Abstract
Hypothesis:
Cochlear implantation may result in an increase in the density of macrophages in vestibular endorgans in the human.
Background:
Vestibular symptoms are a common complication of cochlear implantation. In a previous study, we demonstrated histological evidence of a foreign body response caused by silicon and platinum in the human cochlea following cochlear implantation. The objective of the current study was to seek evidence of a possible immune response in vestibular endorgans after cochlear implantation.
Methods:
The density of macrophages immunostained with anti-Iba1 antibody in the vestibular endorgans (lateral and posterior semicircular canals, utricle and saccule) in ten human subjects who had undergone unilateral cochlear implantation was studied by light microscopy. The densities of macrophages in the neuroepithelium, subepithelial stroma, and among dendritic processes in the mid-stromal zone in four vestibular endorgans in the implanted and the opposite unimplanted ears were compared. The distributions of macrophage morphology (amoeboid, transitional and ramified) were also compared.
Results:
The densities of macrophages in implanted ears in four vestibular endorgans were significantly greater than that in opposite unimplanted ears except in the subepithelial zone of the utricle and posterior semicircular canal. In contrast to the neuroepithelium, the subepithelial distribution of amoeboid macrophages in implanted ears was significantly less than in unimplanted ears.
Conclusion:
An increase in the density of macrophages in four vestibular endorgans after implantation was demonstrated. The transition among phenotype of macrophages suggested possible migration of amoeboid macrophages from the subepithelial stroma into the neuroepithelium.
Keywords: Cochlear implant, Immunohistochemistry, Human temporal bone, Macrophage, Vestibular system
Introduction
Cochlear implantation (CI) is an established treatment for the rehabilitation of profound sensorineural hearing loss. A common postoperative complication is the development of vestibular symptoms (1–3), which in some cases may occur with a delayed onset and episodes of vertigo similar to Meniere’s disease (4, 5). Given the increasing use of bilateral implantation, characterizing the effects of CI surgery on the vestibular system is clinically important. The pathogenesis of vestibular dysfunction during or after CI surgery has been postulated to include direct trauma due to cochleostomy and electrode insertion (6), acute serous labyrinthitis (1), endolymphatic hydrops (1, 5), unintentional spread of electrical stimulation (7) and foreign body reaction with labyrinthitis (5).
Nadol et al. (8) and O’Malley et al. (9) demonstrated evidence of chronic inflammation caused by a foreign body reaction in the cochlea after cochlear implantation. However, to date there has been no report of a possible chronic inflammatory process in the vestibular system caused by a foreign body response after cochlear implantation.
Macrophages play an important role in the immune responses during inflammation, wound healing, disease progression and foreign body reaction (10–13). Macrophages are found in various tissues of the body, and recently macrophages were described in the cochlea and in the vestibular endorgans (14). Since macrophages play important roles in the inflammatory response, we hypothesized that macrophages in the vestibular endorgan after cochlear implantation may be more numerous and activated than in the opposite unimplanted ear. In this study, we undertook a histopathological analysis of the vestibular system in 10 patients with a history of unilateral cochlear implantation. The aim of the study was to evaluate the effects of cochlear implantation surgery on the vestibular system by characterizing the expression of macrophages in implanted and contralateral unimplanted ears. It was assumed that macrophages with ramified, transitional and ameboid morphologies represented an increasing gradient of immune activity (15, 16).
Materials and Methods
Selection of cases
Both temporal bones of 10 patients who in life had undergone unilateral cochlear implantation from the collection of the Otopathology Laboratory of the Massachusetts Eye and Ear Infirmary were studied. All patients were operated by cochleostomy approach including round window enlargement approach. The demographic data of these 10 cases are presented in Table 1. This study was approved by the Institutional Review Board of the Massachusetts Eye and Ear Infirmary.
TABLE1.
Demographics of case studied.
| Case | Age at Death/Sex | Etiology of Hearing Loss | Cochlear Implant-Ear | Approach | Duration of Implantation (months) |
|---|---|---|---|---|---|
| 1 | 84/F | Presbycusis | Nucleus 22-R | RWE | 151 |
| 2 | 76/M | Unknown | Nucleus 22-L | RWE | 210 |
| 3 | 89/M | Unknown | AB Hi Res 90K-R | RWE | 33 |
| 4 | 78/M | Unknown/Noise exposure | Nucleus 24-L | RWE | 83 |
| 5 | 89/F | Genetic (suspected) | Nucleus 24-L | Cochl | 127 |
| 6 | 96/ M | Genetic (suspected)/Noise exposure | Nucleus Freedom-R | Cochl | 61 |
| 7 | 93/F | Unknown | AB Hi Res 90K-L | RWE | 110 |
| 8 | 81/ M | Unknown/Noise exposure | AB Hi Res Advantage 1J-R | RWE | 12 |
| 9 | 70/M | Unknown/Noise exposure | Nucleus hybrid S8-L | Cochl | 75 |
| 10 | 94/F | Genetic (suspected) | AB CII positioner-R | RWE | 143 |
| Average | 85 | 101 |
F, female; M, male; L, left; R, right, AB; Advanced Bionics; Cochl, cochleostomy; RWE, round window enlargement
Histologic Preparation
Temporal bones were removed and fixed in 10% buffered formalin. The specimens were decalcified in ethylene/diamine tetra acetic acid. After decalcification, the cochlear implant electrode was removed. The specimens were then dehydrated in graded alcohols followed by embedment in celloidin. Specimens were then serially sectioned in the axial (horizontal) plane with an average thickness of approximately 20 μm. Every tenth section was stained with hematoxylin and eosin and mounted on glass slides for histologic examination and 2-dimensional graphic reconstruction.
