Abstract
Thyroid uptake of iodide via the sodium-iodide symporter (NIS) is the first step in the biosynthesis of thyroid hormones that are critical for health and development in humans and wildlife. Despite having long been a known target of endocrine disrupting chemicals such as perchlorate, information regarding NIS inhibition activity is still unavailable for the vast majority of environmental chemicals. This study applied a previously validated high-throughput approach to screen for NIS inhibitors in the ToxCast phase I library, representing 293 important environmental chemicals. Here 310 blinded samples were screened in a tiered-approach by an initial single-concentration (100μM) radioactive-iodide uptake (RAIU) assay, followed with 169 samples further evaluated in multi-concentration (0.001μM-100μM) testing in parallel RAIU and cell viability assays. A novel chemical ranking system that incorporates multi-concentration RAIU and cytotoxicity responses was also developed as a standardized method for chemical prioritization in current and future screenings. Representative chemical responses and thyroid effects of high-ranking chemicals are further discussed. This study significantly expands current knowledge of NIS inhibition potentials in environmental chemicals, and provides critical support to U.S.EPA’s Endocrine Disruptor Screening Program (EDSP) initiative to expand coverage of thyroid molecular targets as well as the development of thyroid adverse outcome pathways (AOPs).
Graphical Abstract
Introduction
The presence of endocrine disrupting chemicals (EDCs) in the environment continues to be a top public health concern not only for effects on the regulation of androgen and estrogen pathways, but also thyroid hormone homeostasis. Thyroid hormones [TH; i.e., thyroxine (T4) and triiodothyronine (T3)] regulate an array of physiological processes that are essential for metabolism, cardiovascular function, bone maintenance, as well as fetal and post-natal neurodevelopment.1–3 Over the past two decades, a number of structurally diverse xenobiotics have been shown to interfere with TH homeostasis and result in physiological and morphological perturbations.4–7 Further work in this area has identified multiple molecular targets of chemical-mediated thyroid disruption including the regulation of circulating TH through feedback mechanisms within the hypothalamic-pituitary-thyroid (HPT) axis, TH synthesis and secretion, TH distribution and transport, TH metabolism, and TH receptor binding and action.8–13 These studies demonstrate a need for a better understanding of the structural characteristics of chemicals with thyroid disrupting activity, the potential for environmental exposures, and the possible health risks to humans and wildlife. To address these concerns, the U.S. EPA’s Endocrine Disruptor Screening Program (EDSP21, https://www.epa.gov/endocrine-disruption) in concert with the U.S. EPA’s Office of Research and Development (ORD) recently expanded the coverage of molecular targets for thyroid disruption by developing and implementing the use of high-throughput screening (HTS) assays to identify inhibitors of TH synthesis (e.g., sodium/iodide symporter (NIS) and thyroid peroxidase) and T4 metabolism (e.g., deiodinases). Methods for a thyroid peroxidase assay were previously described and used to screen chemicals in ToxCast chemical libraries.14, 15 In addition, our laboratory recently demonstrated the utility of another HTS assay to detect chemicals that disrupt NIS-mediated transport of extracellular iodide across the cellular membrane.16
NIS is a glycoprotein with 13 transmembrane helices that actively transports iodide (I−) into thyroid follicular cells.17–19 This activity relies on the Na+ electrochemical gradient maintained by Na+/K+ ATPases with an electrogenic stoichiometry of 2 Na+ per I−.20, 21 Under normal physiological conditions, NIS can concentrate iodide into the thyroid gland 20- to 40-fold greater than serum levels.22 Malfunction of the NIS protein is known to disrupt TH homeostasis; patients with various gene mutations leading to aberrant NIS protein have been diagnosed with hypothyroidism.23 NIS activity can also be disrupted by xenobiotic chemicals. A well-known example is perchlorate (ClO4−), which is a widespread environmental contaminant that has been detected in soil, ground/surface water, food, and consumer products across the world24–28. Perchlorate, along with several other environmental anions including thiocyanate (SCN−) and nitrate (NO3−), have been demonstrated to competitively inhibit I− uptake by NIS and disrupt TH synthesis in humans29–31 and multiple species of vertebrates32, 33. Despite having long been a known target of endocrine disruption, studies on NIS inhibition activity have been largely restricted to only a few environmental chemicals. However, two studies have recently identified triclosan, triclocarban, BDE-47, bisphenol A34, and several small drug-like organic molecules as NIS-mediated iodide transport inhibitors35, suggesting that NIS inhibition activity extends beyond traditionally known anions to the more complex organic compounds. Considering the pivotal role of NIS in the thyroid hormone system, there is an urgent need to expand the knowledge of NIS inhibition potentials to a broader range of environmental chemicals.
In this study, we applied a previously validated HTS approach to screen the 293 ToxCast (https://www.epa.gov/chemical-research/toxicity-forecasting) phase I chemicals and developed a specific chemical ranking approach to assist with chemical prioritization in current and future screenings. ToxCast phase I library contains a diverse collection of environmental chemicals (mostly pesticides and antimicrobials) that are currently under the U.S. EPA’s regulatory purview36, as well as a subset of chemicals that have been extensively tested in the Agency’s EDSP Tier I Screening Battery.37 This study significantly expands our current knowledge of environmentally-relevant chemicals with NIS inhibition potential and contributes to a broader understanding of thyroid disruptive mechanisms. In addition, the results support the development of adverse outcome pathways (https://aopwiki.org/) related to thyroid hormone disruption and Organization for Economic Co-operation and Development (OECD) test guidelines38 to assess thyroid disruptive chemicals. With an accompanied R package made publicly available, the newly developed chemical ranking approach could also be extended as a standardized method to prioritize chemicals for other HTS studies.
Materials and Methods
Chemicals
Chemical names, CAS numbers, and maximum concentrations tested are shown in Table S1 (test chemicals) and Table S2 (assay controls). All control chemicals were initially solubilized in DMSO (EMD Millipore Corp., Darmstadt, Germany) at 20mM and included sodium perchlorate (NaClO4; RAIU assay positive control), sodium nitrate (NaNO3; RAIU assay EC80 control), sodium thiocyanate (NaSCN; RAIU assay EC20 control), 2,4-dichlorophenoxyacetic acid (2,4-D; RAIU assay negative control), and 2,3-dichloro-1,4-napthoquinone (DCNQ; cell viability assay positive control) (Sigma Aldrich, St. Louis, MO).
Chemicals of the ToxCast phase I_v2 library were obtained from the National Center of Computational Toxicology (NCCT), US EPA, Research Triangle Park, NC, USA.36 Each chemical was solubilized in DMSO (≤ 20mM, Table S1) and provided as 310 blinded samples in five 96-well plates (62 samples per plate) (Evotec Inc., South San Francisco, CA). Stock chemical plates were visually examined under microscope to check for solubility/precipitate in each well (Table S1). Of the 310 samples, there were 293 unique chemicals; the remaining 17 served as internal quality control replicates of 12 chemicals randomly distributed among the five plates. All chemicals were transferred to bioassay plates using a BioMek FX Automated Laboratory Workstation (Beckman Coulter, Indianapolis, IN) equipped with a stainless steel high density replicating (HDR) tool (96-pin) to transport 0.35μL per well for the RAIU and cell viability assays.
