ABSTRACT
The innervation of bone has been described for centuries, and our understanding of its function has rapidly evolved over the past several decades to encompass roles of subtype‐specific neurons in skeletal homeostasis. Current research has been largely focused on the distribution and function of specific neuronal populations within bone, as well as their cellular and molecular relationships with target cells in the bone microenvironment. This review provides a historical perspective of the field of skeletal neurobiology that highlights the diverse yet interconnected nature of nerves and skeletal health, particularly in the context of bone anabolism and pain. We explore what is known regarding the neuronal subtypes found in the skeleton, their distribution within bone compartments, and their central projection pathways. This neuroskeletal map then serves as a foundation for a comprehensive discussion of the neural control of skeletal development, homeostasis, repair, and bone pain. Active synthesis of this research recently led to the first biotherapeutic success story in the field. Specifically, the ongoing clinical trials of anti‐nerve growth factor therapeutics have been optimized to titrated doses that effectively alleviate pain while maintaining bone and joint health. Continued collaborations between neuroscientists and bone biologists are needed to build on this progress, leading to a more complete understanding of neural regulation of the skeleton and development of novel therapeutics. © 2019 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals, Inc.
Keywords: BONE‐BRAIN‐NERVOUS SYSTEM INTERACTIONS; SYSTEMS BIOLOGY ‐ BONE INTERACTORS, OTHER; ANALYSIS/QUANTITATION OF BONE, OTHER; THERAPEUTICS, ANABOLICS; THERAPEUTICS
Development of a Unified Theory for Nerves in Bone
The field of skeletal neurobiology emerged with a great debate surrounding the relationship between sensory nerve damage and joint disease that began in the mid‐19th century. In 1868, French neurologist Jean‐Martin Charcot detailed joint pathology with progressive degeneration of bones and soft tissues in patients with tabes dorsalis, a complication of untreated syphilis resulting in degeneration of sensory nerves in the dorsal column of the spinal cord1 (Fig. 1 A, D). Charcot speculated that nerves in bone were of a trophic nature. He reasoned that their destruction disrupted the supply of growth factors to the bone and joint, leading to their collapse. Thus, Charcot laid the foundation for the neurotrophic theory of bone and joint health. Almost immediately, this was met with opposition by the neurotraumatic theorists, led by Volkman and Virchow, who asserted that nerve damage results in loss of peripheral sensation leading to repetitive trauma that outpaces healing. This theory was supported by a series of sensory denervation experiments performed in cats by Eloesser in 1917 and Corbin and Hinsey in 1939. They concluded that loss of sensation alone was insufficient to induce neuropathic osteoarthropathy, since only denervated cats exhibiting unnatural gait and inappropriate loading developed bone and joint lesions2, 3 (Fig. 1 B–D). In the 1980s, a third theory emerged following the observation that blood flow was increased in joints of patients with diabetic neuropathy.4 This neurovascular theory was based on the sympathetic control of vascular tone, which when compromised by autonomic neuropathy, allows excess blood flow to affected joints leading to inflammation‐induced bone resorption and susceptibility to minor trauma.
Figure 1.
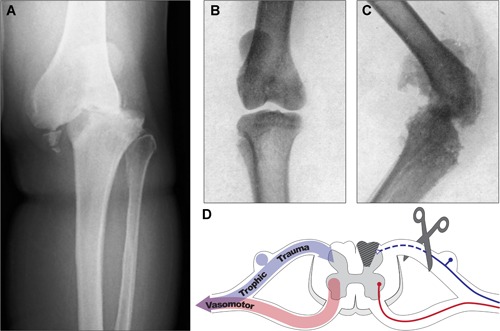
Multifactorial neural regulation of bone and joint. (A) Anteroposterior X‐ray of the knee joint of a 62‐year‐old patient with unilateral Charcot neuroarthropathy with characteristic joint collapse, bone loss, and fragmentation. Patient presented with chronic polyneuropathy and total sensorineural loss following a failed spinal stenosis surgery. Reprinted with permission from Cıvan and colleagues.182 (B, C) X‐ray of the knee joints of Eloesser's cat showing a frontal projection of the control side (B) and lateral view of the affected side (C); the affected knee was subjected to an acute thermocautery trauma following sensory posterior root resection as depicted in D (scissors) and subsequently developed deformity and grating of joint surfaces within 3 weeks. Reprinted with permission from Eloesser.3 (D) Conceptual representation of neurotrophic, neurotraumatic, and neurovascular theories of neural regulation of bone and joint, where sympathetic nerves regulate vascular tone (red), and sensory nerves provide trophic signals and mediate protective pain perception, as well as vasoregulation (blue). Collectively, the peripheral nerve carries different types of neurons that contribute to bone and joint homeostasis; destruction of these, eg, upon dorsal column degeneration (gray hatched region of spinal cord) with tabes dorsalis or dorsal root resection (scissors) and degeneration of the central primary sensory axon, contributes to joint collapse and bone loss.
Now, after more than a century of investigation, the collective model points to a multifactorial relationship between nerves and bone which synthesizes the neurotrophic, neurotraumatic, and neurovascular theories (Fig. 1 D). Indeed, trophic signals and protective pain, as well as regulation of blood flow, are all indispensable components of neural regulation of bone and joint. In recognition of this synergy, this review will first summarize what is known regarding the neuronal subtypes found in the skeleton, their distribution within bone compartments, and their central projection pathways. This neuroskeletal map will then guide a comprehensive discussion of the neural control of skeletal development, homeostasis and repair, and last, bone pain.
Classification of Skeletal Axons
The peripheral nervous system (PNS) relays information between the central nervous system (CNS) and the skeleton. Neurons of the PNS are located in sensory and autonomic ganglia, collections of neural cell bodies that disseminate their peripheral axons to target tissues through nerve bundles (eg, sciatic nerve, tibial nerve, etc) (Fig. 2). The consensus in the field is that the fine nerve branches that target the skeleton are primarily composed of small and medium diameter axons, both unmyelinated and myelinated.5, 6, 7, 8, 9, 10 These morphological parameters are consistent with primary sensory neurons and postganglionic autonomic neurons and have been further classified by neurochemical profiling and electrophysiological properties as discussed below (Fig. 3).
Figure 2.
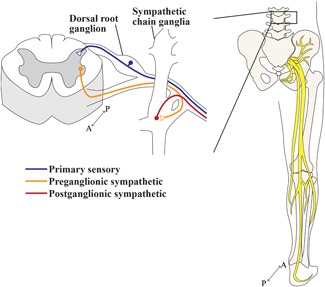
Peripheral neurons’ route to the skeleton. A cross‐section of the lumbar spinal cord, where peripheral neurons that innervate the lower limbs either originate or terminate. Primary sensory neurons (blue) are pseudo‐unipolar: cell bodies reside in dorsal root ganglia from which a single axonal process bifurcates into a centrally projecting axon that targets the dorsal horn of the spinal cord and a long peripheral axon that projects out to the target tissue. The sympathetic system communicates to peripheral targets via a two‐neuron relay. Preganglionic sympathetic axons (orange) originate in the intermediolateral cell column of the spinal cord and project to sympathetic ganglia, mostly the sympathetic chain ganglia for innervation of the lower limb, to synapse with postganglionic sympathetic neurons. Postganglionic sympathetic neurons (red) project long axons towards their targets. The sensory and sympathetic axons are carried through major mixed nerves, as depicted for the right lower limb in relation to the skeleton, posterior view. Proximal to the bone, fine nerve branches containing the small sensory and autonomic axons described leave to supply the periosteum, bone, and bone marrow.
Figure 3.
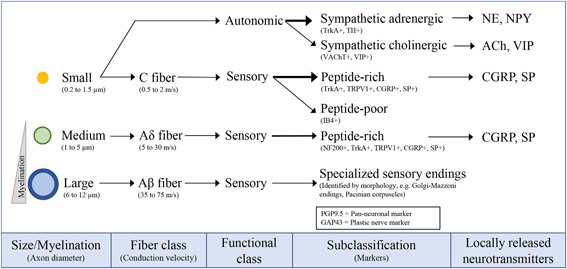
Classification of skeletal axons. The axons in peripheral nerves supplying the bone can be classified on the basis of their size and myelination status, both of which contribute to conduction velocity.14 The majority of skeletal fibers are unmyelinated, small diameter axons (yellow, 0.2 to 1.5 µm) that conduct slowly (0.5 to 2 m/s). These consist of sympathetic fibers, which can have either an adrenergic or cholinergic phenotype, and sensory C fibers. The second most prevalent axon type found in the bone are Aδ fibers: lightly myelinated, medium diameter axons (green, 1‐µm to 5‐µm diameter) that conduct at medium speeds (5 to 30 m/s). Last, Aβ fibers are large‐diameter, thickly myelinated axons (6 to 12 µm) that conduct much faster (35 to 75 m/s) but are relatively scarce or absent in bone. Each fiber subclass has a unique set of markers that are often used to define it immunohistochemically. Small‐sized and medium‐sized neurons of both sensory and/or autonomic origin also have the capacity to release specific neurotransmitters to the local environment. TrkA = tyrosine kinase receptor type 1; TH = tyrosine hydroxylase; VAChT = vesicular acetylcholine transporter; VIP = vasoactive intestinal peptide; TRPV1 = transient receptor potential cation channel subfamily V member 1; CGRP = calcitonin gene‐related peptide; SP = substance P; IB4 = isolectin‐B4; NF200 = neurofilament 200; PGP9.5 = protein gene product 9.5; GAP43 = growth associated protein 43; NE = norepinephrine; NPY = neuropeptide Y; ACh = acetylcholine.
Sensory nerves in bone
Primary sensory neurons in bone house their cell bodies in dorsal root ganglia (DRG) alongside the spinal cord (Fig. 2), or cranial nerve ganglia for those that innervate the craniofacial skeleton. They have a pseudo‐unipolar morphology, meaning that the neuron extends one axon which bifurcates into a central projection toward the brain and a peripheral projection which innervates the target tissue. This connects their receptive fields in the periphery to the dorsal horn of the spinal cord for central processing of pain, pressure, and other perceived stimuli. In addition, these neurons can generate efferent signals, often in the form of dorsal root reflexes, that are directed toward their peripheral targets. In general, this antidromic activity can arise from collateral axon branches within or proximal to the target tissue, from the dorsal root ganglia, or from central or local circuits in the dorsal horn.11 The efferent activity of primary sensory neurons is most evident in neurogenic inflammation following tissue injury, where release of neuropeptides such as calcitonin gene‐related peptide (CGRP) and substance P (SP) promotes vasodilation and plasma extravasation, respectively.12 The presence of neurogenic inflammation within bone is supported by a study in rats in which chemical sensory denervation suppressed vasodilation and inflammatory cell recruitment within the bone marrow after induction of adjuvant‐induced arthritis.13 This capacity for local neuropeptide release also speaks to the trophic nature of sensory nerves in bone, elaborated below in the Molecular Signals From Neurons to Bone‐Neurotransmitters section.
