Abstract
Voltage-gated calcium channels play a critical role in regulating the Ca2+ activity that mediates many aspects of neural development, including neural induction, neurotransmitter phenotype specification, and neurite outgrowth. Using Xenopus laevis embryos, we describe the spatial and temporal expression patterns during development of the 10 pore-forming alpha1 subunits that define the channels’ kinetic properties. In situ hybridization indicates that CaV1.2, CaV2.1, CaV2.2, and CaV3.2 are expressed during neurula stages throughout the neural tube. These, along with CaV1.3 and CaV2.3, beginning at early tail bud stages, and CaV3.1 at late tail bud stages, are detected in complex patterns within the brain and spinal cord through swimming tadpole stages. Additional expression of various alpha1 subunits was observed in the cranial ganglia, retina, olfactory epithelium, pineal gland, and heart. The unique expression patterns for the different alpha1 subunits suggests they are under precise spatial and temporal regulation and are serving specific functions during embryonic development.
Keywords: Xenopus, calcium channel, alpha1 subunit, CaV1.1, CaV1.2, CaV1.3, CaV1.4, CaV2.1, CaV2.2, CaV2.3, CaV3.1, CaV3.2, CaV3.3, nervous system, embryo
INTRODUCTION
Changes in intracellular Ca2+ are responsible for regulating a diverse array of cellular and physiological processes, including release of neurotransmitter and hormone, muscle contraction, chemotaxis, pacemaker activity, synapse formation, and gene expression (Perez-Reyes and Schneider, 1995; Yamakage and Namiki, 2002; Catterall et al., 2005; McKeown et al., 2006; Calin-Jageman and Lee, 2008). While the role of Ca2+ fluctuations and their underlying molecular mechanisms are well characterized in mature cells, Ca2+ activity plays an equally important role during development (Borodinsky and Spitzer, 2006; Webb and Miller, 2006). During early embryogenesis, Ca2+ fluxes are essential for coordinating cellular events following fertilization (Horner and Wolfner, 2008) and have also been implicated in the regulation of cell division (Li et al., 2008), convergent-extension (Wallingford et al., 2001), and dorsoventral patterning of the mesoderm (Palma et al., 2001). Ca2+ activity also regulates virtually every phase of neural development, including the induction of the nervous system (Leclerc et al., 2006; Webb and Miller, 2006; Moreau et al., 2008), maturation of the potassium current and other electrical properties of the cell (Spitzer and Ribera, 1998; Spitzer et al., 2002), and neurite outgrowth (Conklin et al., 2005). Recent work has shown that the specification of neurotransmitter phenotype is associated with Ca2+ activity, with higher frequencies of Ca2+ spiking during a critical developmental period linked to the specification of inhibitory phenotypes and lower levels of spiking resulting in excitatory phenotypes (Borodinsky et al., 2004; Spitzer, 2006; Root et al., 2008).
The molecular mechanisms governing Ca2+ concentrations within cells include a wide array of both intracellular and extracellular channels, pumps, and sensors; the superfamily of voltage-gated calcium channels (VGCCs) plays a particularly critical role in regulating intracellular Ca2+ levels both temporally and spatially (Catterall et al., 2005; Kisilevsky and Zamponi, 2008). VGCCs consist of an α1 subunit, which contains the major pore-forming region and voltage sensor and largely determines the channel’s physiological characteristics, as well as auxiliary α2-δ, β, and γ subunits which are able to modulate the channel’s properties (Catterall, 2000; Obermair et al., 2008). In vertebrates, 10 different α1 subunits have been identified along with 4 α2-δ subunits, 4 β subunits, and 2 γ subunits. The expression patterns of many of these subunits have been studied in the adult due to their critical role in regulating the physiology of excitable cells and their growing association with several pathologies (McKeown et al., 2006). Despite the clear importance of the role of VGCCs during development (Spitzer et al., 2005; Leclerc et al., 2006), the expression and function of these channels during embryogenesis is less well investigated. While there are detailed analyses of the β subunits in zebrafish (Zhou et al., 2006, 2008), there are only limited data on selected α1 subunits in zebrafish (Sanhueza et al., 2009) and amphibians (Palma et al., 2001; Jimenez-Gonzalez et al., 2003; Zhou et al., 2006, 2008). In mammals, there are reports describing expression of specific channels in defined tissues, usually for the later stages of embryogenesis (Son et al., 2002; Xu et al., 2003; Niwa et al., 2004). However, there is currently is no comprehensive description of the α1 subunits during vertebrate embryogenesis.
Here, we have chosen to examine the expression patterns of the 10 α1 sub-units during embryogenesis in Xenopus laevis. The choice of Xenopus is particularly suitable given the large size of the eggs, which are accessible at early developmental stages, the ability to conduct embryological manipulations and transgenesis, and the sequenced genome of X. tropicalis. Perhaps most importantly, many of the roles for Ca2+ activity during embryonic development (e.g., activity dependent neurotransmitter phenotype specification) were first reported in X. laevis and continue to be widely studied in this species. Here, we show that the α1 subunits are expressed in complex, unique, and dynamic patterns that are tightly regulated both temporally and spatially, suggesting specific roles for these subunits during vertebrate embryogenesis.
RESULTS
Voltage-Gated Calcium Channel α1 Subunit Sequences
Ten different VGCC α1 subunits corresponding to CaV1.1 (GenBank accession no. GQ120633), CaV1.2 (GQ120626), CaV1.3 (GQ120627), CaV1.4 (GQ120629), CaV2.1 (GQ120624), CaV2.2 (GQ120625), CaV2.3 (GQ120628), CaV3.1 (GQ120630), CaV3.2 (GQ120631), and CaV3.3 (GQ120632) were cloned from X. laevis cDNA using reverse transcriptase-polymerase chain reaction (RT-PCR). In all cases, BLAST N and BLAST X searches confirmed the identity of the cloned subunit with E values consistently less than 6.0−25 for BLAST N and 5.0−51 for BLAST X.
Early Expression of CaV1.1, CaV1.2, CaV2.1, CaV2.2, and CaV3.2
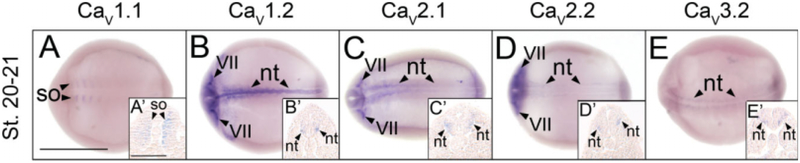
The first subunits to be detected in the developing X. laevis embryo are CaV1.1, CaV1.2, CaV2.1, CaV2.2, and CaV3.2. Transcripts for these subunits are observed beginning at neurula stages. With the exception of CaV1.1, which is expressed in the somites (Fig. 1A,A′), these subunits are all expressed in the neural tube (Fig. 1B–E,B′–E′). Transcripts for CaV1.2, CaV2.1, and CaV2.2 are also detected in cranial placode VII (Fig. 1B–D).
Fig. 1.
Spatial expression patterns of VGCC α1 subunits in stage 20–21 (neurula) Xenopus laevis embryos using whole-mount in situ hybridization. Embryos were viewed dorsally, with anterior to the left. A,A′: CaV1.1 expression. B,B′: CaV1.2 expression. C,C′: CaV2.1 expression. D,D′: CaV2.2 expression. E,E′: CaV3.2 expression. Arrowheads indicate regions of expression. nt, neural tube; so, somite; VII, cranial placode VII, geniculate (facialis) placode. Scale bar = 1.0 mm in A–E; 0.25 mm in A′–E′.
Expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 in the Forebrain
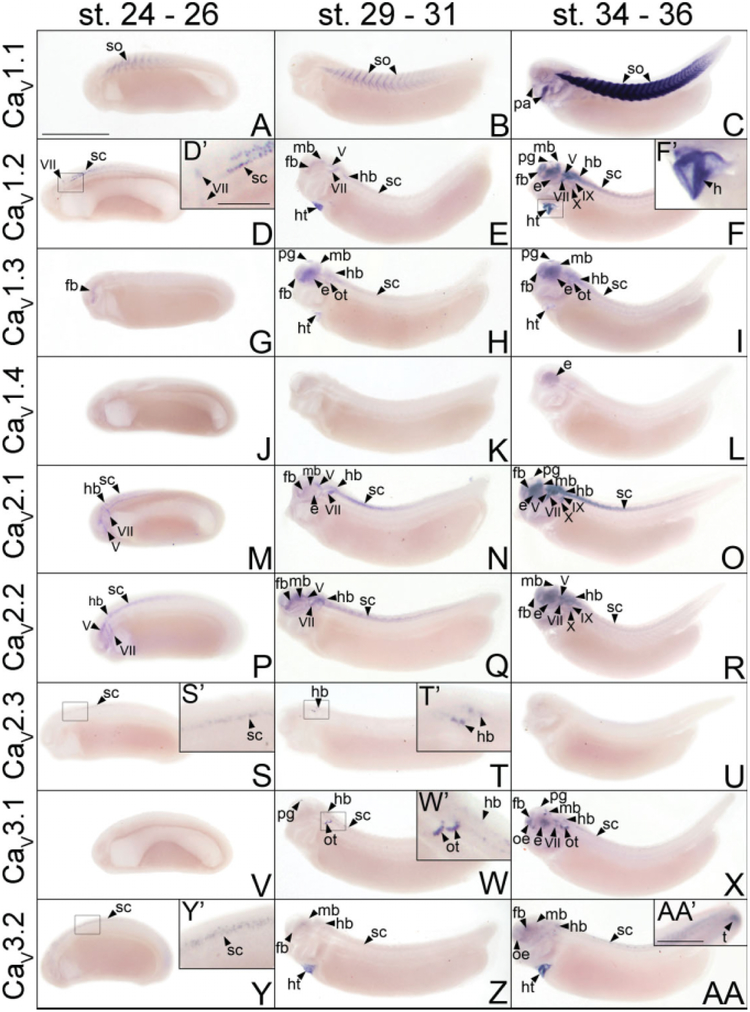
By swimming tadpole stages of development, the VGCC α1 subunits CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 are all expressed in discrete patterns throughout the forebrain. CaV1.3 is the first transcript to be detected at early tail bud stages in the diencephalon (Fig. 2G). This expression spreads to the telencephalon and pineal gland by late tail bud stages (Fig. 2H) and persists into swimming tadpole stages (Fig. 2I). Expression of CaV1.3 at these stages is most prominent in the pineal gland, but transcripts can be detected at low levels throughout the forebrain, particularly in the ventral domain (Fig. 3C).
Fig. 2.
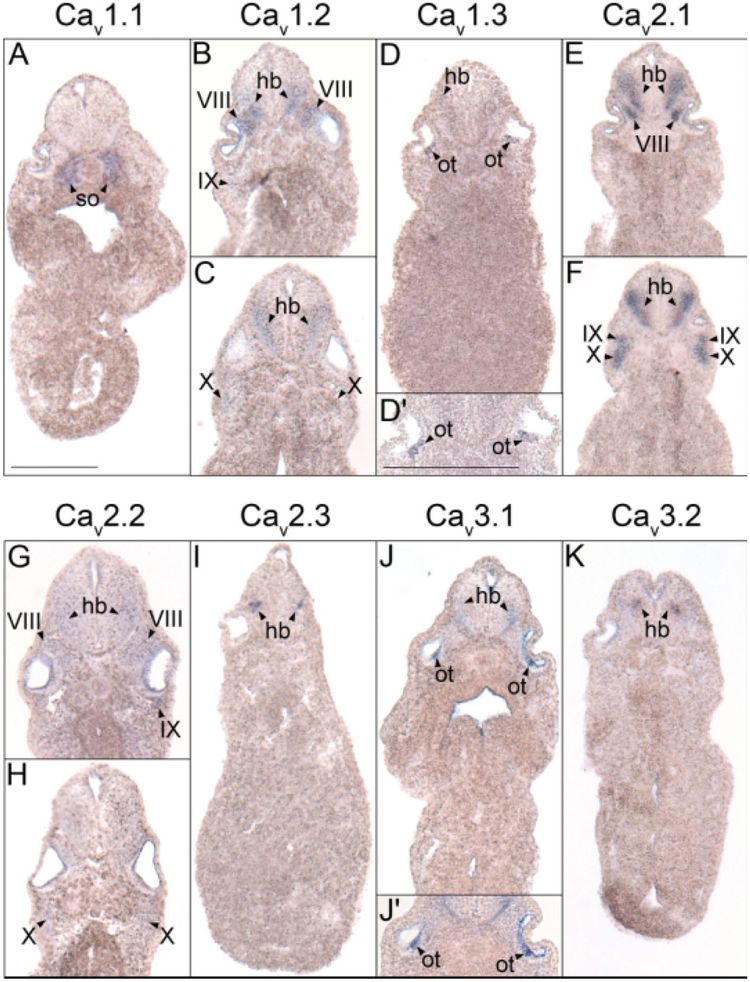
Spatial and temporal expression patterns of VGCC α1 subunits in developing X. laevis embryos using whole-mount in situ hybridization. Embryos were viewed laterally, with anterior to the left and dorsal to the top. A,D,G,J,M,P,S,V,Y: Stages 24–26 (early tail bud). B,E,H,K,N,Q,T,W,Z: Stages 29–31 (late tail bud). C,F,I,L,O,R,U,X,AA: Stages 34–36 (swimming tadpole). A–C: CaV1.1 expression. D–F: CaV1.2 expression. G–I: CaV1.3 expression. J–L: CaV1.4 expression. M–O: CaV2.1 expression. P–R: CaV2.2 expression. S–U: CaV2.3 expression. V–X: CaV3.1 expression. Y–AA: CaV3.2 expression. D′,F′,S′,T′,W′,Y′: Magnified images of portions of the embryos indicated by the hollow boxes. AA′: Magnified image of the tail of an embryo at swimming tadpole stage showing CaV3.2 expression. Arrowheads indicate regions of expression. fb, forebrain; e, eye; hb, hindbrain; ht, heart; mb, midbrain; oe, olfactory epithelium; ot, otic vesicle; pa, pharyngeal arches; pg, pineal gland; sc, spinal cord; so, somite; t, tail bud; V, cranial ganglion V, trigeminal ganglion; VII, cranial ganglion VII, geniculate (facialis) ganglion; IX, cranial ganglion IX, glossopharyngeal ganglion; X, cranial ganglion X, vagal ganglion. Scale bars = 1.0 mm in A–AA, 0.4 mm in AA′; 0.2 mm in D′,F′,S′,T′,W′,Y′.
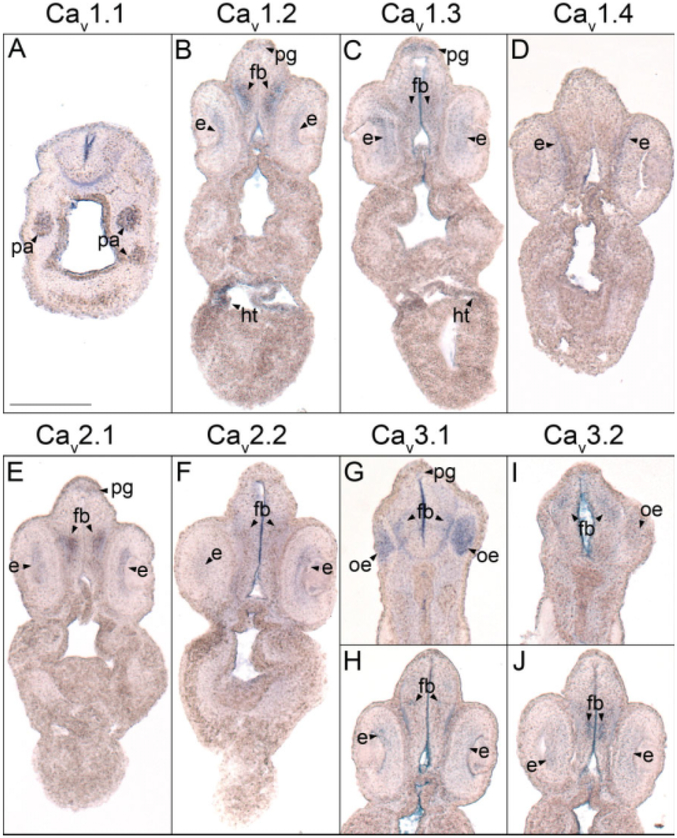
Fig. 3.