Radioactive Iodide Uptake (RAIU) and Cell Viability Assays
RAIU and cell viability assays were conducted with low passage hNIS-HEK293T-EPA cells (< 25 passes) as previously described16. In brief, cells (4×104 per well) were seeded and grown for 40 hours in ScintiPlate-96 microplates (Perkin Elmer, Waltham, MA) and 96-well solid white assay plates (Corning, Corning, NY) for the RAIU and cell viability assays, respectively. Cells were then washed and exposed to test chemicals at room temperature for 2 hours (in addition to radioactive iodide 125I in the RAIU assay). Chemical exposure was terminated by washing cells with ice-cold uptake buffer. Intracellular 125I counts per minute (CPM) were quantified with a MicroBeta2 microplate scintillation counter (Perkin Elmer, Waltham, MA). Cell viability was measured via CellTiter-Glo (Promega, Fitchburg, WI) and BMG FLUOstar Omega where luminescent signal was quantified as relative light units (RLU) indicative of ATP concentration.
Chemical Screening
Chemicals were tested in a tiered approach to maximize resources and testing efficiency. Initial RAIU assays were performed as a single-concentration screen to identify potentially active samples for subsequent concentration-response test. Each of the 310 blinded samples was tested at 100μM with some exceptions owing to solubility (Table S1) in three independent RAIU assays using separate passages of hNIS-HEK293T-EPA cells (i.e., bioreplicates). Chemical samples were further tested in multi-concentration format if the median RAIU inhibition was greater than a pre-determined activity threshold (20% in this study, refer to the data analysis section). Multi-concentration tests were then performed in parallel RAIU and cell viability assays at six concentrations (obtained from 10-fold serial dilutions beginning with the maximum concentration, Table S1) in three independent runs (i.e. 3 bioreplicates). The assay plate maps for single and multi-concentration screening are provided in Figure S1.
Data analysis
Data analysis was performed in R (ver 3.3.0) and documented in R markdown (see SI).
Single-concentration screening analysis
RAIU assay raw readings were measured as counts per minute (CPM) and normalized per 96-well plate as the percent activity of the median DMSO control CPM value (n = 12). The median of normalized percent activity from the 3 bioreplicates for each chemical was used to determine whether the chemical would be further evaluated in multi-concentration testing. The median value was chosen over the mean value in the analysis for its resistance to the impact of extreme values or outliers. The activity threshold was set based on 3 times the baseline median absolute deviation (3bMAD) calculated using DMSO wells from all 15 assay plates in single-concentration screening. As 3bMAD was calculated to be 20.5%, the value for the activity inhibition threshold was rounded to 20% as a more conservative approach for moving chemicals forward for additional testing in the multi-concentration RAIU and cell viability assays.
Multi-concentration screening analysis
Multi-concentration RAIU and cell viability assay readings were quantified as CPM and relative light units (RLU), respectively. Both RAIU and cell viability assay raw readings were normalized the same way as in the single-concentration assay. Dose-response curves were fitted using the Hill model provided in U.S. EPA’s ToxCast Pipeline (tcpl v1.2.2) R package.39 Details of curve fitting and plotting are in SI.
Calculation of Z’, CV of DMSO, AC50, absEC50, Cytotox-point
To monitor the performance of the assay, quality control measures including Z’ scores, coefficients of variation (CV) of DMSO, and AC50 of positive controls (NaClO4 for RAIU, DCNQ for cell viability) were calculated for each 96-well assay plate. CV of DMSO was calculated by the equation: CV = SDDMSO/μDMSO, where SDDMSO and μDMSO are the standard deviation and mean of the raw response value for DMSO control wells, respectively. Z’ scores40 were calculated as: , where σpos and μpos are the standard deviation and mean of normalized positive control (NaClO4 for RAIU assay) values at the highest concentration (100 μM) tested. σDMSO and μDMSO are the standard deviation and mean of normalized DMSO control values. AC50 (the log concentration where the modeled activity equals 50% of the chemical’s modeled maximal activity) was reported for all chemicals with fitted dose-response curves. For chemicals that inhibited iodide uptake by >50%, absolute EC50 (absEC50) were also reported. absEC50 was determined as the log concentration where the modeled activity equals 50% of control activity. absEC80 was determined as the log concentration where the modeled activity equals 80% of control activity.
For chemicals that demonstrated significant cytotoxicity (exceeding 3bMAD of cell viability assay, 17.7%), the concentration where a significant reduction in cell viability for each chemical was determined and referred to as the cytotox-point. The cytotox-point is the equivalent of absEC82.3, the log concentration where the modeled activity equals the cutoff value for significant cytotoxicity (82.3% of control viability).
Chemical ranking score
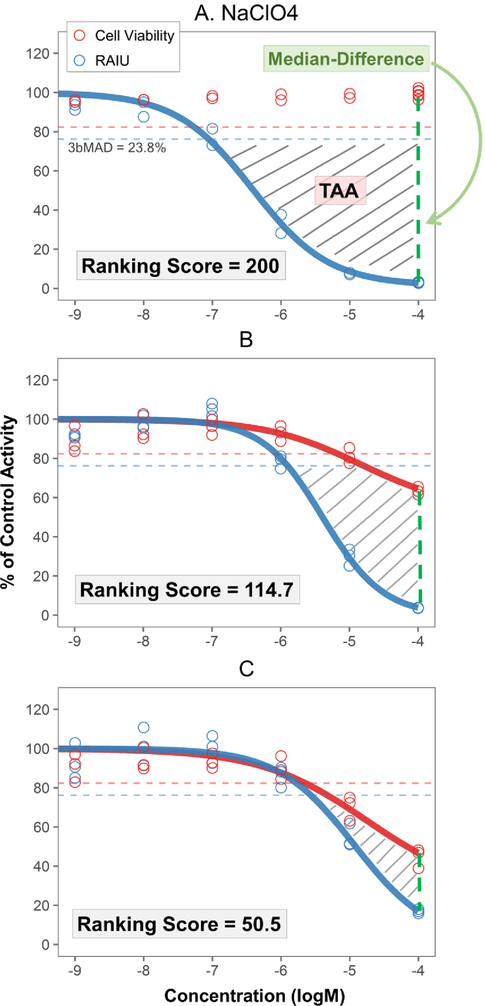
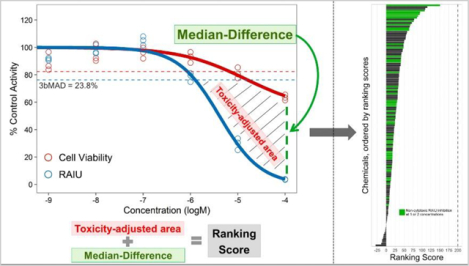
To prioritize the chemicals for potential NIS inhibition activity and further evaluation, a new scoring system was developed based on two metrics that take into account the confounding impact of cytotoxicity on identifying RAIU inhibition activity: 1) toxicity-adjusted area (TAA) and 2) the difference of median responses of RAIU and cell viability at maximum tested concentration (Median-Difference) (Figure1). Ranking analysis was only performed if a chemical produced significant RAIU inhibition in multi-concentration screening. To obtain TAA (gray stripe area illustrated in Figure 1), RAIU inhibition area and cytotoxicity area were first calculated. RAIU inhibition area was defined by the RAIU 3bMAD (23.8%) significant threshold horizontal line (top border), maximum concentration vertical line (right border), and the RAIU dose-response curve. Cytotoxicity area was defined with the same top and right borders and the cell viability dose-response curve. TAA was then obtained by subtracting the cytotoxicity area from the RAIU inhibition area. Therefore, the numeric value of TAA is penalized when a chemical demonstrates strong cytotoxicity. Median-Difference was calculated using the median of cell viability responses minus the median of RAIU responses at the maximum tested concentration (usually 100 μM). Larger Median-Difference values represent larger separations between RAIU and cell viability.
