Abstract
Glioblastoma Multiforme (GBM) is the most common and invasive form of malignant brain tumors and despite advances in surgery, radiotherapy and chemotherapy, the survival of patients with GBM still remains poor. Temozolomide (TMZ) is the chemotherapy drug that is most commonly given orally after surgical resection of these tumors. In this study, the effects of solvents (i.e. dichloromethane and acetonitrile) used for the fabrication of electrosprayed TMZ-loaded poly(lactic-co-glycolic acid) (PLGA) on drug loading, loading efficiency, drug release kinetics, surface morphology and particle size were investigated. The results from this study demonstrated that by using a larger volume of a solvent with higher polarity (i.e. acetonitrile) which allows for a higher amount of hydrophilic TMZ to dissolve into the polymer solution, higher drug loading could be achieved. However, the particles fabricated with high amount of acetonitrile, which has a lower vapor pressure, had large pores and a smaller diameter which led to an initial burst release and high cumulative release at the end of the study. An optimal combination of the two solvents is needed to result in particles with a good amount of loading and minimal initial burst release. The electrosprayed microparticles were able to illicit a cytotoxic response in U-87 MG glioblastoma cells at a lower concentration of drug compared to the free drug. This work indicated that electrospraying is a promising method for the fabrication of TMZ-loaded PLGA microparticles for the treatment of GBM and solvent composition can be altered to control drug loading and release kinetics.
Keywords: Temozolomide, PLGA microparticles, drug delivery, electrospraying, glioblastoma
INTRODUCTION
Gliomas are the most common malignant brain tumors (70–80% of primary malignant brain tumors) and Gliobastoma Multiforme (GBM) is the most prevalent, aggressive and high-grade form of these brain tumors (82% of malignant gliomas) [1]. Each year, around 10,000 patients in the US are diagnosed with GBM and currently the median survival for this disease is around 21 months after diagnosis despite treatment with surgery, radiotherapy and chemotherapy [2]. Gliomas are usually treated by resection surgery and external beam radiation and occasionally by systemic chemotherapy or a combination of these methods [3]. For brain cancer, metastasis to other sites outside of the central nervous system (CNS) does not occur very frequently and local recurrence is the main cause of death for this disease [4]. Due to the infiltrative nature of these tumors, it is difficult for surgeons to completely remove the cancer cells without the risk of neurological deficits [5]. Thus, finding advances in treating the residual disease to prevent local recurrence in brain cancer patients after tumor resection should be an emphasis to increase the long term survival or possibly the complete cure of patients [6].
Systemic delivery of chemotherapy and other anti-neoplastic agents, which can inhibit or halt the progression of tumors, such as immunotherapy or anti-angiogenic drugs, have long been a method of treatment for cancer, However, many shortcomings associated with systemic delivery of these agents, including low local drug concentration at the targeted tumor site, non-target cell and organ toxicity as well as low efficacy of the delivered drug due to its short half-life, has emphasized the need for an alternative approach to replace this method of delivery. For brain malignancies, an added challenge is present for systemic delivery. Drugs for the treatment of these tumors also have the obstacle of having to cross the Blood Brain Barrier (BBB). Although some chemotherapy agents, such as the classes of anti-proliferation alkylating agents temozolomide (TMZ) and nitrosoureas (BCNU and lomustine, are able to cross the BBB to some extent and have been used clinically [7], the efficacy of these drugs even as a concurrent treatment to radiotherapy has been modest [8–10]. The insufficient improvement in patient prognosis with these drugs is partly due to the low local drug concentrations at the targeted site due to the inadequate delivery of the drugs in spite of being able to cross the BBB [11, 12].
By delivering drugs locally, the drug concentration at the tumor environment can be maximized, non-target systemic exposure and organ toxicity can be minimized and the need of finding a method to cross the BBB can be avoided. Furthermore, local delivery can increase the efficacy of the drug as it can bypass the harsh environment and longer journey it has to take to reach the targeted site of interest. The development of new drugs is associated with high cost and difficulty. Thus, it would be beneficial if new treatment methods are developed to successfully deliver currently available therapeutic agents that have been shown to be promising in vitro, however, failed or resulted in modest outcomes when delivered in vivo systemically. When designing a successful drug delivery system, different factors such as the drug concentration, exposure time and drug administration schedule and sequence of delivery have to be taken into account and all these factors will differ based on the drug of interest. Biomaterials, such as disc, wafers and microparticles, can be optimally designed and utilized to accomplish this for different drugs. Compared to scaffold materials such as disc and wafers, microparticles can be beneficial as it can be easily implanted to discrete areas by stereotaxy instead of requiring open surgery [13].
