Abstract
Rationale:
Peptidylarginine deiminase type IV (PAD4), an enzyme essential for NET formation (NETosis), is released together with neutrophil extracellular traps (NETs) into the extracellular milieu. It citrullinates histone and holds the potential to citrullinate other protein targets. While NETosis is implicated in thrombosis, the impact of the released PAD4 is unknown.
Objective:
This study tests the hypothesis that extracellular PAD4, released during inflammatory responses, citrullinates plasma proteins, thus affecting thrombus formation.
Methods and Results:
Here we show that injection of r-huPAD4 in vivo induces formation of von Willebrand factor (VWF)-platelet strings in mesenteric venules, and that this is dependent on PAD4 enzymatic activity. VWF-platelet strings are naturally cleaved by a disintegrin and metalloproteinase with thrombospondin type-1 motif-13 (ADAMTS13). We detected a reduction of endogenous ADAMTS13 activity in the plasma of wild-type mice injected with r-huPAD4. Using mass spectrometry and in vitro studies, we found that r-huPAD4 citrullinates ADAMTS13 on specific arginine residues, and that this modification dramatically inhibits ADAMTS13 enzymatic activity. Elevated citrullination of ADAMTS13 was observed in plasma samples of patients with sepsis or non-infected patients who were elderly (e.g. age >65 years) and/or had underlying co-morbidites (e.g. diabetes, hypertension) as compared to healthy donors. This shows that ADAMTS13 is citrullinated in vivo. VWF-platelet strings that form on venules of Adamts13−/− mice were immediately cleared after injection of r-huADAMTS13, while they persisted in vessels of mice injected with citrullinated r-huADAMTS13. Next, we assessed the effect of extracellular PAD4 on platelet plug formation after ferric chloride-induced injury of mesenteric venules. Administration of r-huPAD4 decreased time to vessel occlusion and significantly reduced thrombus embolization.
Conclusion:
Our data indicate that PAD4 in circulation reduces VWF-platelet string clearance and accelerates formation of a stable platelet plug after vessel injury. We propose that this effect is, at least in part, due to ADAMTS13 inhibition.
Keywords: PAD4, citrullination, ADAMTS13, VWF-platelet strings, thrombosis, von Willebrand factor, inflammation
Subject Terms: Animal Models of Human Disease, Basic Science Research, Inflammation, Thrombosis
Graphical Abstract
INTRODUCTION
Venous thrombosis is a common cardiovascular disorder associated with high morbidity and mortality. Von Willebrand factor (VWF), an important regulator of haemostasis, thrombus formation, and inflammation, plays an important role in venous thrombosis.1, 2 In fact, not only do VWF-deficient mice not develop thrombi in the inferior vena cava stenosis model,3 but VWF is also found to be enriched in thrombi from venous thromboembolism patients.1 VWF, a large multimeric glycoprotein produced by endothelial cells and stored in Weibel-Palade bodies,4 is secreted in plasma through a basal mechanism and is released in larger quantities upon activation of the endothelium.5-7 Microscopy studies show that, upon endothelium stimulation, secreted ultra-large VWF multimers (UL-VWF) remain attached to the cell surface and bind platelets, forming VWF-platelet strings.8 Unstimulated cells also release UL-VWF multimers in the lumen of the vessel. This basal mechanism has been suggested as the main source of circulating plasma VWF.9 In normal conditions, the secreted UL-VWF-platelet strings are cleared from the vessel wall and are converted into smaller, less thrombogenic fragments by a disintegrin and metalloproteinase with thrombospondin type-1 motif-13 (ADAMTS13).8 Reduced activity or absence of ADAMTS13 prolongs the VWF interactions with the vessel wall. This leads to excessive platelet and leukocyte recruitment and microvascular thrombosis that can eventually culminate in severe multi-organ failure and mortality.10 Deficiency of ADAMTS13 is linked to thrombotic thrombocytopenic purpura (TTP), a genetic or autoimmune disease characterized by autoantibodies to ADAMTS13.11, 12 However, reduction in ADAMTS13 activity has also been observed in several thrombo-inflammatory disorders, such as disseminated intravascular coagulation (DIC), stroke, and deep vein thrombosis (DVT).13-16 Use of r-huADAMTS13 in mice can prevent microthrombosis and reduce inflammation, showing the beneficial effects of ADAMTS13 in stroke and myocardial infarction models.17, 18
In addition to platelets and fibrin, neutrophils and neutrophil extracellular traps (NETs) are also observed in venous thrombi.19, 20 NETs promote coagulation, thrombosis, and inflammatory responses.21 A driving force in NETosis is the nuclear citrullination of histones by peptidylarginine deiminase type IV (PAD4).22 PAD4 is a member of a family of PAD enzymes that, upon Ca2+-induced activation, citrullinate their protein substrates, many of which contribute to vital cellular processes, including gene regulation, cellular differentiation, and inflammatory responses.23 During protein citrullination, positively charged arginine residues are deiminated to generate the neutral citrulline. PAD4 is then released into the extracellular environment along with decondensed chromatin.24 Dysregulated PAD activity and abnormal levels of protein citrullination are associated with inflammation and disease. In fact, increased concentrations of extracellular PAD enzyme with citrullinated proteins are found in samples of rheumatoid arthritis (RA), multiple sclerosis, and Alzheimer’s patients. 25-27 The concentration in plasma has been found to be approximately 2.4 to 7.5 fold higher than in healthy controls.28, 29 Increased plasma levels of PAD4 have also been found in septic patients30 and in patients with malignant tumors.31
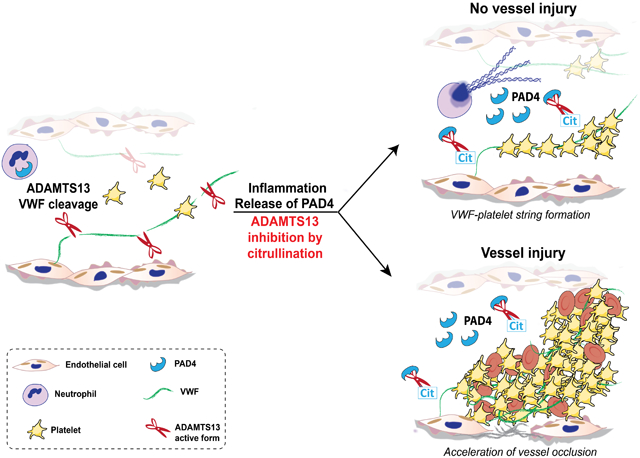
Although citrullinated plasma proteins, such as citrullinated fibrinogen and anti-thrombin, have been identified in plasma samples of RA and cancer patients,31-33 the role of circulating PAD4 and its ability to regulate thrombosis has not been addressed. Therefore, we evaluated, by intravital microscopy of the mesenteric venules, the effect of injected PAD4 in quiescence and after venule injury. Our results show that PAD4 promotes VWF-platelet string formation and accelerates and stabilizes the development of occlusive thrombi in injured venules. Furthermore, we observed that PAD4 reduces ADAMTS13 activity. This behavior may explain the pro-thrombotic effects of plasma PAD4.
METHODS
All supporting data are available within the article and its online supplementary files. For details on experimental procedures, see supplemental material.
RESULTS
Elevated r-huPAD4 in circulation leads to formation of VWF-platelet strings and reduction of plasma ADAMTS13 activity.
