Abstract
Objective:
Cardiovascular disease (CVD) is one of the most prevalent chronic conditions and leading causes of death. Although CVD clinically manifests in adulthood, underlying processes of CVD begin in the earlier decades of life. Inflammation has been shown to play a key role, but relatively little is understood about how inflammation changes over time among young individuals. Additionally, how psychosocial factors like stress may influence changes in inflammation earlier in the lifespan is not entirely clear. Thus, the current three-wave longitudinal study examined the developmental trajectory of CRP, a marker of systemic inflammation, over a four-year period from mid-adolescence into young adulthood. Between- and within-person differences in stress in relation to changes in CRP were also examined.
Methods:
A sample of 350 individuals was recruited during mid-adolescence and participated in one to three assessments, two years apart. At each assessment, participants provided dried blood spots for the assessment of CRP and reported on recent major life events, perceived stress, and daily interpersonal stress.
Results:
Multi-level modeling indicated that CRP increased with age, and within-person increases in perceived stress, but not life events or daily stress, were associated with higher CRP. Between-person differences in average levels of stress from mid-adolescence into young adulthood were not associated with changes in CRP.
Conclusion:
These findings suggest that the link between stress and systemic inflammation between mid-adolescence and young adulthood may be most affected by contemporaneous experiences of perceived stress. There was little evidence to suggest that CRP trajectories varied by between-person differences in overall average levels of perceived stress, life events, and daily stress.
Keywords: inflammation, CRP, perceived stress, life events, daily stress
Cardiovascular disease (CVD) is one of the most common chronic diseases that afflicts Americans. Currently, 36.6% of US adults have some form of CVD and this rate is projected to rise to 43.9% by 2030 (Benjamin et al., 2017). CVD also remains the leading cause of mortality, with approximately one of every three deaths in the US being attributed to CVD (Benjamin et al., 2017). Although CVD clinically manifests in midlife and older adulthood, its foundation (e.g., atherosclerosis) begins developing much earlier in the lifespan, namely during childhood and adolescence (McGill Jr. et al., 2000). Adolescence may be a particularly important focal point for understanding early CVD risk given that CVD precursors (e.g., elevated blood pressure, adiposity, and plaque buildup) are evident by adolescence (Berenson et al., 1998). Furthermore, only half of US adolescents currently have ideal cardiovascular health based on traditional risk factors (Shay et al., 2011). This suggests that a significant portion of the adolescent population will go on to face substantial risk for CVD in adulthood (Shay et al., 2011) and highlights the importance of understanding early pathophysiological processes that give rise to later disease.
Inflammation has emerged as a particularly important risk factor for CVD, as it plays a central role in the development and progression of atherosclerosis and precipitates cardiovascular events (Libby, 2006; Rost et al., 2001). Higher levels of systemic inflammation, as indexed by C-reactive protein (CRP), increase risk for CVD in adults. In adolescents, higher CRP has been linked to greater cardiometabolic risk, suggesting that CRP may be a useful screening tool even among youth (Agostinis-Sobrinho et al., 2018; DeBoer, 2013). However, there still remain gaps in our understanding of inflammatory processes during adolescence.
First, the developmental trajectory of inflammation during adolescence is not fully understood. Studies of adults have established that inflammation increases with age, but much less work has been conducted on adolescent samples. Nevertheless, several extant studies point to age-related increases in inflammation even during adolescence. In cross-sectional analyses of the National Health and Nutrition Examination Surveys, median CRP was lower among 12 to 13-year-olds compared to 18 to 19-year-old youth (De Ferranti, Gauvreau, Ludwig, Newburger, & Rifai, 2006). Similarly, prospective studies examining adversity and inflammation associations in youth provide descriptive data showing that average levels of CRP during earlier assessments were lower than levels of CRP assessed approximately five years later (Copeland et al., 2014; Slopen, Kubzansky, McLaughlin, & Koenen, 2013). Although these studies provide initial evidence that inflammation increases with age among youth, they did not prospectively model the growth trajectory or rate of increase of systemic inflammation.
Another gap in our understanding centers on the psychosocial contributions to adolescent inflammatory biology. Psychosocial stress is known to increase risk for poor health outcomes and has been linked to heightened inflammation in adults (Cohen, Janicki-Deverts, & Miller, 2007; Gruenewald, Cohen, Matthews, Tracy, & Seeman, 2009; Kiecolt-Glaser, Gouin, & Hantsoo, 2010; G. E. Miller, Chen, & Parker, 2011). Given that adolescence may be a time of heightened exposure and sensitivity to stress (American Psychological Association, 2014; Blakemore & Mills, 2014), it may be a sensitive period during which stress can have long-term impacts on neurobiological systems (Romeo, 2017; Tottenham & Galván, 2016) with implications for downstream inflammatory processes (Chiang, Bower, & Taylor, 2015). Despite this, relatively few studies have examined stress-inflammation associations in adolescents. Moreover, existing studies have yielded mixed findings (e.g., Chiang, Bower, Irwin, Taylor, & Fuligni, 2017; Copeland et al., 2014; Fuligni et al., 2009; Low, Matthews, & Hall, 2013; Slopen, Koenen, & Kubzansky, 2012), and whether stress impacts the developmental trajectory of inflammatory processes during the earlier decades of life, including adolescence, remains unclear.
