Abstract
Corneal Epithelial Stem Cells (CESCs) and their proliferative progeny, the Transit Amplifying Cells (TACs), are responsible for maintaining the integrity and transparency of the cornea. These stem cells (SCs) are widely used in corneal transplants and ocular surface reconstruction. Molecular markers are essential to identify, isolate and enrich for these cells, yet no definitive CESC marker has been established. An extensive literature survey shows variability in the expression of putative CESC markers among vertebrates; being attributed to species-specific variations, or other differences in developmental stages of these animals, approaches used in these studies and marker specificity. Here, we expanded the search for CESC markers using the amphibian model Xenopus laevis. In previous studies we found that long-term label retaining cells (suggestive of CESCs and TACs) are present throughout the larval basal corneal epithelium. In adult frogs, these cells become concentrated in the peripheral cornea (limbal region). Here, we used immunofluorescence to characterize the expression of nine proteins in the corneas of both Xenopus larvae and adults (post-metamorphic). We found that localization of some markers change between larval and adult stages. Markers such as p63, Keratin 19, and β1-integrin are restricted to basal corneal epithelial cells of the larvae. After metamorphosis their expression is found in basal and intermediate layer cells of the adult frog corneal epithelium. Another protein, Pax6 was expressed in the larval corneas, but surprisingly it was not detected in the adult corneal epithelium. For the first time we report that Tcf7l2 can be used as a marker to differentiate cornea vs. skin in frogs. Tcf7l2 is present only in the frog skin, which differs from reports indicating that the protein is expressed in the human cornea. Furthermore, we identified the transition between the inner, and the outer surface of the adult frog eyelid as a key boundary in terms of marker expression. Although these markers are useful to identify different regions and cellular layers of the frog corneal epithelium, none is unique to CESCs or TACs. Our results confirm that there is no single conserved CESC marker in vertebrates. This molecular characterization of the Xenopus cornea facilitates its use as a vertebrate model to understand the functions of key proteins in corneal homeostasis and wound repair.
Keywords: Xenopus, Cornea, Stem cells, Markers, Eyelid, Metamorphosis, Gene expression
1. Introduction
Homeostasis and repair of adult tissues, such as skin, intestinal epithelium, skeletal muscle, and bone marrow, is driven by populations of resident stem cells (SCs) (Costamagna et al., 2015; Krieger and Simons, 2015; Li and Clevers, 2010; Mimeault and Batra, 2008). Among such tissues the vertebrate cornea has been an instrumental system to study stem cell biology. The corneal epithelium, under both normal conditions and following injury is replenished by Corneal Epithelial Stem Cells (CESCs), which help maintain clear vision (Cotsarelis et al., 1989; Davanger and Evensen, 1971; Thoft and Friend, 1983). Despite the long known presence of CESCs and their more highly proliferative progeny, the Transit Amplifying Cells (TACs) (Lehrer et al., 1998), their anatomical location in various vertebrate species has not been conclusively determined, and remains somewhat controversial for certain species (Cotsarelis et al., 1989; Majo et al., 2008; Schermer et al., 1986). In humans these corneal stem cells have been shown to reside in the basal epithelium of the limbus, a unique structure located at the peripheral edge of the cornea (Davanger and Evensen, 1971; Di Girolamo, 2011). On the other hand, some investigators have proposed that CESCs are distributed throughout the basal corneal epithelial layer in other vertebrates, such as mice and pigs (Majo et al., 2008; Mort et al., 2012).
Partial or complete damage, or deficiency of these stem cells leads to deleterious effects on the surface integrity of the cornea and impairs vision as well as its wound healing abilities (Chen and Tseng, 1991; Dua et al., 2003). For long term restoration of visual function, clinicians have successfully replaced the stem cell populations either through autologous or allogenic grafting of limbal tissue (Kenyon and Tseng, 1989), or transplantation of cultured, expanded cells (Rama et al., 2010). One of the key difficulties concerning the applicability of corneal stem cells is their isolation as a pure cell population. Establishment of specific biomarkers to identify, isolate, and characterize corneal stem cells is therefore crucial for both understanding their basic biology and for clinical applications involving these SCs.
Over the past few decades a number of proteins have been proposed as markers of corneal stem cells (Castro-Muñozledo, 2015; Chen et al., 2004; Gonzalez et al., 2017; Mort et al., 2012). As a unique and universal marker of CESCs remains to be identified, current putative markers are mostly candidate molecules that have proven useful as markers of adult SCs in a wider array of tissues. For example, p63, ABCG2, and BMI1 are used as markers of adult SCs in skin, blood and intestinal tissues (Pellegrini et al., 2001; Yan et al., 2012; Zhou et al., 2001). With a range of CESC markers already proposed, the biggest challenge lies in finding a definitive biological marker that is useful for identifying and purifying CESCs. Traditionally, studies have examined the presence of markers in corneas of various vertebrates like mice (Li et al., 2017; Majo et al., 2008), rats (Umemoto et al., 2005), rabbits (Hsueh et al., 2004), dogs (Morita et al., 2015), pigs (Notara et al., 2011), horses (Moriyama et al., 2014), as well as fetal and adult humans (Chen et al., 2004; Davies et al., 2009; Lyngholm et al., 2008; Seeker and Daniels, 2008). However, molecular characterization of markers during vertebrate corneal morphogenesis and maturation is hampered by the availability of fetal vs. adult corneas. So far, only a few studies have examined the localization of these markers during different stages of corneal development (Chung et al., 1992; Davies et al., 2009; Dhouailly et al., 2014; Lyngholm et al., 2008). Furthermore, a comprehensive literature survey reveals a great deal of variation in the expression profile of these proteins among vertebrates, particularly with respect to their localization in different layers or regions of the corneal epithelium (see Table 1 and Supplementary Table S1). This exercise reveals that no single biomarker has been shown to characterize individual corneal epithelial cells (e.g., CESCs or TACs). The observed variations can be attributed to differences that are either species-specific, age-related, or due to the methodologies used in these studies.
Table 1.
List of putative molecular markers for CESCs and TACs as studied in different vertebrates.
| Molecular markers | Vertebrates examined | Selected references |
|---|---|---|
| ABCB5 | Human, Mice | 1, 2 |
| ABCG2 | Human, Mice, Rat, Dog | 3, 4, 5, 6, 7 |
| α-enolase | Human, Rat, Rabbit | 8, 9, 10 |
| BMI1 | Human, Mice | 11, 12 |
| β-catenin | Human, Mice, Rat, Rabbit | 13, 14, 15 |
| C/EBPδ | Human | 11 |
| E-cadherin | Human, Mice, Rat, Pig, Rabbit | 8, 15, 16, 17 |
| EGFR | Human, Rat | 14, 18 |
| Fzd7 | Human | 19 |
| Hes1 | Human, Mice | 20, 21, 22 |
| α9-integrin | Human, Mice | 8, 23 |
| β1-integrin | Human, Mice, Rat, Pig | 8, 23, 24, 25 |
| Keratin 19 | Human, Mice, Rat, Guinea pig | 8, 12, 26, 27 |
| Keratin 15 | Human, Mice | 28, 29 |
| Keratin 14 | Human, Mice, Rat, Rabbit, Horse | 13, 28, 29, 30 |
| KLF4 | Mice | 15 |
| N-cadherin | Human | 31 |
| NGFR p75 | Human | 32 |
| NGFR TrkA | Human, Mice, Dog | 2, 33, 34 |
| Notch 1 | Human, Mice, Rat | 21, 35, 36 |
| p63 | Human, Mice, Rat, Rabbit, Dog, Horse | 3, 13, 30, 37, 38, 39 |
| PAX6 | Human, Mice, Rat, Rabbit, Chick, Monkey | 2, 36, 40 |
| SOX9 | Human | 41 |
| TCF4 (TCF7L2) | Human | 42 |
| Vimentin | Human, Mice, Guinea pig, Cat, Rabbit, Chick | 27, 43, 44, 45, 46 |
A survey on the localization of these markers in the corneal epithelium of various animals is included in Supplemental Table S1. Proteins marked in ‘bold’ letters were examined in the current study.
(1) Ksander et al., 2014; (2) Li et al. (2017); (3) Morita et al. (2015); (4) Watanabe et al. (2004); (5) Umemoto et al. (2005); (6) Budak et al. (2005); (7) Priya et al. (2013); (8) Chen et al. (2004); (9) Chung et al. (1995); (10) Zieske et al. (1992); (11) Barbaro et al. (2007); (12) Kalha et al. (2018); (13) Hsueh et al. (2004); (14) Schlotzer-Schrehardt et al. (2005); (15) Tiwari et al. (2017); (16) Suzuki et al. (2000); (17) Guindolet et al. (2017); (18) Zieske et al. (1993); (19) Mei et al. (2014); (20) Nakamura et al. (2008); (21) Djalilian et al. (2008); (22) Ueno et al. (2012); (23) Pajoohesh-Ganji et al. (2006); (24) Notara et al. (2011); (25) Stepp et al. (1993); (26) Kasper (1991); (27) Kasper (1992); (28) Yoshida et al. (2006); (29) Figueira et al. (2007); (30) Linardi et al. (2015); (31) Hayashi et al. (2007); (32) Di Girolamo et al. (2008); (33) Woo et al. (2005); (34) Touhami et al. (2002); (35) Thomas et al. (2007); (36) Zhao et al. (2002); (37) Pellegrini et al. (2001); (38) Moore et al. (2002); (39) Wang et al. (2003); (40) Koroma et al. (1997); (41) Menzel-Severing et al. (2018); (42) Lu et al. (2012); (43) Ritchey et al. (2011); (44) SundarRaj et al. (1992); (45) Jester et al. (1994); (46) Sidney et al. (2015).
An excellent vertebrate model, which is widely used for molecular, cellular and developmental biology studies, is the South African clawed frog, Xenopus laevis (Lee-Liu et al., 2017; Slack et al., 2008; Tandon et al., 2017). The Xenopus tadpole also serves as a well-established model for studying vertebrate eye tissue regeneration, including the lens (Barbosa-Sabanero et al., 2012; Henry et al., 2008; Tseng, 2017). For example, these anurans can regenerate lenses during larval stages of development (Freeman, 1963). However, the competency and extent of lens regeneration decreases as the larva grows older and metamorphosis proceeds (Filoni et al., 1997; Henry and Tsonis, 2010). Currently, Xenopus is being developed as a valuable model for studying corneal stem cells and eye tissue repair (Hamilton and Henry, 2016; Hu et al., 2013; Kha et al., 2018; Perry et al., 2013). Both the larvae and adult (post-metamorphic) frogs have great potential as a classical laboratory model for multiple reasons. First, Xenopus embryos have autonomous, external development that facilitates accessibility and ease of manipulation. Second, they display rapid growth that generates tadpoles in a few weeks, and froglets in approximately two months. Third, they are easy to maintain with relatively low costs. Finally, the anatomy and development of the Xenopus cornea is highly similar to that of the human cornea (Hu et al., 2013).
In Xenopus larvae (stages 46–54) (Nieuwkoop and Faber, 1956) the corneal epithelium consists of two cell layers – an outer apical layer and an inner basal layer (Fig. 1A–B). This stratified squamous corneal epithelium is transparent (devoid of melanophores) and continuous with the more opaque skin of the head (Perry et al., 2013). The boundary between cornea and surrounding skin is clearly demarcated by the presence of pigment cells in the skin epithelium. During the early larval period of development, the corneal epithelium and deeper endothelium mostly remain free of one another, apart from a small central point of connection (the “stroma-attracting center”) (Hu et al., 2013). During these early stages the stroma is not well-developed and contains relatively few keratocytes. As the frog approaches metamorphosis the cornea matures to consist of three principal cellular layers – a stratified epithelium composed of about 13 cell layers at the center (the peripheral region has approximately 10 cell layers), a thick collagenous stroma interspersed with keratocytes, and a deeper single cell layer, the endothelium (Hu et al., 2013) (Fig. 1D–E). The adult corneal epithelium contains flat squamous epithelial cells in the apical layers, and cuboidal cells in the more basal layers. Towards the completion of metamorphosis in adult frogs (stage 66) (Nieuwkoop and Faber, 1956) the ventral eyelid has formed. A dorsal eyelid is also present, though more reduced in size. Later development and metamorphosis marks an important step in corneal maturation, as the corneal epithelium rapidly thickens during this time (Hu et al., 2013). In addition, studies by Hu et al. (2013), and Hamilton and Henry (2016) propose the existence of a limbal region, a wavy structure in the peripheral cornea of these post-metamorphic frogs, similar to the “Palisades of Vogt” observed in human corneas (Goldberg and Bron, 1982).
Fig. 1.
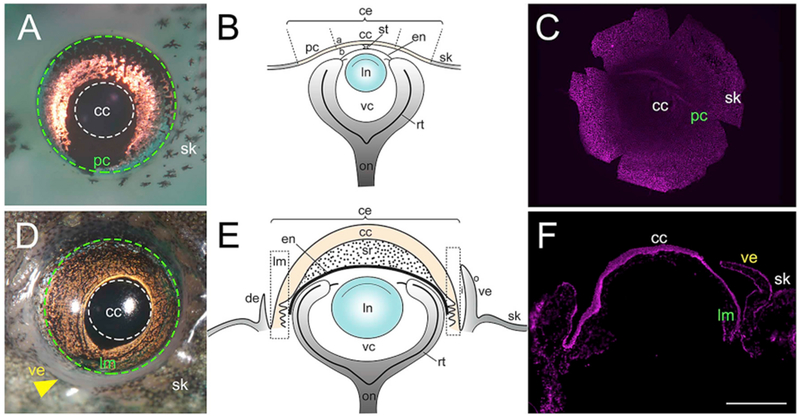
Larvae (stage 50–52) and adult (stage 66) Xenopus eyes. (A) Tadpole eye showing regions of the cornea. Region enclosed by white dotted line represents the central cornea (cc), green dotted line represents the region enclosing the peripheral cornea (pc), and the pigmented skin epithelium (sk) lies outside this line. (B) Schematic drawing of the larval Xenopus eye. The corneal epithelium is shaded light yellow, while the skin is shaded light grey. (C) Hoechst labeling in a larval corneal whole-mount. (D) Adult Xenopus eye. White dotted line encloses the central cornea (cc), green dotted line encloses the limbal region (lm), and yellow arrowhead points to the ventral eyelid (ve). The pigmented skin (sk) surrounds the eye. (E) Schematic drawing of the adult frog eye. The corneal epithelium is shaded light yellow, while the eyelid and skin is shaded light grey. (F) Hoechst labeling in a cross-section of adult Xenopus cornea, (a) apical surface of corneal epithelium, (b) basal surface of corneal epithelium, (ce) corneal epithelium, (de) dorsal eyelid, (en) corneal endothelium, (i) inner portion of eyelid, (In) lens, (o) outer surface of eyelid, (on) optic nerve, (rt) retina, (st) corneal stalk, (sr) stroma, (vc) vitreous chamber. Scale bar in F equals 470 μm for A, 520 μm for C, 800 μm for D, and 400 μm for F. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We have previously reported that “oligopotent stem cells” are distributed throughout the basal corneal epithelial layer of frog larvae (Perry et al., 2013). On the other hand, long-term BrdU or EdU label retaining cells (LRCs) are concentrated in the basal epithelium of the peripheral cornea (the limbal region) in adult frogs, suggesting that more quiescent stem cells come to reside in the limbal region as the cornea undergoes maturation (Hamilton and Henry, 2016). In the present study we used immunofluorescence staining to examine the expression of various molecular markers (including putative corneal stem cell markers, and pluripotency factors) in X. laevis corneas, during larval and post-metamorphic stages of development. Additionally, we consider the usefulness of these markers in the context of previously published studies for a variety of vertebrate systems.
This study represents the first detailed characterization of cell layers and changes in protein expression within the frog cornea. Here, we show that some of the molecular markers exhibit differential expression in the basal corneal epithelium of larval Xenopus. However, as metamorphosis proceeds in these amphibians, the localization of some markers in the mature corneal epithelium changes relative to the larval cornea. Furthermore, in adult frogs the inner and the outer surface of the eyelid have distinct patterns of marker expression. Our immunolabeling studies also demonstrate that Tcf7l2 is a biomarker that distinguishes cornea vs. skin in frogs, being only present in the skin of these animals. Collectively, our results show that the expression patterns for some of the proteins examined in the frog cornea are consistent with their localization in other vertebrates. However, the distribution pattern for other markers is in striking contrast. Furthermore, none of the tested markers is neither unique to larval or adult stages of corneal development, nor specifically distinguishes cells of the limbal region from the central cornea in tadpoles or adult frogs. Like other vertebrates, no single marker is specific to characterize the corneal SCs or TACs in the frog. Perhaps, a combination of molecular markers might be more useful to identify and study the behavior of these cells, and this needs further investigation.
The molecular characterization of different cell types in the Xenopus cornea sets the stage for further investigations to study the functional roles of these markers in corneal homeostasis and wound healing. One can also determine whether these proteins play a crucial role in lens regeneration, a phenomenon unique to larval stages of this vertebrate, in which new lenses arise from the basal corneal epithelial cells (e.g. stem cells and/or TACs) (Freeman, 1963; Perry et al., 2013).
2. Materials and methods
2.1. Animals
Adult Xenopus laevis were procured from Nasco (Fort Atkinson, WI). Fertilized eggs were prepared, and larvae were raised to specific stages according to previously published protocols (Hamilton and Henry, 2016; Henry and Grainger, 1987; Henry and Mittleman, 1995). The developmental staging for animals was based on Nieuwkoop and Faber (1956). The animals were anesthetized prior to all surgical and fixation procedures using a 1:2000 dilution of MS222 (ethyl 3-aminobenzoate methanesulfonate, Sigma, St. Louis, MO). Both animal care and experiments performed in this study were approved and monitored by the University of Illinois Institutional Animal Care and Use Committee and the Division of Animal Resources.
2.2. Antibody selection
There are relatively few antibodies that have been validated in Xenopus. Some of the listed antibodies (Vimentin, E-cadherin, β1-integrin, see Supp. Table S2) have been previously used in frogs (Chen et al., 1997; Edwards-Faret et al., 2018; Huang and Niehrs, 2014; Quigley et al., 2011). In addition, we obtained non-Xenopus validated commercial antibodies where the target epitope was conserved in Xenopus (with percent identity of 65% and greater; listed in Supp. Table S2). The specificity of each of these commercial antibodies in Xenopus was assessed by Western blotting. Only those antibodies which had a clear target band of the expected size on immunoblots were used for the immunofluorescence (IF) studies.
