Abstract
We performed a phase I trial of vorinostat (VOR) given on days 1 to 5 with R-EPOCH (rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin hydrochloride) in patients with aggressive HIV-associated non-Hodgkin lymphoma. VOR was tolerable at 300 mg and seemingly efficacious with chemotherapy with complete response rate of 83% and 1-year event-free survival of 83%. VOR did not significantly alter chemotherapy steady-state concentrations, CD4+ cell counts, or HIV viral loads.
Introduction:
Vorinostat (VOR), a histone deacetylase inhibitor, enhances the anti-tumor effects of rituximab (R) and cytotoxic chemotherapy, induces viral lytic expression and cell killing in Epstein-Barr virus-positive (EBV+) or human herpesvirus-8-positive (HHV-8+) tumors, and reactivates latent human immunodeficiency virus (HIV) for possible eradication by combination antiretroviral therapy (cART).
Patients and Methods:
We performed a phase I trial of VOR given with R-based infusional EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin hydrochloride) (n = 12) and cART in aggressive HIV-associated B-cell non-Hodgkin lymphoma (NHL) in order to identify safe dosing and schedule. VOR (300 or 400 mg) was given orally on days 1 to 5 with each cycle of R-EPOCH for 10 high-risk patients with diffuse large B-cell lymphoma (1 EBV+), 1 EBV+/HHV-8+ primary effusion lymphoma, and 1 unclassifiable NHL. VOR was escalated from 300 to 400 mg using a standard 3 + 3 design based on dose-limiting toxicity observed in cycle 1 of R-EPOCH.
Results:
The recommended phase II dose of VOR was 300 mg, with dose-limiting toxicity in 2 of 6 patients at 400 mg (grade 4 thrombocytopenia, grade 4 neutropenia), and 1 of 6 treated at 300 mg (grade 4 sepsis from tooth abscess). Neither VOR, nor cART regimen, significantly altered chemotherapy steady-state concentrations. VOR chemotherapy did not negatively impact CD4+ cell counts or HIV viral loads, which decreased or remained undetectable in most patients during treatment. The response rate in high-risk patients with NHL treated with VOR(R)-EPOCH was 100% (complete 83% and partial 17%) with a 1-year event-free survival of 83% (95% confidence interval, 51.6%–97.9%).
Conclusion:
VOR combined with R-EPOCH was tolerable and seemingly efficacious in patients with aggressive HIV-NHL.
Keywords: AIDS-related malignancies, Chemotherapy, Epstein-Barr virus, Histone deacetylase inhibitors, Lytic-inducing therapies
Introduction
Individuals infected with human immunodeficiency virus (HIV) are at an increased risk of developing highly aggressive non-Hodgkin lymphoma (NHL). Recent studies have demonstrated improved outcomes in patients with HIV-NHL approaching that of the general population after the introduction of combination antiretroviral therapy (cART) and newer chemotherapy paradigms.1 A large retrospective pooled analysis describing the outcome of patients with HIV-NHL in the contemporary cART era reported 2-year survival rates of 67% for HIV-diffuse large B-cell lymphoma (DLBCL), as compared with 24% in the pre-cART era.2 Recent advancements in the treatment of HIV-DLBCL might be attributed to the efficacy of infusional regimens, such as EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin), and the addition of rituximab (R) to standard curative NHL regimens.3 R-EPOCH is the preferred regimen for treating HIV-DLBCL and HIV-primary effusion lymphoma (PEL) under current National Comprehensive Cancer Network guidelines based on multiple phase II clinical trials and retrospective studies.3 Despite these advancements, treatment of HIV-NHL remains challenging in severely immune-compromised patients and aggressive NHL variants that carry poorer prognosis, such as plasmablastic lymphoma (PBL), PEL, and activated B-cell (ABC) type DLBCL.4–7
Differences in clinical spectrum and biology of HIV-NHL might be exploited therapeutically. For example, the high expression of the multidrug resistance (MDR-1) gene might be overcome by infusional regimens like EPOCH by prolonged continuous drug exposure.8,9 Alternatively, despite their oncogenic potential, latent γ–herpesviruses (Epstein-Barr virus [EBV] and human herpesvirus-8 [HHV-8]) can be targeted therapeutically, as doxorubicin and etoposide (EPOCH drugs), and histone deacetylase (HDAC) inhibitors disrupt viral latency.10–13 Moreover, in preclinical B-cell lymphoma and hematologic malignancy models, the potent HDAC inhibitor vorinostat (VOR) was highly synergistic with R, anthracyclines, and etoposide.14–16 VOR given with R, cyclophosphamide, etoposide, and prednisone was effective in elderly patients with relapsed/refractory DLBCL.17 VOR induced HHV-8 lytic gene expression and p53 acetylation leading to apoptosis and increased survival in a PEL xenograft mouse model.18 VOR also re-activates HIV, suggesting its potential role in eradicating latently infected reservoirs in human hosts via HIV cytopathic effects and immune-mediated mechanisms.19–21
Based on these concepts, the National Cancer Institute (NCI)-funded AIDS Malignancy Consortium (AMC) performed a phase I/II clinical trial (AMC-075) using VOR with R-EPOCH in aggressive, non-Burkitt, HIV-NHL. The primary objectives were to test the safety and the efficacy of VOR when combined with R-based chemotherapy and cART using complete response rate as the primary study endpoint. We report the phase I portion here. To evaluate toxicity, 2 VOR dose levels (300 mg or 400 mg given orally on days 1 through 5 during each chemotherapy cycle) were tested using a 3 + 3 design. This enabled us to compare directly the plasma steady-state concentrations of etoposide, doxorubicin, and vincristine achieved at the 2 VOR dose levels during cART. This trial is registered at http://clinicaltrials.gov as .
Patients and Methods
Eligibility Criteria
Twelve AMC sites in the United States enrolled patients after written informed consent according to the Declaration of Helsinki. Patients with HIV and absolute CD4+ count ≥50 cells/mm3, with DLBCL or aggressive non-Burkitt NHL variants, were eligible. Patients were untreated or had received a maximum of 1 cycle of chemotherapy at time of enrollment.
Patients with any Ann Arbor stage (I-IV), age ≥18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2, and adequate organ function were eligible. Non-zidovudine based cART was required. For antiretroviral-naive subjects at study entry, cART was started after cycle 1 to avoid confounding side effects. Patients who had active hepatitis B virus (surface antigen, core antigen, or viremia), or active hepatitis C infection were ineligible. Patients who were only hepatitis B core antibody-positive required prophylactic anti-hepatitis B virus therapy. Patients with known central nervous system involvement by lymphoma were ineligible.
