Abstract
Background:
Increasing evidence from rodent studies indicates that inhaled multi-walled carbon nanotubes (MWCNTs) have harmful effects on the lungs. In this study, we examined the effects of inhalation exposure to MWCNTs on allergen-induced airway inflammation and fibrosis. We hypothesized that inhalation pre-exposure to MWCNTs would render mice susceptible to developing allergic lung disease induced by house dust mite (HDM) allergen.
Methods:
Male B6C3F1/N mice were exposed by whole body inhalation for 6 hours a day, 5 days a week, for 30 days to air control or 0.06, 0.2 and 0.6 mg/m3 of MWCNTs. The exposure atmospheres were agglomerates (1.4–1.8 μm) composed of MWCNTs (average diameter 16 nm; average length 2.4 μm; 0.52% Ni). Mice then received 25 μg of HDM extract by intranasal instillation 6 times over 3 weeks. Necropsy was performed at 3 and 30 days after the final HDM dose to collect serum, bronchoalveolar lavage fluid (BALF), and lung tissue for histopathology.
Results:
MWCNT exposure at the highest dose inhibited HDM-induced serum IgE levels, IL-13 protein levels in BALF, and airway mucus production. However, perivascular and peribronchiolar inflammatory lesions were observed in the lungs of mice at 3 days with MWCNT and HDM, but not MWCNT or HDM alone. Moreover, combined HDM and MWCNT exposure increased airway fibrosis in the lungs of mice.
Conclusions:
Inhalation pre-exposure to MWCNTs inhibited HDM-induced TH2 immune responses, yet this combined exposure resulted in vascular inflammation and airway fibrosis, indicating that MWCNT pre-exposure alters the immune response to allergens.
Keywords: carbon nanotubes, house dust mite allergen, asthma, inhalation, atopy, nanoparticles
Introduction
Carbon nanotubes (CNTs) are a unique type of engineered nanomaterial with diverse applications in structural engineering, electronics and medicine (Baughman et al., 2002; Shvedova et al., 2009). With the numbers of products containing nanomaterials on the rise, there is concern over their toxic potential, and CNTs in particular have been shown to cause lung injury upon inhalation in rodent models (Nel et al., 2006). Materials in the nanoscale are defined as having at least one dimension under 100 nm; single-walled CNTs typically have diameters of 1 to 3 nm whereas multi-walled CNTs have diameters of 10 to 100 nm. Their lengths are highly variable and can be up to tens of microns (Donaldson et al., 2006). This fiber-like structure contributes to CNT toxicity, while their size allows them to penetrate deep into the lung where they are taken up by macrophages and cause inflammation leading to injury and systemic effects (Bonner, 2010). A number of studies have demonstrated that delivery of CNTs to the lungs of mice can cause fibrosis, systemic immunological effects and exacerbation of allergic responses (reviewed in Ihrie and Bonner, 2018). While exposure to CNTs alone can have pathological outcomes, it is likely that individuals with pre-existing conditions will be more susceptible to CNT-induced lung injury compared to healthy individuals.
Asthma is a chronic airway disease which is growing in prevalence and affects roughly 26 million people in the U.S. (Akinbami et al., 2012). The key features of allergic asthma are airway hyper-responsiveness, eosinophilic inflammation, increased airway mucus production and airway fibrosis (Holgate, 2009). Asthma arises from the immune response to a variety of different environmental allergens derived from pollen, mold, or invertebrates such as house dust mites (Kim et al., 2010). In the classic paradigm for the pathogenesis of allergic asthma, these allergens cause allergic sensitization by being taken up by antigen presenting cells in the airways, which then present them to helper T cells which initiate a TH2 inflammatory response (Holgate, 2009; Kim et al., 2010). This results in production of TH2 cytokines such as interleukin (IL)-4 and IL-13, as well as antigen-specific immunoglobulin E (IgE) that bind the allergen and crosslink Fc receptors on mast cells in the lungs, causing degranulation and initiation of inflammation (Holgate, 2009). Eosinophils are a key cell type in allergic asthma that are recruited to the lung by IL-5 released from mast cells or TH2 cells and mediate the production of inflammatory mediators such as histamine, leukotrienes, and prostaglandins (Holgate, 2009). Chronic airway remodeling in asthma also features subepithelial airway fibrosis, which is mediated by pro-fibrotic cytokines such as transforming growth factor (TGF)-β1 (Bonner, 2010). Since CNTs cause lung inflammation and fibrosis, it is likely that exposure either before or after the development of asthma could impact the progression of the disease and the immunological mechanisms discussed above.
A number of recent studies have examined the effects of multi-walled CNTs (MWCNTs) in ovalbumin and house dust mite (HDM) allergen models of asthma in mice and found that MWCNT exposure enhances airway inflammation, fibrosis and production of pro-inflammatory cytokines (Shipkowski et al., 2015; Ryman-Rasmussen et al., 2009; Ronzani et al., 2014; Inoue et al., 2009). The existing studies on the effects of MWCNTs in asthma models either used high doses of MWCNTs administered intratracheally or high concentrations given by inhalation. Studies addressing the effects at occupationally relevant concentrations (<100 μg/m3) are therefore lacking (Erdely et al., 2013). There is also poor understanding of the consequences of the timing and order of MWCNT and allergen exposure.
In the present study, we sought to examine the effect of inhalation pre-exposure to MWCNTs on the development of allergic lung disease induced by intranasal aspiration of HDM allergen. We hypothesized that mice pre-exposed to MWCNTs by inhalation would be more susceptible to HDM-induced allergic lung disease. Mice were exposed by inhalation to a dose-response of MWCNTs (0.06, 0.2, or 0.6 mg/m3) 5 days a week over 30 days, then administered HDM extract over 3 weeks, and tissues collected 3 days later (acute response) or 30 days later (sub-chronic response). Surprisingly, MWCNT inhalation pre-exposure caused a dose-dependent inhibition in the TH2 immune response to HDM extract, including reduced IgE in serum, decreased IL-13 in BALF, and lower mucus production in airways. However, acute inflammatory lesions around airways and blood vessels, as well as chronic airway fibrosis were only observed in mice exposed to both MWCNTs and HDM. These findings suggest that MWCNT inhalation pre-exposure alters the allergic immune response to allergens, but nonetheless renders mice susceptible to airway inflammation and fibrosis.