2.3. Western blot analysis
Corneas and skin tissue from 60 to 75 larvae (stages 50–52) were excised using fine iridectomy scissors, pooled together and flash-frozen in a dry ice/ethanol bath at −80 °C, as previously described (Henry et al., 2002). Protein was extracted from the samples by homogenizing in RIPA lysis buffer (25 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 0.005% sodium deoxycholate, 1% NP-40 and 1× protease inhibitor (GB Biosciences, Houston, TX)) using a disposable pestle. The protein concentration of each lysate was measured using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA) and assayed using a Nanodrop spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE). Next, the tissue homogenate was centrifuged for 15 min at 13,000 rpm at 4 °C, the supernatant was solubilized in Laemmli buffer, and boiled for 10 min. 20 μg protein sample was run in the well of 10% (or 12%) pre-cast sodium dodecyl sulfate-polylacrylamide gels (SDS-PAGE). Proteins separated by gel electrophoresis were electrophoretically transferred onto 0.45 μm PVDF membranes (Immobilon-P, Millipore Sigma, Burlington, MA) at 30 V overnight at 4 °C. Immunoblots were incubated in blocking buffer made of 5% nonfat milk in 1× TBST (Tris buffered saline; 10 mM Tris-HCl, pH 7.5, 100 mM NaCl with 0.1% Tween-20) for 1 h at room temperature. Following this, the blots were incubated with the primary antibody (listed in Supp. Table S2) diluted in blocking buffer overnight at 4 °C. On the next day, blots were washed with 1× TBST three times for 10 min each. After incubation with peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) diluted in the blocking solution for 1 h, the membranes were washed twice with 1× TBST for 10 min each. Protein bands were detected using HyGLO chemiluminescent substrate (Denville Scientific, Holliston, MA) and developed on X-ray films.
2.4. Immunofluorescent staining
2.4.1. Larval corneas
Following a modified version of the previously published protocol (Perry et al., 2013), the histological analyses of whole corneas were carried out by fixing the larval specimens in Dent’s fixative (80% methanol, 20% dimethyl sulfoxide, DMSO) (Dent et al., 1989). For immunostaining, the eyes were excised from each larvae taking care to keep the cornea attached to these eyes. Eyes were washed six times for 10 min each in 1× PBST (Phosphate buffered saline; 1.86 mM NaH2PO4, 8.41 mM Na2HPO4, 175 mM NaCl, pH7.4, containing 0.5% Tween-20), and blocked in 10% normal goat serum/1× PBST for 2h at room temperature. Next, the eyes were incubated with primary antibodies diluted in the blocking solution (for concentration used see Supp. Table S2) overnight at 4 °C. Six more washes with 1× PBST were done prior to incubating the eyes with secondary antibodies. Goat anti-mouse Alexa-Fluor 488 or 546, and goat anti-rabbit Alexa-Fluor 488 or 546 (Invitrogen, Rockford, IL) were used as secondary antibodies at a concentration of 1:300 diluted in blocking solution for 2 h. Following this, the eyes were washed twice with 1× PBST and incubated with 1 μg/ml solution of Hoechst 33342 (Molecular Probes, Eugene, OR) in 1× PBS for nuclear counter staining. Final washing of the eyes was done in 1× PBST. The labeled corneas were removed from the whole eyes, and small, peripheral, radial incisions were made to allow the corneas to flatten on the slide during mounting. The flattened corneas (pelts) were placed on RainX (ITW Global Brands, Houston, TX) treated slides, and mounted in SlowFade antifade reagent (Life Technologies, Eugene, OR) under coverslips (see Fig. 1C).
2.4.2. Adult corneas
Unlike the simpler organization of the larval stage cornea, the adult Xenopus cornea has many more cellular layers. Therefore, to better facilitate investigations of the expression patterns of markers in each cell layer, we performed immunofluorescent studies in cross-sections of the corneal tissue (Fig. 1F). To isolate fresh corneas, the froglets were euthanized by immersing in 250mg/L (wt/v) of benzocaine hydrochloride (Henry et al., 2018). The freshly harvested corneas were quickly embedded in Optimal Cutting Temperature media (Tissue-Tek, Sakura, Finetek, Japan) in a suitable tissue mold on dry ice and frozen. Transverse serial sections of 8 μm thickness were cut using a cryostat (Leica CM3050S, Leica Microsystems, Wetzlar, Germany), and collected on treated glass slides (Colorfrost Plus, ThermoFisher Scientific, Waltham, MA). Slides were stored at −80 °C and warmed to room temperature prior to use for immunostaining. For the purpose of immunostaining, a modified version of a previously reported method from zebrafish eyes was followed (Uribe and Gross, 2007). Slides were hydrated in 1× PBST (PBS containing 0.1% Tween-20) for 10 min, fixed with 4% paraformaldehyde (PFA) diluted in 1× PBS for 15 min, and washed three times with 1× PBST. Next, the slides were incubated in blocking solution (10% normal goat serum/1× PBST) for 1 h in a humidified chamber at room temperature to block the non-specific protein interactions. Following this, the slides were incubated with primary antibodies made in blocking solution (for concentration used see Supp. Table S2) overnight at 4 °C. Three more washes with 1× PBST were performed prior to secondary antibody incubation. All secondaries were used at a concentration of 1:500 diluted in blocking solution for 2h. Following this, slides were washed again with 1× PBST and nuclei were labeled with a 1 μg/ml solution of Hoechst 33342 in 1× PBS for 30 min. The sections were mounted in SlowFade antifade reagent (Life Technologies, Eugene, OR) under cover slips, and observed under fluorescence illumination.
As controls for each immunofluorescence labeling experiment, we omitted the primary antibody and incubated the larvae whole cornea and adult corneal cryosections with the secondary antibodies alone (diluted in blocking solution). For all of these secondary antibody controls no immunostaining was detected (data not shown).
2.5. Microscopy
Confocal imaging of labeled larval corneas was done using an inverted LSM 700 microscope (Carl Zeiss, Munich, Germany). The Z-stacks were processed with Zen software (Carl Zeiss, Munich, Germany). Immunofluorescence staining of cryosectioned-labeled corneas of adult frogs was captured using an Imager M2 microscope (Carl Zeiss, Munich, Germany). The images were compiled using Adobe Photoshop (Version CC, 2015.5).
3. Results
Using X. laevis corneas, we aimed to examine the expression pattern of 17 molecular markers that have been proposed to label either CESCs and/or TACs (Castro-Munozledo, 2013), or are involved in the maintenance of these cells (Higa et al., 2009; Kitazawa et al., 2016; Menzel-Severing et al., 2018; Nakatsu et al., 2011). In each case, commercial antibodies (Supp. Table S2) were obtained for the protein, and the antibody specificity was investigated by immunoblotting. Investigation of some other proposed markers (e.g. Keratin 14, Keratin 15, Hes1) was not possible due to the unavailability of antibodies with predicted immunoreactivity in Xenopus. Similarly, for some antibodies (e.g., Bmi1, N-Cadherin, C/EBPδ, Fzd7, KLF4, SOX2, ABCG2, ABCB5) specificity was a concern, as we did not detect a specific band of the expected size on immunoblots (Supp. Table S2). Hence, these markers were not considered further in this investigation. The following study demonstrates the localization of nine molecular markers, in the epithelial cells of larval and adult frog corneas and skin (adjoining the cornea).
3.1. Expression of transcription factor markers in corneal epithelia of larval and adult frogs
Various transcription factors (TFs) are key players in development, and are involved in SC functions such as establishing pluripotency, as well as in committing cells towards new lineages (Tam and Lim, 2008). Many of these TFs are known to play important roles in ocular surface maintenance and restoration. For instance, SOX9 is known to regulate the proliferation and differentiation of human CESCs (Menzel-Severing et al., 2018), KLF4 helps to maintain corneal epithelial integrity (Delp et al., 2015; Tiwari et al., 2017), and p63 expression is crucial for successful treatment of limbal stem cell deficiency (LSCD) (Rama et al., 2010).
3.1.1. p63
Given the essential role in epithelial proliferation and development, we first assessed the expression of p63. The nuclear transcription factor p63, a homologue of p53 and ancestral member of this family, is one of the most promising epidermal SC markers (Pellegrini et al., 2001). p63 is expressed in basal cells of different stratified epithelia, and also regulates proliferative potential in human keratinocytes (Parsa et al., 1999; Pellegrini et al., 1998). Loss of function of p63 in knockout mice leads to failure of epithelial stratification, and impaired development of the limb, tail, face and external genitalia (Yang et al., 1999). In most vertebrates including humans, p63 is predominantly expressed by the basal epithelial cells in the cornea (Chen et al., 2004; Moriyama et al., 2014; Pellegrini et al., 2001) (Supp. Table S1). Here, we extended these observations in an amphibian cornea. We used monoclonal anti-p63 (clone 4A4; see Supp. Table S2), and this antibody clone binds all p63 isoforms. The antibody has been used extensively in other studies, including in reports on Xenopus ocular tissue (Hu et al., 2013; Moriyama et al., 2014; Perry et al., 2013; Yang et al., 1999). Immunoblotting detected a specific band of ~ 75 kDa (Supp. Fig. S1A), which is in agreement with the previously reported molecular weight of p63 in Xenopus skin lysate (Tomimori et al., 2004). No p63 label was observed in the apical cells of the central and peripheral cornea in larvae (Fig. 2A and C, respectively). However, nuclear localization of p63 was detected in all basal cells throughout the central and peripheral corneal epithelium (Fig. 2B and D, respectively); consistent with our previous report (Perry et al., 2013). Basal staining of p63 was also seen in the surrounding pigmented skin epithelium, which is continuous with the transparent cornea (data not shown). In frozen sections of adult Xenopus corneas, p63 nuclear staining was found in the epithelial cells in the basal and intermediate layers of central and limbal regions (Fig. 2E and F, respectively), inner and outer surface of the eyelid (Fig. 2G), and the adjoining skin epithelium (Fig. 2H). Maximal expression was detected in basal cells of these regions when compared to the intermediate layer cells; however, no staining was found in apical cells. Distribution of p63-positive cells in the periphery of the Xenopus cornea (the limbal region) is akin to an earlier report showing p63 immunostaining in these mature frogs (Hu et al., 2013). However, Hu and his group (2013) describe that p63 immunostaining is undetected in the central cornea of mature frogs, which is contrary to our observations here.
Fig. 2.
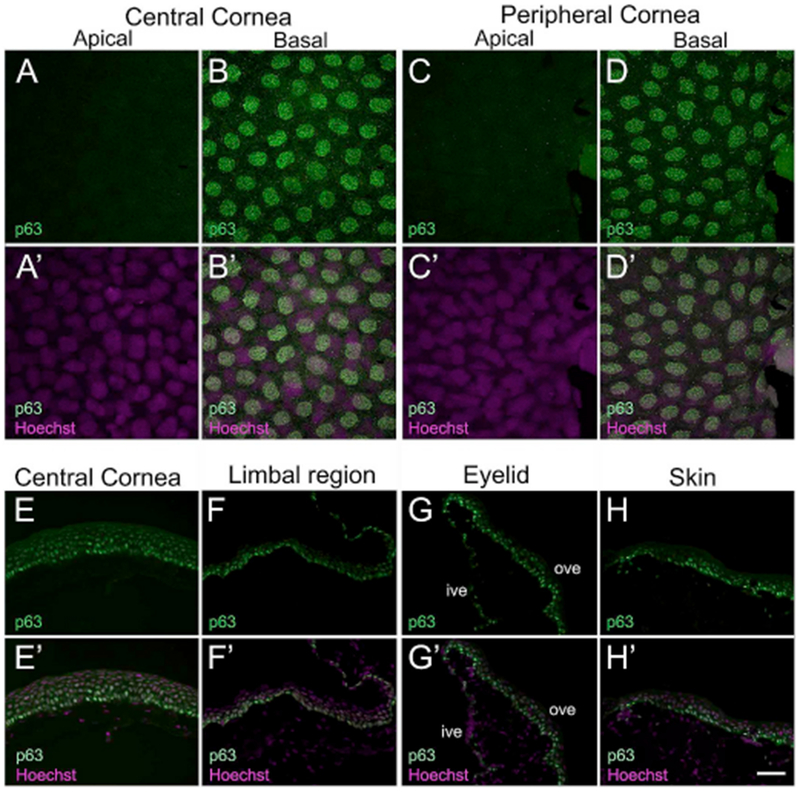
Confocal and fluorescence light microscopic images showing p63 antibody staining in X. laevis epithelial tissues. (A–D) Larval cornea whole-mounts showing p63 expression (green) is restricted to all basal cells (nuclei) of the central and peripheral cornea, as labeled. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) Apical cells of central cornea exhibit no p63 expression. (B-B′) Localized p63 labeling is seen in all nuclei of the basal epithelium. (C-C′) p63 is not expressed in apical cells of peripheral corneal epithelium. (D-D′) All nuclei of basal cells in the peripheral cornea have uniform expression for p63. (E–H) Localization of p63 (green) in cryosections of adult cornea, eyelid and skin. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (E′-H′) Merged images for E-H with Hoechst labeled nuclei (magenta). (E-E′) p63 labeling is detected in nuclei of basal and intermediate cells of the central cornea, but not in the nuclei of apical cells. The nuclei in the basal epithelium have relatively higher levels of p63 staining than those in the intermediate layer. (F-F′) In the limbal region, p63 label is primarily detected in nuclei of basal epithelial cells along with moderate expression in intermediate layer cells. (G-G′) Ventral eyelid showing p63 staining is localized to the nuclei of cells present mainly in the outer surface of the eyelid, with the cells in basal layer showing higher staining intensity. Some weak expression maybe seen in scattered basal cells within the inner surface of the eyelid. (H-H′) p63 expression is also detected in the surrounding skin epithelium, with nuclei in the basal layer showing higher expression compared to those of the intermediate layer cells. No staining is detected in cells of the outermost, apical layer of the skin, ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′, and 50 μm for E-H, E′-H′. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1.2. Pax6
The second transcription factor investigated in the frog cornea was Paired box 6 (Pax6). The Pax6 gene is highly conserved across vertebrates, and is critical for vertebrate eye development including the corneal epithelium (Davis and Piatigorsky, 2011; Shaham et al., 2012) (Supp. Table S1). In the anuran Xenopus, Pax6 represents a central transcriptional regulator of eye development and is involved in the remarkable phenomenon of lens regeneration (Gargioli et al., 2008; Henry and Tsonis, 2010). However, Pax6 localization in the frog corneal epithelium has not been extensively examined. We used a polyclonal Pax6 antibody that detected bands of ~47- and 54-kDa in the larval corneal lysate (Supp. Fig. S2B); consistent with bands of similar size that were observed using whole Xenopus embryos (Rungger-Brandle et al., 2010). However, no bands corresponding to the molecular weight of Pax6 were present in skin lysate. In the larval cornea, we did not detect Pax6 immunoreactivity in apical epithelial cells of central and peripheral cornea (Fig. 3A and C, respectively). On the other hand, we observed nuclear as well as cytoplasmic expression of Pax6 in basal epithelial cells of both the central and peripheral corneal regions (Fig. 3B and D, respectively). Fluorescence intensity of Pax6-positive cells in the basal epithelium of the central cornea (Fig. 3B) was slightly higher and these cells had a tight packing arrangement, compared to the fewer Pax6-positive basal cells in the peripheral cornea (Fig. 3D). Basal cells in the skin were negative for Pax6 (data not shown). In contrast to the larval cornea, no Pax6 staining was detected in the entire thickness of the adult frog cornea; both the central (Fig. 3E), and limbal region (Fig. 3F). The inner surface of the ventral eyelid (shown as “ive” in Fig. 3G) was also negative for Pax6. Surprisingly, cells in the apical layer of the outer ventral eyelid and surrounding skin show positive immunoreactivity for Pax6 (“ove” in Fig. 3G and H, respectively).
Fig. 3.
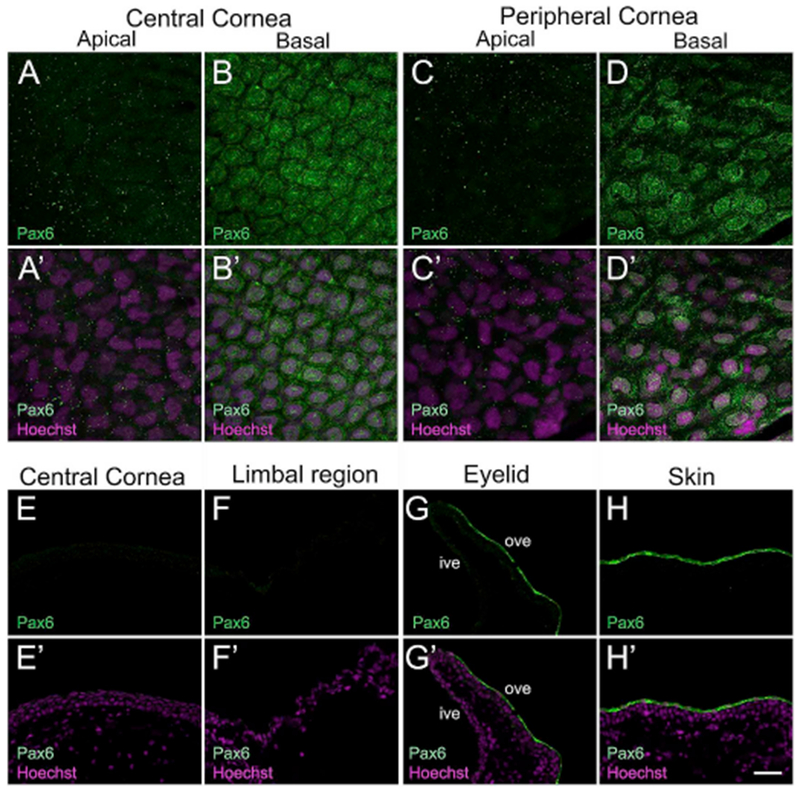
Confocal and fluorescence light microscopic images showing immunofluorescent staining for Paired box protein 6 (Pax6) in larval and adult frog epithelia. (A–D) Pax6 (green) localizes to the basal epithelial cells of central and peripheral cornea in the tadpoles, as labeled. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) Pax6 expression is undetectable in apical cells of the central cornea. (B-B′) Both the nucleus and cytoplasm of basal cells in the central corneal epithelium show uniform Pax6 staining. However, expression is excluded from the peri-nuclear region in these cells. Pax6-positive cells have a tight packing arrangement. (C-C′) Pax6 is not expressed in the apical cells of peripheral corneal epithelium. (D-D′) Basal cells in the peripheral cornea also express Pax6. Fewer Pax6-positive cells were detected in the peripheral region. (E–H) Cross-sections of adult frog cornea, eyelid and skin stained with Pax6 (green) antibody, as labeled. The apical surface is located towards the top of each image and the basal surface towards the bottom. (E′-H′) Merged images for E-H with Hoechst labeled nuclei (magenta). (E-E′) Pax6 staining is not observed in the central cornea. (F-F′) Pax6 expression is also undetected in the limbal region. (G-G′) Pax6 staining is noted only in apical cells of the outer surface of the ventral eyelid, where expression is primarily localized to the cytoplasm of these cells. (H-H′) A similar pattern of Pax6 expression is seen in apical cells in the skin epithelium, outside the cornea, ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′, and 50 μm for E-H, E′-H’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.1.3. Tcf7l2 (Tcf4)
We also examined the localization of another protein, Transcription factor 7 like 2 (Tcf7l2). A member of the high mobility group (HMG) transcription factors, Tcf7l2 plays an essential role in canonical Wnt signaling (Clevers and Nusse, 2012). Also referred to as “Tcf4”, it is among the four sub-families of vertebrate Tcf genes (Tcf7/Tcf1, Tcf7l1/Tcf3, Tcf7l2/Tcf4, and Lef) (Arce et al., 2006; Hoppler and Kavanagh, 2007). Tcf7l2 is involved in the long-term maintenance of various adult SCs, including those in the skin and cornea (Lu et al., 2012; Nguyen et al., 2009; van Es et al., 2012). The Western blot analysis with the Tcf7l2 antibody labeled a single band (~68kDa) in the tadpole skin lysate, while no band was detected in corneal lysate (Supp. Fig. S1C). In tadpoles, Tcf7l2 was expressed in the cytoplasmic compartment of apical epithelial cells of the skin surrounding the cornea (Fig. 4A–C). We noted that both the apical and basal cells in the central and peripheral region of the cornea were negative for this marker (Fig. 4D–G). Similarly, Tcf7l2 staining was undetected in the central cornea and limbal region of the adult frog (Fig. 4H and I, respectively). While the inner surface of eyelid did not express Tcf7l2 (shown as “ive” in Fig. 4J), the basal and intermediate cells in the outer surface of the eyelid (shown as “ove” in Fig. 4J), as well as the adjoining skin epithelium (Fig. 4K), were positive for this antibody. Interestingly, while in the larval cornea Tcf7l2 localizes to apical cells of skin epithelium (Fig. 4B), it is primarily restricted to basal and intermediate layer epithelial cells in the adult frog skin (Fig. 4K).