Immunohistochemistry methods
A total of 80 sections including the four vestibular endorgans (cristae of lateral and posterior semicircular canals and the two maculae) were selected for immunostaining. Evaluation of the crista of the superior semicircular canal was not included because of the sectioning angle in these horizontal sections. An antibody against the ionized calcium-binding adaptor molecule 1 (Iba1), made in rabbit, (Wako chemicals USA, Inc, Richmond, VA) was used to label macrophages in the same manner as was previous reported (14).
Quantitative assessment of macrophages
Macrophages were counted using Excelis HD camera & monitoring system (AccuScope, Inc., Commack, NY), coupled to an Olympus BX51 light microscope and imaging software (CaptaVision: AccuScope Inc.) to capture the images at 40× (1920 × 1080 pixels). One axial (horizontal) section through each of the lateral and posterior cristae, macula utriculi, and macula sacculi were examined in both temporal bones in all 10 subjects (Table.1).
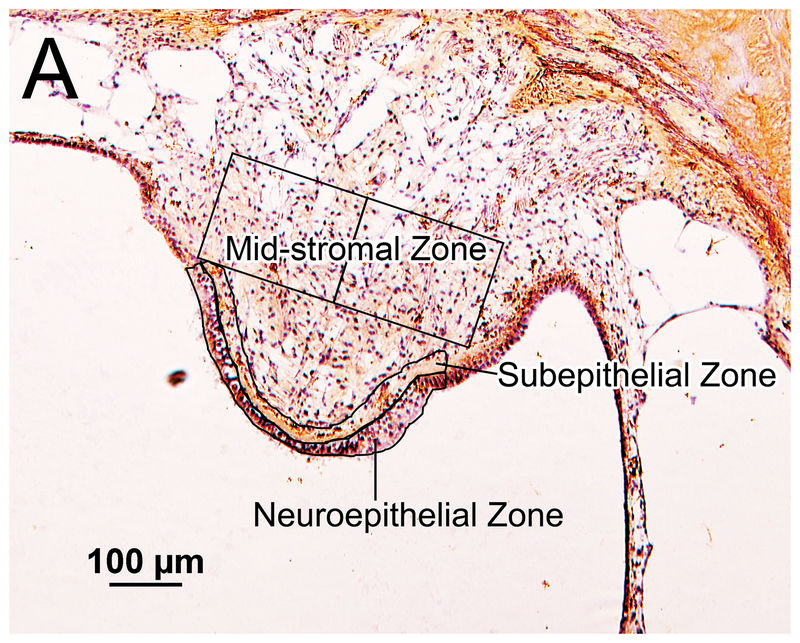
Neuroepithelial, Subepithelial and Mid- Stromal Zones
The densities of cells immunostained by anti-Iba1 in the neuroepithelium, in the subepithelial stroma, and in the mid-stromal zone containing peripheral vestibular dendrites were calculated in each representative section through the endorgans. Only cells containing nuclei were counted as seen focusing through the 20 μm sections.
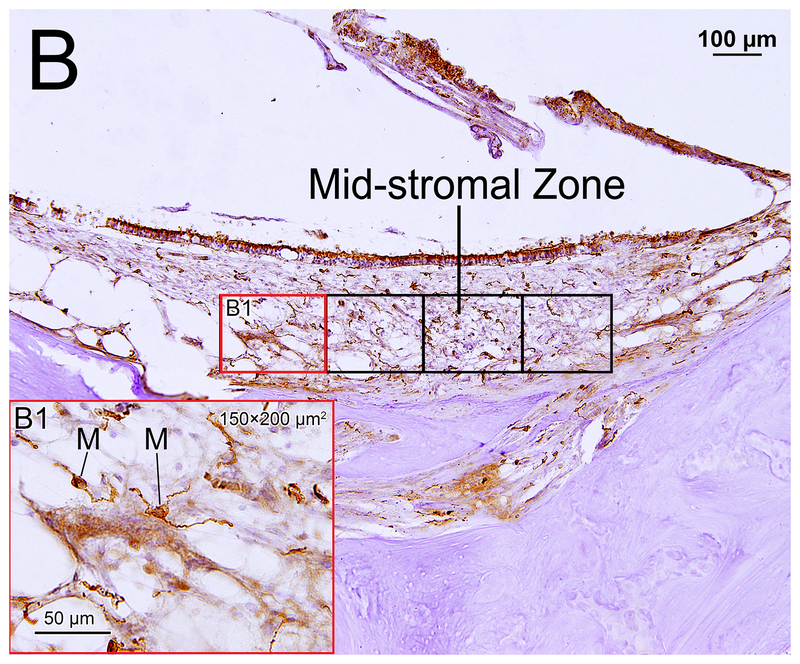
The area of the entire neuroepithelial zone was outlined using CaptaVision imaging software. A subepithelial zone of equivalent area was then outlined (Fig.1A). In the mid-stromal zone, counting frames approximately 150 μm × 200μm resulting in an area of 30,000 μm2 were outlined (Fig. 1B). The number of counting frames used in the mid-stromal zones was 2–3 in the lateral crista, 2–3 in the posterior crista, 3–7 in the macula utricli, and 3–5 in the macula sacculi. The number of Iba1-positive cells and total areas in the neuroepithelial and subepithelial zones and in the counting frames in the mid-stromal zone were calculated. The density of Iba1-positive macrophage cells was expressed as the number of cells per area of 30,000 μm2 in each of the four vestibular endorgans and three zones.