Figure 1. Demonstration of ranking score, TAA and Median-Difference.
Examples of chemical responses: A) sodium perchlorate, B) triphenyltin hydroxide, C) folpet. Cell viability and RAIU assay results are illustrated in red and blue respectively. The red and blue horizontal dotted lines represent the 3bMAD threshold for cell viability and RAIU assay respectively. Toxicity-adjusted area (TAA) is the gray striped area defined by 1) maximum concentration vertical line (right border), 2) the 3bMAD significant threshold horizontal line for RAIU assay (top border; blue dashed line), 3) the dose-response curve of RAIU and 4) if present, the dose-response curve of cell viability. The difference of median responses of RAIU and cell viability at maximum tested concentration (Median-Difference) is indicated by the green vertical dotted line. Sodium perchlorate (A) was chosen as the reference NIS inhibitor to normalize test chemical TAA and Median-Difference as percentage values. Ranking score is obtained as the sum of normalized TAA and Median-Difference values. Therefore, sodium perchlorate has a ranking score of 200. B and C show three test chemicals with less RAIU inhibition and more cytotoxicity relative to sodium perchlorate. As the TAA and Median-Difference values decrease, their ranking scores also decrease.
To rank test chemicals, the well-documented NIS inhibitor, NaClO4, was chosen as the reference chemical to normalize the TAA and Median-Difference of each test chemical. Specifically, the TAA and Median-Difference values of the NaClO4 positive control included on each of the 54 multi-concentration testing plates were first calculated to obtain the median of NaClO4 TAA and Median-Difference (150.03 and 95.67 respectively). The TAA and Median-Difference of test chemicals were normalized as the percentage of the median NaClO4 TAA and Median-Difference separately and then summed to obtain a chemical ranking score. The ranking score of 200 represents the potency of the reference NaClO4 (Figure 1A). Figure 1B and 1C show the dose-response of two test chemicals with lower level ranking scores due to less RAIU inhibition and increased cytotoxicity level.
Functions for the calculation of the ranking score, TAA, and Median-Difference, along with dose-response modeling and visualization are made available in the R package ‘toxplot’ (https://cran.r-project.org/package=toxplot).
Results and Discussion
Assay performance and quality control
Performance of the RAIU and cell viability assays was monitored for each assay plate through CV of DMSO vehicle control, Z’ and AC50 for the positive control chemicals NaClO4/DCNQ (Table S3). Fifteen plates in single-concentration RAIU assay and 54 plates were used in multi-concentration RAIU and cell viability assays, respectively. Both RAIU and cell viability assays performed well with excellent dynamic range, reproducibility, and reliability during both single and multi-concentration screening. CV of DMSO were ≤ 11.5% and the standard deviation of control AC50 were ≤0.13 (logM). The Z’ scores were also consistently above 0.64 in RAIU screening. Additional controls including NaNO3, NaSCN, and 2,4-D (RAIU assay EC80, EC20 and negative controls) also showed great consistency with expected responses in the RAIU and cell viability assays with small variances across the entire screening (Table S4).
The phase I_v2 chemical library consisted of 310 blinded samples that included 293 unique chemicals. Twelve of these chemicals were internally replicated (7 chemicals replicated twice and 5 chemicals replicated three times) to assess assay reproducibility. The robustness of the RAIU assay is shown in Figure S2a, where all 12 replicated chemicals, excluding bisphenol A, produced highly reproducible results. Of the 12 replicated chemicals, 10 exceeded the 20% inhibition threshold and were subsequently tested in multi-concentration screening. The AC50 for each replicate of the 10 chemicals was calculated to assess the reproducibility of the RAIU assay in the multi-concentration setting (Figure S2b, Table S5). These 10 internal replicate samples had highly reproducible AC50 (logM) values with the maximum variation range less than 0.25 (logM).
Single-concentration screening
To facilitate the screening process, the 310 blinded samples were first tested in the RAIU assay at their maximum permissible concentration (typically 100μM, Table S1) to select potentially active compounds for multi-concentration evaluation. Single-concentration RAIU screening results for all samples were ordered by increasing inhibitory median responses and plotted as median and maximum/minimum responses for each sample (Figure S3). These samples demonstrated a wide range of iodide uptake inhibition activity in the assay with median responses ranging from 2.8% to 116.6% of maximum iodide uptake relative to the DMSO control. Of the 310 samples, 169 samples (54.5%) produced over the 20% inhibition threshold and were subjected to multi-concentration testing (Table S1).
All chemical samples were also tested with Sandell-Kolthoff reactions41 to determine the presence of any contaminant iodide that may lead to potential false positive RAIU inhibition (see SI Part I). Only two chemicals (iodosulfuron-methyl-sodium and 3-iodo-2-propynyl-N-butylcarbamate) produced positive SK reactions, suggesting that overall iodide contamination was not introducing false positive results in the RAIU assay.
Multi-concentration testing
The 169 blinded chemical samples with ≥20% inhibition in single-concentration RAIU screening were further tested at 6 concentrations (0.001μM – 100μM) in parallel RAIU and cell viability assays. Dose-response curves for test chemicals and positive controls (NaClO4 and DCNQ) are available in SI. The significant activity threshold (3bMAD, calculated across all 54 assay plates in multi-concentration screening) for the cell viability and RAIU assays were 17.7% and 23.8%, respectively. Among the 169 tested samples, 137 showed significant RAIU inhibition activity (>23.8% inhibition). Within the 137 samples with significant RAIU inhibition activity, 26 samples exhibited no change in cell viability, while 111 displayed a cytotoxic response at one or more concentrations tested.
In reporting the RAIU and cell viability responses of each test chemical, several activity metrics, including AC50, absEC50, and cytotox-point (Table 1 and Table S1), were used together to aid in the interpretation of the assay results. AC50 and absEC50 indicate the chemical potency from two different perspectives, as they differ in the inhibition activity level each represents. absEC50 is the concentration that causes 50% inhibition, while AC50 is the concentration that triggers half-maximal inhibition, which is <50% inhibition for chemicals that do not fully inhibit NIS activity at the maximal concentration tested. AC50 is a useful metric to rank potencies even if the test chemical has low efficacy, provided an activity curve can be fit. However, AC50 is not suitable to compare chemicals with different maximum inhibition activities, as their AC50 values represent the effective concentrations at different absolute activity levels. Moreover, the AC50 value of any given chemical may shift if it can produce stronger inhibition at higher test concentrations. To avoid these two issues, absEC50 was also reported. A reported absEC50 value indicates that the chemical exhibited ≥50% inhibition in the RAIU assay. For quick reference for the occurrence of cytotoxicity, the cytotox-point, which represents the concentration where significant cytotoxicity was observed, was reported along with the AC50 and absEC50 values. AC50 and absEC50 values for cell viability assay are provided in Table S1.
Table 1.