Primary sensory neurons are categorized by size, myelination status, neuropeptide content, growth factor dependence, and conduction velocity (Fig. 3).14 Peptide‐rich fibers expressing CGRP and SP are prevalent in bones across vertebrate species.5, 7, 10, 15, 16, 17 These exist as both small unmyelinated (0.2 to 1.5 µm) or medium myelinated fibers (1 to 5 µm). The myelinated axons are generally identified by their immunoreactivity for the high molecular weight (200 kD) neurofilament protein NF200.10 Peptide‐poor C fibers have not yet been detected within bone,10, 18 but isolectin‐B4 (IB4)‐binding has been demonstrated in 11% to 34% of DRG neural cell bodies innervating the rat tibia, suggesting that they may represent a larger population than previously recognized.19 Survival and recruitment of peptidergic fibers is nerve growth factor (NGF)‐dependent and peptide‐poor fibers are glial‐derived neurotrophic factor (GDNF)‐dependent.20, 21 Upon noxious mechanical stimulation, conduction velocities recorded from fine nerves innervating the rat bone are consistent with small, unmyelinated C fibers (<2 m/s) and medium sized, myelinated Aδ axons (2 to 12.5 m/s),22 which are well matched with reported conduction velocities of somatic sensory afferents (C fibers: 0.5 to 2 m/s; Aδ fibers: 5 to 30 m/s; Aβ fibers: 35 to 75 m/s).14 Large‐diameter neurons are generally absent within bone marrow; however, large sensory fibers with encapsulated endings specialized for detecting vibration and pressure have been described in the interosseous membranes between bones,23 in the jaws anterior to the mental foramen in cats,24 and in the human long bone periosteum.25
Autonomic nerves in bone
The sympathetic and parasympathetic divisions of the autonomic nervous system send messages to the periphery through a two‐neuron relay. Autonomic outflow is communicated through a preganglionic neuron in the spinal cord that synapses onto the cell body of a postganglionic neuron within an autonomic ganglion (Fig. 2); this largely occurs in the sympathetic chain ganglia for the trunk and the cervical ganglia for the head. Both divisions send small, unmyelinated neurons to peripheral targets. Postganglionic autonomic neurons have slow conduction velocities, in the range of C fibers, and are similarly small in size and devoid of myelination (Fig. 3). The autonomic nervous system coordinates involuntary functions in response to internal and external stressors to maintain whole‐body homeostasis, including vascular tone, and is always active at a basal level. Energy expenditure, circadian clock rhythms, and stress are just a few possible signals that have been shown to influence sympathetic outflow with subsequent impacts on bone metabolism.26, 27, 28
Postganglionic autonomic neurons are identified by the presence of cognate small molecule neurotransmitters or enzymes. Sympathetic adrenergic neurons expressing tyrosine hydroxylase (TH), the rate‐limiting enzyme for synthesis of norepinephrine (NE), are abundant in bone.7, 8, 10, 15, 16, 17, 29 Cholinergic neurons containing acetylcholine (ACh), as evidenced by expression of vesicular ACh transporter (VAChT), and the neuropeptide vasoactive intestinal peptide (VIP), also innervate bone.7, 8, 16, 30, 31 Although cholinergic fibers in the periphery are mostly derived from parasympathetic postganglionic neurons, there is in fact a subset of postganglionic sympathetic neurons that also have a cholinergic neurochemical profile.30, 32, 33 For example, in the periosteum, a subset of sympathetic neurons acquires a cholinergic phenotype upon contact with their target.31, 34 Furthermore, sympathectomy abolishes VIP staining in bone,8, 35 supporting a sympathetic origin for the VIP + cholinergic fibers targeting the skeleton. At present, evidence for a parasympathetic innervation of the bone is lacking (there is no evidence for direct tracing from autonomic postganglionic nerves in bone to parasympathetic ganglia).36
Other biomarkers of both neuronal and non‐neuronal cells in bone
Several additional biomarkers often appear in the skeletal neurobiology literature. First, neurotrophic tyrosine kinase receptor type 1 (TrkA) is a member of the Trk receptor family and one of two cognate receptors for NGF. Many peptidergic sensory nerves and the majority, if not all, TH + autonomic neurons in bone express TrkA.37 This implies that axon guidance, survival, sprouting, and activity of these neurons could be influenced by NGF signaling.37 Vascular endothelial cells, periodontal fibroblasts, and osteoblasts have also been reported to express TrkA.38, 39, 40, 41 Second, the vast majority of small sensory and autonomic nerve fibers express growth associated protein 43 (GAP43), a protein that has been related to an axon's capacity for growth, regeneration, and plasticity.29, 37, 42 GAP43 is also expressed by non‐myelinating Schwann cells.43 Third, the transient receptor potential cation channel subfamily V member 1 (TRPV1) is expressed by most unmyelinated and some small diameter myelinated sensory neurons, including those in bone and joint.44, 45, 46 TRPV1 + neurons in bone can be sensitized by the TRPV1 agonist capsaicin, a potent extract of the chili pepper.22 High systemic doses of capsaicin have also been used to induce cell death selectively in small sensory, primarily unmyelinated, neurons in bone and other tissues.13, 47 Though originally thought to be specific to nerve, recent reports have suggested that TRPV1 is also expressed in cells of the chondrocyte, osteoblast, and osteoclast lineages.48, 49 Last, protein gene product 9.5 (PGP9.5) is a ubiquitin‐protein hydrolase that is often used as a pan‐neuronal marker for purposes of quantification of nerves in and on the bone.29, 42, 50
Nerve Entry and Distribution Within the Skeleton
Sensory, and less so autonomic, nerves form subtype‐specific meshwork patterns within the periosteum that descend through the outer connective tissue layers and inner cellular layers into the bone itself7, 10, 17 (Fig. 4 A, B). Sensory and autonomic fibers penetrate the cortical bone alongside vessels through nutrient canals in mice and through Volkmann's canals and Haversian systems in humans, cats, and other large vertebrates.51, 52, 53 In the distal femur of an adult mouse, 26% of the nutrient canals are occupied by CGRP + sensory axons whereas nearly double the proportion of canals contain TH + sympathetic fibers.15 Within the canals, sensory nerves are generally linear, whereas sympathetic fibers spiral around vessels, reminiscent of perivascular patterning in the periosteum. Once in the marrow space, unmyelinated CGRP + sensory fibers branch and project linear, varicose‐rich endings, particularly in the vicinity of the epiphyseal trabecular bone, and less frequently in the metaphysis and diaphysis10, 17 (Fig. 4 A, C). Myelinated NF200 + sensory axons, most of which also contain CGRP, share a similar pattern of distribution, but tend to have a relatively longer and more linear morphology.10 Small, unmyelinated SP + sensory fibers are less abundant.17 In some cases, CGRP and SP are also co‐expressed. In the marrow space, large‐caliber vessels are frequently wrapped by sympathetic TH + fibers rich in varicosities, and like sensory terminals, sympathetic axons can dissociate from vasculature to terminate as free‐nerve endings in the bone marrow (Fig. 4 A, D).10, 17
Figure 4.
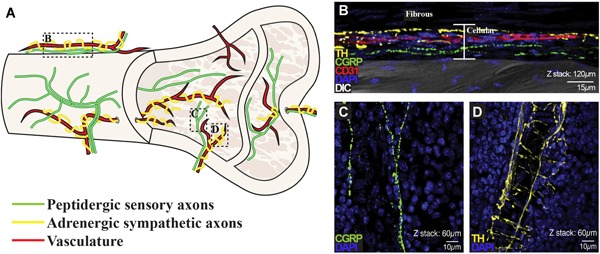
Distribution and patterning of nerves in bone. (A) Schematic of the peptidergic sensory axons and adrenergic sympathetic axons, highlighting their relationship to the vasculature, as well their relative distributions and typical patterning in the periosteum, as they enter the cortical bone, and within the marrow space. (B–D) Representative confocal micrographs of the distal mouse femur with immunostaining for TH + sympathetic fibers (yellow), CGRP + peptidergic sensory fibers (green), CD31 + endothelial cells of blood vessels (red), showing cell nuclei (DAPI, blue) and overlaid on an image captured by DIC microscopy to provide orientation in the periosteum (B) and marrow (C, D). Z‐stack thickness and scale bars as indicated. B–D reprinted and adapted with permission from Chartier and colleagues.15 DIC = differential interference contrast.
Systematic analysis of fiber types innervating bone compartments has been estimated by the number or total length of fibers normalized to tissue area or volume, respectively, of periosteum, mineralized bone, and marrow space of the mouse femur.10, 15 By fiber number in the distal femur, sensory axons densely innervate the periosteum (177.4 CGRP + sensory fibers/mm2, 154.1 NF200 + myelinated sensory fibers/mm2) and are markedly less abundant in the mineralized bone (15.9 CGRP + fibers/mm2, 7.6 NF200 + fibers/mm2) and bone marrow (9.9 CGRP + fibers/mm2, 3.3 NF200 + fibers/mm2).10 Sympathetic fiber density is likewise greatest in the periosteum (128.4 TH‐positive fibers/mm2), though less abundant than sensory fibers. In contrast to sensory innervation of deeper bone compartments, sympathetic axons have a striking presence in mineralized bone (60.2 TH + fibers/mm2) and marrow (45.3 TH + fibers/mm2).10 These relative distributions are consistent with measurements of total fiber length for sensory and sympathetic fibers.15 Although these studies are powerful for comparing relative distribution of skeletal innervation, the high degree of fiber branching and winding through the tissue and the limited working depth in standard histological sections remain obstacles to quantifying the absolute number of neurons that innervate different bone compartments. Advances in tissue clearing techniques and whole‐mount preparations are beginning to be applied to bone that will improve quantitative mapping of skeletal innervation and secondary architecture such as glial cells.54, 55, 56
Pathways to the Brain
There have only been a few studies that have mapped skeletal sensory input to and within the CNS. Retrograde labeled sensory neurons innervating rat tibial periosteum, medullary cavity, and trabecular bone have been observed in ipsilateral DRG spanning lumbar regions L1 to L6, but are concentrated in L3.19 Sensory neurons send their centrally projecting axons from the DRG to synapse with second order projection neurons or interneurons within distinct layers of the dorsal horn of the spinal cord. One study combined retrograde labeling from brain regions implicated in pain processing, with markers of neuronal activity in the spinal cord following noxious mechanical stimulation of bone, to further identify central pathways that may transmit information about bone pain toward the brain.57 They found that acute noxious mechanical stimulation of the rat tibial diaphysis activated neurons in the superficial dorsal horn (laminae I and II) that projected to the lateral parabrachial nucleus (LPB), but not the ventroposterolateral (VPL) or gracile nucleus. This suggests that painful stimuli in the bones of at least the lower limbs of rats are conveyed through the spinoparabrachial pathway (SPB), but not the spinothalamic tract (STT) or the postsynaptic dorsal column (PSDC), respectively. Interestingly, the spinoparabrachial pathway carries a broad range of nociceptive inputs from diverse tissues, and the lateral parabrachial nucleus further connects with brain regions implicated in emotional aspects of pain and homeostatic adaptation in response to pain.58 Only a single study has attempted to identify cortical somatosensory projections of sensory inputs derived from bone.59 It showed that electrical stimulation of nerves that enter the bone marrow of the cat humerus generates evoked potentials in topographically relevant areas of the primary and secondary somatosensory cortices.