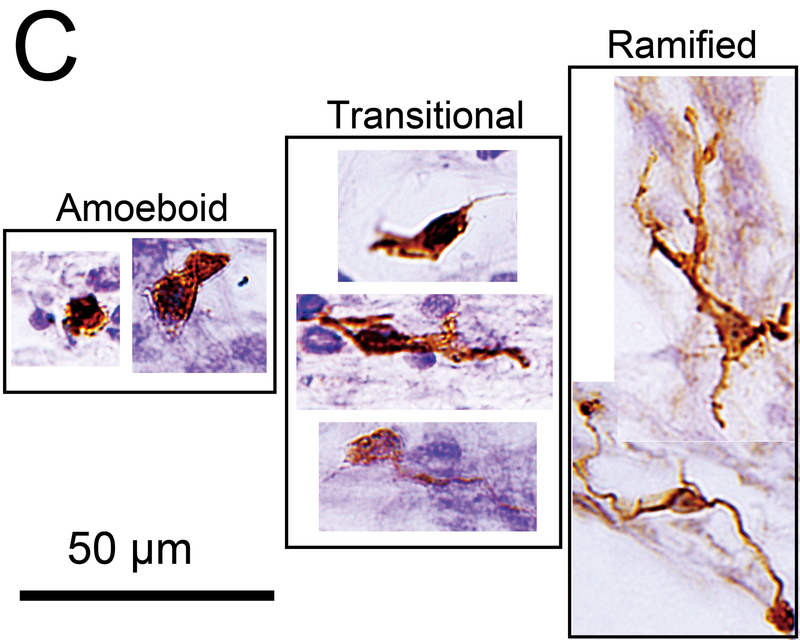
Figure 1.
Iba1- immunostaining of vestibular endorgans: quantification and subtyping of macrophages. (A) Crista ampularis of posterior semicircular canal of unimplanted right ear in case 7. The neuroepithelial and subepithelial zones are outlined. The areas studied in the mid-stromal zone are outlined into the two boxes, each boxed area measuring 150×200 μm2. The densities of Iba1-positive macrophages in these areas were calculated.
(B) Saccule of implanted left ear in case 2. The stromal areas studied within the mid-stromal zone are outlined by the six boxes. (B1) A higher magnification of left red box seen in (B) is shown. Immunostained macrophages (M) were seen.
(C) Macrophage subtypes. The amoeboid type has few processes and a relatively round cell body with a large nucleus. The ramified type has long branching processes. The transitonal type displays intermediate morphology.
Macrophage Morphology
Macrophages were morphologically classified into three types: amoeboid, transitional and ramified (Fig.1C) (15, 16). The amoeboid type was defined by an amoeba-like shape with few branched processes. The ramified type was defined as containing a cell body and highly branched processes. The transitional type included all other macrophages. The proportions of these three types were calculated in the areas of the neuroepithelial, subepithelial, and mid-stromal zones.
Statistical Analysis
The density of Iba1-positive macrophages in each of the four vestibular endorgans in the implanted ear and the contralateral unimplanted ear was statistically compared using the paired-t test or Wilcoxon matched-pairs signed rank test. The densities of Iba1-positive macrophages in the neuroepithelial, subepithelial, and mid-stromal zones were compared using one-way repeated measures ANOVA followed by Tukey’s multiple comparisons test or Friedman test followed by Dunn’s multiple comparisons test. The distribution of each of the three types of macrophage was statistically compared between implanted and unimplanted ears using paired-t test or Wilcoxon matched-pairs signed rank test. To evaluate the correlation of duration of implantation (months after implantation) with the density of macrophages, Pearson’s or Spearman’s coefficients of correlation test was used. All statistical analyses were performed using GraphPad software (GraphPad Prism version 7.02; GraphPad Software, Inc., LaJolla, CA).
Results
Clinical variables
The demographics of the ten studied cases are presented in Table1. There were four females and six males with an average age at death of 85 years and ranging from 70 to 96 years. There were four Advanced Bionics electrodes (Sylmar, CA, USA) and six Cochlear Nucleus electrodes (Cochlear Corp., Sydney, Australia). The average duration of implantation was 101 months, ranging from 12 to 210 months. There were no re-implantation cases. In case 1, there was post-operative dizziness lasting two days after implantation, and in the other nine cases, there were no recorded vestibular symptoms.
Densities of Macrophages
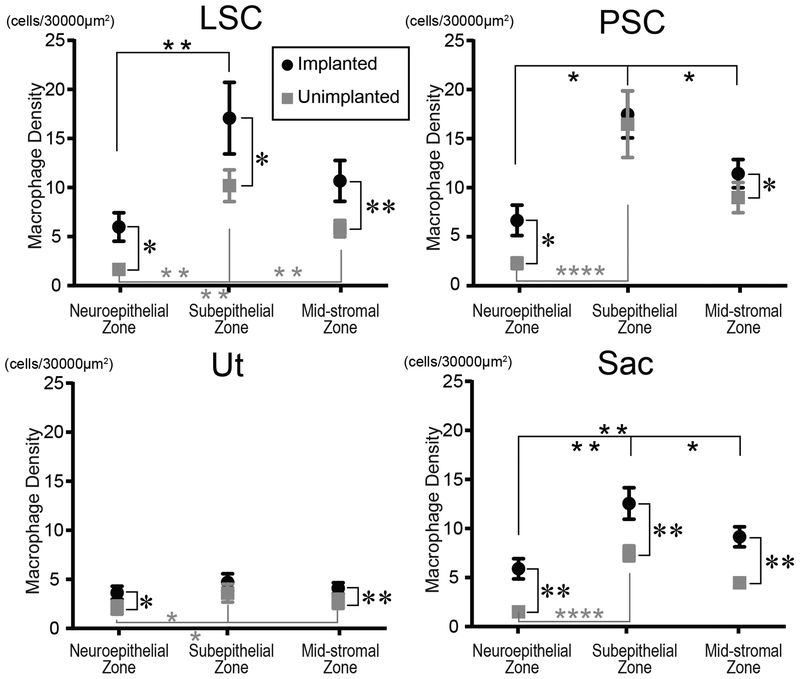
Macrophages were found within or adjacent to the neuroepithelium in both ears (Fig.2). Macrophages were commonly found immediately beneath the basement membrane in the subepithelial zone. There were many macrophages in the mid-stromal zone in both ears. The densities of macrophages in the three zones (neuroepithelial, subepithelial and mid-stromal) in four vestibular endorgans are shown Fig.3. The densities in the implanted ears were significantly greater than those in unimplanted ears in all three zones and in all endorgans except for the subepithelial zone in the posterior crista ampularis and in the utricle. The densities in the subepithelial zone were significantly greater than in the neuroepithelial zone except for the utricle of the implanted ears.