Results for the top 20 ranked chemical samples that demonstrated significant RAIU inhibition in multi-concentration screening
| Chemical | CAS NO. | Max Conc(M)a | AC50 | absEC50 | Cytotox-pointb | Ranking Score | Non-cytotoxic RAIU inhibition at 1 or 2 concentrationsc | |
|---|---|---|---|---|---|---|---|---|
| 1 | Etoxazole | 153233-91-1 | 1.00E-04 | −5.92 | −5.88 | −4.32 | 150.41 | + |
| 2 | Triphenyltin hydroxide | 76-87-9 | 1.00E-04 | −5.39 | −5.39 | −5.17 | 114.70 | − |
| 3 | Niclosamide | 50-65-7 | 5.00E-05 | −6.95 | −6.79 | −6.91 | 109.38 | − |
| 4 | 3-Iodo-2-propynyl-N-butylcarbamate | 55406-53-6 | 1.00E-04 | −5.09 | −5.30 | −4.80 | 107.61 | + |
| 5 | PFOS | 1763-23-1 | 8.00E-05 | −4.75 | −4.78 | −4.09 | 94.75 | + |
| 6 | PFOS | 1763-23-1 | 8.00E-05 | −4.72 | −4.74 | NA | 93.63 | + |
| 7 | Cyprodinil | 121552-61-2 | 1.00E-04 | −4.43 | −4.43 | NA | 89.12 | + |
| 8 | Rotenone | 83-79-4 | 1.00E-04 | −7.15 | −6.22 | −7.30 | 86.15 | + |
| 9 | Pyridaben | 96489-71-3 | 1.00E-04 | −8.64 | −7.49 | −7.60 | 78.17 | − |
| 10 | Methoxyfenozide | 161050-58-4 | 1.00E-04 | −4.78 | −4.72 | −4.68 | 76.97 | + |
| 11 | 2-(Thiocyanomethylthio)benzothiazole | 21564-17-0 | 1.00E-04 | −4.33 | −4.37 | −4.91 | 71.48 | − |
| 12 | Oxyfluorfen | 42874-03-3 | 1.00E-04 | −4.55 | −4.39 | NA | 68.27 | + |
| 13 | Captan | 133-06-2 | 9.50E-05 | −4.49 | −4.50 | −4.81 | 65.79 | − |
| 14 | Fipronil | 120068-37-3 | 1.00E-04 | −4.57 | −4.63 | −4.92 | 64.32 | + |
| 15 | Fluroxypyr-meptyl | 81406-37-3 | 1.00E-04 | −4.77 | −4.74 | −4.74 | 64.10 | + |
| 16 | Cyhalofop-butyl | 122008-85-9 | 9.50E-05 | −5.47 | −4.75 | −5.07 | 58.47 | − |
| 17 | Fenpyroximate (Z,E) | 111812-58-9 | 1.00E-04 | −6.79 | −6.07 | −7.09 | 57.34 | − |
| 18 | Thiobencarb | 28249-77-6 | 1.00E-04 | −4.38 | −4.34 | −4.70 | 55.20 | − |
| 19 | Emamectin benzoate | 155569-91-8 | 1.00E-04 | −4.88 | −4.99 | −5.18 | 54.82 | − |
| 20 | Diphenylamine | 122-39-4 | 1.00E-04 | −4.74 | −4.28 | −5.59 | 54.01 | − |
Results for all test chemicals are in Table S1.
Max Conc: the maximum permissible concentration tested in single-concentration screening. The Max Conc was obtained by 200X dilution of the supplied stock chemicals (concentrations ≤ 20mM). Serial dilution of samples for multi-concentration assay started with the Max Conc.
Cytotox-point: the log concentration where the chemical started to show significant toxicity (absEC82.3). NA: not available, as no significant cytotoxicity was observed.
Non-cytotoxic RAIU inhibition at 1 or 2 concentrations: show chemicals that have significant RAIU inhibition without significant cytotoxicity at one or two concentrations tested in multi-concentration screening. +: yes, -: no.
Chemical potency ranking
An important goal of this HTS study was to identify candidates for further investigations as NIS inhibitors. As estimating chemical RAIU inhibition potency using a cell-based assay without knowing cytotoxicity can cause high false positive rate, parallel cell viability assays were conducted using the same batch of cells, treatments, and experimental conditions as the RAIU assays to provide the best possible estimation of cytotoxicity. A novel scoring system was developed to maximize usage of the rigorous cytotoxicity data and rank potential NIS inhibitors for further analysis (Figure 1). Of the 169 chemical samples tested in multi-concentration, 32 did not produce significant RAIU inhibition and hence were excluded in the ranking analysis. The ranking scores of the remaining 137 chemical samples, along with the TAA and Median-Difference values are provided in Table S1. The results of the top 20 ranked chemicals are listed in Table 1.
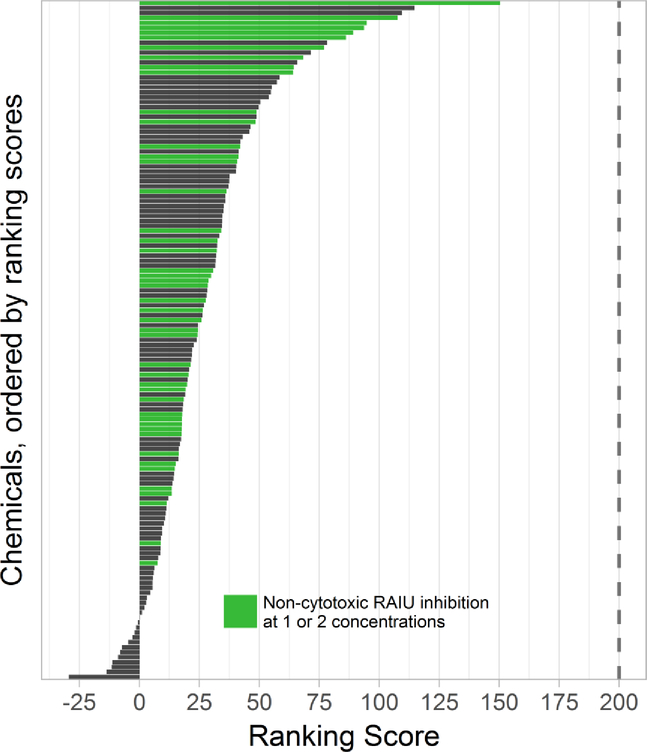
The 137 test chemical samples demonstrated various levels of responses with ranking scores that ranged from 150.41 to −29.52 (Figure 2), much lower than the reference chemical NaClO4, which received a ranking score of 200 (both TAA and Median-Difference valued at 100). Most chemicals received low ranking scores, with only 4 had ranking scores >100, 17 had ranking scores between 50 and 100, and 116 had ranking scores <50. In comparison, several other reference NIS inhibitors including KPF6, KClO4, NaBF4, and NaSCN previously tested in the same assays16 were reanalyzed using the same approach and received ranking scores of 225.8, 213.6, 211.5, 172.2 and 104.3, respectively (see SI Part V). All chemicals tested in this study demonstrated lower potency ranking than these reference chemicals except NaSCN. The rank order for these reference chemicals (KPF6>KClO4>NaClO4>NaBF4>NaSCN) is also in full agreement with previously reported potencies.16
Figure 2. Ranking scores for 137 chemical samples that produced significant RAIU inhibition in multi-concentration screening.
The vertical dotted line is the ranking score of sodium perchlorate, which was used as the reference chemical in the ranking analysis. The ranking score of the 137 test chemical samples ranged from 150.41 to −29.52, with 4 having ranking score >100, 17 having ranking score between 50 and 100, and 116 having ranking score <50. The 46 chemicals that produced significant RAIU inhibition without any significant cytotoxicity at one or two concentrations are marked in green.