To address these issues, we evaluated the trajectory of change in CRP over a four-year period from mid-adolescence to young adulthood. We then examined whether CRP trajectories varied according to between-person differences in overall experiences of stress over the four-year period, and whether within-person changes in stress covaried with changes in CRP. We examined several types of stressors: perceived stress, major life events, and daily negative social interactions. These stressors are distinct in that perceived stress represents subjective stress whereas life events and daily negative social interactions represent more objective stress. Additionally, compared to perceived stress and major life events, daily negative social interactions capture more acute, mundane experiences of stress in everyday life. Despite these distinctions, each of these types of stress has been linked to heightened systemic inflammation in adults (Gouin, Glaser, Malarkey, Beversdorf, & Kiecolt-Glaser, 2012; Hostinar, Lachman, Mroczek, Seeman, & Miller, 2015; McDade, Hawkley, & Cacioppo, 2006). Based on adult work demonstrating age- and stress-related increases in inflammation, we hypothesized that CRP would increase with age, that between-person differences in psychosocial stress would accelerate age-related increases in CRP, and that within-person increases in stress would co-vary with increases in CRP. Given some prior work suggesting that stress responsivity and inflammatory processes differ by gender and ethnicity (Liu et al., 2017; O’Connor et al., 2009; Slopen et al., 2010), we also explored whether CRP trajectories and stress-CRP associations differed by these demographic characteristics.
Method
Participants
Data were obtained from an ethnically diverse sample of 350 adolescents participating in a three-wave longitudinal study. During Wave 1, 316 10th and 11th grade adolescents from four Los Angeles high schools and their primary caregivers participated. Of these original 316 participants, 214 (68%) also participated in Wave 2 when they were in 12th grade and one year out of high school. An additional 34 participants from one of the original four high schools or from one of our previous studies (Tsai, Telzer, Gonzales, & Fuligni, 2013) were recruited to refresh the sample during Wave 2, raising the total number of participants to 248. Two years later during Wave 3, when participants were two and three years out of high school, 180 of the individuals who participated in Wave 1 and/or Wave 2 again provided data. Of the total 350 participants, 148 provided three waves of data, 98 provided two waves of data, and 104 provided one wave of data. The majority of adolescents (70.3%) participated in at least two waves of data collection and the average number of waves completed was 2.13 (SD = .84). Sample characteristics are displayed in Table 1.
Table 1.
Descriptive data of participant characteristics and study variables.
| Variable | Wave 1 | Wave 2 | Wave 3 | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Age | 16.40 (.74) | 14.50 – 20.50 | 18.31 (0.77) | 14.50 – 22.17 | 20.29(.74) | 16.50 – 22.09 |
| Female (n (%)) | 180 (57.0) | 138 (57.0) | 106 (60.2) | |||
| Ethnicity (n (%)) | ||||||
| European | 92 (29.1) | 81 (32.7) | 61 (33.9) | |||
| Latino | 132 (41.7) | 110 (44.4) | 82 (45.6) | |||
| Asian | 73 (23.1) | 38 (15.3) | 26 (14.4) | |||
| Other | 19 (6.0) | 19 (7.7) | 11 (6.1) | |||
| Parent education | 2.72 (0.89) | 1.00 – 4.00 | 2.83 (0.90) | 1.00 – 4.00 | 2.77 (0.92) | 1.00 – 4.00 |
| Waist circumference (cm) | 80.17 (13.13) | 60.00 – 138.20 | 80.46 (13.65) | 59.50 – 139.50 | 83.92 (13.83) | 61.8–144.10 |
| Smoking behavior | 1.03 (0.36) | 0.00 – 7.00 | 1.17 (0.67) | 0.00 – 6.00 | 1.32 (1.13) | 0.00 – 7.00 |
| Depressive symptoms | 15.70 (10.51) | 0.00 – 48.00 | 15.36 (10.12) | 0.00 – 46.00 | 14.89 (10.25) | 0.00 – 50.00 |
| Perceived stress | 1.94 (0.62) | 0.33 – 3.60 | 1.85 (0.62) | 0.30 – 3.50 | 1.81 (0.59) | 0.00 – 3.40 |
| Major life events | 2.41 (1.72) | 0.00 – 8.00 | 3.42 (2.33) | 0.00 – 12.00 | 3.12 (2.36) | 0.00 – 14.00 |
| Daily interpersonal stress | 6.11 (6.85) | 0.00 – 45.00 | 5.07 (5.76) | 0.00 – 39.99 | 3.11 (4.41) | 0.00 – 30.00 |
| CRP (mg/L) | 0.69 (1.27) | 0.03 – 8.45 | 0.89 (1.29) | 0.03 – 7.26 | 1.36 (1.88) | 0.04 – 9.60 |
| < 1.0 (n(%)) | 253 (82.7) | 166 (71.9) | 102 (65.0) | |||
| 1.0 – 3.0 (n(%)) | 38 (12.4) | 49 (21.2) | 30 (19.1) | |||
| > 3.0 (n(%)) | 15 (4.9) | 16 (6.9) | 25 (15.9) | |||
Parent education coded on a scale where 1 = less than high school, 2 = completed high school, 3 = some college, and 4 = four-year college degree of higher. Smoking behavior indicates number of days smoked cigarettes during past 30 days coded on a scale where 1 = 0 days, 2 = 1 or 2 days, 3 = 3 to 5 days, 4 = 6 to 9 days, 5 = 10 to 19 days, 6 = 20 to 29 days, and 7 = all 30 days. CRP values reflect raw values.
Compared to those who participated in only a single wave, participants completing at least two waves had slightly higher levels of perceived stress (single wave: M = 1.82, SD = .61; multiple waves: M = 1.99, SD = .62); t(313) = −2.02, p = .028, d = -.27) and daily interpersonal stress (single wave: M = 4.80, SD = 5.43; multiple waves: M = 6.63, SD = 7.27); t(311) = −2.14, p = .033, d = −.27) during the first wave of data collection. There were no differences for major life events (t(312) = 2.54, p = .385, d = .11) and CRP (t(306) = −.65, p = .518, d = −.08). With respect to sociodemographic characteristics, those with multiple waves of data had parents who were slightly more educated (single wave: M = 2.51, SD = .09; multiple waves: M = 2.81, SD = .06); t(344) = −2.79, p = .01, d = −.33). There were no gender differences in participation (χ2 = .49; p = .49), and Asian Americans were less likely to participate in more than one wave (single wave: 34% Asian American; multiple waves: 16% Asian American; χ2 = 13.80; p = .003).