Fig. 4.
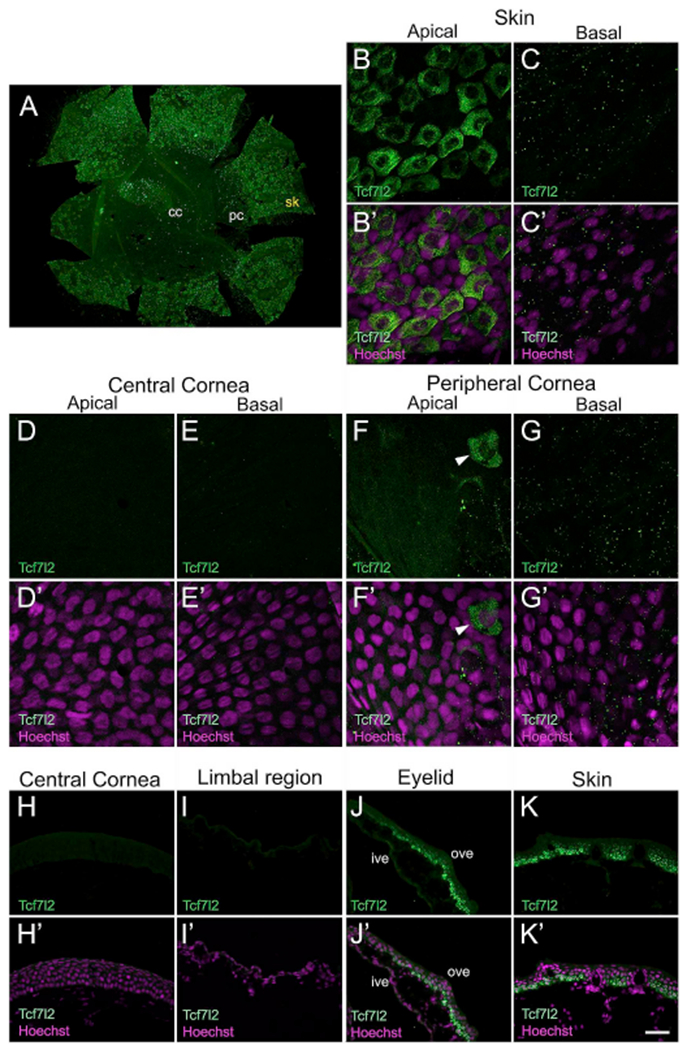
Antibody localization for Transcription factor 7 like 2 (Tcf7l2). Representative images from confocal and fluorescence light microscopy for larval and post-metamorphic frog corneas, as labeled. (A) Whole mount of larval Xenopus cornea showing presence of Tcf7l2 (green) labeled cells in the skin epithelium, surrounding the cornea. Tcf7l2-positive cells are rarely seen in the peripheral cornea. Regions labeled are those shown in B-G and B′-G’. (B–G) Higher magnification confocal images showing the distribution of Tcf7l2 (green) labeled cells in skin and cornea of larval animals, as labeled. (B′-G′) Merged images for B-G with Hoechst labeled nuclei (magenta). (B-B′) Apical cells of the skin epithelium exhibit Tcf7l2 antibody labeling. Tcf7l2 has cytoplasmic expression in a majority of these apical epithelial cells. (C-C′) Skin basal cells do not express Tcf7l2. (D-D′) No expression is observed in the apical cells of the central cornea. (E-E′) Basal cells in the central corneal epithelium also do not show presence of Tcf7l2. (F-F′) The apical epithelium of the peripheral cornea is predominantly devoid of Tcf7l2-positive cells. Tcf7l2 expressing cells (shown by white arrowhead) are rarely detected in the apical layer of the peripheral cornea. (G-G′) Basal epithelial cells of the peripheral cornea are devoid of antibody labeling. (H–K) Immunofluorescent staining for Tcf7l2 (green) on frozen sections of adult frog epithelia, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (H′-K′) Merged images for H-K with Hoechst labeled nuclei (magenta). (H-H′) Tcf7l2 staining is not present in the epithelial cell layers of the central cornea. (1–10 No Tcf7l2 expression is detected in cells of the limbal epithelium. (J-J′) Cells in the basal and intermediate layers of the outer surface of the ventral eyelid show Tcf7l2 staining, with no expression noted in apical cells. The inner surface epithelium of the eyelid is negative for this protein. (K-K′) Tcf7l2 expression is observed in the basal and intermediate cells of the surrounding skin epithelium. Superficial cells of skin epithelium do not label with this antibody, cc, central cornea; ive, inner ventral eyelid; ove, outer ventral eyelid; pc, peripheral cornea; sk, skin. Scale bar in K′ equals 200 μm for A, 25 μm for B-G, B′-G′, and 50 μm for H-K, H′-K’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. Expression of cytoskeletal proteins in corneal epithelia of larval and adult frogs
Cytoskeletal proteins are involved in regulating various cellular processes in epithelial tissues, such as mechanical support, motility, epithelial permeability, intracellular transport, and adhesion (Bragulla and Homberger, 2009; Fuchs and Yang, 1999). Numerous cytoskeletal elements, including the intermediate filament proteins, are expressed within the cornea and play important roles in maintaining intracellular epithelial architecture (Pitz and Moll, 2002). Alterations in the expression patterns of cytoskeletal proteins have been reported during various corneal pathological disorders (Elder et al., 1997; Ikeda et al., 2003).
3.2.1. Keratin 19
In the current study, we examined the expression of Keratin 19 (Krt19; also referred to as Cytokeratin 19, CK19). It belongs to the class ‘keratin’, which are epithelial-specific intermediate filaments expressed during development and differentiation of tissues, including the cornea (Bragulla and Homberger, 2009; Merjava et al., 2011). Krt19 is a known biochemical marker of cutaneous SCs in humans (Michel et al., 1996; Stasiak et al., 1989). It has also been suggested as a biomarker of SCs present in limbal basal epithelium in humans, and other vertebrates (Eghtedari et al., 2016; Yoshida et al., 2006) (Supp. Table S2). To characterize Krt19 in the frog cornea, a polyclonal Krt19 antibody was used. We detected a single band of ~ 50 kDa in corneal lysate, which corresponds to the protein’s predicted molecular weight in Xenopus (Supp. Fig. S1D). In tadpoles, Krt19 expression was cytoplasmic and lower in the apical cells of the central and peripheral cornea (Fig. 5A and C, respectively), compared to the basal cells of these regions (Fig. 5B and D, respectively). There was no significant difference in Krt19 labeling in basal epithelial cells of skin compared to that in the cornea (data not shown). Likewise, adult expression of Krt19 was observed in all cell layers of the central cornea (Fig. 5E), limbal region (Fig. 5F), and the inner surface of the ventral eyelid (shown as “ive” in Fig. 5G). However, expression was restricted only to basal cells lining the outer surface of the ventral eyelid (shown as “ove” in Fig. 5G), and surrounding skin epithelium (Fig. 5H).
Fig. 5.
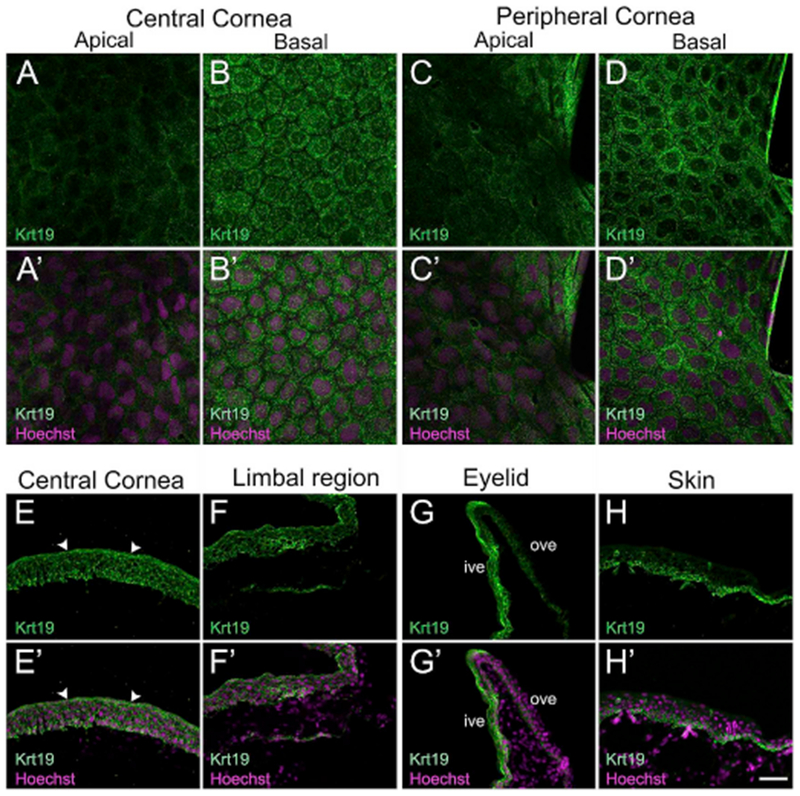
Confocal and fluorescence light microscopic images showing cellular localization of Keratin 19 (Krt19) in larval and post-metamorphic frog epithelia. (A–D) Antibody labeling in whole corneas showing the distribution and appearance of Krt19 (green) labeled cells in larval stages, as labeled. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) Apical cells of the central corneal epithelium exhibit very faint Krt19 labeling. (B-B′) Krt19 is primarily localized in the cytoplasm of cells in the basal epithelium of the central cornea. Krt19 labeling looks uniform throughout the basal layer. (C-C′) Apical epithelium of the peripheral cornea with very weak staining for Krt19. (D-D′) Localized Krt19 labeling in the cytoplasm of all cells in the basal epithelium of the peripheral cornea. (E–H) Krt19 (green) immunostaining in mature Xenopus cornea, eyelid and skin, as labeled. The apical surface is located towards the top of each image and the basal surface towards the bottom. (E′-H′) Merged images for E-H with Hoechst labeled nuclei (magenta). (E-E′) Krt19 is detected in all the epithelial layers of the central cornea. Krt19 predominantly localizes to the apical epithelial cells, where it forms a layer on the surface of the corneal epithelium (indicated by white arrowheads in E and E′). (F-F′) Krt19 localization is observed in all epithelial layers of the limbal area. (G-G′) Ventral eyelid showing Krt19 labeling. Inner surface of the eyelid has Krt19-positive cells in all the layers. The staining is restricted to cells in the basal layer of the outer surface of the eyelid. (H-H′) Skin epithelium showing Krt19 staining. The labeled cells are largely localized to the basal layer, with few positive cells found in the intermediate layers. The outermost layer of skin does not express Krt19. ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′, and 50 μm for E-H, E′-H′). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2.2. Vimentin
Next, we tested the localization of another cytoskeletal protein, vimentin, in the Xenopus corneal epithelium. Expression of vimentin, a widely occurring intermediate filament protein, is considered typical for mesenchymal-derived cells (Lazarides, 1980). It functions as a key player in cellular processes such as cell adhesion, migration, cytoskeletal arrangements, and regulation of cell morphology (Ivaska et al., 2007; Mendez et al., 2010). Vimentin is the major structural protein in corneal keratocytes (Jester et al., 1994), and is known to be expressed in some basal cells of corneal epithelia (Kasper, 1992; Lauweryns et al., 1993b) (Supp. Table S1). A Xenopus specific antibody was used to examine corneal localization of this filamentous protein. The antibody used has been reported in other studies involving frog spinal cord and skin (Edwards-Faret et al., 2018; Mescher et al., 2007). The specificity of this antibody was further validated by Western blotting and a single band of ~55kDa was observed (Supp. Fig. S1E). In larval cornea, neither the apical nor basal epithelial cells stain for vimentin (Fig. 6A, B, D, E). However, vimentin-positive cells were distributed below the basal epithelial layer in both the central and peripheral cornea, which are likely the keratocytes (Fig. 6C and F). Consistent with other reports (Jester et al., 1994), vimentin shows a filamentous cytoplasmic distribution around the nucleus, and also extends out along the cell processes of these keratocytes. Likewise, in adult Xenopus the corneal epithelial cells of central (Fig. 6G) and limbal zones (Fig. 6H) did not show presence of vimentin. However, the antibody labeled keratocytes located within the stromal layer of these regions. In addition, vimentin-positive cells with dendritic processes were seen in the dermis layer beneath the eyelid (Fig. 6I). In the skin of adults, we detected vimentin in the basal cells of the epidermis, as well as in the dermis (Fig. 6J). The presence of vimentin-positive cells with dendritic morphology in frog skin is consistent with a previous report (Mescher et al., 2007).
Fig. 6.
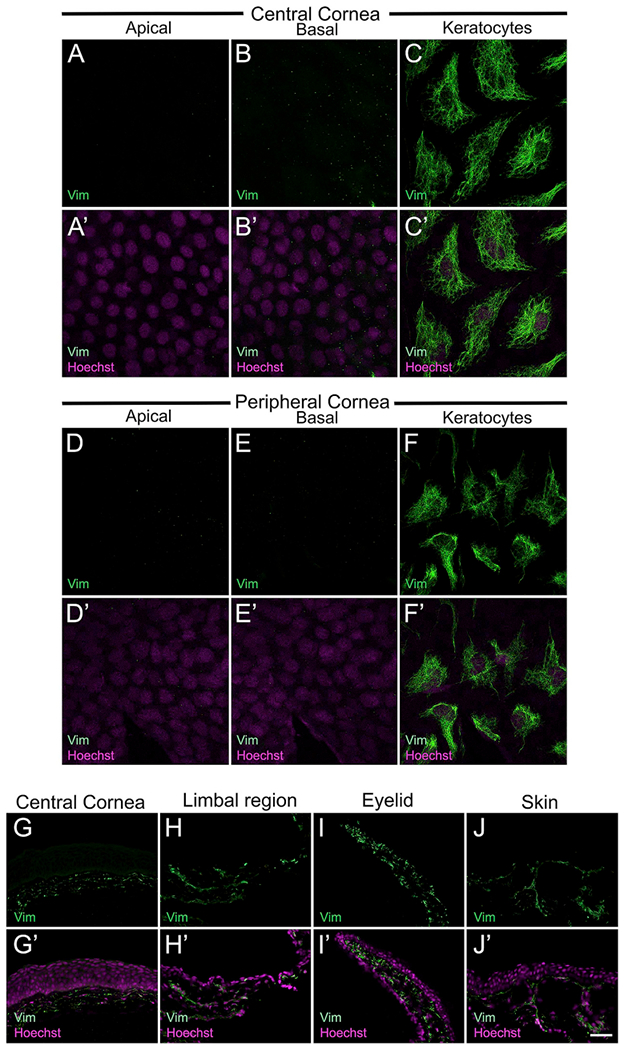
Immunolocalization of vimentin in larval and adult frog epithelia and deeper tissues. (A–F) Confocal images showing presence of vimentin (green) expressing cells, which are the keratocytes found below the basal layer of the larval corneal epithelium. (A′-F′) Merged images for A-F with Hoechst labeled nuclei (magenta). (A-A′) No vimentin-positive cells were observed in the apical layer of the central cornea. (B-B′) Basal cells in the central cornea also do not express vimentin. (C-C′) In the central cornea region, vimentin expression is found in keratocytes, which have a dendritic morphology. (D-D′) Apical cells of the peripheral cornea do not show stain with vimentin antibody. (E-E′) No vimentin localization is detected in basal epithelial cells of the peripheral cornea of larvae. (F-F′) Keratocytes of the peripheral cornea also express vimentin. (G–J) Fluorescence light microscopic images showing vimentin (green) labeling in cross-sections of adult Xenopus epithelia, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (G′-J′) Merged images for G-J with Hoechst labeled nuclei (magenta). (G-G′) Vimentin expression is detected in the keratocytes that are present in the stromal layer. No staining is noted in central corneal epithelium. (H-H′) Vimentin-positive cells are also present in the stroma underlying the limbal epithelium. (I-I′) The eyelid has vimentin labeled cells in the dermis beneath the epithelial layer. (J-J′) Skin epithelium has moderate expression of vimentin, only in the basal cells. The antibody also labels a much higher number of cells in the dermal layer of the skin, ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in J′ equals 25 μm for A-F, A′-F′, and 50 μm for G-J, G′-J’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. Expression of adhesion proteins in corneal epithelia of larval and adult frogs
Adhesion molecules are cell surface receptors, which play important roles in cell regulatory processes. Different classes of adhesion molecules promote cell-cell and cell-extracellular matrix interactions within the cornea, and thereby regulate homeostasis and immunobiology of the cornea (Vorkauf et al., 1995). Studies in humans and animal models of disease have reported that decreased expression of adhesion molecules in corneas is associated with various ocular degenerative disorders (Zhu and Dana, 1999).
3.3.1. β1-integrin
A key cell adhesion molecule investigated in our study was β1-integrin. Integrins are cell surface molecules that function as heterodimers and mediate (a) attachment of basal keratinocytes to extracellular matrix proteins in the basement membrane, (b) mediate intercellular adhesion, and (c) facilitate signaling from the extracellular environment (Li et al., 1998). Some members of the integrin family, including β1-integrin have been implicated as potential SC markers of epidermal keratinocytes (Jones and Watt, 1993). In vertebrate corneas including those of humans, mice and rats, β1-integrin is abundantly expressed by limbal basal cells (Chen et al., 2004; Pajoohesh-Ganji et al., 2006; Stepp et al., 1993) (Supp. Table S1). However, there are no reports of integrin expression in the amphibian cornea. The monoclonal antibody used was Xenopus specific (Supp. Table S2), and has been widely reported in β1-integrin examinations in frog tissues such as the retina (Li and Sakaguchi, 2002; Womble et al., 2018). Western blot analysis of corneal lysate detected a single band of ~115kDa (Supp. Fig. S1F), which corresponds to β1-integrin molecular weight reported in Xenopus epithelial cell extracts (Chen et al., 1997). In larval stages, no staining was detected in the apical cells of the entire corneal epithelium (Fig. 7A and C). In the basal corneal epithelium, β1-integrin immunolocalization was primarily membranous, although weak cytoplasmic expression was also seen (both central and peripheral corneal regions; Fig. 7B and D, respectively), β1-integrin staining pattern and intensity in the basal epithelial cells of the skin resembled that of the basal corneal cells (data not shown). Similarly, the epithelial cells in the central cornea, limbus, eyelid, and skin of adult frogs showed a membranous localization of β1-integrin (Fig. 7E–H). A gradient of β1-integrin expression was seen in all these regions; basal cells show a higher staining intensity compared to the cells in the intermediate and superficial layers. In addition, the stromal cells in the cornea also moderately express the protein, β1-integrin is known to be expressed by the keratocytes in the stromal layer, and is required for maintaining the structural integrity of developing mouse corneas (Parapuram et al., 2011). Based on our findings, the β1-integrin positive cells beneath the corneal epithelium (Fig. 7E–F) are most likely keratocytes.
