Abstract
Study Design.
An experimental laboratory study.
Objective.
To investigate the pathogenesis of intervertebral disc degeneration (IDD) in a murine model of type 1 diabetes mellitus (DM), namely non-obese diabetic (NOD) mouse.
Summary of Background Data:
IDD is a leading contributor of low back pain, which represents one of the most disabling symptoms within the adult population. DM is a chronic metabolic disease currently affecting one in ten adults in the United States. It is associated with an increased risk of developing IDD, but the underlying process remains poorly understood.
Methods.
Total disc glycosaminoglycan (GAG) content, proteoglycan synthesis, aggrecan fragmentation, glucose transporter (mGLUT1) gene expression and apoptosis were assessed in NOD mice and wild-type euglycemic control mice. Spinal structural and molecular changes were analysed by micro-computed tomography (CT), histological staining (Safranin-O and fast green) and quantitative immunofluorescence (anti-ADAMTS-4 and −5 antibodies).
Results.
Compared to euglycemic controls, NOD mice showed increased disc apoptosis and matrix aggrecan fragmentation. Disc GAG content and histological features of NOD mice did not significantly differ from those of euglycemic littermates.
Conclusions.
These data demonstrate that DM may contribute to IDD by increasing aggrecan degradation and promoting cell apoptosis, which may represent early indicators of the involvement of DM in the pathogenesis of IDD.
Keywords: intervertebral disc, intervertebral disc degeneration, Type 1 diabetes mellitus, diabetic mouse model, Apoptosis
Introduction
Intervertebral disc degeneration (IDD) is a major cause of low back pain,1 which affects 80% of the adult population at least once in their life.
The pathophysiological conditions underlying IDD are still poorly understood. Genetic makeup, aging,2 smoking,3 physical inactivity and mechanical overloading, especially due to obesity,4 are among the strongest risk factors involved. Moreover, IDD is often associated with chronic inflammation within disc tissues, which increases matrix breakdown, glycosaminoglycan (GAG) loss and cell death.5 This micro-inflammatory environment is typical of several metabolic disorders, including diabetes mellitus (DM). Indeed, an association between DM and IDD has been reported in both humans and animal models.6 DM is a chronic illness affecting 422 million individuals worldwide, according to recent reports from WHO.7 DM is a multi-organ disorder affecting several connective tissues, including bone, cartilage and the intervertebral disc (IVD).8
As the etiopathogenesis of IDD in diabetic subjects remains scarcely understood, we hypothesised that this may be driven by a DM-induced inflammation leading to a combination of reduced GAG levels, decreased proteoglycan synthesis and increased matrix breakdown within the disc. To evaluate this, we analysed a murine model of type 1 DM (T1DM), namely the non-obese diabetic (NOD) mouse. NOD mice carry specific polymorphisms in insulin-dependent DM loci that lead to autoimmune insulitis and frank T1DM. The strict correlations between autoimmune diabetogenesis in humans and NOD mice make this model relevant to investigate the role of T1DM pathophysiology in driving IDD.9 The aim of this study was to identify morphological and molecular changes in IVDs of NOD mice compared to euglycemic controls to investigate the link between DM and IDD.
Materials and Methods
Mice
Female NOD mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 8 weeks of age and were housed in a specific pathogen-free animal facility until some of them spontaneously developed diabetes. Spines and IVDs were isolated from NOD mice and age-matched female euglycemic mice (19–22 weeks of age) for analyses. DM in NOD mice was confirmed by consecutive blood glucose monitoring (see below). All the experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Blood glucose
Glycemia was monitored using the Ascensia Contour blood glucose monitoring system (Bayer HealthCare LLC, Mishawaka, IN). DM was defined after two consecutive readings of blood glucose levels above 250 mg/dl. To examine the impact of hyperglycemia on IDD, NOD mice were maintained for 4–6 weeks after the onset of DM. If the animals showed blood glucose levels above 500 mg/dl, or displayed symptoms that were associated with severe DM, such as rough hair, excessive water consumption and rapid loss of body weight, one LinBit slow-release insulin implant (LinShin Canada, Inc.) was inserted subcutaneously under the mid-dorsal skin, following the manufacturer’s suggested protocol. The insulin dose from the implant helped keep mice alive but was not sufficient to normalize blood glucose levels.
Isolation of mouse IVDs, nucleus pulposus and annulus fibrosus
The spines were harvested from euthanized mice: IVDs were removed en bloc from the surrounding vertebral bodies by performing an incision between the bony endplate and the discs themselves. To harvest nucleus pulposus (NP) tissue, an axial cut was made on the disc side of the endplate to expose the NP, which was removed using a P-10 pipette tip under a dissecting microscope (MD900E, AmScope), as previously described.10
If not otherwise specified, IVDs from the last 5 lumbar segments (L2–L6) and the first 10 tail vertebrae were used in the experiments.
