Abstract
Objective:
To improve measurement of stiffness in rheumatic disease.
Methods:
Data presented included: 1) Two qualitative projects; 2) The RA Stiffness patient-reported outcome measure (RAST); 3) three items assessing stiffness severity, duration, and interference. 3)
Results:
Stiffness is multidimensional and includes aspects of stiffness experience such as duration, severity, and impact. Stiffness items showed construct validity in RA. Further efforts are required to develop an instrument that will be taken through OMERACT Filter 2.1 for instrument selection.
Conclusion:
The future research agenda for the group includes domain content voting for individual diseases, and development of stiffness item banks and disease-specific short forms.
Keywords: Outcome assessment, Morning Stiffness, OMERACT
Introduction
In 2016, the OMERACT Stiffness special interest group (SIG) was formed to develop an appropriate instrument to measure stiffness in rheumatoid arthritis (1). The SIG reviewed results from a systematic literature review of stiffness patient-reported outcome measures (PROMs), and noted a dearth of data regarding content validity, construct validity, responsiveness, or reliability of any of these instruments (2). Thus, the available measures did not pass the OMERACT filter 2.0 “eyeball test” for good match with the domain of interest. A long-term research agenda was proposed including development of stiffness content mapping through qualitative research followed by development and validation of stiffness assessment tools in RA.
Stiffness is a common and important symptom in rheumatic diseases, including rheumatoid arthritis (RA), spondyloarthritis, and polymyalgia rheumatica (PMR) (3–5), and is present even in low disease activity states (6). Stiffness was initially included in the 1987 American College of Rheumatology classification criteria for RA, and is included in the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), RA Flare Questionnaire, and OMERACT core domain sets for RA Flare, ankylosing spondylitis (AS), psoriatic arthritis, and PMR (7–11). Stiffness has been identified by patients as a hallmark of the RA experience associated with flares, decreased physical function, and impact upon daily functioning (12). Prospective observational studies and survey data have demonstrated that stiffness duration is independently associated with decreased work productivity and early retirement (13–15).
Considerable qualitative work has been performed by our working group to improve our understanding of the patient experience of stiffness. Independent qualitative work in the UK and US identified that in RA, stiffness is a variable and complex symptom that impacts on patients’ daily lives and is not exclusive to the morning (6,12). Conceptual models developed in these studies have been discussed, synthesized and expanded on across rheumatic conditions (1,2).
Despite its central role in patient experience, measurement of stiffness remains suboptimal in clinical trials, and no validated instruments exist for its measurement.
At the 2018 OMERACT stiffness SIG, 37 participants were present, including 22 healthcare providers, 7 fellows, 4 industry representatives, and 4 patient research partners. The primary aims of the OMERACT 2018 Stiffness SIG were to: 1) gain stakeholder input regarding comprehensive understanding of the domain of stiffness. 2) review data regarding preliminary stiffness scales from two cohorts of patients with RA, and 3) obtain stakeholder feedback on other areas of development, including contextual factors affecting stiffness.
Furthering understanding of the domain of stiffness
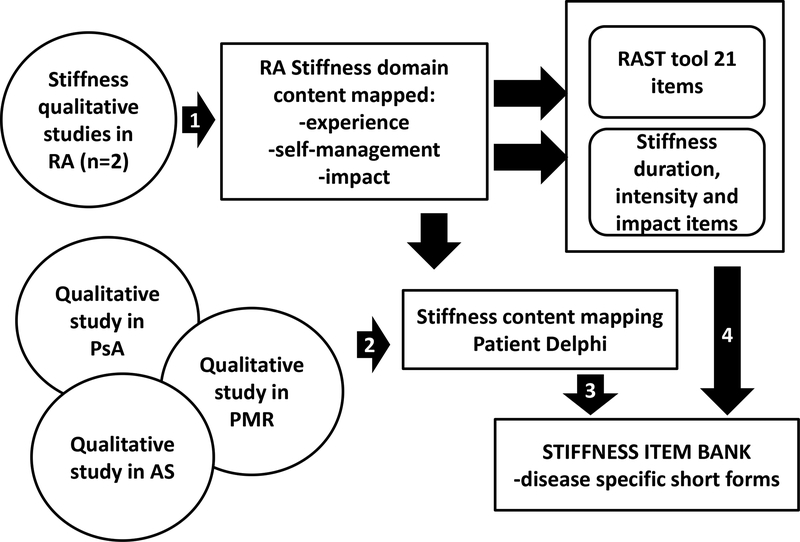
The dimensions (or subdomains) of the experience of stiffness that are relevant for stiffness measurement have yet to be endorsed by the OMERACT community. Stiffness occurs in many disease states, but patient experience among these diseases may differ. There remains, therefore, a fundamental question of whether stiffness is optimally measured using disease-specific or universal measures. The first task will be to identify themes of commonality and divergence among diseases. To accomplish this, we will revisit qualitative data to identify themes which were repeated across diseases, and those which were unique to specific diseases. These themes will be used to generate items, and a Delphi process will be undertaken with patients with each disease of interest. The Delphi process will aim to identify items that achieve consensus among patients from varied disease states, and those which achieve consensus among only patients with individual diseases. Figure 1 summarizes the proposed domain generation and Delphi process.