Fig. 7.
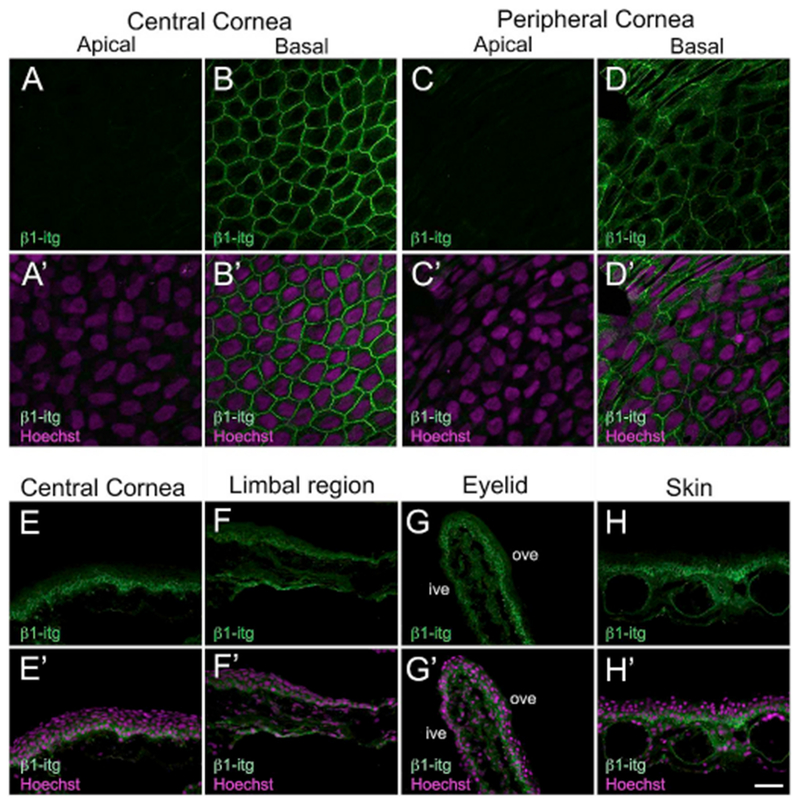
Immunolabeling of larval and adult Xenopus epithelia with β1-integrin (β1-itg). (A–D) Confocal images showing that expression of β1-itg (green) is restricted to basal epithelial cells of the tadpole cornea. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) Apical epithelial cells of the central cornea with no β1-itg label detected. (B-B′) β1-itg staining is predominantly membranous in the basal epithelial cells of the central cornea. Weak cytoplasmic expression is also observed in these cells. (C-C′) β1-itg staining is undetectable in the apical cells of the peripheral cornea. (D-D′) β1-itg stained basal epithelial cells in the peripheral cornea. (E–H) Fluorescence light microscopic images displaying β1-itg (green) antibody labeling on cryosections of adult Xenopus tissues, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (H′-K′) Merged images for H-K with Hoechst labeled nuclei (magenta). (E-E′) β1-itg is expressed throughout the entire thickness of the central corneal epithelium. A gradient in expression is observed, with the basal epithelial cells showing the highest expression. (F-F′) Limbal epithelial cells express β1-itg, with the maximum expression present in the basal layer. Moderate expression of β1-itg is seen in stromal cells underneath the corneal epithelium. (G-G′) Ventral eyelid showing β1-itg immuno reactivity. Basal cells in the outer surface of the ventral eyelid have higher expression of β1-itg. (H-H′) Epithelial cells in the skin display β1-itg labeling, with basal cells having the highest expression, ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′ and 50 μm for E-H, E′-H’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3.2. E-cadherin
We also examined the corneas for expression of E-cadherin. A key member of the Ca2+-dependent transmembrane receptors, E-cadherin plays an important role in mediating cell-cell adhesion (Gumbiner, 2000). This homophilic glycoprotein is crucial for maintenance of cell proliferation, survival and differentiation in various adult epithelial tissues, including the corneal epithelium in humans (Scott et al., 1997; Zelenka and Arpitha, 2008). Here, we used a Xenopus specific E-cadherin antibody to examine its expression in the frog cornea (Supp. Table S2); the antibody has been tested as highly specific in Xenopus embryonic tissue (Huang et al., 2016). Moreover, in Western blots using larval corneal lysate, we detected a band of ~140 kDa (Supp. Fig. S1G), which is in agreement to previous reports (Burns et al., 2013). In the larval stages, the antibody primarily labeled the membrane of apical corneal cells (both central and peripheral cornea regions; Fig. 8A and C, respectively). However, in the basal layer the immunostaining was membranous as well as faintly cytoplasmic (Fig. 8B and D). Similar E-cadherin expression was found in skin epithelial cells surrounding the cornea (data not shown). In the adult animals we noticed a uniform membranous expression of E-cadherin in the cells of the central cornea, limbal region, eyelid and the adjoining skin (Fig. 8E–H).
Fig. 8.
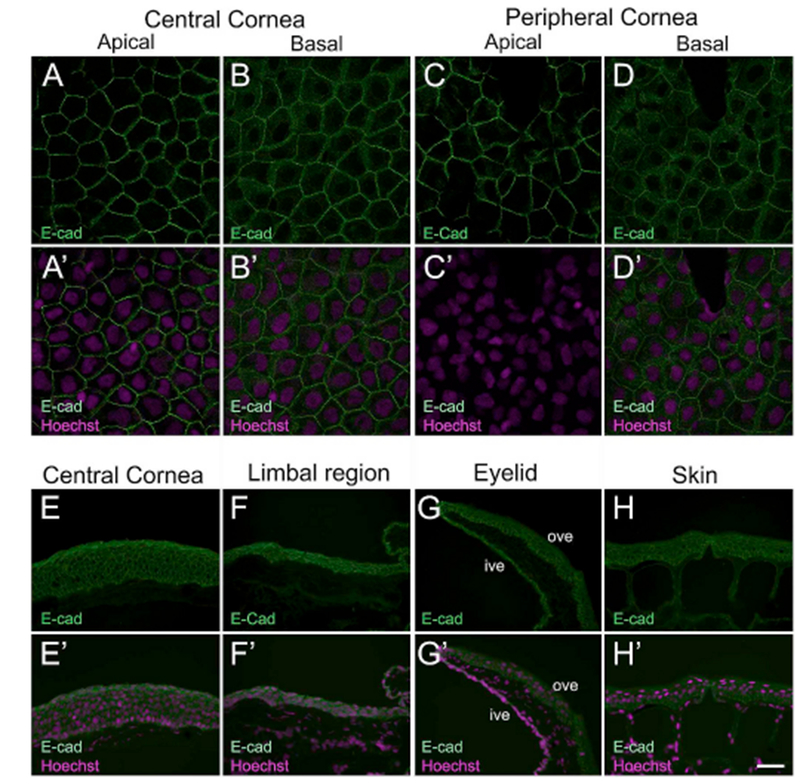
Larval and adult Xenopus epithelia stained with E-cadherin (E-cad) antibody. (A–D) Confocal images showing uniform distribution of E-cad (green) in the membranes of epithelial cells in a larval Xenopus cornea. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) E-cad localizes to the membranes in apical cells of the central corneal epithelium. (B-B′) Basal cells of the central corneal epithelium show E-cad expression predominantly associated with the membranes. Weak E-cad expression is also detected in the cytoplasm of the basal cells. (C-C′) Membrane bound E-Cad in apical cells of the peripheral corneal epithelium. (D-D′) E-cad labeling is associated with the membranes of basal epithelial cells of the peripheral cornea. (E-H) Fluorescence light microscopic images showing E-cad (green) staining on frozen sections of adult Xenopus epithelia, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (H′-K′) Merged images for H-K with Hoechst labeled nuclei (magenta). (E-E′) Epithelial cells throughout the entire thickness of the central cornea have uniform expression of E-cad in the membrane. (F-F′) E-cad localizes to the membrane of all epithelial cells in the limbal region. (G-G′) E-cad uniformly labels the membranes of epithelial cells in the inner and outer surface of the ventral eyelid. (H-H′) Cross-section of skin epithelium with E-cad membrane associated staining in the basal, intermediate and superficial layers, ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′, and 50 μm for E-H, E′-H’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3.3. β-catenin
In addition, we examined the expression pattern for β-catenin in the corneal epithelium of frogs, β-catenin, a key player in the canonical Wnt/β-catenin pathway, is an essential regulator of adult stem cells, tissue homeostasis and regeneration (Nusse, 2005; Ring et al., 2014). It is a central component of the cell adherens junction complex that controls cell-cell adhesion and influences cell migration (Nelson and Nusse, 2004). Along with multiple components of the Wnt signaling pathway, β-catenin is involved in ocular surface development, including that of the corneal epithelium (Nakatsu et al., 2011). To characterize the expression of β-catenin in Xenopus corneas, a monoclonal antibody was used (Supp. Table S2). The antibody used has been previously reported in studies on X. laevis embryos (Chiu et al., 2014; Zhu et al., 2015). The specificity was further confirmed by Western blot analysis, and a band size corresponding to the predicted molecular eight was observed (~ 100 kDa; Supp. Fig. S1H). Membrane bound β-catenin was evenly expressed in the apical layer of the tadpole corneal epithelium (central and peripheral regions; Fig. 9A and C), with no staining detected in the basal cells (Fig. 9B and D). No nuclear localization of the protein was observed in these larval corneal epithelial cells. Unlike the differential localization found in tadpoles, we noted uniform expression of β-catenin in the adult frog cornea. The protein was detected in the membrane of all epithelial cells of the central cornea, limbal region, eyelid, and surrounding skin (Fig. 9E–H).
Fig. 9.
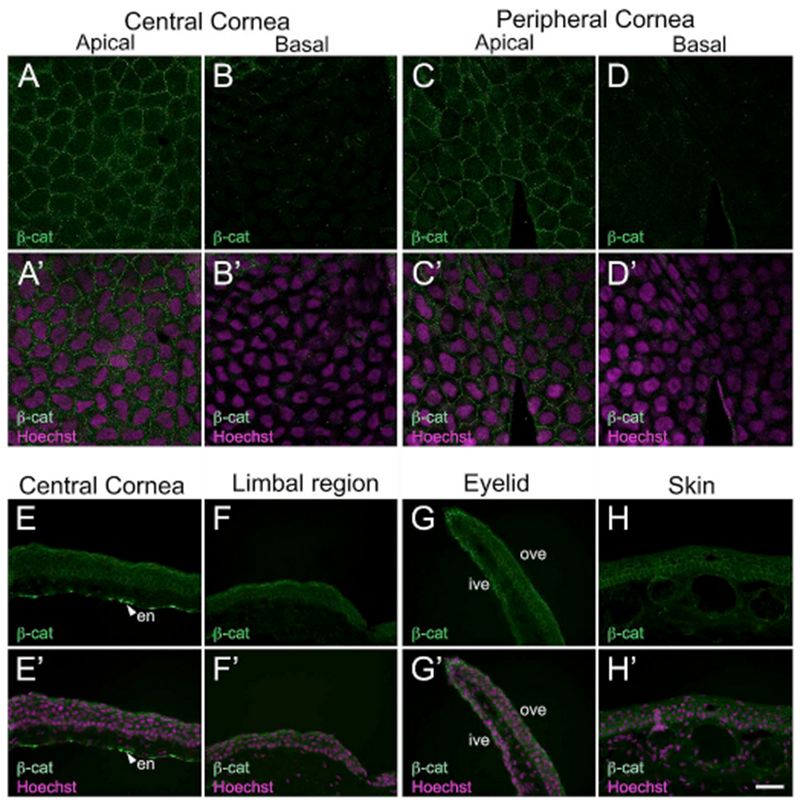
Immunofluorescent localization of β-catenin (β-cat) in larval and adult Xenopus epithelia. (A–D) Confocal images showing restricted localization of β-cat (green) in apical epithelial cells of the larval cornea. (A′-D′) Merged images for A-D with Hoechst labeled nuclei (magenta). (A-A′) β-catenin is associated with the membranes of epithelial cells found in the apical layer of the central cornea. (B-B′) No label is detected in the basal epithelial cells of the central cornea. (C-C′) Apical epithelial cells in the peripheral cornea have membrane-bound expression of β-catenin. (D-D′) Basal epithelial cells show no immunoreactivity with the β-catenin antibody. (E–H) Fluorescence light microscopic images display β-catenin (green) localization in cross-sections of adult frog epithelial tissues, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (H′-K′) Merged images for H-K with Hoechst labeled nuclei (magenta). (E-E′) β-catenin is widely expressed throughout the full thickness of the central cornea. Staining is also found in the corneal endothelium (en) and is denoted by white arrowheads in E and E′. (F-F′) Epithelial cells in the limbal region also show positive reactivity to the β-catenin antibody. (G-G′) Epithelial cells lining the inner and outer portion of ventral eyelid uniformly express β-catenin. (G-G′) Skin epithelial cells in all layers have membranous β-catenin expression, similar to that found in the cornea, en, corneal endothelium; ive, inner ventral eyelid; ove, outer ventral eyelid. Scale bar in H′ equals 25 μm for A-D, A′-D′, and 50 μm for E-H, E′-H’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Expression of growth factor receptors in corneal epithelia of larval and adult frogs
Growth factor (GF) receptors play an important role in corneal epithelial maintenance and wound healing. The SCs in the corneal epithelium have been proposed to express these receptors, which facilitate the maintenance of their proliferative potential (Nakamura et al., 2001; Zieske and Wasson, 1993) (Supp. Table S1).
3.4.1. p75
In the current study, we examined expression of the low-affinity nerve growth factor receptor (p75). Nerve Growth Factor (NGF), and its transmembranous receptors (p75 and tyrosine kinase receptor A (TrkA)) are neurotrophic factors which primarily regulate development, survival, and maintenance of cells of neuronal origin (Barbacid, 1995). In addition, p75 regulates properties of the ocular surface cells (Barbacid, 1995; You et al., 2000). p75, a 75- to 80-kDa glycoprotein belongs to the superfamily of cytokine receptors, and is a low affinity receptor for all members of the neurotrophin family, including NGF (Bothwell, 1991). p75 has been classified as a SC marker in a wide variety of human tissues, including those of epidermis, hair follicles, and cornea (Akiyama et al., 1996; Di Girolamo et al., 2008; Tomellini et al., 2014). For immunolocalization, a monoclonal p75 antibody was used (Supp. Table S2). The specificity was tested by Western blot analysis using the tadpole skin lysate. A specific band of ~75kDa was detected (Supp. Fig. S1I), which corresponds to other reports involving Xenopus tissue (Kanning et al., 2003; Yang et al., 2009). In corneal whole-mounts, we detected higher number of p75 brightly labeled cells in apical layer of skin (Fig. 10A–B). However, the basal skin cells expressed low levels of p75 (Fig. 10C). We did not detect p75 in apical epithelial cells of central cornea (Fig. 10D), while basal cells had moderate p75 expression (Fig. 10E). In the peripheral cornea, a few cells that labeled strongly with p75 (cytoplasmic expression) were found in the apical layer (Fig. 10F). Expression of p75 in peripheral basal cells (Fig. 10G) was similar to that observed in central basal corneal cells, and was continuous in the skin as well (data not shown). In the adult corneal cross-sections, p75 was widely expressed in the epithelial cells of central region (Fig. 10H), limbal region (Fig. 10I), eyelid (Fig. 10J), and skin (Fig. 10K). Interestingly, apical cells in these regions had slightly higher expression than cells in basal and intermediate layers.
Fig. 10.
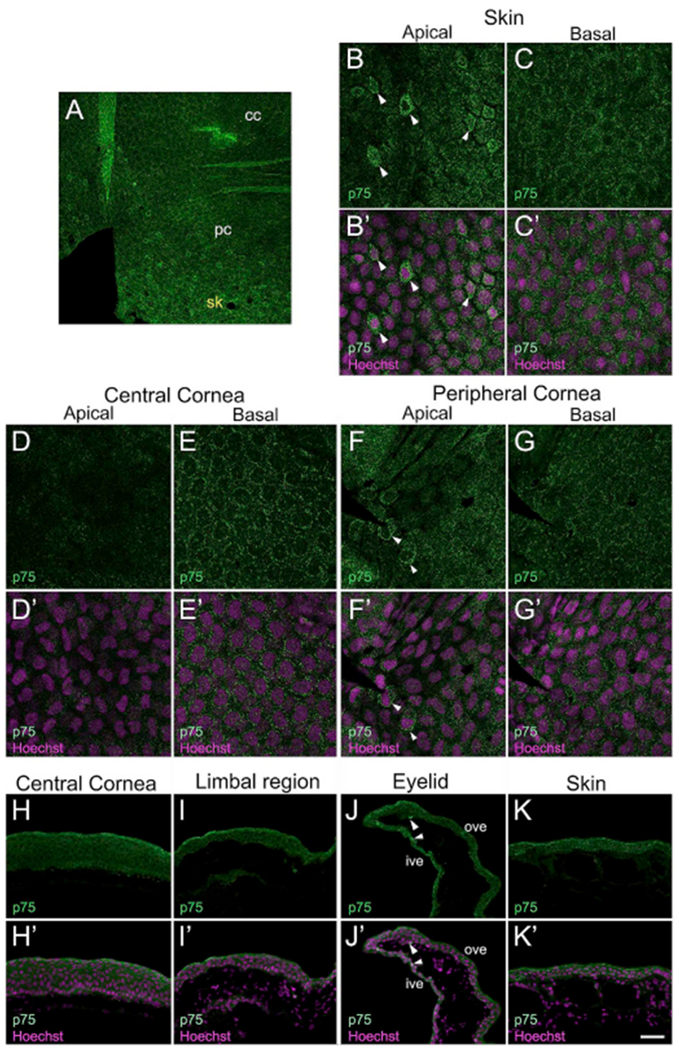
Cellular localization of the transmembrane receptor, p75 in frog epithelial tissues. Confocal and fluorescence light microscopic images show p75 (green) labeling in larval and mature frog corneas. (A) Representative region of a larval whole-mount cornea showing a few scattered p75 more brightly labeled cells in the peripheral cornea (pc), and a higher number of p75 brightly labeled cells distributed in the skin (sk) outside of the cornea. No p75 brightly labeled cells were observed in the central cornea (cc). Higher magnification confocal images of these regions are shown in B-G. (B–G) Distribution of p75 (green) labeled cells in the skin and the cornea of larval animals. (B′-G′) Merged images for B-G with Hoechst labeled nuclei (magenta). (B-B′) The apical epithelium of the skin has cytoplasmic localization of p75 in a higher number of cells (marked by white arrowheads). (C-C′) Moderate expression of p75 is detected in the perinuclear region of basal skin epithelial cells. (D-D′) p75 expression is undetectable in the apical epithelium of the central cornea. (E-E′) Basal cells of the central cornea have peri-nuclear expression of p75. (F-F′) High p75 expression is noted in a few apical epithelial cells of the peripheral cornea (marked by white arrowheads). (G-G′) Basal cells in the peripheral cornea have faint expression of p75 in the peri-nuclear region. (H–K) Immunofluorescent staining for p75 (green) on frozen sections of the adult frog epithelial tissues, as labeled. The apical surface is located towards the top of each image, and the basal surface towards the bottom. (H′-K′) Merged images for H-K with Hoechst labeled nuclei (magenta). (H-H′) p75 is expressed throughout the full thickness of the central corneal epithelium. The localization is membranous, as well as cytoplasmic. (I-I′) Epithelial cells present in all the layers of the limbal region have p75 staining. (J-J′) Ventral eyelid showing p75 localization. The epithelia of both inner and outer surfaces of the ventral eyelid have p75 expression. A few cells more brightly labeled with p75 are also detected (marked by white arrowheads). (K-K′) The skin epithelium has p75 expression in all the cellular layers, cc, central cornea; ive, inner ventral eyelid; ove, outer ventral eyelid; pc, peripheral cornea; sk, skin. Scale bar in K′ equals 60 μm in A, 25 μm for B-G, B′-G′, and 50 μm for H-K, H′-K’. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.5. Expression of Pax6 and Tcf7l2 in corneal epithelia of larval and adult frogs
To better characterize the boundary of cornea and skin in the anurans, we performed immunolocalization of Pax6 and Tcf7l2. Co-staining larval corneas with these antibodies showed that Pax6 is expressed only in the basal corneal epithelial cells and gradually decreases from cornea into the skin; simultaneously, Tcf7l2 is expressed by apical epithelial cells of skin outside the corneal region (Fig. 11A–C). In the adult corneal sections we did not detect Pax6 and Tcf7l2 label in either the central or limbus region (Fig. 11D–E). Both Pax6 and Tcf7l2 labeling were observed in the outer surface of the ventral eyelid and continued in the skin epithelium (Fig. 11F–G).