Cytotoxicity information is critical for interpreting the observed inhibition in the RAIU assay. A traditional toxicological approach would eliminate testing results for all concentrations with significant cytotoxicity. If using this approach, 46 chemicals in this study produced significant RAIU inhibition without significant cytotoxicity at one or two concentrations (Table 1, S1 and Figure 2). Among the 46 chemicals, 5 of them produced >50% RAIU inhibition at concentrations where no cytotoxicity was observed. However, simply excluding RAIU data points where significant cytotoxicity is observed may also cause high false negative rate that is undesirable in HTS, especially for chemicals that only produced borderline cytotoxicity. Although it is impossible to completely dissociate RAIU and cytotoxic activities, a chemical with stronger cytotoxicity at lower concentrations is less likely to be an RAIU inhibitor than one with less or no cytotoxicity, given the same level of observed RAIU inhibition. The new ranking system was designed with this rationale to provide a continuous metric that adjusts cytotoxic chemicals without completely excluding them, thereby reducing the chance of false negatives. For example, a chemical such as triphenyltin hydroxide (Figure 3a) that had significant and strong RAIU inhibition but with low cytotoxicity (at 1E-5M and 1E-4M) will not only be included, but also receive a high ranking score.
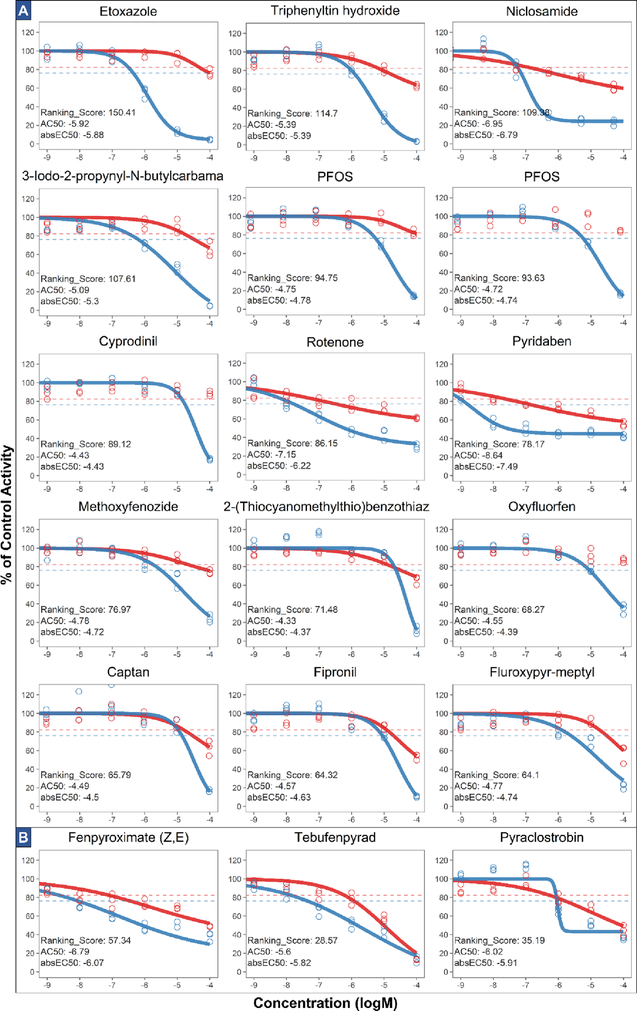
Figure 3. Dose response, ranking score, AC50 and absEC50 of (A) top 15 ranked samples (14 chemicals) and (B) chemicals that disrupt electron transport chain.
Chemical responses (for three bioreplicates) are shown as circles with red representing cell viability assay results and blue representing RAIU assay results. The red and blue horizontal dotted lines represent the 3bMAD threshold for cell viability and RAIU assay, respectively. Dose-response curve was drawn only if significant activity was present. AC50 and absEC50 are expressed as logM.
As described previously, the ranking score incorporates cytotoxicity responses when calculating the two underlying metrics, TAA and Median-Difference. Unlike other single-point metrics such as AC50, the TAA incorporates levels of RAIU inhibition and cytotoxicity at all tested concentrations and the shape of the dose-response curves. The addition of Median-Difference as a component in the ranking score further promotes chemicals with high RAIU inhibition and low cytotoxicity at the highest concentration so that chemicals like PFOS, oxyfluorfen, and cypronidil (Figure 3a) were ranked higher than using TAA alone. These features of the ranking system enhance the quality of chemical prioritization and it produced a vastly different ranking compared to using the AC50 or absEC50 of RAIU response, as ranking scores had only moderate correlation with AC50 or absEC50 for the 137 chemicals (Spearman’s rank correlation, r = 0.46 and 0.41, p <0.01). Since the two underline metrics (TAA and Median-Difference) were normalized in reference to the well-documented NIS inhibitor NaClO4, the ranking score is compatible for comparisons with future NIS inhibitor screenings. As the ranking metrics calculation is publicly accessible in the accompanied R package ‘toxplot’, this ranking approach may also be extended for other HTS studies in which the primary assay produces inhibition of signals, such as the androgen/estrogen antagonist assays42, 43 and steroidogenesis inhibition assay44. In such applications, a well-known active compound could be chosen as the normalization reference, and TAA alone or the ranking score could be used selectively.
Based on the ranking, selected test chemicals that demonstrated strong or representative responses are shown in Figure 3. The highest ranked chemical, etoxazole is an organofluorine acaricide-insecticide that displayed a typical sigmoidal RAIU inhibition response and minimal cytotoxicity (25.2%) at 100μM. The U.S. EPA ToxRef database45, a collection of curated in vivo regulatory toxicity information previously submitted to the agency, reveals that etoxazole increased thyroid gland weight in both subchronic and chronic rat oral toxicity studies. Our finding is the first to show that etoxazole disrupts NIS-mediated iodide uptake. However, whether etoxazole can directly interact with the NIS protein requires further investigation, as a study on fish Oreochromis niloticus found that etoxazole exposure at high concentration led to an increase in Na+/K+ ATPase activities in gill and muscle and a decrease of this activity in kidney.46 Since Na+/K+ ATPase maintains the critical cross-membrane sodium gradient necessary for NIS functionality, disruption of Na+/K+ ATPase activity can indirectly affect NIS activity. Another high-ranking chemical, niclosamide, may also have interfered with NIS function indirectly, as it is a pharmaceutical that uncouples oxidative phosphorylation in mitochondria47, thereby disrupting ATP production and hence Na+/K+ ATPase activity. To date, there is no report of niclosamide regarding thyroid toxicity, except its anti-proliferative effect in human thyroid cancer cells.48
Five insecticides in this study have been shown to disrupt the electron transport chain and demonstrated similar atypical RAIU dose-response curves that may implicate an alternative NIS inhibition mechanism. These chemicals include rotenone and pyridaben (Figure 3a), which are both mitochondrial complex-I inhibitors that cause reduced ATP production.49 Rotenone can inhibit iodide uptake in sheep thyroid slices50, and pyridaben has been associated with reduced thyroid gland weight in a subchronic rat study45. Three lower ranked chemicals (Figure 3b) including fenpyroximate, tebufenpyrad (mitochondrial NADH-CoQ reductase inhibitors), and pyraclostrobin (quinone inhibitors) may also similarly disrupt ATP production.51–53 All of these chemicals share similar atypical dose-responses in the RAIU assay with flat slopes and produced significant effects on cell viability. Since the Cell-Titer Glo viability assay quantifies ATP as an indicator of viable cells, a follow-up assay that measures another endpoint of cytotoxicity could differentiate between energy metabolism disruption and other mechanisms of cell death.
