SUMMARY
Regenerative medicine promises to meet two of the most urgent needs of modern organ transplantation, namely immunosuppression-free transplantation and an inexhaustible source of organs. Ideally, bioengineered organs would be manufactured from a patient’s own biomaterials—both cells and the supporting scaffolding materials in which cells would be embedded and allowed to mature to eventually regenerate the organ in question. While some groups are focusing on the feasibility of this approach, few are focusing on the immunogenicity of the scaffolds that are being developed for organ bioengineering purposes. This review will succinctly discuss progress in the understanding of immunological characteristics and behavior of different scaffolds currently under development, with emphasis on the extracellular matrix scaffolds obtained decellularized animal or human organs which seem to provide the ideal template for bioengineering purposes.
Keywords: B cell, extracellular matrix, macrophage, neutrophil, regenerative medicine, T cell
Introduction
Regenerative medicine (RM), tissue engineering and organ bioengineering are terms used interchangeably – yet erroneously –to indicate a field of health sciences that aims, among other objectives, at manufacturing organs from patients’ own cells to replace their diseased counterparts and restore function. When this technology reaches the bedside, organ transplantation, as it is currently practiced, may become obsolete. In fact, it would enable transplant providers to deliver custom made organs and allow organ-on-demand, whereby patients suffering from end stage disease could receive a new functioning organ when indicated without registering on a waiting list. Moreover, and very importantly, patients could be immunosuppression-free because organs would be manufactured from their own cells which the host immune system would recognize as “self” [1].
Organs, however, not only consist of cellular components, but also have an extracellular compartment, which is fundamental for their well-being and ultimately their ability to function [2]. Therefore, if we want to build organs, we must also manufacture that which is referred to in RM jargon as supporting scaffolding materials. These scaffolds have become quite popular in RM in the past few decades, during which extracellular matrix (ECM)-based, as well as non-ECM-based (otherwise said, synthetic) scaffold technology has advanced dramatically, leading to a variety of preclinical and clinical applications [3]. Despite their acellular nature and lack of HLA antigens, scaffolds are not immunologically inert: an innate, foreign body-like immune response is activated immediately after implantation. Interestingly, there is a wealth of data in the literature on the ability of the immune system to mount a response against components of the ECM [4–6]. For example, antibodies against critical ECM components like perlecan, fibronectin, collagen IV and VI, vimentin and agrin, can be detected in patients with chronic renal rejection [3].
The purpose of this review paper is to summarize the current knowledge on the immunogenicity of the scaffolds that are currently being developed for organ bioengineering purposes, with an emphasis on ECM scaffolds.
Utility of scaffolding materials
The rationale for utilizing scaffolds in organ bioengineering is that ECM innately serves as the framework that allows the existence of multicellular organisms, by providing physical support to cells, as well as all molecular and physical cues necessary for cells to live, maintain viability and function [3]. Moreover, the ECM functions as reservoir of proteins (cytokines and analogues) critical for the tissue’s welfare that are released or stored depending on the tissue’s metabolic needs. Interestingly, the ECM retains [1,3,7] most of these factors throughout decellularization, a process by which ECM scaffolds are generated. This process, first described in the 60’s [1–3,7], aims to completely eliminate the cellular compartment of tissues and organs, including its DNA and RNA content, and can nowadays be successfully and consistently applied to virtually all mammal tissues and organs of clinical interest [1,3,7]. Because cytokines and growth factors are retained, ECM scaffolds are extremely bioactive. For example, when ECM scaffolds are implanted within the chorioallantoic membrane, a strong angiogenic response represented by the generation of new capillaries around and above the scaffolds can be observed [1,3]. Moreover, innate ECM can dictate a tissue-organ specific cell phenotype [7]. In fact, when progenitor cells are seeded on ECM, they are induced to express genes specific to the ontogenesis of the organ from which the ECM originated [2]. Finally, the ultrastructure of the ECM is tissue specific and the molecules are arranged in way that is ideally suited for said tissue cell type [8].
Scaffolds can be manufactured de novo using synthetic or biological materials, or can be obtained through the decellularization of human (allogeneic) or animal (xenogeneic) organs (ECM-based). A variety of factors, including graft complexity, molecular fingerprint and content, and type of graft can affect the host immune response to the graft [1].
Synthetic and natural scaffolds
“Biomaterial” is a term that describes synthetic or natural polymers, lipids, cells, and an array of self-assembled structures, and refers to any substance that has been engineered to interact with biological systems for a medical purpose - either a therapeutic (treat, augment, repair or replace a tissue function of the body) or a diagnostic one. These biomaterials are often used to build implantable scaffolds or devices [9,10], or to formulate nanoparticles or microparticles that can be delivered or conjugated to cells ex vivo or in vivo [11].
In general, synthetic biomaterials trigger a foreign body reaction inducing the formation of granulation tissue, in which neutrophils and macrophages are the predominant contributors [12]. Surface characteristics of the biomaterial and its composition may affect the course and the extent of the immune reaction. Flat and smooth surfaces generally lead to the formation of fibrosis, whereas, implants with a rough surface, such as vascular prostheses, become covered by a layer of macrophages and giant cells with variable amounts of granulation tissue which can persist around the implant and potentially isolate it from the local tissue [13].
The initial phase of foreign body reaction starts with the activation of the coagulation and complement cascades which eventually leads to neutrophil infiltration, and, after 2–3 days, macrophages. Subsequently, macrophages act as the main regulatory cells, activating keratinocytes, fibroblasts, and endothelial cells [14,15]. Lymphocytes are the last cells recruited, arriving in the late inflammatory period 72 hours after the onset of the foreign body reaction. [13].
