Abstract
Prader-Willi syndrome (PWS) is a rare genetic neurodevelop-mental disorder associated with maladaptive social behavior, hyperphagia and morbid obesity. Orexin A is a hypothalamic neuropeptide important as a homeostatic regulator of feeding behavior and in energy metabolism through actions in the lateral hypothalamus. Dysregulation of orexin signaling may contribute to behavioral problems and hyperphagia seen in PWS and we sought to assess orexin A levels in PWS relative to controls children. Morning fasting plasma orexin A levels were analyzed in 23 children (aged 5–11 years) with genetically confirmed PWS and 18 age and gender matched healthy unrelated siblings without PWS. Multiplex immune assays utilized the Milliplex Human Neuropeptide Magnetic panel and the Luminex platform. Natural log-transformed orexin A data were analyzed using general linear model adjusting for diagnosis, gender, age, total body fat, and body mass index (BMI). Plasma orexin A levels were significantly higher (P < 0.006) in children with PWS (average ±SD = 1,028 pg/ml ± 358) compared with unrelated siblings (average ±SD = 609pg/ml ± 351; P < 0.001). Orexin A levels correlated with age in females and were significantly elevated in PWS even after these effects were controlled. These findings support the hypothesis that dysregulation of orexin signaling may contribute to behavioral problems and hyperphagia in PWS. Further studies are warranted to better understand the complex relationship between orexin A levels and the problematic behaviors consistently found in individuals with PWS.
Keywords: abnormal behavior, hyperphagia, neuropeptide, orexin A, Prader-Willi syndrome
INTRODUCTION
Prader-Willi syndrome (PWS) is a genetic neurodevelopmental disorder characterized by failure to thrive during infancy, hypo-gonadotropic hypogonadism, hyperphagia-induced obesity, growth hormone deficiency, and mental deficiencies with behavioral problems. About 70% of PWS cases result from a paternal deletion of the chromosome 15q11-q13 region, and less frequently, uniparental disomy of the maternal chromosome 15 (25% of cases), or imprinting defects within this chromosome region [Butler, 1990, 2011; Bittel and Butler, 2005; Cassidy et al., 2011; Angulo et al., 2015]. PWS affects approximately 1 in every 15,000 live births with an estimated 400,000 people affected by PWS worldwide [Butler and Thompson, 2000; Butler and Lee, 2006].
The classic phenotype of PWS is illustrative of relative growth hormone deficiency, as evidenced by an affected individual’s short stature and small hands and feet. Sex hormone defects also lead to hypogonadism, hypogenitalism, and underdeveloped secondary sex characteristics. Growth hormone is utilized as a treatment to increase stature and improve body composition [e.g., de Souza et al., 2013; Angulo et al., 2015]. PWS is the most common genetic cause of morbid obesity in children [Butler, 1990]; contributing factors include hyperphagia, reduced metabolic rate and decreased physical activity [Butler et al., 2007]. Interventions to decrease the incidence of childhood obesity in patients with PWS include locking refrigerators, constant supervision during food-related activities, and rewarding desirable behavior with non-food items [Butler and Lee, 2006; Butler, 2011]. The morbid obesity often seen in children and adults with PWS can lead to significant health problems, if not managed properly.
Appetite and feeding behavior are regulated by neuropeptides within the hypothalamus, particularly orexin A, through a complex network involving physiological effects both within the hypothalamus and at other appetite centers in the brain [Parker and Bloom, 2012; Sakurai, 2014a,b]. Compared to neurotransmitters, neuropeptides are able to elicit biological effects at low concentrations and travel to more distant sites within the CNS [Parker and Bloom, 2012]. In addition to appetite regulation, orexin signaling is involved in arousal, sleep and wakefulness, metabolism and energy expenditure, pleasure and reward-seeking behavior as well as stress response [Kuru et al., 2000; DiLeone et al., 2003; Yamanaka et al., 2003; Winsky-Sommerer et al., 2005; Borgland et al., 2006,2009; Ganjavi and Shapiro, 2007; Hoyer and Jacobson, 2013; Jalewa et al., 2014]. Changes in peripheral metabolic signals (i.e., glucose, ghrelin, leptin, and blood pH) impact the activity of orexin A (or hypocretin 1) neurons in the lateral hypothalamus which increase eating behaviors through binding to orexin receptor type 1 (OX1R) in the ventromedial hypothalamus, hippocampus, locus coeruleus and limbic centers [Sakurai, 2014a,b]. Decreased brain orexin A signaling has been observed in patients with functional gastrointestinal disorders in addition to secondary symptoms including appetite loss, sleep disturbance, and inhibition of gut function consistent with disruption of multiple endocrine systems [Okumura and Nozu, 2011].
