Abstract
Purpose
Breast fibroglandular tissue (FGT), as visualized on a mammogram (mammographic density, MD), is one of the strongest known risk factors for breast cancer. FGT is also visible on breast MRI, and increased background parenchymal enhancement (BPE) in the FGT has been identified as potentially a major breast cancer risk factor. The aim of this exploratory study was to examine the biologic basis of BPE.
Methods
We examined the unaffected contra-lateral breast of 80 breast cancer patients undergoing a prophylactic mastectomy before any treatment other than surgery of their breast cancer. BPE was classified on the BI-RADS scale (minimal/ mild/moderate/marked). Slides were stained for microvessel density (MVD), CD34 (another measure of endothelial density), glandular tissue within the FGT and VEGF. Spearman correlations were used to evaluate the associations between BPE and these pathologic variables.
Results
In pre-menopausal patients, BPE was highly correlated with MVD, CD34 and glandular concentration within the FGT, and the pathologic variables were themselves highly correlated. The expression of VEGF was effectively confined to terminal duct lobular unit (TDLU) epithelium. The same relationships of the four pathologic variables with BPE were seen in post-menopausal patients, but the relationships were much weaker and not statistically significant.
Conclusion
The strong correlation of BPE and MVD together with the high correlation of MVD with glandular concentration seen in pre-menopausal patients indicates that increased breast cancer risk associated with BPE in pre-menopausal women is likely to result from its association with increased concentration of glandular tissue in the FGT. The effective confinement of VEGF expression to the TDLUs shows that the signal for MVD growth arises directly from the glandular tissue. Further studies are needed to understand the basis of BPE in post-menopausal women.
Keywords: Background parenchymal enhancement, Breast MRI, Fibroglandular tissue, Immunohistochemistry, Microvessel density, VEGF
Introduction
Breast magnetic resonance imaging (MRI) is used for screening and clinical management of breast cancer patients. Normal breast fibroglandular tissue (FGT), as visualized on a mammogram (mammographic density, MD), is one of the strongest known risk factors for breast cancer [1]. FGT is also visible on breast MRI (MRI-FGT) and signal intensity in the FGT increases to varying extents after administration of MRI-contrast medium [2]; this increase is referred to as background parenchymal enhancement (BPE) and is classified on the American College of Radiology’s (ACR) Breast Imaging Reporting and Data System (BI-RADS) four-point BPE scale (minimal/mild/moderate/marked) [2]. In a study of 39 breast cancer cases and 78 age-matched controls, King et al. [3] found that there was an increasing risk of breast cancer with increasing levels of BPE with the relative risk (RR) increasing from 1.0 with a minimal/ mild BPE, to 4 or greater with a moderate/marked BPE. The increased risks were found in pre-menopausal and post-menopausal women. Large relative risks were also found in age-matched case-control studies reported by Dontchos et al. [4] and by Telegrafo et al. [5], and in the study of Wu et al. [6] although they did not report their findings using the BI-RADS BPE coding scheme. In their age-matched case-control study, Grimm et al. [7] found a smaller effect—RR of 2.5 for mild/moderate/marked compared to minimal. Telegrafo et al. [5] reported that similar risks were seen in pre-menopausal and post-menopausal women; the reports on the other studies did not describe their results separately for pre-menopausal or post-menopausal women. Although Bennani-Baiti et al. [8] reported that they found no effect of BPE on risk of breast cancer in their case-control study, their cases were much older than their controls and no details of how they accounted for this were given in the paper. BPE is distinct from the amount of FGT [9] and the studies that have reported on this have found that the risk from high BPE was independent of the risk associated with FGT [3, 4, 6, 7].
BPE is highly responsive to changes in the hormonal environment of the breast. It decreases with menopause [10, 11] and with tamoxifen [12] or aromatase inhibitor treatments [13, 14], all of which decrease breast cancer risk. It increases with menopausal hormone therapy [15–17], but it is not clear whether the effect is greater with estrogen-progestin therapy, which increases risk of breast cancer more than estrogen-alone therapy [18]. These changes in BPE are more pronounced than the hormonally-mediated changes seen in MRI-FGT [12, 13].
The BI-RADS classification of BPE is made with reference to “glandular tissue”: minimal is defined as “less than 25% of glandular tissue demonstrating enhancement”, mild as “25–50% of glandular tissue demonstrating enhancement”, moderate as “50–75% of glandular tissue demonstrating enhancement”, and marked as “more than 75% of glandular tissue demonstrating enhancement” [2]. In practice, “glandular tissue” is interpreted as “fibroglandular tissue” [2, 19]. It is assumed that the enhancement must come from microvessels in the FGT [20] and that these must be generated by the glandular tissue within the FGT [2, 8], but there is no direct evidence of this.