Fig. 11.
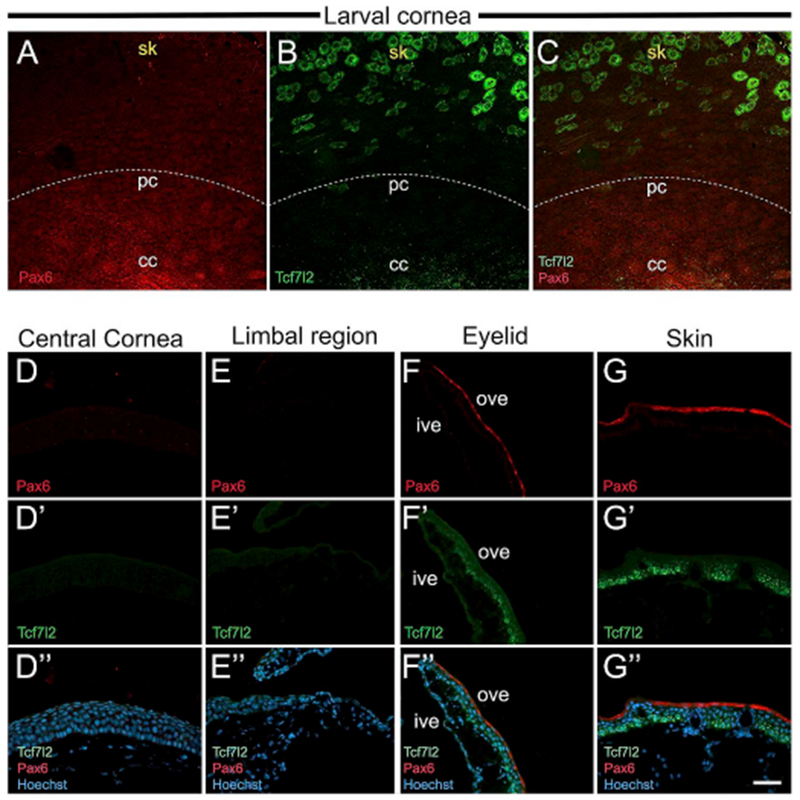
Larval and adult frog epithelia immunostained with Pax6 and Tcf7l2 antibodies. (A–C) Lower magnification confocal images of larval whole-mount cornea showing Pax6 (red), and Tcf7l2 (green) co-staining. The white dotted line represents the boundary demarcating the cornea and skin. (A) A gradient of Pax6 expression is higher in the central cornea (cc), and is lost progressively towards the peripheral cornea (pc), and skin (sk). (B) Tcf7l2-positive cells are abundant in the skin region. (C) Merged image showing Pax6 and Tcf7l2 expression. (D-G, D′-G′ and D”-G″) Cross-sections of adult frog cornea, eyelid and skin immunolabeled with Pax6 (red) and Tcf7l2 (green). The apical surface is located towards the top of each image, and the basal surface towards the bottom. D″, E″, F″ and G″ are merged images for D, D’; E, E’; F, F′ and G, G′, respectively, with Hoechst labeled nuclei (blue). (D, D′ and D″) No Pax6 or Tcf7l2 expression is detected in the central cornea. (E, E′, and E″) The limbal region has undetectable Pax6 or Tcf7l2. (F, F′ and F″) Ventral eyelid region showing Pax6 and Tcf7l2 expression. The inner surface of the eyelid does not stain for either antibody. Outer surface of the eyelid has Pax6 in the apical cells, and Tcf7l2 mainly in the basal layer of cells. (G, G′ and G″) Skin of the adult frog has Pax6 in apical cells of the epithelium, and Tcf7l2 in the basal and intermediate layers of cells, cc, central cornea; ive, inner ventral eyelid; ove, outer ventral eyelid; pc, peripheral cornea; sk, skin. Scale bar in G″ equals 60 μm for A-C, 50 μm for D-G, D′-G′, D”-G″. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Our study revealed that the Xenopus corneal epithelium expresses a range of molecular markers belonging to different functional groups. Many of these proteins have been proposed to identify CESCs and TACs, and/or involved in the maintenance of these cells. We have shown a detailed expression of these proteins in the cellular layers of central and peripheral/limbal regions of the frog cornea (summarized in Fig. 12). We identified significant similarities, as well as key differences in the localization of each of these proteins between the frog and earlier studies in the corneas of other vertebrate species (Supp. Table S1). In addition, we examined the localization of these markers in the skin epithelium of these amphibians. The majority of these factors (except Tcf7l2 and Pax6) have a uniform expression pattern in epithelial cells of the cornea as well as the skin.
Fig. 12.
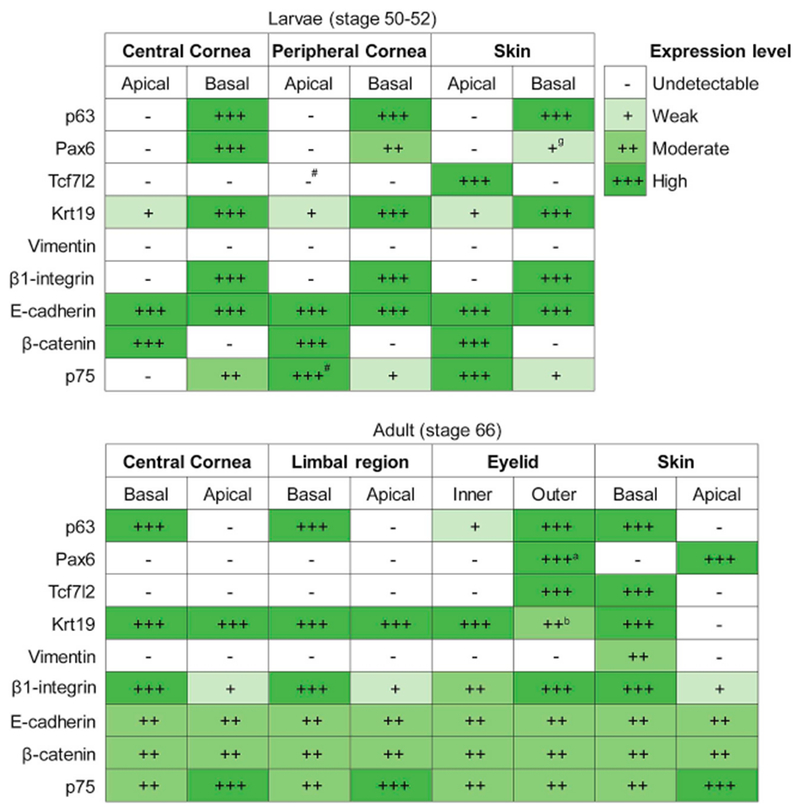
Summary of protein localization detected in the epithelial tissues of larval and adult Xetiopus, as indicated. Relative expression levels are represented as undetectable, weak, moderate and high, as shown in the key. ‘#’ denotes few positive cells; ‘a’ denotes expression only in the apical layer; ‘b’ denotes expression only in the basal layer; ‘g’ denotes gradient in expression.
4.1. p63 is not a unique corneal stem cell marker in frogs
The transcription factor p63 plays a significant role in corneal development, and maintains the proliferative potential of corneal stem cells (Di Iorio et al., 2005; Shalom-Feuerstein et al., 2011). It is essential for maintenance of corneal transparency, and p63 mutations lead to multiple eye abnormalities including LSCD (Di Iorio et al., 2012). In the initial studies of p63 in the human ocular surface (Chen et al., 2004; Pellegrini et al., 2001), it was immunodetected only in the nuclei of limbal basal epithelial cells, but not in basal epithelial cells covering the central cornea. These p63-positive cells were interspersed with p63-negative cells in the limbus. More specifically, the α isoform of ΔNp63 (N-terminal truncated p63) expressed in the limbus, is known to be essential for supporting the proliferative potential of corneal SCs (Di Iorio et al., 2005). These observations led investigators to propose that p63 is a marker of human corneal stem cells. Interestingly, in fetal human corneas, equivalent levels of p63 expression were found in clusters of basal cells in both the limbal and central corneal epithelia (Davies et al., 2009). Analogous to the developing fetal cornea, another study using frozen and formalin-fixed adult human corneas revealed that p63 was found not only in limbal basal cells but also in basal cells of the central cornea (Dua et al., 2003).
The pattern of p63 immunostaining observed by Dua et al. (2003) in human corneas was similar to that reported in mice. In adult mice, p63 isoforms were detected throughout the basal layer of the corneal epithelium (Collinson et al., 2002; Moore et al., 2002). These reports provided support for the arguments made later by Majo et al. (2008), that SCs may not be restricted to the limbus, but are rather distributed throughout the corneal epithelial basal layer. A recent study by Li et al. (2017) confirms that cells expressing ΔNp63 are localized throughout the basal layer of the adult murine corneal epithelium. Contrary to this, p63 expression in the corneas of the adult rat were found to be largely restricted to basal cells in the limbal region (Moore et al., 2002; Umemoto et al., 2005). However, another study in the rat corneas revealed age-related changes in the distribution of p63-positive cells (Hsueh et al., 2004). Initially, the p63-positive cells are present in the central cornea and limbal region of young rats, whereas these cells become confined to basal and suprabasal layers of the limbus in adult rats. The same study also highlighted the dependence of p63 staining on experimental procedures; a higher percentage of p63 labeled cells was noted in basal and suprabasal layers of the limbus when a more sensitive antigen retrieval method was used.
Likewise, one finds significant variations in corneal p63 expression among other vertebrates. Immunohistochemical examination in rabbit corneas has shown that p63-positive cells are restricted to the nuclei of the basal and suprabasal cells of the limbal epithelium, whereas no signal was detected in the central cornea (Hsueh et al., 2004; Wang et al., 2003). p63 labeled cells in the cornea of this mammal are largely distributed in a manner similar to that seen in the human limbus (Pellegrini et al., 2001). Studies on pig eyes provided evidence for anatomical similarities between the porcine and human limbus (Notara et al., 2011; Patruno et al., 2017). Although these reports reveal the presence of p63 in the basal cells of crypts in the porcine limbal region, they do not include data on whether p63 is expressed in the central cornea. On the other hand, in the ocular surface of domestic animals including horses (Linardi et al., 2015; Moriyama et al., 2014) and beagles (Morita et al., 2015), the localization of p63 was widely detected in basal and suprabasal cells of both the central cornea and limbus, with no staining found in apical layers. The strongest expression of p63 was observed in the basal layer of the limbus in both of these species.
Our IF results (Fig. 2) confirm age-related differences in the distribution of p63-positive cells in Xenopus corneas; being restricted to the basal layer in larvae, and present in both basal and intermediate layer cells in post-metamorphic frogs. Localization of p63 labeled basal cells in the larval cornea predominantly mirrors the p63 expression pattern in mice (Moore et al., 2002). However, p63 expression in the cornea epithelium of adult frogs is highly similar to some reports in the corneas of humans (Dua et al., 2003), horses, and dogs, but contradicts observations reported for adult rats, and rabbits (see Supp. Table S1). Our findings corroborate the notion that basal p63-positive cells do not uniquely represent, but at best include the SCs of the corneal epithelium. The presence of p63 labeled cells is rather indicative of the proliferating cells in the corneal epithelium, which would include both CESCs and TACs. Although p63 is one of the widely cited CESC markers (Mort et al., 2012), none of the studies so far has conclusively demonstrated it as a unique marker for corneal SCs. Taking account of our own results, and interspecies differences in p63 expression patterns, it is evident that p63 is not evolutionarily conserved enough to be regarded as an exclusive CESC marker.
4.2. Pax6 label is restricted to basal epithelial cells of the larval cornea, and is lost upon maturation
Pax6 is known to play prominent roles during the development and homeostasis of corneal epithelium (Ouyang et al., 2014). It regulates the expression of the corneal keratin 12 (KRT12) gene (Liu et al., 1999), controls the expression of corneal adhesion-related molecules (Davis et al., 2003), as well as regulates corneal epithelial cell migration and proliferation (Collinson et al., 2004). Furthermore, Pax6 is crucial for the maintenance and proliferation of corneal SCs, as well as their commitment to the corneal epithelial lineage (Li et al., 2015). Mutations in the human PAX6 gene lead to ocular conditions like aniridia and Peter’s anomaly, which are characterized by corneal opacification (Glaser et al., 1994; Hanson et al., 1994). Similar corneal abnormalities are also seen in mutant Pax6 mouse models (Ramaesh et al., 2003).
Immunohistochemical studies in fetal and adult human corneas have shown that Pax6 is expressed in the nucleus of all cells distributed throughout the central and limbal epithelium (Kitazawa et al., 2016; Lyngholm et al., 2008; Nishina et al., 1999; Ouyang et al., 2014) (see Supp. Table S1). Similarly, Pax6 is expressed in nuclei of epithelial cells found in the central cornea, and limbal regions of adult mouse and cynomolgus monkey eyes, and is likely required for maintenance and proliferation of corneal stem cells (Davis and Piatigorsky, 2011; Koroma et al., 1997). Early immunohistochemical studies on chick embryos also support the presence of Pax6-positive nuclei in the corneal epithelium; however, the study did not examine later stages of chicken corneal development (Koroma et al., 1997). In contrast, Pax6 is expressed only in the basal cells of the limbal epithelium of adult rat corneas (Zhao et al., 2002). However, that study does not provide evidence about whether Pax6 staining is in the central corneal epithelium in rats.
Akin to other vertebrates, the expression and function of Pax6 in X. laevis ocular growth has been studied extensively, particularly in the context of retina and lens development (Rungger-Brandle et al., 2010; Yoshii et al., 2007). However, little is known regarding Pax6 expression in this genus during corneal development. In Xenopus larvae, the corneal epithelium is known to be the source of regenerating lenses following lentectomy. In addition, pax6 gene expression, found only in the cornea but not observed in the epidermis, was shown to be a crucial factor for this lens-regeneration ability (Gargioli et al., 2008; Henry et al., 2002). Our results (Fig. 3) in the larval stages showing restricted Pax6 expression in the basal corneal epithelium supports these findings. Predominantly, Pax6 is present in the nuclear compartment of basal corneal epithelial cells. However, our IF results show that in addition to nuclear localization, Pax6 is found in the cytoplasm of these basal cells. Similar cytoplasmic Pax6 localization has been observed by some investigators in the adult mouse cornea, being present only in the basal cells of the limbal epithelium (Pearton et al., 2005). Furthermore, our observations showing Pax6 expression being restricted to the apical epithelial cells of the adult frog skin are in striking contrast to reports of Pax6 being predominantly absent in the skin of other species (Li et al., 2008; Pearton et al., 2005). This is the first report of Pax6 expression in post-metamorphic frogs, and the reasons for this aberrant expression are still unknown. However, we speculate that the absence of Pax6 expression in the corneal epithelium of mature frogs might be an underlying cause of its greatly diminished lens regenerative competency (Filoni et al., 1997; Hamilton and Henry, 2016).
4.3. Tcf7l2 (Tcf4) expression distinguishes cornea vs. skin in larval and adult frogs
Tcf7l2 is required for maintaining the properties of ocular stem cells, including those in the cornea and conjunctiva (Lu et al., 2011; Quan et al., 2016). An in vitro study by Lu et al. (2012) has shown that Tcf4 maintains human CESCs in a less differentiated state, and mediates corneal wound healing. Using IF staining on corneal frozen sections, that study also demonstrated that Tcf4 is largely localized to the cytoplasm and nuclei of limbal basal cells. It was not expressed in the apical layer of the limbal epithelium, or in any layer of the central corneal epithelium. Furthermore, other studies using cultured corneal epithelial stem cells provide evidence for expression of Tcf4, suggesting its use as a positive marker for selection of these cells (Mikhailova et al., 2016). However, little is known about the expression of this protein in corneal epithelial cells of other vertebrate species.
Prior studies in Xenopus have examined the transcript level expression of tcf7l2 in different tissues (Bandin et al., 2015; Michiue et al., 2017). In fact, in a study examining Xenopus cornea to lens transdifferentiation, the tcf7l2 gene was found to be upregulated during lens regeneration (Day and Beck, 2011). However, in our study we did not detect expression of the Tcf7l2 in the cornea epithelium of either tadpoles or adult frogs (Fig. 4). Rather, it was localized in the epithelial cells of skin surrounding the cornea, in both the larvae and adult frogs. The presence of Tcf7l2 in basal epithelial cells of the skin and outer surface of the eyelid in post-metamorphic frogs is somewhat consistent with a previous observation in mice, where Tcf7l2 antibody is known to label hair follicle stem cells in the basal layer of skin (Nguyen et al., 2009). Our finding illustrates that although Tcf7l2 is not suitable as a biomarker of corneal epithelial cells in frogs, it can be used as a molecular marker to demarcate cornea vs. skin in these amphibians.