1,9-dimethylmethylene blue (DMMB) colorimetric assay for sulfated GAGs
NP tissue was isolated separately from five lumbar and ten tail IVDs of each sample from both NOD and control mice and digested using papain at 60°C for 2 hours. GAG content was measured in duplicates by DMMB procedure11 using chondroitin-6-sulfate (C-8529, Sigma, Milwaukee, WI) as a standard. The DNA concentration of each sample was measured using the PicoGreen® assay (P-11496, Molecular Probes, Sunnyvale, CA) to normalize GAG values.
Histological analysis
Tail and lumbar IVDs from two groups of age-matched mice (control n=6, NOD n=6) were isolated and fixed with 2% paraformaldehyde overnight at 4°C. Isolated spines were decalcified and embedded in paraffin (Tissue Tek processors and Leica embedder). 4-μm sections were stained with Safranin-O and fast green dyes (Fisher Scientific, Pittsburgh, PA) by standard procedure and photographed under 40–200x magnification (Nikon Eclipse Ts100), as previously described.12
Proteoglycan synthesis
Organotypic disc cultures were performed on isolated functional spine units (FSUs) each consisting of vertebra, disc, vertebra, from lumbar spines of both NOD and control mice, as previously described.13 Lumbar FSUs were cultured in complete growth medium (F-12/D-MEM containing 10% FCS, 1% PS, and 25 μg/ml L-ascorbic acid) for two days to equilibrate after surgical removal, followed by a 3-day incubation with 35S-sulfate (20 μCi/ml). Individual discs were isolated from FSUs under a 5x magnifier, crushed using a micro-pestle and a 1.5 ml microcentrifuge tube in 0.1 ml of homogenizing buffer (20 mM Tris-HCl, 200 mM NaCl, 100 mM glycine, 0.1% Triton X-100, 50 μM DTT, 0.1 mg/ml soybean trypsin inhibitor) with 4 M guanidine-HCl and extracted for two days with rotation at 4°C. Incorporated 35S-sulfate was separated from free sulfate utilizing size exclusion PD-10 columns, and radioactivity in the fixed fraction measured by liquid scintillation counting as previously described.14 Proteoglycan (PG) synthesis rate was calculated as pmoles of sulfate incorporated per μg of DNA.
Western Analysis
Tail discs were removed as mentioned above and placed into 1.5-ml microcentrifuge tubes with T-PER Tissue Protein Extraction Reagent (78510, Thermo Fisher, Waltham, MA). Samples were homogenized using a Bullet Blender® Storm Homogenizer and orbital shaker. Protein concentration of tissue extracts was measured by bicinchoninic acid (BCA) assay (23225, Pierce™ BCA Protein Assay Kit, Thermo Fisher). 15 μg of proteins were loaded in a 4–20% gradient gel (0025204, Thermo Fisher) for electrophoresis, washed (TBS-T, T9039, Sigma), blocked and incubated with the anti-aggrecan primary antibody (rabbit, 1:1000 dilution, ab36861, Abcam, Cambridge, UK) and, subsequently, with the secondary antibody (goat, 1:10000 dilution, 31460, Thermo Fisher).
Quantitative Immunofluorescence
Tissues were cryoprotected with 30% sucrose in PBS overnight at 4°C, embedded in OCT (Tissue-Tek) and cut at −26°C. 5-μm frozen tissue sections were permeabilized with 0.25% Triton-X100 in PBS for 10 minutes, blocked with 10% serum and 1% BSA in PBS for 30 minutes, followed by overnight incubation with the primary antibodies (anti-ADAMTS4 polyclonal antibody, rabbit, 1:500 dilution, PA1–33177, Thermo Fisher; anti-ADAMTS5 antibody, rabbit, 1:100 dilution, ab41037, Abcam). After washing, sections were incubated in a fluorescent-labelled secondary antibody (Alexa Fluor 488 conjugated donkey-rabbit IgG(H+L), A-21206, Thermo Fisher) for 30 minutes at room temperature, then washed and mounted in Prolong Gold anti-fade reagent with DAPI (P36935, Invitrogen, Carlsbad, CA) to stain for nuclei. Results were analysed using quantitative fluorescent confocal microscopy (40x magnification, Nikon A1 spectral).