Treatment Administration and Supportive Care
R was given at 375 mg/m2 intravenously (IV) for CD20+ lymphomas on day 1. R-EPOCH was given to patients with high-risk NHL every 21 days for 6 cycles. Cyclophosphamide IV on day 5 was administered at initial dose of 375 mg/m2 when baseline CD4+ count was 50 to 200 cells/mm3, or 750 mg/m2 if baseline CD4+ count was > 200 cells/mm3. For subsequent cycles, cyclophosphamide was dose-adjusted based on nadir counts according to specified guidelines (see Supplemental Tables 1 and 2 in the online version). Patients received VOR orally once on days 1 to 5. Treatment and supportive care options are summarized in Table 1.
Table 1.
Treatment and Supportive Care
| Drug or Treatment | Dosing and Schedule |
|---|---|
| Vorinostat (VOR) | PO on days 1–5 every 3 wk with rituximab-EPOCH Dose level was assigned according to 3 + 3 design: starting level +1 (300 mg), level −1 (200 mg), level +2 (400 mg) |
| Rituximab (if CD20+ only) | 375 mg/m2 IV on day 1 every 3 wk with chemotherapy (CHOP or EPOCH) |
| EPOCH | Every 3 wk × 6 cycles |
| Etoposide | 50 mg/m2 IVCI on days 1–4 × 96 hr |
| Prednisone | 60 mg/m2 PO on days 1–5 |
| Doxorubicin | 10 mg/m2 IVCI on days 1–4 × 96 hr |
| Vincristine | 0.4 mg/m2 IVCI on days 1–4 × 96 hr |
| Cyclophosphamide (dose-adjusted) | Starting dose: 375 mg/m2 if CD4 = 50–200/mm3 with subsequent dose-adjustment, or maximum dose of 750 mg/m2 if CD4 >200/mm3 IVPB on day 5 |
| Supportive medications and treatment | |
| G-CSF | Started 24–48 hr after completion of chemotherapy |
| Pegfilgastrim | 6 mg SC once |
| Or | |
| Filgastrim | 300 mg or 480 mg SC daily for 10 d or longer until ANC ≥1000/mm3 |
| Infection prophylaxis | Continuous |
| Trimethoprim-sulfamethoxazole (160–800 mg) | 3 times weekly |
| or dapsone | 100 mg PO daily |
| or atovoquone | 1500 mg P0 daily |
| Fluoroquinolone (anti-bacterial agent) | No later than day 8 of chemotherapy until ANC ≥1000/mm3 |
| Anti-herpetic agent (recommended) | |
| CNS prophylaxis: intrathecal methotrexate or depocyte or cytarabine (per drug package specifications)a | For a total of 4–6 doses |
| cART non—zidovudine-based regimen required | Patients not on cART at study entry were required to start AFTER cycle 1 of chemotherapy |
Abbreviations: ANC = absolute neutrophil count; cART = combination antiretroviral therapy; CHOP = etoposide, cyclophosphamide, doxorubicin, vincristine, and prednisolone; CNS = central nervous system; EPOCH = prednisone, vincristine, cyclophosphamide, and doxorubicin; IV = intravenously; IVCI = intravenous continuous infusion; IVPB = intravenous piggyback; PO =orally; SC = subcutaneously.
CNS prophylaxis was required in patients who had lymphomatous involvement of bone marrow, testes, sinuses, or epidural regions.
Clinical and Response Assessments
Response was assessed by standard whole body computerized tomographic (CT) scan criteria22 after cycle 4, and posttreatment (4–8 weeks, and months 6, 12, 18, and 24). Positive emission tomographic (PET) or CT-PET were required after the final treatment cycle to confirm a complete response (CR). Subjects with bone marrow involvement had a repeat biopsy to confirm CR. Subjects with CR after cycle 4 received up to 2 additional chemotherapy cycles (total, 6 cycles). Subjects who achieved only a partial response (PR) after cycle 4 had the option to continue at the discretion of the treating physician. Subjects were followed every 3 months for 2 years post-treatment, and then every 6 months for years 3 to 5.
Central Pathology Review, Immunohistochemistry, and EBV-encoded Small RNA (EBER) in Situ Hybridization
Central pathology review was conducted at Weill Cornell Medical College as previously described.23 Cases with adequate tissue were categorized as germinal center (GC)-derived versus ABC (none–GC)-type according to the tissue microarray classification algorithm published by Hans et al.24 Monoclonal antibodies to the following antigens were used: CD10 (56C6; Leica Microsystems), BCL-2 (124), BCL-6 (PG-B6p), MUM-1 (MUM1p) and Ki-67 (MIB-1) (DakoCytomation, Carpinteria, CA). EBV Probe ISH Kit (Leica Microsystems, Wetzlar, Germany; Vision BioSystems Novocastra, Newcastle-upon-Tyne, UK) was used for in situ hybridization for EBER. The cases were interpreted as positive for CD10, BCL2, and MUM-1 when more than 30% of neoplastic cells were immunoreactive, and BCL2 positivity was defined when ≥50% of cells had moderate to strong positivity. Nuclear Ki-67 expression was determined semi-quantitatively as a percentage of positive tumor cells. Cases were considered EBER-positive when a hybridization signal was identified in the majority of neoplastic cells.
Correlative Studies
EBV expression was assessed by immunohistochemical detection of LMP-1 or EBER by in-situ hybridization of diagnostic tumor specimens at local and/or central pathology laboratories. T-cell (CD4+) subset analysis, and HIV viral load (VL) by quantitative RNA polymerase chain reaction, were performed by local laboratories at baseline, after cycle 2, and posttreatment (months 1, 6, and 12).
Pharmacokinetics (PK)
Serial plasma samples for PK analysis were collected at 24 to 48, 48 to 72, and 72 to 96 hours after the start of the first chemotherapy infusion. Doxorubicin, etoposide, and vincristine concentrations were determined using a validated liquid chromatography-tandem mass spectrometry method based on prior methods with minor modifications.25–27 The clearance was determined by dividing the drug-infusion rate by the steady-state concentrations, which was the average of the 3 time points.
Definition of Dose-limiting Toxicity (DLT) and Adverse Event (AE)
A DLT was defined as an AE possibly, probably, or definitely attributed to VOR-chemotherapy in the first cycle (21 days) and meeting criteria defined in Table 2. Classification of grade was determined using the NCI Common Terminology Criteria for Adverse Events, Version 4.0. For subsequent cycles, VOR doses were modified according to the above-mentioned guidelines listed in Supplemental Table 1 (in the online version).