Materials and Methods
Carbon Nanotubes
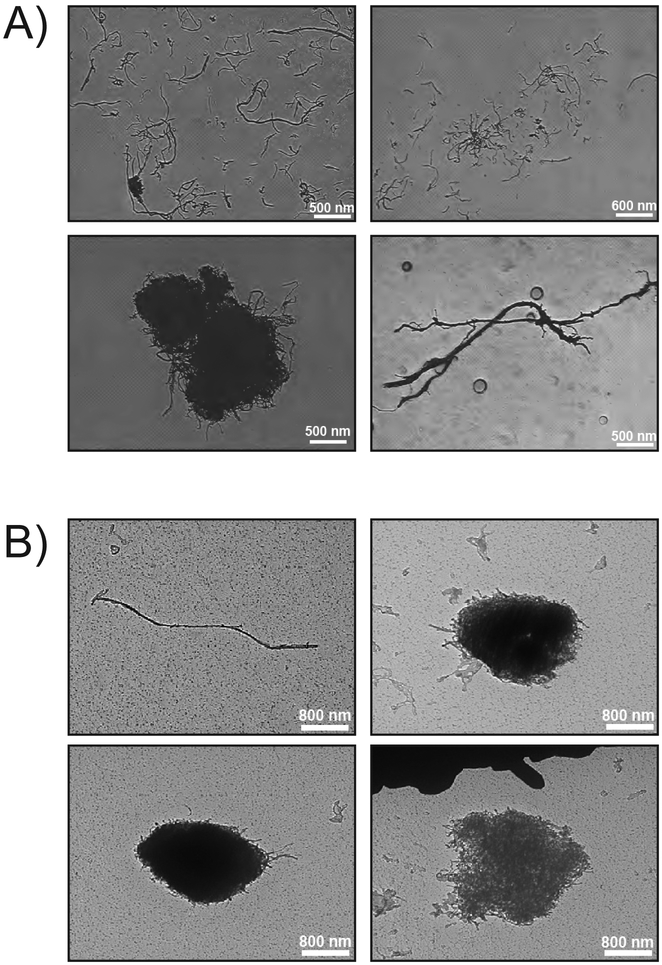
CNTs (Item# SN9847; 10–20 nm nominal outer diameter, 10–30 μm nominal length) were purchased from Sun Innovations, Inc. (Fremont, CA; http://nanomaterialstore.com). Detailed characterization data is presented in Table 1. The MWCNTs have an average diameter of 16 nm and average length of 2.4 μm. These MWCNTs are more “tangled” forming “cotton-ball” agglomerates as opposed to “rigid” or “rod-like” MWCNTs (e.g., Mitsui-7) (Duke and Bonner, 2018). These MWCNTs also have very little trace metal contamination (96% C), which is one of the reasons they were chosen for this study. Representative TEM images of the bulk MWCNTs can be found in Fig. 1A.
Table 1.
Bulk MWCNT characterization data.
| Physicochemical Parameter | Measurement | Testing Location |
|---|---|---|
| Average Diameter (TEM) | 16 nm (n=300) | EMSL Analytical, Inc. (Cinnaminson, NJ) |
| Average Length (TEM) | 849 nm (n=300) | EMSL Analytical, Inc. (Cinnaminson, NJ) |
| Average Length (SEM) | 2.4 μm (n=105) | Battelle Columbus Operations (Columbus, OH) |
| Purity (TGA) | 98% | Battelle Columbus Operations (Columbus, OH) |
| Elemental Analysis | ||
| C (Combustion) | 96% | Galbraith Laboratories (Knoxville, TN) |
| H (Combustion) | 0.6% | Galbraith Laboratories (Knoxville, TN) |
| N (Combustion) | < 0.5% | Galbraith Laboratories (Knoxville, TN) |
| S (Combustion) | < 0.05% | Galbraith Laboratories (Knoxville, TN) |
| Ni (NAA) | 0.52% | Elemental Analysis Inc (Lexington, KY) |
| Surface Composition | ||
| C (XPS) | 95% | EMSL Analytical, Inc. (Cinnaminson, NJ) |
| O (XPS) | 4.4% | EMSL Analytical, Inc. (Cinnaminson, NJ) |
| BET Average Surface Area | 172 m2/g | Micromeritics Analytical Services (Norcross, GA) |
| Skeletal Density (helium gas picnometry) | 2.13 g/cm3 | Micromeritics Analytical Services (Norcross, GA) |
| Zeta Potential (electrophoretic velocity, H2O) | −18 mV | Micromeritics Analytical Services (Norcross, GA) |
Fig. 1.
Representative photomicrographs of MWCNTs. A) TEM images depicting microscale morphometry of bulk MWCNTs, both entangled (bottom left) and unentangled (bottom right and top). B) TEM images depicting microscale morphometry of MWCNTs collected from the 0.06 mg/m3 chamber; entangled (top right and bottom) and unentangled (top left).