Apoptosis assay
Frozen disc sections were treated with TUNEL reagents (11684795910, Roche, Branchburg, NJ) to detect apoptotic cells as described by the manufacturer and counterstained with the Hoechst nuclear stain to detect total cells in the specimens. At least three random areas (140 × 140 pixels) in three sections from each tissue sample were imaged at 40x and 200x magnification. Percent apoptotic cells was calculated by dividing TUNEL-positive fluorescent cells into the total number of cells stained by DAPI. Results were obtained by averaging the percentages measured in each area.
microCT
Micro-computed tomography scans of lumbar spines harvested from 6 mice were acquired using a VivaCT 40 (Scanco Medical) as previously described.15 Trabecular bone parameters were evaluated using the software provided by the manufacturer which calculates bone volume (BV), trabecular thickness and trabecular bone volume (BV/TV; TV: total volume). Vertebral porosity was calculated as 1-BV/TV.
mGLUT1 gene expression
Entire cervical (C4–C7) and thoracic discs (T1–T13) were removed as mentioned above from both NOD and control mice and placed into 1.5-ml microcentrifuge tubes with 100 μl RLT Lysis Buffer (1015762, Qiagen, Hilden, Germany) and BME (63689, Sigma). Samples were homogenized using a Bullet Blender® Storm Homogenizer and proteins were digested using Proteinase K (19131, Qiagen) and incubating samples for 15 minutes at 55°C. RNA was subsequently purified using the RNeasy Fibrous Tissue Mini Kit (74704, Qiagen). RNA concentration was assessed utilizing a ND 1000 Spectrophotometer (77321, NanoDrop Technologies, Wilmington, DE). RT-PCR was performed as previously described.16 Validated PCR primers used in these experiments were GAPDH (forward:5’-GAGGCCGGTGCTGAGTAT-3’, reverse: 5’-GCGGAGATGATGACCCTTTTGG-3’) and mGLUT1 (forward: 5’-GCTGTGCTTATGGGCTTCTC-3’, reverse: 5’-CACATACATGGGCACAAAGC-3’). The expression level of each target gene was calculated as 2−ΔCt.
Statistical analysis
Values are represented as average of 3–5 samples ± standard error for each measurement, with 95% confidence intervals calculated to determine statistical significance (p-value < 0.05). The confidence intervals were calculated based on the t-test distribution because of the small sample size.
Results
GAG content
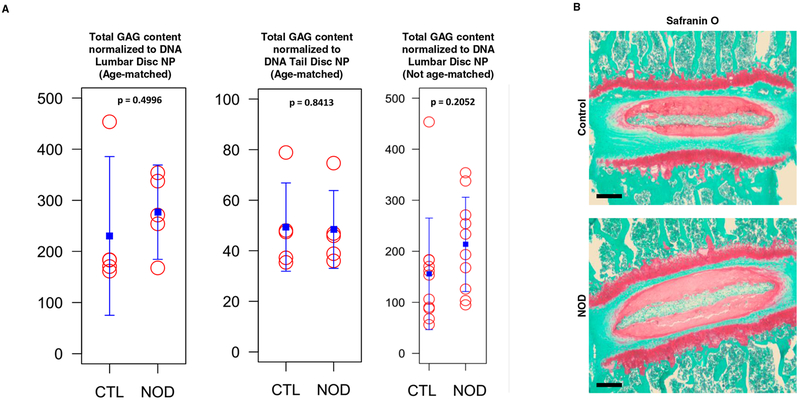
The DMMB assay was performed to measure disc GAG content (Fig. 1a). The GAG amount in lumbar discs of NOD mice was not statistically significantly different from that of age-matched euglycemic littermates. Similarly, GAG levels in both NOD and age-matched control mice tail disc NPs were approximately similar. This finding was qualitatively confirmed by Safranin-O/fast green histological staining, where NOD mice did not show significant changes compared to controls (Fig. 1b).
Fig. 1.
Diabetic mice did not show a significant loss of PG and GAG in disc matrix.
a DMMB assay for total GAG content normalized to DNA in NP tissue of control and NOD mice.
b A representative Safranin O/fast green histological staining of thoracic disc for matrix PG in control and NOD mice. Red, proteoglycan staining.