Table 2.
Definition and Summary of DLT Events
| DLT Definition | ||||
|---|---|---|---|---|
| Hematologic toxicity | • ANC <500/mm3 that did not improve to a level above 750/mm3 within 14 d | |||
| • Febrile neutropenia (ANC <1000/mm3) requiring hospitalization or IV antibiotics | ||||
| • Platelet count <25/mm3 of any duration grade 4 anemia of any duration | ||||
| Non-hematologic | • Any toxicity causing a dose delay of >2 wk for the next treatment cycle | |||
| • Any adverse event ≥ grade 3 except: fatigue, grade 2 alopecia, or grade 3 or 4 nausea, vomiting, and/or diarrhea that was responsive to treatment | ||||
| • Grade 3 or 4 non-hematologic laboratory abnormalities that resolved to grade 1 or baseline within 14 d | ||||
| Vorinostat Dose Level | Dosage and Schedule | No. of Evaluable Patients | No. of Patients With Cycle 1 DLT | Type of DLT |
| +1 | 300 mg QD days 1 −5 | 6 | 1 | Grade 4 sepsis from tooth abscess |
| +2 | 400 mg QD days 1 −5 | 6 | 2 | Grade 4 thrombocytopenia (n = 2) |
Abbreviations: ANC = absolute neutrophil count; DLT = dose-limiting toxicity; IV = intravenously; QD = daily.
Statistical Analyses
Patients were enrolled to establish the recommended phase II dose (RPTD) of VOR using a standard 3 + 3 design (Table 2). The study planned to enroll between 9 and 18 participants during the dose escalation phase, plus 3 additional participants in order to assure that a total of 6 participants were treated at the recommended phase II dose. After determining the RPTD, a phase II study was planned with sample size of 90 participants equally divided between those with and those without VOR, which would be sufficient to detect the difference between the CR rate proportions (ie, 70% for EPOCH alone vs. 88% for VOR-EPOCH) with an odds ratio of 3.27 at the one-sided 0.10 significance level with power of 0.80 using the Normal approximation for a 2-sample test of proportions. Summary statistics were used to describe phase I patient baseline characteristics listed in Table 3. Safety evaluation and tumor response evaluations were tabulated by dose level. The tumor response rates with 95% confidence intervals were estimated for each dose group.
Table 3.
Baseline Demographics and Characteristics of Evaluable Patients With High-risk NHL
| Characteristic | N | Value | % |
|---|---|---|---|
| Gender | |||
| Female | 1 | 8 | |
| Male | 11 | 92 | |
| Race/ethnicity | |||
| White/non-Hispanic | 8 | 67 | |
| White/Hispanic | 2 | 17 | |
| African American | 2 | 17 | |
| Age, y | |||
| Median (range) | 48 (27−65) | ||
| CDC risk group | |||
| Homosexual/bisexual contact | 10 | 83 | |
| Heterosexual contact | 3 | 25 | |
| Multiple risks | |||
| Homosexual/bisexual + heterosexual contact | 8 | ||
| Homosexual/bisexual contact + IV drug use | 2 | 17 | |
| Absolute CD4 count, cells/mm3 | |||
| Median (range) | 222(91−754) | ||
| HIV viral load, copies/ml | |||
| Undetectable or below institutional limit | 5 | 42 | |
| Positive | 7 | 58 | |
| Median (range) | 22,400 (25−482,000) | ||
| Ann Arbor stage | |||
| I–II | 3 | 25 | |
| III–IV | 9 | 75 | |
| ECOG PS | |||
| 0–1 | 10 | 83 | |
| 2 | 2 | 17 | |
| aa-IPI risk | |||
| 0–1 | 4 | 33 | |
| 2–3 | 8 | 67 | |
| LDH elevation | |||
| Yes | 9 | 75 | |
| No | 3 | 25 | |
| Pathologic diagnosis | |||
| DLBCL | |||
| GC type | 5 | 42 | |
| ABC type | 5 | 42 | |
| Other | 2 | 17 | |
| B-cell lymphoma, unclassifiable, with features between DLBCL and BL | 1 | 8 | |
| PEL-extracavitary or solid variant | 1 | 8 | |
| Ki-67 expression (n = 11 available) | |||
| ≥80% | 10 | 91 | |
| <80% | 1 | 9 | |
| EBV expression | |||
| Positive | 2 (1 HHV−8+) | 17 | |
| Negative | 10 | 83 | |
Abbreviations: aa-IPI = age-adjusted International Prognostic Index; ABC = activated B-cell; BL = Burkitt lymphoma; CDC = Center for Disease Control and Prevention; DLBCL = diffuse large B-cell lymphoma; EBV = Epstein Barr virus; ECOG PS = Eastern Cooperative Oncology Group performance status; GC = germinal center; HIV = human immunodeficiency virus; IV = intravenous; LDH = lactose dehydrogenase; NHL = non-Hodgkin lymphoma; PEL = primary effusion lymphoma.
The effect of concurrent VOR and etoposide, doxorubicin, and vincristine clearance as part of R-EPOCH was assessed. Summary statistics were used to describe drug clearance by dose level. CYP3A4, a cytochrome P450 enzyme, is involved in the metabolism of doxorubicin (Pharm GKB https://www.pharmgkb.org/pathway/PA165292177),28 etoposide (Pharm GKB https://www.pharmgkb.org/pathway/PA2025),29 and vincristine (Pharm GKB https://www.pharmgkb.org/pathway/PA150981002).30 In a post-hoc analysis, patients were stratified into groups based on the known drug-drug interaction potential via CYP3A4: concurrent CYP3A4 inhibitors, concurrent CYP3A4 inducers, and no interaction potential or not on cART. Correlations between drug clearance and VOR dose or CYP3A4 interaction potential and between drug clearance and toxicity were performed by Kruskal-Wallis analysis of variance by ranks with post-hoc analysis using an all-pairs Tukey-Kramer test. Mann-Whitney U tests were used to assess correlations between drug exposure and toxicity. For each dose group, the Wilcoxon signed rank test was used to evaluate the changes in HIV viral load and CD4+ cell count from baseline to end of cycle 2, and 1, 6, and 12 months after completion of chemotherapy.
Results
Patient Characteristics, Tumor Classification, and Associated Risk Features
Between December 2010 and November 2012, 14 patients were enrolled. Eligible patients had at least 1 of the following high-risk characteristics: age-adjusted international prognostic index (aa-IPI) 2–3, Ki-67 ≥80%, post-GCB (also known as ABC) subtype DLBCL, or any other aggressive non-GCB, non-Burkitt B-cell NHL. Each of the following was considered an adverse factor for the aa-IPI: stage III to IV, elevated serum lactate dehydrogenase, or ECOG PS of 2.
