Abstract
Purpose
In view of mounting attention related to possible brain retention of gadolinium-based contrast agents (GBCAs) in patients with normal renal function, our purpose was to detail results from a survey of pituitary experts to assess: 1) the timing interval and frequency of pituitary magnetic resonance imaging (MRI) following surgical and/or medical and/or radiation therapy of pituitary tumors, 2) awareness of the types of GBCAs used and their possible safety issues.
Methods
The Pituitary Society Education Committee composed a survey with 12 multiple choice questions, 8 of which specifically addressed the time interval and frequency of MRI in the longitudinal management of pituitary tumors. The survey was distributed at two meetings; the International Pituitary Neurosurgeons Society conference in San Diego, CA, on February 18th, 2018, and the Pituitary Society Membership and Career Development Forum, Chicago, IL on March 18th, 2018.
Results
There is consensus among pituitary endocrinologists and neurosurgeons that long-term repeated imaging is recommended in most pituitary tumors, although the precise strategy of timing varied depending on the specialist group and the specific clinical context of the adenoma. The data also suggest that International Pituitary Neurosurgeons Society neurosurgeons, as well as Pituitary Society neuroendocrinologists, are sometimes unaware of which contrast agents are used by their institution, and many are also unaware that evidence of long-term brain retention has been reported with the use of GBCAs in patients with normal function.
Conclusions
International pituitary endocrinologists and pituitary neurosurgeons experts suggest ongoing MRIs for the management of pituitary tumors; strategies vary based on clinical context, but also on individual experience and practice.
Keywords: Contrast, Gadolinium, Safety, Pituitary, Tumor, Imaging, Adenoma, Neuroendocrinologists, Neurosurgeons, Survey
Background
It has been well established that gadolinium-based contrast agents (GBCAs) may cause nephrogenic systemic fibrosis in patients with renal dysfunction [1]. However, recent studies also reveal that GBCAs administration results in GBCAs brain retention in patients with normal renal function and furthermore even in patients with absent intracranial abnormalities [2]. This information has introduced potential concerns among patients, regulatory agencies, as well as the healthcare, scientific and radiology communities [3].
It is recognized that GBCAs are retained in minute amounts throughout the brain parenchyma, but whether or not such deposition causes harm remains unknown [3–5]. A NIH multidisciplinary conference produced a roadmap to investigate if tissues are affected by GBCAs deposition, if short or long-term clinical manifestations result from GBCAs exposure, and if fetuses and children could be more susceptible [6]. Since long-term serial brain imaging is usually requested by pituitary-focused neurosurgeons and endocrinologists, it is important for this group to assess the relative risk and benefit of using GBCAs in the imaging of pituitary tumors. While more data are required before fully excluding or confirming potential risk, surveys from endocrinologists and neurosurgeons who focus on pituitary disorders shed light on current suggested patterns of pituitary magnetic resonance imaging (MRI) timing and the prevalence of awareness of GBCAs brain retention among pituitary experts. Understanding what imaging strategies are utilized now is a first step toward a possible reappraisal of the approach if and when clinical safety concerns related to GBCAs retention are confirmed.