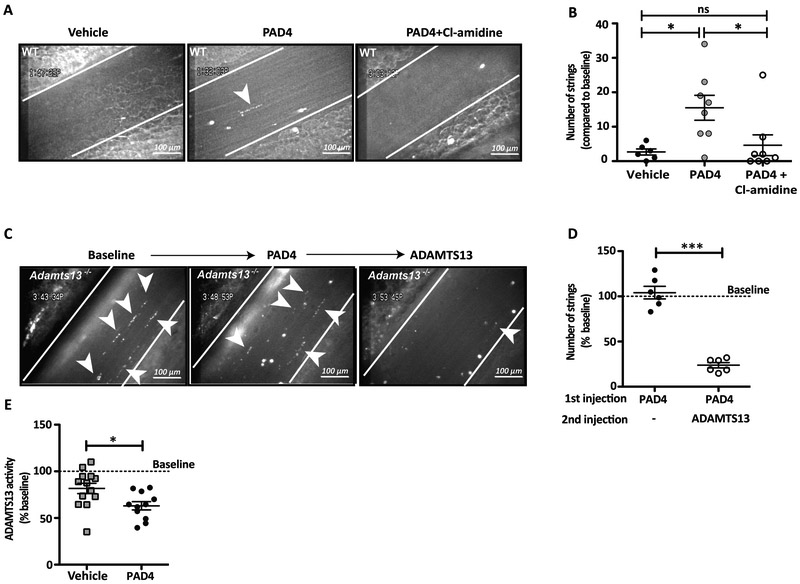
Endothelial cells release UL-VWF molecules in the vessel lumen in the absence of stimuli through a basal secretion mechanism.9 These are normally cleared from the endothelial surface by ADAMTS13 to prevent pro-thrombotic platelet string formation. To determine the effect of PAD4 in circulation on this process, we injected WT mice with r-huPAD4 or vehicle and analyzed mesenteric venules by intravital microscopy under basal conditions. We observed spontaneous formation of VWF-platelet strings in mice treated with r-huPAD4 (Figure 1A). Although most of these strings were short-lived (<30 seconds), their number was significantly higher than in mice injected with buffer (Figure 1B; mean values 2.6 for vehicle and 15.5 for PAD4). To determine if spontaneous formation of VWF-platelet strings was induced by PAD4 activity, mice were co-administered PAD4 and Cl-amidine, an irreversible small molecule PAD-inhibitor.34 As shown in Figure 1A and 1B (mean value 4.62), very few strings formed in the mesenteric venules of the inhibitor-treated mice, indicating that inhibition of PAD4 preserved the natural clearance of the VWF-platelet strings.
Figure 1. Injection of PAD4 leads to VWF-platelet string formation and reduces ADAMTS13 activity.
A, Representative intravital microscopy images of VWF-platelet strings (indicated with arrowheads) anchoring to venules of WT mice. Vessels were recorded for 5 minutes (baseline), then mice were injected with vehicle, r-huPAD4, or r-huPAD4 together with Cl-amidine, and the same vessel was monitored. B, Quantification of number of strings compared to baseline (One-way ANOVA, Tukey’s multiple comparison test; * p= 0.02 buffer vs r-huPAD4 and p=0.03 r-huPAD4 vs Cl-amidine). Data represent the mean ± SEM (n= 6–8 mice). C, VWF-platelet strings were monitored for 5 minutes (baseline) in Adamts13−/− mice. Mice were injected with r-huPAD4 and recorded for an additional 5 minutes. Next, r-huADAMTS13 was administered intravenously and the same mesenteric vessel was recorded again. D, Quantification of percentage of strings compared to baseline (unpaired t test; *** p= 0.0001). Data represent mean ± SEM (n= 6 mice). E, Plasma from WT mice was collected before (baseline) or after infusion of vehicle or r-huPAD4. ADAMTS13 activity in mouse plasma was measured by FRETS-VWF73 assay. The slope of each cleavage reaction was calculated and compared to baseline (unpaired t test; * p= 0.018). Data represent mean ± SEM (n= 11–13 mice).
Next, to evaluate whether PAD4 could render the VWF platelet strings uncleavable by ADAMTS13, we used Adamts13−/− mice. ADAMTS13 deficiency is associated with pre-activation of endothelial cells of mesenteric vessels in mice.35 Therefore, released UL-VWF accumulates on mesenteric venules of Adamts13−/− mice, binds platelets, and can be visualized by intravital microscopy after labelling with rhodamine 6G. We first treated Adamts13−/− mice with r-huPAD4. This had no effect on platelet string formation or persistence of the strings in these mice (Figure 1C and D; mean value 104.11). Subsequent injection of high concentrations36 of r-huADAMTS13 (3200 U/kg) cleared most of the strings (Figure 1C and D; mean value 23.75), indicating that VWF-platelet strings are still susceptible to cleavage by ADAMTS13. Since the number of VWF-platelet strings in mice injected with r-huPAD4 did not increase compared to baseline, we excluded the possibility that r-huPAD4 might stimulate VWF release from the endothelium (Figure 1D). Therefore, we hypothesized that, in circulation, PAD4 might be directly affecting ADAMTS13 activity. To test this hypothesis, we collected plasma samples of WT mice before and after injection of r-huPAD4 or vehicle and assessed endogenous ADAMTS13 activity by FRETS-VWF73 assay. Figure 1E shows that the presence of r-huPAD4 reduced endogenous ADAMTS13 activity (approximately of 20%; mean values 81.7 and 63.1 for vehicle and PAD4 respectively), suggesting that circulating PAD4 might citrullinate and partially inactivate ADAMTS13, leading to the appearance of VWF-platelet strings.
PAD4 citrullinates ADAMTS13 in vitro and in vivo and reduces its capacity to cleave VWF.
To determine whether PAD4 citrullinates ADAMTS13, we incubated r-huPAD4 with r-huADAMTS13; r-huADAMTS13 was then immunopurified using an anti-ADAMTS13 antibody and citrullination was detected by western blot (Figure 2A). A band of approximately 190 kDa, corresponding to ADAMTS13, was observed only when r-huADAMTS13 and r-huPAD4 were incubated together (Figure 2A; upper panel). As expected, using an anti-ADAMTS13 antibody, r-huADAMTS13 was found in all samples to which it had been added (Figure 2A, lower panel). To verify citrullination, immunopurification was also carried out using an anti-pan-citrulline antibody. Again, a band of 190 kDa was detected only in the sample where r-huADAMTS13 had been incubated with r-huPAD4 (Figure 2A), indicating that r-huPAD4 can citrullinate r-huADAMTS13. Next, we tested whether citrullination of ADAMTS13 could occur in the physiological environment of plasma. Adamts13−/− mice were injected with 3200 U/kg of r-huADAMTS13. Plasma was collected and incubated ex vivo with r-huPAD4. ADAMTS13 was then immunopurified and analyzed by western blot. As shown in Figure 2B, a band of approximately 190 kDa was observed in plasma samples collected from mice injected with r-huADAMTS13 and treated with r-huPAD4 ex vivo, whereas no band was detected with the anti-pan-citrulline antibody in plasma to which r-huPAD4 was not added. A faint band corresponding to r-huADAMTS13 was detected when lower concentrations of r-huPAD4 were used in the assay. Membranes were then stripped and re-probed with an anti-ADAMTS13 antibody. A band of approximately 190 kDa was present in all lanes. Our data indicate that plasma does not interfere with citrullination of ADAMTS13 and, thus, citrullination may occur in circulation.
Figure 2. PAD4 citrullinates and inactivates ADAMTS13 in vitro.
