | 9.8 | 13.9 | 18.4** |
| Statin | 58.7 | 36.9 | 60.3 | 68.8 | 73.6** |
| Other lipid-lowering drug | 5.6 | 3.1 | 5.7 | 6.7 | 7.5** |
Linear trend p<0.001
Impact Statement.
We certify that this work is novel. Our study uniquely included a large sample of patients with confirmed heart failure borderline ejection fraction. Our findings are likely to be generalizable to the large fraction of patients with heart failure due to the breadth of geographic and demographic diversity in our community-based population.
Acknowledgements
Conflicts of Interest: Dr. Go reports receiving grant funding through his institution (Kaiser Permanente Northern California Division of Research) from Astra-Zeneca, Novartis and GlaxoSmithKline, as well as from NHLBI, NIDDK, NIA and PCORI. Dr. Smith reports receiving grant funding through his institution (Center for Health Research, Kaiser Permanente Northwest) from Novartis to undertake an FDA mandated drug safety study. Dr. Reynolds reports receiving grant funding through her institution (Department of Research & Evaluation, Kaiser Permanente Southern California) from Novartis. The other authors report no conflicts.
Funding: The study was supported by R24 AG045050 from the National Institute on Aging, as well as RC1 HL099395 and U19 HL91179 from the National Heart, Lung, and Blood Institute and by the Yale Claude D. Pepper Older Americans Independence Center (NIH/NIA P30AG021342).
Sponsor’s Role: The National Institute on Aging and the NIH had no role in the preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication.
References
- 1.Kupari M, Lindroos M, Iivanainen AM, Heikkila J, Tilvis R. Congestive heart failure in old age: prevalence, mechanisms and 4-year prognosis in the Helsinki Ageing Study. J Intern Med 1997; 241(5):387–394 [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA 2010; 304(17):1950–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saczynski JS, Darling CE, Spencer FA, Lessard D, Gore JM, Goldberg RJ. Clinical features, treatment practices, and hospital and long-term outcomes of older patients hospitalized with decompensated heart failure: The Worcester Heart Failure Study. J Am Geriatr Soc 2009;57 (9):1587–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masoudi FA, Krumholz HM. Polypharmacy and comorbidity in heart failure. BMJ 2003; 327(7414):513–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braunstein JB, Anderson GF, Gerstenblith G et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol 2003; 42(7):1226–1233 [DOI] [PubMed] [Google Scholar]
- 6.Alter DA, Ko DT, Tu JV et al. The average lifespan of patients discharged from hospital with heart failure. J Gen Intern Med 2012; 27(9):1171–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahluwalia SC, Gross CP, Chaudhry SI et al. Impact of comorbidity on mortality among older persons with advanced heart failure. J Gen Intern Med 2012; 27(5):513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45(6):613–619 [DOI] [PubMed] [Google Scholar]
- 9.Havranek EP, Masoudi FA, Westfall KA, Wolfe P, Ordin DL, Krumholz HM. Spectrum of heart failure in older patients: results from the National Heart Failure project. Am Heart J 2002; 143(3):412–417 [DOI] [PubMed] [Google Scholar]
- 10.Bhuiyan T, Maurer MS. Heart Failure with Preserved Ejection Fraction: Persistent Diagnosis, Therapeutic Enigma. Curr Cardiovasc Risk Rep 2011; 5(5):440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisminetzky M, Goldberg R, Gurwitz JH. Magnitude and impact of multimorbidity on clinical outcomes in older adults with cardiovascular disease: a literature review. Clin Geriatr Med 2016; 32(2): 227–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA 2005; 294(6):716–724 [DOI] [PubMed] [Google Scholar]
- 13.Rich MW, Chyun DA, Skolnick AH et al. ; American Heart Association Older Populations Committee of the Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursing, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council; American College of Cardiology; and American Geriatrics Society. Knowledge Gaps in Cardiovascular Care of the Older Adult Population: A Scientific Statement From the American Heart Association, American College of Cardiology, and American Geriatrics Society. Circulation. 2016;133:2103–22. [DOI] [PubMed] [Google Scholar]