Forebrain histological analysis of voltage-gated calcium channel (VGCC) α1 subunit expression in stages 35–37 (swimming tadpole) Xenopus laevis using whole-mount in situ hybridization. A–J: Transverse 18-μm sections within the forebrain region of the anterior–posterior axis, with dorsal to the top. A: CaV1.1 expression. B: CaV1.2 expression. C: CaV1.3 expression. D: CaV1.4 expression. E: CaV2.1 expression. F: CaV2.2 expression. G,H: CaV3.1 expression. I,J: CaV3.2 expression. Arrowheads indicate regions of expression. fb, forebrain; e, eye; ht, heart; oe, olfactory epithelium; pa, pharyngeal arches; pg, pineal gland. Scale bar = 0.25 mm.
After the initial appearance of CaV1.3 expression, transcripts for CaV1.2, CaV2.1, and CaV3.2 are all detected in the diencephalon (Fig. 2E,N,Z) and CaV2.2 mRNA is observed in both the telencephalon and diencephalon by late tail bud stages of development (Fig. 2Q). Like CaV1.3, the expression domains of these α1 subunits each expand throughout the forebrain as development progresses to swimming tadpole stages (Fig. 2F,O,R,AA). (Swimming tadpole stages are defined as Nieuwkoop Faber stages 34–36, when the embryo exhibits spontaneous swimming movements and reflexively swims away from adverse stimuli). CaV1.2 and CaV2.1 transcripts also appear in the pineal gland, albeit at lower levels than CaV1.3 (Figs. 2F,O, 3B,E). Within the forebrain, CaV1.2, CaV2.1, and CaV2.2 are all expressed primarily in postmitotic domains (as opposed to domains lining the ventricle) midway along the dorsal–ventral axis (Fig. 3B,E,F). CaV3.2, however, displays a distinct expression pattern. In the telencephalon, CaV3.2 transcripts appear in dorsal postmitotic cells (Fig. 3I), while a distinct domain of expression is detected midway along the dorsal–ventral axis in the ventricular layer of the diencephalon (Fig. 3J).
The onset of CaV3.1 expression coincides with that of CaV1.2, CaV2.1, CaV2.2, and CaV3.2 in the forebrain at late tail bud stages, but it is initially localized in a discrete region of the pineal gland rather than in the forebrain (Fig. 2W). CaV3.1 transcripts persist in the pineal gland into swimming tadpole stages, and they appear in a ventral domain of postmitotic cells in the telencephalon and in a small, slightly dorsal postmitotic region of the diencephalon (Figs. 2X, 3G,H).
Expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 in the Midbrain
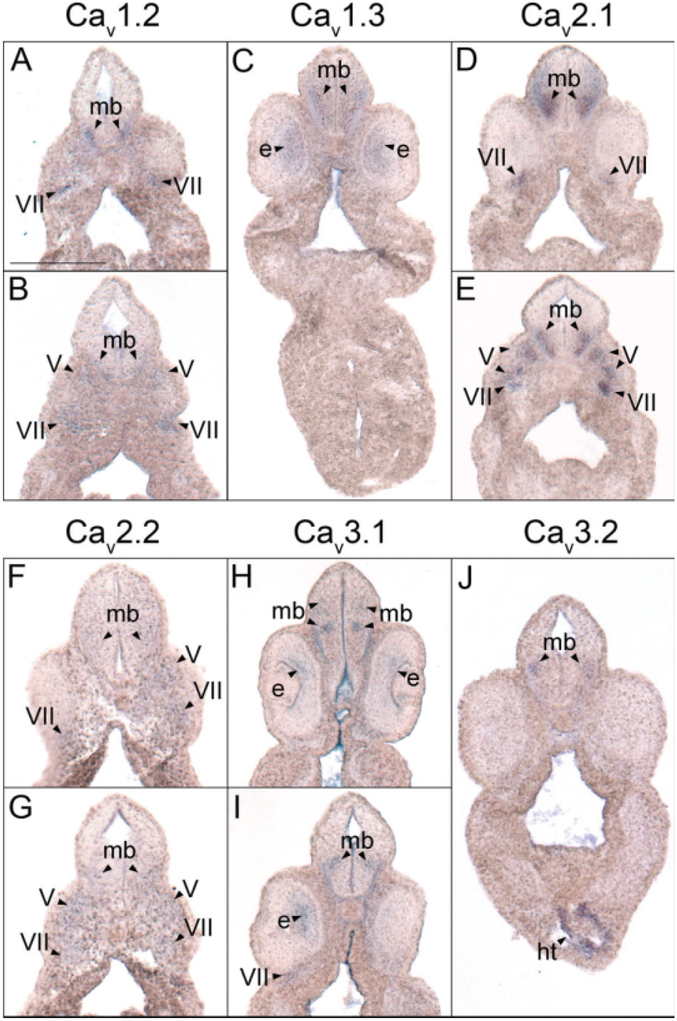
The same array of VGCC α1 subunits expressed in the forebrain during embryonic development of X. laevis also appears in a range of patterns within the midbrain. Transcripts for CaV1.2, CaV1.3, CaV2.1, CaV2.2, and CaV3.2 simultaneously first appear in the midbrain during late tail bud stages (Fig. 2E,H,N,Q,Z). The expression of each of these subunits strengthens in partially overlapping domains throughout development into swimming tadpole stages (Fig. 2F,I,O,R,AA). Expression of CaV1.2 is restricted to a small ventral region of postmitotic cells within the midbrain (Fig. 4A,B), while CaV1.3 and CaV2.1 transcripts are detected in the same region but extend further dorsally (Fig. 4C–E). CaV2.2 appears to be expressed in an area similar to CaV1.3 and CaV2.1 but at much lower levels (Fig. 4F,G). CaV3.2 mRNA is detected in a slightly more dorsal region than CaV1.2 but is still contained within the expression domains of CaV1.3, CaV2.1, and CaV2.2 (Fig. 4J).
Fig. 4.
Midbrain histological analysis of voltage-gated calcium channel (VGCC) α1 subunit expression in stages 35–37 (swimming tadpole) X. laevis using whole-mount in situ hybridization. A–J: Transverse 18-μm sections within the midbrain region of the anterior–posterior axis, with dorsal to the top. A,B: CaV1.2 expression. C: CaV1.3 expression. D,E: CaV2.1 expression. F,G: CaV2.2 expression. H,I: CaV3.1 expression. J: CaV3.2 expression. Arrowheads indicate regions of expression. mb, midbrain; e, eye; ht, heart; V, cranial ganglion V, trigeminal ganglion; VII, cranial ganglion VII, geniculate (facialis) ganglion. Scale bar = 0.25 mm.
Unlike the other five α1 subunits, the initial expression of CaV3.1 in the midbrain is delayed until swimming tadpole stages of development (Fig. 2X). The spatial pattern of expression for this α1 subunit also differs from the others, appearing in three small, distinct regions of postmitotic cells (Figs. 2X, 4H, I). The two anterior domains flank the midline of the dorsal–ventral axis of the midbrain, while the posterior domain is slightly ventral and extends farther laterally (Fig. 4H,I), much like the CaV3.2 pattern of expression in this region.
Expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV2.3, CaV3.1, and CaV3.2 in the Hindbrain
As in the fore- and midbrain, the VGCC α1 subunits CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 are all expressed in the hindbrain during X. laevis embryogenesis. The onset of hindbrain expression for CaV2.1 and CaV2.2 occurs during early tail bud stages (Fig. 2M,P), while CaV1.2, CaV1.3, CaV3.1, and CaV3.2 transcripts appear during late tail bud stages (Fig. 2E,H,W,Z). Expression of each increases in prominence within the hindbrain through swimming tadpole stages (Fig. 2F,I,O,R,X,AA), but in varying spatial patterns. Similar to the midbrain, CaV1.2 mRNA is expressed in a ventrally restricted domain of postmitotic cells near the anterior portion of the hindbrain (Fig. 5B), but transcripts extend farther dorsally in the posterior hindbrain (Fig. 5C). CaV1.3, CaV2.2, and CaV2.1 are all expressed in a wider ventral region along the entire anterior–posterior range of the hindbrain (Fig. 5D–G). The domains of CaV3.1 and CaV3.2 expression are more medial along the dorsal–ventral axis of the hindbrain, with CaV3.1 mRNA detectable more laterally than CaV3.2 (Fig. 5J,K). Coincident with hindbrain expression at late tail bud stages, CaV1.3 and CaV3.1 appear along the ventral side of the otic vesicle (Fig. 2H,W,W′), where they persist into swimming tadpole stages of development (Figs. 2I,X, 5D,D′,J,J′).
Fig. 5.
Hindbrain histological analysis of voltage-gated calcium channel (VGCC) α1 subunit expression in (A–G,J,K) stage 35–37 (swimming tadpole) or (I) stage 30 (late tail bud) X. laevis using whole-mount in situ hybridization. A–K: Transverse 18-μm sections of embryos within the hindbrain region of the anterior–posterior axis, with dorsal to the top. A: CaV1.1 expression. B,C: CaV1.2 expression. D: CaV1.3 expression. E,F: CaV2.1 expression. G,H: CaV2.2 expression. I: CaV2.3 expression. J: CaV3.1 expression. K: CaV3.2 expression. Arrowheads indicate regions of expression. hb, hindbrain; ot, otic vesicle; so, somite; VIII, cranial ganglion VIII, acousticus ganglion; IX, cranial ganglion IX, glossopharyngeal ganglion; X, cranial ganglion X, vagal ganglion. Scale bar represents 0.25 mm.
The α1 subunit CaV2.3 is expressed transiently within the hindbrain. Like CaV1.2, CaV1.3, CaV3.1, and CaV3.2, its onset of expression in this region of the central nervous system (CNS) is during late tail bud stages of development (Fig. 2T,T′), appearing in a small, slightly ventral region of postmitotic cells (Fig. 5I). However, unlike these other α1 subunits, CaV2.3 expression is transient, with transcripts disappearing in the hindbrain by swimming tadpole stages (Fig. 2U).
Expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV2.3, CaV3.1, and CaV3.2 in the Spinal Cord
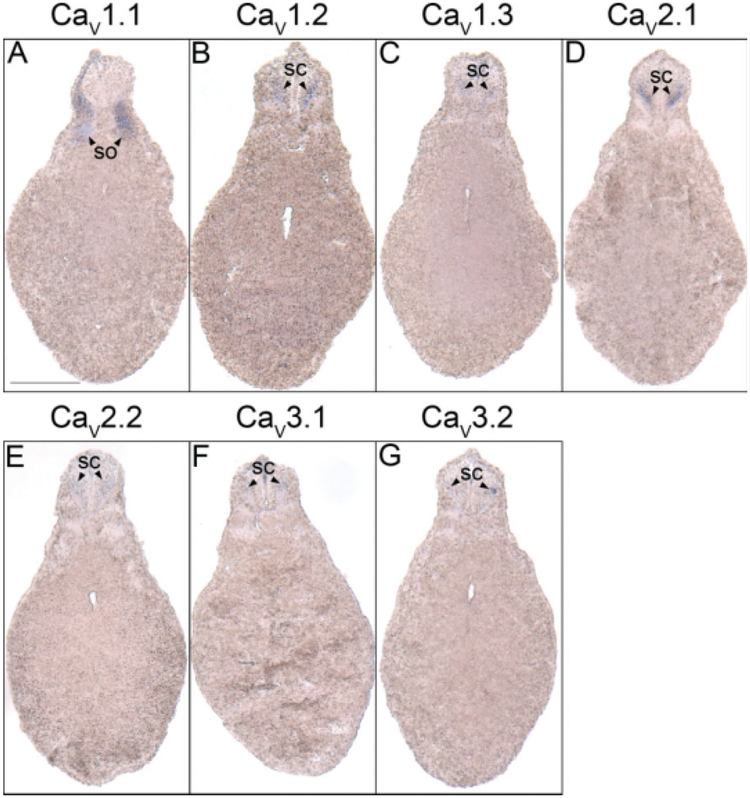
The same VGCC α1 subunits expressed in the hindbrain are also observed in the spinal cord. During neurula stages, transcripts for CaV1.2, CaV2.1, CaV2.2, and CaV3.2 are all detected in the developing neural tube (Fig. 1B–E,B′–E′). The expression of each of these subunits persists in the spinal cord throughout development into swimming tadpole stages (Fig. 2D–F, D′,M–R,Y–AA,Y′). CaV2.3 expression begins later, during early tail bud stages, but it disappears by late tail bud stages (Fig. 2S,S′,T). CaV1.3 and CaV3.1 transcripts also appear in the anterior spinal cord beginning at stage 29, and they persist into swimming tadpole stages (Fig. 2H,I,W,X). CaV1.2 and CaV2.1 are expressed most prominently in the ventral half of the postmitotic domain of the spinal cord (Fig. 6B,D), while CaV1.3 and CaV2.2 are expressed in the same region along the dorsal–ventral axis but more posteriorly (Fig. 6C,E). CaV1.3 mRNA also appears restricted to the anterior spinal cord (Fig. 2I). CaV3.1 and CaV3.2 are expressed in smaller postmitotic regions that are more medial along the dorsal–ventral axis of the spinal cord than the other α1 subunits (Fig. 6F,G). Finally, unlike the other subunits, CaV3.2 is expressed in a small area at the tip of the tail bud during swimming tadpole stages (Fig. 2AA′).
Fig. 6.
Spinal cord histological analysis of voltage-gated calcium channel (VGCC) α1 subunit expression in stages 35–37 (swimming tadpole) Xenopus laevis using whole-mount in situ hybridization. A–G: Transverse 18-μm sections within the spinal cord region of the anterior–posterior axis, with dorsal to the top. A: CaV1.1 expression. B: CaV1.2 expression. C: CaV1.3 expression. D: CaV2.1 expression. E: CaV2.2 expression. F: CaV3.1 expression. G,H: CaV3.2 expression. Arrowheads indicate regions of expression. Abbreviations: sc, spinal cord; so, somite. Scale bar = 0.25 mm.
Expression of CaV1.2, CaV2.1, CaV2.2, and CaV3.1 in the Cranial Ganglia
The VGCC α1 subunits CaV1.2, CaV2.1, CaV2.2, and CaV3.1 are expressed at different times and in various combinations in the cranial ganglia and their developmental precursors, the cranial placodes, of X. laevis. CaV1.2, CaV2.1, and CaV2.2 transcripts are all detected in cranial placode VII beginning at neurula stages (Fig. 1B–D). While CaV2.1 and CaV2.2 continue to be expressed in cranial placode VII and appear in cranial placode V during early tail bud stages (Fig. 2M,P), CaV1.2 mRNA remains detectable only in cranial placode VII (Fig. 2D). By late tail bud stages, CaV1.2 transcripts appear in cranial ganglion V (Fig. 2E) but at lower levels than CaV2.1 and CaV2.2 (Fig. 2N,Q). The expression of each of these subunits persists in cranial ganglia V and VII and is also observed in cranial ganglia VIII, IX, and X as well in swimming tadpole stages (Figs. 2F,O,R, 4A,B,D–G, 5B,C,E–G). At this time, cranial ganglion VII also begins to express CaV3.1 (Figs. 2X, 4I). Expression of CaV3.1 and, to a much lesser degree, CaV3.2 are observed in the olfactory epithelium by swimming tadpole stages of development (Figs. 2X,AA, 3G,I). No VGCC α1 subunit transcripts are detected in presumptive cranial nerves II, III, or XI.