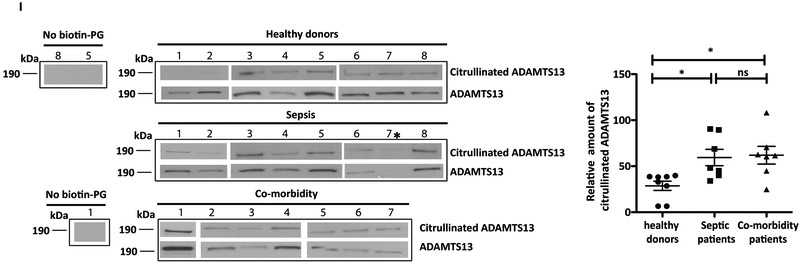
A, B, r-huADAMTS13 was incubated with r-huPAD4. A, ADAMTS13 was immunopurified using an anti-ADAMTS13 antibody or B, citrullinated proteins were isolated using an anti-pan-citrulline antibody. Membranes were blotted with the indicated antibody (left side of blots) and stripped and re-probed with an anti-ADAMTS13 antibody (A; lower panel). The band at approximately 190 kDa indicates ADAMTS13. C, Plasma from Adamts13−/− mice injected with r-huADAMTS13 was collected and incubated with r-huPAD4. ADAMTS13 was immunopurified with an anti-ADAMTS13 antibody and detected by Western blot using an anti-pan-citrulline antibody (upper panel; lower molecular weight plasma proteins shown to non-specifically bind to Sepharose beads were excluded from figure). Membranes were stripped and re-probed using an anti-ADAMTS13 antibody (lower panel). C, D, E, Citrullination of ADAMTS13 was performed by incubating r-huADAMTS13 with r-huPAD4 for 15, 90, and 180 minutes. C, Citrullination of r-huADAMTS13 was detected by Western blot with an anti-pan-citrulline antibody as a band of 190 kDa (upper arrow). The band at 74 kDa (lower arrow) corresponds to auto-citrullinated r-huPAD4. D, E, Activity of r-huADAMTS13 was determined by FRETS-VWF73 assay (60 minutes). D, Fluorescent counts changes in FRETS-VWF73 as a function of time. E, ADAMTS13 activity of the different samples expressed as percentage of that observed for r-huADAMTS13 in the absence of PAD4 (One-way ANOVA, Tukey’s multiple comparison test; ** p=0.0054, *** p=0.0001). F, G, H, Citrullination of ADAMTS13 was performed by incubating r-huADAMTS13 with r-huPAD4 for 180 minutes with or without Cl-amidine. F, Citrullination of r-huADAMTS13 observed by Western blot. G, H, Activity of r-huADAMTS13 determined by FRETS-VWF73 assay (One-way ANOVA, Tukey’s multiple comparison test; *** p=0.0001). Data are representative of 3 independent experiments and expressed as mean ± SEM. I, Western blot of citrullinated-ADAMTS13 and ADAMTS13 from plasma of young healthy donors, septic patients, and co-morbidity patients. Citrullinated ADAMTS13 was labeled with biotin-PG and immunopurified using streptavidin beads. ADAMTS13 was then detected by western blot with anti-ADAMTS13 antibody. As control, healthy donor samples 8 and 5, and co-morbidity donor sample 1, were immunopurified without modification by biotin-PG. Graph represents the relative amount of citrullinated ADAMTS13 for each sample. Septic patient number 7* was excluded from analysis because ADAMTS13 levels could not be detected. (One-way ANOVA, Tukey’s multiple comparison test, p value 0.01; n=7–8 donors).
To assess whether citrullination of ADAMTS13 interferes with its activity, as suggested by the results depicted in Figure 1E, we incubated r-huADAMTS13 with r-huPAD4 over time (for 15, 60, and 180 minutes). Citrullination was then evaluated by western blot, using the anti-pan-citrulline antibody as described above, and ADAMTS13 activity was assessed by VWF-FRETs assay (activity assay was performed for 60 minutes per the manufacturer’s instructions). Interestingly, the intensity of the 190 kDa band increased over time (Figure 2C, upper arrow). The second band of 74 kDa (Figure 2C, lower arrow) corresponds to r-huPAD4, which undergoes auto-citrullination and is therefore detected here by the anti-pan-citrulline antibody.37
To investigate the impact of citrullination on the function of ADAMTS13, we assessed the activity of r-huADAMTS13 with different levels of citrullination by FRETS-VWF73 assay.38 As shown in Figure 2D and 2E, citrullination of r-huADAMTS13 dramatically reduced its capacity to cleave FRETS-VWF73 substrate. Moreover, decreased activity correlated with incubation time (Figure 2C, 2D, and 2E; mean values of 69.5, 15.4, and 0.35 for 15, 90, and 180 minutes, respectively). Next, we determined if we could preserve ADAMTS13 activity by inhibiting PAD4 with Cl-amidine.34 Pre-incubation of PAD4 with Cl-amidine reduced citrullination of ADAMTS13 and auto-citrullination of PAD4 (Figure 2F; upper and lower arrow, respectively). Furthermore, ADAMTS13 activity was partially preserved when ADAMTS13 was incubated with PAD4 in the presence of Cl-amidine (Figure 2G and 2H; mean values of 2.87 for PAD4 incubation and 42.8 when Cl-amidine was added), further indicating that citrullination inactivates the proteolytic activity of ADAMTS13.
To determine whether citrullination of ADAMTS13 could occur during inflammatory events, we immunopurified citrullinated ADAMTS13 from the plasma of septic patients and older patients (>65 years) that had underlying co-morbidities (e.g. diabetes, hypertension) and compared the citrullinated ADAMTS13 levels to those of young and healthy donors. Excessive NET formation has been found in patients affected by sepsis and has been associated with severity of the disease.39-41 In fact, in vitro and animal experiments indicate that NETs contribute to the pathogenesis of intravascular thrombosis, disseminated intravascular coagulation (DIC), and multiple organ dysfunction in septic patients.42-44 Interestingly, severe sepsis is also characterized by a reduction in ADAMTS13 activity.13, 45 Similarly, an increase in NET formation is also found in chronic inflammatory conditions, such as heart disease46, diabetes,47, 48 and in aged mice, where it has been suggested to promote cardiac fibrosis and organ dysfunction.49
To determine whether citrullination of ADAMTS13 occurs in vivo, low abundant plasma proteins were enriched in plasma by the removal of high abundant proteins using spin columns. After a 10-fold sample concentration, citrullinated proteins were labelled with biotin-PG and immunopurified using streptavidin Sepharose beads. Samples were then loaded on gel, and ADAMTS13 was detected by western blot. As observed in figure 2I, bands of 190 kDa corresponding to ADAMTS13 were detected in nearly all of the samples analyzed, indicating that citrullination occurs in vivo. The intensity of the bands was normalized to the plasmatic levels of ADAMTS13 for each individual. Notably, the intensity of the citrullinated ADAMTS13 bands from the plasma of septic patients was higher when compared to healthy controls (mean value 59.4 and 28.7 respectively).
However, the levels were not significantly different from donors with other co-morbidities (mean value 61.9), indicating that elevation in ADAMTS13 citrullination is not specific to sepsis. As a control, plasma samples were immunopurified without the addition of biotin-PG. No band was observed in these samples (Figure 2I).
Identification of citrullinated arginine residues in ADAMTS13 by tandem mass spectrometry.
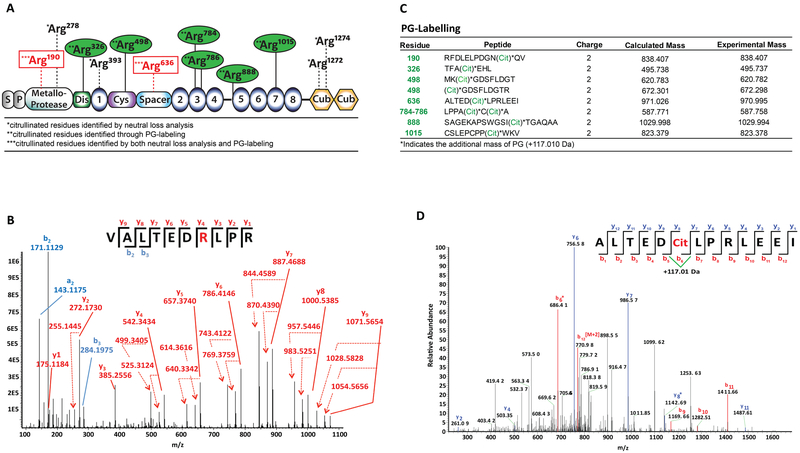
Citrullination of r-huADAMTS13 was confirmed by high-performance tandem mass spectrometry, using both the manual searches for neutral losses of isocyanic acid and ammonia in the obtained spectra and the recent biochemical approach of citrulline labelling by phenylgloxal (PG)50. For detection of ADAMTS13 residues modified by in vitro citrullination, proteins were separated by PAGE, and the 190 kDa band was dissected into slices. Non-citrullinated r-huADAMTS13 was used as control. After in-gel tryptic digestion, peptides were eluted and analyzed by nUPLC-MS/MS to confirm the identity of the protein and determine the positions of the citrullinated residues (citrullination was set as one of the variable modifications to be considered during the searches). The fragmentation pattern of each peptide was manually examined to confirm citrullination of the candidate residue (increase of mass of 0.9840 Da) and exclude the possibility of false positive assignments, e.g., in cases of deamination of asparagine or glutamine (which also increases the mass by 0.98 Da). In addition to backbone cleavage products (b and y ions), the neutral losses of isocyanic acid (HNCO, 43 Da) and ammonia (NH3, 17 Da), abundant features in collision-induced dissociation (CID) MS/MS spectra of citrullinated peptides,51 were considered to identify citrullinated residues. Citrullination was detected on Arg190 and Arg278 in the metalloproteinase domain, Arg393 in the thrombospondin-1 repeat, Arg636 in the spacer domain, and Arg1274 and Arg1276 in the CUB-1 domain (Figure 3A). Figure 3B shows the CID MS/MS spectrum that corresponds to the peptide 630VALTEDrLPR639(r= citrullinated Arg). The increased mass of the intact peptide, together with the identification of the isocyanic acid and ammonia neutral losses (e.g., y4-HCNO, y4-NH3), allowed for the assignment of citrullination at Arg636. There was some evidence for low-level citrullination at additional sites; however, our confidence in the assignment of citrullination remained quite weak and, therefore, they are not listed. A recent deep proteome investigation of citrullination certified that a high degree of caution must be exercised in making assignments for citrullination based on database searches, since it was found that only a low percentage of assigned sites were actually citrullinated.52
Figure 3. Identification of citrullinated arginine residues by tandem mass spectrometry.