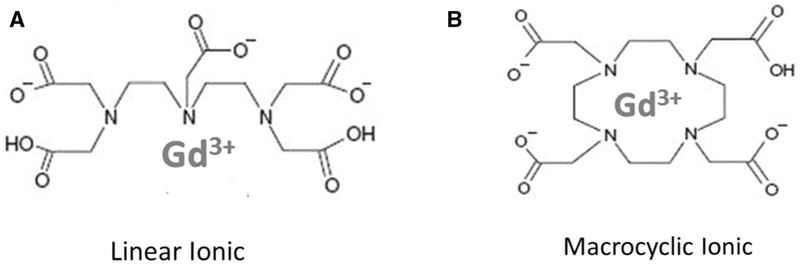
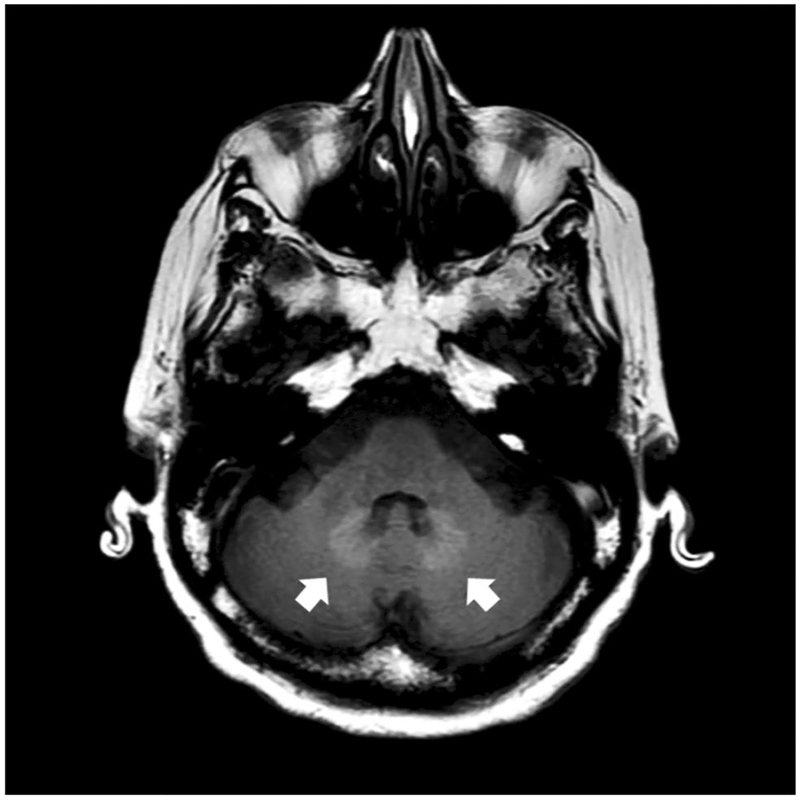
Gadolinium is a rare element from the lanthanide series, which due to its paramagnetic properties, is used for contrast enhancement in MRI. In its free form, gadolinium is toxic, and it competes with calcium in various physiological processes; for this reason, it must be bound to a ligand to form a stable complex. Gadolinium-based contrast agents consist of a central paramagnetic gadolinium ion chelated to a ligand (Table 1) and fall into two classes, depending on their chemical structure: linear, with an open chain, and macrocyclic, with a closed structure (Fig. 1). The bulk of data associating linear agents with brain retention relies on studies reporting a positive correlation between exposure and signal intensity in the dentate nucleus and globus pallidus [7], (Fig. 2). Furthermore, in a study of five deceased patients, each of whom had at least four lifetime gadolinium exposures (using linear agents), vs. ten controls who had received non-contrast MRIs, gadolinium deposition was detected by electron microscopy, which showed cellular localization within the dentate nucleus of the brain. In this study, changes in gadolinium ion signal intensity detected with mass spectrometry were associated with cumulative intravenous gadolinium exposure within the areas of the globus pallidus and dentate of cadaveric tissue [2].
Table 1.
Structure of gadolinium-based contrast agents
| Generic name | Trade name | Structure | Type |
|---|---|---|---|
| Gadolinium-based contrast agents | |||
| Gadopentetate dimeglumine | Magnevist | Linear | Ionic |
| Gadodiamide | Omniscan | Linear | Nonionic |
| Gadoversetamide | Optimark | Linear | Nonionic |
| Gadobenate dimeglumine | MultiHance | Linear | Ionic |
| Gadoxetate disodium | Eovist/Primovist | Linea | Ionic |
| Gadofosveset trisodium | Ablavar/Vasovist | Linear | Ionic |
| Gadoterate meglumine | Dotarem | Macrocyclic | Ionic |
| Gadobutrol | Gadavist | Macrocyclic | Nonionic |
| Gadoteridol | ProHance | Macrocyclic | Nonionic |
Fig. 1.
Gadolinium-based contrast agents (GBCAs) consist of a central paramagnetic Gadolinium (Gd 3+) chelated to a ligand, preventing direct toxicity from free Gd3+. The biochemical structure of the types of ligands are illustrated: a Linear agents, with an elongated organic molecular ligand wrapping around the ion and b Macrocyclic agents, which form a cage-like ligand structure with the ion trapped in the central cavity
Fig. 2.