Inhalation Exposure System
The aerosol generation system used a linear feed dust-metering device (Battelle, West Jefferson OH) to meter neat MWCNT into a particle attrition chamber (PAC) and a single jet disperser for aerosolization. The aerosol was diluted with primary dilution air (filtered, compressed, humidified air). A cyclone separator was used to remove the larger particles from the distribution system. From the cyclone separator, aerosol was directed to the distribution line where it was diluted with secondary dilution air (filtered, compressed, humidified air), then conveyed through a high-velocity distribution line made of stainless steel, bonded and grounded to prevent build-up of electrostatic charge. At each whole-body exposure chamber (H-2000; Lab Products, Inc.; Seaford, DE), aerosol was metered to a chamber inlet duct where it was further diluted with conditioned air (humidified, Purafil-, charcoal-, and high-efficiency particulate air [HEPA]-filtered) to achieve the desired exposure concentration. Aerosol concentrations were measured gravimetrically. Both the mass median aerodynamic diameter (MMAD) and the count median diameter (CMD) were measured in the exposure chambers. MMAD was measured using a Cascade impactor and probit analysis. CMD and the concentration of particles were measured using an electrical low-pressure impactor ([ELPI]; Dekati; Tampere, Finland). The CMD and particle number concentration were analyzed using the ELPIVI 4.0 Data Analysis Software provided by the manufacturer. Monitoring of the aerosol concentration in chambers was performed using real-time aerosol monitors (RAMs) that were calibrated prior to exposing animals by correlation of the measured RAM response (voltage) with gravimetrically determined MWCNT concentrations (mg/m3) during animal exposure (Table 2). Representative images of MWCNT collected from the exposure chamber are shown in Fig. 1B.
Table 2.
Aerosol monitoring in inhalation chambers.
| Target Exposure Concentration (mg/m3) | Measured Exposure Concentration (mg/m3) | Percent of Target ± RSD | Mass Median Aerodynamic Diameter (nm) | Geometric Standard Deviation | Count Median Diameter (nm) |
|---|---|---|---|---|---|
| 0 (Control) | 0.000 ± 0.000 | - | - | - | - |
| 0.06 | 0.061 ± 0.004 | 101 ± 6 | 1.5 | 2.3 | 80 |
| 0.2 | 0.20 ± 0.01 | 100 ± 4 | 1.8 | 1.7 | 84 |
| 0.6 | 0.60 ± 0.03 | 100 ± 5 | 1.4 | 2.5 | 82 |
Animals and Experimental Design
Pathogen-free 8 to 9-week-old male B6C3F1/N mice were purchased from Taconic Farms (Hudson, NY). As illustrated in Fig. 2, mice were exposed by whole body inhalation to MWCNTs at target concentrations of 0.06, 0.2 and 0.6 mg/m3 or air only as a control for 6 hours a day, 5 days per week, over 30 days (twenty, 6-hour exposures per group). Exposure concentrations were chosen based on predicted deposition from a prior 90-day study which utilized the same MWCNTs. Inhalation exposure was performed at Battelle (West Jefferson, OH) as part of a GLP study conducted under contract for the National Toxicology Program/NIEHS (Durham, NC), after which mice were transferred to an AAALAC-accredited animal facility at North Carolina State University (Raleigh, NC). Mice were allowed to acclimate for one week prior to any procedures and were housed 1–5 per cage and provided food (LabDiet 5001 rodent diet) and water ad libitum. As shown in Fig. 2, mice then were exposed to 25 μg of house dust mite allergen (Greer Laboratories Inc., Lenoir, NC) or PBS vehicle by intranasal aspiration (25 μl per nare) on days 0, 2, 4, 14, 16 and 18 (n=5 per treatment group). Mice were euthanized at 3 and 30 days after the last HDM exposure by intraperitoneal injection of pentobarbital (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI).
Fig. 2.
Illustration of exposure protocol. Mice were pre-exposed to MWCNTs (0.06, 0.2 or 0.6 mg/m3) by whole body inhalation for 30 days (5 days/week, 6 hr/day). After a 1 week acclimation period, the animals were sensitized with HDM extract (25 μg) via intranasal aspiration (days 0, 2, 4) and challenged with HDM extract (days 14, 16, 18). Mice were euthanized and necropsy performed on day 21 (3 days post-HDM) or 48 (30 days post-HDM).
Tissue Collection
Bronchoalveolar lavage fluid (BALF) was obtained by lavaging the lungs twice with 0.5 ml sterile DPBS. A Thermo Scientific Cytospin 4 Cytocentrifuge (Thermo Fisher Scientific, Waltham, MA) was immediately used to spin cells from 100 μl of BALF onto glass slides. These cells were then fixed and stained with the Diff-Quik Stain Set (Dade Behring, Inc., Newark, DE). The remaining BALF was stored at −80° C. After BALF collection, the middle and caudal lobes of the right lung, as well as the heart, spleen, and a section of the liver, were placed in RNAlater (Ambion, Austin, TX), according to the manufacturer’s instructions and stored at −80° C. The cranial lobe of the right lung was flash frozen in liquid nitrogen and stored at −80° C. The left lung was infused with 10% neutral buffered formalin, fixed for 24 hours, dehydrated in 70% ethanol, and embedded in paraffin. Whole blood was collected from the jugular veins, allowed to coagulate for 15 minutes in Serum Separator Tubes (BD Microtainer, Franklin Lakes, NJ), then centrifuged to obtain serum. Serum was stored at −80° C.
Cell Counting and Histology
Total inflammatory BALF cell counts were obtained from Diff-Quik stained BALF slides by counting 3 frames per slide at 100x magnification. Differential cell counts of neutrophils, macrophages, eosinophils, and lymphocytes were obtained from Diff-Quik stained BALF slides by counting a total of 500 cells per slide at 200x magnification. Numbers of macrophages with MWCNT inclusions were obtained by counting 500 total macrophages per slide at 200x magnification and noting how many had visible inclusions. Paraffin embedded tissues were cut into three sections and stained with hematoxylin and eosin (H&E), Alcian blue/periodic acid-Schiff (AB/PAS) and/or Gomori trichrome stain.