The final stage of tissue healing at the site of implantation is reparation, i.e. the proliferation of connective tissue cells and the formation of a fibrous capsule, which isolates the implant; or the regeneration of damaged tissue in which parenchymal cells are replaced to as they were before the injury [15].
Virtually all synthetic implants induce such a response, to a different degree. The immunogenicity of synthetic implants is also used to enhance immune response in cancer therapy. Injection of nanoparticles coated with toll like receptor agonists results in enhanced antitumor response [16]. On the other hand, hydrogels, plastics, polystyrene and gold, that are commonly used to create synthetic biomaterials can also be manipulated to mitigate the host immune response. For example, these biomaterials can be coated with peptides possessing immunomodulatory properties in order to reduce the intrinsic immunogenicity of synthetic biomaterials [17].
Natural biomaterials composed of collagen, fibrinogen, hyaluronic acid, glycosaminoglycans, hydroxyapatite, chitosan, silk, or starch, mimic ECM components and may be less immunogenic. They can, however, also trigger an immune response, causing monocytes to release interleukins (IL)-1B and IL-6 [18]. Therefore, alternative scaffold materials are needed for the generation of fully functional bioengineered organs.
Scaffolds from Xenogenic and Allogeneic Organs
ECM-based scaffolds may be considered the ideal scaffolding material for the bioengineering and regeneration of transplantable organs because they retain most of their innate 3D architecture, molecular fingerprint and composition. Importantly, after decellularization, the framework of the native vasculature is preserved which is critical for in vivo implantation and revascularization [1,3,7]. On the contrary, synthetic scaffolds cannot reflect the complete array of native ECM components required for cell attachment, expansion, viability, and function. Moreover, the extraordinary complexity of the innate vasculature of complex organs has never been reproduced [1].
Xenogeneic scaffolds obtained from clinically relevant animal organs hold promise to become the biomaterial of choice for organ bioengineering. In fact, bred animals – likely the pig – may one day become the potentially inexhaustible source of organ scaffolds required to achieve organ-on-demand transplantation. Nevertheless, they also carry a risk of immunogenicity like de novo biological biomaterials. Additionally, the intrinsic risk for transmission of zoonosis remains debated. Pig tissues express several immunogenic proteins, notably galactosylated cell surface glycoproteins originating from alpha 1,3 galactosyltransferase (α-gal) activity, that could plausibly prevent effective use of porcine derived ECM scaffolds. In a recent study, Platz et al. demonstrated that decellularized ECM scaffolds obtained from wild-type pig lungs contained 25% more residual proteins when compared to their α-gal knock out counterpart [19]. Despite robust initial recellularization, subsequent growth and proliferation observed in all cell types with no obvious differences between cells seeded onto wild-type versus α-gal KO lungs, these findings certainly raise concerns for clinical translation. A recent study on face subunit bioengineering, however, showed that ECM scaffolds obtained from the porcine ear may be non-immunogenic [20]. In fact, when implanted in discordant pigs, such scaffolds did not trigger any specific anti-swine leukocyte antigen (SLA) humoral response, as demonstrated by a lack of donor-specific antibodies. Interestingly, no humoral response was detected despite the few scattered cells remaining in the cartilage. Moreover, these scaffolds did not induce any inflammatory process, as evidenced by the absence of signs of inflammation in the site of implantation and by a lack of remodeling within the scaffolds at time of euthanasia (one month after implantation).
Researchers are also proposing the use of scaffolds obtained from the decellularization of allogeneic organs [1,21–27]. In discussing the clinical translation of this technology, one could posit a scenario in which a patient in end stage renal failure needs a transplant. Here, one of the patient’s native kidneys can be removed laparoscopically and processed to obtain an acellular ECM-based scaffold that would be used as template to manufacture a custom-made kidney. Because the cellular compartment of the new organ would be regenerated from patient’s cells, the patient would receive an organ fabricated from his or her own biomaterials which would arguably render immunosuppression after implantation unnecessary. Nevertheless, as with porcine ECM scaffolds, initial protein content may not be completely cleared at the end of decellularization. In the very first report of a transplanted bioengineered trachea, a scaffold was obtained from the decellularization of a deceased donor trachea [28]. Importantly, the scaffold was used despite the small amount of focal MHC class II expression seen in a few areas of the ECM scaffold. The impact of these findings, however, is difficult to determine. In fact, the 5-year outcome of this milestone surgery was marked by narrowing and collapse of the airway requiring stenting in which the stent was replaced multiple times. Because the trachea was implanted without any vascular pedicle and therefore without anastomosis to the bloodstream of the recipient, ischemia may have played a critical role. Nevertheless, a chronic humoral response triggered by persistent donor antigens cannot be discounted.
Overall, in both xenogeneic and allogeneic materials, the tissue remnants present after decellularization may still provoke an innate immune response. For example, damage-associated molecular pattern proteins (DAMPs) may still be present after decellularization. These DAMPs are not only found in the native tissue, but are actively secreted during cell necrosis and by macrophages that respond to the acute tissue injury. DAMPs, including HMGB1, mediate a pro-inflammatory response by activation of TLRs on macrophages and other innate immune cells5, which could result in rejection of the graft.
