Abstract
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability, typically due to CGG-repeat expansions in the FMR1 gene leading to lack of expression. We identified a rare FMR1 gene mutation (c.413G>A), previously reported in a single patient and reviewed the literature for other rare FMR1 mutations. Our patient at 10 years of age presented with the classical findings of FXS including intellectual disability, autism, craniofacial findings, hyperextensibility, fleshy hands, flat feet, unsteady gait, and seizures but without the typical CGG-repeat expansion. He had more features of FXS than the previously reported patient with the same mutation. Twenty individuals reported previously with rare missense or nonsense mutations or other coding disturbances of the FMR1 gene ranged in age from infancy to 50 years; most were verbal with limited speech, had autism and hyperactivity, and all had intellectual disability. Four of the 20 individuals had a mutation within exon 15, three within exon 5, and two within exon 2. The FMR1 missense mutation (c.413G>A) is the same as in a previously reported male where it was shown that there was preservation of the post-synaptic function of the fragile X mental retardation protein (FMRP), the encoded protein of the FMR1 gene was preserved. Both patients with this missense mutation had physical, cognitive, and behavioral features similarly seen in FXS.
Keywords: FMR1 gene, FMRP, fragile X syndrome, genetics, rare mutations, review
1 |. INTRODUCTION
Fragile X syndrome (FXS) is the most commonly inherited cause of intellectual disability and as an X-linked genetic disorder occurs more frequently in males. Social impairment, autism spectrum disorder, speech and language delay, neurological dysfunction (seizures and abnormal sleep patterns) and a characteristic physical appearance are also observed. FXS occurs in 1 in 5,000 males (Coffee et al., 2009; Hagerman & Hagerman, 2002) caused by expansions of a CGG triplet repeat in the 5′ untranslated region of the familial mental retardation 1 (FMR1) gene located on the X chromosome. The expansion of trinucleotide CGG repeats leads to transcriptional silencing of FMR1 with consequent absence of the encoded fragile X mental retardation protein (FMRP), a synaptic RNA-binding protein that functions to modulate action potential duration and synaptic plasticity (Myrick, Hashimoto, Cheng, & Warren, 2015). This repeat expansion is seen in greater than 99% of affected individuals. Rare mutations have been reported in the promoter or coding regions of the gene in individuals with features of FXS (Collins et al., 2010; De et al., 1993; Grozeva et al., 2015; Handt et al., 2014; Hu et al., 2016; Lugenbeel, Peier, Carson, Chudley, & Nelson, 1995; Myrick et al., 2014; Myrick, Hashimoto, et al., 2015; Okray et al., 2015; Quartier et al., 2017; Siomi, Choi, Siomi, Nussbaum, & Dreyfuss, 1994). Typically, greater than 200 hypermethylated CGG trinucleotide repeats are observed in patients with FXS while unaffected individuals have 6–44 CGG repeats. Individuals with a premutation harbor an allele with 55–200 CGG trinucleotide repeats in FMR1, and are at risk for further expansion in subsequent generations, as well as for primary ovarian failure and tremor ataxia syndrome (Garber, Visootsak, & Warren, 2008; Hagerman & Hagerman, 2013; Myrick et al., 2014).
Patients with FXS and the CGG expansion in FMR1 typically present with large, protruding ears, elongated face, highly arched palate, hyperextensible finger joints, flat feet, soft skin, postpubescent macroorchidism, and hypotonia (Regezi, Sciubba, & Jordan, 2008; Santoro, Bray, & Warren, 2012). They also have an increased risk of developing seizures and mitral valve prolapse with features of a connective tissue disorder (Berry-Kravis et al., 2010; Hagerman et al., 2009; McLennan, Polussa, Tassone, & Hagerman, 2011). The FMR1 gene is highly expressed in brain and testicular tissue consistent with abnormalities observed in FXS including intellectual disability, behavioral problems, seizures, characteristic facial features, autism spectrum disorder, and macroorchidism.
Although FXS is usually caused by an expansion of the CGG repeat of FMR1, rare mutations leading to FXS have been reported to date. These mutations include deletions, splicing errors, missense, and nonsense mutations and are found in the Human Gene Mutation Database for FXS (http://www.hgmd.cf.ac.uk/ac/gene.php?gene=FMR1). In particular, one mutation observed in one patient included a change of arginine to glutamine at protein position 138 (Arg138Gln) resulting from a single DNA base pair change of G to A at gene position 413 and led to partial loss of function of FMRP (Myrick et al., 2014). This reported patient had intellectual disability and intractable seizures associated with FXS. Animal models demonstrated that this mutation preserves the translational regulation abilities of FMRP and also post-synaptic regulation of the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor trafficking, polyribosome association, and mRNA binding (Myrick, Deng, et al., 2015). However, pre-synaptic functions, such as rescuing synaptic overgrowth and action potential in the hippocampus and pyramidal neurons combined with regulation of the BK channels are disrupted by this missense mutation suggesting a differential and distinct functional FMRP role in pre-synaptic versus post-synaptic regions (Myrick, Deng, et al., 2015. Additional information on FMRP function could be gained by reporting subjects with rare mutations of FMR1 other than the typical triplet CGG repeat expansion as the causation of FXS. In a literature review, 20 affected individuals were found with mutations of the coding sequence of FMR1 consisting of 17 exons spanning 38 kb (Collins et al., 2010; De et al., 1993; Grønskov, Hallberg, Brøndum-Nielsen, Dedic, & Hjalgrim, 2011; Grozeva et al., 2015; Handt et al., 2014; Hu et al., 2016; Lugenbeel et al., 1995; Myrick et al., 2014; Myrick, Hashimoto, et al., 2015; Quartier et al., 2017; Suhl & Warren, 2015; Wang, Lin, Lin, Li, & Li, 1997; Wright et al., 2015).