We report here, the results of a study we conducted of the histopathologic correlates of BPE to obtain direct evidence related to these assumed effects. We studied the contra-lateral cancer-free breast of women with breast cancer who underwent a bi-lateral contrast-enhanced breast MRI and prophylactic mastectomy prior to any treatment other than surgery for their breast cancer. We hypothesized that higher levels of BPE would be associated with higher levels of microvessel density (MVD) and with the concentration of glandular tissue in the FGT. As a secondary aim, we also studied the concentration of markers of inflammation as inflammation is thought to play a role in breast cancer etiology and may be related to the extent of BPE [21, 22], and estrogen receptor α (ERα) and progesterone receptor (PR), and a marker of cancer stem cells.
Materials and methods
In this exploratory study, we identified 80 uni-lateral breast cancer patients, who had a prophylactic contra-lateral mastectomy before they had received any treatment other than surgery. We studied a variety of aspects of their contra-lateral breast tissue and related these findings to their level of BPE.
Patients
The Memorial Sloan Kettering Cancer Center (MSK) Institutional Review Board granted permission to conduct this Health Insurance Portability and Accountability Act compliant retrospective study. A waiver of patient consent was granted to conduct a retrospective review of the radiology and pathology records of breast cancer patients who (1) had consented to the MSK global biospecimen ascertainment protocol, and (2) had a contrast-enhanced bi-lateral breast MRI before breast surgery or any adjuvant breast cancer treatment. By searching the medical records over the period 2008 through 2014, we identified the subset of women who had a uni-lateral breast cancer and a subsequent prophylactic mastectomy of their contra-lateral breast that showed no evidence of breast cancer. The waiver of consent covered our immunohistochemical study of the paraffin-embedded tissues obtained from the non-cancer breast of such patients and the correlation of the immunohistochemistry with the BPE findings in the non-cancer breasts.
Patients were excluded if, at the time of MRI or breast surgery, they had breast implants; were taking oral contraceptives, post-menopausal hormone therapy, or other hormonal medications (e.g., tamoxifen); or were pregnant or breast feeding within 6 months; or were < 21 or ≥ 70 years of age. Women with a history of any cancer (apart from non-melanoma skin cancer) were also excluded as prior systemic treatments may confound any measurements we made.
We reviewed hospital charts and reports to obtain information on prior surgeries, biopsies, diagnoses of benign breast disease, and hormonal medication use. In this exploratory study, we sought to identify 80 patients meeting the initial eligibility criteria: 20 patients from each of the following four groups: pre-menopausal with minimal/mild BPE; pre-menopausal with moderate/marked BPE; post-menopausal with minimal/mild BPE; and post-menopausal with moderate/marked BPE.
MRI readings
All MRI readings were first carried out by a single radiologist (JSS). MRI-FGT was assessed using a T1-weighted non-fat-suppressed series and categorized using the four-point BI-RADS FGT scale (predominantly fatty/scattered/ heterogeneously dense/dense). We excluded patients with fatty MRI-FGT breasts to help ensure that there was sufficient FGT for the pathology analyses. BPE was assessed using a T1-weighted fat-suppressed sequence from the pre-contrast, the first post-contrast series and the subtraction image (subtraction of the pre-contrast image from the first post-contrast image). BPE was categorized using the four-point BI-RADS BPE scale.
The BPE readings were also carried out by a second radiologist (DMK) completely independently of the initial BPE readings using the same methods on the same MRIs. The main results in the paper are given using the readings of JSS, and comparisons are made between the results using the readings of JSS to the readings obtained by DMK, which are given in Supplementary Tables 1 and 2.
Histology
The following markers were selected for study: MVD, CD34 [23] and VEGF [24] to quantify the degree of vascularization and ongoing angiogenesis; the glandular concentration in the FGT; COX2 [25] and TGFβ [26] as markers of inflammation and macrophage-adipocyte complexes (crown-like structures) for their possible association with release of inflammatory cytokines [27]. We also studied estrogen receptor α (ERα) and progesterone receptor (PR); and the presence of CD44 cells in the absence of CD24 cells as a marker of cancer stem cells [28].
Immunohistochemical (IHC) studies were performed using the formalin-fixed paraffin-embedded tissue blocks that appeared to contain the most FGT, one to two tissue blocks from each patient were chosen with no knowledge of the breast BPE or any clinical factors. All quantifications were made restricting attention to the FGT. Multiple sequential sections from the chosen blocks were cut at 5 μm, and followed the standard MSK Pathology Department protocol for IHC processing. IHC stains for were performed for CD24, CD34, CD44, CD31/PECAM-1, COX2, ERα, PR, TGFβ, and VEGF, with appropriate positive and negative controls for each stain. Sources and dilutions of the primary antibodies were: CD24—polyclonal CD24 antibody, Biorbyt, at 1:50 dilution; CD34—clone 4H11, LSBio, ready to use (RTU); CD44—clone 156–3C11, Neomarkers, at 1:200 dilution; CD31/PECAM-1—clone TLD-3A12, Novus, RTU; COX2—clone COX229, LSBio, RTU; ERα—clone 6F11, Leica, RTU; PR—clone 16, Leica, RTU; TGFβ—clone TB21, Abcam, at 1:50 dilution; and VEGF—clone JH, Millipore Sigma, RTU.