Figure 1.
Stiffness in Rheumatic Diseases study design
Step 1 designates the qualitative analysis step that led to determining the stiffness domain content specifically in RA and two draft instruments for their use. Step 2 illustrates qualitative studies existing in other diseases which we will use to define stiffness content in other rheumatic diseases, followed by a patient Delphi. We will then develop disease specific items and together with the existing RA stiffness items we will create a stiffness item bank. Short forms from this item bank can then be individually assessed through OFISA.
Review of measurement properties of stiffness instruments
Data from two instruments were considered. These instruments have been developed based on qualitative studies of stiffness in RA and consist of the RA stiffness PRO measure (RAST) and 3 stiffness numerical rating scale (NRS) items. Their measurement properties as presented during the SIG are briefly described below.
Measurement properties of the RAST
Work in the UK has focused on the development of an instrument guided by the patient experience. This study was approved by the University of West of England, Bristol IRB, approval # 14/WA/1162. Written informed consent was obtained from all patients.
This body of work has included qualitative investigation into the patient perspective of stiffness (6), followed by patient focus groups, an iterative process of item development involving clinicians, researchers, and patients, and subsequent cognitive interviews to refine draft items (16). A postal survey and analysis to develop the new instrument called the RA stiffness PRO measure (RAST) was presented at OMERACT 2016. This 21-item questionnaire addresses three components of stiffness: severity, physical impact, and psychosocial impact.
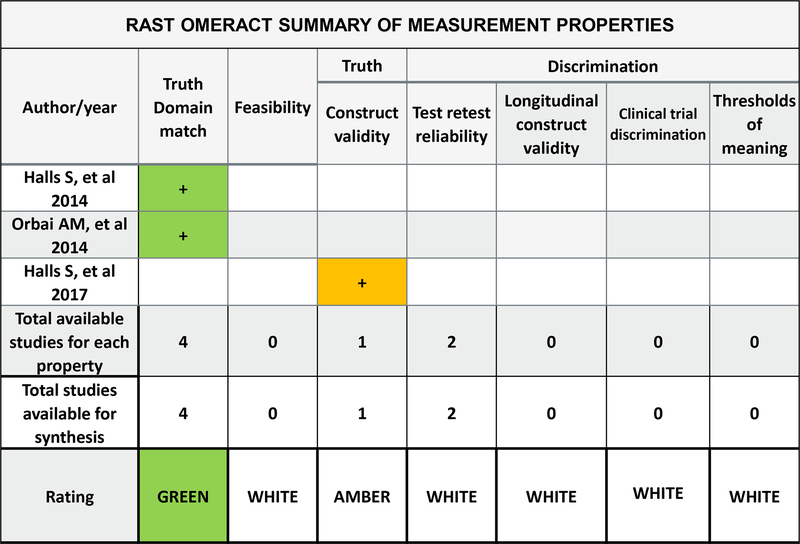
A key element of outcome measure development and testing is ensuring that outcome measures have appropriate measurement properties. One approach to ensure that outcome measures are developed appropriately and have suitable measurement properties is the OMERACT Filter 2.1. The OMERACT Filter recommends that outcome measures are evaluated against the concepts of truth (is the measure capturing what is intended in an unbiased manner?), discrimination (is the measure sensitive and reliable?) and feasibility (is the measure understandable and time efficient for use in clinical and research environments?) (17,18). Work presented at the OMERACT 2018 meeting focused the match between the RAST and the OMERACT Filter 2.1 to identify the measurement properties that are currently met and those that require further development. Figure 2 demonstrates the match between the OMERACT filter 2.0 instrument selection algorithm (OFISA) and the RAST evidence from the presented development study. The RAST data provided new evidence for the concepts of truth and feasibility. Data supporting discrimination is the target of future work.