Herein, we report on the clinical findings of a second male with the same missense mutation at position 413 (c.413G>A) of FMR1 leading to a change in protein position 138 from arginine to glutamine (p.Arg138Gln). We also summarize the literature regarding other rare mutations of FMR1 in affected individuals with the FXS phenotype.
2 |. MATERIALS AND METHODS
2.1 |. Clinical report
Our patient with an FMR1 allele in the normal size range, presented with FXS due to a missense mutation in exon 5 of FMR1. He was the product of a full-term uncomplicated pregnancy with the mother and father being first cousins of Portuguese decent. His birth weight was 7 lb, 7 oz (3.3 kg). No physical abnormalities were noted at birth following a full term vaginal delivery. There were no neonatal or feeding problems. He was unable to sit independently until 9 months of age and began walking at 16 months but did not develop recognizable speech and relied on six signs for communication. At 27 months of age due to developmental delays and behavioral concerns, cognitive, and behavioral tests were performed and an autism spectrum disorder (ASD) diagnosis made. At 3 years of age, corrective surgery was performed for strabismus and bilateralesotropia. His tonsils and adenoids were removed at age 4 years. He had an abscess of a tooth which was surgically removed at 8 years of age followed by a second procedure to remove an abscess on his back at 9 years of age. An evaluation at 4 years 8 months of age revealed a nonverbal status and cognitive functioning at a 14–16 months level. His verbal cognitive score was determined to be less than 12 months of age. The Autism Diagnostic Observation Schedule, Childhood Autism Rating Scale, and Social Responsiveness Scale were administered and all indicated a diagnosis of ASD.
He was also diagnosed with attention deficit hyperactivity disorder (ADHD) and oral motor dyspraxia. Complex partial seizures were noted at 8 years of age when Vyvanse was prescribed due to ADHD with postictal response. An EEG indicated a posterior dominant rhythm that was slow for his age and represented an unorganized pattern with demonstrated electrographic seizures lasting 10–20 s indicating possible encephalopathy. He was treated with Trileptal but the seizures returned after a few months. He was toilet trained at about 8 years of age but within two months of developing seizures he had lost this skill.
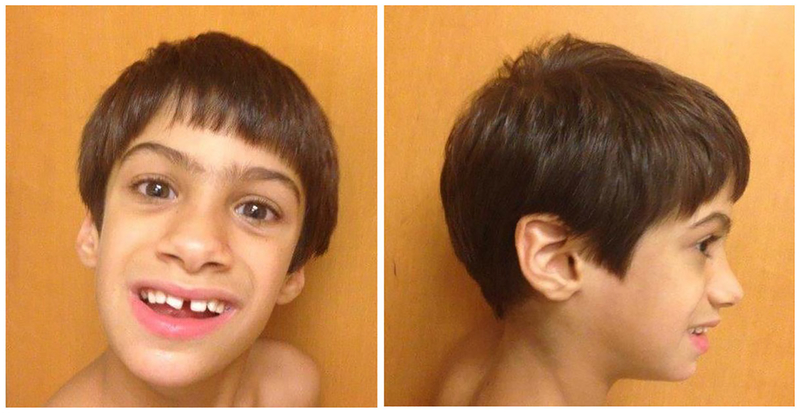
At 10 years of age he was slender with a height of 136 cm (35th centile), weight 25.7 kg (10th centile), head circumference 51.8 cm (25th centile), inner canthal distance 3 cm (50th centile), outer canthal distance 8.1 cm (30th centile), interpupillary distance 5.2 cm (30th centile), palpebral fissure length 2.6 cm (30th centile), ear length 6 cm (60th centile), and ear width 3.8 cm (20th centile). He was rated at a Tanner stage I level of pubertal development. He had multiple features associated with FXS including a large wide mouth with drooling, a large jaw, elongated face, broad ears, soft fleshy hands, hyperextensible digits (metacarpo-phalangeal extension greater than 90° for most digits with double jointed thumbs), and a single palmar crease. His genito-urinary examination showed a normal sized penis without hypospadias, normal testicular volume (3–4 ml), and a mild shawl scrotum. He exhibited hyperactivity and an unsteady gait with toe walking and was unable to tandem walk. His feet were flat with a mild degree of pronation. His resting motor tone was decreased except when excited and exhibited hand flapping. He did not show tremors. Additionally, his large ears showed unusual folding of the pinna bilaterally. He did not have epicanthal folds, ptosis, scoliosis, pectus excavatum, dental abnormalities, hernias, heart murmurs, plantar creases, or self-injury sites (see Figure 1). He also exhibited the groaning or moaning characteristic of non-verbal children with FXS. However, overall he was more affected cognitively and behaviorally than the typical child with FXS as he was non-verbal with severe behavioral problems.
FIGURE 1.
Frontal and profile facial views of the male patient at 10 years of age with an FMR1 gene exon 5 mutation showing an elongated face, a large nose, wide mouth with large jaw and prominent broad ears. [Color figure can be viewed at wileyonlinelibrary.com]
On examination at 10 years of age, he was assessed using the Vineland Adaptive Behavior Scales and his communication standard score was 42, daily living skills 48, socialization 50, and motor score of 50. Overall, his Aberrant Adaptive Behavior score was 46. His IQ was tested using Stanford Binet and revealed a non-verbal IQ of 42, verbal 43, full scale 40, fluid reasoning score of 47, knowledge-based of 49, quantitative reasoning of 58, and visuospatial and working memory scores were 48. On the Leiter International Performance scale his full scale IQ was 30 and brief IQ was 36. During this assessment his ADOS-2 module 1 social affect score was 18, repetitive and restrictive behavior was 6 and his total score was 24 in the severe ASD range. He was treated with risperidone, which can be helpful for those with both ASD and FXS and he responded well with a decrease in hyperactivity. Guanfacine has also been helpful to calm his behavioral problems and he also continues to take Trileptal for seizures.