Central sympathetic networks influencing bone were recognized when it was discovered hat leptin‐deficient mice have high vertebral trabecular bone mass.60 Follow‐up studies revealed that leptin acts as an anti‐osteogenic signal mediated centrally by glucose responsive neurons in the ventromedial hypothalamus (VMH) that is relayed peripherally through sympathetic pathways.27 A hierarchical circuit controlling sympathetic innervation of rat femoral epiphyseal bone marrow has also been inferred by multisynaptic tract‐tracing.61 Several autonomic projection pathways from the brainstem and the hypothalamus demonstrated connections to the femur via a sympathetic relay through preganglionic neurons concentrated in the intermediolateral cell column (IML) of the spinal cord (lower thoracic and upper lumbar segments T4 to L1) and postganglionic neurons in paravertebral chain ganglia (lumbar levels). Central networks labeled by this approach collectively illustrate that the bone does not appear to be innervated by unique cell groups, but rather is regulated by circuitry common to sympathetic control of other peripheral targets—likely in part due to shared mechanisms underlying autonomic mediation of vascular tone.
Together, these few functional and anatomical studies have only just begun to identify the central projection pathways that carry signals to and from the bone to brain and back again. Expanding the map of the brain regions involved in coding sensory signals as they pass toward the brain, and controlling efferent signals to bone, will be invaluable to understanding bone pain and neural regulation of bone metabolism.
Neural Regulation of Skeletal Development, Homeostasis, and Repair
Neural involvement in endochondral ossification during skeletal development was inferred as early as 1925 by Fernando de Castro. At the site of endochondral ossification, de Castro detected nerve fibers terminating near osteoblasts that atrophied upon calcification, suggesting a precise spatial and temporal influence of nerves in skeletal development (referenced in Sherman62). In the mouse and rat, neurons appear first in the perichondrium of the diaphysis almost simultaneous with the first signs of mineralization.29, 42, 50, 63 Nerves accompanying blood vessels invade through the cartilage canals to reach the primary, and later the secondary, ossification centers, where they maintain a close relationship to the bone‐cartilage interface and the endosteum, sites of high osteogenic activity. At each site of bone formation, the appearance of sensory nerve fibers coincides with vascularization and ossification, and precedes the appearance of sympathetic nerve fibers,50, 63 hinting at a predominant role of the sensory nervous system in bone development. Ablation of sensory innervation by genetic or pharmacological approaches does not inhibit bone development, but consistently results in decreased bone mass in adult mice,47, 64, 65 suggesting that sensory nerves contribute to skeletal homeostasis and mineralization during development.
Bone readily adapts to functional demands in response to mechanical strain to maintain the structural integrity of the adult skeleton. It is clear that local skeletal cells sense and transduce mechanical stimulation to drive adaptive remodeling66; however, this does not fully account for bone formation observed at sites far from the region of maximal strain. For example, in the male rat, ulnar end‐loading induces bone formation in the loaded bone, as well as in the unloaded contralateral and adjacent axial bones.67 Brachial plexus anesthesia during loading, which temporarily blocks nerve activity in the sensory, sympathetic, and motor neurons that innervate the forelimb, significantly diminishes adaptive bone modeling in the loaded ulna and completely abolishes adaptive mineral deposition at distant sites.67 This may be due to interneuron connections within the spinal cord to sensory neurons targeting the contralateral limb and hind limbs68; sensory neuron interconnectivity between limbs is understudied, but provides an interesting avenue for future work. Preliminary evidence further suggests that mechanical loading increases synaptic connections between limbs68 and induces periosteal nerve sprouting.55 Thus, sensory neurons in bone are poised to detect, potentiate, and propagate adaptation to mechanical loads.
Nerves also dynamically rearrange to innervate bone tissue following fracture, in a sequence reminiscent of development. Following fracture, the appearance of sprouting fibers into areas of chondrogenesis precedes vascularization and ossification. New or regenerating GAP43 + axons robustly sprout into the fracture hematoma and the surrounding hypertrophic periosteum.69 Numerous CGRP + and SP + sensory fibers emerge from the deep layers of the periosteum to terminate as fine, varicose endings in the cartilage callus and penetrate newly formed woven bone around the fracture site.70, 71, 72 Neuropeptide Y–positive (NPY +) autonomic fibers are initially diminished at the fracture site, but exhibit sprouting during the inflammatory phase and the bone remodeling phase late in fracture healing.73 As healing progresses, many nerve fibers withdraw. In contrast, disordered and exuberant sprouting of sensory and sympathetic fibers into the marrow, mineralized bone, and periosteum remain in excess of 80 days in a non‐healed fracture, characterized by persistent fracture callus and nonunion, and aberrant mineralization in the marrow space adjacent to pathological nerve sprouting.74 Nerves are likely recruited to fracture sites to promote healing, both through release of vasoregulatory and osteogenic factors and through adaptive pain signals that prevent further injury.
Molecular Signals From Neurons to Bone—Neurotransmitters
Nerves in bone integrate central relays controlling vascular tone and pain with peripheral regulation of skeletal cells such as osteoblasts and osteoclasts. These diverse functions utilize the same neurons and the same neurotransmitter pathways, facilitating simultaneous control of multiple aspects of skeletal homeostasis. True synapses have not been found within the bone.75 Instead, nonsynaptic vesicular fusion in axon varicosities releases signaling chemicals into the extracellular space that diffuse to target‐cell receptors by volume transmission. Ultrastructural analysis has provided evidence of free varicose nerve fibers in the vicinity of putative skeletal target cells: peptidergic sensory fibers running along the periosteal cellular layer and at the osteochondral junction at sites of mineralization, contacting osteoblasts and their precursors, osteoclasts, hematopoietic cells, and endothelial cells of intramedullary blood vessels17, 75, 76 and sympathetic nerve fibers contacting osteoblasts, osteoclasts, and bone marrow adipocytes.27, 75, 77 Several lines of evidence have teased out the influence of key neurotransmitters—the neuropeptides CGRP, SP, and NPY and the biogenic amine NE. Throughout this discussion of the effects of neurotransmitters on bone, it is important to consider that many of these can be expressed by other, non‐neural, cell types in bone. It is also worth cautioning against overinterpretation of in vitro effects on isolated bone cells, as receptors for these neurotransmitters are often expressed by a wide variety of bone cells as well as vascular and inflammatory cells, and thus this approach may not always translate in vivo.
α‐CGRP
α‐CGRP, referred to from here simply as CGRP, is a sensory neuropeptide expressed throughout the central and peripheral nervous systems.78 CGRP is a potent vasodilator and is an important molecule in pain transmission and sensitization and inflammation.79, 80, 81 CGRP signals through a G‐protein coupled receptor (GPCR) complex of calcitonin receptor‐like receptor (CALCRL) and receptor activity‐modifying protein 1 (RAMP1).82 In vitro, CGRP is osteoanabolic with potential for direct and indirect inhibition of catabolism. CGRP increases proliferation and reduces apoptosis of osteoprogenitors83 and enhances osteogenic gene expression and osteoblast activity.84 Osteogenic consequences of CGRP are mediated by cAMP/PKA85, 86 through CREB1 activation of osterix87 and through activation of Wnt/β‐catenin.83, 88 CGRP may also promote osteoblast differentiation indirectly by inducing osteogenic signals from endothelial cells.89 High concentrations of CGRP suppress osteoclast maturation and activity in vitro,90, 91, 92 and CGRP regulates osteoblast production of RANKL and osteoprotegerin (OPG) to indirectly modulate osteoclastogenesis.84, 85, 93, 94
Of the sensory neuropeptides identified in bone, CGRP is the most well studied in vivo, with consistent osteoanabolic effects and some evidence for a neural origin. CGRP‐deficient mice exhibit reduced bone formation and decreased trabecular bone mass with no change in cortical bone or strength.95 The mice in this latter study had decreased CGRP reactivity in the DRG and loss of CGRP + nerve fibers in the bone, suggesting at least some of this effect might be of neural origin, but this remains to be confirmed. Conversely, mice overexpressing CGRP under the control of the osteocalcin promoter have increased trabecular bone formation and density96; of interest, calvarial cultures from osteocalcin‐CGRP mice produced CGRP, whereas cultures from nontransgenic mice did not, suggesting an alternative endogenous source for this neuropeptide. Activation of cells expressing TRPV1 suppresses alveolar bone resorption by a mechanism including osteoclast suppression by CGRP.91 CGRP promotes bone accrual during fracture repair.87 Magnesium rod implant into the medullary cavity of rat femur induces cortical bone formation and accelerates fracture healing.87 Mechanistically, magnesium induces CGRP release from local sensory fibers that in turn stimulates osteogenic differentiation of periosteum‐derived stem cells.