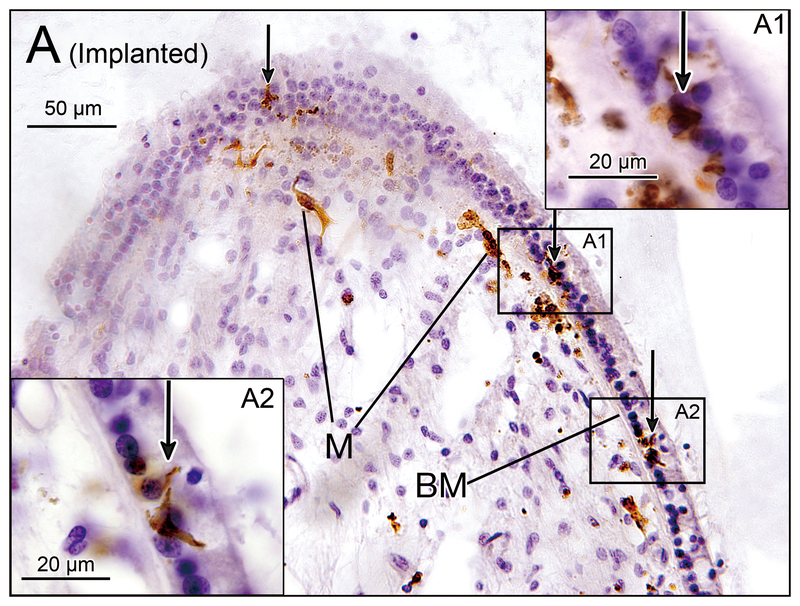
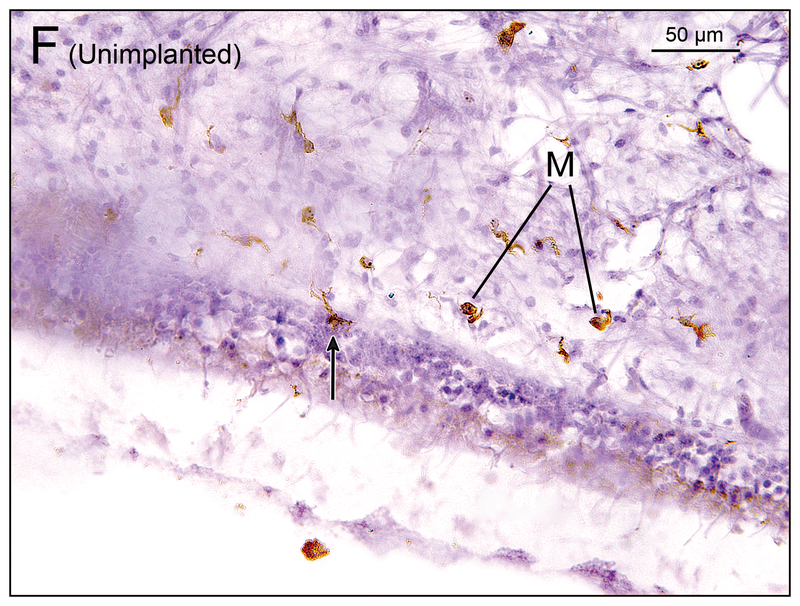
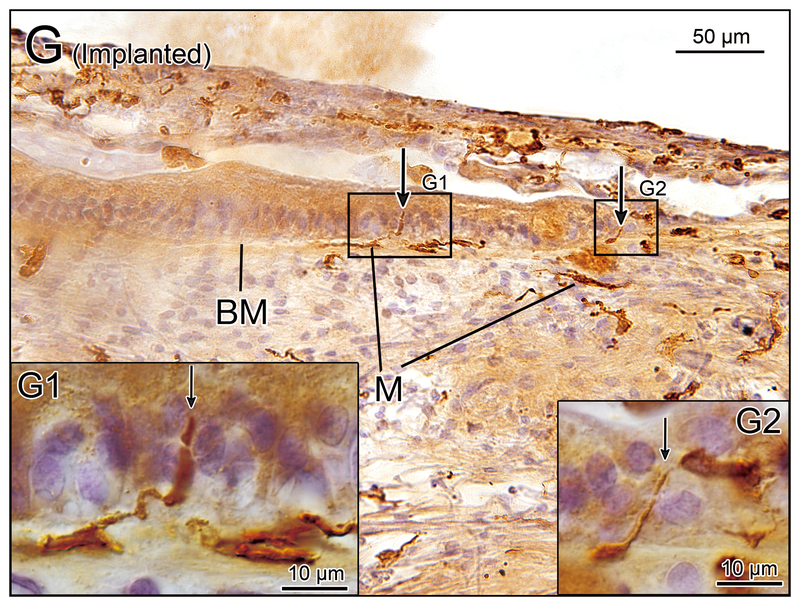
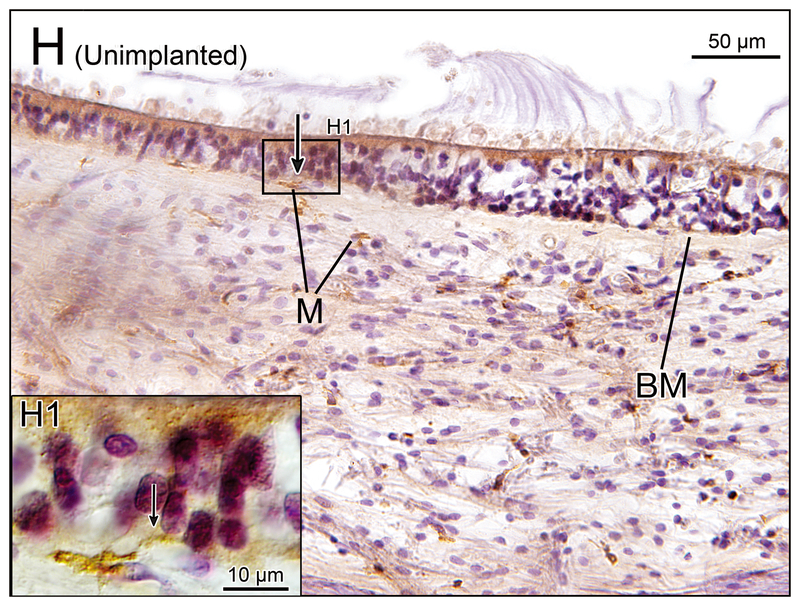
Figure 2.