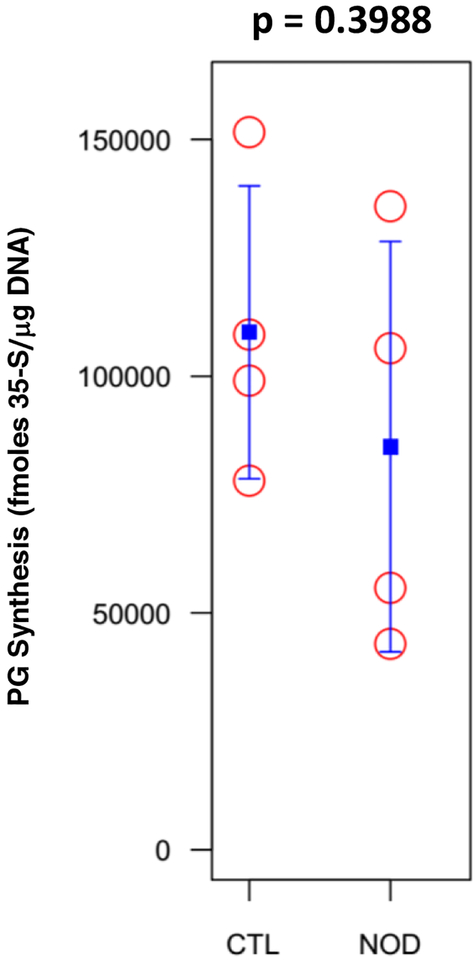
Proteoglycan synthesis
Loss of disc PG was explored in NOD mice for its possible role in driving IDD: PG synthesis was assessed by measuring 35S-sulfate incorporation into disc tissue using a disc organotypic culture system (Fig. 2). NOD mice did not display a statistically significant reduction of PG synthesis compared to euglycemic mice.
Fig. 2.
PG synthesis was measured by 35S-sulfate incorporation in control and NOD mice IVDs and was not significantly affected.
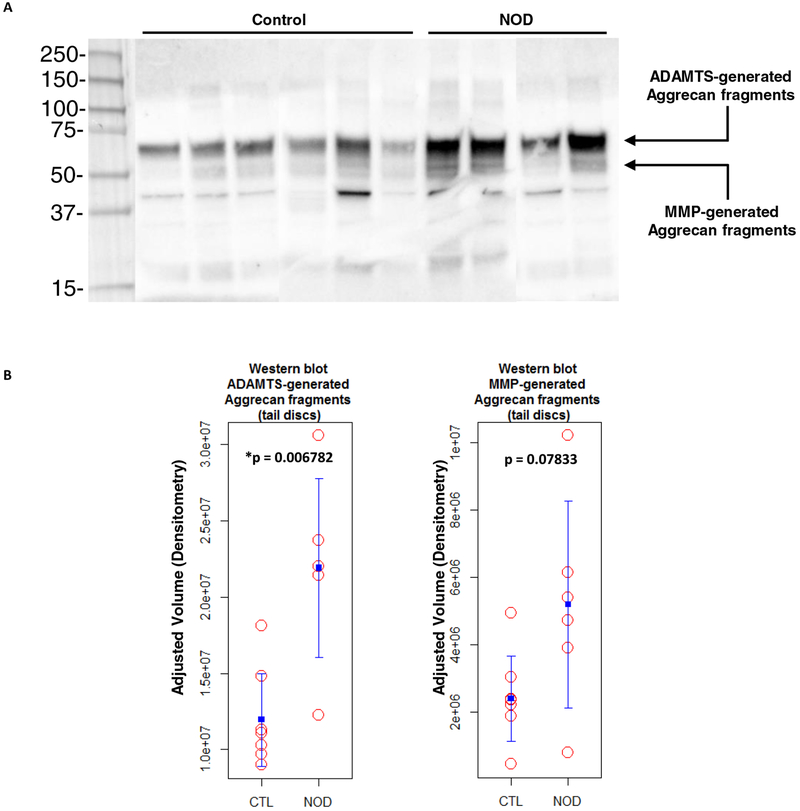
Immunoblot analysis of aggrecan fragments
To investigate if disc matrix degradation was caused by increased aggrecan cleavage, Western Blot analysis was performed on tail IVDs of both NOD and control mice, using antibodies against the aggrecan G1 domain (Fig. 3a). Levels of IVD aggrecan fragments generated by ADAMTS-mediated cleavage were significantly (p < 0.05) higher in NOD mice compared to euglycemic littermates. MMP-generated aggrecan fragments were found to be moderately increased in NOD mice, but this did not reach statistical significance (Fig. 3b), suggesting that ADAMTS might be more substantially involved in disc matrix breakdown in NOD mice.
Fig. 3.
T1DM increased aggrecan fragmentation within IVD matrix.
a A representative immunoblot with corresponding quantitative analysis showing the levels of tail IVD aggrecan fragments generated by ADAMTS and MMP activity within the aggrecan interglobular domain.
b Values of ADAMTS-mediated cleavage were higher and statistically significant in NOD mice (*p < 0.05) compared to euglycemic littermates. MMP-mediated fragmentation was increased in diabetic mice as well (p > 0.05).