TMZ is currently the chemotherapy drug that is most commonly given after surgical resection of malignant glioma tumors through oral administration [2, 14] and has replaced BCNU as the standard therapy for GBM as it is associated with less side effects and better bioavailability compared to BCNU [15]. TMZ is an imidazotetrazine class DNA alkylating agent that functions by methylating guanine and adenine bases of DNA which results in the breaking of DNA double-strands and cell cycle arrest, leading to cell death [16]. Because TMZ is an unstable molecule with a short half-life and dose-limiting side effects [17], the current method of delivering TMZ alone systemically still produces modest benefits [18]. Thus, new strategies such as those employing biomaterials to protect the drug and enhance the delivery of bioactive drug to the cancer cells are much needed.
The co-polymer poly(lactic-co-glycolic acid) (PLGA) is an α-hydroxy acid-derived polyester that has been widely used due to its inert properties, great biocompatibility and versatility and thus, making it the most common material that is used for the fabrication of biodegradable microparticles. PLGA has been shown to have biocompatibility in the brains of rodents and human [19, 20]. The degradation rate of PLGA can be easily tailored from days to years by tuning the lactic to glycolic acid ratio, molecular weight and the end cap groups and thus, providing versatility in the development of microparticles to deliver different drugs where the release kinetics need to be tailored to fit the drug of interest. Because of its versatility and biosafety, PLGA has been widely used for the fabrication of TMZ-loaded particles for the treatment of glioblastoma [21]. There are many different methods of producing PLGA microparticles including emulsion solvent extraction/evaporation [22], electrospraying [23] and microfabrication [24]. Among the different fabrication methods, the emulsion solvent extraction/evaporation method is the most widely used method for the fabrication of drug loaded microparticles as it is associated with an easy and low cost setup [25–27]. However, the disadvantage of this technique is that it results in a large loss of drug to the surrounding solutions during the fabrication process which results in low entrapment efficiency and drug loading [24]. To overcome this, Zhang et al. saturated the aqueous emulsion phase with 0.5 wt/vol % of drug (i.e. 400 mg of drug per synthesis of a standard batch of particles), resulting in the use of a lot of drugs which can be very costly [18]. Lee et al. who tried altering different parameters of fabrication of nanoparticles using the emulsion solvent extraction/evaporation method were not successful at obtaining high entrapment efficiency and drug loading and thus, concluded that the emulsion solvent evaporation method of fabrication of TMZ-loaded microparticles is not efficient and other method should be utilized to fabricate these particles [28]. Anata et al. investigated different emulsion solvent extraction/evaporation techniques (i.e. single and double emulsion) for the fabrication of TMZ/PLGA nanoparticles. They found that by using a single emulsion technique with TMZ saturation in the aqueous phase, they were able to increase loading efficiency by 90–700% and the encapsulation efficiency by 2–15-fold. However, even with this significant increase in loading efficiency with their method, they concluded that the loading efficiency of the particles still needs to be significantly improved to make it suitable for TMZ delivery to treat glioblastoma patients [29]. Zhang et al have shown that TMZ loaded PLGA microparticles synthesized by the emulsion solvent extraction/evaporation have been shown to improve the survival time of animals bearing intracranial tumor [30]. However, to achieve this, a lot of drug was needed to fabricate the particles as the aqueous phase has to be saturated with the drug [30]. Thus, there is a need to find new methods to fabricate TMZ loaded PLGA particles to result in high drug loading and entrapment efficiency in order to improve the response of glioblastoma cells to these type of particles.
Thus, the objective of this study was to investigate the utilization of the electrospraying technique to fabricate TMZ-loaded PLGA microparticles with high drug loading and loading efficiency. This method requires a pump, a syringe and needle with high electric potential and a grounded electrode. A high voltage potential on the needle forces the polymer solution out of the syringe and results in the formation of a jet that breaks into monodisperse droplets which form the microparticles. Electrospraying is associated with many advantages including the ability to fabricate particles with narrow size distribution, its experimental setup requires low investment and it allows for scalable fabrication [31, 32]. A main reason for using electrospraying instead of the common emulsion solvent extraction/evaporation technique is that electrospraying is associated with high entrapment efficiency [32]. The effects of solvent used for fabrication (i.e. different volume ratio of dicholoromethane (DCM) and acetonitrile) of TMZ-loaded PLGA microparticles fabricated by electrospraying on drug loading, loading efficiency, drug release kinetics, surface morphology and particle size were determined. The particles were also tested on U-87 MG glioblastoma cells to determine their ability to induce cytotoxicity in these cells. To the best of our knowledge, this is the first manuscript that investigated the electrospraying method for the fabrication of TMZ-loaded PLGA microparticles.