Axial brain MRI showing T1 signal hyperintensity associated with gadolinium deposition as indicated by arrows pointing to dentate nucleus gadolinium accumulation in a patient who had received a linear GBCA
While it has been mostly linear GBCAs that have been associated with gadolinium deposits in postmortem evaluation of human brains, such an effect has also been reported, albeit less commonly, in association with exposure to macrocyclic agents [4]. Macrocyclic agents are thought to be more stable, and there is less data on long-term retention in the brain [7, 8]. Chemical structural differences account for differences in kinetic and thermodynamic differences in stability. The non-ionic linear chelates are the more unstable and the ionic macrocyclic chelates are the most stable [7] (Table 1; Fig. 1).
The European Medicine Agency (EMA) suspended marketing of four linear chelate agents. As yet, there is no restricted use of macrocyclic agents. The US Food and Drug Administration (FDA) published a safety update regarding evaluation of the risk of gadolinium accumulation associated with repeated administration of contrast agents. They concluded that despite retention of these agents, no adverse health effects had been identified [9]. An association between gadolinium accumulation in the brain and clinically significant pathology has not yet been demonstrated, and, therefore, remains unknown. However, the finding of long-term retention of GBCAs in the brain, despite the lack of evidence of a clinical risk associated with it, raises questions that may have an impact on the frequency with which contrast-based MRI are recommended in the field of neuroendocrinology. While an international survey of radiologists reported a practice change in 24 of 87 (28%) of respondents [10], surveys addressing possible concerns about GBCAs brain retention among pituitary experts have not previously been reported.
Purpose
In view of recent safety concerns, as described above, and mounting attention related to risks of gadolinium use, the goal of this manuscript is to detail results from a survey that was completed by pituitary experts (neuroendocrinologists and neurosurgeons) to assess the timing interval and frequency of pituitary MRI following surgical and/or medical and/or radiation therapy of pituitary tumors. A secondary goal was to assess awareness of gadolinium potential safety issues among pituitary experts involved in the care of patients with pituitary tumors.
Methods
Members of the Pituitary Society Education Committee composed a questionnaire with 12 multiple choice questions, 8 of which specifically addressed the time interval and frequency of MRI under the following conditions; (1) first post-operative scan after uncomplicated pituitary surgery, (2) initial post-operative follow-up timing after pituitary surgery for a nonfunctioning macroadenoma, (3) timing of long-term post-surgical follow-up for a nonfunctioning macroadenoma with no recurrence, (4) timing of long-term post-surgical follow-up for functioning macroadenomas not achieving biochemical control, (5) follow-up for macroadenomas with large residual tumor, (6) follow-up for functioning microadenomas that are well controlled biochemically, (7) follow-up for functioning microadenomas that are uncontrolled biochemically, and (8) following radiation therapy (See Online Appendix Questionnaire). For each of these 8 questions, there were 4 multiple choice answers and a write in comment option for “other”.
The questionnaire was distributed at the Pituitary Society Membership and Career Development Forum, Chicago, IL on March 18th, 2018 to all pituitary experts (n = 97 (39 women); from 16 countries including Argentina (n = 2), Australia (n = 2), Brazil (n = 6), Canada (n = 1), Colombia (n = 1), Germany (n = 1), Israel (n = 1), Italy (n = 4), Japan (n = 6), Netherlands, (n = 4) Poland (n = 1), Spain (n = 1), Sweden (n = 1), United Arab Emirates (n = 1), United Kingdom (n = 5), Uruguay (n = 1) and the remaining 60 were from the USA) who attended the meeting and the response rate was 45% (n = 44). One of the 44 respondents was a neurosurgeon and the other 43 were endocrinologists, most of whom had a focus or interest in pituitary disease and were affiliated with academic medical centers.
The same questionnaire was distributed (n = 54) at the International Pituitary Neurosurgeons Society conference in San Diego, CA, on February 18th, 2018, just 1 month prior and the response rate was 26% (n = 14). Of the 54 neurosurgeons, 36 came from US and 18 from outside of the US, including 4 from Japan, 3 from Canada, and the remainder from Europe and South America. None of the respondents attended both meetings (Table 2).
Table 2.