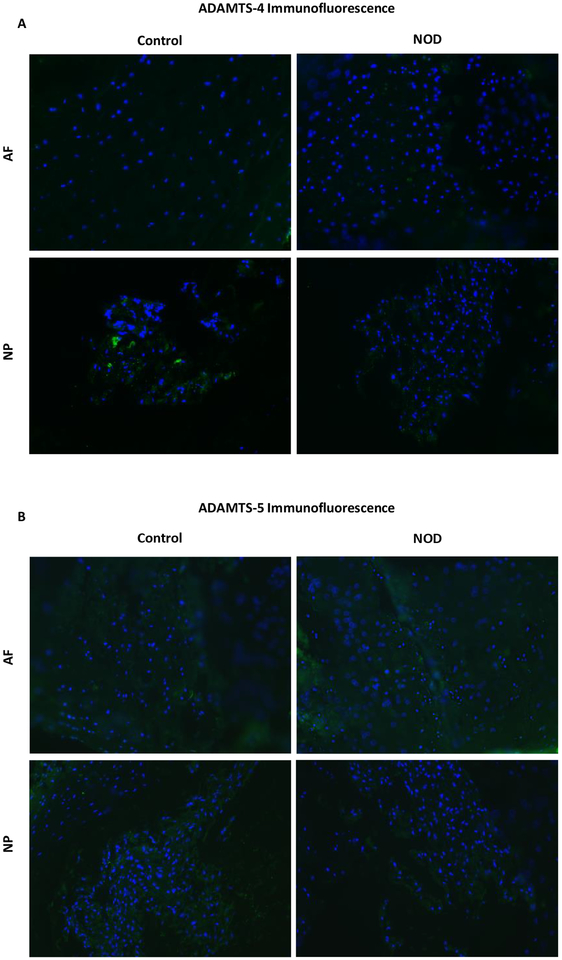
Quantitative immunofluorescence
As ADAMTS seemed to be primarily involved in proteoglycan breakdown within the disc, and thus potentially implicated in IDD, quantitative immunofluorescence with anti-ADAMTS antibodies was performed, in order to identify which isoform could be mainly responsible for aggrecan degradation. More specifically, ADAMTS4 (Fig. 4a) and ADAMTS5 (Fig. 4b) protein expression was investigated in disc sections, which showed no significant differences in both annulus fibrosus (AF) and NP tissues between NOD and control mice.
Fig. 4.
ADAMTS quantitative immunofluorescence assay with anti-ADAMTS-4 (a) and −5 antibodies (b) did not show metalloproteinase upregulation within disc tissues.
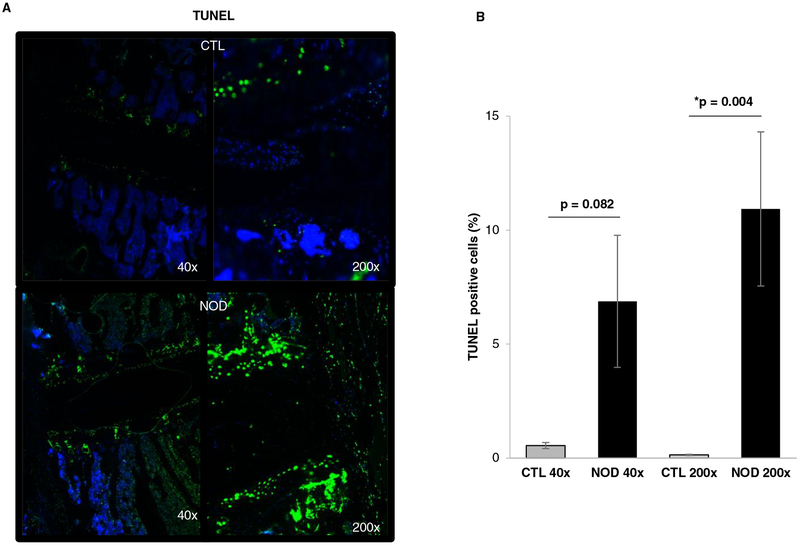
TUNEL assay
Apart from increased metalloproteinase-mediated aggrecan fragmentation, another major cause of reduced PG levels in the disc is loss of functional cells. TUNEL assay demonstrated higher levels of disc apoptosis in NOD mice compared to control mice (Fig. 5a). At 200x magnification, in control mice 0.14% of disc cells were TUNEL positive compared to 10.9% in NOD mice (p < 0.05) (Fig. 5b).
Fig. 5.
TUNEL assay to identify apoptotic cells in disc tissue of control and NOD mice.
a Histological sections including vertebral bodies, cartilaginous endplates and intervertebral disc of both wild-type and NOD mice were analyzed under 40x and 200x magnification. Blue, DAPI (nuclei); green, apoptotic cells.
b TUNEL assay quantification was calculated as the percentage of TUNEL positive cells in control animals and NOD mice in nine random fields. Apoptosis was significantly enhanced (*p < 0.05) in NOD disc tissue under high magnification (200x).