Reward-based feeding behavior beyond homeostatic necessity contributes significantly to obesity and is driven in part by palatability, taste, and texture of food [Erlanson-Albertsson, 2005]. Activation of the orexin pathway stimulates reward-seeking behavior in animals and can lead to overconsumption of palatable foods (i.e., high fat) [Choi et al., 2010, 2012]. The lateral hypothalamus and the orexin pathway also regulates food anticipation and feeding motivation presumably leading to morbid obesity associated with PWS [Erlanson-Albertsson, 2005]. Orexin and its receptors have also been implicated in brain reward pathways involved with addictive behaviors and measures in animal models [DiLeone et al., 2003; Georgescu et al., 2003; Borgland et al., 2006, 2009]. In addition, heighten arousal states associated with psychological stress can elevate orexinergic neurotransmission and cause or prolong binge-eating episodes [Piccoli et al., 2012]. Bittel et al. [2007] also reported significantly increased expression of the orexin A gene in RNA isolated from lymphoblasts derived from adult males with PWS when compared with non-syndromic obese males which may indicate a primary dysfunction in hypothalamic signaling in PWS related to orexin A activity.
Understanding the role of orexin A in individuals with PWS could be considered an important step in elucidating the underlying psychopathology of PWS and development of potential treatments to address significant health risks associated with this overeating syndrome. We measured fasting morning plasma orexin A levels in a cohort of 23 children with PWS compared with 18 healthy unrelated children at similar ages to examine the role of orexin A in hypothalamic dysfunction.
MATERIALS AND METHODS
Subjects
Plasma was obtained for study from 23 children with PWS, diagnosed clinically and confirmed with cytogenetic and molecular genetic testing and methylation analysis. Those included 10 females (mean age ± SD = 8.5 ± 1.9 years, age range = 5–11 years) and 13 males (mean age ± SD = 7.7 ± 1.9 years, age range = 5–11 years). Fifteen of the children with PWS had the 15q11-q13 deletion and eight had maternal disomy 15. The control group consisted of 18 unaffected siblings selected from families where at least one child has PWS but data from that affected child was not included in the current sample. Due to the precise nature and specificity of the effects of the PWS genetic finding which is well-characterized, unaffected and unrelated siblings without PWS should be representative of the normal population and suffice as control subjects. Additionally, siblings of an individual with PWS are likely to live in a similar food-restricted environment unlike traditional childhood rearing environments and as such offer advantages over typically developing control subjects. The control siblings included eight females (mean age ± SD = 8.2 ± 2.1 years; age range = 5–11 years) and 10 males (mean age ± SD = 8.3 ± 2.5 years; age range = 5–11 years). All children recruited in this study were consented using approved forms by the local Human Subjects Committee with oversight from the Institutional Review Board of the University of Kansas Medical Center and the University of Florida School of Medicine as part of a large ongoing multi-site rare disease consortium on PWS. All children were Caucasian-American and those with PWS were treated with growth hormone.
The PWS and control subjects received dietary intervention (60–80% of the average caloric intake for their age) and engaged in exercise programs to maintain caloric intake and weight control. No children received sex steroids or were treated for adrenal insufficiency. Four children with PWS had a history of insulin resistance and three were being treated for hypothyroidism. One child with PWS was prescribed an atypical antipsychotic medication. Height to the nearest 0.1 cm and weight to the nearest 0.1 kg were obtained for each individual in the clinical setting using standing stadiometer and electronic scales. Body mass index (BMI) was calculated (i.e., kg/m2). Body composition and total body fat percentages were determined using dual-energy X-ray absorptiometry (DEXA) and the Lunar DXA Scanner (Atlanta, GA). Demographic and descriptive data on each subject are found in a previously reported study on plasma cytokine levels in this cohort of subjects [Butler et al., 2015]. Peripheral blood was collected in the morning at time of fasting in anti-coagulant vacutainer tubes (e.g., EDTA) and plasma immediately separated then stored at − 80°C.