Semi-Quantitative Morphometric Analysis
Mucous cell metaplasia was assessed by imaging all airways under 500×500 μm (HxW) in each AB/PAS-stained tissue. The AB/PAS-positive area was measured using NIH ImageJ software (National Institutes of Health) expressing the integrated density of the area of AB/PAS+ mucosubstances per unit length of airway basement membrane (Harkema et al., 1987; Zhu et al., 2015). Airway fibrosis was assessed by imaging trichrome stained lung sections and obtaining area to perimeter ratios as described previously (Duke et al., 2017). Briefly, all round to oval shaped airways under approximately 500×500 μm (HxW) were imaged at 100x. The lasso tool in Adobe Photoshop CS5 was used to surround trichrome positive collagen around the airways, giving the outer area, and to surround the basement membrane, giving the inner area and circumference (perimeter). The difference between the outer and inner area was divided by the circumference, giving the area/perimeter ratio. All measurements were performed in a blinded manner. For statistical analysis airways were grouped into small (under 300×300 μm) and large (above 300×300 μm) airways.
ELISA
DuoSet ELISA kits (R&D Systems, Inc.) were used to measure total IgE in serum and IL-13, CCL2, IL-1β, osteopontin and TGF-β1 in BALF. Serum was diluted 1:10 in DPBS for the IgE ELISA. BALF was diluted 1:10 for the osteopontin ELISA. ELISAs were carried out according to manufacturer protocol and concentration interpolated from a standard curve.
Statistical Analysis
All data was graphed and analyzed using GraphPad Prism version 5 (GraphPad Software Inc., San Diego, CA). Significance between treatment groups was determined by two-way ANOVA with a Bonferroni post-test or unpaired Student’s t-test. All data is represented as the mean + SEM of three to five animal replicates.
Results
Inhaled MWCNTs are sequestered in macrophages and persistent over time.
MWCNT inclusions were easily observable in Diff-Quik stained BALF macrophages (Fig. 3). The numbers of macrophages containing MWCNTs significantly increased as a function of MWCNT air concentration. The numbers of macrophages with inclusions decreased >50% between the 3 day and 30 day time points, indicating the high persistence of MWCNTs in the lung (Fig. 3). Treatment of mice with HDM allergen did not affect numbers of macrophages with MWCNT inclusions.
Fig. 3.
MWCNT inclusions in alveolar macrophages. A) Representative images of alveolar macrophages from the 0.6 mg/m3 exposure group at 3 days post-HDM vehicle isolated from BALF by Cytospin centrifugation (left panel 200x, right panel 1000x). B) Numbers of macrophages with MWCNT inclusions per 500 cells at 3 days. C) Images of macrophages from the 0.6 mg/m3 exposure group at 30 days post-HDM vehicle (left panel 200x, right panel 1000x). D) Numbers of macrophages with MWCNT inclusions per 500 cells at 30 days.
Inhaled MWCNTs are visible in macrophages in situ in AB/PAS stained lung sections.
Alveolar macrophages containing MWCNTs inclusions were readily visible in AB/PAS-stained lung sections (Fig. 4). These macrophages were well dispersed throughout the lungs at both the 3 day and 30 day post-HDM treatment time points, as well as among individual animals in each group. Interestingly, occasional MWCNT-containing macrophages were observed clustered in groups within alveoli in the lungs of HDM treated mice at 3 and 30 days, but not in lungs from mice treated with MWCNTs alone (Fig. 4).
Fig. 4.
MWCNT inclusions in alveolar macrophages in situ. MWCNTs were observed in alveolar macrophages in Alcian blue/periodic acid-Schiff stained lung sections at both 3 days (A) and 30 days post-HDM treatment (B). M1–M3 refers to images from 3 individual mice out of a total of 5 per group. Clusters of macrophages with MWCNT inclusions in alveolar spaces were seen only in the lungs of HDM-treated mice (see M3 in panel A).
MWCNT and HDM-induced changes in BALF inflammatory cell profiles.
At 3 days post-HDM exposure, numbers of eosinophils in BALF were elevated by HDM treatment alone, but were not altered by MWCNT inhalation pre-exposure (Fig. 5A). At 30 days post-HDM treatment, eosinophilia was largely resolved. Numbers of neutrophils were induced at 3 days only by the lowest dose of MWCNTs in the absence of HDM extract or at 30 days by the lowest dose of MWCNTs with or without HDM extract (Fig. 5B). Numbers of macrophages remained largely unchanged at 3 and 30 days post-HDM treatment (Fig. 5C).
Fig. 5.
Differential cell counts in BALF from mice at 3 or 30 days post-HDM treatment following inhalation pre-exposure to MWCNTs. A) Eosinophil numbers were elevated by HDM treatment with or without MWCNT inhalation pre-exposure. B) Neutrophils numbers marginally increased at the lowest dose of MWCNTs at 3 or 30 days. C) Numbers of alveolar macrophages remained largely unchanged at 3 or 30 days post-HDM treatment.
MWCNT pre-exposure inhibits in HDM-induced serum IgE and lung IL-13 levels.
Exposure of mice to HDM allergen induced an allergic lung phenotype, as shown by a significant increase in total serum IgE levels at 3 days (Fig. 6). MWCNTs alone did not increase IgE levels in serum, but baseline levels of IgE were inhibited by higher doses of MWCNTs. Surprisingly, HDM-induced IgE production was significantly inhibited by MWCNT inhalation pre-exposure at the highest dose of MWCNTs (Fig. 6A). IgE levels at 30 days were not significantly altered by HDM treatment with or without MWCNT inhalation pre-exposure (data not shown). The protein level of IL-13 in BALF was not significantly increased by HDM treatment at 3 days (Fig. 6B) or at 30 days post-HDM treatment (data not shown). However, higher doses of MWCNT, with or without HDM treatment, inhibited IL-13 in BALF.
Fig. 6.
Serum IgE and BALF levels of IL-13 measured by ELISA at 3 days post-HDM treatment. A) HDM significantly enhanced IgE levels over corresponding controls, while MWCNT pre-exposure decreased IgE levels at the highest dose (0.6 μg/m3). *p<0.05 vs. vehicle, **p<0.01 vs. vehicle, #p<0.05 vs 0 mg/m3, ##p<0.01 vs. 0 mg/m3, ^p<0.05 vs. 0 mg/m3+HDM. B) IL-13 in BALF was suppressed by MWCNT pre-exposure (0.2 μg/m3). #p<0.05 vs 0 mg/m3, ^p<0.05 vs. 0 mg/m3+HDM.