4.4. Expression of Keratin 19 in Xenopus corneal epithelium changes during maturation
The intermediate filament protein Krt19 constitutes a minor cytoskeletal component of the human corneal epithelium (Pitz and Moll, 2002), and plays an important role in regulating epithelial cell shape and migration. Kasper et al. (1988) reported for the first time a “mosaiclike” staining pattern of Krt19 in the peripheral region of adult human corneal epithelium, whereas the central cornea was completely negative for this marker. Notably, the superficial cells in the limbal epithelium were also uniformly positive for Krt19. That study also reported homogeneous distribution of Krt19 in the fetal corneal epithelium. Different patterns of Krt19 expression have been reported in subsequent studies (Supp. Table S1). Some investigators report that Krt19 is located in all layers of the limbal epithelium (staining intensity decreases from basal to superficial layers), and undetectable in the central corneal epithelium (Elder et al., 1997; Lauweryns et al., 1993a; Merjava et al., 2011). On the other hand, a few studies reveal no difference in staining intensity between limbal and central corneal epithelium (Chen et al., 2004; Yoshida et al., 2006). Despite these variations, the studies share similar observations regarding cytoplasmic localization of Krt19 in limbal basal epithelial cells.
Like humans, disparities exist regarding Keratin 19 staining patterns in the mouse cornea. Based on one study, Krt19 was not expressed in the central cornea but was found in basal limbal epithelial cells in adult mice (Yoshida et al., 2006). On the other hand, Krt19 expression was found to be uniform throughout the embryonic mouse corneal epithelium, and Krt19 was used by investigators to assess corneal maturation (Kalha et al., 2017). Another study (Pajoohesh-Ganji et al., 2016) focusing on Krt19 expression during postnatal developmental stages in the mouse revealed that at 7 days after birth Krt19 is found in basal and suprabasal cells throughout the central and limbal epithelium; however, the protein localizes to fewer central corneal cells and primarily becomes restricted towards the limbal region as the animals grow older. Unlike mice, the fetal rat cornea exhibits uniform Krt19 reactivity, whereas the corneal epithelium in adult rats was negative for the protein (Kasper, 1991). In the guinea pig, Krt19 immunoreactivity was noted in basal epithelial cells of the central cornea, and this gradually decreased towards the basal cells of the limbal zone (Kasper, 1992).
In Xenopus we detected age-related variability in Krt19 localization (Fig. 5). While expression was largely seen in basal epithelial cells of the tadpole cornea, it was observed in all the layers of the corneal epithelium following metamorphosis. Immunolabeling of frog corneas matches the cytoplasmic distribution of Krt19 in corneal epithelial cells reported in other vertebrates (Supp. Table S1). Interestingly, the inner and the outer surface of the adult Xenopus eyelid stained differently for Krt19. While the inner side of the eyelid had expression closely resembling the cornea, the outer surface was similar to that found in the skin. Characterization of Krt19 in these anurans suggests that it can be used as a marker to distinguish cornea vs. skin epithelia.
4.5. Vimentin specifically labels keratocytes beneath the corneal epithelium
The structural intermediate filament protein, vimentin, is essential for integrity of the cornea, and plays a central role in corneal wound healing (Ishizaki et al., 1993; Ritchey et al., 2011). Earlier studies on the adult human cornea demonstrated that vimentin is expressed only in the keratocytes – cells of mesenchymal origin found within the corneal stroma (Fini, 1999; Hayashi et al., 1986). Intriguingly, other reports in human, as well as the guinea pig (Kasper, 1992; Lauweryns et al., 1993a, 1993b; Schlotzer-Schrehardt et al., 2007; Schlotzer-Schrehardt and Kruse, 2005) showed that some clusters of limbal basal epithelial cells express this filamentous protein, in addition to its expression found in the stroma (see Supp. Table S1). In those reports, the vimentin expressing epithelial cells were found to co-express Keratin 19. The atypical expression of vimentin within the corneal epithelium has been proposed as an indicator of the proliferative activity of these epithelial cells (Kasper, 1991).
Immunohistochemical observations about the absence of vimentin in corneal epithelial cells have been reported from studies in other vertebrates, including chick (Ritchey et al., 2011), mouse (Das et al., 2014), cat (Jester et al., 1994), and the rabbit (SundarRaj et al., 1992). Interestingly, the study by SundarRaj et al. (1992) in rabbits demonstrated that corneal epithelial cells transiently express vimentin during wound healing. A more recent study using cultured corneal epithelial cells from the rabbit also suggests that vimentin expression might be a hallmark of early differentiating corneal epithelial cells (Castro-Munozledo et al., 2017).
Our immunolocalization studies on Xenopus corneas (Fig. 6) support the presence of vimentin expressing cells underneath the corneal epithelium in both tadpoles and adult frogs. However, we did not detect any vimentin staining within the frog corneal epithelium itself. In an earlier study, we described the presence of a fibrillar layer located below the basal epithelium of the cornea in frog larvae (Perry et al., 2013). This is the same layer we find was positive for vimentin expressing cells, and these likely represent corneal keratocytes of the tadpole eye. Likewise, the presence of keratocytes in the Xenopus adult corneal stroma has been described by Hu et al. (2013), as we have verified here. In addition, the existence of vimentin stained cells within the basal cells of the epidermis and dermis of the skin is in agreement with other reports (Mescher et al., 2007), and identifies cells of mesenchymal origin.
4.6. Differential expression of β1-integrin is detected in larval and adult frog cornea
β1-Integrin plays an essential role in mediating adhesion of corneal epithelial cells to the extracellular matrix, and facilitates corneal wound healing (Carter, 2009; Stepp, 2006). It is abundantly expressed in the central corneal and limbal epithelium of humans; the expression being more intense in the basal cells, with a progressive loss seen in more apical layers (Chen et al., 2004; Schlotzer-Schrehardt and Kruse, 2005). Despite some similar observations, there are differences in expression patterns reported by certain investigators (Supp. Table S1). For example, Chen et al. (2004) showed that in human corneas the staining intensity of all central corneal epithelial cells was similar, but the limbal basal cells had a stronger expression of β1-integrin compared to suprabasal cells. However, immunohistochemical studies by Schlotzer-Schrehardt and Kruse (2005) revealed no pronounced differences in membrane staining between basal cells of central and limbal regions.
Likewise, studies on mouse corneas showed that variabilities exist for β1-integrin localization in central and limbal regions. A recent study revealed β1-integrin expression by some cells in the basal layer of the mouse corneal epithelium (both limbal and central regions), with no staining detected in the apical cells (Li et al., 2017). However, in species of rats, β1-integrin was prominently observed in the membrane of all basal epithelial cells in the cornea (Stepp et al., 1993). Immunostaining studies in pig corneas have revealed that β1-integrin is uniformly expressed in the basal cells of the limbus, including both crypt and noncrypt limbal regions (Notara et al., 2011).
As shown in Fig. 7, we detected differential expression of β1-integrin in the corneal epithelium of tadpoles and mature frogs. The restricted localization of β1-integrin within the basal epithelial cells suggests its possible use as a differential marker to characterize larval corneal layers. In the mature Xenopus cornea, β1-integrin expression largely resembles that of humans and mice (see Supp. Table S1), with intense staining observed in basal cells of both the central cornea, and the limbal region. The observed higher expression of this adhesion molecule in basal cells advocates that it might play a key role in retaining SCs and their proliferative progeny in their microenvironment, as suggested in an earlier report in mouse corneas (Pajoohesh-Ganji et al., 2004).
4.7. E-cadherin is expressed in all cell layers of larval and adult frog corneal epithelia
The cell adhesion protein E-cadherin plays a pivotal role in maintaining corneal thickness, and regulating corneal epithelial homeostasis (Davis et al., 2003; Zhang and Tseng, 2007). Expression studies for E-cadherin in human corneas has yielded variable patterns for its localization. In one of the earliest studies examining E-cadherin in humans, the corneal epithelium did not stain for the protein (Scott et al., 1997). In contrast, a study by Chen et al. (2004) on frozen human corneas revealed that E-cadherin membrane staining was found in all layers of the central corneal epithelium, while only the superficial and supra-basal layers of the limbus stained for the antibody. That later study suggested that E-cadherin is expressed by differentiated corneal epithelial cells, occupying the superficial layers. Later on, other studies using frozen human corneas detected weak E-cadherin expression in the basal layer of the limbal epithelium, along with strong expression in superficial limbal epithelial cells, as well as in all layers of the central cornea (Hayashi et al., 2007; Schlotzer-Schrehardt and Kruse, 2005). Taking into account the very low expression of E-cadherin in the limbal basal cells, some investigators regard E-cadherin as one of the negative markers of limbal stem cells (Figueira et al., 2007; Mort et al., 2012). Unlike humans, studies in rat and rabbit corneas (Suzuki et al., 2000) have shown that E-cadherin is immunolocalized to all cell layers of the corneal epithelium. In mice, in situ hybridization and IF studies have shown that E-cadherin is expressed in all cell layers of embryonic, as well as mature corneal epithelia (Davis et al., 2003; Xu et al., 2002). Our immunostaining results showing membranous localization of E-cadherin in corneal epithelial cells of larvae and adult frogs (Fig. 8), more closely resemble the E-cadherin expression pattern found in rats and rabbits (see Supp. Table S1). The uniform distribution of E-cadherin in the aquatic frog cornea may relate to its more generalized barrier function in the epithelial tissue, as indicated in studies using other vertebrates (Bardag-Gorce et al., 2016).
4.8. β-catenin is associated with cell membranes in larval and adult corneal epithelia
Wnt/β-catenin signaling coordinates several vital processes during eye tissue development. Recent studies have suggested a prominent role of β-catenin in the proliferation of human corneal stem cells, and in maintaining the corneal epithelium in a less differentiated state (Lu et al., 2011, 2012; Nakatsu et al., 2011). In addition, active Wnt/β-catenin signaling is involved in the corneal wound healing process (Kawakita et al., 2005; Lyu and Joo, 2005, 2006). In an immunohistochemical study on human corneoscleral tissues (Nakatsu et al., 2011), β-catenin was seen to localize at the membrane of epithelial cells in both central corneal and limbal regions. Additionally, a small subset of basal epithelial cells in the limbus had nuclear as well as cytoplasmic localization of the protein. This nuclear localization has been suggested as an indicator of active Wnt/β-catenin signaling in the limbal region, and may help to regulate proliferation of SCs in limbal epithelium. Furthermore, that study proposed that the absence of β-catenin nuclear localization in the central corneal epithelial cells signifies relatively inactive signaling during corneal homeostasis. Observations about nuclear localization of β-catenin by Nakatsu et al. (2011) differ from a previous report where uniform β-catenin labeling of the membranes was observed throughout the corneal epithelium in humans (Schlotzer-Schrehardt and Kruse, 2005). Likewise, uniform expression of β-catenin in cell membrane of all corneal layers throughout different stages of postnatal corneal stratification has been reported in mice (Zhang et al., 2015). Studies in both rat and rabbit corneas detected stronger immunoreactivity of β-catenin in the basal layer of the limbus relative to expression in the basal epithelium of the central cornea (Hsueh et al., 2004) (also see Supp. Table S1).
Much like other vertebrates, the activity of Wnt/β-catenin during Xenopus eye development has been closely examined (Fujimura, 2016). In fact, a previous study from our lab illustrated that Wnt/β-catenin signaling is active in the corneal epithelium of larval Xenopus, and is suppressed during early stages of lens regeneration (Hamilton et al., 2016). However, that report lacked immunostaining studies for determining the expression pattern of β-catenin in normal and regenerating corneas. Our current study demonstrated membrane localization of β-catenin in corneas of both tadpoles and mature frogs (Fig. 9). Similar observations have been reported in other vertebrates, where no nuclear signal was detected in resting corneas (Supp. Table S1). Interestingly, the expression of this adherens junction molecule within the corneal layers changes during maturation. Taken together, these observations suggest an important role of β-catenin in cell adhesion. It also suggests that the Wnt/β-catenin signaling may be relatively inactive during the homeostatic state in the Xenopus cornea.
4.9. Distribution and expression pattern of p75-positive cells changes during corneal maturation
The transmembrane protein p75, along with its ligand NGF serve as important molecules in corneal epithelial maintenance and pathology. They modulate processes of cell proliferation, differentiation, apoptosis, and wound repair (Lambiase et al., 2000; Woo et al., 2005). In an earlier report, p75 immunoreactivity was detected only in the superficial cells of the limbal region, but was present throughout the central corneal epithelium (Touhami et al., 2002). However, Di Girolamo et al. (2008) using whole adult corneal specimens reported intense and specific p75 expression in the basal limbal epithelium, while the central corneal region did not exhibit any p75-positive epithelial cells. That study also reported on the absence of p75 staining in human fetal corneas (both central and limbal regions). The group’s findings support the notion that p75 may be a phenotypic marker to identify limbal stem cells and TACs (Di Girolamo et al., 2008). Contrary to this, a contemporary study found that p75 was expressed in basal and suprabasal epithelial cells in both limbal and central corneal regions, with no reactivity in superficial layer cells (Qi et al., 2008) (see Supp. Table S1). In comparison to studies on p75 in the human corneal epithelium, there are lack of reports about p75 expression in other vertebrates.
In Xenopus ocular tissue, the role of p75 has been described in the retina, where it promotes the survival of developing retinal neurons (Hutson and Bothwell, 2001). However, the expression and role of p75 in the frog cornea remained unexamined. Our observations (Fig. 10) support a differential expression of p75 in the epithelial layers of tadpole cornea. In line with findings in the human cornea (Touhami et al., 2002), the higher expression of p75 in the apical cells of peripheral cornea and skin of larval frogs suggests that apoptosis may dominate in these superficial cells. In addition, the distribution of p75-positive cells in frog cornea changes during maturation. As these p75 labeled cells are also found in skin, the protein cannot be uniquely categorized as a cornea-specific marker.
4.10. Differential immunolocalization of Pax6 and Tcf7l2 molecularly demarcates cornea vs. skin in frogs
Based on our co-immunostaining studies (Fig. 11), we infer that a combination of biomarkers would be better suited to identify specific corneal epithelial layers, as well as the CESCs and TACs. Although our current study does not reveal any such possible combination of cornea-specific proteins for CESCs or TACs, we show that Pax6 and Tcf7l2 distinguish the cornea and skin of these anurans.
5. Conclusion
The relative lack of good stem cell-specific markers has been an impediment in understanding corneal homeostasis, and progress in developing corneal regenerative treatments. Using an amphibian corneal model, the current study sheds new insights into several key proteins involved in the formation and maturation of the corneal epithelium. Though we have not identified a specific marker of Xenopus (larvae or adult) corneal stem cells in the present study, specific differences in protein localization helps to characterize different layers and regions of the corneal epithelium in this anuran (Fig. 12). We also found that unlike the outer surface, the inner surface of the eyelid is molecularly similar to the adult cornea. Significantly, our comparative analysis of expression patterns of TFs and other marker proteins through both an extensive literature search, and by examination of developing and mature corneas in Xenopus indicates that the deployment of these proteins is not evolutionarily conserved in corneas of vertebrates. Based on these observations, a combination of molecular markers may be more useful in defining corneal cell types, including the CESCs and TACs.
Supplementary Material
Fig. S1. Western blot analyse of various antibodies in Xenopus larval epithelial tissue lysate, as labeled. Numbers to the right represent molecular mass standards in kilodaltons (kDa). Arrowheads on the left indicate the positions corresponding to the predicted molecular weights of the proteins. Additional bands were detected in p63 (A), Pax6 (B), E-cadherin (G), consistent to described in other studies. See text for details.
Acknowledgements
The authors would like to thank Dr. Ratnakar Singh, and Dr. Maryna Lesoway for the comments on the manuscript. Some antibodies used in this study were obtained from the Developmental Studies Hybridoma Bank (DSHB), a resource created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. This research was supported by NEI grant EY023979 to JJH.
Abbreviations
- X.laevis
Xenopus laevis
- SCs
Stem Cells
- LRCs
Label Retaining Cells
- CESCs
Corneal Epithelial Stem Cells
- TACs
Transit Amplifying Cells
- LSCD
Limbal Stem Cell Deficiency
- IF
Immunofluorescence
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.exer.2019.04.010.