MATERIALS AND METHODS
Materials
U-87 MG glioblastoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, ATCC® HTB-14™). Dulbecco’s Modified Eagle Media (DMEM) with high glucose, l-glutamine and sodium pyruvate was purchased from Caisson’s Lab (Smithfield, UT). Fetal bovine serum (FBS) Premium Select was obtained from Atlanta Biologicals. Penicillin-streptomycin was obtained from GE Healthcare Life Sciences HyClone Laboratories (Logan, UT). Methyl tetrazolium (MTT) powder and DCM were obtained from Sigma-Aldrich (St. Louis, MO). Acetonitrile was purchase from VWR (Radnor, PA). Dimethyl sulfoxide (DMS) was obtained from Fisher Scientific (Hampton, NH). PLGA 50:50 and (LP-779, Internal Viscosity = 0.43 dL/g, MW = 61 kDa) with an acid end group was obtained from Evonik Industries (Birmingham, AL). TMZ was purchased from TCI America (Portland, OR).
Fabrication of Electrosprayed PLGA Microparticles
PLGA microparticles loaded with TMZ were fabricated by electrospraying. Two different solvents, DCM and acetonitrile were investigated by themselves or in combination. The four different solvent combination that were fabricated are listed in Table 1 which are denoted as 1) “TM-0” which was fabricated with 0% DCM and 100% acetonitrile, 2) “TM-25” which was fabricated with 25% DCM and 75% acetonitrile, 3) “TM-50” which was fabricated with 50% DCM and 50% acetonitrile and 4) “TM-100” which was fabricated with 100% DCM and 0% acetonitrile. The electrospraying setup consists of a Legato 100 Single Syringe Pump (KD Scientific, Holliston, MA), ES30P-5W power supply (Gamma High Voltage Research, Ormond Beach, FL), a copper collector plate and a glass syringe with a 21-gauge blunt needle tip (Hamilton, Reno, NV). A 10 mm needle-tip to copper plate distance was used. The pump speed of 2 mL/hr was used with a voltage of 6 kV. Briefly, for the four different types of microparticles, 200 mg PLGA (5 wt/vol %) and the amount of drug shown in the table were dissolved in a total of 4 mL of solvent. The particles were fabricated with a temperature and humidity range of 22–24°C and 45–50%, respectively. The amount of drug used for fabricating the microparticles were based on the maximum amount of TMZ that would dissolve into the different solvent combinations as shown in Table 1 (i.e. 20, 15, 10 and 4 mg in 4 mL of solvent for TM-0, 25, 50 and 100, respectively). The electrosprayed microparticles were collected directly on the copper plate and dried under vacuum for 24 hours to ensure complete removal of the solvent.
Table 1.
Electrosprayed microparticles parameters and drug loading (%), loading efficiency (%) and mean diameter (μm) of microparticles fabricated with different parameters. The results are expressed as mean ± standard deviation for n = 3.
| Groups | Solvent ratio DCM : Acetonitrile |
Amount of Drug Used for Fabrication (mg) | Drug Loading (%) | Loading Efficiency (%) | Mean Diameter (μm) |
|---|---|---|---|---|---|
| TM-0 | 0 : 100 | 20 | 6.60 ± 0.20* | 72.61 ± 2.30 | 2.94 ± 0.76 |
| TM-25 | 25 : 75 | 15 | 5.25 ± 0.21* | 75.30 ± 3.12 | 3.51 ± 0.71 |
| TM-50 | 50 : 50 | 10 | 3.88 ± 0.21* | 81.52 ± 4.49 | 4.28 ± 1.13* |
| TM-100 | 100 : 0 | 4 | 1.38 ± 0.14* | 70.58 ± 7.40 | 11.50 ± 3.39* |
indicates groups that are significantly different (p < 0.05) from all other groups.
Drug Loading and Loading Efficiency
The amount of drug loaded into the microparticles was determined using a previous protocol with some modification [18]. 5 mg of microparticles were completely dissolved in 2.5 mL of DMSO (100%). The amount of TMZ loaded into the microparticles was determined by measuring the absorbance at 327 nm with a microplate reader equipped with the Magellan data analysis software (Tecan Infinite Pro 200, Switzerland). The drug loading and loading efficiency analyses were done in triplicate. The drug loading and loading efficiency were determined with the equations below.