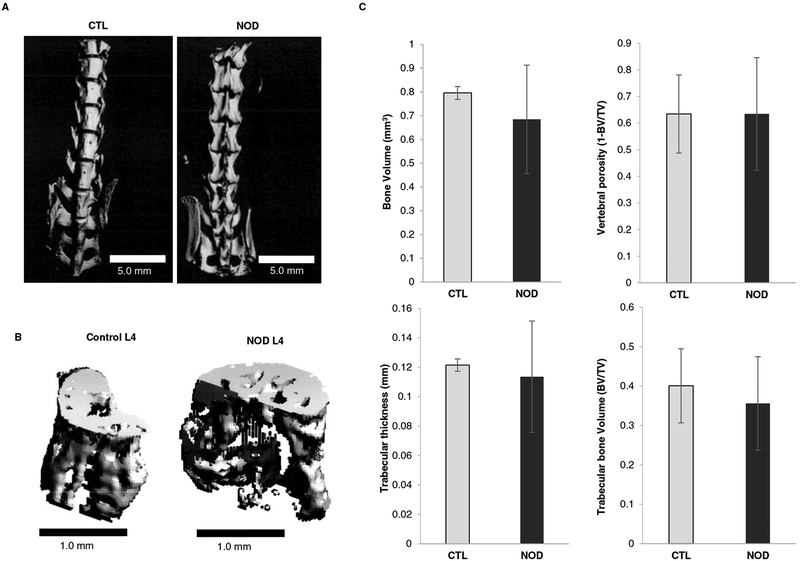
microCT
Given that disc matrix proteoglycan breakdown as well as cellular apoptosis were significantly enhanced in NOD mice, vertebral structure was also investigated using mCT to detect changes in bone integrity. Overall, vertebral bone structure of NOD mice was similar to that of euglycemic littermates (Fig. 6a); likewise, a sample L4 vertebra showed to be macroscopically similar between the two animal models (Fig. 6b). Bone volume of NOD mice was 0.685 ± 0.228 mm3 compared to 0.796 ± 0.027 mm3 in controls. Vertebral bone porosity of diabetic mice was unchanged comparing to euglycemic littermates (0.635 ± 0.212 and 0.634 ± 0.147 respectively). Trabecular bone thickness showed to be 0.114 ± 0.038 mm in NOD mice and 0.121 ± 0.004 mm in control mice. Total trabecular bone volume seemed to be not significantly different in the two models withal: 0.356 ± 0.119 mm3 in diabetic mice and 0.401 ± 0.094 mm3 in euglycemic animals (Fig. 6c). These values suggest that vertebral bone microstructure was largely unaffected in NOD mice.
Fig. 6.
Diabetes did not affect vertebral bone microarchitecture.
a Representative 3D reconstruction of the micro-computed tomographical images of the lumbar spine and L4 vertebra (b) in both control and NOD mice.
c Quantitative bone parameters did not significantly differ from wild type mice and diabetic littermates.
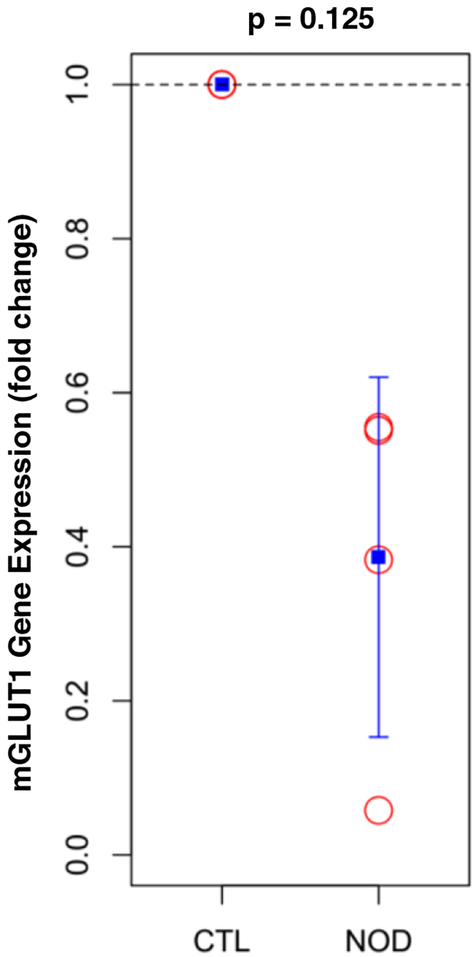
mGLUT1 gene expression
mGLUT1 glucose transporter gene expression in cervical and thoracic discs was assessed through RT-PCR (Fig. 7): mGLUT1 gene expression was 60% lower in NOD mice compared to euglycemic littermates, but this was not statistically significant.