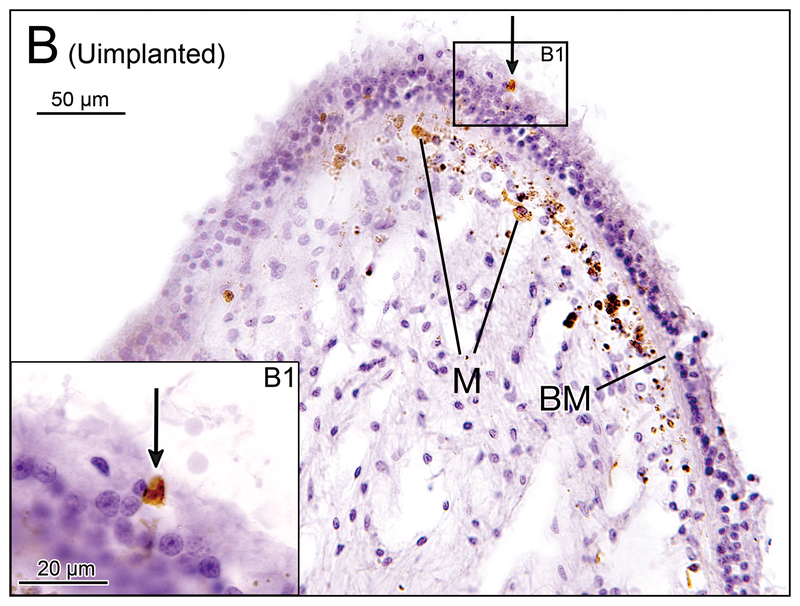
(A) Crista ampularis of the lateral semicircular canal of implanted left ear in case 4. Iba1-immunostaining of vestibular endorgans: implanted (A, C, E, G) and unimplanted (B, D, F, H) ears. There were several macrophages (M) immediately beneath the basement membrane (BM) and a few macrophages were seen in the neuroepithelium (arrows). (A1) and (A2) A higher magnification of boxes in (A).
(B) Crista ampularis of the lateral semicircular canal of unimplanted right ear in case 4. There were several macrophages (M) seen beneath the basement membrane and one macrophage was seen in the neuroepithelium (arrow). (B1) A higher magnification of box in (B).
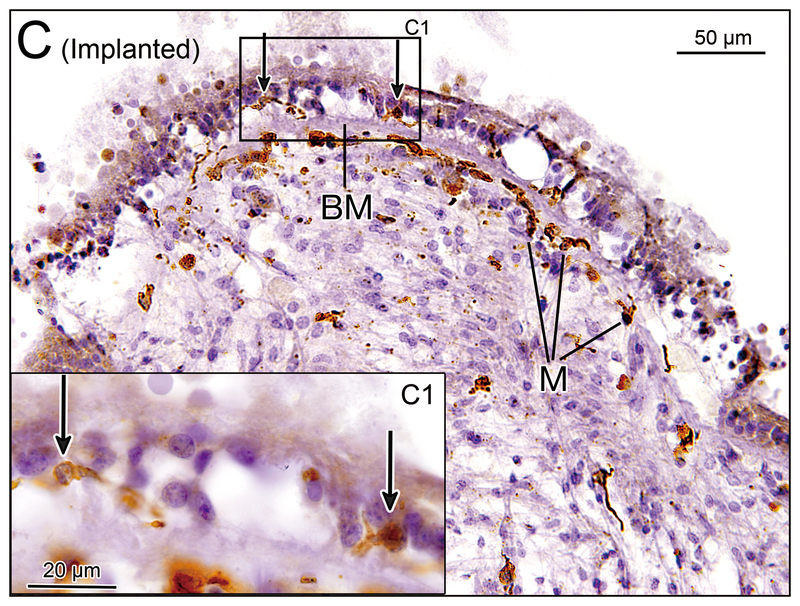
(C) Crista ampularis of the posterior semicircular canal of implanted left ear in case 2. There were several macrophages (M) seen beneath the basement membrane (BM) and a few macrophages were seen in the neuroepithelium (arrows). [See (C1)]. (C1) A higher magnification of box in (C).
(D) Crista ampularis of the posterior semicircular canal of unimplanted right ear in case 2. There were several macrophages (M) seen beneath the basement membrane (BM), and a few macrophages were seen in the neuroepithelium.
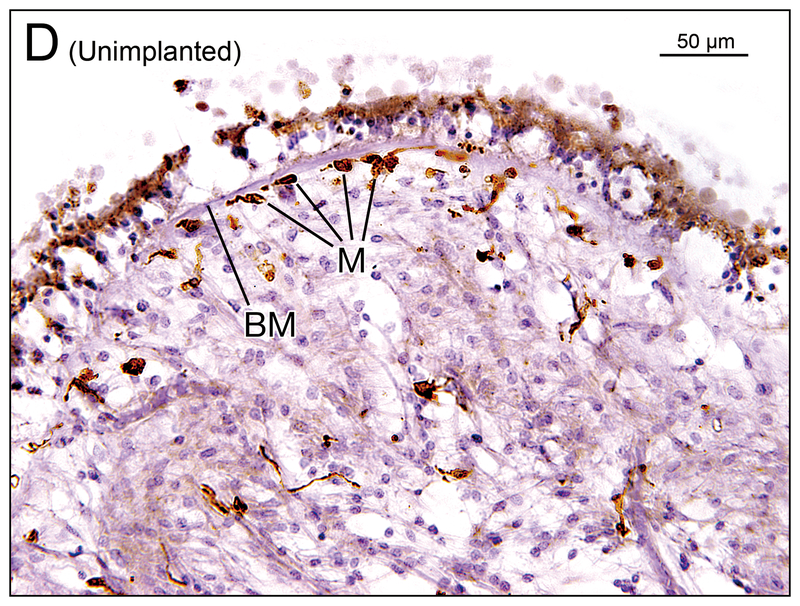
(E) Utricle of implanted right ear in case 1. Many macrophages (M) were seen in the neuroepithelium (arrows).