HDM-induced airway mucous cell metaplasia is inhibited by MWCNT treatment.
Airway mucous cell metaplasia and mucus production in the lungs of mice detected by AB/PAS staining was induced at 3 days post-HDM treatment, but was not induced by MWCNT inhalation pre-exposure alone (Fig 7). Semi-quantitative morphometry revealed that the highest dose of MWCNTs significantly inhibited HDM-induced AB/PAS+ staining at the 3-day post-HDM treatment time point. At 30 days post-HDM treatment, there was no significant increase in mucous cell metaplasia (data not shown).
Fig. 7.
Alcian Blue/Periodic Acid-Schiff staining for airway mucus. A) Representative AB-PAS-stained lung sections at 3 days post-HDM treatment. HDM treatment caused induced airway mucus production (arrows), while MWCNT pre-exposure at the highest dose blunted HDM-induced AB-PAS staining. B) Quantification of the AB-PAS+ area of mucosubstances normalized to airway length (units for area and length are arbitrary pixels). *p<0.05, HDM alone compared to HDM + MWCNT (0.6 mg/m3) as determined by Student’s t-test.
HDM treatment after MWCNT inhalation pre-exposure causes acute airway and vascular inflammation.
Inflammatory lesions around airways and blood vessels, composed of eosinophils and lymphocytes, were observed at 3 days in hematoxylin and eosin-stained lung sections from mice exposed to HDM extract after inhalation pre-exposure to MWCNTs at all doses (Fig 8). HDM treatment alone or MWCNT inhalation exposure alone did not cause the formation of inflammatory lesions at 3 days. No inflammatory lesions were observed in any of the experimental groups at 30 days post-HDM treatment.
Fig. 8.
Representative hematoxylin and eosin stained lung sections at 3 days post-HDM treatment. Inflammatory lesions were observed around the terminal bronchioles (TB) and vessels in the lungs of mice treated with MWCNTs and HDM as indicated by the arrows. Images were captured at 100x and 400x.
MWCNT inhalation increases pro-fibrotic cytokines in the lungs of mice and produces airway fibrosis with HDM treatment.
Pro-fibrotic cytokines (IL-1β and TGF-b1) in BALF were increased in a dose-dependent manner at 30 days by MWCNT inhalation (Fig. 9). HDM extract treatment after MWCNT inhalation did not significantly increase these levels of these cytokines. Semi-quantitative morphometry of collagen-positive lesions at 30 days post-HDM treatment, visualized by Gomori’s trichrome staining, showed a significant increase in airway fibrosis at the highest dose of MWCNTs with HDM treatment (Fig. 10). This increase was observed only in airways with diameters >300 μm.
Fig. 9.
Levels of pro-fibrotic cytokines in BALF measured by ELISA at 30 days post-HDM treatment. A) IL-1β was increased at 30 days by the highest dose of MWCNT (**p<0.01 vs 0 mg/m3). B) TGF-β1 was increased at 30 days and showed an increasing trend with MWCNT exposure (*p<0.05 vs 0 mg/m3).
Fig. 10.
Assessment of airway fibrosis at 30 days post-HDM exposure. A) Representative images of lung sections stained with Gomori’s trichrome showing blue collagen deposition around airways and blood vessels (arrows). B) Semi-quantitative morphometry of collagen thickness around airways larger than 300 × 300 μm accomplished using an area/perimeter ratio method. *p<0.05 vs 0.6 mg/m3 MWCNT + HDM.
Discussion
In this study, we investigated the effects of whole body inhalation pre-exposure to MWCNTs on the development of HDM-induced allergic airway disease in mice. To our knowledge this is the first time MWCNT inhalation pre-exposure has been conducted to evaluate the immune response to allergen sensitization and challenge. The key finding of this study was that exposure to MWCNTs prior to HDM allergen inhibited the acute allergic immune response to HDM extract as shown by decreased serum IgE, reduced IL-13 in BALF, and suppression of mucus production in the airways of mice, yet MWCNT pre-exposure followed by HDM treatment produced acute inflammatory lesions around airways and vessels and chronic airway fibrosis that were not observe with either MWCNT or HDM exposure alone. Therefore, this study provides novel insights and a deeper understanding of MWCNT effects on allergen-induced lung inflammation.
Previous work by others showed evidence for MWCNT-induced immune suppression at similar doses (0.3 and 1 mg/m3) to our present study (Mitchell et al., 2007, 2009). In that study, MWCNTs given by full body inhalation did not cause significant pulmonary inflammation but caused systemic immune dysfunction as determined by reduced T cell proliferation to Concanavalin A and reduced T cell-dependent antibody response to sheep erythrocytes (Mitchell et al., 2007). Though we did not assess these endpoints in our study, the dose-dependent reduction in HDM-induced serum IgE caused by MWCNT exposure in the present study suggests a similar dampened antibody response to HDM. A subsequent study by the same investigators established that the observed immunosuppression was dependent on a mechanism involving TGF-β1 production in the lungs and subsequent COX-2 activation and IL-10 production in the spleen (Mitchell et al., 2009). We also observed a significant increase in TGF-β1 in BALF induced by the highest dose of inhaled MWCNTs (0.6 mg/m3) in the present study, suggesting that similar downstream immunosuppressive signaling events may have occurred. We also examined spleen IL-10 mRNA by qRT-PCR but did not find any changes between treatment groups (data not shown). This may be due to different timing of exposure scenarios in our study that involved a 3-week HDM allergen post-exposure after a 30 day MWCNT inhalation exposure, compared to the previous MWCNT inhalation study that used an exposure scenario of 0.3 or 1 mg/m3 for 6 hr/dy for 14 consecutive days (Mitchell et al., 2009).