Drug Release Kinetics
2.5 mg of microparticles were placed in 1 mL of PBS in a scintillation vial at 37°C and shaken at 70 rpm. At each time point (0, 1, 4, 7, 10, 14 d), the PBS was removed from the scintillation vials and the amount of TMZ was obtained as described above. Due to the instability of the TMZ in the release solution, the TMZ release in the solution was not measured and instead, the amount of TMZ remaining in the microparticles was used to calculate the cumulative release at each time point [33]. This study was conducted at n = 3.
Scanning Electron Microscopy (SEM) Imaging
The microparticle surface morphology was observed by Scanning Electron Microscopy (SEM) using a JSM-7100FA Analytical Field Emission SEM and Energy Dispersive X-Ray analysis System (EDS) (JEOL USA Inc., Peabody, MA). SEM images were taken using an electron beam with 5 kV and 6 kV. The microparticles were sputter coated with a 5 nm gold layer using a smart coater (JEOL) to generate electrical conduction coating on the sample surfaces.
Microparticle Size Determination
The diameter of the microparticles was determined from 2000× SEM images using the NIH Image J Software. For each image, the Feret diameter [34] of particles that the whole particle could be clearly identified in the images (i.e. not covered by other particles) was determined.
Cell Viability
An MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma Aldrich, St. Louis, MO) was used to analyzed the cytotoxicity of TMZ in the human U-87 MG glioblastoma cell line as previously described for testing of microparticles [33]. The U-87 MG glioblastoma cells were cultured in DMEM media supplemented with 10% FBS, 100 U/mL penicillin and 100 μg streptomycin at 37°C, 5% CO2 and 90% relative humidity. The cells were plated in a 96-well plate at a cell density of 1 × 104 cells/100 μL per well. After allowing the cells to attach overnight, the media in each well was replaced with 100 μL of media containing free TMZ powder, empty PLGA microparticles or TMZ-loaded PLGA microparticles. Wells containing only media was used as a control. After 72 hrs, an MTT assay was performed to determine the ability of the drugs to induce cytotoxicity in glioblastoma cells. Briefly, the media in each well was replaced with 100 μL of 0.5 mg/mL of MTT solution. After 3 hours, the MTT solution was removed and 100 μL of DMSO was added to each well to dissolve the formazan salts that was formed by live cells by metabolizing the MTT. The absorbance at 570 nm was determined with a microplate reader equipped with the Magellan data analysis software (Tecan Infinite Pro 200, Switzerland). Four wells were used for each condition (n=4) and the following equation was used to determine cell viability (%).
Statistical Analysis
Multiple-factor analysis of variance (ANOVA) was conducted to identify if there were any significant differences among groups (p < 0.05) followed by the use of Tukey’s Honestly Significantly Different (HSD) test to identify the specific groups that differed statistically significantly.
RESULTS
Drug Loading and Loading Efficiency
The drug loading and loading efficiency of the four different types of microparticles are reported as percentages in Table 1. Particles fabricated with 0, 50, 75 and 100% DCM (i.e. TM-0, TM-50, TM-75 and TM-100) resulted in a drug loading of 6.60 ± 0.20, 5.25 ± 0.21, 3.88 ± 0.21 and 1.38 ± 0.14%, respectively. The drug loading of each of the different types of microparticles were significantly different from one another. The loading efficiencies of the different microparticles were between 70–82% and were not significantly different from each other.