IMMUNE RESPONSE AGAINST XENOGENEIC AND ALLOGENEIC SCAFFOLDS
A]. Innate Immune Response
Implantation of acellular xenogeneic and allogeneic scaffolds in both humans and mice leads to an acute cellular infiltrate, as would be expected with introduction of a foreign body [29]. The composition of cellular infiltrates varies depending on the source and processing of the scaffold. Both neutrophilic and giant cell infiltrations have been seen after implantation of porcine and human scaffolds into rats [30,31]. Mononuclear cells invade the peri-implant space as early as at 24 hours of implantation and this process can continue for several months leading to chronic inflammation and encapsulation or scar formation [32]. On the other hand, when circulating monocytes are absent, normal remodeling of the ECM scaffold does not occur, implying that mononuclear cells play an important role in the constructive remodeling process [32].
Neutrophils
Neutrophils are polymorphonuclear lymphocytes produced daily by the body in large quantities (1011 produced by the bone marrow each day) and reside mainly in the circulation [33]. Although classically considered to be ‘only’ effector cells, neutrophils interact with other cells, influencing, recruiting, and secreting signals for surrounding immune and humoral cells [34]. Neutrophils are highly capable phagocytes that release lytic enzymes and reactive oxigen species (ROS) to clear pathogens [34,35]. Neutrophils also exude a meshwork of chromatin fibers decorated with granule-derived antimicrobial peptides and enzymes., such as neutrophil elastase and myeloperoxidase (MPO), in a process known as NETosis [36].
Within 24–48 h after implantation of a synthetic biomaterial or a decellularized organ, neutrophils begin to infiltrate the material [12,37]. Neutrophils remain at the site of implantation, invading the scaffold as the host response progresses [4]. In addition, they begin the degradation process of the decellularized materials utilizing proteinase 3 (PR3) and matrix metalloproteinases (MMPs) [38]. They also clear the implant of any pathogens and release cytokines that modulate the subsequent responses to the implant. Although their persistence in the implanted tissue is transient, neutrophils are crucial in shaping the immune response.
Macrophages
Three to four days after implantation, the macrophage becomes the dominant immune cell in the host response. There is no unanimously accepted nomenclature for monocytes [39], but it is generally agreed that M1-like macrophage subtype has proinflammatory effects in contrast to the M2-like subtype which can lead to tissue repair [40]. M1s are known to be induced by IFN-γ alone or in combination with LPS, TNF and GM-CSF, while M2, macrophages are induced by a variety of signals including the cytokines IL-4, IL-13, and IL-10, immune complexes, and glucocorticoids [41].
In general, decellularized scaffolds have been noted to induce macrophages towards an anti-inflammatory state [42]. Although the mechanisms are not yet well understood, it is thought that a transition from M1 phenotype to the anti-inflammatory M2 phenotype is important for constructive tissue remodeling and prevention of scar tissue formation. Thus, modulation of macrophages may contribute to improved outcomes in regenerative medicine.
Multiple studies have shown that transition from an M1 to an M2 phenotype occurs one to two weeks after implantation of the biological scaffold [43–45]. In the case of suboptimal decellularization, presence of cellular debris within the scaffold leads to an extended M1 type immune response, poor remodeling outcomes, encapsulation and scarring. Degradation of the ECM scaffold appears necessary for the switch from an M1 to M2 phenotype, thus implying that the breakdown products may be important for this transition. Further support of the immunomodulatory capabilities of the ECM is found in studies which have demonstrated that hydrogels composed of ECM bioscaffolds facilitate a transition to an M2 type response compared to the M1 response elicited in the absence of ECM [46]. These protolerogenic effects of ECM also seem to extend to scaffolds obtained from diseased organs. Petrosyan et al. [47] studied the effects of monocytes on different ECM in mice including, acellular ECM scaffolds obtained from wild type kidneys as well as from kidneys of mice affected by Alport syndrome at different time-points of disease progression. Results showed that both healthy and diseased ECM scaffolds induce differentiation of macrophages into reparative M2 macrophages, whereas artificial biomaterials favored differentiation into the M1 phenotype. Therefore, even ECM scaffolds obtained from diseases organs offer a more protoleroegnic profile compared to synthetic materials.
B]. Adaptive Immune Response
Despite the major role played by neutrophils and macrophages, other cellular and humoral immune responses are likely to occur after implantation of acellular ECM scaffolds. Data exist that show there is an attenuated T-helper 1 (Th1) cell-mediated immune response against acellular ECM scaffolds compared to fresh tissue [45]. This effect could be driven both by the absence of the cells, which can trigger the immune response, and by the decellularization process which promotes anti-inflammatory and immunosuppressive effects both in vitro and in vivo. Evidence for the latter hypothesis is provided by animal studies that demonstrate decellularized xenogeneic scaffolds inhibiting in vitro T-cell proliferation and release of proinflammatory cytokines IL-2 and IFN-γ, while increasing production of the inhibitory cytokine IL-10. In vivo, transplant of allogeneic mouse cells seeded onto decellularized rabbit muscle scaffolds in rats prolonged survival compared to donor cells transplanted within poly(ε-caprolactone) scaffolds [48]. The mechanisms by which decellularized scaffolds modulate the host adaptive immune response remains to be determined. One potential explanation is that the process of decellularization unmasks certain surface peptides and molecules on the scaffold that modulate the immune response [49–51], but the nature of such mediators is still unclear.