Procedures
During the first wave of data collection, participants were recruited via in-class presentations and mailings of study flyers and recruitment forms to students’ homes. Families indicating interest in the study were contacted via phone by study staff and given more information about the study. Families providing parental verbal consent were scheduled for an in-home visit. Previously-participating families were re-contacted at the second and third waves of data collection for continued participation.
Each wave of data collection consisted of a home visit and a two-week daily diary protocol (Supplemental Figure 1). Home visits included the completion a set of online questionnaires by adolescents and by primary caregivers. Anthropometric measures (i.e., height, weight) and whole blood spots for the assessment of CRP were then collected. After the in-home visit, participants completed a two-week daily diary protocol. Using daily diary checklists, participants reported on their social and emotional experiences each night before going to bed for 15 consecutive days. To ensure compliance, reminder text messages were sent participants each day. Additionally, participants were provided with pre-programmed electronic time stampers that they used to indicate the dates and times that diary checklists were completed. At each wave of data collection, the majority of adolescents (93.2% - 95.6%) completed daily checklists for at least 14 days.
At the end of the daily diary protocol, study staff collected completed materials and compensated participants. Adolescents were compensated $50 at Wave 1, $75 at Wave 2, and $120 at Wave 3. They also received two movie theater passes at each wave if their diaries were completed correctly and on time. All study procedures were approved by the UCLA Institutional Review Board and all study participants provided written consent and assent.
Measures
Stress.
Three types of stressors were assessed: global levels of perceived stress, major life events, and daily interpersonal stress. These measures were examined because they have been previously linked to circulating markers of inflammation and other CVD-relevant outcomes (Dixon, Meng, Goldberg, Schneiderman, & Delamater, 2009; Fuligni et al., 2009; McDade et al., 2006; Pyykkönen et al., 2010; Räikkönen, Matthews, & Kuller, 2007).
Perceived stress.
Adolescent participants completed the 10-item Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983), which assessed how often participants experienced feelings of stress during the past month on a 5-point scale (0 = never, 4 = very often). Items include how often participants felt “nervous or stressed” and “unable to control the important things in your life.” The PSS has been shown to be valid and reliable (Cohen & Williamson, 1988). Internal reliability of the measure was good in the present sample (αs = .84 - .89 across waves).
Major life events.
Adolescents completed a checklist of events across the domains of family, friends, and school to assess the number of major life events at each Wave. During Wave 1, participants indicated whether they had experienced any one of 14 events during the previous three months. Five additional items were included in subsequent waves and the temporal frame was extended to the previous twelve months. Items were adapted from previous measures of stressful events that have been associated with negative outcomes (Conger et al., 2002; Hammen, 1991). Example items included parents divorced or separated, a family member became seriously ill, a close friend moved quite far away, you had a serious falling out or ended a friendship with a close friend, you were suspended or expelled in school, and your grades in school went down a lot. Affirmative responses were summed across items, such that higher scores reflected greater exposure to stressful events.
Daily interpersonal stress.
Each night during the daily diary period, youth indicated on bedtime diary checklists whether they had experienced any of eight negative social interactions across the domains of family, peers, and school. Items were argued with a parent, argued with another family member, argued with a friend, punished by a parent, parents argued, something bad happened to a family member, had an argument or was punished by an adult at school, and was insulted, threatened, or made fun of by someone at school. The number of endorsed items each day was summed across days to index cumulative stress exposure.
Inflammation.
CRP was assessed via dried blood spots (DBS), a relatively non-invasive procedure that has been well validated against standard methods for whole blood collected via venipuncture (Crimmins et al., 2014; McDade, Burhop, & Dohnal, 2004). Blood spots were collected during in-home visit at each wave of data collection. Participants’ fingers were first cleaned with alcohol and subsequently punctured with a sterile, disposable microlancet commonly used by diabetics. Five drops of capillary blood were collected onto standardized filter paper, and blood spot samples were covered and dried overnight. DBS samples were then stored at -80°C until shipped to the Laboratory for Human Biology Research at Northwestern University. Samples were assayed for CRP using high-sensitivity enzyme-linked immunosorbent assay. All samples were run in duplicate, and intra- and inter-assay coefficients of variation were <6.4% and <9.3%, respectively, across all waves of data collection. Twenty-eight samples across waves fell under the lower detection of limit of .03 mg/L and were thus assigned a value of .03. Ten samples across waves yielded CRP levels greater than 10 mg/L and were excluded from analyses under the assumption that they likely represent the effects of acute infection rather than chronic inflammation (O’Connor et al., 2009). Natural log transformations were applied to CRP levels at each wave to correct for their positively skewed distributions.
Covariates.
Sociodemographic characteristics (age, gender, ethnicity, parent education), biobehavioral factors (waist circumference, smoking behavior), and depressive symptoms were included as covariates, as these factors have previously been associated with inflammation (Festa et al., 2001; A. H. Miller & Raison, 2016; O’Connor et al., 2009). At study entry, youth self-reported their gender and participants’ parents reported participants’ date of birth, from which age was computed. Information on ethnicity and parent education was also collected at the time of study entry. Participants’ ethnicities were based on self-reports and the birth countries of their parents and grandparents as reported by participants’ parents. Parents also reported the highest level of education they and their spouses completed on an 11-point scale (1 = some elementary school, 11 = graduated from medical, law, or graduate school). Educational attainment for each parent was recoded as less than high school, high school diploma, some college, or 4-year college degree or higher, and subsequently averaged together. Depressive symptoms, smoking behavior, and waist circumference, which has been shown to be a valid measure adiposity (Pietrobelli et al., 1998; Taylor, Jones, Williams, & Goulding, 2000), were assessed at each wave of data collection during home visits. Participants indicated how often they experienced cognitive, affective, and somatic symptoms of depression using the Center for Epidemiologic Studies Depression Scale (CESD; Radloff, 1977) and reported on the number of days they smoked cigarettes during the past 30 days on a 7-point scale (1 = 0 days; 7 = all 30 days). Waist circumference was assessed twice at the mid-point between iliac crest and lower rib. Measurements were taken, averaged, and then adjusted for gender by standardizing within gender at each wave.