Other top-ranking chemicals including PFOS, triphenyltin hydroxide, fipronil and captan have been shown to disrupt TH levels in previous studies and had significant effects on iodide uptake by NIS in this screening. Among these four chemicals, PFOS has been the most studied due to its widespread presence and persistence in the environment. PFOS was reported to decrease serum T4 levels in rat, mouse, and monkey.54–56 Aquatic exposure to PFOS has also been shown to increase T3 level, upregulate NIS expression, and alter expression of other HPT genes in zebrafish larvae.57 In addition, higher concentrations of serum PFOA and PFOS have been associated with current thyroid disease in the U.S. adult population.58 In this study, both PFOS samples produced significant RAIU inhibition with similar dose-responses (Figure 3a) and absEC50 values (−4.78 and −4.74). Triphenyltin hydroxide is a fungicide that can cause reduced thyroid weight in rats59 and decreased serum T4 levels in Japanese quail60 through dietary exposure. In our study, triphenyltin hydroxide produced a typical RAIU inhibition dose-response (absEC50: −5.39) with moderate cytotoxicity at 100μM. Fipronil is a broadly used insecticide that has been associated with elevated rates of T4 elimination in the rat, and increased hepatic enzyme activity was suggested to be the cause.61 A follow-up fipronil study in sheep found that the thyroid disruption activity previously observed in the rat could not be replicated in rams, and only a moderate level of increased T4 clearance was observed in ewes.62 Here, fipronil demonstrated significant RAIU inhibition between 10μM and 100μM and significant cytotoxicity at 100μM. Captan showed strong RAIU inhibition only at 100μM with moderate cytotoxicity in this study, but it has been shown to cause does-dependent decrease of serum T4 levels in felmale pubertal rats assay, with significant thyroid histological changes observed at the highest dose.37
A few top-ranking chemicals in Figure 3a were only associated with thyroid disruption activity in the U.S. EPA ToxRef database, but no other published literature. 3-iodo-2-propynyl-N-butylcarbamate, cyprodinil, and methoxyfenozide were associated with pathological changes in thyroid gland in subchronic rat toxicity studies.45 Oxyfluorfen was report with increased thyroid gland weight in chronic rat toxicity studies.45 Fluroxypyr-meptyl and 2-(thiocyanomethylthio)benzothiazole were not previously associated with thyroid disruption, though similarly structured benzothiozoles have been identified as thyroid peroxidase inhibitors.11
Although some of the top-ranking chemicals have been shown to disrupt thyroid hormones in the literature, linking NIS inhibition activity to in vivo thyroid effects still requires further investigation. First, a putative NIS inhibitor could also interfere other molecular targets of the thyroid hormone pathways. Also, considerations of absorption, distribution, metabolism, excretion (ADME) as well as potential compensatory feedback are needed before assuming that a chemical would alter thyroid hormone synthesis in vivo. For these reasons, additional targeted studies using short-term in vivo assays, such as those that employ amphibian or weanling rat models, are warranted to investigate the thyroid-disruptive potential. Overall, the findings reported here confirm that the cell-based screening can be effectively used to identify chemicals that directly compete with iodide for intracellular transport through the symporter, as well as those chemicals that alter NIS function by disruption of energy production required to maintain the sodium gradient. Because of the likely alternative RAIU inhibition mechanisms among these chemicals, close examination of the dose-response is suggested rather than relying on a single metric such as the AC50 or absEC50 to draw conclusions regarding NIS inhibition. In addition, for high ranking chemicals that produced borderline cytotoxicity, additional testing is recommended. For example, retesting potential inhibitors in a secondary assay such as the rat thyroid FRTL-5 cell line based RAIU assay would further confirm these HTS findings.
As public concern increases over the effects of environmental chemicals on thyroid hormone signaling, there is an urgent need to expand the current understanding of the risks of thyroid disruption by a broader range of compounds. Iodide uptake via NIS is the initial step of thyroid hormone biosynthesis and is a known target of thyroid disruption in humans and wildlife. As the first large-scale effort to investigate NIS as a molecular target of environmental chemicals, the screening of 293 ToxCast chemicals significantly expands the current knowledge of NIS inhibition. The new chemical ranking approach fulfills the need for chemical prioritization in large-scale HTS assays and will be deployed for the imminent screening of the ToxCast phase II and e1K chemical libraries for putative NIS inhibitors. Moreover, these data provide an integral component of the U.S.EPA’s EDSP initiative to expand the coverage of known molecular targets of chemical-induced thyroid disruption, and can support the development of OECD test guidelines38 to assess chemicals for thyroid disrupting activity.
Supplementary Material
Acknowledgements
This research was funded by the Office of Research and Development, U.S. EPA, Washington, DC. The authors thank U.S. EPA scientists Drs. Ann Richard and Katherine Coutros for their assistance with obtaining the ToxCast chemical library, and Dr. Michael Hornung, Dr. William Mundy, and Dr. Katie Paul-Friedman for their scientific and editorial contributions to this manuscript.
Footnotes
DISCLAIMER: The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency.
Supporting Information Available
The SI file contains six sections. Part I: Supplemental Materials and Methods. Part II: Figure S1–S3, Table S1–S5. Part III: Dose-response of all chemicals tested in multi-concentration with plots ordered by ranking scores. Part IV: Dose-response of sodium perchlorate and DCNQ positive controls in multi-concentration screening. Part V: Dose-response of reanalyzed reference chemicals. Part VI: Documented data analysis in R markdown. This information is available free of charge via the Internet at http://pubs.acs.org.