SP
SP is a member of the tachykinin peptide hormone family encoded by the tachykinin precursor 1 (TAC1) gene. SP is expressed in the brain and peripheral nerves and is often co‐released with CGRP; thus, it is involved in many overlapping processes including vasodilation, inflammation, and pain.97, 98 SP is also broadly expressed in non‐neural tissues including macrophages and neutrophils, epithelial cells, and endothelial cells,99, 100 and SP secretion from osteoblast‐ and osteoclast‐lineage cells has been detected in vitro.101, 102 SP binds and activates neurokinin‐1 receptor (NK‐1R), expressed in osteoblasts, osteocytes, and osteoclasts, as well as their precursors.103, 104, 105 In vitro, SP promotes viability and proliferation of osteoblast precursors and stimulates osteogenesis, in part through MAPK‐Erk and Wnt/β‐catenin signaling.101, 105, 106, 107 SP can drive osteoclastogenesis directly by inducing nuclear translocation of NF‐κB in osteoclast precursors, independent of RANKL, and indirectly by stimulating RANKL production in osteoblast lineage cells.105 In vivo studies of SP have shown differential effects on bone metabolism dependent on the bone under study and the physiological status of the bone.98, 108, 109 It is perhaps particularly difficult to isolate the effects of SP on bone cells because of its pervasive role in inflammation and its broad expression profile. Tissue‐specific genetic manipulation of SP and its receptor would be beneficial to resolve the roles of SP on bone metabolism and bone pain as detailed in the following section under Bone Pain.
NE
NE is the canonical neurotransmitter of postganglionic noradrenergic sympathetic nerves. Adrenergic receptors (ARs) including β1, β2, α1B, α2A, α2B, and α2C, have been detected in osteoblast lineage cells,27, 110, 111, 112, 113, 114 and β2, α2A, α2B, and α2C are expressed in osteoclast lineage cells.111 There is an abundance of pharmacological agents targeting ARs owing to their therapeutic value against life‐threatening conditions such as hypertension, asthma, and cardiac arrest.115 Thus, these drugs have been exploited to great lengths for mechanistic studies into noradrenergic influence on bone and the potential for their use to improve bone mass, and are thoroughly reviewed elsewhere.116 The effects of sympathetic signaling through NE have been extensively studied following the discovery of the central leptin‐sympathetic outflow‐bone axis (reviewed in Elefteriou and colleagues117). Briefly, bone mass is increased by genetically disrupting the noradrenergic signaling axis, either by knockout of the NE‐synthetic enzyme dopamine β‐hydroxylase or β2‐AR.27, 118 Mechanistically, sympathetic activity induces osteopenia as a result of activation of the β2‐AR on osteoblasts, which suppresses osteoblast proliferation and promotes release of RANKL to support osteoclastogenesis.27, 113, 118 Although chronic stimulation of sympathetic outflow may have detrimental effects on bone, β2‐AR signaling in osteoblasts and osteocytes also disrupts their capacity to maintain the endosteal hematopoietic stem/progenitor cell (HSPC) niche; and thus, upon physiological activation can positively regulate hematopoiesis.110, 119 In the context of cancer, both progression and metastasis are associated with chronic stress, a condition that stimulates sympathetic outflow.120, 121, 122 In bone metastasis, sympathetic signaling via activation of β2‐AR on osteoblasts leads directly to cancer cell recruitment and indirectly to vascular changes that promote colonization of metastatic cancer cells in the bone marrow.121, 123, 124 Last, NE uptake by bone and immune cells may also contribute to extraneuronal control of local NE homeostasis and actions on the skeleton.56, 125, 126
NPY
NPY is the most abundant neuropeptide in the mammalian CNS and is also released from peripheral sympathetic nerves with NE. NPY contributes to neural control of a wide variety of physiological processes including stress and anxiety, appetite, circadian rhythm, cardiovascular activity, immune function, and pain.127 Peripherally, NPY is a potent vasoconstrictor. In addition to neural sources of NPY, it is also found in the adrenal medulla, and Npy mRNA has recently been detected in calvaria and femur.128 NPY and its related peptides, peptide YY (PYY) and pancreatic polypeptide (PP), can act on several common receptors, Y1, Y2, Y4, Y5, and Y6, which all couple to Gi proteins resulting in decreased cAMP production upon activation.127 NPY acts both centrally and peripherally to influence bone metabolism as deletion of NPY results in increased bone formation that is only partially rescued by re‐addition of NPY to the hypothalamus.129 Central control of bone metabolism mediated by NPY is through an inhibitory effect on Y2 expressing neurons; hypothalamic deletion of Y2 receptors stimulates osteoblast activity and increases cortical and cancellous bone formation.130 Knockout or pharmacological antagonism of Y1 increases bone formation resulting in high bone mass, though this is attributed in part to Y1 expression in osteoblasts providing evidence of local control of bone metabolism by NPY.131, 132, 133 In vitro, Y1 is expressed in induced bone marrow mesenchymal stromal cells (BMSCs) and gene expression increases with differentiation into osteoblasts; it is not detected in bone marrow macrophages (BMMs),128 suggesting a predominant local action of NPY on osteoblasts.
A key feature of all neurotransmitters identified to date in bone is their reliance on GPCR‐mediated signaling. Specifically, neurotransmitters bind their cognate GPCRs to stimulate or inhibit second messenger signaling cascades, as determined by coupling to cytoplasmic G‐proteins. This modulates a variety of downstream responses including protein secretion, ion channel opening, and gene transcription. In this way, volume transmission of neuropeptides and neurotransmitters from peripheral sensory and sympathetic fibers has the potential to produce summative, wide‐reaching, and potentially long‐lasting effects on bone including maintenance of skeletal homeostasis, vasoregulation, and peripheral sensitization. Though challenging, more studies are needed to investigate the duality and cumulative effects of neural signals on bone and their context and location‐specific functions.
Molecular Signals From Bone to Neuron—Growth Factors, Guidance Molecules, and Inflammatory Mediators
Recent work has interrogated the relationships between local cell‐derived factors, nerve function, and bone health. Three key molecules, and their impact on skeletal homeostasis through neural and non‐neural mechanisms, are discussed in the following sections.
NGF
During skeletal development, osteochondral lineage cells express NGF at the surfaces of newly forming bone, sites of incipient TrkA + nerve invasion.134 A functional NGF‐TrkA signaling axis is critical for proper neurovascular invasion and subsequent bone accrual.134 Similarly, following axial compression, periosteal and endosteal cells from the osteoprogenitor lineage acutely upregulate expression of NGF, which is followed by transient nerve sprouting at the periosteum.55 Disruption of NGF‐TrkA signaling abrogates load‐induced bone formation and downstream Wnt/β‐catenin signaling in osteocytes, whereas exogenous NGF enhances bone adaptation. Periosteal mesenchymal osteoprogenitor cells are the only NGF + skeletal cells in rib, but NGF is induced in a variety of skeletal cells in the fracture callus that resolves to mirror naive bone by 6 weeks postfracture.135 In vitro, NGF mRNA is expressed in BMSCs, calvarial osteoblasts, and osteoblastic cell lines and is upregulated during proliferative growth, upon loading, and with the addition of proinflammatory cytokines,40, 55, 134, 136 supporting a role for skeletal NGF signaling at spatially and temporally innervated sites coincident with high bone turnover. Aside from the classical role of NGF in nerve outgrowth and innervation of target tissues during development, precisely how NGF‐TrkA signaling contributes to bone development, anabolism, and repair is unclear. NGF has been shown to regulate nerve sprouting, activation, and neuropeptide expression and release.137 Additionally, NGF may modulate the vasculature by inducing neural expression of the pro‐angiogenic vascular endothelial growth factor (VEGF),38 directly activating TrkA receptors on vascular endothelial cells to promote angiogenesis,39 or potentially even acting directly on osteoblasts.40 Finally, there is evidence that the majority of bone nociceptors express receptors for NGF,15, 37, 46, 138, 139, 140, 141, 142, 143, 144, 145, 146 and blocking signaling through NGF and its receptors reduces pain in animal models of skeletal pathology and prevents inflammation‐induced changes in activity and sensitivity of bone nociceptors. This suggests NGF signaling may also be relevant to afferent functions of sensory neurons in bone, including pain as detailed in the following section under Bone Pain.
Semaphorin 3a
Semaphorin 3a (Sema3a) is a vital cue for neural migration and axon guidance during development.147, 148 Loss of Sema3a leads to gross bone and cartilage formation abnormalities, such as fusion of vertebral bones, rib duplications, and poor alignment of the rib‐sternum junction.147 Conversely, Sema3a derived from synapsin‐expressing neurons enhances bone formation during development.65 Sema3a is also expressed by osteochondral lineage cells at future sites of ossification prior to or coincident with invasion of vessels and nerve fibers.149 Sema3a can inhibit osteoclastogenesis and promote osteoblast differentiation. Exogenous Sema3a decreases bone loss following ovariectomy and accelerates bone regeneration after cortical bone injury.150 In the mandible neurotrophism, sympathetic signals stimulate release of NGF and maintain Sema3a expression in bone cells and resident immune cells; these factors balance sympathetic and sensory neuron infiltration.151 Together, this illustrates an emerging signaling network that includes nerves, bone cells, and immune cells which converge to influence bone homeostasis through local secretion and maintenance of Sema3a.
Prostaglandin E2
Prostaglandin E2 (PGE2) is a lipid metabolite and the most abundant prostaglandin in the body. PGE2 is a vasodilator, a potent inflammatory mediator, and a neuromodulator that can sensitize peripheral sensory neurons, contribute to inflammatory pain, and inhibit sympathetic neuronal release of NE.152, 153, 154 Administration of PGE2 has long been known to regulate both bone formation and resorption, typically with an imbalance in favor of formation.155 PGE2's anabolic potential is largely attributed to stimulation of osteogenesis through PGE receptor 4 (EP4) in osteoprogenitors.156, 157 Recent work has suggested that the osteoanabolic properties of endogenous PGE2 are also mediated by skeletal neurons. Specifically, PGE2 secreted from osteoblasts at active sites of remodeling signals through EP4 receptors in advillin‐expressing nerve fibers to promote bone formation.64 Loss of this signaling axis results in bone loss, whereas boosting PGE2 activation of advillin + nerves enhances bone regeneration following surgical ablation.64 Taken together, PGE2 signaling is suggestive of a novel converging mechanism for nerves to “sense” both bone pain and bone remodeling.