Statistical Approach
A series of multi-level models were estimated using Stata 14 to examine the developmental trajectory of CRP and between- and within-person associations between psychosocial stress and CRP. More specifically, we tested two-level models in which within-person variations in CRP were modeled at level 1 and between-person differences were modeled at level 2. Both intercepts and slopes were modeled as random parameters. We first mapped CRP trajectories by entering age as the primary level 1 predictor while controlling for sociodemographic characteristics (i.e., gender, ethnicity, and parent education), biobehavioral factors (waist circumference, smoking behavior), and depressive symptoms. Age was centered around 14.5 (the youngest age across waves), and parent education was grand-mean centered. Waist circumference, smoking behavior, and depressive symptoms were person-mean centered and included as time-varying covariates at level 1 while sociodemographic characteristics were entered as level 2 predictors of the intercept. We next examined whether psychosocial stress predicted within- and between-person variations in CRP. For within-person differences, repeatedly-assessed measures of stress, centered within a person, were added as level 1 predictors to the base model described above. For between-person differences, reported experiences of stress were averaged across all three waves in order to reflect overall levels of stress across the four-year period. These composite measures of stress were then grand-mean centered and added as level 2 predictors of the random intercept and slope of CRP in the base model described above. The parameter of interest was the cross-level interaction between average levels of stress and age in the prediction of CRP, which indicates whether the developmental trajectory of CRP varied according to individual differences in overall levels of stress. Separate models were estimated for each measure of stress.
Results
Descriptive statistics for study variables at each wave are displayed in Table 1. Overall, participants perceived modest levels of perceived stress and relatively few major life events and stressful days in their everyday lives. However, it should be noted that there was variability around the averages, with 32–39% of participants reporting perceived stress some of the time on at least one occasion, 45–59% reporting two or more significant life events on at least one occasion, and 17–41% reporting endorsing at least 5 daily stressors across the two-week period on at least one occasion. Similarly, CRP levels were relatively low across waves, though there was some variance around these averages. Between 17% and 34% of the sample showed a CRP > 1 on at least one occasion. Average levels of CRP increased over the course of the study, as did the percentage of participants with CRP greater than 3, the threshold indicating greater CVD risk in adults (Pearson et al., 2003). Bivariate correlations between stress variables and CRP at each wave are presented in Table 2.
Table 2.
Bivariate correlations between psychosocial stressors and natural log-transformed CRP.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. W1 perceived stress | |||||||||||
| 2. W2 perceived stress | 0.52*** | ||||||||||
| 3. W3 perceived stress | 0.39*** | 0.57*** | |||||||||
| 4. W1 life events | 0.29*** | 0.26*** | 0.22** | ||||||||
| 5. W2 life events | 0.18* | 0.30*** | 0.15* | 0.34*** | |||||||
| 6. W3 life events | 0.26*** | 0.21** | 0.23** | 0.26** | 0.46*** | ||||||
| 7. W1 daily stress | 0.20*** | 0.20** | 0.13 | 0.19*** | 0.23** | 0.19* | |||||
| 8. W2 daily stress | 0.09 | 0.19** | 0.12 | 0.10 | 0.29*** | 0.13 | 0.37*** | ||||
| 9. W3 daily stress | 0.06 | 0.05 | 0.14 | 0.17* | 0.13 | 0.10 | 0.25** | 0.24** | |||
| 10. W1 lnCRP | 0.04 | −0.11 | 0.05 | −0.06 | 0.01 | −0.01 | −0.002 | −0.01 | 0.05 | ||
| 11. W2 lnCRP | −0.03 | −0.04 | −0.09 | 0.05 | 0.12 | −0.09 | 0.12 | −0.02 | 0.07 | 0.55*** | |
| 12. W3 lnCRP | 0.06 | 0.10 | 0.17** | −0.05 | 0.09 | −0.08 | −0.06 | −0.03 | 0.02 | 0.49*** | 0.47*** |
p < .05
p < .01
p < .001.
Development of CRP
As depicted in Figure 1, CRP increased significantly with age (b(SE) = .16(.02), p < .001). In addition, levels of CRP were higher on occasions when waist circumference was higher than one’s average level of waist circumference (b(SE) = .33(.11), p = .002). Asian American ethnicity (b(SE) = -.61(.18), p = .001) and higher parental education (b(SE) = -.16(.07), p = .023), but not gender (b(SE) = .20(.12), p = .107), were associated with lower levels of CRP during Wave 1.1
Figure 1.
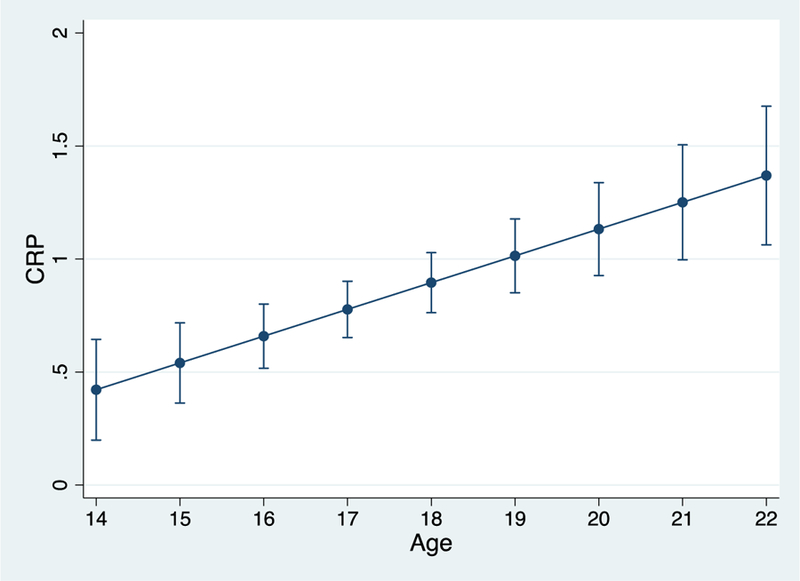
Age-related increases in raw CRP. Bars reflect 95% confidence intervals.