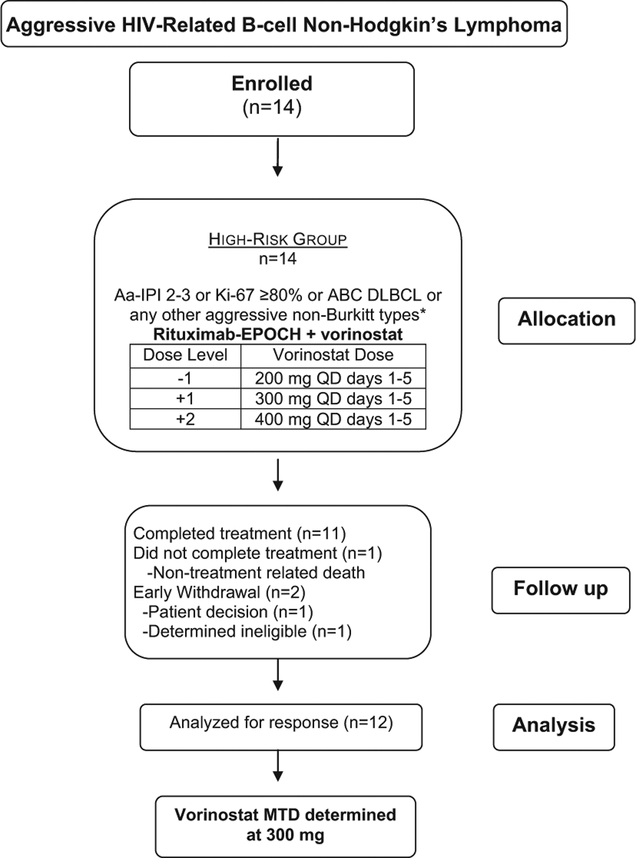
Twelve patients of 14 patients enrolled were evaluable for treatment response (Figure 1); 1 patient withdrew from the study during cycle 1, and 1 patient with B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and Burkitt lymphoma (BL) not otherwise specified was registered but not treated on protocol after he was determined to be ineligible. The protocol was later amended to include patients with B-cell lymphoma with features intermediate between DLBCL and BL.
Figure 1.
Consortium Diagram. *Histologic DLBCL Subtypes: Defined as Either GCB or ABC (Also Known as Post-GCB). By Immunophenotypic Criteria (GCB Type Was Considered When CD10 Was Expressed in > 30% of Cells, or if CD10−, BCL6+, and IRF4/MUM1−; All Others Were Considered to be ABC Type or Non-GC) Abbreviations: aa-IPI = age-adjusted International Prognostic Index; ABC = activated B–cell-like; DLBCL = diffuse large B-cell lymphoma; EPOCH = etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; GCB germinal center B–cell-like; MTD = maximum tolerated dosage; QD = daily.
Patient characteristics, tumor classification, and disease features of 12 patients assessable for treatment response treated with VOR-R-EPOCH are listed in Table 3. Ninety-two percent were male, 67% non-Hispanic white, 17% Hispanic, and 17% African American. The median age was 48 years (range, 27–65 years). Two (17%) patients were injection drug users. Ten (83%) patients were on cART prior to enrollment; 2 patients began cART after cycle 1. The median baseline absolute CD4+ count was 222 cells/mm3 (range, 91–754 cells/mm3), and < 100 cells/mm3 in 2 (17%) patients. Five (42%) patients had an undetectable HIV VL. Seven (58%) had HIV viremia with a median VL of 22,400 copies/ml (range, 25–482,000 copies/ml). Pathology was confirmed in 9 available cases by stains performed at the central pathology laboratory. Two cases submitted did not yield enough diagnostic tissue, and 1 case was not available; local institution pathology reports were reviewed and diagnoses made accordingly. Ten cases were classified as DLBCL (5 GC type, and 5 ABC type), 1 case as B-cell lymphoma, unclassifiable, with features of DLBCL and BL, and 1 case as HHV-8+/EBV+ PEL. One DLBCL case (ABC type) was EBV+. Ki-67 expression was 80% in 10 (91%) of 11 cases analyzed. Nine (75%) patients had advanced stage lymphoma (III-IV by Ann Arbor criteria), 9 (75%) had high baseline lactate dehydrogenase, 8 (67%) had high IPI risk, and 2 (17%) had an ECOG PS of 2.
Cycle I DLTs
One patient treated with R-EPOCH at VOR dose level 1 (300 mg) withdrew from the study early during cycle 1 of treatment, and was not evaluable. One of 6 evaluable patients treated with R-EPOCH at VOR 300-mg dose level experienced a DLT during cycle 1 with grade 4 sepsis, arising from a tooth abscess; all 6 patients completed therapy. Two of 6 patients treated at the VOR 400-mg dose level had DLTs during cycle 1; both had grade 4 thrombocytopenia with concurrent grade 4 neutropenia, and one of them had bone marrow involvement. Grade 4 thrombocytopenia did not occur at the 300-mg level. One patient with bone marrow involvement treated at the 300-mg level did not experience significant neutropenia either. Grade 4 neutropenia also occurred in a patient without bone marrow involvement at 300 mg after cycle 1, and in another patient with limited stage disease at the 400-mg level. The RPTD was 300 mg. At the VOR 400-mg dose, 5 patients completed treatment; 1 patient died of unrelated cause (illicit drug overdose) before completing therapy. Other AEs for all cycles are described in Table 4.
Table 4.