Bone Pain
Pain is associated with most bony pathologies, and depending on the site of pathology, the quality of the resulting pain can be distinct. With fracture that involves the periosteum, pain responses are often described as splitting, excruciating, generally localized to the site of injury, and often radiating “up and down” throughout the body.158 By contrast, for pathology confined within the bone marrow, such as skeletal metastases, pain is often described as dull, diffuse, and difficult to localize. In some patients with osteoarthritis, resting bone pain has been found to be better correlated with intraosseous venous stasis than the presence of degenerative changes in the joint.159 Intraosseous varicose veins, or “bone perforators,” have also been reported to cause intense pain with qualities that combine both periosteal and marrow responses, likely due to their location, which spans the cortical bone.160 In addition to resting pain, patients with skeletal metastases or venous stasis often have bouts of intense “breakthrough” pain that are associated with movement or weight bearing.161, 162 Small‐diameter mechanosensitive sensory afferents covering the periosteal surface can be selectively activated by high‐threshold mechanical stimuli,163, 164 and Aδ nociceptors in bone marrow respond to high‐threshold intraosseous pressure,22 providing the physiological foundation for perceived pain in response to mechanical distortions. In addition, peripheral sensory endings in bone can be activated by growth factors NGF and the GDNF family of ligands, as well as mixture of inflammatory mediators including histamine, serotonin, bradykinin, and PGE2,46, 164, 165 and can directly contribute to pain associated with inflammation and bone metastases.
As noted above, both the periosteum and the bone marrow contain neurons that are capable of detecting and transmitting information about noxious stimuli. These can be enhanced during pathophysiologic states in multiple ways. First, local processes can induce sprouting of sensory neurons. From the perspective of bone healing, this may be adaptive as discussed for fracture where nerve recruitment may promote repair by augmenting protective pain detection as well as efferent anabolic cues. However, it can also contribute to increased pain. Metastatic bone tumors in particular are known to induce increases in the number of sensory fibers during tumor growth,138 and blockade of nerve fiber sprouting significantly decreases tumor‐associated pain in rodent.139 Beyond de novo sprouting, chronic, particularly inflammatory, processes can cause peripheral and/or central sensitization of skeletal nociceptors. Specifically, sensory nerves that innervate both periosteum and the bone marrow can be sensitized by a number of different inflammatory mediators, including NGF, artemin, neurturin, GDNF, histamine, serotonin, bradykinin, and PGE2.46, 164, 165, 166 Thus, even in the absence of a change in innervation, inflammation can elicit pain in response to normally innocuous stimuli (allodynia) or increase sensitivity to noxious stimuli (hyperalgesia). In many cases bone pain also presents as cutaneous sensitivity at sites surrounding or distant to the injury (referred pain).167, 168, 169, 170 This fact has been leveraged in preclinical studies for the relative ease of studying thermal and mechanical sensitivity of the skin near the affected bone as a surrogate for bone pain (reviewed in Nencini and Ivanusic171). However, preclinical studies that that use cutaneous sensitivity as a surrogate for bone pain may overestimate analgesic efficacy, impacting potential for clinical translational success. It is noteworthy that many preclinical studies are now incorporating alternative, more clinically relevant assays (eg, changes in weight distribution and activity levels) that better reflect the patient experience of bone pain and provide information about functional status.46, 165, 166, 172, 173, 174, 175, 176, 177
Relationships between the nervous system, bone pain, and anabolism
It is interesting that the nerves that innervate skeletal tissues appear to function in both skeletal pain and anabolism, and influence a variety of different skeletal cell types. This implies that manipulating sensory or sympathetic neurons with drug therapies targeted to bone pain could have effects on anabolism and vice versa. A case to illustrate this is the emerging success story of the development of therapies directed against NGF signaling to treat bone pain. NGF‐TrkA signaling is an important mediator of both acute nociception and chronic pain.178 In rodents, inhibition of NGF‐TrkA signaling is effective in reducing pain associated with arthritis, fracture, and tumor growth.37 Tanezumab®, a humanized anti‐NGF antibody that blocks NGF‐TrkA and NGF‐p75 mediated signaling, is currently in phase III clinical trials for treatment of pain from osteoarthritis.178 During phase II trials in 2009, tanezumab was successful in treating pain, but patients within the high‐dose treatment groups developed rapidly progressive osteoarthritis,179, 180 a joint condition that resembles neurogenic arthropathy. Osteonecrosis and subchondral insufficiency fractures were also reported. This caused the drug to be placed on clinical hold. However, after modification of treatment regimens, including significant reductions in dose, tanezumab received fast‐track designation for treatment of chronic lower back pain and pain from osteoarthritis in 2017. In late 2018, Pfizer and Eli Lilly and Company reported the results from the first phase III study for patients with osteoarthritis treated for 16‐weeks.181 Pain management was significantly improved in the tanezumab groups and the incidence of rapidly progressing osteoarthritis was low (only 1.3%). Development of this therapeutic provides a modern‐day example of the relationship between neurotrophism and neurotrauma that harkens back to the days of Charcot and highlights the complex nature of interactions of the nervous system with skeletal tissues.
Prospectus and Conclusions
The origins of the field of neuroskeletal biology were rooted in a great debate between the neurotrophic and neurotraumatic theorists. One hundred and fifty years later, the field has come together with the realization that nerves in bone can influence a number of important processes related to skeletal homeostasis, including anabolism and pain. Current research is evolving to consider both in order to define clinically relevant regulators of neuroskeletal health. The first success story is exemplified by the ongoing clinical trials of anti‐NGF therapeutics at doses that have been customized for both inhibition of pain with simultaneous maintenance of bone and joint health. However, more studies are needed to uncover the regulatory factors that mediate interactions between skeletal nerve fibers, bone cells, and other local cell populations, including vascular and inflammatory cells. It is important to recognize that many of the neural receptors and transmitters that might constitute druggable targets for a variety of disease processes are expressed by and signal in many of these different cell types in bone. Additional collaborations between neuroscientists and bone biologists will undoubtedly support identification of new interactions between bone and the nervous system, promoting the discovery and success of novel therapies.
Disclosures
All authors state that they have no conflicts of interest.
Acknowledgments
We are grateful for support from the NIH including National Institute of Dental and Craniofacial Research (NIDCR) R00‐DE024178 to ELS; National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) U01‐DK116317 to ELS; and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) T32‐AR060719 to JMB.
Authors’ roles: Conception and first draft written by JMB and ELS with contributions from ATB, CSC, and JJI. JMB, ATB, CSC, JJI, and ELS revised the manuscript and approved the final version of the manuscript.
References
References
- 1. Sanders LJ. The Charcot foot: historical perspective 1827–2003. Diabetes Metab Res Rev. 2004;20 Suppl 1:S4–8. [DOI] [PubMed] [Google Scholar]
- 2. Corbin KB, Hinsey JC. Influence of the nervous system on bone and joints. Anat Rec. 1939;75(3):307–17. [Google Scholar]
- 3. Eloesser L. On the nature of neuropathic affections of the joints. Ann Surg. 1917;66(2):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edmonds ME, Clarke MB, Newton S, Barrett J, Watkins PJ. Increased uptake of bone radiopharmaceutical in diabetic neuropathy. Q J Med. 1985;57(224):843–55. [PubMed] [Google Scholar]
- 5. Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M. Substance P‐ and CGRP‐immunoreactive nerves in bone. Peptides. 1988;9(1):165–71. [DOI] [PubMed] [Google Scholar]
- 6. Duncan CP, Shim SS. J. Edouard Samson Address: the autonomic nerve supply of bone. An experimental study of the intraosseous adrenergic nervi vasorum in the rabbit. J Bone Joint Surg Br. 1977;59(3):323–30. [DOI] [PubMed] [Google Scholar]
- 7. Hill EL, Elde R. Distribution of CGRP‐, VIP‐, D beta H‐, SP‐, and NPY‐immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264(3):469–80. [DOI] [PubMed] [Google Scholar]
- 8. Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide‐containing nerve fibers. Science. 1986;232(4752):868–71. [DOI] [PubMed] [Google Scholar]
- 9. Ivanusic JJ, Mahns DA, Sahai V, Rowe MJ. Absence of large‐diameter sensory fibres in a nerve to the cat humerus. J Anat. 2006;208(2):251–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mach DB, Rogers SD, Sabino MC, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113(1):155–66. [DOI] [PubMed] [Google Scholar]
- 11. Sorkin LS, Eddinger KA, Woller SA, Yaksh TL. Origins of antidromic activity in sensory afferent fibers and neurogenic inflammation. Semin Immunopathol. 2018;40(3):237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai S, Hukuda S, Maeda T. Neonatal capsaicin pretreatment suppresses intramedullary inflammation in adjuvant‐induced spondylitis. Clin Exp Immunol. 1994;95(1):108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia A‐S, White LE. Neuroscience. 5th edition Sunderland, MA: Sinauer Associates; 2012. [Google Scholar]
- 15. Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cherruau M, Morvan FO, Schirar A, Saffar JL. Chemical sympathectomy‐induced changes in TH‐, VIP‐, and CGRP‐immunoreactive fibers in the rat mandible periosteum: influence on bone resorption. J Cell Physiol. 2003;194(3):341–8. [DOI] [PubMed] [Google Scholar]
- 17. Imai S, Tokunaga Y, Maeda T, Kikkawa M, Hukuda S. Calcitonin gene‐related peptide, substance P, and tyrosine hydroxylase‐immunoreactive innervation of rat bone marrows: an immunohistochemical and ultrastructural investigation on possible efferent and afferent mechanisms. J Orthop Res. 1997;15(1):133–40. [DOI] [PubMed] [Google Scholar]