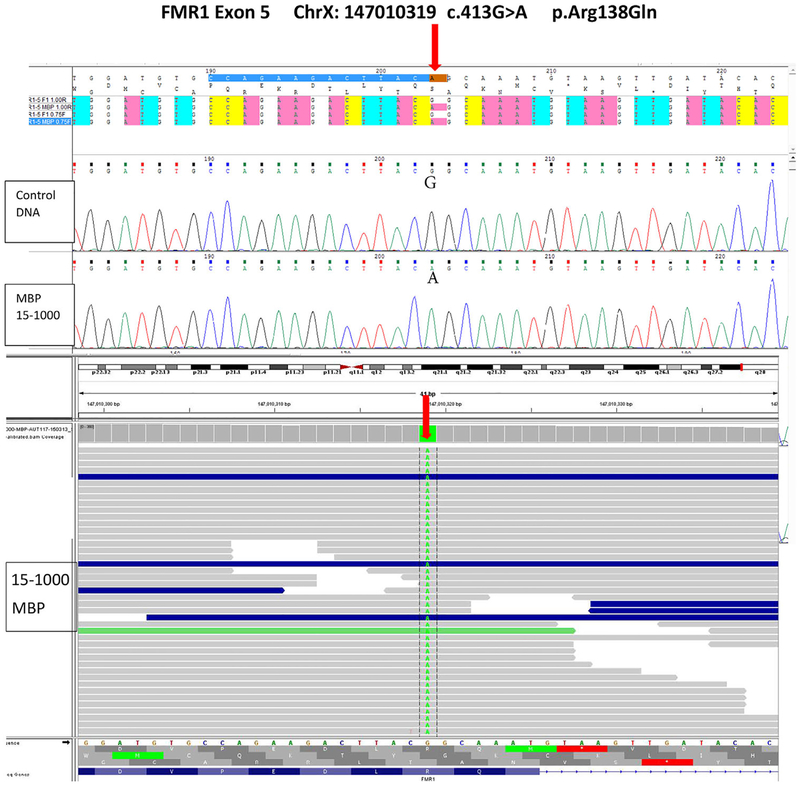
As a young child, the patient underwent FXS testing for the typical CGG-repeat length expansion of FMR1 and was reported to have a normal allele of24 CGG repeats. He had a normal male 46, XY karyotype, as well as a normal brain MRI scan. A 180k chromosomal microarray was performed previously and interpreted as normal. DNA methylation analysis was negative for Angelman syndrome. At 10 years of age, because of previous negative genetic testing along with a history of consanguinity, a panel of genes (N = 117) associated with intellectual disability and ASD was ordered for next generation sequencing at the Munroe–Meyer Institute, University of Nebraska Medical Center Human Genetics Laboratory in Omaha. The list of genes can be obtained at the genetics laboratory website (https://www.unmc.edu/mmi/geneticslab/catalog/postnatal-testing/post-p-autism.html). The results showed the presence of a hemizygous guanine to adenine transition (c.413G>A) causing a missense mutation coding for a change at amino acid arginine to glutamine at position 138 (Arg138Gln) of the FMR1 gene (see Figure 2). DNA testing of the mother and older sister showed the same c.148 G>A mutation in FMR1.
FIGURE 2.
DNA sequencing of FMR1 gene showing the exon 5 mutation (ChrX: 147,010,319 c.413G>A p.Arg138Gln) using Sanger confirmation (top). Next generation sequencing image showing the G to A change at position 413 of the FMR1 gene in exon 5 (bottom). [Color figure can be viewed at wileyonlinelibrary.com]
3 |. RESULTS AND DISCUSSION
We identified a male child with features of FXS who presented without the typical CGG triplet repeat expansion of the 5′ end of FMR1 gene but DNA sequencing revealed a mutation at position c.148 G>A in FMR1. This point mutation has been reported previously in one patient presenting with seizures and intellectual disability (Myrick, Hashimoto, et al., 2015). Our patient also presented with intellectual disability and seizures consistent with the previously reported patient with the same missense mutation. Additionally, he had ASD, ADHD, and several typical features of FXS including characteristic facial features (large upper face, small lower face, and prominent broad ears), hyperextensible digits, soft fleshy hands, flat feet, unsteady gait, hypotonia, and strabismus.