Treatment-related Adverse Events at the Patient Level
| Dose Level Toxicities/Grade | All Cycles | |||
|---|---|---|---|---|
| VOR(R)-EPOCH (300 mg) (n = 6) | VOR(R)-EPOCH (400 mg) (n = 6) | |||
| Grade ≤2 | Grade ≥3 | Grade ≤2 | Grade ≥3 | |
| Hematologic | ||||
| Anemia | 2 | 1 | 1 | 3 |
| Febrile neutropenia | n/a | 2 | n/a | 2 |
| Lymphopenia | 0 | 1 | 1 | 2 |
| Neutropenia | 1 | 3 | 0 | 4 |
| Thrombocytopenia | 2 | 2 | 1 | 3 |
| Non-hematologic | ||||
| Alopecia | 0 | 0 | 0 | 0 |
| Bone pain | 0 | 0 | 0 | 0 |
| Cardiac | 1 | 1 | 0 | 1 |
| Death-NOS | n/a | 1 | n/a | 0 |
| Dermatologic | 3 | 0 | 2 | 0 |
| Dyspnea | 0 | 0 | 0 | 0 |
| Eye disorders | 1 | 0 | 0 | 0 |
| Fatigue | 0 | 0 | 0 | 0 |
| Gastrointestinal | ||||
| Abdominal pain | 1 | 1 | 1 | 0 |
| Anal mucositis | 0 | 0 | 1 | 0 |
| Colitis | 0 | 1 | 0 | 0 |
| Constipation | 3 | 0 | 2 | 0 |
| Diarrhea | 1 | 2 | 1 | 0 |
| Dyspepsia | 1 | 0 | 0 | 0 |
| Esophagitis | 1 | 0 | 1 | 0 |
| Intra-abdominal hemorrhage | 0 | 1 | 0 | 0 |
| Muscositis oral | 1 | 0 | 2 | 0 |
| Nausea | 5 | 0 | 1 | 0 |
| Vomiting | 1 | 1 | 1 | 0 |
| Generalized symptoms | 4 | 0 | 5 | 1 |
| Hepatic | 1 | 1 | 1 | 1 |
| Infection | 2 | 4 | 2 | 0 |
| Infusion reaction | 1 | 0 | 0 | 0 |
| Metabolic | 4 | 1 | 5 | 1 |
| Musculoskeletal | 4 | 0 | 1 | 0 |
| Neurologic | 5 | 0 | 3 | 0 |
| Oligospermia | 0 | 0 | 0 | 0 |
| Paresthesia | 0 | 0 | 0 | 0 |
| Procedural complication | 0 | 1 | 0 | 0 |
| Psychiatric | 1 | 0 | 1 | 0 |
| Respiratory, thoracic, and mediastinal | 4 | 0 | 0 | 0 |
| Vascular | 3 | 0 | 0 | 0 |
Abbreviations: NOS = not otherwise specified; R = rituximab; VOR = vorinostat.
Treatment Efficacy
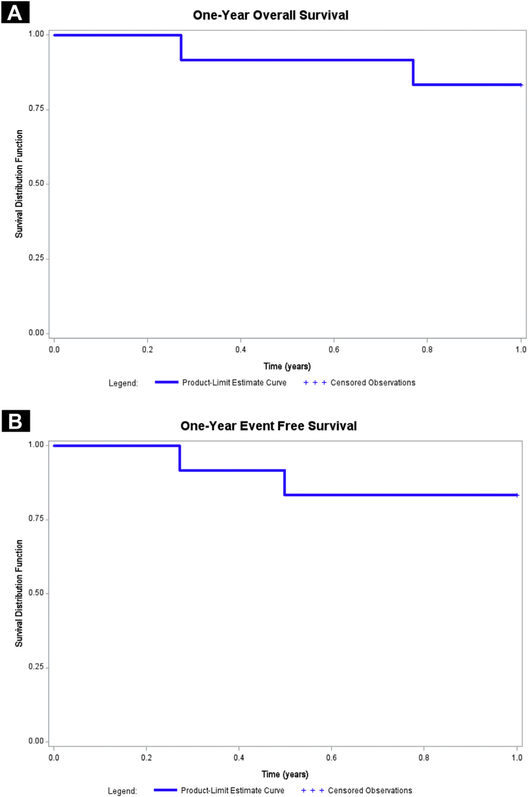
The overall response rate in 12 evaluable patients with high-risk NHL treated with VOR(R)-EPOCH was 100% (CR + PR rates of 83% and 17%, respectively). The median follow-up time for the survivors was 228 weeks (range, 34–278 weeks). To date, 3 patients have died: one of illicit intravenous drug use after cycle 4 after achieving a PR, and 2 CR patients after lymphoma relapse at months 8 and 23 after starting treatment. The 1-year overall survival (OS) and event-free survival (EFS) were both 83% (95% confidence interval [CI], 51.6%–97.9%) (Figure 2).
Figure 2.
Survival for the Study Population. A, One-year Event-free Survival for the Entire Study Population (n = 12). B, One-year Overall Survival for the Entire Study Population (n = 12)
Treatment Effect on CD4+ Cell Count and HIV Viral Load
Absolute CD4+ cell counts did not statistically differ from baseline to end of cycle 2, 1 month, 6 months, and 12 months posttreatment after VOR-chemotherapy (Table 5). During or after VOR-R-EPOCH, the HIV VL became undetectable in 4 patients, remained undetectable in 2 patients, and decreased initially in 1 patient before exacerbating after cycle 6 (300 mg VOR level). Transient “blips” in HIV viremia, characterized by detectable levels, occurred once either during VOR-chemotherapy or post-treatment in 5 patients: 3 patients after 2 cycles, and 2 patients 1-month posttreatment.
Table 5.