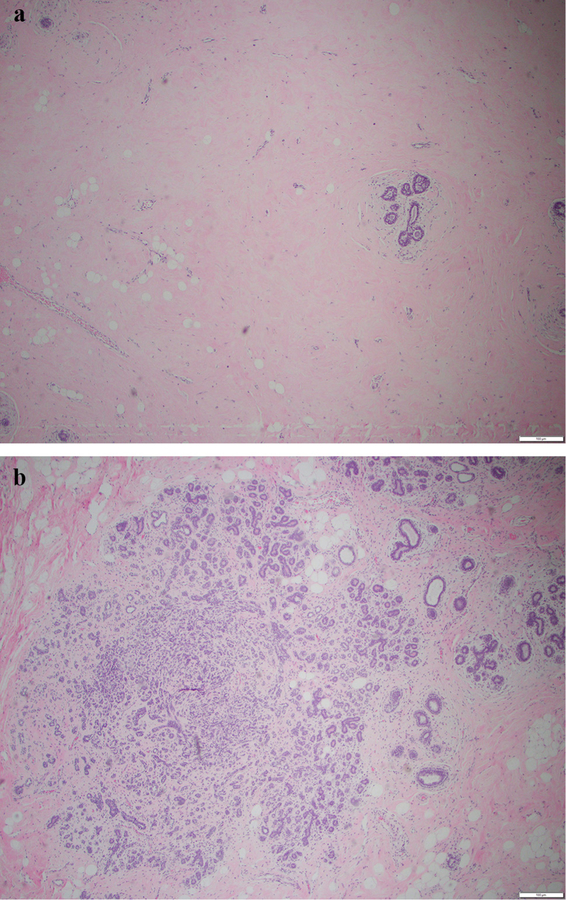
MVD was recorded as the number of CD31/PECAM-1-staining microvessels per 200 × HPF in the stroma in five 200 × HPFs containing the highest number of TDLUs. Other IHC stains were assessed in 10 microscopic fields containing the highest number of TDLUs, at multiple magnifications (2×, 4×, 10× and 20× HPFs). CD34 was recorded semi-quantitatively from weak to strong (+, ++, +++) in membranous staining of endothelial cells (Supplementary Fig. 1a, Fig. 1b). We found that VEGF staining was almost completely confined to TDLU epithelium and was recorded semi-quantitatively from weak to strong (+, ++, +++) in membranous staining of TDLU epithelial cells (Fig. 1, Supplementary Fig. 2a).
Fig. 1.
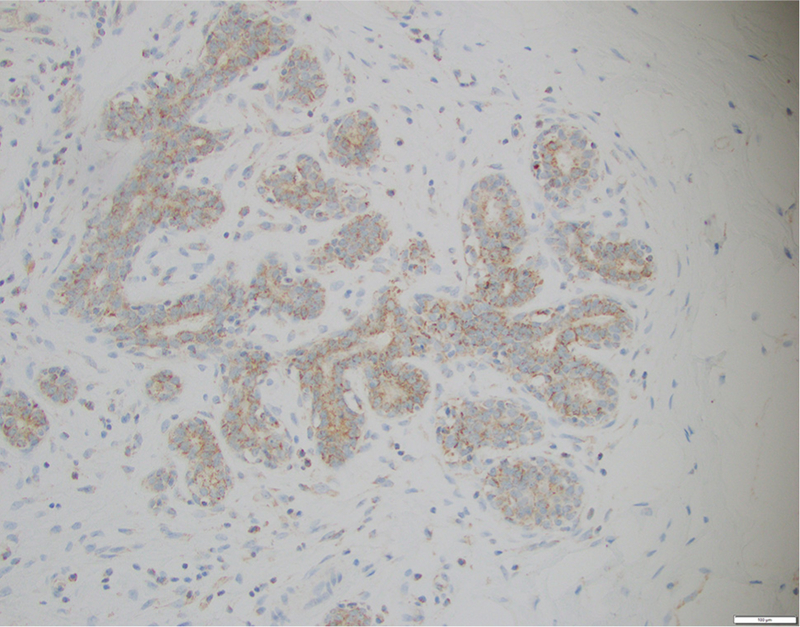
Strong (+++) VEGF staining of TDLU epithelium
The glandular concentration within the FGT, i.e., glandular tissue as a proportion of the FGT, was estimated on the H&E slides by visualizing 15 microscopic fields containing the most FGT at multiple magnifications (2×, 4× and 10× HPFs), and was recorded as low, medium or high (Fig. 2a, b).
Fig. 2.
a Low (+) glandular concentration. b High (+++) glandular concentration
The other IHC stains were recorded as follows: ERα and PR—% nuclear staining of TDLU epithelial cells; CD24, CD44, COX2, TGFβ—positive (+) and negative (−) membranous staining of TDLU epithelial cells.