Drug Release Kinetics
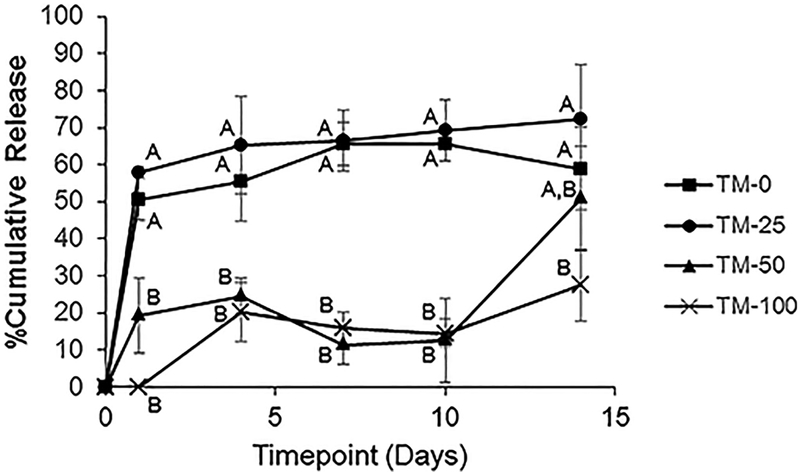
The drug release kinetics of the different types of microparticles that were fabricated are shown in Figure 2. The microparticles that were fabricated with a higher volume of acetonitrile resulted in significantly higher % cumulative release (65.7 ± 8.2 and 69.3 ± 4.6% for TM-0 and TM-25, respectively) at the end of the release study (i.e. Day 14) compared to those fabricated with a lower amount of acetonitrile (27.4 ± 9.6% for TM-100). Furthermore, TM-0 and TM-25 microparticles which were fabricated with a higher volume of acetonitrile had a higher initial burst release (50.6 ± 1.6 and 57.9 ± 5.7%, respectively) compared to TM-50 and TM-100 (19.4 ± 10.1 and 0 ± 0%, respectively).
Figure 2.
Release kinetics of the different electrosprayed particles fabricated with different solvents. The results are expressed as mean ± standard deviation for n = 3. Groups not connected with the same letter are significantly different from each other (p < 0.05).
Microparticle Size and Morphology
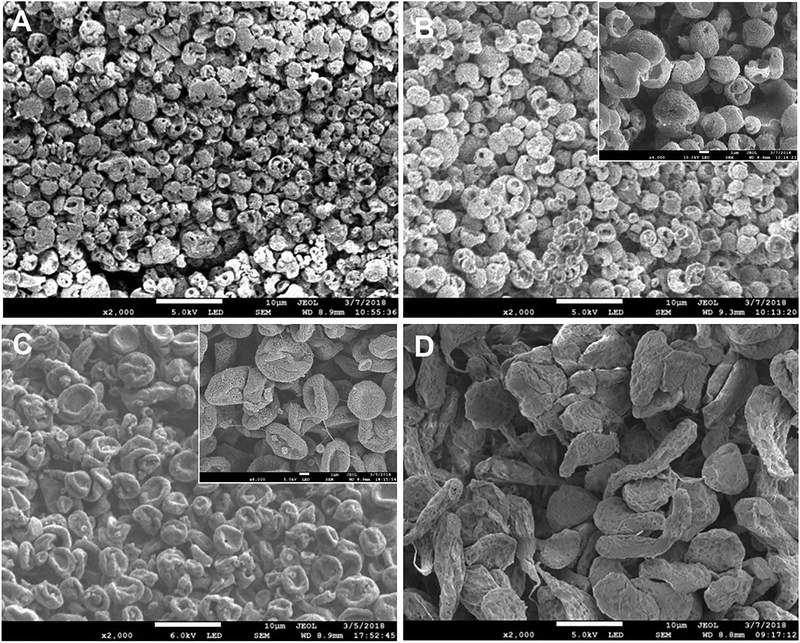
SEM images of TM-0 and TM-25 show spherical microparticles with a porous surface of size reaching up to 1 micron (Figure 1A and B). TM-25 in addition to spherical particles shows traces of deformed biconcave disc microparticles indicating the beginning of a transformation process. TM-50 microparticles (Figure 1C) had a biconcave disc shape while further magnification show a nano-porous surface and TM-100 microparticles (Figure 1D) had irregular and stretched out shapes with a rough non-porous surface. Xie et al. saw similar morphologies for their PLGA and PCL microparticles that were fabricated by electrohydrodynamic atomization (EHDA). As polymer concentration decreased, the shape of the particles they fabricated changed from sphere to biconcave to an irregular shape [13]. Overall, with an increase in the amount of DCM and a decrease in the amount of acetonitrile used for the solvent, the size of the particles increased (2.94 ± 0.76, 3.51 ± 0.71, 4.28 ± 1.13 and 11.50 ± 3.39 μm for TM-0, TM-25, TM-50 and TM-100, respectively). The particle size of TM-50 and TM-100 were significantly larger compared to TM-0 and TM-25.
Figure 1.
SEM images of the different microparticles: A) TM-0, B) TM-25, C) TM-50 and D) TM-100. The main images were taken at 2000× and the scale bar of these images represents 10 μm. The embedded images in B) and C) were taken at 6000× and the scale bar of these images represents 2 μm.