Expression of CaV1.2, CaV1.3, CaV1.4, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 in the Retina
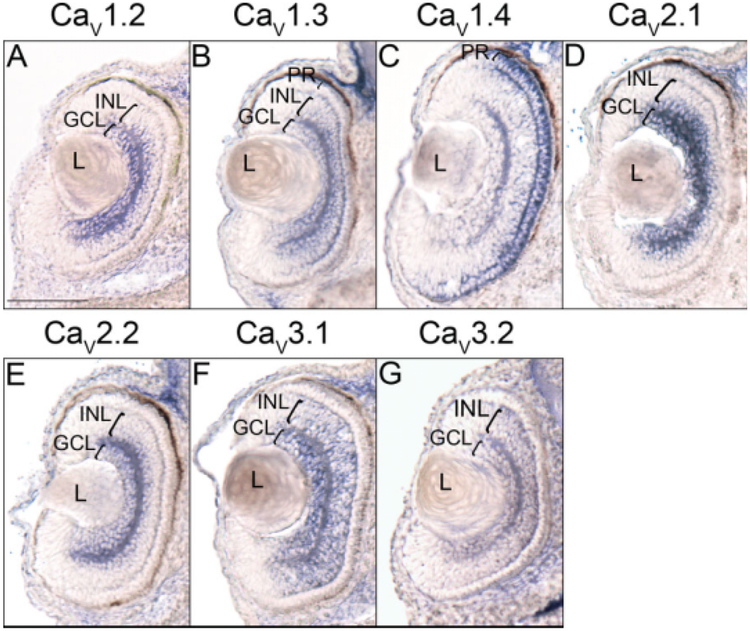
With the exception of CaV2.3, all of the VGCC α1 subunits that are expressed in the X. laevis embryonic brain and spinal cord are also detected in the developing retina. CaV1.3 and CaV2.1 are the first transcripts to appear at low levels in the retina during late tail bud stages (Fig. 2H,N), but by swimming tadpole stages, expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 is observed (Fig. 2F,I,O,R,X,AA). Expression of CaV1.4, which is not observed in any region of the embryo before this stage, also appears in the retina (Fig. 2L). CaV1.2, CaV2.1, and CaV2.2 all appear to be expressed in layers in the central retina (Fig. 3B,E,F); histological analysis of embryos at stage 40 shows transcripts for these subunits to be expressed in the ganglion cell layer (GCL) and the inner portion of the inner nuclear layer (INL; Fig. 7A,D,E). CaV1.3, CaV3.1, and to a lesser extent CaV3.2 are expressed in a wider area of the retina (Fig. 3C,H,J), and appear later, at stage 40, to encompass all of the GCL and INL (Fig. 7B,F,G). CaV1.4 is expressed in a narrow outer layer of the retina (Fig. 3D), which analysis at stage 40 identifies as photoreceptor cells (PR; Fig. 7C). CaV1.3 expression is also observed at low levels in the PR at this later stage (Fig. 7B).
Fig. 7.
Retina histological analysis of voltage-gated calcium channel (VGCC) α1 subunit expression in stage 40 (swimming tadpole) X. laevis using whole-mount in situ hybridization. A–G: Transverse 18-μm sections of the retina, with dorsal to the top. A: CaV1.2 expression. B: CaV1.3 expression. C: CaV1.4 expression. D: CaV2.1 expression. E: CaV2.2 expression. F: CaV3.1 expression. G: CaV3.2 expression. Brackets indicate retinal layers with expression. GCL, ganglion cell layer; INL, inner nuclear layer; L, lens; PR, photoreceptor cells. Scale bar = 0.1 mm.
Expression of CaV1.2, CaV1.3, and CaV3.2 in the Heart
Beginning at late tail bud stages, the VGCC α1 subunits CaV1.2, CaV1.3, and CaV3.2 are all expressed in the myocardium (Fig. 2E,H,Z). CaV1.3 expression is noticeably weaker than CaV1.2 and CaV3.2 at this stage and appears to diminish toward swimming tadpole stages (Figs. 2H,I, 3C). CaV1.2 and CaV3.2 expression, on the other hand, appears to strengthen as development progresses (Figs. 2F,F′,AA, 3B, 4J).
Non-neural Expression of CaV1.1
Unlike the other eight α1 subunits, neither CaV1.1 nor CaV3.3 mRNA is detected in the X. laevis CNS through swimming tadpole stages of development. Using in situ hybridization, CaV1.1 transcripts are first observed in the somites during neurula stages (Fig. 1A,A′) and continue to be expressed in the somatic and skeletal muscle tissue through swimming tadpole stages (Figs. 2A–C, 5A, 6A). By this stage of development, CaV1.1 mRNA is also present in the pharyngeal arches (Figs. 2C, 3A). CaV3.3 expression, on the other hand, is not observed at levels detectable using in situ hybridization in developing X. laevis embryos through swimming tadpole stages (data not shown).
DISCUSSION
Although most of the VGCC α1 subunits are expressed predominantly in the CNS, they all display remarkable diversity in their unique patterns of expression. Even those that appear similar show subtle differences temporally and spatially. For example, whole-mount in situ hybridization shows the expression of CaV2.2 and CaV2.1 to be nearly identical throughout development, but CaV2.2 transcripts appear in the telencephalon by late tail bud stages, slightly earlier than CaV2.1. Differences are also observed between genes within specific regions where two or more are expressed. For example, CaV2.1 mRNA is detected in the mid- and hindbrain in a domain that extends farther dorsally than CaV1.2, while CaV3.1 and CaV3.2 transcripts appear in more discrete regions of the brain and spinal cord contained within the expression realms of CaV1.2, CaV1.3, CaV2.1, and CaV2.2. This variability in expression among the various α1 subunits suggests that each is serving a distinct function within the developing X. laevis embryo. While there are no other detailed, comprehensive studies of VGCC α1 subunits in the vertebrate embryo, several reports have focused on subunit expression (Table 1) and calcium activity during early development. In zebrafish, CaV1.1 and CaV1.3 mRNA is observed beginning at the two-cell stage and becomes restricted to the somites and CNS, respectively, later in development (Sanhueza et al., 2009). CaV2.1, CaV3.1, and CaV3.2 transcripts are also detected during zebrafish gastrula stages using whole-embryo RT-PCR. In X. laevis, Palma et al. initially detect CaV1.1 transcripts at blastula stages using whole-embryo RT-PCR. Using in situ hybridization, they observe CaV1.1 mRNA in the dorsal mesoderm at gastrula stages, where it is implicated in the dorsalization of the embryo, and in the somites at neurula stages (Palma et al., 2001). An additional study has detected an L-type calcium channel in ectodermal cells in the gastrulating Pleurodeles waltl through labeling with a fluorescent derivative of dihydropyridine (Leclerc et al., 1995). A study by Baccaglini and Spitzer demonstrated the importance of calcium-regulated action potentials in Rohon-Beard cells of the developing Xenopus spinal cord (Baccaglini and Spitzer, 1977); however, no VGCC α1 subunit transcripts were detected in this region.
TABLE 1.