T Cells
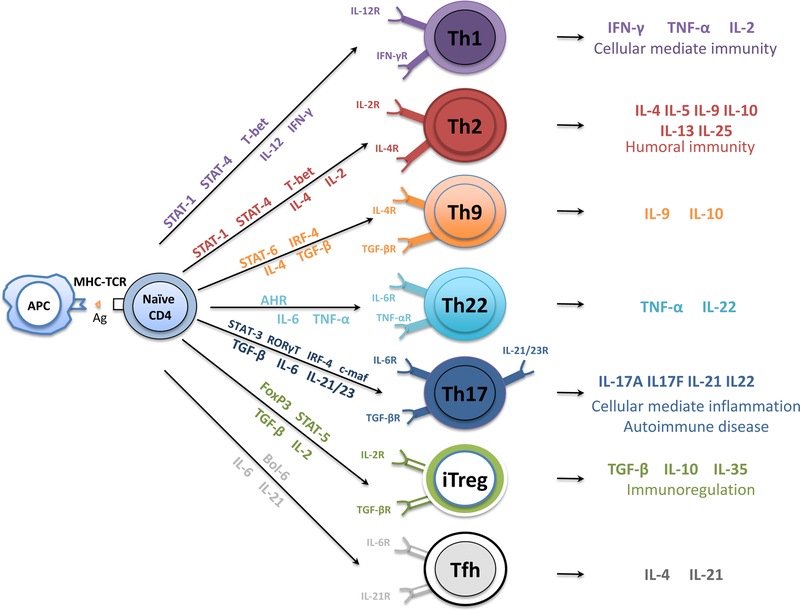
T cells are broadly defined by the cell surface markers CD4 and CD8. CD4+ T cells are activated by HLA class II molecules expressed on APCs. Functionally, CD4+ T cells are usually helper T cells because they “help” activate other T and B cells. The human immune system is fine-tuned in such a way that the antigen in question stimulates a particular cytokine environment, which in turn activates precise transcriptional networks that induce differentiation toward a specific T helper (Th) cell pathway, including Th1, Th2, Th9, Th17, Th22, and T follicular helper cells. T cells can also present regulatory features (regulatory T cells, or Tregs) and counteract the activity of conventional T cells [52] (Figure 1).
Figure 1.
Differentiation of naïve CD4+ T cells into CD4+ T cell subsets. On T cell receptor activation by antigen-presenting dendritic cells, naïve CD4+ T cells differentiate into T helper (Th)1, Th2, Th9, Th17, Th22, T follicular helper, and T regulatory cells controlled by their respective transcription factors under unique cytokine-polarized milieus. AHR = aryl hydrocarbon receptor; GATA-3 = transcription factor GATA-3; c-Maf= protooncogene c-Maf; IFN-γ = interferon gamma; IL = interleukin; IRF = interferon regulatory factor; iTreg = induced regulatory T cells; LT-α = lymphotoxin- α; STAT = signal transducer and activator of transcription; TGF-β = transforming growth factor–β; TNF-α = tumor necrosis factor–α.
In general terms, T helper 1 (Th1) polarization is associated with a pro-inflammatory response, while Th2 polarization is generally associated with wound healing and constructive remodeling response, analogous to M2 macrophage polarization [53]. The implantation of porcine small intestinal submucosa (SIS) into mice led to graft acceptance and increased production of IL-4 and an absence of IFN-γ leading to an anti-inflammatory, predominantly Th2 associated response [54]. In humans, implantation of porcine SIS was also associated with a Th2 polarized cytokine and antibody isotype profile [55]. Antibodies produced against “non-self” ECM also appear to be limited to a Th2 profile, and repeated exposure to xenogeneic ECM in mice led to an accentuated Th2 response.
In vitro studies showed that human pancreas ECM scaffolds decrease human CD4+ T-cell expansion, induce T-cell apoptosis and promote conversion of CD4+ T-cells into Tregs [56] (Figure 2). Further demonstrating the protolerogenic effects of ECM, decellularized porcine and rat livers did not elicit an inflammatory response at 7 days after implantation into a rat. Serum white blood cell counts did not increase after implantation, and no sign of T-cell activation was reported. Although cells positive for the pan monocyte marker, CD68, did infiltrate the implant, these cells did not express surface markers specific for either M1 or M2 macrophages and their role was unclear [57].
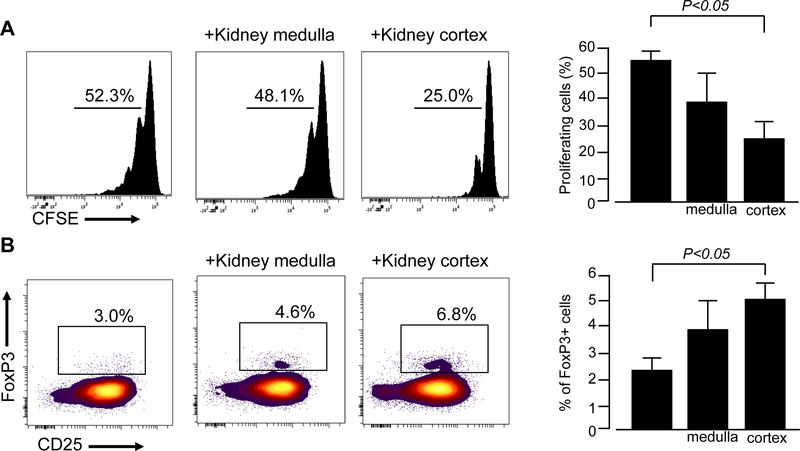
Figure 2.
Human renal ECM inhibits naïve CD4+ T cell expansion and promotes their conversion into regulatory T cells. (A) CFSE-labeled naïve CD4+ T cells were activated with anti-CD3/anti-CD28 mAb without or with ECM from kidney medulla or kidney cortex. Cell division was quantified on day 5 with CFSE dilution by flow cytometry. Representative histograms (left) and data quantification (right) of 6 independent experiments from 6 different donors. (B) Naïve CD4+ T cells activation with anti-CD3/anti-CD28 mAb +IL2 without or with ECM from kidney medulla or kidney cortex. On day 5, the percentage of CD4+CD25+FoxP3+ regulatory T cells was quantified by flow cytometry. Representative histograms (left) and data quantification (right) of 4 independent experiments from 4 different donors.