Many studies have examined the effects of CNTs on allergic airway disease in mice. The ovalbumin (OVA) mouse model of asthma is the most widely used model to test MWCNT exacerbation of allergen-induced airway disease and induces similar pathological endpoints to the HDM model; therefore, both are used as models to study the pathology of asthma. We previously reported that nose-only inhalation exposure of MWCNTs after sensitization and challenge by OVA exacerbated airway fibrosis in mice and synergistically elevated IL-5 mRNA in lung tissue above that observed with either OVA or MWCNT alone (Ryman-Rasmussen et al., 2009). That study contrasted to the present study, since the MWCNT exposure was much higher in concentration (100 mg/m3) yet brief (6 hr duration in 1 day) and importantly the MWCNT inhalation exposure occurred after allergen sensitization and challenge (Ryman-Rasmussen et al., 2009a). Exacerbation of allergen-induced lung inflammation has also been shown with MWCNTs delivered by intratracheal instillation. For example, OVA-induced allergic responses in mice (serum IgE, BALF IL-4, and lung eosinophilia) were exacerbated by subsequent intratracheal instillation of SWCNTs or MWCNTs (Inoue et al., 2009, 2010; Mizutani et al., 2012). Exacerbation of HDM-induced allergic responses and airway remodeling (serum IgE, TH2 cytokines, eosinophilia and mucus production) were also exacerbated by subsequent exposure to MWCNTs (Ronzani et al., 2014; Shipkowski et al., 2015). Collectively, these findings of enhanced TH2 allergic responses by CNT post-exposure contrast with our observed suppression of HDM-induced TH2 allergic responses by pre-exposure to inhaled MWCNTs and emphasize that exposure sequence plays an important role in the immune response and disease outcome to inhaled MWCNTs and allergens.
This study highlights the importance of dose, exposure route and timing of exposure when dealing with MWCNT toxicity. In potential real-world occupational exposures to MWCNTs and allergens, it is possible that individuals may be exposed to CNTs at the same time as allergens, before allergen exposure, after allergen exposure, or a combination of these sequences. To properly assess effects of CNT exposure on allergic airway disease, it is therefore necessary to evaluate a variety of different exposure sequences and scenarios. As discussed above, there are a number of studies that addressed allergen pre-exposure followed by CNT exposure, or allergen and CNT co-exposure. However, until now there have been no studies addressing CNT inhalation pre-exposure followed by allergen. Additionally, asthma can develop later in life (late-onset or intrinsic asthma), in which case an individual could be pre-exposed to CNTs prior to the development of asthma, which could affect the severity of chronic airway remodeling.
The immunotoxic responses to MWCNTs, either alone or in combination with allergens, also depend on specific physical and chemical characteristics such as rigidity, surface charge, and residual metal catalyst content, to name but a few (Duke and Bonner, 2018). The MWCNTs used in the present study are representative of many MWCNTs currently manufactured in bulk; individual tubes are tangled and form ‘cotton ball’ agglomerates. Many MWCNTs contain residual metal catalysts, such as Ni, from the chemical vapor deposition synthesis process. For example, the MWCNTs used in this study contained ~0.5% residual nickel (Table 1). Similar physical characteristics are shared by MWCNTs that have been shown to exacerbate allergen-induced airway inflammation and fibrosis (Ryman-Rasmussen et al., 2009a; Thompson, 2014; Shipkowski et al., 2015). In contrast to the tangled MWCNTs used in the present study, rigid MWCNTs have been shown to directly cause allergic airway inflammation and TH2-like responses including eosinophilia and mucous cell metaplasia in the absence of allergens (Rydman et al., 2014).
Inhalation is the most physiologically relevant route of exposure for MWCNTs. A commonly used and valid alternative route of lung exposure in mice is oropharyngeal aspiration, which involves delivery of a bolus dose of MWCNTs in aqueous suspension. Aspiration and inhalation exposures to MWCNTs have been shown to result in some similar lung inflammatory responses and gene expression profiles (Kinaret et al., 2017). However, even when using surfactant-based dispersion media, aspiration exposure results in agglomerates of MWCNTs deposited in the distal lung that do not accurately represent the deposition pattern of inhaled MWCNTs (Muller et al., 2005; Bonner et al., 2013). Therefore, aspirated MWCNTs produce pathological changes (e.g., granuloma formation) that are not typically observed with inhaled MWCNTs. Furthermore, aspiration exposure involves delivery of a relatively high single dose of MWCNTs, while we delivered MWCNTs by whole body inhalation 5 days a week over a 30-day period. This exposure produced a consistently reproducible distribution of MWCNTs throughout the lungs of mice that were primarily confined to alveolar macrophages. Other inhalation exposure studies have been performed with MWCNTs that produced pathological changes, although these exposures were relatively high dose. For example, we previously reported that mice exposed to MWCNTs by nose-only inhalation at 30 mg/m3 for 6 hr caused subpleural fibrosis and pleural inflammation (Ryman-Rasmussen et al., 2009b). Others have shown that inhalation to a 5 mg/m3 MWCNT aerosol for 5 hours/day for 12 days (4 times/week for 3 weeks) produced a progressive and persistent increase in fibrillar collagen for up to 336 days post-exposure (Mercer et al., 2013). In the present study, we did not observe fibrosis in the lungs of mice exposed to the highest dose in our study: 0.6 mg/m3 for 5 hours/day for 5 days a week for 30 days. However, the cumulative dose used by Mercer et al. was approximately 5 times higher than the highest dose used in our study, so this difference was not surprising. Moreover, the study by Mercer and colleagues used rigid, rod-like MWCNTs (Mitsui-7) and we recently reported that rod-like MWCNTs are more fibrogenic compared to tangled, flexible MWCNTs (Duke et al., 2017).