Stress and CRP
Perceived stress.
As displayed in Table 3 (column 1), there was a significant within-person association between perceived stress and CRP, such that CRP levels were higher on occasions when participants perceived greater stress than their average levels of stress across time (p = .001; Figure 2). By contrast, individual differences in average perceived stress did not moderate age-related increases in CRP across time (p = .149).
Table 3.
Multilevel models examining within and between-person associations between stress and CRP .
| Variable | Perceived Stress | Major Life Events | Daily Interpersonal Stress | |||
|---|---|---|---|---|---|---|
| b (SE) | 95% CI | b (SE) | 95% CI | b (SE) | 95% CI | |
| Intercept | −1.52 (0.16)*** | (−1.83, −1.22) | −1.51 (0.16)*** | (−1.83, −1.20) | −1.49 (0.16)*** | (−1.80, −1.18) |
| Age | 0.16 (0.02)*** | (0.12, 0.21) | 0.15 (0.03)*** | (0.10, 0.20) | 0.15 (0.03)*** | (0.10, 0.20) |
| Female | 0.19 (0.13) | (−0.05, 0.44) | 0.22 (0.12) | (−0.02, 0.46) | 0.20 (0.12) | (−0.04, 0.44) |
| Latino | 0.03 (0.15) | (−0.27, 0.33) | 0.06 (0.15) | (−0.24, 0.36) | 0.042 (0.15) | (−0.26, 0.34) |
| Asian | −0.62 (0.18)*** | (−0.97, −0.28) | −0.64 (0.18)*** | (−0.99, −0.29) | −0.60 (0.18)*** | (−0.96, −0.25) |
| Other | −0.06 (0.27) | (−0.58, 0.46) | −0.03 (0.27) | (−0.56, 0.50) | −0.05 (0.27) | (−0.57, 0.47) |
| Parent education | −0.17 (0.07)* | (−0.31, −0.03) | −0.16 (0.07)* | (−0.31, −0.02) | −0.16 (0.07)* | (−0.30, −0.015) |
| Waist circumference | 0.36 (0.11)*** | (0.15, 0.57) | 0.35 (0.11)*** | (0.4, 0.56) | 0.34 (0.11)** | (0.12, 0.56) |
| Smoking behavior | −0.01 (0.10) | (−0.22, 0.19) | −0.03 (0.11) | (−0.24, 0.18) | −0.03 (0.11) | (−0.24, 0.19) |
| Depressive symptoms | −0.02 (0.01)* | (−0.03 −0.001) | −0.003 (0.01) | (−0.02, 0.01) | 0.002 (0.01) | (−0.01, 0.02) |
| Concurrent stress | 0.47 (0.14)*** | (0.20, 0.74) | 0.03 (0.03) | (−0.03, 0.10) | −0.01 (0.01) | (−0.03, 0.01) |
| Average stress | −0.18 (0.19) | (−0.55, 0.18) | −0.07 (0.061) | (−0.19, 0.05) | −0.01 (0.02) | (−0.05, 0.03) |
| Age x average stress | 0.07 (0.05) | (−0.03, 0.16) | 0.014 (0.02) | (−0.02, 0.04) | 0.002 (0.01) | (−0.01, 0.01) |
p < .05
p < .01
p < .001. Gender was coded as 0 = male and 1 = female. Ethnicity was dummy coded with European-American as the reference group. Concurrent stress reflects time-varying stress measures at each wave that were entered at Level 1. Average stress reflects measures of stress averaged across waves and entered at Level 2.
Figure 2.
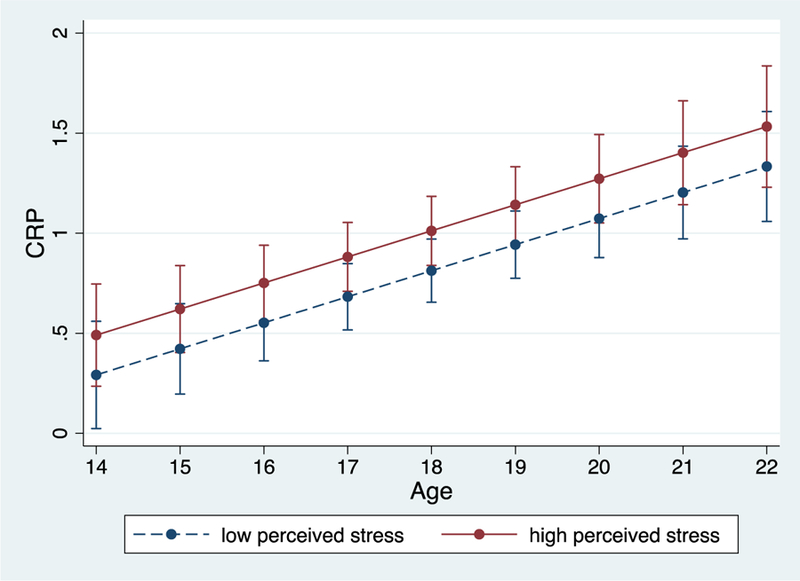
Within-person associations between perceived stress and raw CRP. During times of higher perceived stress, levels of CRP were higher. Bars reflect 95% confidence intervals.