The mechanism through which acellular ECM scaffolds modulate the host immune response remains to be determined. Low or absent levels of MHC classes I and II molecules would explain the decreased T-cell proliferative response seen in vitro, the non-inflammatory effects seen in vivo, and the reductions seen in IL-2 and IFN-γ through mitigation of direct T-cell antigenic presentation mechanisms. This explanation, however, fails to account for the increases seen in IL-10, as well as polarization of the macrophage response toward an M2 phenotype and the protection afforded by such scaffolds in preventing the rejection of xenogeneic donor cells. Clearly, increased levels of IL-10 raises the possibility of a response mediated by regulatory T cells which may play an integral role in the effects seen, as demonstrated in this study [58,59]. As reported above, the ECM functions as reservoir of cytokines and growth factors that persist, can be detected, and quantified after decellularization. In fact, a recent study showed that high levels of TGF-β which can be found within ECM scaffolds obtained from the human pancreas, may be implicated in the induction of T-cell apoptosis and promote conversion of naïve CD4+ T cells into CD4+CD25+Foxp3+ Tregs [60].
Altogether, available data concur that acellular ECM scaffolds polarize host responses away from a classical Th1-proinflammatory profile and appear to downregulate T-cell xenogeneic responses and Th1 effector function by inducing a state of peripheral T-cell hyporesponsiveness (Figure 3). These results have substantial implications for future clinical application of tissue-engineered therapies.
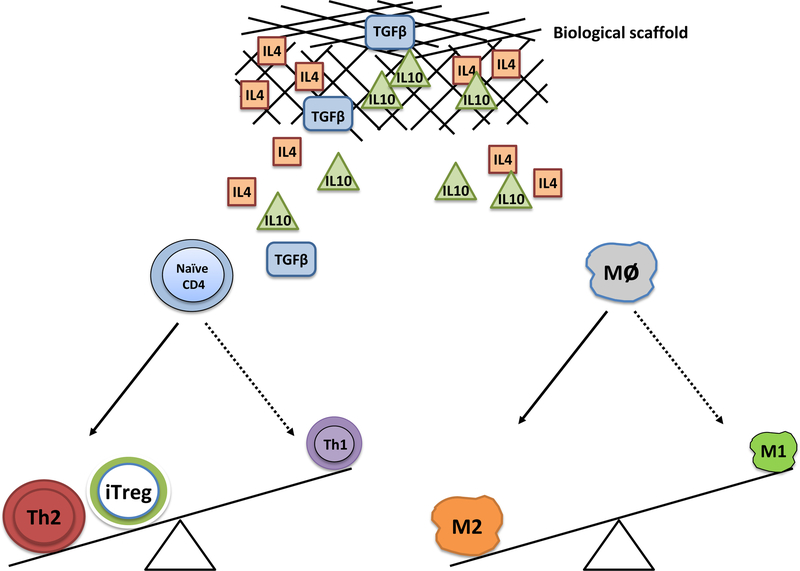
Figure 3.
Cytokines embedded in the biological scaffolds are thought to drive conversion of T cells and monocytes towards anti-inflammatory phenotypes (e.g. Treg, Th2, and M2 monocytes).
C]. Humoral Immunity Against Xenogeneic Scaffolds
Antibodies directed against the well-studied Gal epitope, namely the glycoside hydrolase enzyme that hydrolyses the terminal alpha-galactosyl moieties from glycolipids and glycoproteins expressed by porcine cells, are key players of hyperacute antibody-mediated rejection, while anti-non-Gal epitope antibodies may play a role in chronic rejection of xenogeneic implants from Gal knock-out animals [61].
Anti-Gal Antibodies
In experimental xenotransplantation, hyperacute rejection (HAR) of the graft occurs within minutes. In a model of transplantation from pig to non-human primate, HAR is thought to be mediated by the preformed xenogeneic antibody against a galactose residue expressed on pig vascular endothelium. The α-Gal epitope is a sugar moiety present on all non-primate mammals, New World monkeys, and the human microbiome. All humans produce anti-Gal antibodies.
The α-gal epitope is thought to play a major role in facilitating HAR of xenotransplants, but despite its presence in ECM, its role in the host response to biological scaffolds is controversial. When organs from α-Gal-deficient pigs are transplanted into anti-Gal antibody producing baboons, HAR is prevented and there is no binding of preformed anti-Gal antibody [62].
The α-gal epitope (1–3Galα1–4GlcNAc-R) is abundantly synthesized on glycolipids and glycoproteins of non-primate mammals and New World monkeys by the glycosylation enzyme α−1,3 galactosyltransferase (α−1,3 GT) [63]. In humans, primates, and Old World monkeys, this epitope is absent as the α−1,3 GT gene was inactivated in ancestral Old World primates [64]. Xenografts containing α-Gal epitopes activate B cells and cause a dramatic increase in the circulating levels of anti-Gal IgG [65]. When the epitope is present in the organism, even acellular tissues such as proteoglycans and glycoproteins contain the α-Gal epitope [66]. Although this implies exposure of the xenogeneic α-gal epitope to the human host, the clinical significance of anti-Gal antibodies remains unclear.
One study in old world monkeys found that while implanted porcine SIS containing α-Gal induced a humoral response, the implant had no histologic differences at any time point compared to SIS that was harvested for implantation from a knockout pig without the α-Gal epitope [67]. Another study found that when decellularized skin from pigs lacking the α-Gal epitope was implanted into old world primates, anti-Gal antibodies were absent and significantly fewer T-cells were present in the implant at 3 and 6 months post-implantation [68]. No changes, however, were observed in the remodeling process of the implant. As reported above, similar findings were observed by Platz et al. [19].