FMRP transports mRNA from the neuronal nuclei to the synapses with selectivity specific for many pre- and post-synaptic targets. Individuals with FXS have abnormal dendritic spines in the brain which are important for increasing the surface area of synaptic connections involved in neuroplasticity (Bassell & Warren, 2008; Bhakar, Dölen, & Bear, 2012; Garber et al., 2008). FMRP is also involved in several postsynaptic signaling cascades including acetylcholine, dopamine, and glutamate receptor signaling (Osterweil, Krueger, Reinhold, & Bear, 2010; Volk, Pfeiffer, Gibson, & Huber, 2007; Wang et al., 2008). In addition to facilitating mRNA transport and post-synaptic functions, FMRP is also found in structures in axons and pre-synaptic terminals called fragile-X granules expressed during periods of synaptic plasticity. FMRP regulates expression of dendritic voltage-gated K+ channels in brain circuits and affects the activity of several pre-synaptic voltage-gated ion channels (Myrick et al., 2014). It also regulates the expression of the sodium-activated potassium channel Slack, post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) involved in synaptic plasticity and adaptation, and n-type voltage-gated calcium channels Cav2.2 (Ferron, Nieto-Rostro, Cassidy, & Dolphin, 2014). Finally, FMRP modulates activity of large-conductance calcium-activated potassium (BK) channels found in the hippocampal and cortical excitatory neurons, critical for regulating action potential duration playing a role in seizure propagation (Bhakar et al., 2012; Brenner et al., 2005; Deng et al., 2013; Grønskov et al., 2011; Myrick et al., 2014; Shruti, Clem, & Barth, 2008).
In animal models, the mutation seen in our patient has been linked specifically to impairments of the pre-synaptic functions of FMRP such as regulation of BK channels and prevention of synaptic overgrowth (Brenner et al., 2005; Deng et al., 2013; Myrick, Hashimoto, et al., 2015; Shruti et al., 2008). The role of BK channels in seizures may partially explain why seizures were among the FXS symptoms reported in both of these patients. Interestingly, post-synaptic functions of FMRP such as translational regulation, mRNA binding, and AMPA receptor trafficking were all preserved in the animal models with this missense mutation.
Several mutations affecting FMR1 have been recorded in the literature including those in the promoter region in three males younger than18 years of age with unexplained developmental delay not due to the typical CGG repeat expansion. The three promoter mutations, located at c.−332G>C, c. −293T>C, and c. −254A>G, showed reduced FMR1 promoter activity in luciferase studies at 5.9%, 29.2%, and 36.2%, respectively, of wild-type levels and were likely pathogenic (Collins et al., 2010). In addition, both missense and nonsense mutations and deletions or disturbances of the coding sequence were reported in 20 patients and clinical information supporting FXS or FXS-like symptoms were described in 17. Five separate FMR1 gene missense mutations seen in six described patients included: c.1100T>A in exon 11, p.Ile367Asn; c.1444G>A in exon 14, p.Gly482Ser; c.797G>A in exon 8, p.Gly266Glu; c.1601G>A in exon 15, p.Arg534His in two patients and c.413G>A in exon 5, p.Arg138Gln. Other changes included a two base pair change at genomic position g.23714GG-TA at the intron/exon boundary of exon 2; an insertion in the intron/exon 10 boundary (IVS10+14[C>T]) causing exon 10 skipping; an insertion in exon 15 causing a nonsense mutation 1457insG; three brothers with the same nonsense mutation in exon 17 causing a frameshift change r.1737_1738ins1737+1_?, p.Ile580fs*9; a frameshift causing skipping of exon 10 at c.990+1G>A, p.Lys295Asnfs*11; a nonsense mutation at c.80C>A in exon 2, p.Ser27X resulting in a frameshift and premature termination of the protein and a one base pair change at c.373delA in exon 5, p.Thr126Leu and a nonsense mutation in exon 6 at c.420–8A>G, p.Met140Ilefs*3. A single 3′UTR change of unknown significance at genomic location c.*746T>C has been reported (see Table 1).
TABLE 1.
Demographics, clinical, behavior/cognition, and molecular genetics of individuals with rare FMR1 gene mutations
| Clinical category | De Boulle et al. (1993) | Lugenbeel et al. (1995) | lugenbeel et al. (1995) | Wang et al. (1997) | Gronskov et al. (2011) | Handt et al. (2014) | Handt et al. (2014) | Handt et al. (2014) | Myrick et al. (2014) | Wright et al. (2015) | Myrick, Hashimoto et al. (2015)a | Okray et al. (2015) | Suhl et al. (2015) | Grozeva et al. (2015) | Hu et al. (2016) | Quartier et al. (2017) | Quartier et al. (2017) | Quartier et al. (2017) | Quartier et al. (2017) | Quartier et al. (2017) | Present case |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||||||||||||
| Age (y) | 27 | 3 | ~30 | N/a | 35 | 4 | 11 | 11 | 16 | N/a | 12 | 36 | 10 | N/a | N/a | 50 | 48 | 47 | 10 | 20 | 10 |
| Ethnicity | Caucasian | Caucasian | Caucasian | N/a | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | N/a | Caucasian | Caucasian | Black | N/a | N/a | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian |
| Gender | Male | Male | Male | N/a | Male | Male | Male | Male | Male | N/a | Male | Male | Male | N/a | N/a | Male | Male | Male | Male | Male | Male |
| Current height | 1.85m (90th centile) | N/a | N/a | Male | N/a | 98th centile | WNL | 97th centile | 1.72m (45th centile | N/a | 25th centile | WNL | 1.6m (75th centile) | N/a | N/a | N/a | N/a | N/a | 75th centile (+1 SD) | 1.76m (95th centile) | 1.36m (35th centile) |