Orexin A Assay and Analysis
Neuropeptide levels were determined with the multiplex assays with Milliplex Human Neuropeptide Magnetic panel and the Luminex platform using established protocols following manufacturer’s guidelines. Twenty-five microliter of blood plasma, concentration standard or Milliplex quality control standard was combined with assay buffer and 25 μl of primary antibody. The plate was then foil-wrapped and incubated with agitation on a plate shaker for 2 hr at room temperature (20–25°C). Twenty-five microliter of antibody-immobilized beads were added, followed by overnight incubation and agitation overnight at 4°C. The next day, the plates were washed, detection antibodies added and incubation at room temperature for 1 hr. Streptavidin-Phycoerythrin was added; the plate was covered, incubated and agitated on a plate shaker for 30 min. After incubation, the plates were washed again and 100 μl of Sheath Fluid was added to each sample well. The plate was resuspended on a plate shaker for 5 min at room temperature then read using the Luminex® 200™ xPONENT software. Median fluorescent intensity data were analyzed using a weight 5-parameter curve-fitting method for calculating analyte concentrations in the sample wells. Orexin A levels were analyzed using the Lumi-nex® 200™ software with a minimum detectable concentration of 301 pg/ml. Plasma orexin A levels were calculated using a standard curve derived from reference neuropeptide concentration standards provided by the manufacturer. The inter-assay coefficient of variation for orexin A levels ranged from 0% to 20% while the intra-assay coefficient of variation ranged from 0% to15%. No to very low cross-reactivity was seen between orexin and other neuropeptides (e.g., 0% cross-reactivity with oxytocin), using the immunoassay multiplex method indicating high sensitivity and good selectivity for orexin A. Plasma samples were analyzed using numbers and blinded to gender and control versus PWS during each assay run.
Statistical Analysis
Data were presented as mean and/or median ± standard deviation of raw and/or natural log-transformed orexin A levels by diagnosis (PWS or healthy unrelated siblings) and gender. Natural log-transformed orexin levels were analyzed using analysis of variance (ANOVA) and the general linear model with controls for age, gender, body mass index (BMI) or total body fat. Data falling below the limits of detection for the Milliplex assay were replaced with one half the minimum detectable level of orexin A (150.5 pg/ml) as described previously [Butler et al., 2015]. Final transformed data met statistical criteria for the assumption of normality with equal variance and near linear residual plots. Statistical analyses and descriptive statistics were generated with SAS statistical analysis software version 0.4 (SAS Inc., Cary, NC) with P values <0.05 considered significant.
RESULTS
The 23 PWS and 18 control subjects did not significantly differ by age or BMI, but PWS participants did have significantly more total body fat than controls (see Table I). Plasma orexin A levels for children with PWS (average ± SD = 1,028 ± 358 pg/ml; range 475–1,910 pg/ml) were significantly higher than the unaffected, unrelated sibling controls (average ± SD = 609 ± 351ml; range 165–1,220 pg/ml; t = 2.02, P = 0.0064), and plasma levels of orexin A remained significantly higher in PWS than controls among males when isolated bygender (see Fig. 1). No significant correlations were found between plasma orexin A levels and age, BMI or total body fat for PWS or control subjects. However, plasma orexin A levels were significantly positively correlated with age in females with and without PWS (F = 4.61, P = 0.048; see Fig. 2) but was not correlated with age in males. Orexin A levels were also not correlated with BMI or total body fat. Linear regression modeling showed a significant effect of the PWS diagnosis when age and gender were controlled (see Table II). The effect of diagnosis remained significant among females after controlling for the effects of age and males when age and total body fat were controlled.
TABLE I.
Subject Characteristics and Plasma Orexin A Levels in Prader-Willi Syndrome and Healthy Unrelated Sibling Control Children
| Characteristic | Prader-Willi syndrome (N = 23) [mean ± SD (range)] |
Control subjects (N = 18) [mean ± SD (range)] |
F-value | P-value |
|---|---|---|---|---|
| Age | 8.2 ± 2.0 yr (5–11 yr) | 8.2 ± 2.3 yr (5–11 yr) | 0.07 | 0.79 |
| BMI | 20.7 ± 5.0 (l4–28) | 18.2 ± 3.3 (15–25) | 2.2 | 0.15 |
| BMI z-score | 0.96± 1.4(13–32) | 0.52 ± 1.35 (12–26) | 1.1 | 0.31 |
| Total body fat | 33.1 ± 13.1% (13–54%) | 24.0 ± 10.1% (10–47%) | 5.5 | 0.02* |
| Orexin levels [mean pg/ml ± SD (range, N)] | ||||
| All subjects | 1028 ± 358 (475–1,910, N = 23) | 609 ± 351 (165–1,220, N = 18) | 14.8 | <0.001* |
| Female subjects (N = 18) | 880 ± 458 (165–1,910, N = 10) | 656 ± 345 (165–1,114, N = 8) | 4.0 | 0.06 |
| Male subjects (N = 23) | 1005 ± 257 (475–1,401, N = 13) | 570 ± 359 (165–1,220, N = 10) | 11.1 | 0.003* |
Indicates statistical significance for linear regression analysis at P< 0.05 level of alpha.