Anti-Non-Gal Antibodies
Anti-non-Gal antibodies are antibodies formed to any epitope other than α-Gal. In humans, they may form against ECM peptide sequences that are different from those in homologous human proteins. If binding of these antibodies to a xenograft occurs, activation of the complement cascade can ultimately lead to graft rejection [6]. Antibodies to collagen have been described, as well as antibodies to cartilage, after removal of the α-Gal epitope [45]. Similar humoral responses have been observed with porcine SIS.
Additionally, it has been shown that various decellularization methods may result in materials that contain residual MHC molecules [69]. Although it is unknown whether antibodies to residual MHC molecules have significant effects on the outcomes of the host response to these scaffolds, it is possible that an excessive humoral response to various proteins in the matrix may inhibit the ability of stem cells to bind and penetrate scaffolds due to a lack of available adhesion sites [45]. Like anti-Gal antibodies, anti-non-Gal antibodies can be harmful to ECM implant regeneration, and can hinder stem cell interaction with the ECM which is required to direct stem cell differentiation [70].
CONCLUSIONS
Organ bioengineering is still in its infancy, but the question has now changed from asking whether it is possible to asking how to fully develop its potential and allow for its clinical translation. One of the barriers to the use of xenogeneic, allogeneic, synthetic, and de novo biological tissues is the propensity of the graft to provoke a host immune response. The immunogenicity of grafts has implications both for the scale of production that could be achieved and the outcomes of grafts after implantation. One solution to solving the problem of immunogenicity is to cloak grafts in immune-neutral substances, such as ECM or peptides. Additionally, studies are finding techniques to suppress or harness the immune system to promote graft tolerance. Data also indicate that, while ECM elicits an early innate immune response like that of the foreign body reaction. Over time, however, this response tends to shift to a tolerogenic profile, with generation of M2 macrophages and T regulatory cells. Leveraging these protolerogenic effects of ECM could be crucial to the promotion of tolerance to standard and bioengineered grafts.
Funding sources:
PC is supported by NIH grant 5T32AI078892–07.
Abbreviations
- ECM
Extracellular matrix
- PAMPs
Pathogen associated molecular proteins
- MHC
Major histocompatibility complex
- IL
Interleukin
- TGF-β1
Tissue growth factor-β1
- DAMPs
Damage associated molecular proteins
- IFN-γ
interferon- γ
- Th1
T-helper cell 1
- Th2
T-helper cell 2
- SIS
Small intestinal submucosa
- HAR
hyperacute rejection
- α−1,3 GT
α−1,3 galactosyltransferase
REFERENCES
- [1].Orlando G, Soker S, Stratta RJ: Organ bioengineering and regeneration as the new Holy Grail for organ transplantation Ann Surg 2013, 258:221–232. [DOI] [PubMed] [Google Scholar]
- [2].Hynes RO: The extracellular matrix: not just pretty fibrils Science 2009, 326:1216–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Meng FW, Slivka PF, Dearth CL, Badylak SF: Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages Biomaterials 2015, 46:131–140. [DOI] [PubMed] [Google Scholar]
- [4].Morris AH, Stamer DK, Kyriakides TR: The host response to naturally-derived extracellular matrix biomaterials Semin Immunol 2017,. [DOI] [PubMed] [Google Scholar]
- [5].Leifer CA: Dendritic cells in host response to biologic scaffolds Semin Immunol 2017,. [DOI] [PubMed] [Google Scholar]
- [6].Galili U: Avoiding detrimental human immune response against Mammalian extracellular matrix implants Tissue Eng Part B Rev 2015, 21:231–241. [DOI] [PubMed] [Google Scholar]
- [7].Bharat A, Mohanakumar T: Immune Responses to Tissue-Restricted Nonmajor Histocompatibility Complex Antigens in Allograft Rejection J Immunol Res 2017, 2017:6312514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, et al. : Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends J R Soc Interface 2007, 4:999–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ: Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy Nat Biotechnol 2015, 33:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Singh A, Peppas NA: Hydrogels and scaffolds for immunomodulation Adv Mater 2014, 26:6530–6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P: Nanoparticle-based immunotherapy for cancer ACS Nano 2015, 9:16–30. [DOI] [PubMed] [Google Scholar]
- [12].Anderson JM, Rodriguez A, Chang DT: Foreign body reaction to biomaterials Semin Immunol 2008, 20:86-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Velnar T, Bunc G, Klobucar R, Gradisnik L: Biomaterials and host versus graft response: a short review Bosn J Basic Med Sci 2016, 16:82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Diegelmann RF, Evans MC: Wound healing: an overview of acute, fibrotic and delayed healing Front Biosci 2004, 9:283–289. [DOI] [PubMed] [Google Scholar]
- [15].Hunt TK, Hopf H, Hussain Z: Physiology of wound healing Adv Skin Wound Care 2000, 13:6–11. [PubMed] [Google Scholar]
- [16].Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Preat V, Florindo HF: In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model J Control Release 2015, 198:91–103. [DOI] [PubMed] [Google Scholar]
- [17].Hubbell JA, Thomas SN, Swartz MA: Materials engineering for immunomodulation Nature 2009, 462:449–460. [DOI] [PubMed] [Google Scholar]