A, Schematic representation of the identified citrullinated arginine residues within the different domains of r-huADAMTS13. Arg residues identified through PG-labeling are indicated in green; those identified by both neutral loss analysis and PG-labeling are identified in red. B, CID mass spectrum of peptide 630VALTEDrLPR639. The y ion series (y1-y9, solid lines), together with the neutral losses (dashed lines) of NHCO (43 Da) and NH3 (17 Da) are marked in red. The N-terminal fragments are labeled in blue. Arg190 was found to be citrullinated, as shown by the neutral losses of NHCO and NH3. All sites identified as citrullinated were found in two independent experiments. C, D, Compiled mass spectrometry data of citrullinated ADAMTS13 labeled with PG. Multiple examinations of labeled ADAMTS13, digested with neutrophil elastase, were searched against the human proteome with the additional mass of +117.010 Da on Arg residues. C, ADAMTS13 residues in which we have high confidence of citrullination labeling (cit). D, MS2 fragmentation of the peptide containing Arg636. MS2 spectra were analyzed for ions corresponding to the additional mass of +117.010 Da. The fragmentation pattern for Arg636 shows the mass shift on b6* and y8*.
Protein citrullination increases the mass at each modified site by only 0.98 Da; thus, it is difficult to identify all citrullination sites in a protein. For this reason, we also used a chemical labelling approach to identify additional citrullination sites in ADAMTS13. Here, citrullinated r-huADAMTS13 was labelled with phenylgloxal (PG), which selectively modifies citrullinated residues under acidic conditions; the modified peptide fragments show a mass increase of +117.010 Da.27, 53 For the protein digestion step, we used neutrophil elastase (NE) over trypsin because it cleaves after alanine and valine residues rather than arginine and lysine and can therefore yield enhanced protein coverage. The resulting peptides were then subjected to LC-MS/MS analysis. As shown in Figure 3A and 3C, 8 PG modified ADAMTS13 derived peptides were found, including Arg190 in the metalloproteinase domain, Arg326 in the disintegrin domain, Arg498 in the cysteine-rich domain, Arg636 in the spacer domain, and Arg784, Arg786, Arg888, and Arg1015 in thrombospondin type-1 repeats. Figure 3D depicts a representative high-resolution MS2 fragmentation pattern of the peptide containing the citrullinated Arg636 residue. An additional mass of +117.010 Da was observed on ions b6 and y8 confirming PG modification of this residue. Residues Arg190 and Arg636, which were identified by both methods, are highlighted with red squares in panel A.
Citrullinated ADAMTS13 is unable to cleave VWF-platelet strings from the vessel wall of Adamts13−/− mice.
To define whether citrullination of r-huADAMTS13 affects VWF-platelet string cleavage in vivo, we monitored VWF-platelet strings on externalized mesenteric venules of Adamts13−/− mice before and after injection of non-citrullinated r-huADAMTS13 or citrullinated r-huADAMTS13. As expected, platelet strings were observed in all venules of approximately 150–250 μm of diameter in untreated Adamts13−/− mice (Baseline, Figure 4A). VWF-platelet strings were completely cleared after injection of r-huADAMTS13 (mean value of 12.7); strikingly, they persisted in mice treated with citrullinated r-huADAMTS13. These results indicate that citrullinated r-huADAMTS13 was not functional in vivo, as it was unable to clear VWF-platelet strings from the vessel wall. Subsequent injection of r-huADAMTS13 was indeed able to remove the platelet strings (data not shown), showing that citrullinated r-huADAMTS13 did not interfere with the activity of functional r-huADAMTS13. Since r-huPAD4 was present in the citrullinated r-huADAMTS13 samples, as a control we also injected the same concentration of r-huPAD4 in the Adamts13−/− mice, and no effect on VWF strings was observed. Quantification of VWF-platelet strings obtained from several animals treated as shown in Figure 4A is presented in Figure 4B. Our results indicate that citrullinated r-huADAMTS13 is unable to remove VWF-platelet strings from the vessel wall.
Figure 4. Citrullinated ADAMTS13 fails to cleave VWF-platelet strings in vivo.
A, VWF-platelet strings anchoring to endothelium observed in Adamts13−/− mice were monitored for 5 minutes (Baseline, upper panel). Mice were injected (as indicated) with r-huADAMTS13 (3200U/kg), citrullinated r-huADAMTS13 (3200U/kg), or r-huPAD4 (0.28mU/g), and the mesenteric vessels were recorded for an additional 5 minutes after injection (lower panels). Representative photographs are shown. Arrowheads indicate VWF-platelet strings. B, Quantification of percentage of strings compared to baseline. One-way ANOVA, Tukey’s multiple comparison test (n=6 mice; # p=0.005, ### p=0.0001 and *** p=0.0001; # indicates significance compared to baseline).
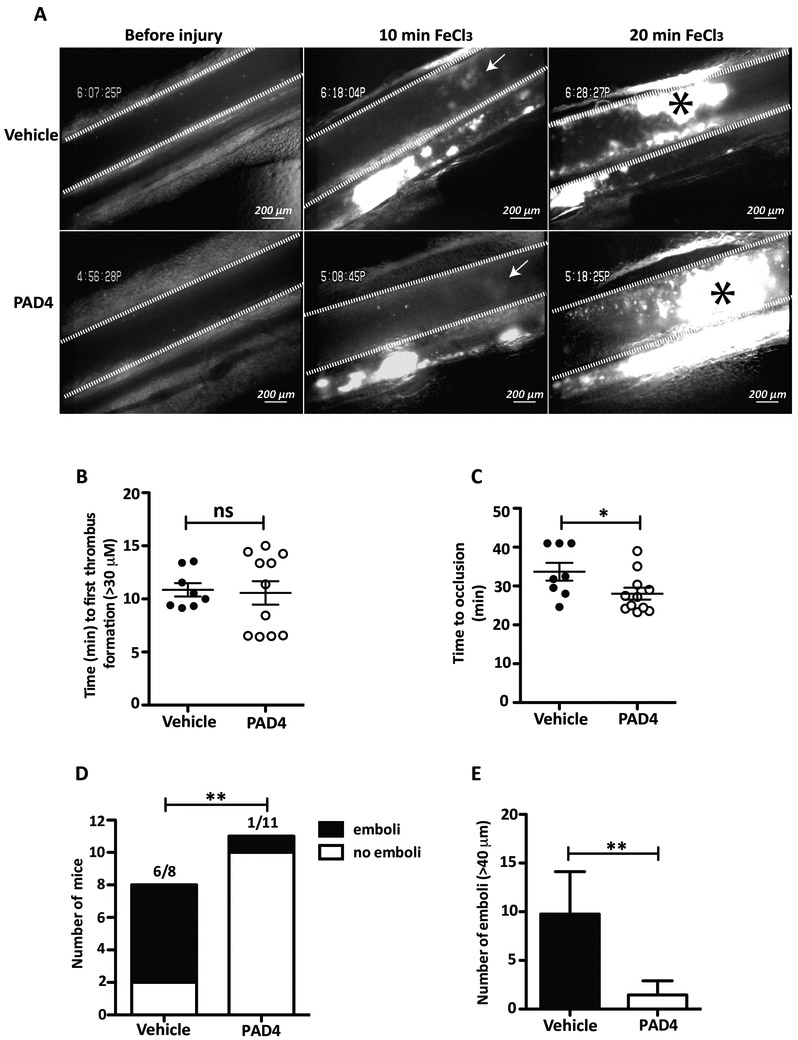
PAD4 injection in WT mice accelerates venule thrombus formation after FeCl3 injury and reduces embolization.