FIG. 1.
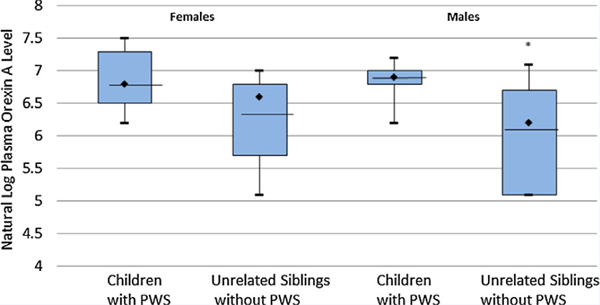
Distribution of plasma or orexin A levels for individuals with Prader-Willi syndrome (PWS) and healthy unrelated sibling control children by gender. Box plots represent mean (line), median (diamond), and interquartile tranges (25% and 75%). Error bars indicate maximum and minimum values form natural log-transformed data for each subject group. *P-value <0.05.
FIG. 2.
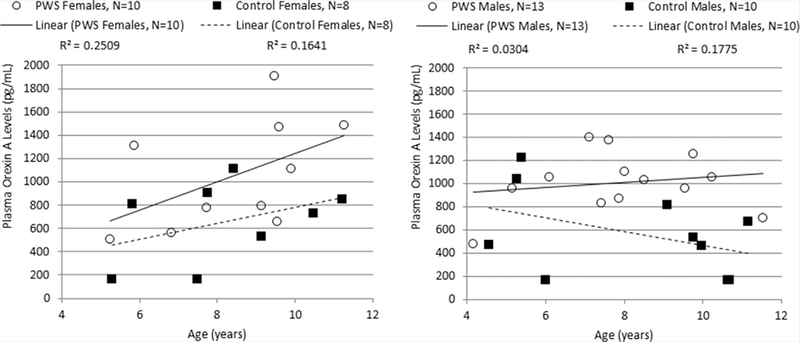
Correlation between orexin A level and age by gender in Prader–Willi syndrome (PWS) and healthy unrelated sibling control children.
TABLE II.
Linear Regression Modeling of Natural Log Plasma Orexin A Levels in Prader-Willi Syndrome and Healthy Unrelated Sibling Control Children
| F-value | P-value | |
|---|---|---|
| Model 1: Total sample | ||
| Diagnosis | 14.1 (df = 1) | 0.001* |
| Gender | 0.18 (df = 1) | 0.68 |
| Age | 0.06 (df = 1) | 0.81 |
| Overall model fit (R2 = 0.27) | 4.8 (df = 3.37) | 0.01* |
| Model 2: Female subjects | ||
| Diagnosis | 4.9 (df = 1) | 0.04* |
| Age | 4.6 (df = 1) | 0.05* |
| Overall model fit (R2 = 0.39) | 4.8 (df = 2.15) | 0.03* |
| Model 3: Male subjects | ||
| Diagnosis | 10.9 (df = 1) | 0.004* |
| Age | 1.5 (df = 1) | 0.23 |
| Total fat | 0.18 (df = 1) | 0.68 |
| Overall model fit (R2 = 0.40) | 4.2 (df = 3.19) | 0.02* |
Indicated statistical significance for controlled linear regression analysis at P< 0.05 level of alpha.