Questionnaire distribution and response rate
| Meeting | Pituitary Society Membership and Career Development Forum |
International Pituitary Neurosurgeons Society |
|---|---|---|
| Questionnaire distribution date | March 18, 2018 | February 18–20, 2018 |
| Distributed to (n) | 97 | 54 |
| Responding endocrinologists (n) | 44 | 0 |
| Responding neurosurgeons (n) | 1 | 14 |
| Response rate (n;%) | 44 (45%) | 14 (26%) |
| Combined response rate (n;%) | 58/151 (38%) |
Analysis
Responses were analyzed to determine areas of consensus and controversy among endocrinologists and neurosurgeons surveyed, and for endocrinologists and neurosurgeons combined. Consensus was defined if > 50% of the respondents in either single specialty group or the combined group agreed upon a single answer. The percentage of neurosurgeons and endocrinologists aware of gadolinium concerns were also calculated.
Results
Initial post-operative follow-up
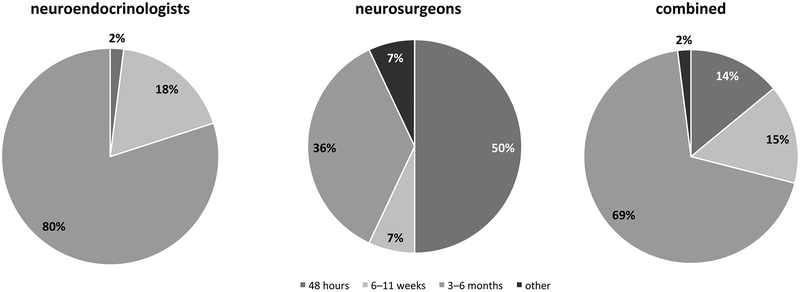
For initial follow-up imaging after uncomplicated pituitary surgery, there was consensus among the Pituitary Society attendees, with 80% (35/44) obtaining a first MRI at 3–6 months and only 1 individual who suggested an MRI within the first 48 h. There was less consensus among International Pituitary Neurosurgeons Society attendees, with 50% (7/14) requesting an MRI within the first 24 h, and 36% (5/14) within 3–6 months. Combining respondents, from both meetings, the majority, 69% (40/58), selected 3–6 months for an initial MRI after pituitary surgery (Fig. 3).
Fig. 3.
When do you recommend the first pituitary MRI to be performed in the postoperative period after uncomplicated transsphenoidal surgery (TSS)?
Surveillance of non-functioning macroadenomas
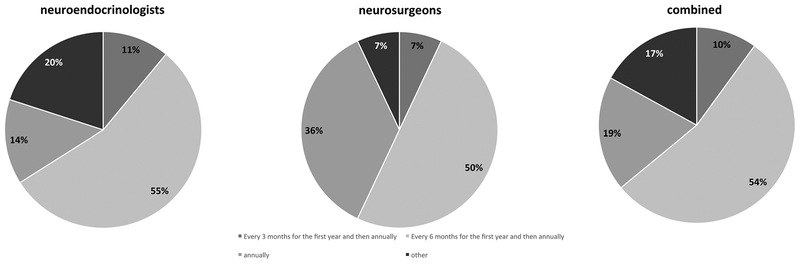
Regarding the timing to follow-up for a non-functioning macroadenoma, most Pituitary Society attendants agreed with annual imaging for at least 2–5 years. There was, however, some controversy regarding MRI frequency within the first year, with 55% (24/44) suggesting every 6 months, 11% (5/44) every 3 months and 14% (6/14) just once at one year (Fig. 2). The neurosurgeons had a similar spread on this issue with 50% (7/44) answering every 6 months, 7% (1/14) every 3 months and 36% (5/14) once at one year and 7% (1/14) specified other. Combining responses from both groups, “every 6 months for the first year and then annually” was most favored with 54% of the respondents selecting this option (Fig. 4).
Fig. 4.
What time interval would you consider for patients with non-functioning pituitary macroadenomas to undergo follow-up imaging after TSS?