| Current weight | 65kg (50th centile) | N/a | N/a | N/a | N/a | 98th centile | WNL | 97th centile | 52.3kg (17th centile) | N/a | 25th centile | WNL | 39.9kg (60th centile) | N/a | N/a | N/a | N/a | N/a | (−0.5 SD) | 59.2kg (50th centile) | 25.7kg (10th centile) |
| Clinical features | |||||||||||||||||||||
| Enlarged testicles | Yes (>100 ml) | No | Yes (75 mL) | N/a | Yes | N/a | N/a | N/a | Yes | N/a | No | Yes | N/a | N/a | N/a | Yes | Yes | Yes | No | No | No (3–4 ml) |
| Dysmorphic features | Yes | Yes | Yes | N/a | Yes | N/a | N/a | N/a | Yes | N/a | No | Yes | Yes | N/a | N/a | Yes | Yes | Yes | Yes | Yes | Yes |
| Epicanthal folds | No | Yes | N/a | Yes | no | N/a | N/a | N/a | N/a | N/a | No | N/a | No | N/a | N/a | No | No | No | N/a | No | No |
| Elongated face | Yes; asymmetric | Yes | Yes | N/a | Yes | N/a | N/a | N/a | Yes | N/a | No | N/a | Yes | N/a | N/a | Yes | Yes | Yes | Yes | Yes | Yes |
| Prognathism | Yes | N/a | Yes | Yes | Yes | N/a | N/a | N/a | N/a | N/a | No | Yes | Yes | N/a | N/a | No | Yes | Yes | Yes | Yes | no |
| Broad high forehead | Yes | Yes | Yes | Yes | Yes | N/a | N/a | N/a | Yes | N/a | No | Yes | N/a | N/a | N/a | Yes | No | Yes | Yes | Yes | Yes |
| Prominent ears | Yes | Yes | Yes | Yes | Yes | N/a | N/a | N/a | Yes | N/a | Yes | Yes | Yes | N/a | N/a | Yes | Yes | Yes | Yes | no | Yes |
| Hypermobility/hyper-extensible joints | N/a | Yes | Yes | Yes | Yes | N/a | N/a | N/a | Yes | N/a | N/a | Yes | Yes | N/a | N/a | Yes | Yes | Yes | Yes | Yes | Yes |
| Hypotonia | N/a | N/a | N/a | N/a | Yes | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | N/a | Yes |
| Seizures | Focal | N/a | N/a | N/a | Tonic-clonic | N/a | N/a | N/a | N/a | N/a | Partial | No | No | N/a | N/a | Yes | No | No | N/a | N/a | Partial complex |
| Behavioral/cognitive | |||||||||||||||||||||
| Autism | N/a | Yes | Yes | N/a | Tactile defensive, limitedinterests | N/a | N/a | N/a | Yes | N/a | No | Yes | Yes | N/a | N/a | Yes | Yes | Yes | Yes | Yes | Yes |
| Verbal ability | No | Perseveration and cluttering of phrases | Rapid speech with classical cluttering | N/a | Few words, out of context | Impaired | N/a | N/a | Yes | N/a | Yes, complete sentences | N/a | Yes | N/a | N/a | NO | Yes | No | N/a | N/a | no |
| ADHD | N/a | Yes | N/a | N/a | N/a | Yes | N/a | Yes | N/a | N/a | N/a | N/a | Yes | N/a | N/a | Yes | Yes | Yes | No | Yes | Yes |
| Developmental delay | Yes | Yes | Yes | Yes | Yes | Mild/moderate | Yes | Yes | Yes | N/a | Yes | N/a | Yes | N/a | N/a | Yes | Yes | Yes | Yes | Yes | Yes |
| Language impairment | Yes | Yes | N/a | N/a | Yes | Yes | Yes | N/a | Yes | N/a | Yes | Yes | Yes | N/a | N/a | Yes | Yes | Yes | No | Yes | Yes |
| Cognitive impairment/Intelligence quotient | IQ<20 | N/a | N/a | Yes | Yes | Psychomotor delay | Learning impairment | Learning disability | N/a | N/a | IQ=42 | IQ=41 | IQ=47 | N/a | N/a | Yes | Yes | Yes | Yes | Yes | IQ=42 |
| Other | |||||||||||||||||||||
| Hepatomegaly, glycogenesis, growth retardation, spastic paraparesis, increased DTRs, flat feet, knee anomaly | Reduced balance | Toe walking, hand flapping, obsessions | Myoclonus | Normal head size, right pre-auricular tag | Agitation, outbursts, macrocephaly, urinary incontinence | Mild macrocephaly | Periodic aggressive outbursts | Birthmarks | Marfanoid habitus, gynecomastia, affected brothers | Marfanoid habitus, affected brothers | Marfanoid habitus, affected brothers | Fifth digit clinodactyly, short toes, shawl scrotum | Third and fifth digit clinodactyly | Strabismus, mitral valve prolapse, wide mouth, hirsutism | |||||||
| Molecular genetics | |||||||||||||||||||||
| Mutation type | Hissense | 1-bp deletion-frameshift | 2-bp change | Intron 10 C>T | Nonsense | Missense | Missense | Missense | Missense | Missense | Missense | Nonsense | 3’UTR | Missense | Missense | Nonsense | Nonsense | Nonsense | frameshift | Nonsense | Missense |
| Genomic location | c.11OOT>A | c.373delA | g.23714GG-TA | IVS10+14 (C>T) | c.80C>A | c.1444G>A | c.1601G>A | c.1601G>A | c.797G>A | c.377T>C | c.413G>A | 1457insG | c.*746T>C | c.677G>A | c.1618G>A | r.1737_1738ins1737+1_? | r.1737_1738ins1737+1_? | r.1737_1738ins1737+1_? | c.990+1G>A | c.420-8A>G | c.413G>A |
| Amino acid protein position | p.11e367Asn | p.Thr126Leu | p.Ser27X | p.Gly482Ser | p.Arg534His | p.Arg534His | p.Gly266Glu | p.Phe126Ser | p.Arg138Gln | frameshift, stop codon | p.Arg226Lys | p.Gly540Glu | p.lle580fs*9 | p.lle580fs*9 | p.lle580fs*9 | p.Lys295Asnfs*11 | p.Met140llefs*3 | p.Arg138Gln | |||
| Exon | Exon 11 | Exon 5 | Intron/Exon 2 boundary | Intron/exon 10 boundary exon 10 skipped | Exon 2 | Exon 14 | Exon 15 | Exon 15 | Exon 8 | Exon 5 | Exon 5 | Exon 15 | Exon 8 | Exon 15 | Exon 17 | Exon 17 | Exon 17 | Exon 10 skipped (r.881_990del) | Exon 6 | Exon 5 | |
| Classification | VUS | LP | IP | LP | P | VUS | VUS | VUS | VUS | VUS | LP | P | VUS | VUS | VUS | LP | LP | LP | LP | LP | LP |
| Protein domain | KH2 | NLS | Agenet1 | KH2 | Agenet1 | RGG | RGG | KH1 | NIS | NLS | RGG | KH1 | R6G | KH2 | NES | NIS | |||||
| Positive family history | N/a | N/a | Yes; mother | N/a | Yes; mother | N/a | Yes; mother, sister | Yes; mother | N/a | N/a | Yes; mother | N/a | Yes; mother, half brother | N/a | N/a | Yes; mother, two brothers | Yes; mother, two brothers | Yes; mother, two brothers | No, de novo | Yes; heterozygous mother | Yes; mother, sister |
DTRs, deep tendon reflexes; LP, likely pathogenic; N/a, not available; P, pathogenic; VUS, variant of uncertain significance; WNL, within normal limits.