DISCUSSION
The results of this study support orexigenic dysfunction in a cohort of children with PWS in relation to unrelated siblings without PWS. These findings are consistent with orexin’s association with serious neurological dysfunction involving consummatory behaviors including feeding and addictions [Wolf, 1998; Dube et al., 1999; Harris et al., 2005; Sakurai, 2014a,b]. Orexin A stimulates appetite and increases food consumption and its genes are expressed bilaterally in the lateral hypothalamus, also termed the “feeding center” of that region [Wolf, 1998; Dube et al., 1999]. The dopamine-rich ventral tegmental area (VTA) and nucleus accumbens (NA) also modulate behaviors motivated by food and drug rewards; both structures are heavily innervated by the orexin neurons and express high levels of orexin receptors [Trivedi et al., 1998]. Overstimulation of orexin signaling in the hypothalamus, as well as the VTA and NA may significantly contribute to hyperphagia by increasing the reward value of food in patients with PWS. Orexin A gene expression in lympho-blastoid cells derived from adult males with PWS was elevated relative to non-syndromic obese males reported by Bittel et al. [2007] further supporting this hypothesis. Hence, the insatiable appetite and unusual food-related problems exhibited by PWS patients suggests an abnormality in the orexin system.
Previous studies have shown significant decreases in orexin A levels (13%, P =0.023) as healthy and unaffected infants aged into young adults [Hunt et al., 2015]. The PWS and control female subjects in our study demonstrated increasing plasma orexin A levels as they aged. Given the accelerated maturation and growth that occurs from infancy to pubescence, very high levels of orexin would be expected. Hence, orexin’s role as a coordinator of energy homeostasis and a long-term satiety factor support an elevation of this neuropeptide in the plasma of young children; a finding consistent with previous research [Tomasik et al., 2004]. Orexin A levels in female children with PWS increased more rapidly than in the unaffected female sibling controls consistent with previously suggested orexigenic dysfunction inpatients with PWS. We did not find a significant correlation between age and plasma orexin levels for the PWS and control male subjects.
The Luminex assay utilizes a capture antibody technique combined with biotinylated detection antibodies but has not been directly compared with standard radioimmunoassays (RIA) or enzyme-linked immunosorbent assays (ELISA) in measuring neuropeptides. Additionally, the present study is limited by a relatively small sample size as an expected restriction due to the nature of rare disorders. This study also lacks data on the behavioral characteristics of the children with PWS, as compared to normal controls which limits our ability to correlate the observed elevation in neuropeptide levels to the expected symptomology (i.e., hyper-phagia, anxiety, repetitive behaviors, etc.). Also, neuropeptide levels may be abnormally increased or decreased in siblings of individuals with PWS compared to children with no family history of PWS. However, strengths of our study would include age-matching with a similar gender ratio and BMI. All children with PWS in our study received a genetically confirmed diagnosis and had no other known or recognizable genetic disorders or chromosomal disorders. Evaluations of the children were similar for both subject groups and laboratory conditions, time to specimen processing, and data analysis were the same across both study groups.
Our findings support further research to examine the relationship between orexin and its effect on the behavior, physical findings, genetic subtypes and gender in PWS. Further studies are also necessary using other biochemistry assays (e.g., ELISA, RIA) to replicate our findings and prior to exploring possible therapeutic options with the use of orexin antagonists and potential effect on the disturbed behavior often seen in PWS which could be related to this neuropeptide.
ACKNOWLEDGMENTS
Partial funding support was received from the Angelman, Rett, and Prader-Willi Syndrome Consortium (U54 HD06122) which is a part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN) and supported through collaboration between the NIH Office of Rare Disease Research (ORDR) at the National Center of Advancing Translational Science (NCATS) and the National Institute of Child Health and Human Development (NICHD). NICHD grant number HD02528 is also acknowledged. The content is solely the responsibility of the authors and does not necessarily represent the office views of the National Institutes of Health.
Grant sponsor: Angelman, Rett and Prader-Willi Syndrome Consortium; Grant number: U54 HD06122; Grant sponsor: NIH Office of Rare Disease Research; Grant sponsor: National Center of Advancing Translational Science; Grant sponsor: National Institute of Child Health and Human Development; Grant number: HD02528.
Footnotes
Conflict of interest: None.