Follow-up on non-functioning pituitary adenoma with no residual tumor after surgery
Both the International Pituitary Neurosurgeons Society and Pituitary Society attendees agreed on decreasing the interval of MRI after several years without tumor recurrence. Only one Pituitary Society attendant and no neurosurgeons suggested yearly follow-up ongoing continually. In both pituitary expert groups, there was a fairly even distribution for the cut-off of how many years after adenoma stability, decreased frequency of imaging should occur. Thirty-nine, 20 and 39% in the Pituitary Society (n = 44) and 36, 43, and 21% of the neurosurgeons (n = 14) opted for a decrease in frequency of MRIs after 2, 3, or 5 years of no recurrence, respectively. The combined responses were also spread with 38, 26 and 20% of all respondents (n = 58) favoring a decrease in the frequency of MRIs after 2, 3 and 5 years respectively.
Follow-up for uncontrolled functioning adenoma
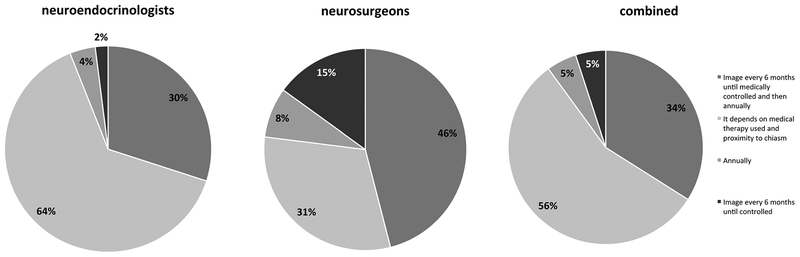
For functioning pituitary macroadenomas with significant non-resectable residual tumor, biochemically uncontrolled, the majority of Pituitary Society respondents 64% (28/44) indicated that follow-up depends on type of medical therapy and proximity to the optic chiasm. In contrast, the neurosurgeons’ most preferred choice in 46% (6/13) was to image every 6 months until control achieved and then annually. When the responder groups were combined, the most common preference in 56% (32/57) was that follow-up management depends on medical therapy used and the proximity of the tumor to the chiasm which would determine the interval of imaging in patients with functioning pituitary macroadenoma with significant residual tumor and are biochemically uncontrolled on medical therapies (Fig. 5).
Fig. 5.
At what time interval would you suggest that patients with a functioning pituitary macroadenoma, with large, non-resectable residual tumor, biochemically uncontrolled under medical therapy, undergo follow-up imaging studies after surgical treatment?
Surveillance of functioning adenomas in clinical and biochemical remission
In this category, there was also variability in responses in both pituitary expert groups. Among the neurosurgeons, 23, 46 and 31% (n = 13) selected; (1) every 6 months for the first year, then annually and less often after several years, (2) annually, or (3) only if concerning symptoms or loss of biochemical control, respectively, while among Pituitary Society respondents 34, 32, and 34% (n = 44) selected each of the latter respective choices. Among the group over all, similarly about a third of respondents (n = 57) selected each choice with 32, 35 and 33% selecting the respective three choices, as aforementioned.
Uncontrolled functioning adenoma follow-up
Concerning uncontrolled functioning pituitary microadenomas follow-up, there was a difference between the neurosurgical and the neuroendocrinology response, where the most selected choice from the Pituitary Society in 39% (17/44) was “every 6 months for the first year, then annually and less often after several years”; whereas only 17% (2/12) of the neurosurgeons answering this question selected that choice. The most popular choice for neurosurgeons in 58% (7/12) was to image every 6 months until biochemical control achieved and then less often, an answer selected by only 25% (11/44) of the Pituitary Society attendants.
Tumor pathology as a determinant of imaging follow-up
The question on whether additional factors were relevant to the MRI follow-up, revealed that there was an agreement among endocrinologists and neurosurgeons that the Ki 67 was a useful index influencing the MRI timeline follow-up. Overall approximately 72% (42/58) of respondents indicated that Ki67 was a significant factor. In addition, 62% (36/58) thought that “silent” ACTH pathology was also relevant factor. 73% and 63% of pituitary respondents (n = 44) reported that Ki67 and silent ACTH were relevant in determining MRI timing and 71 and 57% of neurosurgeons (n = 14) reported relevance of these variables respectively.