Absolute CD4 Counts and HIV Viral Loads From Collected Samples of Study Patients at Baseline, During VOR Chemotherapy, and After Completion of Treatment
| Patient ID/Laboratory Test | Current ART | VOR Dose Level, mg | Baseline | After Cycle 2 | Posttreatment | ||
|---|---|---|---|---|---|---|---|
| 1 mo | 6 mo | 12 mo | |||||
| 075–061-001 | RTV, DRV, FTC, TDF | 300 | |||||
| Absolute CD4 count, cells/μl | 178 | 321 | 234 | 359 | – | ||
| HIV VL, copies/ml | 282,977 | 92 | 54 | UD (<48) | – | ||
| 075–041-002 | EFV, FTC, TDF | 300 | |||||
| Absolute CD4 count, cells/μl | 426 | 384 | 394 | – | – | ||
| HIV VL, copies/ml | UD (<20) | UD (<20) | UD (<20) | – | – | ||
| 075–061-003 | Started after cycle 1 | 300 | |||||
| Absolute CD4 count, cells/μl | 258 | 458 | 212 | 334 | – | ||
| HIV VL, copies/ml | 55,116 | 764 | UD (<48) | UD (<48) | – | ||
| 075–031-013 | FTC, ETR, TDF | 300 | |||||
| Absolute CD4 count, cells/μl | 91 | 100 | 38 | – | – | ||
| HIV VL, copies/ml | 22,400 | 322 | 235,000 | – | – | ||
| 075–150-014 | FTC, NVP, TDF | 300 | |||||
| Absolute CD4 count, cells/μl | 754 | 810 | 445 | – | – | ||
| HIV VL, copies/ml | 9034 | 2076 | UD (<40) | – | – | ||
| 075–120-016 | 3TC, EFV, FTC, TDF | 300 | |||||
| Absolute CD4 count, cells/μl | 279 | 407 | 338 | 354 | 448 | ||
| HIV VL, copies/ml | UD (<20) | 30 | UD (<20) | UD (<75) | UD (<20) | ||
| 075–041-004 | FTC, ETR, RAL | 400 | |||||
| Absolute CD4 count, cells/μl | 134 | 183 | 227 | 341 | 284 | ||
| HIV VL, copies/ml | UD (<48) | 46 | UD (<20) | UD (<20) | UD (<20) | ||
| 075–080-006 | ABC, 3TC, NVP | 400 | |||||
| Absolute CD4 count, cells/μl | 269 | 282 | 237 | 314 | 373 | ||
| HIV VL, copies/ml | UD (<20) | UD (<20) | 549 | UD (<20) | 548 | ||
| 075–060-007 | Started after cycle 1 | 400 | |||||
| Absolute CD4 count, cells/μl | 186 | 125 | 137 | 157 | 150 | ||
| HIV VL, copies/ml | 482,000 | 1000 | UD (<48) | UD (<48) | UD (<20) | ||
| 075–152-009 | EFV, FTC, TDF | 400 | |||||
| Absolute CD4 count, cells/μl | 485 | 412 | 218 | 357 | 350 | ||
| HIV VL, copies/ml | UD (<40) | UD (<40) | 49 | UD (<40) | UD (<40) | ||
| 075–080-011 | RTV, DRV, RAL | 400 | |||||
| Absolute CD4 count, cells/μl | 96 | 64 | 136 | 126 | 126 | ||
| HIV VL, copies/ml | 88 | 22 | 23 | 2419 | 4377 | ||
| 075–041-012 | RTV, MVC, RAL | 400 | |||||
| Absolute CD4 count, cells/μl | 109 | 221 | – | – | – | ||
| HIV VL, copies/ml | 25 | UD (<20) | – | – | – | ||
Abbreviations: ABC = abacavir; ART = antiretroviral therapy; DRV = darunavir; EFV = efavirenz; ETR = etravirine; FTC = emtricitabine; LPV = lopinavir; MVC = maraviroc; NPV = nevirapine; RAL = raltegravir; RTV = ritonavir; 3TC = lamivudine; TDF = tenofovir; UD = undetectable; VL = viral load; VOR = vorinostat.
PKs
PK data were available on 11 patients treated with VOR(R)-EPOCH. Doxorubicin (P = .93 for VOR; P = .55 for cART), etoposide (P = .65 for VOR; P = .89 for cART), and vincristine (P = .52 for VOR; P = .76 for cART) clearance were similar regardless of the dose of VOR or cART regimen (see Supplemental Table 3 in the online version). In addition, doxorubicin, etoposide, and vincristine clearances were not associated with any AEs (P > .05).
Discussion
Although the clinical outcome of patients with HIV-NHL has improved since the advent of cART, management remains challenging in those with high IPI score, ABC type DLBCL, “double hit” DLBCL, PEL, and PBL. The phase I component of this phase I to II trial was designed to determine the appropriate dose of VOR to combine with R-EPOCH for a randomized phase II study of R-EPOCH with or without VOR in HIV-associated B-cell lymphoma (available at http://clinicaltrials.gov as ). In the phase I study, VOR with R-EPOCH was generally well-tolerated and safe at the RPTD. The main reason for using VOR for only 5 days was to ensure safety in this vulnerable and previously untested HIV population. The 5-day exposure was based on existing toxicity data at the time from ongoing clinical studies combining VOR with chemotherapy at higher doses or given for a longer period.17,31 One of these trials was a phase I/II study testing VOR + combination chemotherapy (cyclophosphamide, etoposide, prednisone, and rituximab) in elderly patients with refractory DLBCL.17 In this trial, VOR dose escalation began at 300 mg and increased up to 400 mg daily for 10 days during each 28-day chemotherapy cycle. The MTD for VOR was 300 mg.17 In another phase I/II trial, VOR 400 mg daily on days 1 to 9 combined with R-CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone). for DLBCL (SWOG S0806 trial) resulted in excess rates of febrile neutropenia and sepsis, leading to a reduction in the duration of VOR to days 1 to 5 for the remainder phase II study.31 In AMC-075, we elected to combine VOR on days 1 to 5 with EPOCH (a 5-day regimen) because VOR was known to synergize with rituximab, anthracyclines, and etoposide.14–16 In addition, because in AMC-034 the rate of grade 3 or 4 neutropenia was 43% in patients who received R-EPOCH, with febrile neutropenia occurring in 16%, we did not want to compromise a potentially curative therapy using excessive doses of VOR. In this small study, neither VOR nor cART regimen significantly altered chemotherapy steady-state concentrations. The AMC-075 phase II study recently closed to accruals, and the results will be reported after completion of minimum time for patient follow-up and data analysis.
The preliminary results from AMC-075 phase I trial suggest promising antitumor activity in this high-risk population with HIV and aggressive B-cell NHL subtypes. The CR rate in 12 patients was 83% with 1-year EFS of 83% (95% CI, 51.6%−97.9%). In our previous trial (AMC 034), the 1-year progression-free survival (PFS) in the concurrent R-EPOCH arm was 78% (95% CI, 67%−90%), which compared favorably over PFS observed with R-CHOP (48%; 95% CI, 32%−64%) in the AMC 010 trial, suggesting that infusional EPOCH contributed to a better outcome.3,32 A phase II study performed at the NCI using short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) for newly diagnosed HIV-DLBCL with suspension of cART during therapy demonstrated 5-year PFS and OS of 84% and 68%, respectively, but outcomes were markedly inferior in the non-GCB subtype with 5-year PFS of 44%.33 The AMC-075 phase I study reported here included 12 patients with high-risk NHL features: 5 patients with ABC-type DLBCL, 5 patients with GCB-type who had high age-adjusted IPI scores or tumors with a high Ki-67 proliferative index (≥80%), 1 patient with unclassifiable features between DLBCL and BL, and 1 patient with extracavitary PEL variant. The high CR and 1-year EFS rates are encouraging in this setting. In SWOG S0806, which combined VOR with R-CHOP for DLBCL in the non-HIV setting, the OR rate with was 81% (95% CI, 69%−90%) with an estimated 2-year PFS of 72% (95% CI, 58%–81%), which was slightly more than 68% expected from R-CHOP alone per IPI adjusted historical rate, but less than an IPI-adjusted target of 78%; consequently, the investigators concluded that such results were not sufficient to warrant further investigation of VOR plus R-CHOP in DLBCL.31
We studied VOR in combination with standard chemotherapy in order to augment the anti-tumor effects of chemotherapy, and to target latently infected HIV-harboring reservoirs and γ-herpesviruses (EBV and HHV-8) in positive tumors, which occur at a significantly higher frequency in the setting of HIV infection as compared with the general population.34 HDAC inhibitors alter chromatin state via acetylation of lysine residues in histones, thus facilitating the expression of silenced cellular and viral genes. Acetylation of BCL6, which is a proto-oncogene frequently over-expressed in DLBCL, inhibits its function. In the Eμ-myc B-cell lymphoma mouse model, VOR selectively killed Eμ-myc lymphoma cells mediated by pro-apoptotic proteins Bid and Bim.35 In our study, 2 (17%) patients had γ–herpesvirus-associated NHL; 1 patient had EBV+ DLBCL, and 1 had EBV+ HHV-8+ solid PEL variant. The patient with PEL had a sustained CR. The patient with EBV+ ABC-type DLBCL had a PR after 4 cycles, but was lost to follow-up. Although the number of patients in this study was too small to draw any conclusions about the effectiveness of VOR-containing regimen over standard chemotherapy in γ–herpesvirus-associated NHLs, our recently completed randomized phase 2 study (VOR[R]-EPOCH vs. R-EPOCH alone) included a total of 90 patients with DLBCL and other γ–herpesvirus-associated lymphomas, including PBL and PEL, and may provide further insights in the near future.