Summary of Embryonic and Adult Vertebrate CACNA Expression Patternsa
| Gene | Embryonic Vertebrate Expressionb | Stage of Onsetb | Adult Vertebrate Expression |
|---|---|---|---|
| CACNA1S | Widespread1 | 2-cell (zebrafish) | Skeletal muscle7 |
| CaV1.1 | Dorsal mesoderm2 | Gastrula (X. laevis) | |
| L-type | Dorsal mesoderm/somites1,2 Pharyngeal arches |
Neurula Swimming tadpole |
|
| CACNA1C | Neural tube/spinal cord, CN VII | Neurula | Brain (cortex, hippocampus, cerebellum)8,9, spinal cord motoneurons10,11, retina (IPL, INL, OPL)12,13, heart7, smooth muscle (blood vessels, intestine, lung, uterus) |
| CaV1.2 | Forebrain, midbrain, hindbrain, CN V, heart3 | Late tail bud | |
| L-type | Pineal gland, CN VIII, IX, X, retina (GCL, INL) | Swimming tadpole | |
| Cochlear hair cells4 | E8 (chick) | ||
|
CACNA1D CaV1.3 L-type |
Forebrain1 Pineal gland1, midbrain1, hindbrain1, ventralotic vesicle4, spinal cord1, retina1 (GCL, INL, ONL), heart3 |
Early tail bud Late tail bud | Brain (cortex, hippocampus, cerebellum)8,9, spinal cord motoneurons10,11, cochlear hair cells7, retina (GCL, IPL, INL, OPL, ONL)12, heart7, pancreas |
|
CACNA1F CaV1.4 L-type |
Retina (ONL) | Swimming tadpole | Retina (GCL, ONL)13, spinal cord7, lymphoid tissue |
|
CACNA1A CaV2.1 P/Q-type |
Neural tube/spinal cord, CN VII Hindbrain, CN V Forebrain, midbrain, retina (GCL, INL) Pineal gland, CN VIII, IX, X |
Neurula Early tail bud Late tail bud Swimming tadpole |
Brain (cortex, hippocampus, cerebellum, brainstem, olfactory bulb, thalamus)9,14, spinal cord motoneurons10,15, retina (GCL, IPL, INL, OPL)12,13, heart7, pancreas |
|
CACNA1B CaV2.2 N-type |
Neural tube/spinal cord, CN VII Hindbrain, CN V Forebrain, midbrain Retina (GCL, INL), CN VIII, IX, X |
Neurula Early tail bud Late tail bud Swimming tadpole |
Brain (cortex, hippocampus, cerebellum, brainstem)9, spinal cord motoneurons10,15, retina (GCL, IPL, INL, OPL, ONL)12,13 |
|
CACNA1E CaV2.3 R-type |
Spinal cord (transient) Hindbrain (transient) |
Early tail bud Late tail bud |
Brain (cortex, hippocampus, cerebellum, brainstem, olfactory bulb, thalamus)9,14, spinal cord motoneurons10,16, retina (GCL, IPL, INL, ONL)12,13, heart7, testes |
|
CACNA1G CaV3.1 T-type |
Pineal gland, hindbrain, ventral otic vesicle4, spinal cord Forebrain, olfactory epithelium, midbrain, CN VII, retina (GCL, INL), heart3,5 |
Late tail bud Swimming tadpole |
Brain (cortex, hippocampus, olfactory bulb, thalamus, hypothalamus, midbrain, cerebellum, medulla)7,17, spinal cord motoneurons17, ventral cochlear nucleus, dorsal cochlear nucleus, retina (GCL, INL, OPL)13, heart7, ovary |
|
CACNA1H CaV3.2 |
Neural tube/spinal cord6 Forebrain6, midbrain6, hind-brain6, heart3,6 |
Neurula Late tail bud |
Brain (cortex, hippocampus, olfactory bulb, thalamus, hypothalamus, midbrain)7,17, |
| T-type | Olfactory epithelium6, retina (GCL, INL), tail | Swimming tadpole | spinal cord motoneurons17, dorsal cochlear nucleus, retina (GCL, INL, OPL)13, heart7, smooth muscle, liver, kidney |
|
CACNA1I CaV3.3 T-type |
No expression detected | N/A | Brain (cortex, hippocampus, olfactory bulb, thalamus, hypothalamus, midbrain, cerebellum, medulla)7,17, dorsal cochlear nucleus17, retina (INL, OPL)13 |
Based on studies in mouse, rat, human, turtle, zebrafish, chick, and X. laevis.
References:
Italics in these columns indicate expression patterns not observed in this study.
The discrepancies between these studies and the results presented here may be due to interspecies differences or variations in detection method, probe composition, or stringency of hybridization conditions. To preclude results due to cross-hybridization, our probes were designed to ensure specificity. BLAST N searches for each α1 subunit clone yielded only a single hit for a unique scaffold in the X. tropicalis genome with E values of 0.0. This suggests that cross-hybridization of specific α1 subunit RNA probes with other α1 transcripts during high-stringency in situ hybridization in this study is unlikely, a result supported by the unique expression patterns for each subunit.
Other descriptions of α1 subunit embryonic expression have focused on specific areas of organogenesis at later stages of vertebrate development (Table 1). Using pharmacological and electrophysiological approaches on culture neurons, Li et al. (2001) and Thaler et al. (2001) have shown N, L, R, and even T-type channels are present in the Xenopus spinal cord, specifically at the neuromuscular junction. Localization of CaV3.2 mRNA in the mouse brain at embryonic day (E) 9 and subsequent expansion into the spinal cord and heart by E12 is spatially consistent with X. laevis expression (Son et al., 2002). CaV3.2 has also been shown, in agreement with X. laevis expression, to be the major T-type α1 subunit expressed in E9.5 mice before becoming superseded by CaV3.1 expression by E18 (Niwa et al., 2004). A contrasting study, however, finds CaV3.2 is expressed at lower levels than CaV1.2, CaV1.3, CaV1.1, and especially CaV3.1 from E9.5 to E15.5 (Xu et al., 2003). Lastly, expression of CaV1.2, CaV1.3, and CaV3.1 is detected in E8 cochlear chick hair cells; with the exception of CaV1.2, these findings are consistent with X. laevis expression data (Levic et al., 2007).
The expression patterns of the auxiliary VGCC β subunits are much more thoroughly described in early vertebrate embryonic development, particularly in zebrafish. Isoforms for the β2 and β4 subunits are detected at the four-cell stage (Ebert et al., 2008a,b), and spatial expression analysis of isoforms for β1, β2, β3, and β4 detects these subunits primarily in the skeletal muscle, the CNS, and, in the case of cacnb4a, the heart by 72 hours postfertilization (hpf; Zhou et al., 2006,2008). Each type has at least one isoform that is expressed in the brain, spinal cord, retina, trigeminal ganglion, and spinal cord, suggesting regions of potential coexpression and association with most of the α1 subunits based on X. laevis expression patterns. Other expression domains, such as β2 in the retinal photoreceptor cells or β3 in otic cells, show that, while overlap exists outside the brain and spinal cord, no β subunit expression pattern perfectly matches with any X. laevis α1 subunit. For example, zebrafish cacnb1 is the only β subunit to be expressed in skeletal muscle (Zhou et al., 2006), like X. laevis CaV1.1, but it is also located throughout the CNS, indicating that, if it is functioning as an auxiliary VGCC subunit, it could be associating with more than one α1 subtype. Interestingly, while zebra-fish studies identify cacnb1 as the only β subunit to appear in skeletal muscle (Zhou et al., 2006, 2008), transcripts for a completely different subtype, β3, are located in somites during early X. laevis development (Palma et al., 2001). Finally, there are some areas of β subunit expression, such as the β3-expressing spinal cord Rohon-Beard neurons, that do not appear to show α1 subunit expression in X. laevis embryogenesis (Zhou et al., 2006). This lack of complete overlap between any α1-b subunit pair indicates different associations between the various α1 subunits and their auxiliary β subunits could be a source of Ca2+ activity regulation during embryogenesis. Considering the high concentration of α1 and β subunits in the developing nervous system, this source of regulation could be particularly important in neural development and contribute to temporal and spatial specificity in the wide range of roles Ca2+ signaling has been suggested to play therein.
In addition to embryonic functions, VGCCs clearly play a key role in the regulation of intercellular Ca2+ concentration and activity in excitable cells of the adult. Many of the expression patterns of specific VGCC α1 subunits we observe correlate with those reported in the adult vertebrate (Table 1). For example, CaV1.1 expression in the X. laevis somites is consistent with the skeletal muscle expression detected in adult mammals (Catterall et al., 2005); the predominant outer nuclear layer retinal expression of CaV1.4 in adult vertebrates is likewise detected in X. laevis; and broad neural expression of CaV1.2, CaV1.3, CaV2.1, CaV2.2, CaV3.1, and CaV3.2 is observed in both vertebrate adults and X. laevis embryos. Nevertheless, some adult domains of expression are not present embryonically. Neither the adult neural expression of CaV3.3 nor the adult cardiac expression of CaV3.1 is detected in X. laevis through swimming tadpole stages of development, indicating these VCGGs are not involved in the embryonic development of their respective tissues. More important, however, are those patterns of embryonic expression that are not observed in the adult, such as the embryonic expression of CaV2.3, which appears to be precisely regulated spatially and temporally in a way that does not reflect its broad neural adult expression, suggesting it is playing a role during the formation of the nervous system that differs from its adult function.