References
- Akiyama M, Smith LT, Holbrook KA, 1996. Growth factor and growth factor receptor localization in the hair follicle bulge and associated tissue in human fetus. J. Investig. Dermatol 106, 391–396. [DOI] [PubMed] [Google Scholar]
- Arce L, Yokoyama NN, Waterman ML, 2006. Diversity of LEF/TCF action in development and disease. Oncogene 25, 7492–7504. [DOI] [PubMed] [Google Scholar]
- Bandin S, Morona R, Gonzalez A, 2015. Prepatterning and patterning of the thalamus along embryonic development of Xenopus laevis. Front. Neuroanat 9, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M, 1995. Neurotrophic factors and their receptors. Curr. Opin. Cell Biol 7, 148–155. [DOI] [PubMed] [Google Scholar]
- Barbaro V, Testa A, Di Iorio E, Mavilio F, Pellegrini G, De Luca M, 2007. C/EBP delta regulates cell cycle and self-renewal of human limbal stem cells. J. Cell Biol 177, 1037–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K, 2012. Lens and retina regeneration: new perspectives from model organisms. Biochem. J 447, 321–334. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Hoft RH, Wood A, Oliva J, Niihara H, Makalinao A, Thropay J, Pan D, Meepe I, Tiger K, Garcia J, Laporte A, French SW, Niihara Y, 2016. The role of E-cadherin in maintaining the barrier function of corneal epithelium after treatment with cultured autologous oral mucosa epithelial cell sheet grafts for limbal stem deficiency. J. Ophthalmol 4805986 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M, 1991. Keeping track of neurotrophin receptors. Cell 65, 915–918. [DOI] [PubMed] [Google Scholar]
- Bragulla HH, Homberger DG, 2009. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat 214, 516–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM, 2005. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J. Cell Sci 118, 1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns JC, Collado MS, Oliver ER, Corwin JT, 2013. Specializations of intercellular junctions are associated with the presence and absence of hair cell regeneration in ears from six vertebrate classes. J. Comp. Neurol 521, 1430–1448. [DOI] [PubMed] [Google Scholar]
- Carter RT, 2009. The role of integrins in corneal wound healing. Vet. Ophthalmol 12 (Suppl. 1), 2–9. [DOI] [PubMed] [Google Scholar]
- Castro-Munozledo F, 2013. Review: corneal epithelial stem cells, their niche and wound healing. Mol. Vis 19, 1600–1613. [PMC free article] [PubMed] [Google Scholar]
- Castro-Muñozledo F, 2015. The Mammalian Limbal Stem Cell Niche: A Complex Interaction between Cells, Growth Factors and Extracellular Matrix, pp. 23–56. [Google Scholar]
- Castro-Munozledo F, Meza-Aguilar DG, Dominguez-Castillo R, Hernandez-Zequinely V, Sanchez-Guzman E, 2017. Vimentin as a marker of early differentiating, highly motile corneal epithelial cells. J. Cell. Physiol 232, 818–830. [DOI] [PubMed] [Google Scholar]
- Chen JJ, Tseng SC, 1991. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Investig. Ophthalmol. Vis. Sci 32, 2219–2233. [PubMed] [Google Scholar]
- Chen W, Joos TO, Defoe DM, 1997. Evidence for beta 1-integrins on both apical and basal surfaces of Xenopus retinal pigment epithelium. Exp. Eye Res 64, 73–84. [DOI] [PubMed] [Google Scholar]
- Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ, 2004. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cell 22, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WT, Charney Le R, Blitz IL, Fish MB, Li Y, Biesinger J, Xie X, Cho KW, 2014. Genome-wide view of TGFbeta/Foxh1 regulation of the early mesendoderm program. Development 141, 4537–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EH, Bukusoglu G, Zieske JD, 1992. Localization of corneal epithelial stem cells in the developing rat. Investig. Ophthalmol. Vis. Sci 33, 2199–2206. [PubMed] [Google Scholar]
- Chung EH, DeGregorio PG, Wasson M, Zieske JD, 1995. Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells. Investig. Ophthalmol. Vis. Sci 36, 1336–1343. [PubMed] [Google Scholar]
- Clevers H, Nusse R, 2012. Wnt/beta-catenin signaling and disease. Cell 149, 1192–1205. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Chanas SA, Hill RE, West JD, 2004. Corneal development, limbal stem cell function, and corneal epithelial cell migration in the Pax6( + /−) mouse. Investig. Ophthalmol. Vis. Sci 45, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Collinson JM, Morris L, Reid AI, Ramaesh T, Keighren MA, Flockhart JH, Hill RE, Tan SS, Ramaesh K, Dhillon B, West JD, 2002. Clonal analysis of patterns of growth, stem cell activity, and cell movement during the development and maintenance of the murine corneal epithelium. Dev. Dynam 224, 432–440. [DOI] [PubMed] [Google Scholar]
- Costamagna D, Berardi E, Ceccarelli G, Sampaolesi M, 2015. Adult stem cells and skeletal muscle regeneration. Curr. Gene Ther 15, 348–363. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM, 1989. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell 57, 201–209. [DOI] [PubMed] [Google Scholar]
- Das SK, Gupta I, Cho YK, Zhang X, Uehara H, Muddana SK, Bernhisel AA, Archer B, Ambati BK, 2014. Vimentin knockdown decreases corneal opacity. Investig. Ophthalmol. Vis. Sci 55, 4030–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanger M, Evensen A, 1971. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature 229, 560–561. [DOI] [PubMed] [Google Scholar]
- Davies SB, Chui J, Madigan MC, Provis JM, Wakefield D, Di Girolamo N, 2009. Stem cell activity in the developing human cornea. Stem Cell 27, 2781–2792. [DOI] [PubMed] [Google Scholar]
- Davis J, Duncan MK, Robison WG Jr., Piatigorsky J, 2003. Requirement for Pax6 in corneal morphogenesis: a role in adhesion. J. Cell Sci 116, 2157–2167. [DOI] [PubMed] [Google Scholar]
- Davis J, Piatigorsky J, 2011. Overexpression of Pax6 in mouse cornea directly alters corneal epithelial cells: changes in immune function, vascularization, and differentiation. Investig. Ophthalmol. Vis. Sci 52, 4158–4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RC, Beck CW, 2011. Transdifferentiation from cornea to lens in Xenopus laevis depends on BMP signalling and involves upregulation of Wnt signalling. BMC Dev. Biol 11, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp EE, Swamynathan S, Kao WW, Swamynathan SK, 2015. Spatiotemporally regulated ablation of Klf4 in adult mouse corneal epithelial cells results in altered epithelial cell identity and disrupted homeostasis. Investig. Ophthalmol. Vis. Sci 56, 3549–3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent JA, Poison AG, Klymkowsky MW, 1989. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 105, 61–74. [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Pearton DJ, Michon F, 2014. The vertebrate corneal epithelium: from early specification to constant renewal. Dev. Dynam 243, 1226–1241. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, 2011. Stem cells of the human cornea. Br. Med. Bull 100, 191–207. [DOI] [PubMed] [Google Scholar]
- Di Girolamo N, Sarris M, Chui J, Cheema H, Coroneo MT, Wakefield D, 2008. Localization of the low-affinity nerve growth factor receptor p75 in human limbal epithelial cells. J. Cell Mol. Med 12, 2799–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Barbara V, Ruzza A, Ponzin D, Pellegrini G, De Luca M, 2005. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. U. S. A 102, 9523–9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Iorio E, Kaye SB, Ponzin D, Barbaro V, Ferrari S, Bohm E, Nardiello P, Castaldo G, McGrath JA, Willoughby CE, 2012. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology 119, 74–83. [DOI] [PubMed] [Google Scholar]
- Djalilian AR, Namavari A, Ito A, Balali S, Afshar A, Lavker RM, Yue BY, 2008. Down-regulation of Notch signaling during corneal epithelial proliferation. Mol. Vis 14, 1041–1049. [PMC free article] [PubMed] [Google Scholar]
- Dua HS, Joseph A, Shanmuganathan VA, Jones RE, 2003. Stem cell differentiation and the effects of deficiency. Eye 17, 877–885. [DOI] [PubMed] [Google Scholar]
- Edwards-Faret G, Cebrian-Silla A, Mendez-Olivos EE, Gonzalez-Pinto K, Garcia-Verdugo JM, Larrain J, 2018. Cellular composition and organization of the spinal cord central canal during metamorphosis of the frog Xenopus laevis. J. Comp. Neurol 526, 1712–1732. [DOI] [PubMed] [Google Scholar]
- Eghtedari Y, Richardson A, Mai K, Heng B, Guillemin GJ, Wakefield D, Di Girolamo N, 2016. Keratin 14 expression in epithelial progenitor cells of the developing human cornea. Stem Cell. Dev 25, 699–711. [DOI] [PubMed] [Google Scholar]
- Elder MJ, Hiscott P, Dart JK, 1997. Intermediate filament expression by normal and diseased human corneal epithelium. Hum. Pathol 28, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Figueira EC, Di Girolamo N, Coroneo MT, Wakefield D, 2007. The phenotype of limbal epithelial stem cells. Investig. Ophthalmol. Vis. Sci 48, 144–156. [DOI] [PubMed] [Google Scholar]
- Filoni S, Bernardini S, Cannata SM, D’Alessio A, 1997. Lens regeneration in larval Xenopus laevis: experimental analysis of the decline in the regenerative capacity during development. Dev. Biol 187, 13–24. [DOI] [PubMed] [Google Scholar]
- Fini ME, 1999. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog. Retin. Eye Res 18, 529–551. [DOI] [PubMed] [Google Scholar]
- Freeman G, 1963. Lens regeneration from the cornea in Xenopus laevis. J. Exp. Zool 154, 39–65. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Yang Y, 1999. Crossroads on cytoskeletal highways. Cell 98, 547–550. [DOI] [PubMed] [Google Scholar]
- Fujimura N, 2016. WNT/beta-Catenin signaling in vertebrate eye development. Front. Cell Dev. Biol 4, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Giambra V, Santoni S, Bernardini S, Frezza D, Filoni S, Cannata SM, 2008. The lens-regenerating competence in the outer cornea and epidermis of larval Xenopus laevis is related to pax6 expression. J. Anat 212, 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL, 1994. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat. Genet 7, 463–471. [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Bron AJ, 1982. Limbal palisades of Vogt. Trans. Am. Ophthalmol. Soc 80, 155–171. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Sasamoto Y, Ksander BR, Frank MH, Frank NY, 2017. Limbal Stem Cells: Identity, Developmental Origin, and Therapeutic Potential. Wiley interdisciplinary reviews, Developmental biology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindolet D, Crouzet E, He Z, Herbepin P, Jumelle C, Perrache C, Dumollard JM, Forest F, Peoc’h M, Gain P, Gabison E, Thuret G, 2017. Storage of porcine cornea in an innovative bioreactor. Investig. Ophthalmol. Vis. Sci 58, 5907–5917. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, 2000. Regulation of cadherin adhesive activity. J. Cell Biol 148, 399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PW, Henry JJ, 2016. The lens regenerative competency of limbal vs. central regions of mature Xenopus cornea epithelium. Exp. Eye Res 152, 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton PW, Sun Y, Henry JJ, 2016. Lens regeneration from the cornea requires suppression of Wnt/beta-catenin signaling. Exp. Eye Res 145, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson IM, Fletcher JM, Jordan T, Brown A, Taylor D, Adams RJ, Punnett HH, van Heyningen V, 1994. Mutations at the PAX6 locus are found in heterogeneous anterior segment malformations including Peters’ anomaly. Nat. Genet 6, 168–173. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Sueishi K, Tanaka K, Inomata H, 1986. Immunohistochemical evidence of the origin of human corneal endothelial cells and keratocytes. Albrecht Von Graefe’s Arch. Clin. Exp. Ophthalmol 224, 452–456. [DOI] [PubMed] [Google Scholar]
- Hayashi R, Yamato M, Sugiyama H, Sumide T, Yang J, Okano T, Tano Y, Nishida K, 2007. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cell 25, 289–296. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Carinato ME, Schaefer JJ, Wolfe AD, Walter BE, Perry KJ, Elbl TN, 2002. Characterizing gene expression during lens formation in Xenopus laevis: evaluating the model for embryonic lens induction. Dev. Dynam 224, 168–185. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger, 1987. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev. Biol 124, 200–214. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Mittleman JM, 1995. The matured eye of Xenopus laevis tadpoles produces factors that elicit a lens-forming response in embryonic ectoderm. Dev. Biol 171, 39–50. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Perry KJ, Hamilton PW, 2018. Methods for examining lens regeneration in Xenopus. Cold Spring Harb. Protoc [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Tsonis PA, 2010. Molecular and cellular aspects of amphibian lens regeneration. Prog. Retin. Eye Res 29, 543–555. 10.1101/pdb.prot101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry JJ, Wever JM, Natalia Vergara M, Fukui L, 2008. Chapter 6 - Xenopus, an Ideal Vertebrate System for Studies of Eye Development and Regeneration A2 - Tsonis, Panagiotis A, Animal Models in Eye Research Academic Press, London, pp. 57–92. [Google Scholar]
- Higa K, Shimmura S, Miyashita H, Kato N, Ogawa Y, Kawakita T, Shimazaki J, Tsubota K, 2009. N-cadherin in the maintenance of human corneal limbal epithelial progenitor cells in vitro. Investig. Ophthalmol. Vis. Sci 50, 4640–4645. [DOI] [PubMed] [Google Scholar]
- Hoppler S, Kavanagh CL, 2007. Wnt signalling: variety at the core. J. Cell Sci 120, 385–393. [DOI] [PubMed] [Google Scholar]
- Hsueh YJ, Wang DY, Cheng CC, Chen JK, 2004. Age-related expressions of p63 and other keratinocyte stem cell markers in rat cornea. J. Biomed. Sci 11, 641–651. [DOI] [PubMed] [Google Scholar]
- Hu W, Haamedi N, Lee J, Kinoshita T, Ohnuma S, 2013. The structure and development of Xenopus laevis cornea. Exp. Eye Res 116, 109–128. [DOI] [PubMed] [Google Scholar]
- Huang C, Kratzer MC, Wedlich D, Kashef J, 2016. E-cadherin is required for cranial neural crest migration in Xenopus laevis. Dev. Biol 411, 159–171. [DOI] [PubMed] [Google Scholar]
- Huang YL, Niehrs C, 2014. Polarized Wnt signaling regulates ectodermal cell fate in Xenopus. Dev. Cell 29, 250–257. [DOI] [PubMed] [Google Scholar]
- Hutson LD, Bothwell M, 2001. Expression and function of Xenopus laevis p75(NTR) suggest evolution of developmental regulatory mechanisms. J. Neurobiol 49, 79–98. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Cunningham LA, Boggess D, Hawes N, Hobson CD, Sundberg JP, Naggert JK, Smith RS, Nishina PM, 2003. Aberrant actin cytoskeleton leads to accelerated proliferation of corneal epithelial cells in mice deficient for destrin (actin depolymerizing factor). Hum. Mol. Genet 12, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW, 1993. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Investig. Ophthalmol. Vis. Sci 34, 3320–3328. [PubMed] [Google Scholar]
- Ivaska J, Pallari HM, Nevo J, Eriksson JE, 2007. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp. Cell Res 313, 2050–2062. [DOI] [PubMed] [Google Scholar]
- Jester JV, Barry PA, Lind GJ, Petroll WM, Garana R, Cavanagh HD, 1994. Corneal keratocytes: in situ and in vitro organization of cytoskeletal contractile proteins. Investig. Ophthalmol. Vis. Sci 35, 730–743. [PubMed] [Google Scholar]
- Jones PH, Watt FM, 1993. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724. [DOI] [PubMed] [Google Scholar]
- Kalha S, Shrestha B, Sanz Navarro M, Jones KB, Klein OD, Michon F, 2018. Bmi1 + progenitor cell dynamics in murine cornea during homeostasis and wound healing. Stem Cell 36, 562–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC, 2003. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J. Neurosci. : J. Off. Soc. Neurosci 23, 5425–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper M, 1991. Heterogeneity in the immunolocalization of cytokeratin specific monoclonal antibodies in the rat eye: evaluation of unusual epithelial tissue entities. Histochemistry 95, 613–620. [DOI] [PubMed] [Google Scholar]
- Kasper M, 1992. Patterns of cytokeratins and vimentin in Guinea pig and mouse eye tissue: evidence for regional variations in intermediate filament expression in limbal epithelium. Acta Histochem 93, 319–332. [DOI] [PubMed] [Google Scholar]
- Kasper M, Moll R, Stosiek P, Karsten U, 1988. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry 89, 369–377. [DOI] [PubMed] [Google Scholar]
- Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC, 2005. Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am. J. Pathol 167, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon KR, Tseng SC, 1989. Limbal autograft transplantation for ocular surface disorders. Ophthalmology 96, 709–722 discussion 722–703. [DOI] [PubMed] [Google Scholar]
- Kha CX, Son PH, Lauper J, Tseng KA, 2018. A model for investigating developmental eye repair in Xenopus laevis. Exp. Eye Res 169, 38–47. [DOI] [PubMed] [Google Scholar]
- Kitazawa K, Hikichi T, Nakamura T, Sotozono C, Kinoshita S, Masui S, 2016. PAX6 regulates human corneal epithelium cell identity. Exp. Eye Res 154, 30–38. [DOI] [PubMed] [Google Scholar]
- Koroma BM, Yang JM, Sundin OH, 1997. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci 38, 108–120. [PubMed] [Google Scholar]
- Krieger T, Simons BD, 2015. Dynamic stem cell heterogeneity. Development 142, 1396–1406. [DOI] [PubMed] [Google Scholar]
- Ksander BR, Kolovou PE, Wilson BJ, Saab KR, Guo Q, Ma J, McGuire SP, Gregory MS, Vincent WJ, Perez VL, Cruz-Guilloty F, Kao WW, Call MK, Tucker BA, Zhan Q, Murphy GF, Lathrop KL, Alt C, Mortensen LJ, Lin CP, Zieske JD, Frank MH, Frank NY, 2014. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 511, 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L, 2000. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Investig. Ophthalmol. Vis. Sci 41, 1063–1069. [PubMed] [Google Scholar]
- Lauweryns B, van den Oord JJ, De Vos R, Missotten L, 1993a. A new epithelial cell type in the human cornea. Investig. Ophthalmol. Vis. Sci 34, 1983–1990. [PubMed] [Google Scholar]
- Lauweryns B, van den Oord JJ, Missotten L, 1993b. The transitional zone between limbus and peripheral cornea. An immunohistochemical study. Investig. Ophthalmol. Vis. Sci 34, 1991–1999. [PubMed] [Google Scholar]
- Lazarides E, 1980. Intermediate filaments as mechanical integrators of cellular space. Nature 283, 249–256. [DOI] [PubMed] [Google Scholar]
- Lee-Liu D, Mendez-Olivos EE, Munoz R, Larrain J, 2017. The African clawed frog Xenopus laevis: a model organism to study regeneration of the central nervous system. Neurosci. Lett 652, 82–93. [DOI] [PubMed] [Google Scholar]
- Lehrer MS, Sun TT, Lavker RM, 1998. Strategies of epithelial repair: modulation of stem cell and transit amplifying cell proliferation. J. Cell Sci 111 (Pt 19), 2867–2875. [DOI] [PubMed] [Google Scholar]
- Li A, Simmons PJ, Kaur P, 1998. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl. Acad. Sci. U. S. A 95, 3902–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Xu F, Zhu J, Krawczyk M, Zhang Y, Yuan J, Patel S, Wang Y, Lin Y, Zhang M, Cai H, Chen D, Zhang M, Cao G, Yeh E, Lin D, Su Q, Li WW, Sen GL, Afshari N, Chen S, Maas RL, Fu XD, Zhang K, Liu Y, Ouyang H, 2015. Transcription factor PAX6 (paired box 6) controls limbal stem cell lineage in development and disease. J. Biol. Chem 290, 20448–20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiao Y, Coursey TG, Chen X, Deng R, Lu F, Pflugfelder SC, Li DQ, 2017. Identification for differential localization of putative corneal epithelial stem cells in mouse and human. Sci. Rep 7, 5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H, 2010. Coexistence of quiescent and active adult stem cells in mammals. Science 327, 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Sakaguchi DS, 2002. Expression patterns of focal adhesion associated proteins in the developing retina. Dev. Dynam 225, 544–553. [DOI] [PubMed] [Google Scholar]