It has been shown that ADAMTS13 inhibition in mice, using inhibitory antibodies, promotes thrombi formation.35 To evaluate whether PAD4 might also possess pro-thrombotic effects, we injected r-huPAD4 (0.028U/g) or vehicle intravenously into WT mice prior to injury of the mesenteric venules by FeCl3. The resulting concentration in plasma (approximately 0.28U/ml) was less than seen in RA patients (from 1.5 mU/ml to 111 U/ml).28, 29 To analyze embolization, larger vessels were used (250–350 μm). We then observed the vessel by intravital microscopy (Figure 5A). No difference in the length of time to form the first thrombus of 30 μm in diameter was observed between the two groups (Figure 5B). However, WT mice injected with r-huPAD4 grew thrombi to occlusive size in 28 minutes, whereas mice injected with vehicle occluded 34.2 minutes after injury (Figure 5C). These data indicate that PAD4 accelerates thrombus development by approximately 20%. Next, we analyzed the formation of large emboli (detachment of a part of the thrombus >40 μm reflecting thrombus instability) in the two groups of mice. Most of the mice injected with vehicle formed large emboli within the 40 minutes of observation (5 mice out of 7) contrarily to mice injected with r-huPAD4, where large emboli were rarely observed (1 out of 11 mice; Figure 5D). The mean number of emboli/mouse was 10.8 for mice injected with buffer and 1.5 for mice injected with r-huPAD4 (Figure 5E). Taken together, our data indicate that circulating PAD4 accelerates the formation of stable thrombi in injured veins.
Figure 5. Injection of PAD4 prolongs time to occlusion of mesenteric venules and reduces embolization of platelet-plug thrombi after FeCl3 injury.
A, Photographs of mesenteric venules (delimited by white lines) 5 minutes after injection of vehicle or r-huPAD4 and 10 and 20 minutes after FeCl3 injury. B, Determination of time to 1st thrombus formation (>30 μm) and C, time of occlusion (>60 seconds; asterisk at 20 minutes indicates venous thrombus) after FeCl3 injury of mice injected with vehicle or r-huPAD4. Data are analyzed by Mann-Whitney U test (*p= 0.035; n= 8–11 mice). D, Quantification of number of mice that formed emboli (> 40 μm; Fisher’s exact test p= 0.0063; n= 8–11 mice). E, Number of emboli/mouse (Mann-Whitney U test p=0.0065; n=8–11 mice).
DISCUSSION
Inflammation is a key factor for immuno-thrombosis; it not only activates endothelium and consequently induces UL-VWF release,54 but it also initiates the recruitment, accumulation, and activation of immune cells at sites of inflammation.55 Neutrophils are the first line of defence and, upon activation, they can release highly pro-thrombotic NETs.55 PAD4 is one of the proteins released with NETs.24 In fact, as mentioned above, high levels of PAD4 have been found in the plasma and serum of RA, cancer, and septic patients compared to healthy subjects.28, 29, 31
Although deficiency of PAD4 or treatment with PAD inhibitors reduces disease severity in mouse models of RA,56, 57 neurodegenerative disorders26, and cancer,58 and protects mice from forming stable thrombi in DVT,59 the impact of extracellular PAD4 in circulation has not been addressed.
Here, we show that increased levels of PAD4 in the bloodstream lead to UL-VWF multimer accumulation on mesenteric vessels, an important underlying mechanism of thrombus formation. PAD4 inhibition by Cl-amidine blocked this effect, indicating that the enzymatic activity of PAD4 mediates the observed phenotype. VWF-platelet strings accumulate in circulation when the activity of ADAMTS13 is impaired. Decreased ADAMTS13 activity may result from massive release of VWF into the bloodstream, with subsequent consumption of ADAMTS13, or can be consequential to a total or partial inactivation of the enzyme.12 Our studies indicate that injection of PAD4 in Adamts13−/− mice does not increase the number of VWF-platelet strings anchored onto the vessel wall, demonstrating that PAD4 is not promoting VWF release from endothelial cells. Instead, we observed partial reduction in ADAMTS13 activity in plasma of WT mice treated with PAD4 compared to those treated with vehicle. This suggests that PAD4 modulates ADAMTS13 activity. However, it is important to keep in mind that other plasma proteins can be citrullinated and may contribute to the observed pro-thrombotic effect of PAD4 in plasma.
Both ADAMTS13 and NETs bind VWF,60, 61 possibly facilitating the interaction between ADAMTS13 and PAD4. In agreement with this notion, we observed, using in vitro flow assays on human umbilical vein endothelial cells, that r-huPAD4 binds to UL-VWF (data not shown) again suggesting that PAD4 can accumulate on VWF strings rendering citrullination of ADAMTS13 possible. Moreover, we showed that r-huPAD4 citrullinates ADAMTS13 in vitro and in vivo. In fact, citrullinated ADAMTS13 was found at higher levels in the plasma samples of septic patients and in patients with other co-morbidities compared to healthy controls. The observation that an increase in citrullination of ADAMTS13 is not unique for septic patients could be due to the fact that susceptibility to NETosis can be found in many conditions, such as heart disease46, diabetes,47, 48 and possibly old age49. Finding citrullination of ADAMTS13 in the context of a complex environment, i.e. plasma, where hundreds of other proteins are present, is remarkable given the broad substrate scope of PAD4. In agreement with our PAD4 injection studies, we found that citrullination reduces ADAMTS13 activity both in vitro and in vivo.
Deficiency of ADAMTS13 is a severe condition not solely restricted to congenital or acquired TTP,11, 12 where autoantibodies bind and neutralize ADAMTS13, but also occurs in secondary thrombotic microangiopathies (TMA), such as DIC-sepsis, severe malaria,13 stroke,16 or in DVT patients.15 The reason for decreased ADAMTS13 activity during secondary thrombo-inflammatory conditions is unclear. Such a reduction in ADAMTS13 activity is not attributed to anti-ADAMTS13 antibodies, but rather to other, as yet unknown, factors. We propose that inhibition of the enzyme by a post-translational modification could be the reason for the reduced activity, at least in part. Leukocyte proteases, such as neutrophil elastase62 and plasmin,63 have been shown to proteolytically degrade ADAMTS13 in vitro, thereby providing a potential mechanism for reducing its activity. However, in the presence of plasma, such inactivation was lost,64 suggesting that other mechanisms are likely involved in vivo. Myeloperoxidase (MPO) is also released with NETs. Through hypochlorous acid production, MPO can oxidize methionine residues on ADAMTS13, and thus contribute to inactivation of the enzyme in vitro.64 However, it is likely that this oxidation is limited by the antioxidant systems present in vivo in blood. Our data suggest that increased levels of PAD4 in circulation reduce ADAMTS13 activity and that citrullinated-ADAMTS13 is less active, rendering citrullination a novel mechanism of ADAMTS13 inhibition. However, citrullination of other plasma proteins by circulating PAD4 might contribute to the observed pro-thrombotic effect of PAD4 in plasma. In fact, many plasma proteins, such as fibrinogen and anti-plasmin, have been found to be citrullinated in the plasma of RA and cancer patients.28, 29, 31 However, effects of the modification on their activity are not yet clear.