- 18. Jimenez‐Andrade JM, Mantyh WG, Bloom AP, et al. A phenotypically restricted set of primary afferent nerve fibers innervate the bone versus skin: therapeutic opportunity for treating skeletal pain. Bone. 2010;46(2):306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivanusic JJ. Size, neurochemistry, and segmental distribution of sensory neurons innervating the rat tibia. J Comp Neurol. 2009;517(3):276–83. [DOI] [PubMed] [Google Scholar]
- 20. Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7(7):1484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett DL, Michael GJ, Ramachandran N, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18(8):3059–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nencini S, Ivanusic J. Mechanically sensitive Aδ nociceptors that innervate bone marrow respond to changes in intra‐osseous pressure. J Physiol. 2017;595(13):4399–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zelená J. Survival of Pacinian corpuscles after denervation in adult rats. Cell Tissue Res. 1982;224(3):673–83. [DOI] [PubMed] [Google Scholar]
- 24. Sakada S, Aida H. Localization and shape of Golgi‐Mazzoni corpuscles in the facial bones’ periosteum of the cat. Bull Tokyo Dent Coll. 1971;12:235–53. [Google Scholar]
- 25. Ralston HJ, Miller MR, Kasahara M. Nerve endings in human fasciae, tendons, ligaments, periosteum, and joint synovial membrane. Anat Rec. 1960;136:137–47. [DOI] [PubMed] [Google Scholar]
- 26. Kawai M, Kinoshita S, Shimba S, Ozono K, Michigami T. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm‐dependent manner. J Biol Chem. 2014;289(3):1457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111(3):305–17. [DOI] [PubMed] [Google Scholar]
- 28. Yirmiya R, Goshen I, Bajayo A, et al. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc Natl Acad Sci U S A. 2006;103(45):16876–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gajda M, Litwin JA, Tabarowski Z, et al. Development of rat tibia innervation: colocalization of autonomic nerve fiber markers with growth‐associated protein 43. Cells Tissues Organs. 2010;191(6):489–99. [DOI] [PubMed] [Google Scholar]
- 30. Anderson CR, Bergner A, Murphy SM. How many types of cholinergic sympathetic neuron are there in the rat stellate ganglion? Neuroscience. 2006;140(2):567–76. [DOI] [PubMed] [Google Scholar]
- 31. Asmus SE, Parsons S, Landis SC. Developmental changes in the transmitter properties of sympathetic neurons that innervate the periosteum. J Neurosci. 2000;20(4):1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brodski C, Schnürch H, Dechant G. Neurotrophin‐3 promotes the cholinergic differentiation of sympathetic neurons. Proc Natl Acad Sci U S A. 2000;97(17):9683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Furlan A, Lübke M, Adameyko I, Lallemend F, Ernfors P. The transcription factor Hmx1 and growth factor receptor activities control sympathetic neurons diversification. EMBO J. 2013;32(11):1613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asmus SE, Tian H, Landis SC. Induction of cholinergic function in cultured sympathetic neurons by periosteal cells: cellular mechanisms. Dev Biol. 2001;235(1):1–11. [DOI] [PubMed] [Google Scholar]
- 35. Bataille C, Mauprivez C, Haÿ E, et al. Different sympathetic pathways control the metabolism of distinct bone envelopes. Bone. 2012;50(5):1162–72. [DOI] [PubMed] [Google Scholar]
- 36. Bajayo A, Bar A, Denes A, et al. Skeletal parasympathetic innervation communicates central IL‐1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A. 2012;109(38):15455–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Castañeda‐Corral G, Jimenez‐Andrade JM, Bloom AP, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura K, Tan F, Li Z, Thiele CJ. NGF activation of TrkA induces vascular endothelial growth factor expression via induction of Hypoxia‐Inducible Factor‐1α. Mol Cell Neurosci. 2011;46(2):498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cantarella G, Lempereur L, Presta M, et al. Nerve growth factor‐endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16(10):1307–9. [DOI] [PubMed] [Google Scholar]
- 40. Mogi M, Kondo A, Kinpara K, Togari A. Anti‐apoptotic action of nerve growth factor in mouse osteoblastic cell line. Life Sci. 2000;67(10):1197–206. [DOI] [PubMed] [Google Scholar]
- 41. Kurihara H, Shinohara H, Yoshino H, Takeda K, Shiba H. Neurotrophins in cultured cells from periodontal tissues. J Periodontol. 2003;74(1):76–84. [DOI] [PubMed] [Google Scholar]
- 42. Gajda M, Litwin JA, Cichocki T, Timmermans J‐P, Adriaensen D. Development of sensory innervation in rat tibia: co‐localization of CGRP and substance P with growth‐associated protein 43 (GAP‐43). J Anat. 2005;207(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Curtis R, Stewart HJ, Hall SM, Wilkin GP, Mirsky R, Jessen KR. GAP‐43 is expressed by nonmyelin‐forming Schwann cells of the peripheral nervous system. J Cell Biol. 1992;116(6):1455–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cho WG, Valtschanoff JG. Vanilloid receptor TRPV1‐positive sensory afferents in the mouse ankle and knee joints. Brain Res. 2008;1219:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ghilardi JR, Röhrich H, Lindsay TH, et al. Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci. 2005;25(12):3126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nencini S, Ringuet M, Kim D‐H, Chen Y‐J, Greenhill C, Ivanusic JJ. Mechanisms of nerve growth factor signaling in bone nociceptors and in an animal model of inflammatory bone pain. Mol Pain. 2017;13:1744806917697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Offley SC, Guo T‐Z, Wei T, et al. Capsaicin‐sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J Bone Miner Res. 2005;20(2):257–67. [DOI] [PubMed] [Google Scholar]
- 48. He L‐H, Liu M, He Y, et al. TRPV1 deletion impaired fracture healing and inhibited osteoclast and osteoblast differentiation. Sci Rep. 2017;7:42385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi M, Watanabe K, Yokoyama S, et al. Capsaicin, a TRPV1 ligand, suppresses bone resorption by inhibiting the prostaglandin E production of osteoblasts, and attenuates the inflammatory bone loss induced by lipopolysaccharide. ISRN Pharmacol. 2012;2012:439860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sisask G, Bjurholm A, Ahmed M, Kreicbergs A. Ontogeny of sensory nerves in the developing skeleton. Anat Rec. 1995;243(2):234–40. [DOI] [PubMed] [Google Scholar]
- 51. Huang Y, van Dessel J, Martens W, et al. Sensory innervation around immediately vs. delayed loaded implants: a pilot study. Int J Oral Sci. 2015;7(1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hurrell DJ. The nerve supply of bone. J Anat. 1937;72(Pt 1):54–61. [PMC free article] [PubMed] [Google Scholar]
- 53. Jilka RL. The Relevance of mouse models for investigating age‐related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jing D, Zhang S, Luo W, et al. Tissue clearing of both hard and soft tissue organs with the PEGASOS method. Cell Res. 2018;28(8):803–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tomlinson RE, Li Z, Li Z, et al. NGF‐TrkA signaling in sensory nerves is required for skeletal adaptation to mechanical loads in mice. Proc Natl Acad Sci U S A. 2017;114(18):E3632–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu Y, Ma Y, Elefteriou F. Cortical bone is an extraneuronal site of norepinephrine uptake in adult mice. Bone Rep. 2018;9:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams MC, Ivanusic JJ. Evidence for the involvement of the spinoparabrachial pathway, but not the spinothalamic tract or post‐synaptic dorsal column, in acute bone nociception. Neurosci Lett. 2008;443(3):246–50. [DOI] [PubMed] [Google Scholar]
- 58. Gauriau C, Bernard J‐F. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87(2):251–8. [DOI] [PubMed] [Google Scholar]
- 59. Ivanusic JJ, Sahai V, Mahns DA. The cortical representation of sensory inputs arising from bone. Brain Res. 2009;1269:47–53. [DOI] [PubMed] [Google Scholar]
- 60. Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100(2):197–207. [DOI] [PubMed] [Google Scholar]
- 61. Dénes A, Boldogkoi Z, Uhereczky G, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience. 2005;134(3):947–63. [DOI] [PubMed] [Google Scholar]
- 62. Sherman MS. The nerves of bone. J Bone Joint Surg Am. 1963;45(3):522–8. [Google Scholar]
- 63. Sisask G, Silfverswärd C‐J, Bjurholm A, Nilsson O. Ontogeny of sensory and autonomic nerves in the developing mouse skeleton. Auton Neurosci Basic Clin. 2013;177(2):237–43. [DOI] [PubMed] [Google Scholar]
- 64. Chen H, Hu B, Lv X, et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat Commun. 2019;10(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fukuda T, Takeda S, Xu R, et al. Sema3A regulates bone‐mass accrual through sensory innervations. Nature. 2013;497(7450):490–3. [DOI] [PubMed] [Google Scholar]
- 66. Klein‐Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182–90. [DOI] [PubMed] [Google Scholar]
- 67. Sample SJ, Behan M, Smith L, et al. Functional adaptation to loading of a single bone is neuronally regulated and involves multiple bones. J Bone Miner Res. 2008;23(9):1372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu Q, Sample SJ, Baker TA, Thomas CF, Behan M, Muir P. Mechanical loading of a long bone induces plasticity in sensory input to the central nervous system. Neurosci Lett. 2009;463(3):254–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li J, Ahmad T, Spetea M, Ahmed M, Kreicbergs A. Bone reinnervation after fracture: a study in the rat. J Bone Miner Res. 2001;16(8):1505–10. [DOI] [PubMed] [Google Scholar]
- 70. Hukkanen M, Konttinen YT, Santavirta S, et al. Rapid proliferation of calcitonin gene‐related peptide‐immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience. 1993;54(4):969–79. [DOI] [PubMed] [Google Scholar]
- 71. Li J, Kreicbergs A, Bergström J, Stark A, Ahmed M. Site‐specific CGRP innervation coincides with bone formation during fracture healing and modeling: a study in rat angulated tibia. J Orthop Res. 2007;25(9):1204–12. [DOI] [PubMed] [Google Scholar]
- 72. Li J, Ahmed M, Bergstrom J, Ackermann P, Stark A, Kreicbergs A. Occurrence of substance P in bone repair under different load comparison of straight and angulated fracture in rat tibia. J Orthop Res. 2010;28(12):1643–50. [DOI] [PubMed] [Google Scholar]