All cases were independently reviewed and scored by a single pathologist (ADC), who was blinded to the patient’s age, weight, menopausal status, clinical diagnosis, and the radiologic characteristics of the breast, including the reading of FGT and BPE.
Statistical analysis
We analyzed these data using the statistical package program Stata 14 (Stata Corporation, College Station, TX, USA). Non-parametric Spearman correlations were used to calculate the statistical significance of the relationship between the pathologic factors and BPE, and to investigate the inter-relationships of the various factors studied. All statistical significance levels (p values) quoted are two-sided.
Results
We identified 80 patients who had undergone prophylactic mastectomy of the non-cancer unaffected breast and who met the other inclusion criteria for the study. The distribution of the 80 patients by menopausal status and BPE category was not as precise as we had intended because we were unable to identify 20 post-menopausal patients with moderate/marked BPE (Table 1).
Table 1.
Patient characteristics
| Variable | Pre-menopausal | Post-menopausal |
|---|---|---|
| Number | 48 | 32 |
| Age (median, IQR) | 38 (33, 46) | 54 (53, 59) |
| Race/ethnicity | ||
| White | 43 | 26 |
| African-American | 3 | 4 |
| Other | 2 | 2 |
| BMI (median, IQR) | 23.3 (20.7, 25.3) | 27.7 (23.3, 31.5) |
The relationships between BPE (as read by JSS) and MVD, CD34 and glandular concentration in pre-menopausal patients are shown in Table 2. All three factors were much higher in women with moderate/marked BPE, and the results were all highly statistically significant. VEGF expression was also strongly associated with BPE to approximately the same extent as the other three factors (Table 2). All four pathologic factors were highly correlated with each other (Table 2). The close relationship of VEGF with TDLU cells is illustrated in Fig. 1, and low and high glandular concentration is illustrated in Fig. 2a, b. The relationships of the pathologic variables with BPE were slightly strengthened when the analyses were restricted to Whites.
Table 2.
The relationship of BPE to microvessel density, CD34, glandular concentration and VEGF in pre-menopausal patients
| Variable | Category | BPE |
ra | pa | |||
|---|---|---|---|---|---|---|---|
| Min (n = 12) | Mild (n = 14) | Mod (n = 13) | Marked (n = 9) | ||||
| Microvessel densityb | < 3 | 8 (67%) | 4 (29%) | 0 (0%) | 0 (0%) | 0.71 | < 0.001 |
| 3− | 4 (33%) | 6 (43%) | 6 (38%) | 1 (11%) | |||
| 5+ | 0 (0%) | 4 (29%) | 7 (15%) | 8 (89%) | |||
| Averagec | 1.33 | 2.00 | 2.54 | 2.89 | |||
| CD34d | + | 11 (92%) | 7 (50%) | 2 (69%) | 1 (11%) | 0.71 | < 0.001 |
| ++ | 1 (8%) | 6 (43%) | 8 (26%) | 0 (0%) | |||
| +++ | 0 (0%) | 1 (7%) | 3 (3%) | 8 (89%) | |||
| Averagec | 1.08 | 1.57 | 2.08 | 2.78 | |||
| Glandular concentratione | Low | 11 (92%) | 7 (50%) | 2 (15%) | 1 (11%) | 0.70 | < 0.001 |
| Medium | 1 (8%) | 6 (43%) | 4 (31%) | 1 (11%) | |||
| High | 0 (0%) | 1 (7%) | 7 (54%) | 7 (78%) | |||
| Averagec | 1.08 | 1.57 | 2.38 | 2.67 | |||
| VEGFf | + | 11 (92%) | 9 (64%) | 2 (15%) | 1 (11%) | 0.67 | < 0.001 |
| ++ | 1 (8%) | 4 (29%) | 8 (62%) | 3 (33%) | |||
| +++ | 0 (0%) | 1 (7%) | 3 (23%) | 5 (56%) | |||
| Averagec | 1.08 | 1.43 | 2.08 | 2.44 | |||
| Spearman correlations between pathological variablesg | |||||||
| Variable | (1) | (2) | (3) | (4) | |||
| (1) MVD | 1.00 | 0.78 | 0.78 | 0.79 | |||
| (2) CD34 | 1.00 | 0.95 | 0.89 | ||||
| (3) Glan con | 1.00 | 0.92 | |||||
| (4) VEGF | 1.00 | ||||||
BPE measurements made by JSS
Non-parametric Spearman correlation (r) with associated 2-sided significance level (p)
Per 200 × HPF
Averages scoring 3 levels as 1, 2 and 3 respectively
Membranous staining of endothelial cells
As a proportion of the FGT
Membranous staining of TDLU epithelial cells
All p values < 0.001
BPE is known to vary during the menstrual cycle, with the lowest level being found in week 2 and the highest in weeks 3 and 4 [2, 29]; the BPE level in week 1 is close to the level in week 2. We identified the week of the menstrual cycle when the MRI examinations was carried out for 41 of the 48 pre-menopausal patients. We carried out the above analyses including only the results from MRIs carried out in weeks 1 and 2 combined and then including only the results from MRIs carried out in weeks 3 and 4 combined. The same strong relationships between the pathologic variables and BPE were found: the results are shown in Supplementary Table 1-Weeks 1–2 and Supplementary Table 1-Weeks 3–4.