Molecular genetic findings reported by Collins et al. (2010).
All variants listed above are a mixture of pathogenic, likely pathogenic, and variants of uncertain significance (VUS) as defined by the American College of Medical Genetics and Genomics (ACMG) consensus criteria using standards with guidelines for the interpretation of sequence variants (Richards et al., 2015). In general, rare structural variants, such as nonsense or frameshift, in a gene that has strong congruence with the clinical phenotype can be classified as likely pathogenic or pathogenic. Rare missense variants need more evidence to rise to the level of pathogenicity, such as functional data or presence in multiple patients. Based on this criteria, we have classified each of the 17 gene variants as pathogenic, likely pathogenic or VUS (see Table 1).
The FMR1 gene encodes FMRP which has an amino terminal domain consisting of structural modules, a tandem array of two Agenet/Tudor domains (Agenet 1 and 2) located at amino acid residues 4–50 and 63–115, respectively, with RNA binding activity (Lacoux et al., 2012) and NLS (nuclear localization signal) at amino acid residues 115–252 followed by K homology (KH) domains at KH0 (residues 63–113), KH1 (residues 221–266), and KH2 (residues 285–328). The NES (nuclear export signal) is located at residues 422–446 and followed by C-terminus arginine-glycine-glycine (RGG) residues located at 527–552 which also binds RNA (Myrick, Deng, et al 2015; Myrick, Hashimoto, et al., 2015; Quartier et al., 2017). The interface between Agenet and KH domains involve a large number of aromatic and hydrophobic forces and intra-molecule interactions that confer stability. The Agenet domain contains an aromatic cage with binding potential to methylated lysine and histones (Alpatov et al., 2014).
All subjects reported were male when sex information was available and ranged in age from infancy to 50 years. Two of the males had height and weight at or above the 95th centile indicating a large size reported in some individuals with FXS. Dysmorphic features included craniofacial findings with an elongated face, high broad forehead, prominent ears and hypermobile joints in the majority of reported males consistent with FXS. Most subjects were verbal with limited speech and/or had autism and hyperactivity; all had developmental delays or intellectual disability. Seizures, rapid cluttered speech and enlarged testicles were also seen as common features in FXS (see Table 1). One adult male had a testicular volume of greater than 100 ml.
The first report of a patient with the c.148G>A mutation in FMR1 along with our reported male suggest that a subset of patients with FXS preserved the post-synaptic functions of FMRP at the KH helix alpha A domain. Four of the 20 subjects had disturbances of exon 15, three with exon 5, and two with exon 2. Our identification of a second patient with FXS carrying the same mutation, but with more features of FXS provides further support for the hypothesis of a subset of individuals with FXS having coding sequence mutations as the cause of their phenotype. The findings and clinical history of our patient with a delayed diagnosis were similar to the 20 subjects with missense or nonsense mutations or other changes within the coding sequence of the FMR1 gene reported in the literature and summarized in Table 1. The clinical data reported on each patient were consistent with the clinical phenotype seen in males with FXS due to the classical CGG triplet repeat expansion in FMR1.
Our family had a history of consanguinuity (parents were first cousins) and thus at risk for rare genetic conditions involving an area of homozygosity. Homozygosity mapping followed by exome sequencing to identify homozygous variants in genes associated with autism or intellectueal disability was not undertaken. The gene panel utilized would not have covered all known associated genes nor any new genes. This is a potential limitation and a possible alternative cause of his condition that cannot be ruled out. Our study suggests that additional patients meeting the clinical criteria for FXS but with normal testing without the CGG repeat expansion may have other mutations of the gene. Next generation sequencing and further genetic testing beyond the triplet repeat mutation analysis should be performed as standard DNA test to rule out FXS in our patient when tested at 3 years of age did not identify the typical FMR1 triple repeat mutation. In addition, his mother and teenage sister were tested and found to carry the same rare FMR1 gene mutation with a profound impact on genetic and reproductive counseling and risk estimates.