Cell Viability
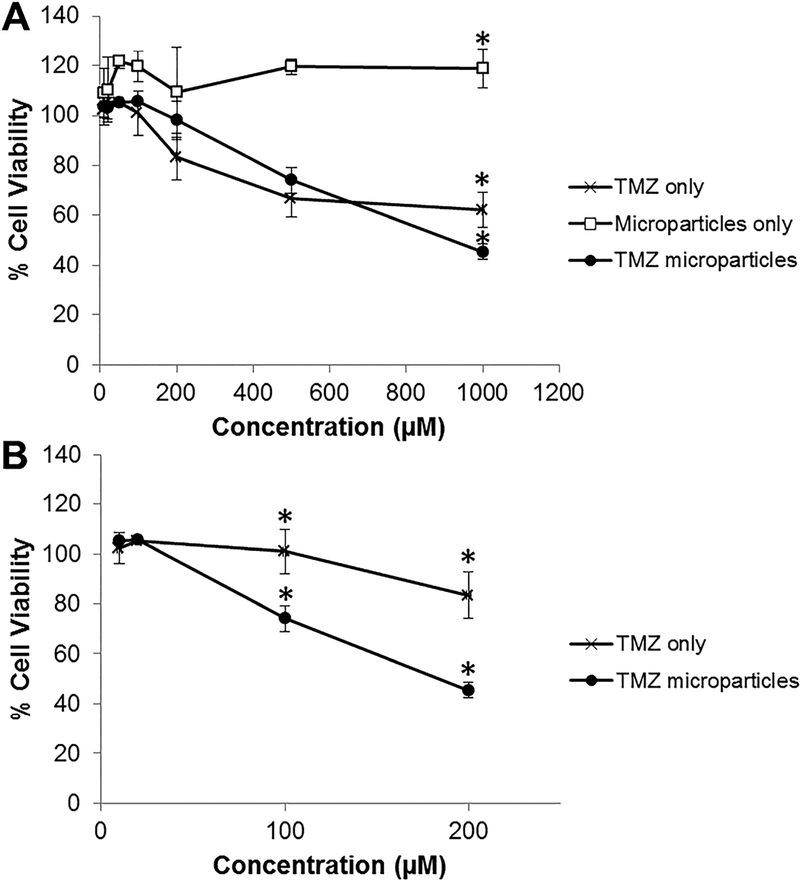
To test the response of glioblastoma U-87 MG cells to TMZ alone, empty PLGA microparticles and TMZ-loaded PLGA microparticles were determined using an MTT assay. TM-25 microparticles were used as an initial proof-of-concept of the bioactivity of the drug loaded in the electrosprayed TMZ-loaded PLGA microparticles. Empty PLGA microparticles did not illicit a negative response on cell viability of the glioblastoma cells (Figure 3A). Cells exposed to TMZ by itself (66.7 ± 7.5 and 62.3 ± 7.2%, respectively) or TMZ-loaded PLGA microparticles (74.1 ± 5.2 and 45.5 ± 3.0%, respectively) had a significantly lower % cell viability compared to empty microparticles (119.5 ± 3.1 and 119.0 ± 7.9%, respectively) at a concentration of 500 and 1000 μM. At a drug concentration of 1000 μM, the TMZ-loaded PLGA microparticles had a significantly lower % cell viability (45.5 ± 3.0%) compared to TMZ delivered by itself (62.3 ± 7.2%). For the results shown in Figure 3A, the drug concentration of the TMZ-loaded microparticles are the amount of drug that was loaded in the particles and not the actual amount that was present in the media and exposed to the cells. Thus, for better comparison, in Figure 3B, we compared the cell viability of cells exposed to TMZ by itself and TMZ-loaded microparticles based on the amount of TMZ that had been released into the media based on the release study. For this comparison, we observed that starting at 100 μM, cells exposed to TMZ-loaded microparticles resulted in significantly lower cell viability (74.1 ± 5.2%) compared to TMZ delivered by itself which had no effect on the cell viability (100.9 ± 8.9%). At a concentration of 200 μM, cells exposed to TMZ loaded microparticles resulted in a cell viability of 45.5 ± 3.0% whereas cells exposed to TMZ by itself resulted in a cell viability of 83.5 ± 9.2%.
Figure 3.
Cell viability of U-87 MG glioblastoma cells exposed to the TMZ only, empty PLGA microparticles only or TMZ loaded PLGA microparticles (i.e. TM-25) A) for 72 hours at different drug concentrations based on drug loaded in the TMZ microparticles and B) at different drug concentrations based on drug release from the TMZ microparticles at Day 4. The results are expressed as mean ± standard deviation for n = 4. * indicates groups that are significantly different (p < 0.05) from all other groups within the same drug concentration.