The same relationships of the four pathologic factors with BPE were seen in post-menopausal patients, but the relationships were much weaker and not statistically significant (Table 3). The pathologic factors were again highly correlated with each other (Table 3).
Table 3.
The relationship of BPE to microvessel density, CD34, glandular concentration, and VEGF in post-menopausal patients
| Variable | Category | BPE |
ra | pa | |||
|---|---|---|---|---|---|---|---|
| Min (n = 11) | Mild (n = 12) | Mod (n = 6) | Marked (n = 3) | ||||
| Microvessel densityb | < 3 | 5 (45%) | 5 (42%) | 2 (33%) | 1 (33%) | 0.22 | 0.22 |
| 3− | 4 (36%) | 3 (25%) | 1 (17%) | 0 (0%) | |||
| 5+ | 2 (18%) | 4 (33%) | 3 (50%) | 2 (67%) | |||
| Averagec | 1.73 | 1.92 | 2.17 | 2.33 | |||
| CD34d | + | 9 (82%) | 7 (58%) | 4 (69%) | 1 (33%) | 0.28 | 0.13 |
| ++ | 2 (18%) | 5 (42%) | 2 (26%) | 1 (33%) | |||
| +++ | 0 (0%) | 0 (0%) | 0 (0%) | 1 (33%) | |||
| Averagec | 1.18 | 1.42 | 1.33 | 2.00 | |||
| Glandular concentratione | Low | 9 (82%) | 7 (58%) | 3 (50%) | 1 (33%) | 0.33 | 0.063 |
| Medium | 1 (9%) | 3 (25%) | 2 (33%) | 0 (0%) | |||
| High | 1 (9%) | 2 (17%) | 1 (17%) | 2 (67%) | |||
| Averagec | 1.27 | 1.58 | 1.67 | 2.33 | |||
| VEGFf | + | 9 (82%) | 8 (67%) | 3 (50%) | 1 (33%) | 0.32 | 0.079 |
| ++ | 2(18%) | 4 (33%) | 3 (50%) | 2 (67%) | |||
| +++ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Averagec | 1.18 | 1.33 | 1.50 | 1.67 | |||
| Spearman correlations between pathological variablesg | |||||||
| Variable | (1) | (2) | (3) | (4) | |||
| (1) MVD | 1.00 | 0.73 | 0.80 | 0.73 | |||
| (2) CD34 | 1.00 | 0.93 | 0.86 | ||||
| (3) Glan con | 1.00 | 0.93 | |||||
| (4) VEGF | 1.00 | ||||||
BPE measurements made by JSS
Non-parametric Spearman correlation (r) with associated 2-sided significance level (p)
Per 200 × HPF
Averages scoring 3 levels as 1, 2 and 3 respectively
Membranous staining of endothelial cells
As a proportion of the FGT
Membranous staining of TDLU epithelial cells
All p values < 0.001
Further analysis of the relationships between each of the four pathologic factors and BPE both in pre-menopausal women and in post-menopausal women showed that the relationships could be satisfactorily fit by linear regression models with the increasing BPE levels taking values 1, 2, 3, and 4, and the categorized pathologic variables taking values 1, 2, and 3. These analyses showed that the slope of the regression lines for the post-menopausal women were much lower than the slopes found for the pre-menopausal women: MVD (80% lower, p = 0.006), CD34 (65% lower, p = 0.005), glandular density (49% lower, p = 0.074), and VEGF (66% lower, p = 0.011). The estimated slopes (standard errors) of the post-menopausal patients were 0.320 (0.418), 0.194 (0.098), 0.291 (0.142), and 0.161 (0.086) for MVD, CD34, glandular density and VEGF respectively.
No relationships were seen between BPE and CD44+/ CD24−, TGFβ, ERα, or PR in pre-menopausal or in post-menopausal patients (Tables 4, 5). COX2 expression was seen in all TDLUs. Crown-like structures were rarely seen.
Table 4.