- [18].Rayahin JE, Gemeinhart RA: Activation of Macrophages in Response to Biomaterials Results Probl Cell Differ 2017, 62:317–351. [DOI] [PubMed] [Google Scholar]
- [19].Platz J, Bonenfant NR, Uhl FE, Coffey AL, McKnight T, Parsons C, Sokocevic D, Borg ZD, Lam YW, Deng B, et al. : Comparative Decellularization and Recellularization of Wild-Type and Alpha 1,3 Galactosyltransferase Knockout Pig Lungs: A Model for Ex Vivo Xenogeneic Lung Bioengineering and Transplantation Tissue Eng Part C Methods 2016, 22:725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Duisit J, Orlando G, Debluts D, Maistriaux L, Xhema D, de Bisthoven YJ, Galli C, Peloso A, Behets C, Lengele B, Gianello P: Decellularization of the Porcine Ear Generates a Biocompatible, Nonimmunogenic Extracellular Matrix Platform for Face Subunit Bioengineering Ann Surg 2017,. [DOI] [PubMed] [Google Scholar]
- [21].Gifford S, Zambon JP, Orlando G: Recycling organs - growing tailor-made replacement kidneys Regen Med 2015, 10:913–915. [DOI] [PubMed] [Google Scholar]
- [22].Peloso A, Petrosyan A, Da Sacco S, Booth C, Zambon JP, O’Brien T, Aardema C, Robertson J, De Filippo RE, Soker S, et al. : Renal Extracellular Matrix Scaffolds From Discarded Kidneys Maintain Glomerular Morphometry and Vascular Resilience and Retains Critical Growth Factors Transplantation 2015, 99:1807–1816. [DOI] [PubMed] [Google Scholar]
- [23].Peloso A, Dhal A, Zambon JP, Li P, Orlando G, Atala A, Soker S: Current achievements and future perspectives in whole-organ bioengineering Stem Cell Res Ther 2015, 6:107–015-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ren X, Moser PT, Gilpin SE, Okamoto T, Wu T, Tapias LF, Mercier FE, Xiong L, Ghawi R, Scadden DT, et al. : Engineering pulmonary vasculature in decellularized rat and human lungs Nat Biotechnol 2015, 33:1097–1102. [DOI] [PubMed] [Google Scholar]
- [25].Guyette JP, Charest JM, Mills RW, Jank BJ, Moser PT, Gilpin SE, Gershlak JR, Okamoto T, Gonzalez G, Milan DJ, et al. : Bioengineering Human Myocardium on Native Extracellular Matrix Circ Res 2016, 118:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, et al. : Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation Sci Rep 2015, 5:13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhou Q, Li L, Li J: Stem cells with decellularized liver scaffolds in liver regeneration and their potential clinical applications Liver Int 2015, 35:687–694. [DOI] [PubMed] [Google Scholar]
- [28].Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. : Clinical transplantation of a tissue-engineered airway Lancet 2008, 372:2023–2030. [DOI] [PubMed] [Google Scholar]
- [29].Wilshaw SP, Kearney J, Fisher J, Ingham E: Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells Tissue Eng Part A 2008, 14:463–472. [DOI] [PubMed] [Google Scholar]
- [30].Muhamed J, Revi D, Rajan A, Geetha S, Anilkumar TV: Biocompatibility and Immunophenotypic Characterization of a Porcine Cholecyst-derived Scaffold Implanted in Rats Toxicol Pathol 2015, 43:536–545. [DOI] [PubMed] [Google Scholar]
- [31].Orlando G, Farney AC, Iskandar SS, Mirmalek-Sani SH, Sullivan DC, Moran E, AbouShwareb T, De Coppi P, Wood KJ, Stratta RJ, et al. : Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations Ann Surg 2012, 256:363–370. [DOI] [PubMed] [Google Scholar]
- [32].Tondreau MY, Laterreur V, Vallieres K, Gauvin R, Bourget JM, Tremblay C, Lacroix D, Germain L, Ruel J, Auger FA: In Vivo Remodeling of Fibroblast-Derived Vascular Scaffolds Implanted for 6 Months in Rats Biomed Res Int 2016, 2016:3762484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER: Neutrophil kinetics in health and disease Trends Immunol 2010, 31:318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Smith JA: Neutrophils, host defense, and inflammation: a double-edged sword J Leukoc Biol 1994, 56:672–686. [DOI] [PubMed] [Google Scholar]
- [35].Chistiakov DA, Bobryshev YV, Orekhov AN: Neutrophil’s weapons in atherosclerosis Exp Mol Pathol 2015, 99:663–671. [DOI] [PubMed] [Google Scholar]
- [36].Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A: Neutrophil extracellular traps kill bacteria Science 2004, 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- [37].Hernigou P, Mathieu G, Poignard A, Manicom O, Beaujean F, Rouard H: Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique J Bone Joint Surg Am 2006, 88 Suppl 1 Pt 2:322–327. [DOI] [PubMed] [Google Scholar]
- [38].Soehnlein O: Multiple roles for neutrophils in atherosclerosis Circ Res 2012, 110:875–888. [DOI] [PubMed] [Google Scholar]
- [39].Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. : Macrophage activation and polarization: nomenclature and experimental guidelines Immunity 2014, 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization Trends Immunol 2004, 25:677–686. [DOI] [PubMed] [Google Scholar]
- [41].Gordon S, Taylor PR: Monocyte and macrophage heterogeneity Nat Rev Immunol 2005, 5:953–964. [DOI] [PubMed] [Google Scholar]
- [42].Pinto ML, Rios E, Silva AC, Neves SC, Caires HR, Pinto AT, Duraes C, Carvalho FA, Cardoso AP, Santos NC, et al. : Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18 Biomaterials 2017, 124:211–224. [DOI] [PubMed] [Google Scholar]