Major life events.
As shown in Table 3 (column 3), there was no evidence that CRP levels were higher on waves when participants reported more life events (p = .357). Paralleling findings for perceived stress, the rate of increase in CRP from mid-adolescence to young adulthood also did not vary as a function of overall average levels of major life events (p = .347).
Daily interpersonal stress.
As displayed in Table 3 (column 5), there was also no within-person association between daily interpersonal stress and levels of CRP (p = .576). Likewise, average levels of negative daily social interactions did not contribute to variation in age-related increases in CRP (p = .442).
Exploratory Analyses
Prior research has documented gender and ethnic differences in inflammation, with females having higher levels of inflammation compared to males, and Asian-Americans having lower inflammation and Latinos having higher inflammation compared to European-Americans (Khera et al., 2005; O’Connor et al., 2009). Therefore, we explored whether the developmental trajectory of CRP and the associations between stress and CRP differed by gender or ethnicity. To do so, we examined the two-way cross-level interactions between age and gender/ethnicity, the three-way interactions among age, average stress (level 2), and gender/ethnicity for between-person differences, and the two-way interactions between stress (level 1) and gender/ethnicity for within-person differences. Results revealed no gender or ethnic differences in the developmental trajectory of CRP (Supplemental Table 1) and in between- and within- person associations between stress and CRP (Supplemental Table 2).
Discussion
The current study examined the development of CRP and associations between psychosocial stress and CRP over a four-year period from mid-adolescence into young adulthood. As expected, levels of CRP increased with age, and within-person changes in perceived stress co-varied with changes in CRP, such that individuals’ levels of CRP were higher during times when they perceived more stress in their lives. By contrast, within-person fluctuations in major life events and daily interpersonal stress were not tied to concurrent levels of CRP. Additionally, between-person differences in all three types of stress (averaged across waves) were not associated with increases in CRP over the next several years. Our findings show that CRP increases steadily from mid-adolescence into young adulthood and suggest that although individual differences in stress may not be consequential for the developmental trajectory of CRP, levels of CRP do rise within adolescents at times when they perceive more stress.
The rise in CRP across adolescence and into young adulthood is somewhat consistent with one of the only other published study (to our knowledge) that explicitly examined trajectories of inflammatory processes. In that study, IL-6 production in response to bacterial stimuli increased over a 1.5-year period in 15 to 19-year-old female adolescents at high risk for depression (Miller & Chen, 2010). However, unlike in the present study, basal inflammation as measured by circulating IL-6 did not increase over time. This discrepancy may be due to a number of factors, including differences in inflammatory markers assessed and in the gender and ethnic makeup of the samples. It may also have to do with the notion that exaggerated inflammatory responses to threat are thought to eventually engender a chronic inflammatory state (G. E. Miller et al., 2011), and it may be that the 1.5 year follow-up in that study was too short to capture increases in basal inflammation. The age-related increases in CRP observed here are more consistent with other prospective studies descriptively showing that average levels of CRP increase from 0.20–0.30 mg/L during early adolescence to 0.70–0.75 mg/L during late adolescence and emerging adulthood (Copeland et al., 2014; De Ferranti et al., 2006). We observed larger increases in CRP over time than these previous studies did, and it will be important for future work to employ longitudinal designs with repeated assessments of inflammatory markers to clarify the rate at which inflammation may increase during the adolescent years.
Results showed that within-person increases in perceived stress, but not life events or negative daily social interactions, were associated with higher levels of CRP. A major difference between the measure of perceived stress and the measures of life events and daily stress is that the former captures individuals’ appraisals of potentially threatening or challenging events in their overall lives and indexes subjective feelings of global stress. By comparison, the measures of life events and daily stress are based on the occurrence of events that are assumed to be stressful. Theoretical work posits that stress is experienced when circumstances are appraised as threatening and availability of resources to cope are limited (Lazarus & Folkman, 1984). From this perspective, it is the emotional and behavioral responses to the event rather than the event per se that impacts health. Thus, it may be that one’s internalized appraisal of stress matters more for whether his or her CRP increases, at least during adolescence and young adulthood. Consistent with this notion, a previous study showed that perceived stress in response to bereavement rather than the event itself was associated with immunologic changes (Irwin, Daniels, & Weiner, 1987). Alternatively, the perceived stress measure’s focus on psychological response (rather than stimulus event) may more effectively capture stressors that may have been missed by the pre-specified checklist assessments used to tap life events and daily stressors. That said, several previous studies assessing systemic inflammation in youth have observed links with adverse life events (Copeland et al., 2014; Dixon et al., 2009; Slopen et al., 2013), and we previously reported CRP links to daily interpersonal stress (Fuligni et al., 2009). Thus, additional studies examining the fluctuations in stress and inflammation during adolescence are needed to confirm whether subjective compared to objective stress has more predictive utility for adolescents’ inflammatory outcomes.
Between-person analyses revealed no association between psychosocial stress averaged across the four-year period and CRP trajectories, which may have to do with the nature of the types of stress measured in the present investigation. Specifically, we assessed stress that was relatively recent and may be considered normative and transient. It may be that such perturbations have minimal impact on CRP trajectories relative to more chronic or severe stress, such as maltreatment or growing up in poverty. The lack of between-person associations may also have to do with the fact that the immune system and other biological systems that regulate inflammatory processes (HPA axis, autonomic nervous system) are relatively intact early in life and may be more effective in adapting to the environment and maintaining homeostasis in the face of threats. As such, during childhood and adolescence, inflammatory responses may be more tightly regulated, terminating quickly in the absence of threat (Miller & Chen, 2010). If so, the effects of stress on the developmental trajectory of systemic inflammation may not emerge until later in life—for instance, after biological systems have become worn down or during later stages of atherosclerosis when accumulation of macrophages secreting proinflammatory cytokines in blood vessel walls has increased.