Figure 2:
OMERACT Summary of evidence for measurement properties of Rheumatoid Arthritis Stiffness (RAST) questionnaire. Color designates quality of evidence: green=good methods used, use this evidence; amber=some cautions but we will use this evidence. In the rating row, color designates overall evidence-based instrument rating for the core instrument set: green= at least 2 pieces of evidence with good methods and consistent findings of adequate or better performance; amber=in between green and red; red= inadequate performance on at least 1 study that used good methods, white=no evidence. Arithmetic signs designate the performance of the instrument according to that study (for each measurement property studied): “+”adequate or better performance, “+/−“ equivocal performance, “-“ less than adequate/poor performance.
Measurement properties of three stiffness items
A complementary approach was employed in the US cohort to further explore the domain of stiffness. Three stiffness items using a 5-point NRS, assessing severity, duration, and interference with daily activities were administered in a cohort of patients with RA. These items were developed based upon the same conceptual model and upon qualitative data (12). A 100 mm visual analog scale (VAS) rating stiffness intensity was subsequently added and completed by a subset of patients.
In the past 7 days, how long did your stiffness last on average? (0: None, 1: Less than 30 min, 2: 30 min-1 hour, 4: More than 1 hour to 2 hours, 5: More than 2 hours to 4 hours, 6: More than 4 hours)
In the past 7 days, how would you rate your stiffness on average? (0: None, 1: Mild, 2: Moderate, 3: Severe, 4: Very severe)
In the past 7 days, how often did your stiffness interfere with your activities? (0: Never, 1: Rarely, 2: Sometimes, 3: Often, 4: Always)
How would you describe your stiffness in the past week (VAS, 0 None to 100 Extreme)
Items were administered as part of an observational study evaluating the use of PROs in routine care at an academic rheumatology clinic, along with selected Patient-Reported Outcome Measurement Information System (PROMIS) measures (Pain Interference, Fatigue, Physical Function, Sleep Disturbance, Depression, Anxiety (all version 1.0), and Ability to Participate in Social Roles (v2.0)), a 100 mm pain VAS, and a health transition item (19,20). This study was approved by the Johns Hopkins IRB, approval # NA_00040493. Written informed consent was obtained from all patients. In total, 196 patients with RA completed the baseline visit and 83 participants completed both VAS and NRS items.
Data offered preliminary evidence of construct validity based upon correlation with other PROMs expected to correlate with stiffness. Responsiveness in states of change in several clinical anchors and longitudinal construct validity was also seen for all three items.
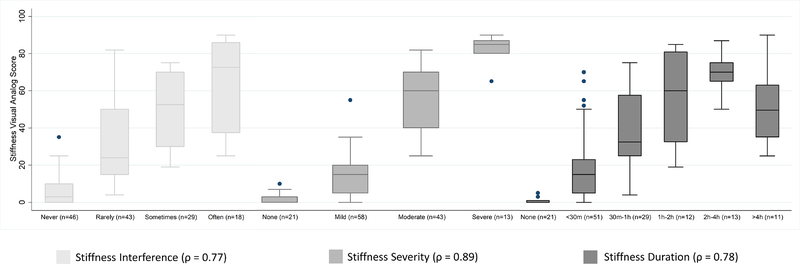
Among the 83 participants who completed both VAS and NRS items, the stiffness VAS, which did not specify a subdomain, correlated most strongly with the stiffness severity score (Figure 3). A score of “none” in severity corresponded to a median VAS of 0, “mild” to a VAS of 15, “moderate” to a VAS of 60, and “severe” to a VAS of 85.