- 73. Long H, Ahmed M, Ackermann P, Stark A, Li J. Neuropeptide Y innervation during fracture healing and remodeling. Acta Orthop. 2010;81(5):639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chartier SR, Thompson ML, Longo G, Fealk MN, Majuta LA, Mantyh PW. Exuberant sprouting of sensory and sympathetic nerve fibers in nonhealed bone fractures and the generation and maintenance of chronic skeletal pain. Pain. 2014;155(11):2323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Serre CM, Farlay D, Delmas PD, Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate‐containing fibers. Bone. 1999;25(6):623–9. [DOI] [PubMed] [Google Scholar]
- 76. Hara‐Irie F, Amizuka N, Ozawa H. Immunohistochemical and ultrastructural localization of CGRP‐positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone. 1996;18(1):29–39. [DOI] [PubMed] [Google Scholar]
- 77. Robles H, Park S, Joens MS, Fitzpatrick JAJ, Craft CS, Scheller EL. Characterization of the bone marrow adipocyte niche with three‐dimensional electron microscopy. Bone. 2018;118:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Crenshaw EB, Russo AF, Swanson LW, Rosenfeld MG. Neuron‐specific alternative RNA processing in transgenic mice expressing a metallothionein‐calcitonin fusion gene. Cell. 1987;49(3):389–98. [DOI] [PubMed] [Google Scholar]
- 79. Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I. Calcitonin gene‐related peptide is a potent vasodilator. Nature. 1985;313(5997):54–6. [DOI] [PubMed] [Google Scholar]
- 80. Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene‐related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987;403(2):350–4. [DOI] [PubMed] [Google Scholar]
- 81. Russell FA, King R, Smillie S‐J, Kodji X, Brain SD. Calcitonin gene‐related peptide: physiology and pathophysiology. Physiol Rev. 2014;94(4):1099–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McLatchie LM, Fraser NJ, Main MJ, et al. RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature. 1998;393(6683):333–9. [DOI] [PubMed] [Google Scholar]
- 83. Mrak E, Guidobono F, Moro G, Fraschini G, Rubinacci A, Villa I. Calcitonin gene‐related peptide (CGRP) inhibits apoptosis in human osteoblasts by β‐catenin stabilization. J Cell Physiol. 2010;225(3):701–8. [DOI] [PubMed] [Google Scholar]
- 84. Wang L, Shi X, Zhao R, et al. Calcitonin gene‐related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF‐κB activation, osteoclastogenesis and bone resorption. Bone. 2010;46(5):1369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. He H, Chai J, Zhang S, et al. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation. Mol Med Rep. 2016;13(5):3977–84. [DOI] [PubMed] [Google Scholar]
- 86. Michelangeli VP, Fletcher AE, Allan EH, Nicholson GC, Martin TJ. Effects of calcitonin gene‐related peptide on cyclic AMP formation in chicken, rat, and mouse bone cells. J Bone Miner Res. 1989;4(2):269–72. [DOI] [PubMed] [Google Scholar]
- 87. Zhang Y, Xu J, Ruan YC, et al. Implant‐derived magnesium induces local neuronal production of CGRP to improve bone‐fracture healing in rats. Nat Med. 2016;22(10):1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zhou R, Yuan Z, Liu J, Liu J. Calcitonin gene‐related peptide promotes the expression of osteoblastic genes and activates the WNT signal transduction pathway in bone marrow stromal stem cells. Mol Med Rep. 2016;13(6):4689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bo Y, Yan L, Gang Z, Tao L, Yinghui T. Effect of calcitonin gene‐related peptide on osteoblast differentiation in an osteoblast and endothelial cell co‐culture system. Cell Biol Int. 2012;36(10):909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cornish J, Callon KE, Bava U, Kamona SA, Cooper GJ, Reid IR. Effects of calcitonin, amylin, and calcitonin gene‐related peptide on osteoclast development. Bone. 2001;29(2):162–8. [DOI] [PubMed] [Google Scholar]
- 91. Takahashi N, Matsuda Y, Sato K, et al. Neuronal TRPV1 activation regulates alveolar bone resorption by suppressing osteoclastogenesis via CGRP. Sci Rep. 2016;6:29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zaidi M, Chambers TJ, Gaines Das RE, Morris HR, MacIntyre I. A direct action of human calcitonin gene‐related peptide on isolated osteoclasts. J Endocrinol. 1987;115(3):511–8. [DOI] [PubMed] [Google Scholar]
- 93. Kauther MD, Xu J, Wedemeyer C. Alpha‐calcitonin gene‐related peptide can reverse the catabolic influence of UHMWPE particles on RANKL expression in primary human osteoblasts. Int J Biol Sci. 2010;6(6):525–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Villa I, Mrak E, Rubinacci A, Ravasi F, Guidobono F. CGRP inhibits osteoprotegerin production in human osteoblast‐like cells via cAMP/PKA‐dependent pathway. Am J Physiol Cell Physiol. 2006;291(3):C529–37. [DOI] [PubMed] [Google Scholar]
- 95. Schinke T, Liese S, Priemel M, et al. Decreased bone formation and osteopenia in mice lacking alpha‐calcitonin gene‐related peptide. J Bone Miner Res. 2004;19(12):2049–56. [DOI] [PubMed] [Google Scholar]
- 96. Ballica R, Valentijn K, Khachatryan A, et al. Targeted expression of calcitonin gene‐related peptide to osteoblasts increases bone density in mice. J Bone Miner Res. 1999;14(7):1067–74. [DOI] [PubMed] [Google Scholar]
- 97. Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–4. [DOI] [PubMed] [Google Scholar]
- 98. Guo T‐Z, Wei T, Shi X, et al. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ho WZ, Lai JP, Zhu XH, Uvaydova M, Douglas SD. Human monocytes and macrophages express substance P and neurokinin‐1 receptor. J Immunol. 1997;159(11):5654–60. [PubMed] [Google Scholar]
- 100. Watanabe M, Nakayasu K, Iwatsu M, Kanai A. Endogenous substance P in corneal epithelial cells and keratocytes. Jpn J Ophthalmol. 2002;46(6):616–20. [DOI] [PubMed] [Google Scholar]
- 101. Niedermair T, Schirner S, Seebröker R, Straub RH, Grässel S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci Rep. 2018;8(1):9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pinto FM, Almeida TA, Hernandez M, Devillier P, Advenier C, Candenas ML. mRNA expression of tachykinins and tachykinin receptors in different human tissues. Eur J Pharmacol. 2004;494(2–3):233–9. [DOI] [PubMed] [Google Scholar]
- 103. Goto T, Yamaza T, Kido MA, Tanaka T. Light‐ and electron‐microscopic study of the distribution of axons containing substance P and the localization of neurokinin‐1 receptor in bone. Cell Tissue Res. 1998;293(1):87–93. [DOI] [PubMed] [Google Scholar]
- 104. Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T. Substance P stimulates late‐stage rat osteoblastic bone formation through neurokinin‐1 receptors. Neuropeptides. 2007;41(1):25–31. [DOI] [PubMed] [Google Scholar]
- 105. Wang L, Zhao R, Shi X, et al. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45(2):309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fu S, Mei G, Wang Z, et al. Neuropeptide substance P improves osteoblastic and angiogenic differentiation capacity of bone marrow stem cells in vitro. Biomed Res Int. 2014;2014:596023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hong HS, Lee J, Lee E, et al. A new role of substance P as an injury‐inducible messenger for mobilization of CD29(+) stromal‐like cells. Nat Med. 2009;15(4):425–35. [DOI] [PubMed] [Google Scholar]
- 108. Wedemeyer C, Neuerburg C, Pfeiffer A, et al. Polyethylene particle‐induced bone resorption in substance P‐deficient mice. Calcif Tissue Int. 2007;80(4):268–74. [DOI] [PubMed] [Google Scholar]
- 109. Niedermair T, Kuhn V, Doranehgard F, et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol J Int. 2014;38:22–35. [DOI] [PubMed] [Google Scholar]
- 110. Asada N, Katayama Y, Sato M, et al. Matrix‐embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell. 2013;12(6):737–47. [DOI] [PubMed] [Google Scholar]
- 111. Fonseca TL, Jorgetti V, Costa CC, et al. Double disruption of α2A‐ and α2C‐adrenoceptors results in sympathetic hyperactivity and high‐bone‐mass phenotype. J Bone Miner Res. 2011;26(3):591–603. [DOI] [PubMed] [Google Scholar]
- 112. Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1‐ and beta2‐adrenergic receptors in human osteoblasts. J Cell Physiol. 2009;220(1):267–75. [DOI] [PubMed] [Google Scholar]
- 113. Kajimura D, Hinoi E, Ferron M, et al. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med. 2011;208(4):841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pierroz DD, Bonnet N, Bianchi EN, et al. Deletion of β‐adrenergic receptor 1, 2, or both leads to different bone phenotypes and response to mechanical stimulation. J Bone Miner Res. 2012;27(6):1252–62. [DOI] [PubMed] [Google Scholar]
- 115. Becker DE. Basic and clinical pharmacology of autonomic drugs. Anesth Prog. 2012;59(4):159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8(2):94–104. [PubMed] [Google Scholar]
- 117. Elefteriou F, Campbell P, Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int. 2014;94(1):140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434(7032):514–20. [DOI] [PubMed] [Google Scholar]
- 119. Katayama Y, Battista M, Kao W‐M, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407–21. [DOI] [PubMed] [Google Scholar]
- 120. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939–44. [DOI] [PubMed] [Google Scholar]
- 121. Campbell JP, Karolak MR, Ma Y, et al. Stimulation of host bone marrow stromal cells by sympathetic nerves promotes breast cancer bone metastasis in mice. PLoS Biol. 2012;10(7):e1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 123. Clément‐Demange L, Mulcrone PL, Tabarestani TQ, Sterling JA, Elefteriou F. β2ARs stimulation in osteoblasts promotes breast cancer cell adhesion to bone marrow endothelial cells in an IL‐1β and selectin‐dependent manner. J Bone Oncol. 2018;13:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mulcrone PL, Campbell JP, Clément‐Demange L, et al. Skeletal colonization by breast cancer cells is stimulated by an osteoblast and β2AR‐dependent neo‐angiogenic switch. J Bone Miner Res. 2017;32(7):1442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pirzgalska RM, Seixas E, Seidman JS, et al. Sympathetic neuron‐associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat Med. 2017;23(11):1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ma Y, Krueger JJ, Redmon SN, et al. Extracellular norepinephrine clearance by the norepinephrine transporter is required for skeletal homeostasis. J Biol Chem. 2013;288(42):30105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Brothers SP, Wahlestedt C. Therapeutic potential of neuropeptide Y (NPY) receptor ligands. EMBO Mol Med. 2010;2(11):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wee NKY, Sinder BP, Novak S, et al. Skeletal phenotype of the neuropeptide Y knockout mouse. Neuropeptides. 2019;73:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Baldock PA, Lee NJ, Driessler F, et al. Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One. 2009;4(12):e8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Baldock PA, Sainsbury A, Couzens M, et al. Hypothalamic Y2 receptors regulate bone formation. J Clin Invest. 2002;109(7):915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Baldock PA, Allison SJ, Lundberg P, et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J Biol Chem. 2007;282(26):19092–102. [DOI] [PubMed] [Google Scholar]