Finally, the existence of other subsets of FXS patients may well occur but they are likely under-reported without the use of advanced genetic testing technology. For example, dozens of FMR1 gene deletions (full, partial, or mosaicism) have been reported in individuals with features of FXS, specifically using microarray analysis and recently reviewed (Ciaccio et al., 2017; Coffee et al., 2009; Gonçalves et al., 2016; Hammond, Macias, Tarleton, & Pai, 1997). Additionally, known noncoding FMR1 polymorphisms or gene variants are summarized in Table 2. These reports and data are beyond the scope of our summary review of coding sequence variation and the reader can refer to these articles for further information on types of genetic lesions involving FMR1. Thus, the advent of next generation DNA sequencing may allow for a much more rapid detection of non-CGG repeat FMR1 mutations and number of affected individuals identified, impacting diagnosis, genetic counseling, and testing of at-risk family members. Ultimately, their discovery and reporting may lead to a better understanding of the function of FMRP, particularly at the synapse, impacting novel treatment approaches for those with rare FMR1 gene mutations. Knowing the relationship between specific mutations and the differential effect of FMRP on pre-synaptic and/or post-synaptic function would be of interest and should be further studied in other clinical cases and animal models.
TABLE 2.
Noncoding variants reported in the FMR1 genea
| Novel mutations | |
|---|---|
| cDNA variants | Position |
| c.105–8A>C | Intron 2 |
| c.630 + 438A>C | Intron 7 |
| c.631–840G>A | Intron 7 |
| c.880+885A>G | Intron 9b |
| c.990+4T>C | Intron 10 |
| c.1472–521C>G | Intron 14b |
| c.*23T>C | 3′UTR |
| c.*746T>C | 3′UTRc |
| c.*1867G>A | 3′UTR |
| c.*2035C>T | 3′UTR |
| c.1189–39A>G | Intron 12 |
| c.*60G>C | 3′UTR |
| c.*68T>C | 3′UTR |
| Single nucleotide polymorphisms (SNPs) | |
| c.52–112A>G | |
| c.271–19A>G | |
| c.801+31C>T | |
| c.990+14C>T | |
| c.991–54A>G | |
| c.1737+8C>A | |
| c.*32C>G | |
Cited from Collins et al. (2010) and Handt et al. (2014).
Predicted splice site.
Predicted miRNA binding site.
ACKNOWLEDGMENTS
The authors thank Naveen Khanzada, MD for assistance in manuscript preparation. The authors acknowledge support from the National Institute of Child Health and Human Development (NICHD) grant numbers HD02528, HD036071, and the University of California—Davis MIND Institute Intellectual and Developmental Disabilities Research Center U54 HD079125. RH has received funding from Alcobra, Neuren, Marinus, and Novartis to carry out treatment studies in individuals with fragile X syndrome. She has also consulted with Ovid and Zynerba regarding treatment of fragile X syndrome. FT received funding from Asuragen for studies on fragile X syndrome.
Funding information
University of California—Davis Intellectual and Developmental Disabilities Research Center, Grant number: U54 HD079125; National Institute of Child Health and Human Development, Grant number: HD02528 HD036071; Alcobra, Neuren, Marinus, and Novartis; Asuragen
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Alpatov R, Lesch BJ, Nakamoto-Kinoshita M, Blanco A, Chen S, Stützer A, … Shi Y (2014). A chromatin-dependent role of the fragile X mental retardation protein FMRP in the DNA damage response. Cell, 157, 869–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, & Warren ST (2008). Fragile X syndrome: Loss of local mRNA regulation alters synaptic development and function. Neuron, 60, 201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis E, Raspa M, Loggin-Hester L, Bishop E, Holiday D, & Bailey DB (2010). Seizures in fragile X syndrome: Characteristics and comorbid diagnoses. American Journal on Intellectual and Developmental Disabilities, 115, 461–472. [DOI] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, & Bear MF (2012). The pathophysiology of fragile X (and what it teaches us about synapses). Annual Review of Neuroscience, 35, 417–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, & Aldrich RW (2005). BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nature Neuroscience, 8, 1752–1759. [DOI] [PubMed] [Google Scholar]
- Ciaccio C, Fontana L, Milani D, Tabano S, Miozzo M, & Esposito S (2017). Fragile X syndrome: A review of clinical and molecular diagnoses. Italian Journal of Pediatrics, 43, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, & Warren ST (2009). Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. American Journal of Human Genetics, 85, 503–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SC, Bray SM, Suhl JA, Cutler DJ, Coffee B, Zwick ME, & Warren ST (2010). Identification of novel FMR1 variants by massively parallel sequencing in developmentally delayed males. American Journal of Medical Genetics Part A, 152A(1), 2512–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boulle K, Verkerk AJ, Reyniers E, Vits L, Hendrickx J, Van Roy B, … Willems PJ (1993). A point mutation in the FMR-1 gene associated with fragile X mental retardation. Nature Genetics, 3, 31–35. [DOI] [PubMed] [Google Scholar]