Figure 3:
Correlation of stiffness VAS with stiffness NRS items. All correlations are Spearman rho.
The data from the US cohort lend additional support toward a multidimensional approach to measurement of stiffness.
Further SIG discussion
Patient research partners emphasized two major trends. First, there was considerable frustration over the ongoing use of morning stiffness duration in the literature and underscored findings from qualitative work that stiffness fluctuated throughout the day and was not restricted to the morning. Second, there is a need to better understand contextual factors related to stiffness. For example, one patient research partner pointed out the role of medications in modifying stiffness. Another patient research partner noted the varied experience of stiffness depending upon the context of the current level of functioning (“It really depends on what I was doing the week before. Was I doing Yoga or in bed in a massive flare?”). Future efforts will also focus upon identifying contextual factors which modify the experience of stiffness.
Research Agenda
The OMERACT 2018 Stiffness SIG included the following items on its research agenda:
Re-evaluate qualitative data to delineate common and distinct domains (or subdomains) of the experience of stiffness across disease states.
Via a Delphi process, gain endorsement from the OMERACT community regarding the essential domains (or subdomains) for the measurement of stiffness.
Further investigate the two proposed stiffness instruments including a longitudinal survey to establish reliability and responsiveness of the RAST.
Conclusion
The stiffness special interest group will delineate the minimum necessary stiffness domain content for RA, and additional rheumatic diseases. Once domain content has been defined we will look for existing instruments to fulfill this need or likely develop a stiffness measurement instrument and build a body of evidence to support it meeting the OMERACT Filter 2.1 framework for instrument selection.
Acknowledgements
The authors acknowledge all OMERACT 2016 Stiffness Special Interest Group participants for their contributions. A travel scholarship from Janssen supported the fellow attendance to the OMERACT conference. A research grant from Horizon pharma provided funding in part for statistical analysis. All analysis was conducted by investigators. All statements in this report including its conclusions are the opinions of the authors and do not necessarily reflect those of PCORI, its board of governors, or its methodology committee or NIAMS. We thank Jennifer Horonjeff, PhD for her collaboration with the SIG.
Disclosures:
AMO is a Jerome L. Greene Foundation Scholar and is supported in part by a research grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award number P30-AR070254 (Core B), a Rheumatology Research Foundation Scientist Development award, and a Staurulakis Family Discovery award. Work from the US Cohort was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under award numbers [P30-AR070254 Core B] and [P30-AR053503 Core D] and Patient Centered Outcomes Research Institute (PCORI) Pilot Project Award number [IP2-PI000737], and the Camille Julia Morgan Arthritis Research and Education Fund. All statements in this report including its conclusions are the opinions of the authors and do not necessarily reflect those of PCORI, its board of governors, or its methodology committee, or of NIH or NIAMS. RH is an employee of Horizon Pharma, LLC; CK is an employee of Janssen Scientific Affairs, LLC.
Footnotes
Publisher's Disclaimer: This article has been accepted for publication in The Journal of Rheumatology following full peer review. This version has not gone through proper copyediting, proofreading and typesetting, and therefore will not be identical to the final published version. Reprints and permissions are not available for this version. Please cite this article as doi 10.3899/jrheum.181074. This accepted article is protected by copyright. All rights reserved.