Several limitations of the current investigation should be addressed in future research. First, we focused only on a single measure of systemic inflammation, CRP, which remains low early in life during childhood and adolescence. Thus, before definitive conclusions can be made about whether adolescent experiences of psychosocial stress contribute to a pro-inflammatory phenotype, a broader range of inflammatory measures should be examined. These should include ex vivo stimulated cytokine production by a range of stimuli (e.g., bacterial, viral) and sensitivity to anti-inflammatory signaling from different molecules (e.g., cortisol, IL-10), in addition to genomic measures of inflammation and other circulating markers of systemic inflammation (e.g., IL-6, TNF-α). Second, although we examined three different forms of stress, our measures of them are not without limitations. For instance, the majority of items in our daily interpersonal stress measure focused on the family domain and the prevalence of certain family stressors has been shown to decline from mid to late adolescence (Laursen, Coy, & Collins, 1998). It will be important for future work to include broader measures of daily stressors before definitively concluding that daily stress has minimal impact on inflammation during the latter period of adolescence. Third, our sample was drawn from communities of European-, Latino-, and Asian-American adolescents in the greater Los Angeles area. Whether findings generalize to young persons of other ethnicities and geographical locations should be examined. For instance, the extent to which the stressors assessed in the present study are distressing may differ for those in rural or more disadvantaged contexts. Lastly, the clinical significance of our findings could not be ascertained, as no direct measures of health outcomes were included in the current study.
In summary, results from the present investigation suggest that CRP levels increase through mid-adolescence and into young adulthood and rise during times of higher perceived stress. However, we found no evidence that differential perceptions of stress and exposure to adverse major life events or more mundane daily events influenced the developmental trajectory of CRP in young, relatively healthy individuals. These data highlight the critical role of subjective perception and interpretation in shaping biological responses to stress. They also underscore the importance of assessing such subjective appraisals when analyzing links between stress and inflammation in adolescence and young adulthood when background levels of systemic inflammation remain relatively low.
Supplementary Material
Acknowledgements:
Preparation of this manuscript was supported by the National Heart, Lung, and Blood Institute (F32-HL134276 to J.J.C.). Collection of data was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01-HD062547; P2C-HD041022), and the National Institute on Aging (P30-AG028748; P30-AG017265).
Footnotes
Conflicts of interest: None.
Analyses limited to participants who completed all three waves of data collection yielded similar results, with CRP increasing significantly with age (b(SE) = .18(.03), p < .001) and greater waist circumference (b(SE) = .28(.12), p = .025). In addition, adolescents from Asian-American backgrounds (b(SE) = -.52(.25), p = .039) and parents with higher education (b(SE) = -.38(.10), p < .001) had lower CRP. In addition, analyses of raw, non-transformed values of CRP revealed similar results. There was a significant increasing linear trend over age (b(SE) = .12(.03), p < .001), and Asian-American ethnicity and higher parental education were associated with lower CRP levels (ps < .005). Contrasting results for natural log-transformed CRP, waist circumference did not co-vary with raw values of CRP within a person (p = .132).
Reference
- Agostinis-Sobrinho C, Ruiz JR, Moreira C, Abreu S, Lopes L, Oliveira-Santos J, … Santos R (2018). Ability of nontraditional risk factors and inflammatory biomarkers for cardiovascular disease to identify high cardiometabolic risk in adolescents: results from the LabMed Physical Activity Study. Journal of Adolescent Health 62(3), 320–326. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. (2014). Stress in America: Are teens adopting adults’ stress habits. Stress in America Surveys (http://www.apa.org/news/press/releases/stress/2013/stress-report.pdf). [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, … & Munter P (2017). Heart disease and stroke statistics–2017 update: a report from the American Heart Association. Circulation, 135(10), e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, & Wattigney WA (1998). Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. New England Journal of Medicine 338(23), 1650–1656. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology 65, 187–207. [DOI] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, Irwin MR, Taylor SE, & Fuligni AJ (2017). Adiposity moderates links from early adversity and depressive symptoms to inflammatory reactivity to acute stress during late adolescence. Brain, Behavior, and Immunity 66, 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang JJ, Bower JE, & Taylor SE (2015). Early adversity, neural development, and inflammation. Developmental Psychobiology 57(8), 887–907. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, & Miller GE (2007). Psychological stress and disease. Journal of the American Medical Association 298(14), 1685–1687. [DOI] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived Stress in a probability sample of the United States. In Spacapan S & Oskamp S (Eds.), The Social Psychology of Health Newbury Park, CA: Sage. [Google Scholar]
- Conger RD, Wallace LE, Sun Y, Simons RL, McLoyd VC, & Brody GH (2002). Economic pressure in African American families: a replication and extension of the family stress model. Developmental Psychology 38(2), 179–193. [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman C, & Costello EJ (2014). Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proceedings of the National Academy of Sciences 111(21), 7570–7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Kim JK, McCreath H, Faul J, Weir D, & Seeman T (2014). Validation of blood-based assays using dried blood spots for use in large population studies. Biodemography and social biology 60(1), 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, & Rifai N (2006). Inflammation and changes in metabolic syndrome abnormalities in US adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clinical chemistry 52(7), 1325–1330. [DOI] [PubMed] [Google Scholar]