- 14.Upadhya B, Pisani B, Kitzman DW. Evolution of a Geriatric Syndrome: Pathophysiology and Treatment of Heart Failure with Preserved Ejection Fraction. J Am Geriatr Soc 2017; 65(11):2431–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Go AS, Magid DJ, Wells B et al. The Cardiovascular Research Network: a new paradigm for cardiovascular quality and outcomes research. Circ Cardiovasc Qual Outcomes 2008; 1 (2):138–147 [DOI] [PubMed] [Google Scholar]
- 16.Magid DJ, Gurwitz JH, Rumsfeld JS, Go AS. Creating a research data network for cardiovascular disease: the CVRN. Expert Rev Cardiovasc Ther 2008; 6(8):1043–1045 [DOI] [PubMed] [Google Scholar]
- 17.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62(16):e147–239 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. Multiple Chronic Conditions—A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, DC: December 2010 [Google Scholar]
- 19.Drye EE, Altaf FK, Lipska KJ et al. Defining Multiple Chronic Conditions for Quality Measurement. Med Care 2018; 56(2):193–201 [DOI] [PubMed] [Google Scholar]
- 20.Saczynski JS, Go AS, Magid DJ et al. Patterns of comorbidity in older adults with heart failure: the Cardiovascular Research Network PRESERVE study. J Am Geriatr Soc 2013; 61 (1):26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA 2006; 296(17):2105–2111 [DOI] [PubMed] [Google Scholar]
- 22.Go AS, Yang J, Ackerson LM et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation 2006; 113(23):2713–2723 [DOI] [PubMed] [Google Scholar]
- 23.Chrischilles E, Schneider K, Wilwert J et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care 2014;52 Suppl 3:S75–84 [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Go AS, Lindquist K, Bertenthal D, Covinsky KE. Chronic conditions and mortality among the oldest old. Am J Public Health 2008;98(7):1209–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Deursen VM, Urso R, Laroche C et al. Comorbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16(1):103–111 [DOI] [PubMed] [Google Scholar]
- 26.Ather S, Chan W, Bozkurt B et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59(11):998–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Go AS, Yang J, Gurwitz JH, Hsu J, Lane K, Platt R. Comparative effectiveness of different beta-adrenergic antagonists on mortality among adults with heart failure in clinical practice. Arch Intern Med 2008; 168(22):2415–2421 [DOI] [PubMed] [Google Scholar]
- 28.Allen LA, Magid DJ, Gurwitz JH et al. Risk factors for adverse outcomes by left ventricular ejection fraction in a contemporary heart failure population. Circ Heart Fail 2013; 6(4):635–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016; 18:891–975 [DOI] [PubMed] [Google Scholar]
- 30.Green AR, Leff B, Wang Y et al. Geriatric Conditions in Patients Undergoing Defibrillator Implantation for Prevention of Sudden Cardiac Death: Prevalence and Impact on Mortality. Circ Cardiovasc Qual Outcomes 2016; 9(1):23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simos K: Bringing Machine Learning to the Point of Care to Improve Suicide Prevention.“Rethinking Clinical Trials. October 31, 2017. Accessed March 6, 2018 http://www.rethinkingclinicaltrials.org/october-27-2017-bringing-machine-learning-to-the-point-of-care-to-improve-suicide-prevention/ [Google Scholar]
- 32.Boyd CM, Ritchie CS, Tipton EF, Studenski SA, Wieland D. From Bedside to Bench: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging Clin Exp Res 2008; 20(3):181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HHS initiative on multiple chronic conditions. Accessed March 14, 2018 https://www.hhs.gov/ash/about-ash/multiple-chronic-conditions/index.html [Google Scholar]
- 34.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making or patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One 2012; 7(8):e41601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stange KC. Challenges of managing multimorbidity. Ann Fam Med 2012;10(1):2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: Medication use for adults with heart failure, overall and stratified by multimorbidity burden
Appendix B: Chronic Conditions Codes