The relationship of BPE to CD44+/CD24−, TGFβ, ERα and PR in pre-menopausal patients
| Variable | Category | BPE |
ra | pa | |||
|---|---|---|---|---|---|---|---|
| Min (n = 12) | Mild (n = 14) | Mod (n = 13) | Marked (n = 9) | ||||
| CD44+/CD24−b | − | 9 (75%) | 5 (36%) | 7 (54%) | 4 (44%) | 0.15 | 0.30 |
| + | 3 (25%) | 9 (64%) | 6 (46%) | 5 (56%) | |||
| TGFβb | − | 10 (83%) | 12 (86%) | 13 (100%) | 7 (78%) | −0.03 | 0.82 |
| + | 2 (17%) | 2 (14%) | 0 (0%) | 2 (22%) | |||
| ERαc | < 5 | 5 (42%) | 5 (36%) | 4 (31%) | 3 (33%) | 0.11 | 0.46 |
| 5− | 6 (50%) | 8 (57%) | 8 (62%) | 5 (56%) | |||
| 10− | 0 (0%) | 1 (7%) | 1 (8%) | 1 (11%) | |||
| 20− | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 30+ | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| PRc | < 5 | 9 (75%) | 11 (79%) | 5 (38%) | 5 (56%) | 0.14 | 0.36 |
| 5− | 2 (17%) | 2 (14%) | 6 (46%) | 3 (33%) | |||
| 10− | 0 (0%) | 1 (7%) | 2 (15%) | 1 (11%) | |||
| 20− | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 30+ | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
BPE measurement made by JSS
Non-parametric Spearman correlation (r) and associated significance level (p)
Membranous staining of TDLU epithelial cells
% nuclear staining of TDLU epithelial cells
Table 5.
The relationship of BPE to CD44+/CD24−,TGFβ, ERα, and PR in post-menopausal patients
| Variable | Category | BPE |
ra | pa | |||
|---|---|---|---|---|---|---|---|
| Min (n = 11) | Mild (n = 12) | Mod (n = 6) | Marked (n = 3) | ||||
| CD44+/CD24−b | − | 3 (27%) | 4 (33%) | 2 (33%) | 0 (0%) | − 0.06 | 0.75 |
| + | 8 (73%) | 8 (67%) | 4 (67%) | 3 (100%) | |||
| TGFβb | − | 1 (9%) | 1 (8%) | 1 (17%) | 0 (0%) | − 0.01 | 0.97 |
| + | 10 (91%) | 11 (92%) | 5 (83%) | 3 (100%) | |||
| ERαc | < 5 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.10 | 0.59 |
| 5− | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 10− | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 20− | 5 (45%) | 5 (42%) | 1 (17%) | 1 (33%) | |||
| 30+ | 6 (55%) | 7 (58%) | 5 (83%) | 2 (67%) | |||
| PRc | < 5 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.10 | 0.57 |
| 5− | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 10− | 2 (18%) | 1 (8%) | 0 (0%) | 0 (0%) | |||
| 20− | 5 (45%) | 9 (75%) | 4 (67%) | 2 (67%) | |||
| 30+ | 4 (36%) | 2 (17%) | 2 (33%) | 1 (33%) | |||
BPE measurement made by JSS
Non-parametric Spearman correlation (r) and associated significance level (p)
Membranous staining of TDLU epithelial cells
% nuclear staining of TDLU epithelial cells
The relationships of BPE as read by DMK to the pathologic factors were very similar to the results found for BPE as read by JSS (Supplementary Tables 2, 3).
Discussion
We found strong correlations of BPE with MVD, CD34, glandular concentration, and VEGF in pre-menopausal patients. As expected the two measures of endothelial density, i.e., MVD and CD34, were highly correlated. MVD and CD34 were also highly correlated with glandular concentration. Independent assessment of BPE by a second radiologist provided further support to these findings, and further analysis controlling for menstrual cycle phase showed that these correlations were not affected by cycle phase at MRI. These results are strongly supportive of the common assumption that BPE is a measure of microvessels in the FGT [20] and that microvessels are closely associated with the glandular tissue within the FGT [2, 8]. The biological basis of the high correlation of glandular concentration with MVD would appear to be that VEGF expression is essentially confined to TDLU cells, which comprise the glandular tissue, and VEGF is a potent mitogen for micro- and macro-vascular endothelial cells and is associated with increased vascular permeability [30–34]. A high correlation between VEGF expression and microvessel density was previously noted in breast cancer samples by Toi and colleagues [35], who reported “frequent appearance of peri-ductal proliferations of microvessels around VEGF positive tumor components”. These results suggest that a significant part of the risk of breast cancer associated with increased BPE in pre-menopausal women is due to an increased concentration of glandular tissue in the FGT.
Increased FGT is likely to increase breast cancer risk due to the association of increased FGT with increased glandular tissue. This was shown in the analysis of Boyd and his colleagues [36] of the extensive data on normal breast gathered by Longacre and Bartow [37]; in further analysis of these data, we found this to hold in both pre-menopausal and post-menopausal women. If our findings are confirmed, it will demonstrate that BPE effectively distinguishes the glandular tissue from the fibrous tissue in FGT of pre-menopausal women. If this is the case, knowledge of BPE measured as an absolute quantity, rather than, as in the BI-RADS system, as a quantity relative to the amount of FGT, would be expected to be a marker of breast cancer risk in pre-menopausal women without there being any requirement to include FGT in the risk assessment. The change in BPE during the menstrual cycle with the least BPE being shown in the second week of the cycle could be in large part due to the “lobular contraction and necrosis” around the peri-menstrual period noted by Longacre and Bartow [37].