MRI imaging following radiotherapy
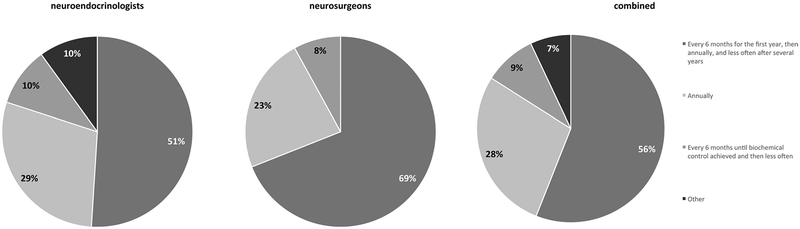
Regarding follow-up after radiotherapy, overall, 56% (30/54) of respondents selected every 6 months and then annually and then less often after several years; including 50% (21/41) of Pituitary Society attendees and 69% (9/13) of neurosurgeons (Fig. 6).
Fig. 6.
At what time interval/frequency do you suggest brain MRIs with contrast after sellar radiation?
Gadolinium retention awareness assessment
Lack of prior knowledge of new possible retention concerns regarding gadolinium in patients with normal renal function were reported by 28% (16/57), including 46% of neurosurgeons (n = 13) and 23% of Pituitary Society attendees (n = 44). Most respondents, 67% (36/54), reported that there had not been a recent change in the MRI contrast agent used at their institution including 64% (27/42) of Pituitary Society attendants and 75% (9/12) of neurosurgeons. Notably, a small minority of respondents (11%, 6/54) reported that they did not know what type of MRI contrast agent was used at their institution including 10% (4/42) of Pituitary Society attendants and 17% (2/12) of neurosurgeons.
Discussion
Magnetic resonance imaging has been a long-standing valuable modality for follow-up of patients with pituitary tumors, especially after surgery. Recently published guidelines on the imaging follow-up of nonfunctioning pituitary adenomas after surgery or radiotherapy recommend MRI with gadolinium contrast and addition of T2- and T1-weighted images with fat suppression sequences [11]. Nonetheless, there is no consensus on the frequency of the MRIs and on the duration of imaging follow-up. Similarly, guidelines on acromegaly and Cushing’s disease management suggest initial 3-month post-operative imaging using T2-weighted MRI with contrast but, again, the frequency of subsequent follow-up is not conclusive [12, 13]. As a result of these, the monitoring scanning protocol varies amongst clinicians, as clearly reflected by the results of this survey.
Gadolinium based contrast agents have been an essential adjunct to MRI studies, and data shows a reassuring safety profile with hypersensitivity reactions reported in 0.078% of patients [14] and nephrogenic fibrosing dermopathy in 0.26–8.8% of patients depending on underlying risk factors and renal function [15, 16]. Recent, evidence reports long term gadolinium retention within the dentate nucleus and globus pallidus of the brain associated with GBCAs, which appears to be more prevalent with use of linear [17] rather than with macrocyclic agents [18, 19]. In addition, in autopsy studies in five patients, without renal impairment, who received 1–4 doses of GBCAs in their lifetime, gadolinium accumulation was evident in brain tissue postmortem [20]. Similar findings were subsequently reported in patients who had received multiple doses of linear GBCAs for brain disease imaging [21]. The clinical significance of gadolinium deposition in the brain has not yet been identified. A survey study of 42 patients revealed that patients who received GBCAs identified central and peripheral pain, headache and bone pain as potential correlates [22]. The study, however, was limited; it was anonymous and heavily biased towards responders with presumed gadolinium toxicity. Considering the location of gadolinium deposition in the basal ganglia, the expected clinical sequelae would include movement disorders and sensory symptoms, including sharp or burning pain, and no study to date has assessed peripheral nervous system symptoms in context of long-term GBCAs administration. Recent pre-clinical studies in rodent models have also demonstrated gadolinium deposits in the brain of the animals without a clear phenotype [23]. The effect on neurodevelopment has not yet been determined, but previous reports of adverse effect with significant neurotoxicity with early-age-exposure to similar rare-earth metal such as lanthanum [24]. The importance of this sort of pre-clinical study would be to investigate potential effects of GBCAs on neurodevelopment as it might relate to gadolinium depositions in the pediatric population.