We identified several citrullinated arginine residues within ADAMTS13. Previous studies have pinpointed residues within the metalloprotease domain of ADAMTS13 that, when mutated, interfered with its activity. Mutation of Arg190, which was found to be citrullinated in our study, has been shown to partially reduce ADAMTS13 activity.65 The ADAMTS13 spacer domain is also important for its activity. In particular, exosite 3, a hydrophobic cluster delimited by arginine residues (Arg636 and Arg660), plays a crucial role in substrate recognition.66 Arg636, identified as citrullinated in our study by two independent methods, is predicted to promote the binding of ADAMTS13 to the α6 helix of VWF A2 domain,66 where the specific cleavage of theTyr1605–Met1606 bond by ADAMTS13 occurs. Deletion of Arg636 and Arg639 in the spacer domain significantly impairs ADAMTS13 proteolytic activity towards VWF.67 This may suggest that citrullination of Arg190 and Arg636 might, at least partially, explain the reduction in ADAMTS13 activity after PAD4 injection in the bloodstream. Importantly, conformational changes in ADAMTS13 were recently shown to be critical for its cleavage of VWF.68 Citrullination of other Arg residues might lead to loss of positive charge and interfere with this process.
We previously showed that ADAMTS13 deficiency increases platelet-plug formation after FeCl3 injury. Therefore, we evaluated the effect of PAD4 during thrombosis. After FeCl3 injury of mesenteric veins, we observed an increase of the time to vessel occlusion in mice treated with r-huPAD4 compared to vehicle, suggesting a pro-thrombotic effect of PAD4 in circulation. Moreover, the thrombi developed in vessels of mice injected with r-huPAD4 were more stable. This effect may be due to accumulation of VWF strings in the forming thrombus, a consequence of reduced ADAMTS13 activity. In addition, other plasma proteins might be citrullinated by PAD4 and contribute to thrombus stability.32
In total, our data suggest that high levels of circulating PAD4 are pro-thrombotic and this effect is due in part to reduced ADAMTS13 activity. Since infusion of r-huADAMTS13 can prevent microthrombosis and reduce inflammation in mice,17, 18 we propose that concomitant use of PAD inhibitors together with r-huADAMTS13 may enhance these beneficial effects of ADAMTS13.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Triggered by inflammation and thrombosis, neutrophils may release their chromatin as neutrophil extracellular traps (NETs) and also the enzyme that decondenses the chromatin, peptidyl arginine deiminase 4 (PAD4).
PAD4 citrullinates proteins, which can alter their structure and function, and is found in the plasma of patients with various diseases.
In many thrombotic microangiopathies where NETs play a role, reduction in the activity of the von Willebrand factor (VWF)-cleaving enzyme, a disintegrin-like and metalloprotease thrombospondin type 1 motif, member 13 (ADAMTS13), is observed.
What New Information Does This Article Contribute?
Ours is the first study demonstrating that PAD4 in the bloodstream is pro-thrombotic and leads to accumulation of VWF-platelet strings on the vessel wall, an important underlying mechanism of pathological thrombus formation.
Citrullination of ADAMTS13 by PAD4, a novel mechanism of inhibition of ADAMTS13 activity, leads to accumulation of VWF-platelet strings.
Inhibition of PAD4 prevents accumulation of VWF-platelet strings, indicating that PAD4 inhibitors together with ADAMTS13 represent a promising new therapy for patients suffering from thrombotic microangiopathies.
In this study, the effect of PAD4 in circulation was analyzed for the first time. PAD4 is an intracellular enzyme that can be released during inflammation by neutrophils together with NETs. We show that PAD4 in plasma reduces endogenous ADAMTS13 activity and causes VWF-platelet strings to accumulate on the vessel wall, an important mechanism initiating pathological thrombus formation and inflammatory cell recruitment. We determined that PAD4 citrullinates specific arginines in ADAMTS13, and this reduces enzymatic activity. Citrullinated ADAMTS13 was identified for the first time in human plasma samples; interestingly, its levels appear to increase with age and during inflammatory conditions, all situations where NETosis occurs. Next, we evaluated the effect of PAD4 during thrombosis. We observed by intravital microscopy that PAD4 in plasma increased the time to vessel occlusion. Our data suggest that circulating PAD4 is pro-thrombotic, and this effect is due, in part, to its negative impact on ADAMTS13 activity. ADAMTS13 is currently being tested as a treatment for diseases driven by release of ultra-large VWF multimers. In many of these conditions, NETs with PAD4 are also generated. We propose that the concomitant use of a PAD4 inhibitor with ADAMTS13 may potentiate the treatment of thrombotic disorders
ACKNOWLEDGMENTS
We thank Dr. Friedrich Scheiflinger (Shire) for kindly providing r-huADAMTS13 and anti-ADAMTS13 antibodies, Dr. Emanuele Pignatti from Boston Children’s Hospital, Harvard Medical School, for valuable scientific input, Tiffany Frary for help in preparation of the manuscript, and Lindsey Ades for helping with the collection of patient samples.
SOURCES OF FUNDING
Work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health grant R35 HL135765 to D.D.W, a postdoctoral fellowship by Deutsche Forschungsgemeinschaft (CH 1734/1–1) to D.C, National Institutes of Health grant P41 GM104603 and contract HHSN268201000031C to C.E.C, and National Institute of Health grant R35 GM109767 to P.R.T.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Nonstandard Abbreviations and Acronyms:
- ADAMTS13
A disintegrin and metalloproteinase with thrombospondin type-1 motif-13
- PAD4
Peptidylarginine deiminase type IV
- NETs
Neutrophil extracellular traps
Footnotes
DISCLOSURE OF CONFLICTS OF INTEREST
D.D.W. received a research grant from Shire and is on the SAB of Neutrolis. P.R.T. was a founder of Padlock Therapeutics, which was acquired by Bristol Myers Squibb in 2016 and is entitled to payments if certain milestones are met. P.R.T. is a consultant for Disarm Therapeutics. P.R.T. and E.W. are consultants for Celgene.