VOR was a logical drug choice for HIV-NHL as it induces HIV transcription in latently infected CD4+ cells.19–21 The term “shock and kill” refers to activating viral transcription leading to viral cytopathic effects and immune-mediated cell death.36 In our study, reduction of HIV VL occurred during treatment in several patients already on cART who had viremia at baseline, but there was no negative overall impact on CD4+ cell counts. Subjects who had HIV VL below detection at baseline generally continued to have suppressed virus, but transient “blips” were observed in some patients. These finding were surprising because the opposite effect (early increase in viremia) was expected with VOR-chemotherapy. In recent clinical studies, VOR disrupted HIV provirus latency in vivo, and induced a transient increase in cell-associated HIV RNA in several patients receiving suppressive cART, followed by reduction during the first few days of treatment.37,38 In our study, it is possible that a transient increase of HIV viremia may have occurred early in patients; however, the first VL after starting treatment was measured after 2 chemotherapy cycles (> 26 days later). Alternatively, our findings suggest either a possible shutdown of HIV transcription early after VOR-chemotherapy, or reduction of HIV harboring reservoirs from cytotoxic effects induced by VOR-chemotherapy, although mathematical models so far suggest VOR treatment does not induce latently infected cell killing.39 Preliminarily, analysis of latent HIV reservoirs before and after completion of treatment using quantitative viral outgrowth assay in 1 patient revealed no significant impact of VOR-chemotherapy (personal communication); additional latent HIV reservoirs studies are underway in patients of the phase II study.
In summary, combining VOR with R-EPOCH and cART is safe in aggressive HIV-associated NHLs. The impact of VOR given concomitantly with R plus cytotoxic chemotherapy on treatment efficacy, HIV VL, and infected reservoirs will be more definitively addressed in our recently enrolled randomized phase II trial. Future directions include the addition of newer targeted agents in specific NHL subsets.
Clinical Practice Points
Our group previously investigated infusional EPOCH in combination with concurrent or sequential R for aggressive B-cell HIV-NHL (AMC-034), demonstrating a 78% 1-year PFS with concurrent R-EPOCH, which compared favorably over R-CHOP (48% 1-year PFS) in AMC-010.
Another NCI phase II study using short-course EPOCH with dose-dense R for newly diagnosed HIV-DLBCL demonstrated a 5-year PFS of 84%, but only 48% in non-GCB DLBCL.
Therefore, treatment of HIV-NHL remains challenging in non-GCB DLBCL, and other aggressive and often viral-associated NHL variants.
We performed a phase I trial (AMC-075) of VOR, a histone deacetylase inhibitor that enhances anti-tumor chemotherapy and R effects, induces viral lytic expression in EBV+ or HHV-8+ tumors, and reactivates latent HIV for possible eradication of infected cell reservoirs, in combination with R-EPOCH on days 1–5 and cART in aggressive HIV-NHL.
Twelve patients (5 ABC-DLBCL [1 EBV+], 5 GCB-DLBCL with high age-adjusted IPI scores or Ki-67 ≥80%, 1 unclassifiable with features between DLBCL and Burkitt lymphoma, and 1 EBV+/HHV-8+ primary effusion lymphoma) were evaluated.
VOR was tolerable and safe at 300 mg with CR rate and 1-yearPFS of 83%, and did not significantly alter chemotherapy steady-state concentrations, CD4+ cell counts, or HIV viral loads.
The clinical impact of adding VOR to R-chemotherapy for the treatment of HIV-NHL will be more definitively addressed in our recently enrolled randomized phase II trial.
Supplementary Material
Acknowledgments
This study received financial support from the following: AIDS Malignancy Consortium grant: UM1 CA121947; Center for AIDS Research grants to University of and Miami: P30AI07396, and UCLA: P30AI028697; Support for drug analysis was supported by National Institutes of Health grants P30CA006973 and UL1TR001079 and the Shared Instrument Grant S10RR026824 to the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center.
Disclosure
All authors are supported by NCI-sponsored AIDS Malignancy Consortium. Erin Reid has received research support from Genentech. The remaining authors have stated that they have no conflicts of interest.
Footnotes
Supplemental Data
Supplemental tables accompanying this article can be found in the online version at https://doi.org/10.1016/j.clml.2018.01.004.