DISCUSSION
The emulsion solvent evaporation technique of fabricating microparticles is often used to fabricate PLGA microparticles due to its low cost and ease in setup. However, this method often results in high loss of drug to the external aqueous phase by diffusion, which leads to low drug loading and loading efficiency. Thus, in this work, we investigated the utilization of the electrospraying technique to fabricate TMZ-loaded PLGA microparticles with higher drug loading and loading efficiency for the treatment of glioblastoma. The effects of altering the solvent used for the fabrication of the microparticles on drug loading, loading efficiency and release kinetics and particle size and morphology were studied. The two solvents that were investigated were DCM and acetonitrile which have a relative polarity of 0.309 and 0.460, respectively. Due to its hydrophilic nature [29], TMZ more readily dissolves in acetonitrile which is a more polar solvent. Solvent combinations with a higher volume of acetonitrile were able to incorporate a higher amount of drug in the electrospraying polymer/drug/solvent solution as TMZ dissolved better in the solvent (Table 1). This resulted in significantly higher drug loading for microparticles fabricated with more acetonitrile compared to microparticles fabricated with less acetonitrile as a higher starting drug concentration could be used to fabricate the particles. The four different microparticles fabricated by electrospraying had loading efficiency of 70–82%. Zhang et al. was able to achieve an entrapment efficiency of around 62–85% with emulsion solvent evaporation method by saturating the aqueous phase and thus, using a lot of drug during the fabrication process [33]. Without saturation, the drug diffuses easily into the aqueous phase and the entrapment efficiency that was achieved by them was only around 30%. Instead of using only 4–20 mg of drug for fabrication with the electrospraying technique to achieve high drug loading and loading efficiency, an additional 400 mg of drug is needed to saturate the aqueous phase if the emulsion solvent evaporation method was used.
Particle porosity (i.e. pore size and amount of pores) can play a role in the release kinetics of drugs from particles [35]. As determined by SEM, the porosity of the microparticles changed with the different solvent used for fabricating the particles. The porosity of particles usually increases with a decrease in solvent extraction rate [25]. In our study, we saw that an increase in acetonitrile volume and decrease in DCM volume resulted in higher pore size. Acetonitrile and DCM have a vapor pressure of 97 and 475 hPa at 20°C, respectively. A solvent with a higher vapor pressure exerts more pressure on the liquid phase resulting in higher volatility or ease of vaporization. During fabrication, the lower volatility of acetonitrile resulted in slower extraction of the solvent, and thus, resulted in the larger pores and porosity for particles with higher portions of acetonitrile. Porosity in microparticles can be created intentionally using porogens such as gelatin, PBS [36] or gas-forming agents or can be created as an artifact of the fabrication technique which can also be created such as the loss of absorbed oil that leaks out of particles after hardening [37] or during the freeze drying step at the end of the fabrication process [38]. In our study, the pores in the microparticles were created as an artifact of the solvent evaporation process as the particles travel to the copper collection plate during fabrication. Several studies have shown that microparticle porosity can result in initial burst release of drugs due to pore diffusion [39, 40]. Rapid initial release of drug often occurs due to the hydration of the outer wall of the particles which leads to leaching of the drug [25]. Surface porosity of PLGA microparticles plays an essential role in determining the magnitude of this initial release due to pore diffusion [39]. Consistent with this, we saw that particles with large pores (TM-0 and TM-25) resulted in a high burst release compared to particles with small pores (TM-50) or no pores (TM-100) on their surface.
Surface morphology and porosity of the microparticles can also affect the rate of erosion of particles [41, 42]. The different microstructure of the microparticles that are created during the fabrication process results in a difference in the accumulation of acidic degradation byproduct of polyesters such as PLGA. These acidic byproducts play a role in further catalyzing degradation of the microparticles. The release of drugs from PLGA microparticles is initially dependent on diffusion through pores and channels, resulting in the initial burst release. As the internal porosity decreases overtime, the polymer matrix becomes denser. The release of the drug then starts to depend on erosion of the polymer and not so much on diffusion.
In addition to facilitating drug release and degradation of the particles, the pores on the surface of these microparticles are advantageous for our applications as they facilitate cell attachment [36]. Increase in interaction of the cells and particles will more likely result in the cancer cells uptaking the drug released from the particles.