Reference
- 1.Taylor PN; Razvi S; Pearce SH; Dayan CM, A Review of the Clinical Consequences of Variation in Thyroid Function Within the Reference Range. J. Clin. Endocrinol. Metab 2013, 98, (9), 3562–3571. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert ME; Sanchez-Huerta K; Wood C, Mild Thyroid Hormone Insufficiency During Development Compromises Activity-Dependent Neuroplasticity in the Hippocampus of Adult Male Rats. Endocrinology 2016, 157, (2), 774–787. [DOI] [PubMed] [Google Scholar]
- 3.Moog NK; Entringer S; Heim C; Wadhwa PD; Kathmann N; Buss C, Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 2017, 342, 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boas M; Feldt-Rasmussen U; Main KM, Thyroid effects of endocrine disrupting chemicals. Mol. Cell. Endocrinol 2012, 355, (2), 240–248. [DOI] [PubMed] [Google Scholar]
- 5.DeVito M; Biegel L; Brouwer A; Brown S; Brucker-Davis F; Cheek AO; Christensen R; Colborn T; Cooke P; Crissman J; Crofton K; Doerge D; Gray E; Hauser P; Hurley P; Kohn M; Lazar J; McMaster S; McClain M; McConnell E; Meier C; Miller R; Tietge J; Tyl R, Screening Methods for Thyroid Hormone Disruptors. Environ. Health Perspect 1999, 107, (5), 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brucker-Davis F, Effects of Environmental Synthetic Chemicals on Thyroid Function. Thyroid 1998, 8, (9), 827–856. [DOI] [PubMed] [Google Scholar]
- 7.Capen CC; Martin SL, The Effects of Xenobiotics on the Structure and Function of Thyroid Follicular and C-Cells. Toxicol. Pathol 1989, 17, (2), 266–293. [DOI] [PubMed] [Google Scholar]
- 8.Murk AJ; Rijntjes E; Blaauboer BJ; Clewell R; Crofton KM; Dingemans MML; David Furlow J; Kavlock R; Köhrle J; Opitz R; Traas T; Visser TJ; Xia M; Gutleb AC, Mechanism-based testing strategy using in vitro approaches for identification of thyroid hormone disrupting chemicals. Toxicol. In Vitro 2013, 27, (4), 1320–1346. [DOI] [PubMed] [Google Scholar]
- 9.Pearce EN; Andersson M; Zimmermann MB, Global Iodine Nutrition: Where Do We Stand in 2013? Thyroid 2013, 23, (5), 523–528. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari SM; Fallahi P; Antonelli A; Benvenga S, Environmental Issues in Thyroid Diseases. Front. Endocrinol. (Lausanne) 2017, 8, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung MW; Kosian PA; Haselman JT; Korte JJ; Challis K; Macherla C; Nevalainen E; Degitz SJ, In Vitro, Ex Vivo, and In Vivo Determination of Thyroid Hormone Modulating Activity of Benzothiazoles. Toxicol. Sci 2015, 146, (2), 254–264. [DOI] [PubMed] [Google Scholar]
- 12.Gore AC; Chappell VA; Fenton SE; Flaws JA; Nadal A; Prins GS; Toppari J; Zoeller RT, Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev 2015, 36, (6), 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jomaa B; Aarts J; de Haan LH; Peijnenburg A; Bovee TF; Murk AJ; Rietjens I, In vitro pituitary and thyroid cell proliferation assays and their relevance as alternatives to animal testing. Altex 2013, 30, (3), 293–307. [DOI] [PubMed] [Google Scholar]
- 14.Paul Friedman K; Watt ED; Hornung MW; Hedge JM; Judson RS; Crofton KM; Houck KA; Simmons SO, Tiered High-Throughput Screening Approach to Identify Thyroperoxidase Inhibitors Within the ToxCast Phase I and II Chemical Libraries. Toxicol. Sci 2016, 151, (1), 160–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul KB; Hedge JM; Rotroff DM; Hornung MW; Crofton KM; Simmons SO, Development of a Thyroperoxidase Inhibition Assay for High-Throughput Screening. Chem. Res. Toxicol 2014, 27, (3), 387–399. [DOI] [PubMed] [Google Scholar]
- 16.Hallinger DR; Murr AS; Buckalew AR; Simmons SO; Stoker TE; Laws SC, Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS). Toxicol. In Vitro 2017, 40, 66–78. [DOI] [PubMed] [Google Scholar]
- 17.Dai G; Levy O; Carrasco N, Cloning and characterization of the thyroid iodide transporter. Nature 1996, 379, (6564), 458–460. [DOI] [PubMed] [Google Scholar]
- 18.Levy O; De la Vieja A; Ginter CS; Riedel C; Dai G; Carrasco N, N-linked glycosylation of the thyroid Na+/I- symporter (NIS). Implications for its secondary structure model. J. Biol. Chem 1998, 273, (35), 22657–63. [DOI] [PubMed] [Google Scholar]
- 19.Darrouzet E; Lindenthal S; Marcellin D; Pellequer JL; Pourcher T, The sodium/iodide symporter: state of the art of its molecular characterization. Biochim. Biophys. Acta 2014, 1838, (1 Pt B), 244–53. [DOI] [PubMed] [Google Scholar]
- 20.Eskandari S; Loo DDF; Dai G; Levy O; Wright EM; Carrasco N, Thyroid Na+/I− Symporter: Mechanism, Stoichiometry, and Specificity. J. Biol. Chem 1997, 272, (43), 27230–27238. [DOI] [PubMed] [Google Scholar]
- 21.Carrasco N, Iodide transport in the thyroid gland. Biochim. Biophys. Acta, Rev. Biomembr 1993, 1154, (1), 65–82. [DOI] [PubMed] [Google Scholar]
- 22.Dohán O; De la Vieja A; Paroder V; Riedel C; Artani M; Reed M; Ginter CS; Carrasco, The Sodium/Iodide Symporter (NIS): Characterization, Regulation, and Medical Significance. Endocr. Rev 2003, 24, (1), 48–77. [DOI] [PubMed] [Google Scholar]
- 23.Pohlenz J; Refetoff S, Mutations in the sodium/iodide symporter (NIS) gene as a cause for iodide transport defects and congenital hypothyroidism. Biochimie 1999, 81, (5), 469–476. [DOI] [PubMed] [Google Scholar]
- 24.Dasgupta PK; Dyke JV; Kirk AB; Jackson WA, Perchlorate in the United States. Analysis of Relative Source Contributions to the Food Chain. Environ. Sci. Technol 2006, 40, (21), 6608–6614. [DOI] [PubMed] [Google Scholar]
- 25.Dyke JV; Ito K; Obitsu T; Hisamatsu Y; Dasgupta PK; Blount BC, Perchlorate in Dairy Milk. Comparison of Japan versus the United States. Environ. Sci. Technol 2007, 41, (1), 88–92. [DOI] [PubMed] [Google Scholar]
- 26.El Aribi H; Le Blanc YJC; Antonsen S; Sakuma T, Analysis of perchlorate in foods and beverages by ion chromatography coupled with tandem mass spectrometry (IC-ESI-MS/MS). Anal. Chim. Acta 2006, 567, (1), 39–47. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y; Zhang P; Wang Y; Shi J; Cai Y; Mou S; Jiang G, Perchlorate in sewage sludge, rice, bottled water and milk collected from different areas in China. Environ. Int 2007, 33, (7), 955–962. [DOI] [PubMed] [Google Scholar]
- 28.Kannan K; Praamsma ML; Oldi JF; Kunisue T; Sinha RK, Occurrence of perchlorate in drinking water, groundwater, surface water and human saliva from India. Chemosphere 2009, 76, (1), 22–26. [DOI] [PubMed] [Google Scholar]
- 29.Leung AM; Pearce EN; Braverman LE, Perchlorate, iodine and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab 2010, 24, (1), 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horton MK; Blount BC; Valentin-Blasini L; Wapner R; Whyatt R; Gennings C; Factor-Litvak P, CO-occurring exposure to perchlorate, nitrate and thiocyanate alters thyroid function in healthy pregnant women. Environ. Res 2015, 143, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greer MA; Goodman G; Pleus RC; Greer SE, Health effects assessment for environmental perchlorate contamination: the dose response for inhibition of thyroidal radioiodine uptake in humans. Environ. Health Perspect. 2002, 110, (9), 927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz-Santaliestra ME; Sparling DW, Alteration of Larval Development and Metamorphosis by Nitrate and Perchlorate in Southern Leopard Frogs (Rana sphenocephala). Arch. Environ. Contam. Toxicol 2007, 53, (4), 639–646. [DOI] [PubMed] [Google Scholar]
- 33.Stoker TE; Ferrell JM; Laws SC; Cooper RL; Buckalew A, Evaluation of ammonium perchlorate in the endocrine disruptor screening and testing program’s male pubertal protocol: Ability to detect effects on thyroid endpoints. Toxicology 2006, 228, (1), 58–65. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y; Beland FA; Fang J-L, Effect of triclosan, triclocarban, 2,2′,4,4′-tetrabromodiphenyl ether, and bisphenol A on the iodide uptake, thyroid peroxidase activity, and expression of genes involved in thyroid hormone synthesis. Toxicol. In Vitro 2016, 32, 310–319. [DOI] [PubMed] [Google Scholar]
- 35.Lecat-Guillet N; Merer G; Lopez R; Pourcher T; Rousseau B; Ambroise Y, Small-molecule inhibitors of sodium iodide symporter function. ChemBioChem 2008, 9, (6), 889–95. [DOI] [PubMed] [Google Scholar]
- 36.Richard AM; Judson RS; Houck KA; Grulke CM; Volarath P; Thillainadarajah I; Yang C; Rathman J; Martin MT; Wambaugh JF; Knudsen TB; Kancherla J; Mansouri K; Patlewicz G; Williams AJ; Little SB; Crofton KM; Thomas RS, ToxCast Chemical Landscape: Paving the Road to 21st Century Toxicology. Chem. Res. Toxicol 2016, 29, (8), 1225–1251. [DOI] [PubMed] [Google Scholar]
- 37.U.S.EPA, Endocrine Disruptor Screening Program Tier 1 screening determinations or weight of evidence assessments. In https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-tier-1-screening-determinations-and, 2015.