- Deng PY, Rotman Z, Blundon JA, Cho Y, Cui J, Cavalli V, … Klyachko VA (2013). FMRP regulates neurotransmitter release and synaptic information transmission by modulating action potential duration via BK channels. Neuron, 77, 696–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferron L, Nieto-Rostro M, Cassidy JS, & Dolphin AC (2014). Fragile X mental retardation protein controls synaptic vesicle exocytosis by modulating N-type calcium channel density. Nature Communications, 5, 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, & Warren ST (2008). Fragile X syndrome. European Journal of Human Genetics, 16, 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves TF, dos Santos JM, Gonçalves AP, Tassone F, Mendoza-Morales G, Ribeiro MG, … Pimentel G (2016). Finding FMR1 mosaicism in fragile X syndrome. Expert Review of Molecular Diagnostics, 16, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønskov K, Hallberg A, Brøndum-Nielsen K, Dedic A, & Hjalgrim H (2011). A nonsense mutation in FMR1 gene causing fragile X syndrome. European Journal of Human Genetics, 4, 489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozeva D, Carss K, Spasic-Boskovic O, Tejada M-I, Gecz J, Shaw M, … Raymond FL (2015). Targeted next-Generation sequencing analysis of 1,000 individuals with intellectual disability. Human Mutation, 36, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, & Hagerman PJ (2002). Fragile x syndrome: Diagnosis, treatment, and research. (3rd ed.). Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- Hagerman R, & Hagerman P (2013). Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurology, 12, 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Lachiewicz A, … Tranfaglia M (2009). Advances in the treatment of fragile x syndrome. Pediatrics, 123, 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LS, Macias MM, Tarleton JC, & Pai GS (1997). Fragile X syndrome and deletions in FMR1: New case and review of the literature. American Journal of Medical Genetics, 72, 430–434. [PubMed] [Google Scholar]
- Handt M, Epplen A, Hoffjan S, Mese K, Epplen JT, & Dekomien G (2014). Point mutation frequency in the FMR1 gene as revealed by fragile X syndrome screening. Molecular and Cellular Probes, 28, 279–283. [DOI] [PubMed] [Google Scholar]
- Hu H, Haas SA, Chelly J, Van Esch H, Raynaud M, de Brouwer APM, … Kalscheuer H-H (2016). X-exome sequencing of 405 unresolved families identifies seven novel intellectual disability genes. Molecular Psychiatry, 21, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoux C, Di Marino D, Boyl PP, Zalfa F, Yan B, Ciotti MT, … Bagni C (2012). BC1-FMRP interaction is modulated by 2′-O-methylation: RNA-binding activity of the tudor domain and translational regulation at synapses. Nucleic Acids Research, 40, 4086–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugenbeel KA, Peier AM, Carson NL, Chudley AE, & Nelson DL (1995). Intragenic loss of function mutations demonstrate the primary role of FMR1 in fragile X syndrome. Nature Genetics, 10, 483–485. [DOI] [PubMed] [Google Scholar]
- McLennan Y, Polussa J, Tassone F, & Hagerman R (2011). Fragile X syndrome. Current Genomics, 12, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Nakamoto-Kinoshita M, Lindor NM, Kimani S, Cheng X, & Warren ST (2014). Fragile X syndrome due to a missense mutation. European Journal of Human Genetics, 22, 1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Deng P-Y, Hashimoto H, Oh YM, Cho Y, Poidevin MJ, … Klyachko VA (2015). Independent role for presynaptic FMRP revealed by an FMR1 missense mutation associated with intellectual disability and seizures. Proceedings of the National Academy of Sciences, 112, 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick LK, Hashimoto H, Cheng X, & Warren ST (2015). Human FMRP contains an integral tandem Agenet (Tudor) and KH motif in the amino terminal domain. Human Molecular Genetics, 24, 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okray Z, de Esch CE, Van Esch H, Devriendt K, Claeys A, Yan J, … Hassan BA (2015). A novel fragile X syndrome mutation reveals a conserved role for the carboxy-terminus in FMRP localization and function. EMBO Molecular Medicine, 7, 423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, & Bear MF (2010). Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 30, 15616–15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartier A, Poquet H, Gilbert-Dussardier B, Rossi M, Casteleyn A-S, Portes des V, … Piton A (2017). Intragenic FMR1 disease-causing variants: A significant mutational mechanism leading to Fragile-X syndrome. European Journal of Human Genetics, 25, 423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regezi JA, Sciubba JJ, & Jordan RCK (2008). Oral Pathology: Clinical Pathologic Correlations. (5th Ed.). St. Louis, MO: Saunders/Elsevier. [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, … ACMG Laboratory Quality Assurance Committee, (2015). Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine, 17, 405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MR, Bray SM, & Warren ST (2012). Molecular mechanisms of fragile X syndrome: A twenty-year perspective. The Annual Review of Pathology: Mechanisms of Disease, 7, 219–245. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL, & Barth AL (2008). A seizure-induced gain-of-function in BK channels is associated with elevated firing activity in neocortical pyramidal neurons. Neurobiology of Disease, 30, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Choi M, Siomi MC, Nussbaum RL, & Dreyfuss G (1994). Essential role for KH domains in RNA binding: Impaired RNA binding by a mutation in the KH domain of FMR1 that causes fragile X syndrome. Cell, 77, 33–39. [DOI] [PubMed] [Google Scholar]
- Suhl JA, & Warren ST (2015). Single-nucleotide mutations in FMR1 reveal novel functions and regulatory mechanisms of the fragile X syndrome protein FMRP. Journal of Experimental Neuroscience, 9, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, & Huber KM (2007). Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27, 11624–11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Lin ML, Lin SJ, Li YC, & Li SY (1997). Novel point mutation within intron 10 of FMR-1 gene causing fragile X syndrome. Human Mutation, 10, 393–399. [DOI] [PubMed] [Google Scholar]
- Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, … Zhuo M (2008). FMRP Acts as a key messenger for dopamine modulation in the forebrain. Neuron, 59, 634–647. [DOI] [PubMed] [Google Scholar]
- Wright CF, Fitzgerald TW, Jones WD, Clayton S, McRae JF, Van Kogelenberg M, … DDD study, (2015). Genetic diagnosis of developmental disorders in the DDD study: A scalable analysis of genome-wide research data. The Lancet, 385, 1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]