Fig. 7.
Diabetic mice showed a downregulation in mouse glucose transporter 1 (mGLUT1) gene expression compared to control littermates.
Discussion
DM is a common condition that causes metabolic disturbances in many tissues, including bone and cartilage. Recently, a longitudinal cohort study concluded that DM was significantly associated with IDD in the upper cervical spine.17 On the other hand, additional risk factors associated with DM, i.e. age and high BMI, may contribute to lumbar spine degeneration. Liu et al. have conducted a retrospective study assessing the severity of IDD in diabetic patients using the Pfirrmann score: they concluded that diabetic subjects, especially those with a long-standing and poorly controlled disease, were more prone to develop IDD.18 In this study, we investigated disc degenerative changes in diabetic NOD mice, an animal model of T1DM. In our diabetic mice, both matrix aggrecan degradation and apoptosis were found to be significantly elevated compared to control animals.
Our findings are consistent with previous studies. Won et al. investigated the effect of hyperglycemia on apoptosis of notochordal cells within the NP. They found that cell death was premature and associated with the transition to a fibrocartilaginous-inducing IDD environment.19 Diminished synthesis and expression of GLUT1, a high affinity transporter which is essential for glucose uptake into cells, has been described in young adolescents affected by T1DM.20 Indeed, mGLUT1 showed a trend toward downregulation in IVDs of diabetic NOD mice, possibly providing an explanation for establishing a low metabolic state in NP cells that could likely lead to cell apoptosis. Alternatively, mGLUT1 downregulation may be interpreted as a protective compensatory response against hyperglycemia, which can negatively impact on cell viability.
Disintegrins and metalloproteinases with thrombospondin motifs (ADAMTSs) and matrix metalloproteinases (MMPs) are the major proteolytic enzymes involved in extracellular matrix breakdown in IDD. Several studies have demonstrated their upregulation in different conditions including inflammation, mechanical overload and genetic predisposition (all consistent with DM etiopathological features).21 Aggrecan fragmentation due to ADAMTS enzymatic activity was found to be significantly increased in this study: this may be due to the pro-inflammatory environment characterizing DM which could upregulate ADAMTS expression.22 Surprisingly, disc ADAMTS-4 and ADAMTS-5 immunofluorescence showed little differences between NOD and control mice. It is possible that enhanced disc aggrecanolysis in NOD mice is due to an increased enzymatic activity of these aggrecanases without upregulation of their protein expression.
On the other hand, Safranin-O histology did not show significant morphological alterations, while disc PG synthesis and GAG/DNA ratio in the NP were not significantly affected in diabetic mice compared to controls; this increase may be due to a higher cell loss caused by apoptosis rather than to a GAG enhanced synthesis. However, DM duration in NOD mice was only 6 weeks at the time of analyses, which might not be long enough to cause substantial impact on disc PG content.
mCT showed no significant differences in NOD mice compared to euglycemic littermates. Several clinical studies have reported a reduced bone density in patients with T1DM explaining that insulin deficiency may lead to decreased bone formation and increased bone resorption.23 On the other hand, some studies have observed that insulin excess in non-insulin-dependent DM is associated with increased bone density, due to the anabolic and mitogenic effects of insulin and higher body weight.24 It is conceivable that no notable morphological vertebral alterations were observed because of the short duration of the disease in NOD mice, as major degenerative spine changes usually occur with long-standing DM.
In conclusion, we demonstrated that diabetic NOD mice showed higher levels of ADAMTS-mediated aggrecan fragmentation in disc matrix and increased disc cell apoptosis, which may promote IDD development in this model of T1DM. Loss of viable cells may be a consequence of a low metabolic state due to reduced glucose uptake in disc cells, which was confirmed by down-regulation of mGLUT1 expression. However, disc GAG content and PG synthesis seemed not to be significantly affected, suggesting that matrix aggrecan breakdown was not extensive enough to cause loss of total disc PG content within the time frame studied. This might be due to the relatively short duration of the DM disease in our model. Nevertheless, the observed changes in NOD mice may represent the earliest effects of DM on disc matrix health, contributing to IDD associated with DM.
ACKNOWLEDGMENTS
The authors thank Ms. Qing Dong and the staff in the Ferguson Laboratory for Spine research in the Department of Orthopaedic Surgery, University of Pittsburgh, for their technical assistance.
This work was supported in part by The Albert B. Ferguson, Jr., M.D. Orthopaedic Fund of The Pittsburgh Foundation and NIH AG044376.
No relevant financial activities outside the submitted work.