- DeBoer MD (2013). Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: a need for screening tools to target interventions. Nutrition 29(2), 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D, Meng H, Goldberg R, Schneiderman N, & Delamater A (2009). Stress and Body Mass Index Each Contributes Independently to Tumor Necrosis Factor-α Production in Prepubescent Latino Children. Journal of Pediatric Nursing 24(5), 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa A, D’Agostino R Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, & Haffner SM (2001). The relation of body fat mass and distribution to markers of chronic inflammation. International journal of obesity 25(10), 1407–1415. [DOI] [PubMed] [Google Scholar]
- Fuligni AJ, Telzer EH, Bower J, Cole SW, Kiang L, & Irwin MR (2009). A preliminary study of daily interpersonal stress and C-reactive protein levels among adolescents from Latin American and European backgrounds. Psychosomatic Medicine 71(3), 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, & Kiecolt-Glaser J (2012). Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychology 31(2), 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy RP, & Seeman TE (2009). Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Social Science & Medicine 69(3), 451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C (1991). Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology 100(4), 555–561. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Lachman ME, Mroczek DK, Seeman TE, & Miller GE (2015). Additive contributions of childhood adversity and recent stressors to inflammation at midlife: Findings from the MIDUS study. Developmental Psychology 51(11), 1630–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Daniels M, & Weiner H (1987). Immune and neuroendocrine changes during bereavement. Psychiatric Clinics of North America 10(3), 449–465. [PubMed] [Google Scholar]
- Khera A, McGuire DK, Murphy SA, Stanek HG, Das SR, Vongpatanasin W, … de Lemos JA (2005). Race and gender differences in C-reactive protein levels. Journal of the American College of Cardiology 46(3), 464–469. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, & Hantsoo L (2010). Close relationships, inflammation, and health. Neuroscience & Biobehavioral Reviews 35(1), 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen B, Coy KC, & Collins WA (1998). Reconsidering changes in parent‐child conflict across adolescence: A meta‐analysis. Child Development 69(3), 817–832. [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, & Folkman S (1984). Stress, appraisal, and coping New York: Springer. [Google Scholar]
- Libby P (2006). Inflammation and cardiovascular disease mechanisms. The American Journal of Clinical Nutrition 83(2), 456S–460S. [DOI] [PubMed] [Google Scholar]
- Liu JJW, Ein N, Peck K, Huang V, Pruessner JC, & Vickers K (2017). Sex differences in salivary cortisol reactivity to the Trier Social Stress Test (TSST): a meta-analysis. Psychoneuroendocrinology 82, 26–37. [DOI] [PubMed] [Google Scholar]
- Low CA, Matthews KA, & Hall M (2013). Elevated CRP in adolescents: roles of stress and coping. Psychosomatic Medicine 75(5), 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Burhop J, & Dohnal J (2004). High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clinical chemistry 50(3), 652–654. [DOI] [PubMed] [Google Scholar]
- McDade TW, Hawkley LC, & Cacioppo JT (2006). Psychosocial and behavioral predictors of inflammation in middle-aged and older adults: the Chicago health, aging, and social relations study. Psychosomatic Medicine 68(3), 376–381. [DOI] [PubMed] [Google Scholar]
- McGill HC Jr., McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP, & Group, P. D. o. A. i. Y. R. (2000). Origin of atherosclerosis in childhood and adolescence–. The American Journal of Clinical Nutrition 72(5), 1307s–1315s. [DOI] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology 16(1), 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, & Chen E (2010). Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychological Science 21(6), 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Parker KJ (2011). Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychological Bulletin 137(6), 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, … Sloan EK (2009). To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain, Behavior, and Immunity 23(7), 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M, … Myers GL (2003). Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3), 499–511. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, & Heymsfield SB (1998). Body mass index as a measure of adiposity among children and adolescents: a validation study. The Journal of Pediatrics 132(2), 204–210. [DOI] [PubMed] [Google Scholar]
- Pyykkönen AJ, Räikkönen K, Tuomi T, Eriksson JG, Groop L, & Isomaa B (2010). Stressful life events and the metabolic syndrome. Diabetes Care 33(2), 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1(3), 385–401. [Google Scholar]
- Räikkönen K, Matthews KA, & Kuller LH (2007). Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women. Diabetes Care 30(4), 872–877. [DOI] [PubMed] [Google Scholar]
- Romeo RD (2017). The impact of stress on the structure of the adolescent brain: implications for adolescent mental health. Brain research 1654, 185–191. [DOI] [PubMed] [Google Scholar]
- Rost NS, Wolf PA, Kase CS, Kelly-Hayes M, Silbershatz H, Massaro JM, … Wilson PWF (2001). Plasma concentration of C-reactive protein and risk of ischemic stroke and transient ischemic attack. Stroke 32(11), 2575–2579. [DOI] [PubMed] [Google Scholar]
- Shay CM, Ning H, Daniels SR, Rooks CR, Gidding SS, & Lloyd-Jones DM (2013). Status of Cardiovascular Health in US Adolescents: Prevalence Estimates from the National Health and Nutrition Examination Surveys (NHANES) 2005–2010. Circulation, 127, 1369–1376. [DOI] [PubMed] [Google Scholar]
- Slopen N, Koenen KC, & Kubzansky LD (2012). Childhood adversity and immune and inflammatory biomarkers associated with cardiovascular risk in youth: a systematic review. Brain, Behavior, and Immunity 26(2), 239–250. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, & Koenen KC (2013). Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology 38(2), 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, Lewis TT, Gruenewald TL, Mujahid MS, Ryff CD, Albert MA, & Williams DR (2010). Early life adversity and inflammation in African Americans and whites in the midlife in the United States survey. Psychosomatic Medicine 72(7), 694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RW, Jones IE, Williams SM, & Goulding A (2000). Evaluation of waist circumference, waist-to-hip ratio, and the conicity index as screening tools for high trunk fat mass, as measured by dual-energy X-ray absorptiometry, in children aged 3–19 y. The American Journal of Clinical Nutrition 72(2), 490–495. [DOI] [PubMed] [Google Scholar]
- Tottenham N, & Galván A (2016). Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neuroscience & Biobehavioral Reviews 70, 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KM, Telzer EH, Gonzales NA, & Fuligni AJ (2013). Adolescents’ daily assistance to the family in response to maternal need. Journal of Marriage and Family 75(4), 964–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