References
- 1.Orbai A-M, Halls S, Hewlett S, Bartlett S, Leong A, Bingham C III, et al. More than just minutes of stiffness in the morning: report from the OMERACT Rheumatoid Arthritis Flare Group stiffness breakout sessions. J Rheumatol 2016;28:1304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halls S, Sinnathurai P, Hewlett S, Mackie SL, March L, Bartlett SJ, et al. Stiffness is the cardinal symptom of inflammatory musculoskeletal diseases, yet still variably measured: report from the OMERACT 2016 stiffness special interest group. J Rheumatol 2016;44. [DOI] [PubMed] [Google Scholar]
- 3.Mackie SL, Arat S, da Silva J, Duarte C, Halliday S, Hughes R, et al. Polymyalgia Rheumatica (PMR) Special Interest Group at OMERACT 11: outcomes of importance for patients with PMR. J Rheumatol 2014;41:819–23. [DOI] [PubMed] [Google Scholar]
- 4.Calin A The individual with ankylosing spondylitis: defining disease status and the impact of the illness. Br J Rheumatol 1995;34:663–72. [DOI] [PubMed] [Google Scholar]
- 5.van Tuyl LHD, Lems WF, Boers M. Measurement of stiffness in patients with rheumatoid arthritis in low disease activity or remission: a systematic review. BMC Musculoskelet Disord 2014;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halls S, Dures E, Kirwan J, Pollock J, Baker G, Edmunds A, et al. Stiffness is more than just duration and severity: A qualitative exploration in people with rheumatoid arthritis. Rheumatol (United Kingdom) 2014;54:615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 8.van der Heijde D, Bellamy N, Calin A, Dougados M, Khan MA, van der Linden S. Preliminary core sets for endpoints in ankylosing spondylitis. Assessments in Ankylosing Spondylitis Working Group. J Rheumatol 1997;24:2225–9. [PubMed] [Google Scholar]
- 9.Orbai A-M, de Wit M, Mease PJ, Callis Duffin K, Elmamoun M, Tillett W, et al. Updating the Psoriatic Arthritis (PsA) Core Domain Set: A Report from the PsA Workshop at OMERACT 2016. J Rheumatol NIH Public Access; 2017;44:1522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackie SL, Twohig H, Neill LM, Harrison E, Shea B, Black RJ, et al. The OMERACT Core Domain Set for Outcome Measures for Clinical Trials in Polymyalgia Rheumatica. J Rheumatol 2017;44:1515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartlett SJ, Barbic SP, Bykerk VP, Choy EH, Alten R, Christensen R, et al. Content and construct validity, reliability, and responsiveness of the rheumatoid arthritis flare questionnaire: OMERACT 2016 workshop report. J Rheumatol 2017;44:1536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orbai A-M, Smith KC, Bartlett SJ, De Leon E, Bingham CO III. “Stiffness has different meanings, I think, to everyone”. Examining stiffness from the perspective of people living with rheumatoid arthritis. Arthritis Care Res 2014;66:1662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva J a P, Phillips S, Buttgereit F. Impact of impaired morning function on the lives and well-being of patients with rheumatoid arthritis. Scand J Rheumatol Suppl 2011;125:6–11. [DOI] [PubMed] [Google Scholar]
- 14.Mattila K, Buttgereit F, Tuominen R. Influence of rheumatoid arthritis-related morning stiffness on productivity at work: results from a survey in 11 European countries. Rheumatol Int Springer Berlin Heidelberg; 2015;35:1791–7. [DOI] [PubMed] [Google Scholar]
- 15.Westhoff G, Buttgereit F, Gromnica-Ihle E, Zink A. Morning stiffness and its influence on early retirement in patients with recent onset rheumatoid arthritis. Rheumatology 2008;47:980–4. [DOI] [PubMed] [Google Scholar]
- 16.Halls S, Dures E, Kirwan JR, Pollock J, Baker G, Edmunds A, et al. Development and testing of candidate items for inclusion in a new rheumatoid arthritis stiffness patient-reported outcome measure. Rheumatology 2017;1–10. [DOI] [PubMed] [Google Scholar]
- 17.Boers M, Kirwan JR, Tugwell P, Beaton D, De Wit M, Gossec L, et al. The OMERACT Handbook. Ottawa, CA: OMERACT; 2017. 52–73 p. [Google Scholar]
- 18.Boers M, Kirwan JR, Wells G, Beaton D, Gossec L, D’Agostino MA, et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0. J Clin Epidemiol 2014;67:745–53. [DOI] [PubMed] [Google Scholar]
- 19.Bingham CO, Bartlett SJ, Merkel PA, Mielenz TJ, Pilkonis PA, Edmundson L, et al. Using patient-reported outcomes and PROMIS in research and clinical applications: experiences from the PCORI pilot projects Qual Life Res Springer International Publishing; 2016;25:2109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartlett SJ, Orbai AM, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, et al. Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PLoS One 2015;10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]