The semi-quantitative measures we have made of the immunochemistry stains are likely to be subject to significant intra- and inter-observer variability. The pathology readings were, however, made completely independently of the radiology readings, so it is likely that the true relationships between BPE and the pathology findings are even more definitive than what we observed. Nevertheless, the interpretations we have made must be regarded as tentative until further measurements are made. It would be helpful to also measure breast cell proliferation, but this would only be of use if the MRI could be done immediately before the prophylactic surgery.
The relationships of the pathologic variables to BPE were also seen in post-menopausal patients, but the relationships were much weaker and not statistically significant. BPE is significantly lower after menopause [10], and our failure to find the planned numbers of post-menopausal patients with moderate/marked BPE decreased our statistical power in this group. Nevertheless, analysis showed that the relationships of the pathologic variables to BPE were considerably weaker in post-menopausal patients. To what extent this may be due to a poorer relationship of the tissues showing BPE to the tissues studied is unknown. Further studies of the biological basis of moderate/marked BPE in post-menopausal women are clearly needed. Prospective studies in which the part of the breast showing high BPE was directly sampled for detailed pathologic analysis are likely to be most informative.
Supplementary Material
Acknowledgements
We would especially like to thank all the patients who gave permission to have their tissues used for research.
Funding This study was funded in part through the National Institutes of Health/National Cancer Institute Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center, which also provided special support through an Interdisciplinary Population Science Research award from the Survivorship, Outcomes and Risk Development Funds within Memorial Sloan Kettering Cancer Center. Additional funding was also provided by the Susan G. Komen Foundation.
Abbreviations
- ACR
American College of Radiology
- BI-RADS
Breast Imaging Reporting and Data System
- BPE
Background parenchymal enhancement
- FGT
Fibroglandular tissue
- IHC
Immunohistochemistry
- MD
Mammographic density
- MRI
Magnetic resonance imaging
- MSK
Memorial Sloan Kettering Cancer Center
- MVD
Microvessel density
- TDLU
Terminal duct lobular unit
- VEGF
Vascular endothelial growth factor
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the Memorial Sloan Kettering Cancer Center institutional national research committee and with the 1964 Helsinki declaration and its later amendments. For this type of study formal consent is not required. This article does not contain any studies with animals performed by any of the authors.
References
- 1.McCormack VA, dos Santos Silva I (2006) Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomark Prev 15(6):1159–1169. 10.1158/1055-9965.epi-06-0034 [DOI] [PubMed] [Google Scholar]
- 2.Morris EA (2010) Diagnostic breast MR imaging: current status and future directions. Magn Reson Imaging Clin N Am 18(1):57–74. 10.1016/j.mric.2009.09.005 [DOI] [PubMed] [Google Scholar]
- 3.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA (2011) Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 260(1):50–60. 10.1148/radiol.11102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR, Peacock S, Lehman CD (2015) Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 276(2):371–380. 10.1148/radiol.2015142304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M (2016) Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn Reson Imaging 34(2):173–176. 10.1016/j.mri.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Berg WA, Zuley ML, Kurland BF, Jankowitz RC, Nishikawa R, Gur D, Sumkin JH (2016) Breast MRI contrast enhancement kinetics of normal parenchyma correlate with presence of breast cancer. Breast Cancer Res 18(1):76 10.1186/s13058-016-0734-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimm LJ, Saha A, Ghate SV, Kim C, Soo MS, Yoon SC, Mazurowski MA (2018) Relationship between background parenchymal enhancement on high-risk screening MRI and future breast cancer risk. Acad Radiol 10.1016/j.acra.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 8.Bennani-Baiti B, Dietzel M, Baltzer PA (2016) MRI background parenchymal enhancement is not associated with breast cancer. PLoS ONE 11(7):e0158573 10.1371/journal.pone.0158573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen NL, Kuhl CK, Barabasch A, Strobel K, Schrading S (2014) Does MRI breast “density” (degree of background enhancement) correlate with mammographic breast density? J Magn Reson Imaging 40(2):483–489. 10.1002/jmri.24495 [DOI] [PubMed] [Google Scholar]
- 10.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA (2012) Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 22(12):2641–2647. 10.1007/s00330-012-2553-8 [DOI] [PubMed] [Google Scholar]
- 11.Scaranelo AM, Carrillo MC, Fleming R, Jacks LM, Kulkarni SR, Crystal P (2013) Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology 267(3):692–700. 10.1148/radiol.13120121 [DOI] [PubMed] [Google Scholar]
- 12.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, Brooks JD, Morris EA (2012) Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J 18(6):527–534. 10.1111/tbj.12002 [DOI] [PubMed] [Google Scholar]