The diverse array of unique expression patterns for several of the VGCC α1 subunits during X. laevis embryonic development suggests these may be playing important roles in the formation of adult structures that are distinct from their adult functions. Several embryonic functions of Ca2+ signaling, such as axis formation and patterning (Palma et al., 2001; Wallingford et al., 2001), neural induction (Leclerc et al., 2006; Webb and Miller, 2006; Moreau et al., 2008), and neurotransmitter phenotype specification (Borodinsky et al., 2004; Spitzer, 2006; Root et al., 2008), have in fact been suggested. The presentation of a full overview and analysis of the embryonic expression patterns of the α1 subunits that control this signaling is a crucial step in defining how these processes may be mediating various aspects of embryonic development.
EXPERIMENTAL PROCEDURES
Animal Use
Embryos were obtained by means of natural matings induced with human chorionic gonadotropin as described (Mills et al., 1999; Sive et al., 2000). Staging of embryos was performed according to Nieuwkoop and Faber (1994). All procedures were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the College of William and Mary.
Cloning and Sequence Analysis
Primers for RT-PCR were designed from genomic sequences of X. tropicalis VGCC α1 subunits. Sequences were obtained from the genome project database of the Joint Genome Institute (transcript ID numbers: Cav1.1, 186038; Cav1.2, 365832; Cav1.3, 425287; Cav1.4, 470861; Cav2.1, 470594; Cav2.2, 189681; Cav2.3, 467646; Cav3.1, 380951; Cav3.2, 311573; Cav3.3, 323418) through BLAST N searches using mouse and human orthologs. Primer pairs for each channel were selected from regions that were unique to that particular channel (to ensure that a specific channel would be cloned) but highly conserved among species (to ensure that the primers would recognize X. laevis sequences). Primers used for the cloning were as follows: Cav1.1, (5′-AACCGCTTTGACTGCTTTGT-3′), and (5′-GTTCTTGTCCAGC TCACAGTT-3′); Cav1.2, (5′-GCCTGTGGGAAGTTATCGAA-3′), and (5′-TT GCTCTGTTTGACCCTGAA-3′); Cav1.3, (5′-TTCCTCATCCCCCTCTTCTT-3′), and (5′-ATCGATGCTGCAAGGCA AG-3′); Cav1.4, (5′-GCCTGCATCAGCATTGTAGA-3′), and (5′-TTCTC CCTCAGCTTTTGGAA-3′); Cav2.1, (5′-GGAGAGCCAGTCTTGGATGA-3′), and (5′-AGTAACCATTGGGCAACCT G-3′); Cav2.2, (5′-GGGATGAACGTCCAGAATTG-3′), and (5′-GCGCTTCTA CTCCTGTGACC-3′); Cav2.3, (5′-TATTTCATTGGGATATTTTGCTTTG-3′), and (5′-GACGTGGACCCATTCCATA C-3′); Cav3.1, (5′-ATCCTCCTCAACTGCGTGAC-3′), and (5′-CCCATGCTG AGGGTGTTTAT-3′); Cav3.2, (5′-CCCACCGTCTTCTTCTGTCT-3′), and (5′-TGATACTCAATCCCCATGCTC-3′); Cav3.3, (5′-CCTTCATCTTCCTCAACT GC-3′), and (5′-AAGTTCTCAACCAC GACACC-3′).
Total RNA was extracted from stage 40 X. laevis embryos using the RNeasy Maxi kit (Qiagen), and cDNA was synthesized using the iScript cDNA Synthesis kit (Bio-Rad) following manufacturers’ instructions. PCR was performed using either Taq DNA polymerase (New England Biolabs) or the Supertaq DNA Polymerase kit (Ambion). PCR products were cloned into the pCRII-TOPO vector and transformed into One Shot DH5α Chemically Competent Escherichia coli (Invitrogen) or into the Strata-Clone pSC-A-amp/kan vector and transformed using StrataClone Solo-Pack Competent Cells (Stratagene). Plasmid DNA containing the cloned PCR products was purified using the Wizard Plus SV Miniprep Kit (Promega) and sequenced on an ABI 3130 Genetic Analyzer sequencer using the BigDye Terminator v3.1 Ready Reaction Cycle Sequencing Kit (Applied Biosystems). The identity of each clone was verified by nucleotide and deduced amino acid BLAST comparisons with orthologous sequences from a range of organisms including X. tropicalis.
Expression Analysis
Antisense mRNA probes were generated for each of the α1 subunits by linearizing the plasmid DNA with a restriction enzyme and transcribing with an RNA polymerase as follows: Cav1.1 (EcoRV, Sp6); Cav1.2 (Not1, T3); Cav1.3 (Not1, T3); Cav1.4 (Not1, T3); Cav2.1 (BamHI, T3); Cav2.2 (Hind III, T7); Cav2.3 (EcoRV, Sp6); Cav3.1 (NotI, T3); Cav3.2 (NotI, T3); Cav3.3 (EcoRV, Sp6). High stringency whole-mount in situ hybridization analysis using NBT/BCIP (nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate) alkaline phosphatase substrate (Promega) was carried out as described with minor modifications (Harland, 1991). Sense probes were used to exclude the possibility of non-specific hybridization.
Following whole-mount in situ hybridization, embryos were dehydrated and cleared as described for whole-mount photography (Sive et al., 2000). For histological analysis, embryos were cryoprotected in 1.6 M sucrose in phosphate buffered saline for at least 12 hr at 4°C, embedded in Tissue Freezing Medium at −20°C, cryosectioned into 18 μm slices, and mounted on slides for photography. Histological analysis of embryos from Figure 1 was performed as previously described (Li et al., 2006).
ACKNOWLEDGMENTS
We thank Lomax Boyd for his suggestions on the manuscript. Support was provided by a grant from the Howard Hughes Medical Institute Undergraduate Science Education Program to the College of William and Mary and from the NIH (R15NS067566).
Grant sponsor: Howard Hughes Medical Institute; Grant number: 52005868.