- 38.OECD, New Scoping Document on in vitro and ex vivo Assays for the Identification of Modulators of Thyroid Hormone Signalling. OECD Publishing: 2017. [Google Scholar]
- 39.Filer DL; Kothiya P; Setzer RW; Judson RS; Martin MT, tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics 2017, 33, (4), 618–620. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J-H; Chung TDY; Oldenburg KR, A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen 1999, 4, (2), 67–73. [DOI] [PubMed] [Google Scholar]
- 41.Sandell EB; Kolthoff IM, Chronometric catalytic method for the determination of micro quantities of iodine. J. Am. Chem. Soc 1934, 56, (6), 1426–1426. [Google Scholar]
- 42.OECD, Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists. OECD Publishing. [Google Scholar]
- 43.OECD, Test No. 458: Stably Transfected Human Androgen Receptor Transcriptional Activation Assay for Detection of Androgenic Agonist and Antagonist Activity of Chemicals. OECD Publishing. [Google Scholar]
- 44.OECD, Test No. 456: H295R Steroidogenesis Assay. OECD Publishing. [Google Scholar]
- 45.Martin M; Judson R; Reif D; Kavlock R; Dix D, Profiling chemicals based on chronic toxicity results from the US EPA ToxRef Database. Environ. Health Perspect 2009, 117, (3), 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Üner N; Oruç E; Sevgiler Y, Oxidative stress-related and ATPase effects of etoxazole in different tissues of Oreochromis niloticus. Environ. Toxicol. Pharmacol 2005, 20, (1), 99–106. [DOI] [PubMed] [Google Scholar]
- 47.Pearson RD; Hewlett EL, NIclosamide therapy for tapeworm infections. Ann. Intern. Med 1985, 102, (4), 550–551. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L; He M; Zhang Y; Nilubol N; Shen M; Kebebew E, Quantitative High-Throughput Drug Screening Identifies Novel Classes of Drugs with Anticancer Activity in Thyroid Cancer Cells: Opportunities for Repurposing. J. Clin. Endocrinol. Metab 2012, 97, (3), E319–E328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hollingworth RM; Ahammadsahib KI; Gadelhak G; McLaughlin JL, New Inhibitors of Complex-I of the Mitochondrial Electron-Transport Chain with Activity as Pesticides. Biochem. Soc. Trans 1994, 22, (1), 230–233. [DOI] [PubMed] [Google Scholar]
- 50.Tyler DD; Gonze J; Lamy F; Dumont JE, Influence of mitochondrial inhibitors on the respiration and energy-dependent uptake of iodide by thyroid slices. Biochem. J 1968, 106, (1), 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel JS; Gudmestad NC; Meinhardt S; Adhikari TB, Pyraclostrobin sensitivity of baseline and fungicide exposed isolates of Pyrenophora tritici-repentis. Crop Protect. 2012, 34, 37–41. [Google Scholar]
- 52.Motoba K; Suzuki T; Uchida M, Effect of a new acaricide, fenpyroximate, on energy metabolism and mitochondrial morphology in adult female Tetranychus urticae (two-spotted spider mite). Pestic. Biochem. Physiol 1992, 43, (1), 37–44. [Google Scholar]
- 53.Dekeyser MA, Acaricide mode of action. Pest Manage. Sci 2005, 61, (2), 103–110. [DOI] [PubMed] [Google Scholar]
- 54.Lau C; Thibodeaux JR; Hanson RG; Rogers JM; Grey BE; Stanton ME; Butenhoff JL; Stevenson LA, Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. II: Postnatal Evaluation. Toxicol. Sci 2003, 74, (2), 382–392. [DOI] [PubMed] [Google Scholar]
- 55.Thibodeaux JR; Hanson RG; Rogers JM; Grey BE; Barbee BD; Richards JH; Butenhoff JL; Stevenson LA; Lau C, Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. I: Maternal and Prenatal Evaluations. Toxicol. Sci 2003, 74, (2), 369–381. [DOI] [PubMed] [Google Scholar]
- 56.Seacat AM; Thomford PJ; Hansen KJ; Olsen GW; Case MT; Butenhoff JL, Subchronic Toxicity Studies on Perfluorooctanesulfonate Potassium Salt in Cynomolgus Monkeys. Toxicol. Sci 2002, 68, (1), 249–264. [DOI] [PubMed] [Google Scholar]
- 57.Shi X; Liu C; Wu G; Zhou B, Waterborne exposure to PFOS causes disruption of the hypothalamus–pituitary–thyroid axis in zebrafish larvae. Chemosphere 2009, 77, (7), 1010–1018. [DOI] [PubMed] [Google Scholar]
- 58.Melzer D; Rice N; Depledge MH; Henley WE; Galloway TS, Association between Serum Perfluorooctanoic Acid (PFOA) and Thyroid Disease in the U.S. National Health and Nutrition Examination Survey. Environ. Health Perspect 2010, 118, (5), 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Golub MS; Doherty JD, Triphenyltin as a potential human endocrine disruptor. J. Toxicol. Environ. Health B 2004, 7, (4), 281–295. [DOI] [PubMed] [Google Scholar]
- 60.Grote K; Niemann L; Gericke C; Selzsam B; Chahoud I, Effects of fentin hydroxide on reproduction of the Japanese quail (Coturnix coturnix japonica). Environ. Res 2006, 101, (1), 81–88. [DOI] [PubMed] [Google Scholar]
- 61.Leghait J; Gayrard V; Picard-Hagen N; Camp M; Perdu E; Toutain P-L; Viguié C, Fipronil-induced disruption of thyroid function in rats is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxicology 2009, 255, (1–2), 38–44. [DOI] [PubMed] [Google Scholar]
- 62.Leghait J; Gayrard V; Toutain P-L; Picard-Hagen N; Viguié C, Is the mechanisms of fipronil-induced thyroid disruption specific of the rat: Re-evaluation of fipronil thyroid toxicity in sheep? Toxicol. Lett 2010, 194, (3), 51–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.