(F) Utricle of unimplanted left ear in case 1. There were some macrophages (M) in the subepithelial stroma and a single macrophage was seen in the neuroepithelium (arrow).
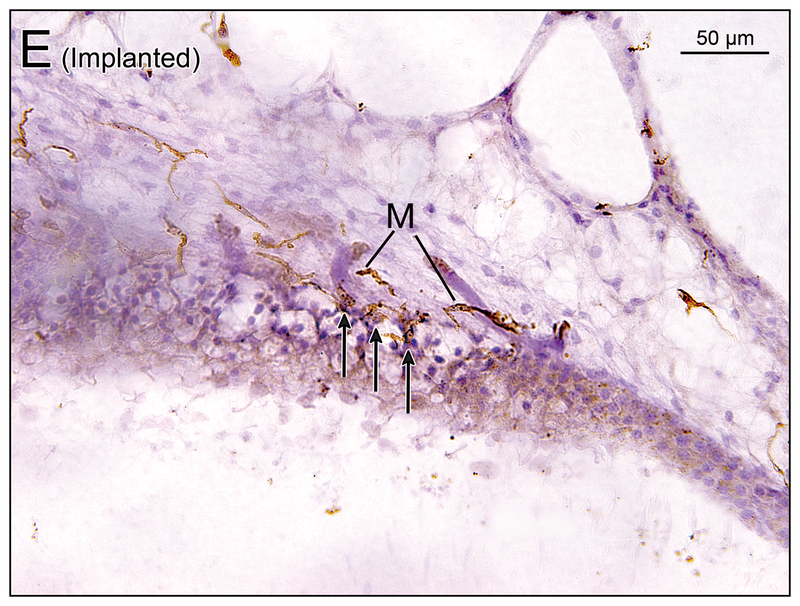
(G) Saccule of implanted left ear in case 7. There were some macrophages (M) below the basement membrane (BM) in the subepithelial stroma and long processes of some macrophages extended into the neuroepithelium (arrows). [See (G1) and (G2)]. (G1) and (G2) A higher magnification of boxes in (G) by Nomarski (differential interference contrast) microscopy.
(H) Saccule of unimplanted right ear in case 7. There were some macrophages (M) in the subepithelial stroma. There is a single macrophage below the basement membrane (BM) and long process of the macrophage extended into the neuroepithelium (arrow). [See (H1)] (H1) A higher magnification of box in (H) by Nomarski microscopy.
Figure 3.
Density of macrophages in vestibular endorgans in the three zones (neuroepithelium, subepithelal stroma, and mid-stroma). Black circles and gray squares indicate implanted and unimplanted ears, respectively. LSC= lateral semicircular canal; PSC= posterior semicircular canal; Ut = utricle; Sac = saccule. Asterisks (*) indicate the statistical significance between implanted and unimplanted ear (*, p<0.05; **, p<0.01; paired-t test or Wilcoxon matched-pairs signed rank test) and asterisks (*) indicate the statistical significance among the three zones (*, p<0.05; **, p<0.01; ****, p<0.0001; one-way repeated measures ANOVA followed by Tukey’s multiple comparisons test or Friedman test followed by Dunn’s multiple comparisons test).
Macrophage Morphology
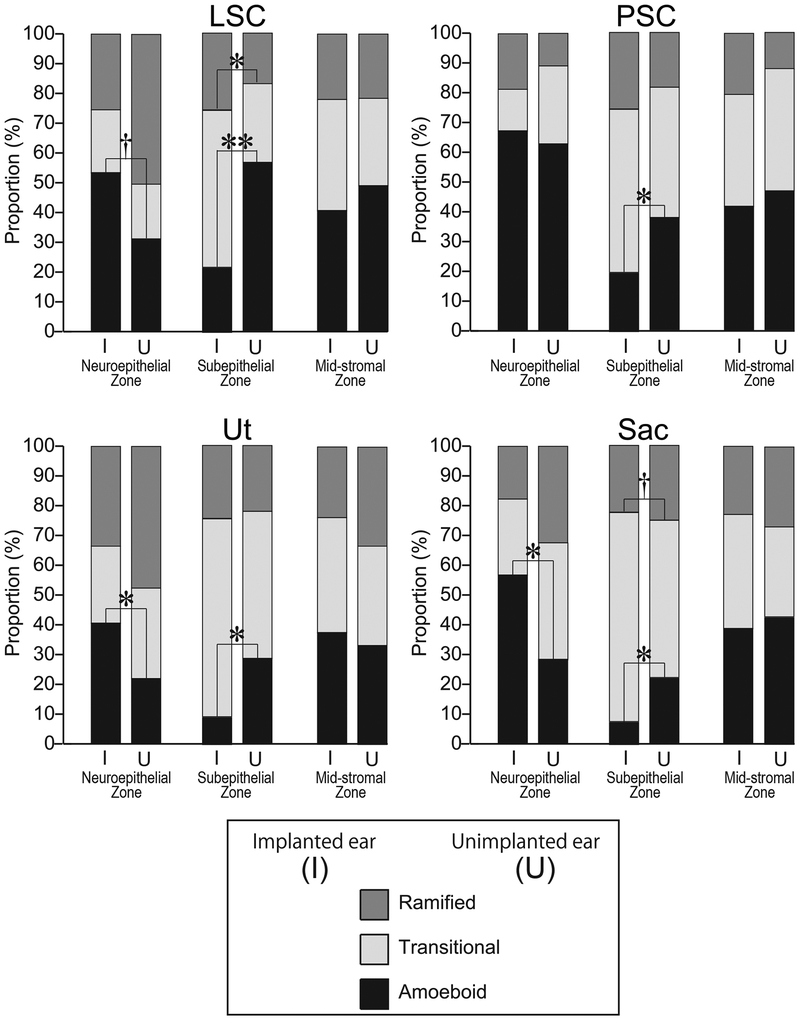
Distributions of the three macrophage morphologies in the three zones are shown Fig.4. The proportion of amoeboid macrophages in the neuroepithelial zone in implanted ears tended to be greater than in the contralateral unimplanted ears. The proportions of amoeboid macrophages in the utricle and the saccule were significantly greater in the neuroepithelial zone of the implanted ears than in the unimplanted ears. In the lateral crista ampularis, the proportion of amoeboid macrophages tended to be greater in implanted ears than unimplanted ears. In contrast, in the subepithelial zone of all organs, the proportions of amoeboid macrophages were significantly less in implanted ears than unimplanted ears. There was no difference in the distributions of the three macrophage morphologies in the mid-stromal zone between implanted and unimplanted ears.