REFERENCES
- Angulo MA, Butler MG, Cataletto ME. 2015. Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. J Endocrinol Invest 38:1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Butler MG. 2005. Prader-Willi syndrome: Clinical genetics, cytogenetics and molecular biology. Expert Rev Mol Med 7:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittel DC, Kibiryeva N, Sell SM, Strong TV, Butler MG. 2007. Whole genome microarray analysis of gene expression in Prader-Willi syndrome. Am J Med Genet Part A 143A:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. 2006. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49:589–601. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29:11215–11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Lee PDK. 2006. Management of Prader-Willi syndrome, 3rd edition In: Whitman BY, editor. New York: Springer. [Google Scholar]
- Butler MG, Thompson T. 2000. Prader-Willi syndrome: Clinical and genetic findings. The Endocrinol 10:3S–16S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Theodoro MF, Bittel DC, Donnelly JE. 2007. Energy expenditure and physical activity in Prader-Willi syndrome: Comparison with obese subjects. Am J Med Genet Part A 143A:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Hossain W, Sulsona C, Driscoll DJ, Manzardo AM. 2015. Increased plasma chemokine levels in children with Prader-Willi syndrome. Am J Med Genet Part A 167A:563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 1990. Prader-Willi syndrome: Current understanding of cause and diagnosis. Am J Med Genet 35:319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG. 2011. Prader-Willi syndrome: Obesity due to genomic imprinting. Curr Genomics 12:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Schwartz S, Miller JL, Driscoll SJ. 2011. Prader-Willi syndrome. Genet Med 14:10–26. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. 2010. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. 2012. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MA, McAllister C, Suttie M, Perrotta C, Mattina T, Faravelli F, Forzano F, Holland A, Hammond P. 2013. Growth hormone, gender and face shape in Prader-Willi syndrome. Am J Med Genet Part A 161A:2453–2463. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. 2003. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73:759–768. [DOI] [PubMed] [Google Scholar]
- Dube MG, Kalra SP, Kalra PS. 1999. Food intake elicited by central administration of orexins/hypocretins: Identification of hypothalamic sites of action. Brain Res 842:473–477. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C 2005. How palatable food disrupts appetite regulation. Basic Clin Pharmacol Toxicol 97:61–73. [DOI] [PubMed] [Google Scholar]
- Ganjavi H, Shapiro CM. 2007. Hypocretin/orexin: A molecular link between sleep, energy regulation, and pleasure. J Neuropsychiatry Clin Neurosci 19:413–419. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. 2003. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. 2005. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437:556–559. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Jacobson LH. 2013. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand? Neuropeptides 47:477–488. [DOI] [PubMed] [Google Scholar]
- Hunt NJ, Rodriguez ML, Waters KA, Machaalani R. 2015. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol Aging 36:292–300. [DOI] [PubMed] [Google Scholar]
- Jalewa J, Wong-Lin K, McGinnity TM, Prasad G, Holscher C. 2014. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav Brain Res 272:196–204. [DOI] [PubMed] [Google Scholar]
- Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H. 2000. Centrally administered orexin/hypocretin activates HPA axis in rats. Neuroreport 11:1977–1980. [DOI] [PubMed] [Google Scholar]
- Okumura T, Nozu T. 2011. Role of brain orexin in the pathophysiology of functional gastrointestinal disorders. J Gastroenterol Hepatol 26:61–66. [DOI] [PubMed] [Google Scholar]
- Parker JA, Bloom SR. 2012. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology 63:18–30. [DOI] [PubMed] [Google Scholar]
- Piccoli L, Micioni Di Bonaventura MV, Cifani C, Costantini VJ, Massagrande M, Montanari D, Martinelli P, Antolini M, Ciccocioppo R, Massi M, Merlo-Pich E, Di Fabio R, Corsi M. 2012. Role of orexin-1 receptor mechanisms on compulsive food consumption in a model of binge eating in female rats. Neuropsychopharmacology 37:1999–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T 2014a. Roles of orexins in the regulation of body weight homeostasis. Obes Res Clin Pract 8:e414–e420. [DOI] [PubMed] [Google Scholar]
- Sakurai T 2014b. The role of orexin in motivated behaviours. Nat Rev Neurosci 15:719–731. [DOI] [PubMed] [Google Scholar]
- Tomasik PJ, Spodaryk M, Sztefko K. 2004. Plasma concentrations of orexins in children. Ann Nutr Metab 48:215–220. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LHT, Guan XM. 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L. 2005. Stress and arousal: The corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol 32:285–294. [DOI] [PubMed] [Google Scholar]
- Wolf G 1998. Orexins: A newly discovered family of hypothalamic regulators of food intake. Nutr Rev 56:172–173. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami Ki, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. 2003. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron 38:701–713. [DOI] [PubMed] [Google Scholar]


