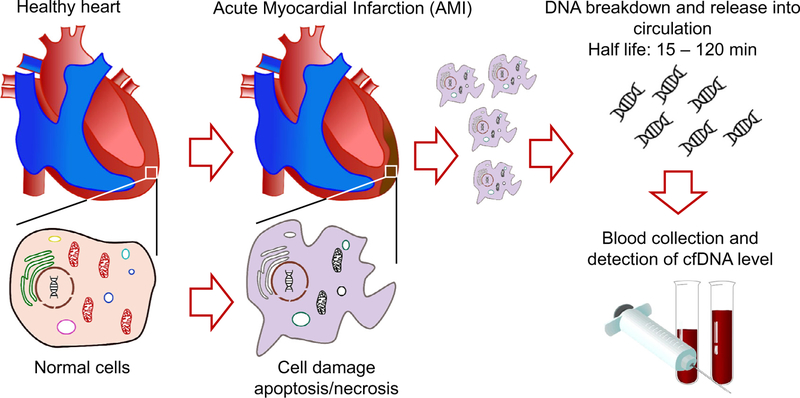
Cardiovascular disease is the number 1 cause of death in the United States.1 In this issue of The American Journal of Medical Sciences, Xie et al examined the use of cell-free DNA (cfDNA) in patients as a diagnosis for clinical features of myocardial infarction (MI).2 Advances in the genetic and proteomic techniques give hope for an increase in the number of new diagnostic markers.3 Identification of biomarkers that have mechanistic implications and accurately and reliably predict the development of heart failure post-MI is needed. Cardiac troponin, a known biomarker for MI, has been shown to be detectable as early as 6 hours; however, troponin does not reflect current cardiovascular status as levels can remain elevated up to 14 hours after MI.4 The half-life of cfDNA is not completely clear, although studies suggest dynamic changes in the levels—from 16 minutes to 2 hours.5,6 Such rapid changes should be considered an advantage rather than a drawback, since they provide a powerful tool to monitor the response immediately after treatment. The authors proposed the use cfDNA would be better suited to serve as a marker for adverse events such as reinfarction or development of heart failure after MI.
Nonencapsulated DNA that can be found in plasma, urine or cerebrospinal fluid and that usually originates from apoptosis or necrosis is classified as cfDNA (Figure 1). Under physiological conditions, macrophages remove cfDNA fragments from the bloodstream; however, under pathologic conditions that lead to overproduction by cells (cancer/tumors) or excessive necrosis and inflammation, larger amounts of cfDNA can be detected in the circulation. Over the past decade, cell-free nucleic acids have become a focal point for molecular pathology research since cfDNA is easily and noninvasively detectable in body fluids, can be assayed multiple times, and most importantly may be used to assess the severity of disease, and predict the efficacy of its treatment.7
FIGURE 1.
Simplified scheme of cfDNA generation in AMI. Xie et al show that measuring circulating cfDNA levels in AMI patients may be an alternative noninvasive approach to monitor the disease, and identify high-risk individuals that may undergo heart failure and have other complications.
There are multiple risk factors for the development of cardiovascular disease including obesity, high blood pressure, smoking and poor nutrition.1 Unsurprisingly, Xie et al showed an increase in secondary risk factors in patients with MI compared to those with cardiovascular disease and healthy controls. The authors, however, did not assess whether these risk factors were elevated in the group with adverse post-MI events. Vora et al showed there was a 1.7% increase in total cfDNA per body mass index unit (kg/m2) indicating that the cfDNA increase post-MI may be due to increased obesity in these patients.8 Though the study itself is relevant and interesting, a larger cohort of patients is needed as well as thorough research into patients’ history, medications, differential diagnosis, accompanying disorders that may result in false positives (such as liver or kidney disease) and the degree of cardiac damage. Wide variability in cfDNA content among the genetically heterogenic cohort of patients introduces a high degree of complexity. Thus, in order to clarify specific effects, studies performed in genetically homogeneous subjects such as rodents will bring more light to the mechanisms behind cfDNA regulation of cardiovascular disease. Also, it would be intriguing to assess fluctuations in the cfDNA levels in a healthy cohort in order to evaluate the effect of sex and gender.
In addition, studies have shown that the different content of telomeric sequences in cfDNA may contribute to the immune response to disease.9 The authors did not evaluate the content of cfDNA and thus it is unclear if the increase in cfDNA was due to a larger injury site or if the cfDNA is actually regulating the wound healing response after MI. Using cfDNA as a biomarker could be looked at as a “chicken and egg problem” since it is yet unclear if complications may be arising from cfDNA itself, or if cfDNA found in circulation is just a simple consequence of cell deterioration. Since there is evidence that cfDNA may induce an immune response by activating specific sensing receptors, additional care should be exercised when interpreting the data.10 Most importantly, thorough basic science experiments that focus on the consequences and origins of cfDNA and the molecular pathways involved are needed. Additional studies that evaluate what genetic sequences are present in the cfDNA found in the post-MI patients should be performed to truly understand the role they play in disease.
While the authors demonstrated that cfDNA can act as a biomarker for adverse events post-MI, whether or not cfDNA plays a role in the development of heart failure after ischemic injury remains unclear. Conclusions about the lack of gender differences are overstated since the number of subjects tested is too small to make such statement confidently. Future studies should be performed evaluating sex differences as the MI group in this study had fewer women enrolled. Monitoring the levels of cfDNA is an attractive alternative to existing tests as it may be a more dynamic tool; even still its predictive capabilities for early screening in AMI may prove challenging. Multiple levels of validation are required in order to consider cfDNA as predictors of MI susceptibility and for clinical implementation of such a test. Joint efforts by the clinical and research community will define the practical considerations of this approach for broad clinical application.
ACKNOWLEDGMENTS
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institutes of Health, the U.S. Department of Health and Human Services, the American Heart Association or the U.S. Department of Veterans Affairs.
This work was supported by the National Institute of Health (NIDDK) R00DK105160 to DVI and the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development Award IK2BX003922 to KYD-P.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: a report from the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie J, Yang J, Hu P. Variations in circulating cell-free DNA and correlates to clinical manifestations in acute myocardial infarction patients. Am J Med Sci 2018;356(2):121–129. [DOI] [PubMed] [Google Scholar]
- 3.Good DM, Thongboonkerd V, Novak J, et al. Body fluid proteomics for biomarker discovery: lessons from the past hold the key to success in the future. J Proteome Res 2007;6:4549–4555. [DOI] [PubMed] [Google Scholar]
- 4.Daubert MA, Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag 2010;6:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med 2008;14:985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riediger AL, Dietz S, Schirmer U, et al. Mutation analysis of circulating plasma DNA to determine response to EGFR tyrosine kinase inhibitor therapy of lung adenocarcinoma patients. Sci Rep 2016;6:33505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart CM, Kothari PD, Mouliere F, et al. The value of cell-free DNA for molecular pathology. J Pathol 2018;244:616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vora NL, Johnson KL, Basu S, et al. A multifactorial relationship exists between total circulating cell-free DNA levels and maternal. BMI Prenat Diagn 2012;32:912–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zinkova A, Brynychova I, Svacina A, et al. Cell-free DNA from human plasma and serum differs in content of telomeric sequences and its ability to promote immune response. Sci Rep 2017;7:2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konecna B, Laukova L. Vlkova B. Immune activation by nucleic acids: A role in pregnancy complications. Scand J Immunol 2018;87:e12651. [DOI] [PubMed] [Google Scholar]