REFERENCES
- 1.Takahashi M, Yamashita A, Moriguchi-Goto S, Marutsuka K, Sato Y, Yamamoto H, Koshimoto C, Asada Y. Critical Role of Von Willebrand Factor and Platelet Interaction in Venous Thromboembolism. Histol Histopathol. 2009;24:1391–1398 [DOI] [PubMed] [Google Scholar]
- 2.Chauhan AK, Kisucka J, Lamb CB, Bergmeier W, Wagner DD. Von Willebrand Factor and Factor Viii Are Independently Required to Form Stable Occlusive Thrombi in Injured Veins. Blood. 2007;109:2424–2429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brill A, Fuchs TA, Chauhan AK, Yang JJ, De Meyer SF, Kollnberger M, Wakefield TW, Lammle B, Massberg S, Wagner DD. Von Willebrand Factor-Mediated Platelet Adhesion Is Critical for Deep Vein Thrombosis in Mouse Models. Blood. 2011;117:1400–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner DD, Olmsted JB, Marder VJ. Immunolocalization of Von Willebrand Protein in Weibel-Palade Bodies of Human Endothelial Cells. J Cell Biol. 1982;95:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sporn LA, Marder VJ, Wagner DD. Inducible Secretion of Large, Biologically Potent Von Willebrand Factor Multimers. Cell. 1986;46:185–190 [DOI] [PubMed] [Google Scholar]
- 6.Giblin JP, Hewlett LJ, Hannah MJ. Basal Secretion of Von Willebrand Factor from Human Endothelial Cells. Blood. 2008;112:957–964 [DOI] [PubMed] [Google Scholar]
- 7.Schillemans M, Karampini E, Kat M, Bierings R. Exocytosis of Weibel-Palade Bodies: How to Unpack a Vascular Emergency Kit. J Thromb Haemost. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. Adamts-13 Rapidly Cleaves Newly Secreted Ultralarge Von Willebrand Factor Multimers on the Endothelial Surface under Flowing Conditions. Blood. 2002;100:4033–4039 [DOI] [PubMed] [Google Scholar]
- 9.Lopes da Silva M, Cutler DF. Von Willebrand Factor Multimerization and the Polarity of Secretory Pathways in Endothelial Cells. Blood. 2016;128:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chauhan AK, Kisucka J, Brill A, Walsh MT, Scheiflinger F, Wagner DD. Adamts13: A New Link between Thrombosis and Inflammation. J Exp Med. 2008;205:2065–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbij FC, Fijnheer R, Voorberg J, Sorvillo N. Acquired Ttp: Adamts13 Meets the Immune System. Blood Rev. 2014;28:227–234 [DOI] [PubMed] [Google Scholar]
- 12.Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic Thrombocytopenic Purpura. Nat Rev Dis Primers. 2017;3:17020. [DOI] [PubMed] [Google Scholar]
- 13.Schwameis M, Schorgenhofer C, Assinger A, Steiner MM, Jilma B. Vwf Excess and Adamts13 Deficiency: A Unifying Pathomechanism Linking Inflammation to Thrombosis in Dic, Malaria, and Ttp. Thromb Haemost. 2015;113:708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denorme F, Kraft P, Pareyn I, Drechsler C, Deckmyn H, Vanhoorelbeke K, Kleinschnitz C, De Meyer SF. Reduced Adamts13 Levels in Patients with Acute and Chronic Cerebrovascular Disease. PLoS One. 2017;12:e0179258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz JA, Fuchs TA, Jackson TO, Hovinga JA, Lammle B, Henke PK, Myers DD Jr., Wagner DD, Wakefield TW, for the Michigan Research Venous G. Plasma DNA Is Elevated in Patients with Deep Vein Thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonneveld MA, de Maat MP, Portegies ML, Kavousi M, Hofman A, Turecek PL, Rottensteiner H, Scheiflinger F, Koudstaal PJ, Ikram MA, Leebeek FW. Low Adamts13 Activity Is Associated with an Increased Risk of Ischemic Stroke. Blood. 2015;126:2739–2746 [DOI] [PubMed] [Google Scholar]
- 17.Zhao BQ, Chauhan AK, Canault M, Patten IS, Yang JJ, Dockal M, Scheiflinger F, Wagner DD. Von Willebrand Factor-Cleaving Protease Adamts13 Reduces Ischemic Brain Injury in Experimental Stroke. Blood. 2009;114:3329–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Meyer SF, Savchenko AS, Haas MS, Schatzberg D, Carroll MC, Schiviz A, Dietrich B, Rottensteiner H, Scheiflinger F, Wagner DD. Protective Anti-Inflammatory Effect of Adamts13 on Myocardial Ischemia/Reperfusion Injury in Mice. Blood. 2012;120:5217–5223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs TA, Brill A, Wagner DD. Neutrophil Extracellular Trap (Net) Impact on Deep Vein Thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savchenko AS, Martinod K, Seidman MA, Wong SL, Borissoff JI, Piazza G, Libby P, Goldhaber SZ, Mitchell RN, Wagner DD. Neutrophil Extracellular Traps Form Predominantly During the Organizing Stage of Human Venous Thromboembolism Development. J Thromb Haemost. 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong SL, Wagner DD. Peptidylarginine Deiminase 4: A Nuclear Button Triggering Neutrophil Extracellular Traps in Inflammatory Diseases and Aging. FASEB J. 2018:fj201800691R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, Wang Y. Pad4 Mediated Histone Hypercitrullination Induces Heterochromatin Decondensation and Chromatin Unfolding to Form Neutrophil Extracellular Trap-Like Structures. Front Immunol. 2012;3:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witalison EE, Thompson PR, Hofseth LJ. Protein Arginine Deiminases and Associated Citrullination: Physiological Functions and Diseases Associated with Dysregulation. Curr Drug Targets. 2015;16:700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spengler J, Lugonja B, Ytterberg AJ, et al. Release of Active Peptidyl Arginine Deiminases by Neutrophils Can Explain Production of Extracellular Citrullinated Autoantigens in Rheumatoid Arthritis Synovial Fluid. Arthritis Rheumatol. 2015;67:3135–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishigami A, Ohsawa T, Hiratsuka M, Taguchi H, Kobayashi S, Saito Y, Murayama S, Asaga H, Toda T, Kimura N, Maruyama N. Abnormal Accumulation of Citrullinated Proteins Catalyzed by Peptidylarginine Deiminase in Hippocampal Extracts from Patients with Alzheimer’s Disease. J Neurosci Res. 2005;80:120–128 [DOI] [PubMed] [Google Scholar]
- 26.Sarswat A, Wasilewski E, Chakka SK, Bello AM, Caprariello AV, Muthuramu CM, Stys PK, Dunn SE, Kotra LP. Inhibitors of Protein Arginine Deiminases and Their Efficacy in Animal Models of Multiple Sclerosis. Bioorg Med Chem. 2017;25:2643–2656 [DOI] [PubMed] [Google Scholar]
- 27.Tilvawala R, Nguyen SH, Maurais AJ, Nemmara VV, Nagar M, Salinger AJ, Nagpal S, Weerapana E, Thompson PR. The Rheumatoid Arthritis-Associated Citrullinome. Cell Chem Biol. 2018;25:691–704 e696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Umeda N, Matsumoto I, Kawaguchi H, Kurashima Y, Kondo Y, Tsuboi H, Ogishima H, Suzuki T, Kagami Y, Sakyu T, Ishigami A, Maruyama N, Sumida T. Prevalence of Soluble Peptidylarginine Deiminase 4 (Pad4) and Anti-Pad4 Antibodies in Autoimmune Diseases. Clin Rheumatol. 2016;35:1181–1188 [DOI] [PubMed] [Google Scholar]
- 29.Zendman AJ, Raijmakers R, Nijenhuis S, Vossenaar ER, Tillaart M, Chirivi RG, Raats JM, van Venrooij WJ, Drijfhout JW, Pruijn GJ. Abap: Antibody-Based Assay for Peptidylarginine Deiminase Activity. Anal Biochem. 2007;369:232–240 [DOI] [PubMed] [Google Scholar]
- 30.Costa NA, Gut AL, Azevedo PS, et al. Peptidylarginine Deiminase 4 Concentration, but Not Padi4 Polymorphisms, Is Associated with Icu Mortality in Septic Shock Patients. J Cell Mol Med. 2018;22:4732–4737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang X, Han J, Pang L, Zhao Y, Yang Y, Shen Z. Increased Padi4 Expression in Blood and Tissues of Patients with Malignant Tumors. BMC Cancer. 2009;9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ordonez A, Martinez-Martinez I, Corrales FJ, Miqueo C, Minano A, Vicente V, Corral J. Effect of Citrullination on the Function and Conformation of Antithrombin. FEBS J. 2009;276:6763–6772 [DOI] [PubMed] [Google Scholar]
- 33.Hermansson M, Artemenko K, Ossipova E, Eriksson H, Lengqvist J, Makrygiannakis D, Catrina AI, Nicholas AP, Klareskog L, Savitski M, Zubarev RA, Jakobsson PJ. Ms Analysis of Rheumatoid Arthritic Synovial Tissue Identifies Specific Citrullination Sites on Fibrinogen. Proteomics Clin Appl. 2010;4:511–518 [DOI] [PubMed] [Google Scholar]