- 13.King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE, Pike MC, Dickler MN, Morris EA (2012) Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 264(3):670–678. 10.1148/radiol.12112669 [DOI] [PubMed] [Google Scholar]
- 14.Mousa NA, Eiada R, Crystal P, Nayot D, Casper RF (2012) The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause 19(4):420–425. 10.1097/gme.0b013e31823772a8 [DOI] [PubMed] [Google Scholar]
- 15.Pfleiderer S, Sachse S, Sauner D, Marx C, Malich A, Wurdinger S, Kaiser W (2004) Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res 6(3):R232 R238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L (2005) Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging—initial observations. Radiology 235(1):36–41. 10.1148/radiol.2351040012 [DOI] [PubMed] [Google Scholar]
- 17.Reichenbach JR, Przetak C, Klinger G, Kaiser WA (1999) Assessment of breast tissue changes on hormonal replacement therapy using MRI: a pilot study. J Comput Assist Tomogr 23(3):407–413 [DOI] [PubMed] [Google Scholar]
- 18.Prentice RL, Chlebowski RT, Stefanick ML, Manson JE, Langer RD, Pettinger M, Hendrix SL, Hubbell FA, Kooperberg C, Kuller LH, Lane DS, McTiernan A, O’Sullivan MJ, Rossouw JE, Anderson GL (2008) Conjugated equine estrogens and breast cancer risk in the Women’s Health Initiative clinical trial and observational study. Am J Epidemiol 167(12):1407–1415. 10.1093/aje/kwn090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewin AA, Kim SG, Babb JS, Melsaether AN, McKellop J, Moccaldi M, Klautau Leite AP, Moy L (2016) Assessment of back-ground parenchymal enhancement and lesion kinetics in breast MRI of BRCA ½ mutation carriers compared to matched controls using quantitative kinetic analysis. Acad Radiol 23(3):358–367. 10.1016/j.acra.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD (2012) Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 198(4):W373–W380. 10.2214/AJR.10.6272 [DOI] [PubMed] [Google Scholar]
- 21.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ (2013) Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res 19(22):6074–6083. 10.1158/1078-0432.CCR-12-2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson ER, Brown KA (2013) Obesity and breast cancer: role of inflammation and aromatase. J Mol Endocrinol 51(3):T51–T59. 10.1530/JME-13-0217 [DOI] [PubMed] [Google Scholar]
- 23.Fina L, Molgaard HV, Robertson D, Bradley NJ, Monaghan P, Delia D, Sutherland DR, Baker MA, Greaves MF (1990) Expression of the CD34 gene in vascular endothelial cells. Blood 75(12):2417–2426 [PubMed] [Google Scholar]
- 24.Matsumoto K, Ema M (2014) Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem 156(1):1–10. 10.1093/jb/mvu031 [DOI] [PubMed] [Google Scholar]
- 25.Sobolewski C, Cerella C, Dicato M, Ghibelli L, Diederich M (2010) The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 10.1155/2010/215158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis MA, Sheppard D (2014) TGF-beta activation and function in immunity. Annu Rev Immunol 32:51–82. 10.1146/annurev-immunol-032713-120257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, Subbaramaiah K, Dannenberg AJ (2011) Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res 4(7):1021–1029. 10.1158/1940-6207.CAPR-11-0110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camerlingo R, Ferraro GA, De Francesco F, Romano M, Nicoletti G, Di Bonito M, Rinaldo M, D’Andrea F, Pirozzi G (2014) The role of CD44+/CD24-/low biomarker for screening, diagnosis and monitoring of breast cancer. Oncol Rep 31(3):1127–1132. 10.3892/or.2013.2943 [DOI] [PubMed] [Google Scholar]
- 29.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L (2005) Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 11(4):236–241. 10.1111/j.1075-122X.2005.21499.x [DOI] [PubMed] [Google Scholar]
- 30.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM (1987) Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 57(6):673–686 [PubMed] [Google Scholar]
- 31.Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van de Water L, Senger DR (1991) Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 174(5):1275–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roberts WG, Palade GE (1995) Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 108(Pt 6):2369–2379 [DOI] [PubMed] [Google Scholar]
- 33.Dellian M, Witwer BP, Salehi HA, Yuan F, Jain RK (1996) Quantitation and physiological characterization of angiogenic vessels in mice: effect of basic fibroblast growth factor, vascular endothelial growth factor/vascular permeability factor, and host microenvironment. Am J Pathol 149(1):59–71 [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara N, Davis-Smyth T (1997) The biology of vascular endothelial growth factor. Endocr Rev 18(1):4–25. 10.1210/edrv.18.1.0287 [DOI] [PubMed] [Google Scholar]
- 35.Toi M, Inada K, Suzuki H, Tominaga T (1995) Tumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expression. Breast Cancer Res Treat 36(2):193–204 [DOI] [PubMed] [Google Scholar]
- 36.Li T, Sun L, Miller N, Nicklee T, Woo J, Hulse-Smith L, Tsao MS, Khokha R, Martin L, Boyd N (2005) The association of measured breast tissue characteristics with mammographic density and other risk factors for breast cancer. Cancer Epidemiol Biomark Prev 14(2):343–349 [DOI] [PubMed] [Google Scholar]
- 37.Longacre TA, Bartow SA (1986) A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol 10:382–393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.