Based on the results from our questionnaire, a patient with a non-functioning completely resected adenoma, associated with low concern for clinically significant recurrence, would still receive ongoing imaging every 2–5 years, and thus would receive 3–6 doses (including pre-operative and post-operative MRI) of GBCAs in 20-year time period. Since patients with uncontrolled and functioning adenomas are likely to have much more frequent images, the lifetime doses of GBCA of these patients is likely well over 10 doses. Considering the recent studies concerning gadolinium deposition in brain basal ganglia, long-term follow-up of pituitary tumors, which are typically benign lesions, must be managed with careful attention to avoid any potential risk imposed by potentially unnecessary long-term repeated radiographic imaging. Further research is needed to establish treatment algorithms, which would balance careful monitoring of the tumor with any potential risk of the imaging process and to limit any unnecessary exposure to GBCAs.
There are multiple guidelines published related to pituitary tumors. The unifying theme between the guidelines is that there is no clear recommendation on the frequency or the length of time of surveillance of the follow-up MRI scans. As per the Joint Guidelines Committee of the American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS) published in 2016 “There is insufficient evidence to make a recommendation on the length of time of surveillance and its frequency” [11]. On the other hand, the Endocrine Society Clinical Practice Guidelines published in 2011 on follow up of pituitary incidentalomas not undergoing surgical intervention recommends MRI at 6 months for macroincidentalomas, 1 year for a microincidentaloma, and thereafter progressively less frequently if unchanged in size. Post-operative follow-up is less clear, making the surveys reported here even more useful. For Cushing’s disease and acromegaly patients, there are no clear guidelines beyond the first 3 months post operatively [12, 13]. The 2015 American Association of Clinical Endocrinologists (AACE) guidelines for postoperative management following pituitary surgery recommend follow-up imaging for nonfunctioning pituitary adenomas to be repeated annually for 3 to 5 years and thereafter per clinical judgment. Pituitary imaging for patients with hormonally hyperfunctioning tumors depends on tumor type, biochemical parameters, and overall disease activity [25].
The data gathered from our questionnaire suggest that pituitary neurosurgeons, as well as Pituitary Society neuroendocrinologists, (comprised of international pituitary specialists), are sometimes unaware of which contrast agents are used by their institution, and many are also unaware that evidence of long-term brain retention has been reported with the use of GBCAs. There is consensus among pituitary endocrinologists and neurosurgeons that long term repeated imaging is recommended in most pituitary tumors, although the precise strategy of timing varied depending on the specialist group and the specific clinical context of the adenoma. These findings may be skewed to represent opinions of thought leaders form academic centers, since that who attended the meetings, rather than those in community practices.
Study limitations include a small sample size, a disproportionate number of Pituitary Society endocrinologists compared to neurosurgeons and an overall response rate of 38% (58/151). Since the surveys were anonymous, we do not have specific information on the demographics of the responders. Strengths include the detailed clinical context included in the questionnaire, the uniform live setting, in which all surveys were completed in real time at the live meeting in which respondents were in the same time and space within each group. A wider survey of other centers globally could provide further valuable information. Nevertheless, this survey is the first to provide detailed information on everyday clinical practice regarding follow-up imaging of pituitary specialists worldwide. Furthermore, this is the first report assessing the awareness within the pituitary community of the potential concern about long-term central nervous system retention of GBCAs.
Conclusion
Pituitary MRI remains a mainstay for follow-up in pituitary disease patients. International pituitary endocrinologists and pituitary neurosurgeons suggest ongoing MRIs for the management of pituitary tumors, and their strategies vary based on the clinical context, but also on individual experience and practice. While further data is needed on the clinical consequences of the contrast agent retention and on the relative toxicities of the different type of agents, practicing physicians, who determine the interval in which imaging is ordered, should be informed of the emerging controversy regarding safety of GBCAs.
Supplementary Material
Funding
No funding has been received for this project.
Footnotes
Conflict of interest The authors have no conflicts of interest for this project.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11102-018-0924-0) contains supplementary material, which is available to authorized users.