- Li W, Chen YT, Hayashi da Y, Blanco G, Kheirkah A, He H, Chen SY, Liu CY, Tseng SC, 2008. Down-regulation of Pax6 is associated with abnormal differentiation of corneal epithelial cells in severe ocular surface diseases. J. Pathol 214, 114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linardi RL, Megee SO, Mainardi SR, Senoo M, Galantino-Homer HL, 2015. Expression and localization of epithelial stem cell and differentiation markers in equine skin, eye and hoof. Vet. Dermatol 26, 213–e247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Kao WW, Wilson SE, 1999. Corneal epithelium-specific mouse keratin K12 promoter. Exp. Eye Res 68, 295–301. [DOI] [PubMed] [Google Scholar]
- Lu R, Bian F, Zhang X, Qi H, Chuang EY, Pflugfelder SC, Li DQ, 2011. The beta-catenin/Tcf4/survivin signaling maintains a less differentiated phenotype and high proliferative capacity of human corneal epithelial progenitor cells. Int. J. Biochem. Cell Biol 43, 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Qu Y, Ge J, Zhang L, Su Z, Pflugfelder SC, Li DQ, 2012. Transcription factor TCF4 maintains the properties of human corneal epithelial stem cells. Stem Cell 30, 753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyngholm M, Hoyer PE, Vorum H, Nielsen K, Ehlers N, Mollgard K, 2008. Immunohistochemical markers for corneal stem cells in the early developing human eye. Exp. Eye Res 87, 115–121. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK, 2005. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J. Biol. Chem 280, 21653–21660. [DOI] [PubMed] [Google Scholar]
- Lyu J, Joo CK, 2006. Expression of Wnt and MMP in epithelial cells during corneal wound healing. Cornea 25, S24–S28. [DOI] [PubMed] [Google Scholar]
- Majo F, Rochat A, Nicolas M, Jaoude GA, Barrandon Y, 2008. Oligopotent stem cells are distributed throughout the mammalian ocular surface. Nature 456, 250–254. [DOI] [PubMed] [Google Scholar]
- Mei H, Nakatsu MN, Baclagon ER, Deng SX, 2014. Frizzled 7 maintains the undifferentiated state of human limbal stem/progenitor cells. Stem Cell 32, 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez MG, Kojima S, Goldman RD, 2010. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. : Off. Publ. Fed. Am. Soc. Exp. Biol 24, 1838–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel-Severing J, Zenkel M, Polisetti N, Sock E, Wegner M, Kruse FE, Schlotzer-Schrehardt U, 2018. Transcription factor profiling identifies Sox9 as regulator of proliferation and differentiation in corneal epithelial stem/progenitor cells. Sci. Rep 8, 10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merjava S, Neuwirth A, Tanzerova M, Jirsova K, 2011. The spectrum of cytokeratins expressed in the adult human cornea, limbus and perilimbal conjunctiva. Histol. Histopathol 26, 323–331. [DOI] [PubMed] [Google Scholar]
- Mescher AL, Wolf WL, Moseman EA, Hartman B, Harrison C, Nguyen E, Neff AW, 2007. Cells of cutaneous immunity in Xenopus: studies during larval development and limb regeneration. Dev. Comp. Immunol 31, 383–393. [DOI] [PubMed] [Google Scholar]
- Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L, 1996. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J. Cell Sci 109 (Pt 5), 1017–1028. [DOI] [PubMed] [Google Scholar]
- Michiue T, Yamamoto T, Yasuoka Y, Goto T, Ikeda T, Nagura K, Nakayama T, Taira M, Kinoshita T, 2017. High variability of expression profiles of homeologous genes for Wnt, Hh, Notch, and Hippo signaling pathways in Xenopus laevis. Dev. Biol 426, 270–290. [DOI] [PubMed] [Google Scholar]
- Mikhailova A, Ilmarinen T, Ratnayake A, Petrovski G, Uusitalo H, Skottman H, Rafat M, 2016. Human pluripotent stem cell-derived limbal epithelial stem cells on bioengineered matrices for corneal reconstruction. Exp. Eye Res 146, 26–34. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Batra SK, 2008. Recent progress on tissue-resident adult stem cell biology and their therapeutic implications. Stem Cell Rev 4, 27–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JE, McMullen CB, Mahon G, Adamis AP, 2002. The corneal epithelial stem cell. DNA Cell Biol 21, 443–451. [DOI] [PubMed] [Google Scholar]
- Morita M, Fujita N, Takahashi A, Nam ER, Yui S, Chung CS, Kawahara N, Lin HY, Tsuzuki K, Nakagawa T, Nishimura R, 2015. Evaluation of ABCG2 and p63 expression in canine cornea and cultivated corneal epithelial cells. Vet. Ophthalmol 18, 59–68. [DOI] [PubMed] [Google Scholar]
- Moriyama H, Kasashima Y, Kuwano A, Wada S, 2014. Anatomical location and culture of equine corneal epithelial stem cells. Vet. Ophthalmol 17, 106–112. [DOI] [PubMed] [Google Scholar]
- Mort RL, Douvaras P, Morley SD, Dora N, Hill RE, Collinson JM, West JD, 2012. Stem cells and corneal epithelial maintenance: insights from the mouse and other animal models. Results Probl. Cell Differ 55, 357–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Ohtsuka T, Sekiyama E, Cooper LJ, Kokubu H, Fullwood NJ, Barrandon Y, Kageyama R, Kinoshita S, 2008. Hes1 regulates corneal development and the function of corneal epithelial stem/progenitor cells. Stem Cell 26, 1265–1274. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Sotozono C, Kinoshita S, 2001. The epidermal growth factor receptor (EGFR): role in corneal wound healing and homeostasis. Exp. Eye Res 72, 511–517. [DOI] [PubMed] [Google Scholar]
- Nakatsu MN, Ding Z, Ng MY, Truong TT, Yu F, Deng SX, 2011. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Investig. Ophthalmol. Vis. Sci 52, 4734–4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R, 2004. Convergence of Wnt, beta-catenin, and cadherin pathways. Science 303, 1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E, 2009. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat. Genet 41, 1068–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J, 1956. Normal Table of Xenopus laevis. Daudin), pp. 162–203. [Google Scholar]
- Nishina S, Kohsaka S, Yamaguchi Y, Handa H, Kawakami A, Fujisawa H, Azuma N, 1999. PAX6 expression in the developing human eye. Br. J. Ophthalmol 83, 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notara M, Schrader S, Daniels JT, 2011. The porcine limbal epithelial stem cell niche as a new model for the study of transplanted tissue-engineered human limbal epithelial cells. Tissue Eng 17, 741–750. [DOI] [PubMed] [Google Scholar]
- Nusse R, 2005. Wnt signaling in disease and in development. Cell Res 15, 28–32. [DOI] [PubMed] [Google Scholar]
- Ouyang H, Xue Y, Lin Y, Zhang X, Xi L, Patel S, Cai H, Luo J, Zhang M, Zhang M, Yang Y, Li G, Li H, Jiang W, Yeh E, Lin J, Pei M, Zhu J, Cao G, Zhang L, Yu B, Chen S, Fu XD, Liu Y, Zhang K, 2014. WNT7A and PAX6 define corneal epithelium homeostasis and pathogenesis. Nature 511, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Ghosh SP, Stepp MA, 2004. Regional distribution of alpha9beta 1 integrin within the limbus of the mouse ocular surface. Dev. Dynam 230, 518–528. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Simmens SJ, Stepp MA, 2006. Integrins in slow-cycling corneal epithelial cells at the limbus in the mouse. Stem Cell 24, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Pajoohesh-Ganji A, Pal-Ghosh S, Tadvalkar G, Stepp MA, 2016. K14 + compound niches are present on the mouse cornea early after birth and expand after debridement wounds. Dev. Dynam 245, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parapuram SK, Huh K, Liu S, Leask A, 2011. Integrin beta 1 is necessary for the maintenance of corneal structural integrity. Investig. Ophthalmol. Vis. Sci 52, 7799–7806. [DOI] [PubMed] [Google Scholar]
- Parsa R, Yang A, McKeon F, Green H, 1999. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Investig. Dermatol 113, 1099–1105. [DOI] [PubMed] [Google Scholar]
- Patruno M, Perazzi A, Martinello T, Blaseotto A, Di Iorio E, Iacopetti I, 2017. Morphological description of limbal epithelium: searching for stem cells crypts in the dog, cat, pig, cow, sheep and horse. Vet. Res. Commun 41, 169–173. [DOI] [PubMed] [Google Scholar]
- Pearton DJ, Yang Y, Dhouailly D, 2005. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl. Acad. Sci. U. S. A 102, 3714–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Bondanza S, Guerra L, De Luca M, 1998. Cultivation of human keratinocyte stem cells: current and future clinical applications. Med. Biol. Eng. Comput 36, 778–790. [DOI] [PubMed] [Google Scholar]
- Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M, 2001. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. U. S. A 98, 3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry KJ, Thomas AG, Henry JJ, 2013. Expression of pluripotency factors in larval epithelia of the frog Xenopus: evidence for the presence of cornea epithelial stem cells. Dev. Biol 374, 281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitz S, Moll R, 2002. Intermediate-filament expression in ocular tissue. Prog. Retin. Eye Res 21, 241–262. [DOI] [PubMed] [Google Scholar]
- Priya CG, Prasad T, Prajna NV, Muthukkaruppan V, 2013. Identification of human corneal epithelial stem cells on the basis of high ABCG2 expression combined with a large N/C ratio. Microsc. Res. Tech 76, 242–248. [DOI] [PubMed] [Google Scholar]
- Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB, Pflugfelder SC, 2008. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp. Eye Res 86, 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y, Zhang X, Xu S, Li K, Zhu F, Li Q, Cai X, Lu R, 2016. Tcf7l2 localization of putative stem/progenitor cells in mouse conjunctiva. Am. J. Physiol. Cell Physiol 311, C246–C254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley IK, Stubbs JL, Kintner C, 2011. Specification of ion transport cells in the Xenopus larval skin. Development 138, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G, 2010. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med 363, 147–155. [DOI] [PubMed] [Google Scholar]
- Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B, 2003. Corneal abnormalities in Pax6 + /− small eye mice mimic human aniridia-related keratopathy. Investig. Ophthalmol. Vis. Sci 44, 1871–1878. [DOI] [PubMed] [Google Scholar]
- Ring A, Kim YM, Kahn M, 2014. Wnt/catenin signaling in adult stem cell physiology and disease. Stem Cell Rev 10, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchey ER, Code K, Zelinka CP, Scott MA, Fischer AJ, 2011. The chicken cornea as a model of wound healing and neuronal re-innervation. Mol. Vis 17, 2440–2454. [PMC free article] [PubMed] [Google Scholar]
- Rungger-Brandle E, Ripperger JA, Steiner K, Conti A, Stieger A, Soltanieh S, Rungger D, 2010. Retinal patterning by Pax6-dependent cell adhesion molecules. Dev. Neurobiol 70, 764–780. [DOI] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT, 1986. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol 103, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Dietrich T, Saito K, Sorokin L, Sasaki T, Paulsson M, Kruse FE, 2007. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp. Eye Res 85, 845–860. [DOI] [PubMed] [Google Scholar]
- Schlotzer-Schrehardt U, Kruse FE, 2005. Identification and characterization of limbal stem cells. Exp. Eye Res 81, 247–264. [DOI] [PubMed] [Google Scholar]
- Scott RA, Lauweryns B, Snead DM, Haynes RJ, Mahida Y, Dua HS, 1997. E-cadherin distribution and epithelial basement membrane characteristics of the normal human conjunctiva and cornea. Eye 11 (Pt 5), 607–612. [DOI] [PubMed] [Google Scholar]
- Seeker GA, Daniels JT, 2008. Limbal Epithelial Stem Cells of the Cornea StemBook, Cambridge (MA). [PubMed] [Google Scholar]
- Shaham 0, Menuchin Y, Farhy C, Ashery-Padan R, 2012. Pax6: a multi-level regulator of ocular development. Prog. Retin. Eye Res 31, 351–376. [DOI] [PubMed] [Google Scholar]
- Shalom-Feuerstein R, Lena AM, Zhou H, De La Forest Divonne S, Van Bokhoven H, Candi E, Melino G, Aberdam D, 2011. DeltaNp63 is an ectodermal gatekeeper of epidermal morphogenesis. Cell Death Differ 18, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney LE, McIntosh OD, Hopkinson A, 2015. Phenotypic change and induction of cytokeratin expression during in vitro culture of corneal stromal cells. Investig. Ophthalmol. Vis. Sci 56, 7225–7235. [DOI] [PubMed] [Google Scholar]
- Slack JM, Lin G, Chen Y, 2008. The Xenopus tadpole: a new model for regeneration research. Cell. Mol. Life Sci. : CM 65, 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak PC, Purkis PE, Leigh IM, Lane EB, 1989. Keratin 19: predicted amino acid sequence and broad tissue distribution suggest it evolved from keratinocyte keratins. J. Investig. Dermatol 92, 707–716. [DOI] [PubMed] [Google Scholar]
- Stepp MA, 2006. Corneal integrins and their functions. Exp. Eye Res 83, 3–15. [DOI] [PubMed] [Google Scholar]
- Stepp MA, Spurr-Michaud S, Gipson IK, 1993. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Investig. Ophthalmol. Vis. Sci 34, 1829–1844. [PubMed] [Google Scholar]
- SundarRaj N, Rizzo JD, Anderson SC, Gesiotto JP, 1992. Expression of vimentin by rabbit corneal epithelial cells during wound repair. Cell Tissue Res 267, 347–356. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Tanaka T, Enoki M, Nishida T, 2000. Coordinated reassembly of the basement membrane and junctional proteins during corneal epithelial wound healing. Investig. Ophthalmol. Vis. Sci 41, 2495–2500. [PubMed] [Google Scholar]
- Tam WL, Lim B, 2008. Genome-wide Transcription Factor Localization and Function in Stem Cells StemBook, Cambridge (MA). [PubMed] [Google Scholar]
- Tandon P, Conlon F, Furlow JD, Horb ME, 2017. Expanding the genetic toolkit in Xenopus: approaches and opportunities for human disease modeling. Dev. Biol 426, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoft RA, Friend J, 1983. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis. Sci 24, 1442–1443. [PubMed] [Google Scholar]
- Thomas PB, Liu YH, Zhuang FF, Selvam S, Song SW, Smith RE, Trousdale MD, Yiu SC, 2007. Identification of Notch-1 expression in the limbal basal epithelium. Mol. Vis 13, 337–344. [PMC free article] [PubMed] [Google Scholar]
- Tiwari A, Loughner CL, Swamynathan S, Swamynathan SK, 2017. KLF4 plays an essential role in corneal epithelial homeostasis by promoting epithelial cell fate and suppressing epithelial-mesenchymal transition. Investig. Ophthalmol. Vis. Sci 58, 2785–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomellini E, Lagadec C, Polakowska R, Le Bourhis X, 2014. Role of p75 neurotrophin receptor in stem cell biology: more than just a marker. Cell. Mol. Life Sci. : CM 71, 2467–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimori Y, Katoh I, Kurata S, Okuyama T, Kamiyama R, Ikawa Y, 2004. Evolutionarily conserved expression pattern and trans-regulating activity of Xenopus p51/p63. Biochem. Biophys. Res. Commun 313, 230–236. [DOI] [PubMed] [Google Scholar]
- Touhami A, Grueterich M, Tseng SC, 2002. The role of NGF signaling in human limbal epithelium expanded by amniotic membrane culture. Investig. Ophthalmol. Vis. Sci 43, 987–994. [PubMed] [Google Scholar]
- Tseng AS, 2017. Seeing the future: using Xenopus to understand eye regeneration. Genesis 55. [DOI] [PubMed] [Google Scholar]
- Ueno H, Ferrari G, Hattori T, Saban DR, Katikireddy KR, Chauhan SK, Dana R, 2012. Dependence of corneal stem/progenitor cells on ocular surface innervation. Investig. Ophthalmol. Vis. Sci 53, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T, Yamato M, Nishida K, Kohno C, Yang J, Tano Y, Okano T, 2005. Rat limbal epithelial side population cells exhibit a distinct expression of stem cell markers that are lacking in side population cells from the central cornea. FEBS Lett 579, 6569–6574. [DOI] [PubMed] [Google Scholar]
- Uribe RA, Gross JM, 2007. Immunohistochemistry on cryosections from embryonic and adult zebrafish eyes. CSH Protoc 10.1101/pdb.prot4779. [DOI] [PubMed] [Google Scholar]
- van Es JH, Haegebarth A, Kujala P, Itzkovitz S, Koo BK, Boj SF, Korving J, van den Born M, van Oudenaarden A, Robine S, Clevers H, 2012. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol. Cell Biol 32, 1918–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorkauf W, Vorkauf M, Nolle B, Duncker G, 1995. Adhesion molecules in normal and pathological corneas. An immunohistochemical study using monoclonal antibodies. Albrecht Von Graefe’s Arch. Clin. Exp. Ophthalmol 233, 209–219. [DOI] [PubMed] [Google Scholar]
- Wang DY, Hsueh YJ, Yang VC, Chen JK, 2003. Propagation and phenotypic preservation of rabbit limbal epithelial cells on amniotic membrane. Investig. Ophthalmol. Vis. Sci 44, 4698–4704. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y, 2004. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett 565, 6–10. [DOI] [PubMed] [Google Scholar]
- Womble M, Amin NM, Nascone-Yoder N, 2018. The left-right asymmetry of liver lobation is generated by Pitx2c-mediated asymmetries in the hepatic diverticulum. Dev. Biol 439, 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HM, Bentley E, Campbell SF, Marfurt CF, Murphy CJ, 2005. Nerve growth factor and corneal wound healing in dogs. Exp. Eye Res 80, 633–642. [DOI] [PubMed] [Google Scholar]
- Xu L, Overbeek PA, Reneker LW, 2002. Systematic analysis of E-, N- and P-cadherin expression in mouse eye development. Exp. Eye Res 74, 753–760. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ, 2012. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc. Natl. Acad. Sci. U. S. A 109, 466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F, 1999. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718. [DOI] [PubMed] [Google Scholar]
- Yang F, Je HS, Ji Y, Nagappan G, Hempstead B, Lu B, 2009. Pro-BDNF-induced synaptic depression and retraction at developing neuromuscular synapses. J. Cell Biol 185, 727–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Shimmura S, Kawakita T, Miyashita H, Den S, Shimazaki J, Tsubota K, 2006. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Investig. Ophthalmol. Vis. Sci 47, 4780–4786. [DOI] [PubMed] [Google Scholar]
- Yoshii C, Ueda Y, Okamoto M, Araki M, 2007. Neural retinal regeneration in the anuran amphibian Xenopus laevis post-metamorphosis: transdifferentiation of retinal pigmented epithelium regenerates the neural retina. Dev. Biol 303, 45–56. [DOI] [PubMed] [Google Scholar]
- You L, Kruse FE, Volcker HE, 2000. Neurotrophic factors in the human cornea. Investig. Ophthalmol. Vis. Sci 41, 692–702. [PubMed] [Google Scholar]
- Zelenka PS, Arpitha P, 2008. Coordinating cell proliferation and migration in the lens and cornea. Semin. Cell Dev. Biol 19, 113–124. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tseng H, 2007. Basonuclin-null mutation impairs homeostasis and wound repair in mouse corneal epithelium. PLoS One 2 e1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh LK, Zhang S, Call M, Yuan Y, Yasunaga M, Kao WW, Liu CY, 2015. Wnt/beta-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development. Development 142, 3383–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Das AV, Thoreson WB, James J, Wattnem TE, Rodriguez-Sierra J, Ahmad I, 2002. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev. Biol 250, 317–331. [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, Sorrentino BP, 2001. The ABC transporter Bcrpl/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat. Med 7, 1028–1034. [DOI] [PubMed] [Google Scholar]
- Zhu SN, Dana MR, 1999. Expression of cell adhesion molecules on limbal and neovascular endothelium in corneal inflammatory neovascularization. Investig. Ophthalmol. Vis. Sci 40, 1427–1434. [PubMed] [Google Scholar]
- Zhu X, Min Z, Tan R, Tao Q, 2015. NF2/Merlin is required for the axial pattern formation in the Xenopus laevis embryo. Mech. Dev 138 (Pt 3), 305–312. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Bukusoglu G, Yankauckas MA, Wasson ME, Keutmann HT, 1992. Alpha-enolase is restricted to basal cells of stratified squamous epithelium. Dev. Biol 151, 18–26. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Wasson M, 1993. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J. Cell Sci 106 (Pt 1), 145–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Western blot analyse of various antibodies in Xenopus larval epithelial tissue lysate, as labeled. Numbers to the right represent molecular mass standards in kilodaltons (kDa). Arrowheads on the left indicate the positions corresponding to the predicted molecular weights of the proteins. Additional bands were detected in p63 (A), Pax6 (B), E-cadherin (G), consistent to described in other studies. See text for details.