- [43].Mimura KK, Moraes AR, Miranda AC, Greco R, Ansari T, Sibbons P, Greco KV, Oliani SM: Mechanisms underlying heterologous skin scaffold-mediated tissue remodeling Sci Rep 2016, 6:35074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF: Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine Biomaterials 2012, 33:3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Badylak SF, Gilbert TW: Immune response to biologic scaffold materials Semin Immunol 2008, 20:109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF: Macrophage polarization in response to ECM coated polypropylene mesh Biomaterials 2014, 35:6838–6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Petrosyan A, Da Sacco S, Tripuraneni N, Kreuser U, Lavarreda-Pearce M, Tamburrini R, De Filippo RE, Orlando G, Cravedi P, Perin L: A step towards clinical application of acellular matrix: A clue from macrophage polarization Matrix Biol 2017, 57–58:334–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fishman JM, Lowdell MW, Urbani L, Ansari T, Burns AJ, Turmaine M, North J, Sibbons P, Seifalian AM, Wood KJ, et al. : Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model Proc Natl Acad Sci U S A 2013, 110:14360–14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE, Standifer NE, Braun KR, et al. : ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors Proc Natl Acad Sci U S A 2011, 108:7938–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT: Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells J Leukoc Biol 2009, 86:567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thomas AH, Edelman ER, Stultz CM: Collagen fragments modulate innate immunity Exp Biol Med (Maywood) 2007, 232:406–411. [PubMed] [Google Scholar]
- [52].Hall BM: T Cells: Soldiers and Spies--The Surveillance and Control of Effector T Cells by Regulatory T Cells Clin J Am Soc Nephrol 2015, 10:2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gordon S: Alternative activation of macrophages Nat Rev Immunol 2003, 3:23–35. [DOI] [PubMed] [Google Scholar]
- [54].Allman AJ, McPherson TB, Badylak SF, Merrill LC, Kallakury B, Sheehan C, Raeder RH, Metzger DW: Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response Transplantation 2001, 71:1631–1640. [DOI] [PubMed] [Google Scholar]
- [55].Aachoui Y, Ghosh SK: Extracellular matrix from porcine small intestinal submucosa (SIS) as immune adjuvants PLoS One 2011, 6:e27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Peloso A, Urbani L, Cravedi P, Katari R, Maghsoudlou P, Fallas ME, Sordi V, Citro A, Purroy C, Niu G, et al. : The Human Pancreas as a Source of Protolerogenic Extracellular Matrix Scaffold for a New-generation Bioartificial Endocrine Pancreas Ann Surg 2016, 264:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mirmalek-Sani SH, Sullivan DC, Zimmerman C, Shupe TD, Petersen BE: Immunogenicity of decellularized porcine liver for bioengineered hepatic tissue Am J Pathol 2013, 183:558–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sun L, Yi S, O’Connell PJ: IL-10 is required for human CD4(+)CD25(+) regulatory T cell-mediated suppression of xenogeneic proliferation Immunol Cell Biol 2010, 88:477–485. [DOI] [PubMed] [Google Scholar]
- [59].Wu J, Yi S, Ouyang L, Jimenez E, Simond D, Wang W, Wang Y, Hawthorne WJ, O’Connell PJ: In vitro expanded human CD4+CD25+ regulatory T cells are potent suppressors of T-cell-mediated xenogeneic responses Transplantation 2008, 85:1841–1848. [DOI] [PubMed] [Google Scholar]
- [60].Oh SA, Li MO: TGF-beta: guardian of T cell function J Immunol 2013, 191:3973–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tazelaar HD, Byrne GW, McGregor CG: Comparison of Gal and non-Gal-mediated cardiac xenograft rejection Transplantation 2011, 91:968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, Wise Y, Liu Y, Xiang Y, Copeman L, et al. : Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys Nat Med 2005, 11:1295–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Galili U, Macher BA, Buehler J, Shohet SB: Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1--−−3)-linked galactose residues J Exp Med 1985, 162:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA: Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells J Biol Chem 1988, 263:17755–17762. [PubMed] [Google Scholar]
- [65].Galili U: Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans Immunol Today 1993, 14:480–482. [DOI] [PubMed] [Google Scholar]
- [66].Maruyama S, Cantu E 3rd, Galili U, D’Agati V, Godman G, Stern DM, Andres G: Alpha-Galactosyl Epitopes on Glycoproteins of Porcine Renal Extracellular Matrix Kidney Int 2000, 57:655–663. [DOI] [PubMed] [Google Scholar]
- [67].Park S, Kim WH, Choi SY, Kim YJ: Removal of alpha-Gal epitopes from porcine aortic valve and pericardium using recombinant human alpha galactosidase A J Korean Med Sci 2009, 24:1126–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xu H, Wan H, Zuo W, Sun W, Owens RT, Harper JR, Ayares DL, McQuillan DJ: A porcine-derived acellular dermal scaffold that supports soft tissue regeneration: removal of terminal galactose-alpha-(1,3)-galactose and retention of matrix structure Tissue Eng Part A 2009, 15:1807–1819. [DOI] [PubMed] [Google Scholar]
- [69].Wong ML, Griffiths LG: Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization Acta Biomater 2014, 10:1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Galili U: Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits Immunology 2013, 140:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Duisit J, Orlando G, Debluts D, Maistriaux L, Xhema D, de Bisthoven YJ, Galli C, Peloso A, Behets C, Lengelé B, Gianello P. Decellularization of the Porcine Ear Generates a Biocompatible, Nonimmunogenic Extracellular Matrix Platform for Face Subunit Bioengineering. Ann Surg 2017; March 1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]