An important issue for using experimental animal studies to assess the risk of MWCNTs on human health is the selection of appropriate inhalation doses that are comparable and relevant to occupational workplace exposures. Other studies have estimated workplace exposures to CNTs. For example, Erdely and coworkers measured elemental carbon (EC) concentrations at the inhalable size fraction from eight MWCNT facilities and found an arithmetic exposure mean of 10.6 μg/m3 with a range from non-detectable to 79.6 μg/m3 (Erdely et al., 2013). Dahm and colleagues assessed the mass EC at the respirable and inhalable aerosol particle fraction in over 100 participants from 12 facilities across the US manufacturing CNTs and nanofibers (Dahm et al., 2018). They reported a mean inhalable EC exposure level of 6.22 μg/m3 with a range from <0.001 to 417.91 μg/m3. Moreover, MWCNT agglomerates (between 2 and 5 μm) were identified by TEM in personal air samples. Therefore, the size of respirable agglomerates (~2 μm) that we generated in our exposures are qualitatively relevant to those seen in workplace exposures. Kuijpers and colleagues reported exposure levels of MWCNTs in production facilities between 20 and 88 μg/m3 (Kuijpers et al., 2016). The lower dose of 60 μg/m3 used in our study falls within the range of these workplace exposures and is therefore quantitatively relevant. Even the high dose of 600 μg/m3 is relevant when considering regulatory decision-making where multiple uncertainty factors (that each can be up to a default value of 10x each in the absence of specific data) may be applied to doses/exposures when extrapolating toxicological effects from rodents to humans, from sub-chronic to chronic exposures, and when accounting for possible inter-individual variability in the human response (Dankovic et al., 2015). While only the highest dose of MWCNTs used in our sub-chronic study inhibited IgE and mucus production in mice, it is possible that chronic exposures to lower doses of MWCNTs in occupational settings could produce similar effects in humans.
In conclusion, our work demonstrates that mice exposed by inhalation to MWCNTs results in inhibition of the allergic TH2 immune response to HDM extract as evidenced by a significant decrease in allergen-inducible serum IgE levels, reduced BALF IL-13, and decreased airway mucous cell metaplasia. These results suggest that MWCNT inhalation pre-exposure might reduce the allergic response to common household allergens such as HDM. Despite reducing allergen-induced TH2 immune responses, MWCNT pre-exposure followed by HDM treatment caused acute airway and vascular inflammatory lesions and an increase in chronic airway fibrosis that was not seen with either MWCNTs or HDM alone. Taken together, our findings emphasize the potential for low-dose MWCNT inhalation exposure to reduce atopic immune responses to allergens, but nevertheless promote a pro-inflammatory and pro-fibrotic environment in the lung that might render exposed individuals chronically susceptible to common allergens or inhaled pathogens. Therefore, these results provide novel insight into the effects of inhaled MWCNTs on susceptibility to allergic lung disease.
Acknowledgments
The authors would like to thank Dr. Srikanth Nadadur at the NIEHS extramural program for logistics and coordination of the study participants.
Funding Information
This work was supported by the National Institute of Environmental Health Sciences [extramural grants R01ES020897, T32ES007046, P30ES025128 and Intramural Research Program Project Z01ES103316–01] and the National Science Foundation [grant 15–022].
Footnotes
Declaration of Interests
The authors do not have any conflict of interest to report.
References
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson C. a, and Liu X (2012). Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Baughman RH, Zakhidov AA, and de Heer WA (2002). Carbon Nanotubes--the Route Toward Applications. Science, 297, 787–792. [DOI] [PubMed] [Google Scholar]
- Bonner JC (2010). Nanoparticles as a potential cause of pleural and interstitial lung disease. Proc. Am. Thorac. Soc, 7, 138–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JC, Silva RM, Taylor AJ, Brown JM, Hilderbrand SC, Castranova V, Porter D, Elder A, Oberdörster G, Harkema JR, Bramble LA, Kavanagh TJ, Botta D, Nel A, and Pinkerton KE (2013). Interlaboratory Evaluation of Rodent Pulmonary Responses to Engineered Nanomaterials: The NIEHS Nano GO Consortium. Environ. Health Perspect, 121, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Bertke S, Beard JD, Erdely A, Fernback JE, Mercer RR, Grinshpun SA. (2018) Exposure assessments for a cross-sectional epidemiologic study of US carbon nanotube and nanofiber workers. Int J Hyg Environ Health. 221:429–440. [DOI] [PubMed] [Google Scholar]
- Dankovic DA, Naumann BD, Maier A, Dourson ML, and Levy LS (2015) The scientific basis of uncertainty factors used in setting occupational exposure limits. J Occup Environ Hygiene. 12, S55–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson K, Aitken R, Tran L, Stone V, Duffin R, Forrest G, and Alexander A (2006). Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci, 92, 5–22. [DOI] [PubMed] [Google Scholar]
- Duke KS and Bonner JC (2018). Mechanisms of carbon nanotube-induced pulmonary fibrosis: a physicochemical characteristic perspective. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology, 10, e1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke KS, Taylor-Just AJ, Ihrie MD, Shipkowski KA, Thompson EA, Dandley EC, Parsons GN, and Bonner JC (2017). STAT1-dependent and -independent pulmonary allergic and fibrogenic responses in mice after exposure to tangled versus rod-like multi-walled carbon nanotubes. Part. Fibre Toxicol, 14, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdely A, Dahm M, Chen BT, Zeidler-Erdely PC, Fernback JE, Birch ME, Evans DE, Kashon ML, Deddens J. a, Hulderman T, Bilgesu S. a, Battelli L, Schwegler-Berry D, Leonard HD, McKinney W, Frazer DG, Antonini JM, Porter DW, Castranova V, and Schubauer-Berigan MK (2013). Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part. Fibre Toxicol, 10, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema JR, Plopper CG, Hyde DM, St George JA (1987) Regional differences in quantitities of histochemically detectable mucosubstances in nasal, paranasal, and nasopharyngeal epithelium of the bonnet monkey. J. Histochem. Cytochem 35, 279–286. [DOI] [PubMed] [Google Scholar]