Figure 4.
Distribution of macrophage morphology. The proportion of the three macrophage morphologies in the three zones of vestibular endorgans. Amoeboid, transitional and ramified types are shown. The columns of implanted ear (I) and unimplanted ear (U) are indicated side by side. The asterisks (*) indicate the statistical significance between implanted and unimplanted ears (*, p<0.05; **, p<0.01; paired-t test or Wilcoxon matched-pairs signed rank test). The daggers (†) indicate a significant tendency (†, 0.05≤ p<0.1).
Correlation coefficients between macrophage density and duration of implantation (months)
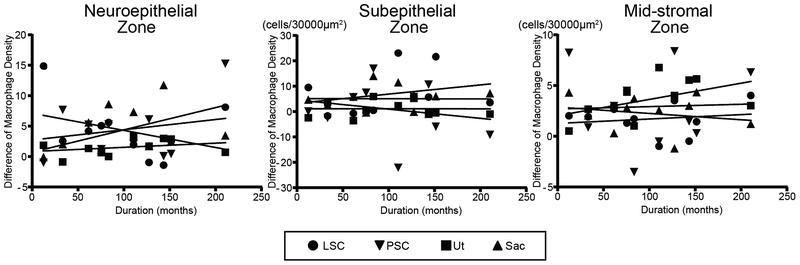
To evaluate the effect of duration of implantation (months after implantation) on the density of macrophages, a comparison of difference of the density between ears (implanted minus unimplanted) from 12 to 210 months was done (Fig. 5). The density of macrophages did not show significant correlation with the duration of implantation in the three zones in the four vestibular endorgans.
Figure 5.
Correlation between density of macrophages vs duration after implantation. Scatter plot of difference of macrophage density (implanted minus unimplanted) verses duration (months after cochlear implantation) in the three zones in the four vestibular endorgans. No significant correlations were found (Pearson’s or Spearman’s coefficients of correlation test).
Discussion
Previous studies have reported that cochlear implantation may cause inflammation and a foreign body response within the cochlea (1, 3–9). Although the possible inflammatory effect in the vestibular system following cochlear implantation is poorly understood, recently Banakis Hartl et al. (6) pointed out the possibility that changes in inner ear fluid pressure during electrode insertion may damage vestibular endorgans as well as the cochlea. Activated macrophages migrate into the damaged tissue in both acute and chronic inflammation (17–21). We hypothesized that there are more macrophages in the vestibular endorgans after cochlear implantation due to a chronic inflammatory response.
Density of Macrophages
This study demonstrated a significantly greater density of macrophages in implanted ears in the neuroepithelial and mid-stromal zones compared to unimplanted ears. In the subepithelial zone of the lateral crista ampularis and saccule, the density of macrophages was significantly greater in implanted ears. These results suggested an increase of immune activity in the vestibular endorgans following cochlear implantation.
Macrophage Activity and Macrophage Morphology
In the neuroepithelial zone of the utricle and saccule, the proportion of amoeboid macrophages in implanted ears was significantly greater than in unimplanted ears and also tended to be greater in the lateral crista ampularis. This result is consistent with activation of macrophage activity in the neuroepithelium in the implanted ear. In contrast, in the subepithelial zone the proportion of amoeboid macrophages in the implanted ear was significantly less than in implanted ears. This inversion of distribution might be explained by migration of activated macrophages from the subepithelial stroma into the neuroepithelium similar to the putative migration of activated macrophages after hair cell damage (16–18).
Function of macrophages in the neuroepithelium
Various possible roles of macrophages, such as phagocytosis (16), antigen presentation (22, 23) and immunoregulation (24, 25), have been described. However, phagocytosis of vestibular hair cells would seem to be inconsistent with a previous study demonstrating no difference in number of hair cells of vestibular endorgans between implanted and unimplanted ears (26). In the current study, some macrophages were present at the basement membrane and extended into the neuroepithelium by long processes (Fig. 2G, H). Although this was more common in implanted ears, it also occurred in unimplanted ears. Therefore, other possible roles of macrophages such as homeostasis and resolution of inflammation may be occurring in the neuroepithelium.
Correlation between macrophage density and duration of implantation
There were neither significant positive nor negative correlations between macrophage density and duration after implantation in the vestibular endorgans. This result is consistent with the finding of Nadol et al. (27), and Mohammad and Nadol (28) reporting no correlation between the degree of foreign body response and the duration of implantation in the cochlea.