- 132. Lee NJ, Nguyen AD, Enriquez RF, et al. Osteoblast specific Y1 receptor deletion enhances bone mass. Bone. 2011;48(3):461–7. [DOI] [PubMed] [Google Scholar]
- 133. Sousa DM, Baldock PA, Enriquez RF, et al. Neuropeptide Y Y1 receptor antagonism increases bone mass in mice. Bone. 2012;51(1):8–16. [DOI] [PubMed] [Google Scholar]
- 134. Tomlinson RE, Li Z, Zhang Q, et al. NGF‐TrkA signaling by sensory nerves coordinates the vascularization and ossification of developing endochondral bone. Cell Rep. 2016;16(10):2723–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Grills BL, Schuijers JA. Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop Scand. 1998;69(4):415–9. [DOI] [PubMed] [Google Scholar]
- 136. Nakanishi T, Takahashi K, Aoki C, Nishikawa K, Hattori T, Taniguchi S. Expression of nerve growth factor family neurotrophins in a mouse osteoblastic cell line. Biochem Biophys Res Commun. 1994;198(3):891–7. [DOI] [PubMed] [Google Scholar]
- 137. Lindsay RM, Harmar AJ. Nerve growth factor regulates expression of neuropeptide genes in adult sensory neurons. Nature. 1989;337(6205):362–4. [DOI] [PubMed] [Google Scholar]
- 138. Bloom AP, Jimenez‐Andrade JM, Taylor RN, et al. Breast cancer‐induced bone remodeling, skeletal pain, and sprouting of sensory nerve fibers. J Pain. 2011;12(6):698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Mantyh WG, Jimenez‐Andrade JM, Stake JI, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171(2):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Jimenez‐Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer‐induced bone pain. J Neurosci. 2010;30(44):14649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chartier SR, Mitchell SA, Majuta LA, Mantyh PW. Immunohistochemical localization of nerve growth factor, tropomyosin receptor kinase A, and p75 in the bone and articular cartilage of the mouse femur. Mol Pain. 2017;13:17448069–17745465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sevcik MA, Ghilardi JR, Peters CM, et al. Anti‐NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain. 2005;115(1–2):128–41. [DOI] [PubMed] [Google Scholar]
- 143. Koewler NJ, Freeman KT, Buus RJ, et al. Effects of a monoclonal antibody raised against nerve growth factor on skeletal pain and bone healing after fracture of the C57BL/6J mouse femur. J Bone Miner Res. 2007;22(11):1732–42. [DOI] [PubMed] [Google Scholar]
- 144. Jimenez‐Andrade JM, Martin CD, Koewler NJ, et al. Nerve growth factor sequestering therapy attenuates non‐malignant skeletal pain following fracture. Pain. 2007;133(1–3):183–96. [DOI] [PubMed] [Google Scholar]
- 145. Jimenez‐Andrade JM, Ghilardi JR, Castañeda‐Corral G, Kuskowski MA, Mantyh PW. Preventive or late administration of anti‐NGF therapy attenuates tumor‐induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Halvorson KG, Kubota K, Sevcik MA, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65(20):9426–35. [DOI] [PubMed] [Google Scholar]
- 147. Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383(6600):525–8. [DOI] [PubMed] [Google Scholar]
- 148. Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75(2):217–27. [DOI] [PubMed] [Google Scholar]
- 149. Gomez C, Burt‐Pichat B, Mallein‐Gerin F, et al. Expression of Semaphorin‐3A and its receptors in endochondral ossification: potential role in skeletal development and innervation. Dev Dyn. 2005;234(2):393–403. [DOI] [PubMed] [Google Scholar]
- 150. Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485(7396):69–74. [DOI] [PubMed] [Google Scholar]
- 151. Mauprivez C, Bataille C, Baroukh B, et al. Periosteum metabolism and nerve fiber positioning depend on interactions between osteoblasts and peripheral innervation in rat mandible. PLoS One. 2015;10(10):e0140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Malik KU, Sehic E. Prostaglandins and the release of the adrenergic transmitter. Ann N Y Acad Sci. 1990;604:222–36. [DOI] [PubMed] [Google Scholar]
- 153. Omori K, Kida T, Hori M, Ozaki H, Murata T. Multiple roles of the PGE2‐EP receptor signal in vascular permeability. Br J Pharmacol. 2014;171(21):4879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. St‐Jacques B, Ma W. Peripheral prostaglandin E2 prolongs the sensitization of nociceptive dorsal root ganglion neurons possibly by facilitating the synthesis and anterograde axonal trafficking of EP4 receptors. Exp Neurol. 2014;261:354–66. [DOI] [PubMed] [Google Scholar]
- 155. Bergmann P, Schoutens A. Prostaglandins and bone. Bone. 1995;16(4):485–8. [DOI] [PubMed] [Google Scholar]
- 156. Weinreb M, Suponitzky I, Keila S. Systemic administration of an anabolic dose of PGE2 in young rats increases the osteogenic capacity of bone marrow. Bone. 1997;20(6):521–6. [DOI] [PubMed] [Google Scholar]
- 157. Yoshida K, Oida H, Kobayashi T, et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci U S A. 2002;99(7):4580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Santy J, Mackintosh C. A phenomenological study of pain following fractured shaft of femur. J Clin Nurs. 2001;10(4):521–7. [DOI] [PubMed] [Google Scholar]
- 159. Arnoldi CC, Djurhuus JC, Heerfordt J, Karle A. Intraosseous phlebography, intraosseous pressure measurements and 99mTC‐polyphosphate scintigraphy in patients with various painful conditions in the hip and knee. Acta Orthop Scand. 1980;51(1):19–28. [DOI] [PubMed] [Google Scholar]
- 160. Rezaie ES, Maas M, van der Horst CMAM. Episodes of extreme lower leg pain caused by intraosseous varicose veins. BMJ Case Rep. 2018;2018:bcr‐2017–223986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Mantyh P. The science behind metastatic bone pain. Eur J Cancer Suppl. 2006;4(8):4–8. [Google Scholar]
- 162. Mercadante S, Arcuri E. Breakthrough pain in cancer patients: pathophysiology and treatment. Cancer Treat Rev. 1998;24(6):425–32. [DOI] [PubMed] [Google Scholar]
- 163. Mahns DA, Ivanusic JJ, Sahai V, Rowe MJ. An intact peripheral nerve preparation for monitoring the activity of single, periosteal afferent nerve fibres. J Neurosci Methods. 2006;156(1–2):140–4. [DOI] [PubMed] [Google Scholar]
- 164. Zhao J, Levy D. The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache‐like behavior. Pain. 2014;155(7):1392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Nencini S, Ringuet M, Kim D‐H, Greenhill C, Ivanusic JJ. GDNF, neurturin, and artemin activate and sensitize bone afferent neurons and contribute to inflammatory bone pain. J Neurosci. 2018;38(21):4899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Nencini S, Thai J, Ivanusic JJ. Sequestration of artemin reduces inflammation‐induced activation and sensitization of bone marrow nociceptors in a rodent model of carrageenan‐induced inflammatory bone pain. Eur J Pain. 2019;23(2):397–409. [DOI] [PubMed] [Google Scholar]
- 167. Kerba M, Wu JSY, Duan Q, Hagen NA, Bennett MI. Neuropathic pain features in patients with bone metastases referred for palliative radiotherapy. J Clin Oncol. 2010;28(33):4892–7. [DOI] [PubMed] [Google Scholar]
- 168. Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4(3):229–38. [DOI] [PubMed] [Google Scholar]
- 169. Moss P, Benson HAE, Will R, Wright A. Patients with knee osteoarthritis who score highly on the PainDETECT questionnaire present with multimodality hyperalgesia, increased pain, and impaired physical function. Clin J Pain. 2018;34(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Scott AC, McConnell S, Laird B, Colvin L, Fallon M. Quantitative Sensory Testing to assess the sensory characteristics of cancer‐induced bone pain after radiotherapy and potential clinical biomarkers of response. Eur J Pain. 2012;16(1):123–33. [DOI] [PubMed] [Google Scholar]
- 171. Nencini S, Ivanusic JJ. The physiology of bone pain. How much do we really know? Front Physiol. 2016;7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. McCaffrey G, Thompson ML, Majuta L, et al. NGF blockade at early times during bone cancer development attenuates bone destruction and increases limb use. Cancer Res. 2014;74(23):7014–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Jimenez‐Andrade JM, Mantyh PW. Sensory and sympathetic nerve fibers undergo sprouting and neuroma formation in the painful arthritic joint of geriatric mice. Arthritis Res Ther. 2012;14(3):R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Jimenez‐Andrade JM, Bloom AP, Mantyh WG, et al. Capsaicin‐sensitive sensory nerve fibers contribute to the generation and maintenance of skeletal fracture pain. Neuroscience. 2009;162(4):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Freeman KT, Koewler NJ, Jimenez‐Andrade JM, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108(3):473–83. [DOI] [PubMed] [Google Scholar]
- 176. Thompson ML, Chartier SR, Mitchell SA, Mantyh PW. Preventing painful age‐related bone fractures: anti‐sclerostin therapy builds cortical bone and increases the proliferation of osteogenic cells in the periosteum of the geriatric mouse femur. Mol Pain. 2016:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Majuta LA, Guedon J‐MG, Mitchell SAT, Kuskowski MA, Mantyh PW. Mice with cancer‐induced bone pain show a marked decline in day/night activity. Pain Rep. 2017;2(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Patel MK, Kaye AD, Urman RD. Tanezumab: therapy targeting nerve growth factor in pain pathogenesis. J Anaesthesiol Clin Pharmacol. 2018;34(1):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Hochberg MC. Serious joint‐related adverse events in randomized controlled trials of anti‐nerve growth factor monoclonal antibodies. Osteoarthritis Cartilage. 2015;23 Suppl 1:S18–21. [DOI] [PubMed] [Google Scholar]
- 180. Roemer FW, Hayes CW, Miller CG, Hoover K, Guermazi A. Imaging atlas for eligibility and on‐study safety of potential knee adverse events in anti‐NGF studies (Part 1). Osteoarthritis Cartilage. 2015;23 Suppl 1:S22–42. [DOI] [PubMed] [Google Scholar]
- 181. ClinicalTrials.gov [Internet] . Bethesda (MD): National Library of Medicine (US). 2016 Mar 16. Identifier NCT02709486, A phase 3 randomized, double‐blind, placebo‐controlled, multicenter study of the analgesic efficacy and safety of the subcutaneous administration of tanezumab in subjects with osteoarthritis of the hip or knee; 2019 Jun 26 [cited 2019 Jan 27] Available from. https://clinicaltrials.gov/ct2/show/NCT02709486 [Google Scholar]
- 182. Cıvan M, Yazıcıoğlu Ö, Çakmak M, Akgül T. Charcot arthropathy of the knee after unsuccessful spinal stenosis surgery: a case report. Int J Surg Case Rep. 2017;36:22–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