References
- 1.Ersoy H, Rybicki FJ (2007) Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 26(5):1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Paolini MA, Murray DL et al. (2017) Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 285(2):546–554 [DOI] [PubMed] [Google Scholar]
- 3.Levine D, McDonald RJ, Kressel HY. Gadolinium retention after contrast-enhanced MRI. JAMA. 2018 [DOI] [PubMed] [Google Scholar]
- 4.Moreno J, Vaz N, Soler J, Carrasco J, Podlipnick S (2018) High signal intensity in the dentate nucleus on unenhanced T1-weighted MR images in melanoma patients receiving macrocyclic gadolinium-based contrast. J Radiol Diagn Methods 1:101 [Google Scholar]
- 5.Stojanov D, Aracki-Trenkic A, Benedeto-Stojanov D (2016) Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents—current status. Neuroradiology 58(5):433–441 [DOI] [PubMed] [Google Scholar]
- 6.McDonald RJ, Levine D, Weinreb J, Kanal E, Davenport MS, Ellis JH et al. Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology. 2018:181151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekkers IA, Roos R, van der Molen AJ (2018) Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur Radiol 28(4):1579–1584 [DOI] [PubMed] [Google Scholar]
- 8.Rasschaert M, Emerit A, Fretellier N, Factor C, Robert P, Idee JM et al. (2018) Gadolinium retention, brain T1 hyperintensity, and endogenous metals: a comparative study of macrocyclic versus linear gadolinium chelates in renally sensitized rats. Investig Radiol 53(6):328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malayeri AA, Brooks K, Bryant LH, Evers R, Kumar P, Reich DS et al. (2016) NIH perspective on reports of gadolinium deposition in the brain. J Am Coll Radiol 13(3):237–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald RT, Agarwal V, Hoang JK, Gaillard F, Dixon A, Kanal E. The impact of gadolinium deposition on radiology practice: an international survey of radiologists. Curr Probl Diagn Radiol. 2018 [DOI] [PubMed] [Google Scholar]
- 11.Ziu M, Dunn IF, Hess C, Fleseriu M, Bodach ME, Tumialan LM et al. (2016) Congress of neurological surgeons systematic review and evidence-based guideline on posttreatment follow-up evaluation of patients with nonfunctioning pituitary adenomas. Neurosurgery 79(4):E541–E543 [DOI] [PubMed] [Google Scholar]
- 12.Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A et al. (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(11):3933–3951 [DOI] [PubMed] [Google Scholar]
- 13.Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO et al. (2015) Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100(8):2807–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung JW, Kang HR, Kim MH, Lee W, Min KU, Han MH et al. (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264(2):414–422 [DOI] [PubMed] [Google Scholar]
- 15.Golding LP, Provenzale JM (2008) Nephrogenic systemic fibrosis: possible association with a predisposing infection. AJR Am J Roentgenol 190(4):1069–1075 [DOI] [PubMed] [Google Scholar]
- 16.Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J et al. (2008) Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 248(3):807–816 [DOI] [PubMed] [Google Scholar]
- 17.Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270(3):834–841 [DOI] [PubMed] [Google Scholar]
- 18.Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kick-ingereder P et al. (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275(3):783–791 [DOI] [PubMed] [Google Scholar]
- 19.Olchowy C, Cebulski K, Lasecki M, Chaber R, Olchowy A, Kalwak K et al. (2017) The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—a systematic review. PloS ONE 12(2):e0171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J et al. (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276(1):228–232 [DOI] [PubMed] [Google Scholar]
- 21.Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49(10):685–690 [DOI] [PubMed] [Google Scholar]
- 22.Semelka RC, Ramalho J, Vakharia A, AlObaidy M, Burke LM, Jay M et al. (2016) Gadolinium deposition disease: Initial description of a disease that has been around for a while. Magn Reson Imaging 34(10):1383–1390 [DOI] [PubMed] [Google Scholar]
- 23.Bussi S, Coppo A, Botteron C, Fraimbault V, Fanizzi A, De Laurentiis E et al. (2018) Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging 47(3):746–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zarros A, Byrne AM, Boomkamp SD, Tsakiris S, Baillie GS (2013) Lanthanum-induced neurotoxicity: solving the riddle of its involvement in cognitive impairment? Arch Toxicol 87(11):2031–2035 [DOI] [PubMed] [Google Scholar]
- 25.Woodmansee WW, Carmichael J, Kelly D, Katznelson L (2015) American association of clinical endocrinologists and american college of endocrinology disease state clinical review: postoperative management following pituitary surgery. Endocr Pract 21(7):832–838 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.