REFERENCES
- Aguilar J, Escobedo L, Bautista W, Felix R, Delgado-Lezama R. 2004. N- and P/Q-type Ca2+ channels regulate synaptic efficacy between spinal dorsolateral funiculus terminals and motoneurons. Biochem Biophys Res Commun 317: 551–557. [DOI] [PubMed] [Google Scholar]
- Baccaglini PI, Spitzer NC. 1977. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol 271:93–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Spitzer NC. 2006. Second messenger pas de deux: the coordinated dance between calcium and cAMP. Sci STKE 2006:pe22. [DOI] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. 2004. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429: 523–530. [DOI] [PubMed] [Google Scholar]
- Calin-Jageman I, Lee A. 2008. Ca(v)1 L- type Ca2+ channel signaling complexes in neurons. J Neurochem 105:573–583. [DOI] [PubMed] [Google Scholar]
- Castro A, Andrade A, Vergara P, Segovia J, Aguilar J, Felix R, Delgado-Lezama R. 2009. Involvement of R-type Ca2+ channels in neurotransmitter release from spinal dorsolateral funiculus terminals synapsing motoneurons. J Comp Neurol 513:188–196. [DOI] [PubMed] [Google Scholar]
- Catterall WA. 2000. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol 16:521–555. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J. 2005. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57:411–425. [DOI] [PubMed] [Google Scholar]
- Conklin MW, Lin MS, Spitzer NC. 2005. Local calcium transients contribute to disappearance of pFAK, focal complex removal and deadhesion of neuronal growth cones and fibroblasts. Dev Biol 287:201–212. [DOI] [PubMed] [Google Scholar]
- Ebert A, McAnelly C, Srinivasan A, Mueller R, Garrity D, Garrity D. 2008a. The calcium channel beta2 (CACNB2) subunit repertoire in teleosts. BMC Mol Biol 9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AM, McAnelly CA, Srinivasan A, Linker JL, Horne WA, Garrity DM. 2008b. Ca2+ channel-independent requirement for MAGUK family CACNB4 genes in initiation of zebrafish epiboly. Proc Natl Acad Sci U S A 105:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland RM. 1991. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol 36:685–695. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA. 1993. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol 123:949–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner VL, Wolfner MF. 2008. Transitioning from egg to embryo: triggers and mechanisms of egg activation. Dev Dyn 237:527–544. [DOI] [PubMed] [Google Scholar]
- Jimenez-Gonzalez C, McLaren GJ, Dale N. 2003. Development of the Ca2+ channel and BK-channel expression in embryos and larvae of Xenopus laevis. Eur J Neurosci 18:2175–2187. [DOI] [PubMed] [Google Scholar]
- Kisilevsky AE, Zamponi GW. 2008. Presynaptic calcium channels: structure, regulators, and blockers. Handb Exp Pharmacol:45–75. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Duprat AM, Moreau M. 1995. In vivo labelling of L-type Ca2+ channels by fluorescent dihydropyridine: correlation between ontogenesis of the channels and the acquisition of neural competence in ectoderm cells from Pleurodeles waltl embryos. Cell Calcium 17: 216–224. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Neant I, Webb SE, Miller AL, Moreau M. 2006. Calcium transients and calcium signalling during early neurogenesis in the amphibian embryo Xenopus laevis. Biochim Biophys Acta 1763:1184–1191. [DOI] [PubMed] [Google Scholar]
- Levic S, Nie L, Tuteja D, Harvey M, Sokolowski BHA, Yamoah EN. 2007. Development and regeneration of hair cells share common functional features. Proc Natl Acad Sci U S A 104: 19108–19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Thaler C, Brehm P. 2001. Calcium channels in Xenopus spinal neurons differ in somas and presynaptic terminals. J Neurophysiol 86:269–279. [DOI] [PubMed] [Google Scholar]
- Li M, Sipe CW, Hoke K, August LL, Wright MA, Saha MS. 2006. The role of early lineage in GABAergic and glutamatergic cell fate determination in Xenopus laevis. J Comp Neurol 495:645–657. [DOI] [PubMed] [Google Scholar]
- Li WM, Webb SE, Chan CM, Miller AL. 2008. Multiple roles of the furrow deepening Ca2+ transient during cytokinesis in Zebrafish embryos. Dev Biol 316: 228–248. [DOI] [PubMed] [Google Scholar]
- McKeown L, Robinson P, Jones OT. 2006. Molecular basis of inherited calcium channelopathies: role of mutations in pore-forming subunits. Acta Pharmacol Sin 27:799–812. [DOI] [PubMed] [Google Scholar]
- Meacham CA, White LD, Barone S, Shafer TJ. 2003. Ontogeny of voltage-sensitive calcium channel alpha(1A) and alpha(1E) subunit expression and synaptic function in rat central nervous system. Brain Res Dev Brain Res 142: 47–65. [DOI] [PubMed] [Google Scholar]
- Mills KR, Kruep D, Saha MS. 1999. Elucidating the origins of the vascular system: a fate map of the vascular endothelial and red blood cell lineages in Xenopus laevis. Dev Biol 209: 352–368. [DOI] [PubMed] [Google Scholar]
- Moreau M, Neant I, Webb SE, Miller AL, Leclerc C. 2008. Calcium signalling during neural induction in Xenopus laevis embryos. Philos Trans R Soc Lond β Biol Sci 363:1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. 1994. Normal table of Xenopus laevis (Daudin). New York: Garland Publishing. [Google Scholar]
- Niwa N, Yasui K, Opthof T, Takemura H, Shimizu A, Horiba M, Lee J-K, Honjo H, Kamiya K, Kodama I. 2004. Cav3.2 subunit underlies the functional T-type Ca2+ channel in murine hearts during the embryonic period. Am J Physiol Heart Circ Physiol 286:H2257–H2263. [DOI] [PubMed] [Google Scholar]
- Obermair GJ, Tuluc P, Flucher BE. 2008. Auxiliary Ca(2+) channel subunits: lessons learned from muscle. Curr Opin Pharmacol 8:311–318. [DOI] [PubMed] [Google Scholar]
- Palma V, Kukuljan M, Mayor R. 2001. Calcium mediates dorsoventral patterning of mesoderm in Xenopus. Curr Biol 11:1606–1610. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Schneider T. 1995. Molecular biology of calcium channels. Kidney Int 48:1111–1124. [DOI] [PubMed] [Google Scholar]
- Plant TD, Schirra C, Katz E, Uchitel OD, Konnerth A. 1998. Single-cell RT-PCR and functional characterization of Ca2+ channels in motoneurons of the rat facial nucleus. J Neurosci 18:9573–9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC, Ribera AB. 2008. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci 28:4777–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza D, Montoya A, Sierralta J, Kukuljan M. 2009. Expression of voltage-activated calcium channels in the early zebrafish embryo. Zygote 17: 131–135. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier JF, Hounsgaard J. 2003. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci 18:258–266. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. 2000. Early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Son WY, Han CT, Lee JH, Jung KY, Lee HM, Choo YK. 2002. Developmental expression patterns of alpha1H T-type Ca2+ channels during spermatogenesis and organogenesis in mice. Dev Growth Differ 44:181–190. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Ribera AB. 1998. Development of electrical excitability in embryonic neurons: mechanisms and roles. J Neurobiol 37:190–197. [PubMed] [Google Scholar]
- Spitzer NC. 2006. Electrical activity in early neuronal development. Nature 444:707–712. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Kingston PA, Manning TJ, Conklin MW. 2002. Outside and in: development of neuronal excitability. Curr Opin Neurobiol 12:315–323. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Borodinsky LN, Root CM. 2005. Homeostatic activity-dependent paradigm for neurotransmitter specification. Cell Calcium 37:417–423. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee J, Daud A, Perez-Reyes E, Bayliss DA. 1999. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci 19:1895–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler C, Li W, Brehm P. 2001. Calcium channel isoforms underlying synaptic transmission at embryonic Xenopus neuromuscular junctions. J Neurosci 21:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Ewald AJ, Harland RM, Fraser SE. 2001. Calcium signaling during convergent extension in Xenopus. Curr Biol 11:652–661. [DOI] [PubMed] [Google Scholar]
- Webb SE, Miller AL. 2006. Ca2+ signaling and early embryonic patterning during the blastula and gastrula periods of zebrafish and Xenopus development. Biochim Biophys Acta 1763:1192–1208. [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Hoskins L, Catterall WA. 1998. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci 18:6319–6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P, Shen C, McRory J. 2006. Differential distribution of voltage-gated calcium channels in dopaminergic neurons of the rat retina. J Comp Neurol 497:384–396. [DOI] [PubMed] [Google Scholar]
- Xu HP, Zhao JW, Yang XL. 2002. Expression of voltage-dependent calcium channel subunits in the rat retina. Neurosci Lett 329:297–300. [DOI] [PubMed] [Google Scholar]
- Xu M, Welling A, Paparisto S, Hofmann F, Klugbauer N. 2003. Enhanced expression of L-type Cav1.3 calcium channels in murine embryonic hearts from Cav1.2-deficient mice. J Biol Chem 278:40837–40841. [DOI] [PubMed] [Google Scholar]
- Yamakage M, Namiki A. 2002. Calcium channels--basic aspects of their structure, function and gene encoding; anesthetic action on the channels--a review. Can J Anaesth 49:151–164. [DOI] [PubMed] [Google Scholar]
- Zhou W, Saint-Amant L, Hirata H, Cui WW, Sprague SM, Kuwada JY. 2006. Non-sense mutations in the dihydropyridine receptor betα1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium 39:227–236. [DOI] [PubMed] [Google Scholar]
- Zhou W, Horstick EJ, Hirata H, Kuwada JY. 2008. Identification and expression of voltage-gated calcium channel beta subunits in Zebrafish. Dev Dyn 237: 3842–3852. [DOI] [PubMed] [Google Scholar]