- Holgate ST (2009). Pathogenesis of Asthma In, Allergy and Allergic Diseases. Wiley-Blackwell, Oxford, UK, pp. 1608–1631. [Google Scholar]
- Ihrie MD and Bonner JC (2018). The Toxicology of Engineered Nanomaterials in Asthma. Curr. Environ. Heal. Reports, 5, 100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, and Takano H (2009). Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol. Appl. Pharmacol, 237, 306–316. [DOI] [PubMed] [Google Scholar]
- Inoue K, Yanagisawa R, Koike E, Nishikawa M, and Takano H (2010). Repeated pulmonary exposure to single-walled carbon nanotubes exacerbates allergic inflammation of the airway: Possible role of oxidative stress. Free Radic. Biol. Med, 48, 924–934. [DOI] [PubMed] [Google Scholar]
- Kim HY, DeKruyff RH, and Umetsu DT (2010). The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat. Immunol, 11, 577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinaret P, Ilves M, Fortino V, Rydman E, Karisola P, Lähde A, Koivisto J, Jokiniemi J, Wolff H, Savolainen K, Greco D, and Alenius H (2017). Inhalation and Oropharyngeal Aspiration Exposure to Rod-Like Carbon Nanotubes Induce Similar Airway Inflammation and Biological Responses in Mouse Lungs. ACS Nano, 11, 291–303. [DOI] [PubMed] [Google Scholar]
- Kuijpers E, Bekker C, Fransman W, Brouwer D, Tromp P, Vlaanderen J, Godderis L, Hoet P, Lan Q, Silverman D, Vermeulen R, Pronk A. Occupational Exposure to Multi-Walled Carbon Nanotubes During Commercial Production Synthesis and Handling. (2016) Ann Occup Hyg. 60, 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Scabilloni JF, Hubbs AF, Battelli LA, McKinney W, Friend S, Wolfarth MG, Andrew M, Castranova V, and Porter DW (2013). Distribution and fibrotic response following inhalation exposure to multi-walled carbon nanotubes. Part. Fibre Toxicol, 10, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, Gao J, Wal R Vander, Gigliotti A, Burchiel SW, and McDonald JD (2007). Pulmonary and Systemic Immune Response to Inhaled Multiwalled Carbon Nanotubes. Toxicol. Sci, 100, 203–214. [DOI] [PubMed] [Google Scholar]
- Mitchell LA, Lauer FT, Burchiel SW, and McDonald JD (2009). Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat. Nanotechnol, 4, 451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani N, Nabe T, and Yoshino S (2012). Exposure to Multiwalled Carbon Nanotubes and Allergen Promotes Early- and Late-Phase Increases in Airway Resistance in Mice. Biol. Pharm. Bull, 35, 2133–2140. [DOI] [PubMed] [Google Scholar]
- Muller J, Huaux F, Moreau N, Misson P, Heilier J-F, Delos M, Arras M, Fonseca A, Nagy JB, and Lison D (2005). Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol, 207, 221–231. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Mädler L, and Li N (2006). Toxic Potential of Materials at the Nanolevel. Science, 311, 622–627. [DOI] [PubMed] [Google Scholar]
- Ronzani C, Casset A, and Pons F (2014). Exposure to multi-walled carbon nanotubes results in aggravation of airway inflammation and remodeling and in increased production of epithelium-derived innate cytokines in a mouse model of asthma. Arch. Toxicol, 88, 489–499. [DOI] [PubMed] [Google Scholar]
- Rydman EM, Ilves M, Koivisto AJ, Kinaret PAS, Fortino V, Savinko TS, Lehto MT, Pulkkinen V, Vippola M, Hämeri KJ, Matikainen S, Wolff H, Savolainen KM, Greco D, and Alenius H (2014). Inhalation of rod-like carbon nanotubes causes unconventional allergic airway inflammation. Part. Fibre Toxicol, 11, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Tewksbury EW, Moss OR, Cesta MF, Wong B. a., and Bonner JC (2009a). Inhaled multiwalled carbon nanotubes potentiate airway fibrosis in murine allergic asthma. Am. J. Respir. Cell Mol. Biol, 40, 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, Cesta MF, Brody AR, Shipley-Phillips JK, Everitt JI, Tewksbury EW, Moss OR, Wong BA, Dodd DE, Andersen ME, Bonner JC (2009b) Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat. Nanotech 4(11), 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkowski KA, Taylor AJ, Thompson EA, Glista-Baker EE, Sayers BC, Messenger ZJ, Bauer RN, Jaspers I, and Bonner JC (2015). An Allergic Lung Microenvironment Suppresses Carbon Nanotube-Induced Inflammasome Activation via STAT6-Dependent Inhibition of Caspase-1. PLoS One, 10, e0128888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Porter D, Schulte P, Kagan VE, Fadeel B, and Castranova V (2009). Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: Two faces of Janus? Pharmacol. Ther, 121, 192–204. [DOI] [PubMed] [Google Scholar]
- Thompson EA (2014). Innate Immune Responses to Nanoparticle Exposure in the Lung. J. Enviromental Immunol. Toxicol, 2, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Wang Z, Cao Y, Cheng S, Chen H, Bunjhoo H, Xie J, Wang C, Xu Y, and Xiong W (2015). CCL2/CCR2-Dependent Recruitment of Th17 Cells but Not Tc17 Cells to the Lung in a Murine Asthma Model. Int. Arch. Allergy Immunol, 166, 52–62. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Abdullah LH, Doyle SP, Nguyen K, Ribeiro CMP, Vasquez PA, Forest MG, Lethem MI, Dickey BF, Davis CW (2015) Baseline goblet cell mucin secretion in the airways exceeds stimulated secretion over extended time periods, and is sensitive to shear stress and intracellular mucin stores. PLoS ONE, 10(5), e0127267. [DOI] [PMC free article] [PubMed] [Google Scholar]