References
- 1.Yanik EL, Katki HA, Engels EA. Cancer risk among the HIV-infected elderly in the United States. AIDS 2016; 30:1663–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barta SK, Samuel MS, Xue X, et al. Changes in the influence of lymphoma- and HIV-specific factors on outcomes in AIDS-related non-Hodgkin lymphoma. Ann Oncol 2015; 26:958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barta SK, Xue X, Wang D, et al. Treatment factors affecting outcomes in HIV-associated non-Hodgkin lymphomas: a pooled analysis of 1546 patients. Blood 2013; 122:3251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castillo JJ, Furman M, Beltrán BE, et al. Human immunodeficiency virus-associated plasmablastic lymphoma: poor prognosis in the era of highly active antiretroviral therapy. Cancer 2012; 118:5270–7. [DOI] [PubMed] [Google Scholar]
- 5.Bayraktar UD, Ramos JC, Petrich A, et al. Outcome of patients with relapsed/refractory acquired immune deficiency syndrome-related lymphoma diagnosed 1999–2008 and treated with curative intent in the AIDS Malignancy Consortium. Leuk Lymphoma 2012; 53:2383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster WR, Bischin A, Dorer R, et al. Human herpesvirus type 8-associated large B-cell lymphoma: a nonserous extracavitary variant of primary effusion lymphoma in an HIV-infected man: a case report and review of the literature. Clin Lymphoma Myeloma Leuk 2016; 16:311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunleavy K, Wilson WH. Role of molecular subtype in predicting outcome of AIDS-related diffuse large B-cell lymphoma. J Clin Oncol 2010; 28:e260. [DOI] [PubMed] [Google Scholar]
- 8.Tulpule A, Sherrod A, Dharmapala D, et al. Multidrug resistance (MDR-1) expression in AIDS-related lymphomas. Leuk Res 2002; 26:121–7. [DOI] [PubMed] [Google Scholar]
- 9.Little RF, Pittaluga S, Grant N, et al. Highly effective treatment of acquired immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH: impact of antiretroviral therapy suspension and tumor biology. Blood 2003; 101:4653–9. [DOI] [PubMed] [Google Scholar]
- 10.Moore SM, Cannon JS, Tanhehco YC, et al. Induction of Epstein-Barr virus kinases to sensitize tumor cells to nucleoside analogues. Antimicrob Agents Chemother 2001; 45:2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klass CM, Krug LT, Pozharskaya VP, et al. The targeting of primary effusion lymphoma cells for apoptosis by inducing lytic replication of human herpesvirus 8 while blocking virus production. Blood 2005; 105:4028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo JS, Cho NY, Kim HR, et al. Cell cycle arrest and lytic induction of EBV-transformed B lymphoblastoid cells by a histone deacetylase inhibitor, Trichostatin A. Oncol Rep 2008; 19:93–8. [PubMed] [Google Scholar]
- 13.Lima RT, Seca H, Bras S, et al. Treatment of Akata EBV-positive cells with doxorubicin causes more EBV reactivation than treatment with etoposide. Chemotherapy 2011; 57:195–203. [DOI] [PubMed] [Google Scholar]
- 14.Nolan L, Crabb S, Beers S, et al. Synergistic cell death elicited with CD20 monoclonal antibodies and vorinostat. Cancer Res 2009. (abstract 3241). [Google Scholar]
- 15.Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, et al. Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood 2006; 108:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shiozawa K, Nakanishi T, Tan M, et al. Preclinical studies of vorinostat (suberoylanilide hydroxamic acid) combined with cytosine arabinoside and etoposide for treatment of acute leukemias. Clin Cancer Res 2009; 15:1698–707. [DOI] [PubMed] [Google Scholar]
- 17.Straus DJ, Hamlin PA, Matasar MJ, et al. Phase I/II trial of vorinostat with rituximab, cyclophosphamide, etoposide and prednisone as palliative treatment for elderly patients with relapsed or refractory diffuse large B-cell lymphoma not eligible for autologous stem cell transplantation. Br J Haematol 2015; 168:663–70. [DOI] [PubMed] [Google Scholar]
- 18.Bhatt S, Ashlock BM, Toomey NL, et al. Efficacious proteasome/HDAC inhibitor combination therapy for primary effusion lymphoma. J Clin Invest 2013; 123: 2616–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Archin NM, Espeseth A, Parker D, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses 2009; 25:207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contreras X, Schweneker M, Chen CS, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem 2009; 284:6782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses 2009; 25:883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007; 25:579–86. [DOI] [PubMed] [Google Scholar]
- 23.Chadburn A, Chiu A, Lee JY, et al. Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma and clinical implications in patients from AIDS Malignancies Consortium Clinical Trials 010 and 034. J Clin Oncol 2009; 27:5039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004; 103:275–82. [DOI] [PubMed] [Google Scholar]
- 25.Stearns V, Mori T, Jacobs LK, et al. Preclinical and clinical evaluation of intraductally administered agents in early breast cancer. Sci Transl Med 2011; 3:106ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennison JB, Renbarger JL, Walterhouse DO, et al. Quantification of vincristine and its major metabolite in human plasma by high-performance liquid chromatography/tandem mass spectrometry. Ther Drug Monit 2008; 30:357–64. [DOI] [PubMed] [Google Scholar]
- 27.Kornblau SM, Estey E, Madden T, et al. Phase I study of mitoxantrone plus etoposide with multidrug blockade by SDZ PSC-833 in relapsed or refractory acute myelogenous leukemia. J Clin Oncol 1997; 15:1796–802. [DOI] [PubMed] [Google Scholar]
- 28.Thorn CF, Oshiro C, Marsh S, et al. Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 2011; 21:440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, Bogni A, Schuetz EG, et al. Etoposide pathway. Pharmacogenet Genomics 2009; 19:552–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whirl-Carrillo M, McDonagh EM, Hebert JM, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 2012; 92:414–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persky DO, Li H, Rimsza LM, et al. A phase I/II trial of vorinostat (SAHA) in combination with rituximab-CHOP in patients with newly diagnosed advanced stage diffuse large B-cell lymphoma (DLBCL): SWOG S0806. Blood 2015; 126:3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sparano JA, Lee JY, Kaplan LD, et al. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood 2010; 115:3008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunleavy K, Little RF, Pittaluga S, et al. The role of tumor histogenesis, FDG-PET, and short-course EPOCH with dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large B-cell lymphoma. Blood 2010; 115:3017–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carbone A, Cesarman E, Spina M, Gloghini A, Schulz TF. HIV-associated lymphomas and gamma-herpesviruses. Blood 2009; 113:1213–24. [DOI] [PubMed] [Google Scholar]
- 35.Lindemann RK, Newbold A, Whitecross KF, et al. Analysis of the apoptotic and therapeutic activities of histone deacetylase inhibitors by using a mouse model of B cell lymphoma. Proc Natl Acad Sci U S A 2007; 104:8071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deeks SG. HIV: shock and kill. Nature 2012; 487:439–40. [DOI] [PubMed] [Google Scholar]
- 37.Archin NM, Liberty AL, Kashuba AD, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012; 487:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott JH, Wightman F, Solomon A, et al. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 2014; 10:e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke R, Lewin SR, Elliott JH, Perelson AS. Modeling the effects of vorinostat in vivo reveals both transient and delayed HIV transcriptional activation and minimal killing of latently infected cells. PLoS Pathog 2015; 11:e1005237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.