- 34.Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Sato M, Thompson PR. Inhibitors and Inactivators of Protein Arginine Deiminase 4: Functional and Structural Characterization. Biochemistry. 2006;45:11727–11736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chauhan AK, Motto DG, Lamb CB, Bergmeier W, Dockal M, Plaimauer B, Scheiflinger F, Ginsburg D, Wagner DD. Systemic Antithrombotic Effects of Adamts13. J Exp Med. 2006;203:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Witsch T, Martinod K, Sorvillo N, Portier I, De Meyer SF, Wagner DD. Recombinant Human Adamts13 Treatment Improves Myocardial Remodeling and Functionality after Pressure Overload Injury in Mice. J Am Heart Assoc. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slack JL, Jones LE Jr., Bhatia MM, Thompson PR. Autodeimination of Protein Arginine Deiminase 4 Alters Protein-Protein Interactions but Not Activity. Biochemistry. 2011;50:3997–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. Frets-Vwf73, a First Fluorogenic Substrate for Adamts13 Assay. Br J Haematol. 2005;129:93–100 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Liu Z, Liu B, Zhao T, Chong W, Wang Y, Alam HB. Citrullinated Histone H3: A Novel Target for the Treatment of Sepsis. Surgery. 2014;156:229–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular Histones Are Major Mediators of Death in Sepsis. Nat Med. 2009;15:1318–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien XM, Biron BM, Reichner JS. Consequences of Extracellular Trap Formation in Sepsis. Curr Opin Hematol. 2017;24:66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delabranche X, Stiel L, Severac F, Galoisy AC, Mauvieux L, Zobairi F, Lavigne T, Toti F, Angles-Cano E, Meziani F, Boisrame-Helms J. Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock. 2017;47:313–317 [DOI] [PubMed] [Google Scholar]
- 43.Liaw PC, Ito T, Iba T, Thachil J, Zeerleder S. Damp and Dic: The Role of Extracellular DNA and DNA-Binding Proteins in the Pathogenesis of Dic. Blood Rev. 2016;30:257–261 [DOI] [PubMed] [Google Scholar]
- 44.Maruchi Y, Tsuda M, Mori H, Takenaka N, Gocho T, Huq MA, Takeyama N. Plasma Myeloperoxidase-Conjugated DNA Level Predicts Outcomes and Organ Dysfunction in Patients with Septic Shock. Crit Care. 2018;22:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin JJ, Chan OW, Hsiao HJ, Wang Y, Hsia SH, Chiu CH. Decreased Adamts 13 Activity Is Associated with Disease Severity and Outcome in Pediatric Severe Sepsis. Medicine (Baltimore). 2016;95:e3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer B. Elevated Levels of Circulating DNA and Chromatin Are Independently Associated with Severe Coronary Atherosclerosis and a Prothrombotic State. ATVB. 2013;33:2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB, Kahn CR, Wagner DD. Diabetes Primes Neutrophils to Undergo Netosis, Which Impairs Wound Healing. Nat Med. 2015;21:815–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Menegazzo L, Ciciliot S, Poncina N, Mazzucato M, Persano M, Bonora B, Albiero M, Vigili de Kreutzenberg S, Avogaro A, Fadini GP. Netosis Is Induced by High Glucose and Associated with Type 2 Diabetes. Acta Diabetol. 2015;52:497–503 [DOI] [PubMed] [Google Scholar]
- 49.Martinod K, Witsch T, Erpenbeck L, Savchenko A, Hayashi H, Cherpokova D, Gallant M, Mauler M, Cifuni SM, Wagner DD. Peptidylarginine Deiminase 4 Promotes Age-Related Organ Fibrosis. J Exp Med. 2017;214:439–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewallen DM, Bicker KL, Subramanian V, Clancy KW, Slade DJ, Martell J, Dreyton CJ, Sokolove J, Weerapana E, Thompson PR. Chemical Proteomic Platform to Identify Citrullinated Proteins. ACS Chem Biol. 2015;10:2520–2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao G, Wang D, Gu J, Shen Q, Gross SS, Wang Y. Neutral Loss of Isocyanic Acid in Peptide Cid Spectra: A Novel Diagnostic Marker for Mass Spectrometric Identification of Protein Citrullination. J Am Soc Mass Spectrom. 2009;20:723–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee CY, Wang D, Wilhelm M, Zolg DP, Schmidt T, Schnatbaum K, Reimer U, Ponten F, Uhlen M, Hahne H, Kuster B. Mining the Human Tissue Proteome for Protein Citrullination. Mol Cell Proteomics. 2018;17:1378–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemmara VV, Tilvawala R, Salinger AJ, Miller L, Nguyen SH, Weerapana E, Thompson PR. Citrullination Inactivates Nicotinamide- N-Methyltransferase. ACS Chem Biol. 2018;13:2663–2672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen J, Chung DW. Inflammation, Von Willebrand Factor, and Adamts13. Blood. 2018;132:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engelmann B, Massberg S. Thrombosis as an Intravascular Effector of Innate Immunity. Nat Rev Immunol. 2013;13:34–45 [DOI] [PubMed] [Google Scholar]
- 56.Kawalkowska J, Quirke AM, Ghari F, Davis S, Subramanian V, Thompson PR, Williams RO, Fischer R, La Thangue NB, Venables PJ. Abrogation of Collagen-Induced Arthritis by a Peptidyl Arginine Deiminase Inhibitor Is Associated with Modulation of T Cell-Mediated Immune Responses. Sci Rep. 2016;6:26430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willis VC, Gizinski AM, Banda NK, et al. N-Alpha-Benzoyl-N5-(2-Chloro-1-Iminoethyl)-L-Ornithine Amide, a Protein Arginine Deiminase Inhibitor, Reduces the Severity of Murine Collagen-Induced Arthritis. J Immunol. 2011;186:4396–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McElwee JL, Mohanan S, Griffith OL, Breuer HC, Anguish LJ, Cherrington BD, Palmer AM, Howe LR, Subramanian V, Causey CP, Thompson PR, Gray JW, Coonrod SA. Identification of Padi2 as a Potential Breast Cancer Biomarker and Therapeutic Target. BMC Cancer. 2012;12:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil Histone Modification by Peptidylarginine Deiminase 4 Is Critical for Deep Vein Thrombosis in Mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA Traps Promote Thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grassle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaria C, Baldauf C, Grater F, Schneppenheim R, Obser T, Schneider SW. Von Willebrand Factor Directly Interacts with DNA from Neutrophil Extracellular Traps. ATVB. 2014;34:1382–1389 [DOI] [PubMed] [Google Scholar]
- 62.Ono T, Mimuro J, Madoiwa S, Soejima K, Kashiwakura Y, Ishiwata A, Takano K, Ohmori T, Sakata Y. Severe Secondary Deficiency of Von Willebrand Factor-Cleaving Protease (Adamts13) in Patients with Sepsis-Induced Disseminated Intravascular Coagulation: Its Correlation with Development of Renal Failure. Blood. 2006;107:528–534 [DOI] [PubMed] [Google Scholar]
- 63.Crawley JT, Lam JK, Rance JB, Mollica LR, O’Donnell JS, Lane DA. Proteolytic Inactivation of Adamts13 by Thrombin and Plasmin. Blood. 2005;105:1085–1093 [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Chen J, Ling M, Lopez JA, Chung DW, Fu X. Hypochlorous Acid Generated by Neutrophils Inactivates Adamts13: An Oxidative Mechanism for Regulating Adamts13 Proteolytic Activity During Inflammation. J Biol Chem. 2015;290:1422–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Groot R, Lane DA, Crawley JT. The Adamts13 Metalloprotease Domain: Roles of Subsites in Enzyme Activity and Specificity. Blood. 2010;116:3064–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fang X, Lin J, Fang Y, Wu J. Prediction of Spacer-Alpha6 Complex: A Novel Insight into Binding of Adamts13 with A2 Domain of Von Willebrand Factor under Forces. Sci Rep. 2018;8:5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veronica Casina HH, Anastasia Lyalenko, and X.long Zheng. Identification of a Novel Exosite (Glu634-Arg639) in the Spacer Domain of Adamts13 Required for Recognition of Von Willebrand Factor. Abstract Blood. 2012:120:2214 [Google Scholar]
- 68.Ercig B, Wichapong K, Reutelingsperger CPM, Vanhoorelbeke K, Voorberg J, Nicolaes GAF. Insights into 3d Structure of Adamts13: A Stepping Stone Towards Novel Therapeutic Treatment of Thrombotic Thrombocytopenic Purpura. Thromb Haemost. 2018;118:28–41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.