Footnotes
The device(s)/drug(s) is/are FDA-approved or approved by corresponding national agency for this indication.
References
- 1.Vadala G, Russo F, Ambrosio L, et al. Biotechnologies and Biomaterials in Spine Surgery. J Biol Regul Homeost Agents. 2015;29(4 Suppl):137–47. [PubMed] [Google Scholar]
- 2.Vadala G, Russo F, Di Martino A, et al. Intervertebral disc regeneration: from the degenerative cascade to molecular therapy and tissue engineering. J Tissue Eng Regen Med 2015;9(6):679–90. [DOI] [PubMed] [Google Scholar]
- 3.Nasto LA, Ngo K, Leme AS, et al. Investigating the role of DNA damage in tobacco smoking-induced spine degeneration. Spine J 2014;14(3):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russo F, Hartman RA, Bell KM, et al. Biomechanical Evaluation of Transpedicular Nucleotomy With Intact Annulus Fibrosus. Spine (Phila Pa 1976). 2017;42(4):E193–E201. [DOI] [PubMed] [Google Scholar]
- 5.Vadala G, Russo F, Ambrosio L, et al. Stem cells sources for intervertebral disc regeneration. World J Stem Cells. 2016;8(5):185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Illien-Junger S, Lu Y, Qureshi SA, et al. Chronic ingestion of advanced glycation end products induces degenerative spinal changes and hypertrophy in aging pre-diabetic mice. PLoS One. 2015;10(2):e0116625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Global report on diabetes. 2016.
- 8.Robinson D, Mirovsky Y, Halperin N, et al. Changes in proteoglycans of intervertebral disc in diabetic patients. A possible cause of increased back pain. Spine (Phila Pa 1976). 1998;23(8):849–55; discussion 56. [DOI] [PubMed] [Google Scholar]
- 9.Pearson JA, Wong FS, Wen L. The importance of the Non Obese Diabetic (NOD) mouse model in autoimmune diabetes. J Autoimmun. 2016;66:76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vo N, Seo HY, Robinson A, et al. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res 2010;28(12):1600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–7. [DOI] [PubMed] [Google Scholar]
- 12.Alp E, Koc RK, Durak AC, et al. Doxycycline plus streptomycin versus ciprofloxacin plus rifampicin in spinal brucellosis [ISRCTN31053647]. BMC Infect Dis 2006;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Vo NV, Sowa GA, et al. Bupivacaine decreases cell viability and matrix protein synthesis in an intervertebral disc organ model system. Spine J 2011;11(2):139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Studer RK, Aboka AM, Gilbertson LG, et al. p38 MAPK inhibition in nucleus pulposus cells: a potential target for treating intervertebral disc degeneration. Spine (Phila Pa 1976). 2007;32(25):2827–33. [DOI] [PubMed] [Google Scholar]
- 15.Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 2010;25(7):1468–86. [DOI] [PubMed] [Google Scholar]
- 16.Nasto LA, Robinson AR, Ngo K, et al. Mitochondrial-derived reactive oxygen species (ROS) play a causal role in aging-related intervertebral disc degeneration. J Orthop Res 2013;31(7):1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teraguchi M, Yoshimura N, Hashizume H, et al. Progression, incidence, and risk factors for intervertebral disc degeneration in a longitudinal population-based cohort: the Wakayama Spine Study. Osteoarthritis Cartilage. 2017. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Pan F, Ba Z, et al. The potential effect of type 2 diabetes mellitus on lumbar disc degeneration: a retrospective single-center study. J Orthop Surg Res 2018;13(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won HY, Park JB, Park EY, et al. Effect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic rats. J Neurosurg Spine. 2009;11(6):741–8. [DOI] [PubMed] [Google Scholar]
- 20.Garg M, Thamotharan M, Becker DJ, et al. Adolescents with clinical type 1 diabetes display reduced red blood cell glucose transporter isoform 1 (GLUT1). Pediatr Diabetes. 2014;15(7):511–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vo NV, Hartman RA, Yurube T, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J 2013;13(3):331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Illien-Junger S, Grosjean F, Laudier DM, et al. Combined anti-inflammatory and anti-AGE drug treatments have a protective effect on intervertebral discs in mice with diabetes. PLoS One. 2013;8(5):e64302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krakauer JC, McKenna MJ, Buderer NF, et al. Bone loss and bone turnover in diabetes. Diabetes. 1995;44(7):775–82. [DOI] [PubMed] [Google Scholar]
- 24.Barrett-Connor E, Kritz-Silverstein D. Does hyperinsulinemia preserve bone? Diabetes Care. 1996;19(12):1388–92. [DOI] [PubMed] [Google Scholar]