Conclusion
This study demonstrated an increase in the density of macrophages in the vestibular endorgans after cochlear implantation. Our findings suggest that activation of the immune system after cochlear implantation is evident within the vestibular endorgans and can remain so for several years.
Acknowledgements
We thank Diane Jones, Barbara Burgess, and Meng Yu Zhu for their expert preparation of the temporal bone specimens, and Garyfallia Pagonis for technical assistance in creating digitized images of the temporal bone sections and figures. This work was supported by grants #U24-DC013983 and R01-DC000152–34 from the National Institute of Deafness and Other Communication Disorders (NIDCD).
(source of support)
Grants #U24DC013983 and R01DC000152–34, National Institute on Deafness and Other Communication Disorders (NIH)
Footnotes
(disclosure)
All authors declare no conflict of interest related to this manuscript.
BIBLIOGRAPHY
- 1.Fina M, Skinner M, Goebel JA, Piccirillo JF, Neely JG. Vestibular dysfunction after cochlear implantation. Otol Neurotol. 2003, 24: 234–242. [DOI] [PubMed] [Google Scholar]
- 2.Terry B, Kelt RE, Jeyakumar A. Delayed complications after cochlear implantation. JAMA Otolaryngol Head Neck Surg. 2015, 141: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim I, Da Silva SD, Segal B, Zeitouni A. Effect of cochlear implant surgery on vestibular function: Meta-analysis study. J Otolaryngol Head Neck Surg. 2017, 46: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ito J Influence of the multichannel cochlear implant on vestibular function. Otolaryngol Head Neck Surg. 1998, 118: 900–902. [DOI] [PubMed] [Google Scholar]
- 5.Kubo T, Yamamoto KI, Iwaki T, Doi K, Tamura M. Different forms of dizziness occurring after cochlear implant. Eur Arch Otorhinolaryngol. 2001, 258: 9–12. [DOI] [PubMed] [Google Scholar]
- 6.Banakis Hartl RM, Greene NT, Jenkins HA, Cass SP, Tollin DJ. Lateral Semicircular Canal Pressures during Cochlear Implant Electrode Insertion: A Possible Mechanism for Postoperative Vestibular Loss. Otol Neurotol. 2018, 39: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bance ML, O’Driscoll M, Giles E, Ramsden RT. Vestibular Stimulation by Multichannel Cochlear Implants. Laryngoscope. 1998, 108: 291–294. [DOI] [PubMed] [Google Scholar]
- 8.Nadol JB, O’Malley JT, Burgess BJ, Galler D. Cellular immunologic responses to cochlear implantation in the human. Hear Res. 2014, 318: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Malley JT, Burgess BJ, Galler D, Nadol JB. Foreign body response to silicone in cochlear implant electrodes in the human. Otol Neurotol. 2017, 38: 970–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005, 4: 281–286. [DOI] [PubMed] [Google Scholar]
- 11.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005, 308: 1314–1318. [DOI] [PubMed] [Google Scholar]
- 12.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011, 11: 723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vijaya Bhaskar TB, Ma N, Lendlein A, Roch T. The interaction of human macrophage subsets with silicone as a biomaterial. Clin Hemorheol Microcirc. 2015, 61: 119–133. [DOI] [PubMed] [Google Scholar]
- 14.O’Malley JT, Nadol JB, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol Neurotol. 2016, 37: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres-Platas SG, Comeau S, Rachalski A, et al. Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation. 2014, 11: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirose K, Rutherford MA, Warchol ME. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hear Res. 2017, 352: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaur T Macrophage recruitment and epithelial repair following hair cell injury in the mouse utricle. Front Cell Neurosci. 2015, 9: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladrech S, Wang J, Simonneau L, Puel JL, Lenoir M. Macrophage contribution to the response of the rat organ of Corti to amikacin. J Neurosci Res. 2007, 85:1970–1979. [DOI] [PubMed] [Google Scholar]
- 19.Okano T, Nakagawa T, Kita T, et al. Bone marrow-derived cells expressing Iba1 are constitutively present as resident tissue macrophages in the mouse cochlea. J Neurosci Res. 2008, 86:1758–1767. [DOI] [PubMed] [Google Scholar]
- 20.Frye MD, Yang W, Zhang C, Xiong B, Hu BH. Dynamic activation of basilar membrane macrophages in response to chronic sensory cell degeneration in aging mouse cochleae. Hear Res. 2017, 344: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frye MD, Zhang C, Hu BH. Lower level noise exposure that produces only TTS modulates the immune homeostasis of cochlear macrophages. J Neuroimmunol. 2018, 323: 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang W, Vethanayagam RR, Dong Y, Cai Q, Hu BH. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience. 2015, 303: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kämpfe Nordström C, Danckwardt-Lillieström N, Laurell G, Liu W, Rask-Andersen H. The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression. Front Immunol. 2019, 9: 3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warchol ME, Schwendener RA, Hirose K. Depletion of Resident Macrophages Does Not Alter Sensory Regeneration in the Avian Cochlea. PLoS One. 2012, 7: e51574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Molnar M, Garnham C, Benav H, Rask-Andersen H. Macrophages in the human cochlea: Saviors or predators-A study using super-resolution immunohistochemistry. Front Immunol. 2018, 9: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handzel O, Burgess BJ, Nadol JB. Histopathology of the peripheral vestibular system after cochlear implantation in the human. Otol Neurotol. 2006, 27: 57–64. [DOI] [PubMed] [Google Scholar]
- 27.Nadol JB, Eddington DK. Histologic evaluation of the tissue seal and biologic response around cochlear implant electrodes in the human. Otol Neurotol. 2004, 25: 257–262. [DOI] [PubMed] [Google Scholar]
- 28.Seyyedi M, Nadol JB. Intracochlear inflammatory response to cochlear implant electrodes in humans. Otol Neurotol. 2014, 35: 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]