Besides a different in porosity characteristic with change of solvent ratio, the size of the microparticles fabricated in this study also differed, which can also play a role in the release kinetics of the drug loaded. Particles that are smaller in size usually result in a high initial burst and/or fast release of the drug as these particles have higher surface area [43, 44]. Xie et al demonstrated that smaller PLGA microparticles fabricated by electrohydrodynamic atomization were obtained when acetonitrile was used instead of DCM [13]. We similarly saw that a higher ratio of acetonitrile compared to DCM resulted in smaller particle size. DCM and acetonitrile have a dielectric constant (indicating conductivity of the solvent) of 9.1 and 37.5, respectively [13]. There is a positive correlation between dielectric constants and conductivity and thus, acetonitrile, which has a higher dielectric constant, has a higher conductivity compared to DCM which has a lower dielectric constant. As seen by Xie et al, fabrication of particles with polymer solution with a high dielectric constant and conductivity results in particles with smaller size [13]. This is explained by the scaling laws which when rewritten, explains the inverse relationship between particle diameter (d) and liquid conductivity (K) as shown in the equation below [13]:
Besides the surface porosity, the size of the microparticles also plays a role in determining the degradation rate of the microparticles which in turn affects release kinetics. Dunne et al demonstrated that there was a linear relationship between PLGA microparticles size and degradation rate [45]. It is more difficult for acidic byproducts to diffuse out of larger microparticles, thus, resulting in a higher accumulation of the autocatalytic degradation byproducts. The low pH in the polymer matrix results in an increase in the concentration of protons which can catalyze ester hydrolysis which leads to an increase in autocatalytic degradation of the remaining polymer matrix. The increase in degradation of the polymer matrix results in an increase in drug release rates for these larger particles. In the release study, we saw a drastic release at Day 14 for TM-50. This could have result from the change of release from diffusion to release through erosion of the polymer at this point.
The electrosprayed TMZ-loaded microparticles were able to better induce glioblastoma cell death compared to TMZ delivered by itself which indicates that the biomaterial is able to protect the bioactivity of the drug. The empty PLGA microparticles did not illicit a negative response to the cells and thus, indicating, as expected, the polymer itself is not toxic to the cells. MTT is often the first method used to evaluate the cytotoxic effect of drug (such as TMZ) loaded particles on glioblastoma cell lines [18, 33]. In the future, other methods, such as analysis with flow cytometry with annexin V for cell apoptosis, will be performed to better understand glioblastoma cell response to the optimized particles.
CONCLUSIONS
The electrospraying method of fabricating PLGA microparticles resulted in particles with high loading efficiency. Furthermore, this method resulted in ease in obtaining the particles as tedious separation of the particles from the aqueous phase is not needed as in the emulsion solvent evaporation method [13]. By using a larger volume of a solvent with higher polarity (i.e. acetonitrile) which allows for a higher amount of hydrophilic TMZ to dissolve into the polymer solution, higher drug loading could be achieved. However, the change in solvents (which have different polarity and vapor pressure) used to fabricate the microparticles not only changed the amount of drug loading, it also affected the surface morphology, porosity and size of the particles which in turn affected the release kinetics of the particles. Microparticles that were fabricated with high amount of acetonitrile and low amount of DCM resulted in a high drug loading and had large pores and a smaller diameter which led to an initial burst release and high cumulative release at the end of the study. Microparticles that were fabricated with low amount of acetonitrile and high amount of DCM had a low drug loading and had smaller pores or no pores and a larger diameter, resulting in a more sustained release. The electrosprayed microparticles were able to illicit a cytotoxic response in U-87 MG glioblastoma cells at a lower concentration of drug compared to the free drug. In conclusion, electrospraying is a promising method to fabricate TMZ-loaded PLGA microparticles with high drug loading and loading efficiency that is able to better sustain the bioactivity of loaded drugs compared to drugs that are delivered by themselves. These particles can be used to delivery TMZ locally at a targeted site by incorporating them into a biomaterial scaffold or by redesigning them to form a colloidal gel which can be injected at the site of interest to form a scaffold by themselves [46]. These particles can be also implanted to discrete areas by stereotaxy which will not require open surgery [13].
ACKNOWLEDGEMENT
Research reported in this publication was supported by the National Institute of General Medical Sciences (NIGMS) under award number R25GM065925. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Omuro A and DeAngelis LM, Glioblastoma and other malignant gliomas: a clinical review. JAMA, 2013. 310(17): p. 1842–50. [DOI] [PubMed] [Google Scholar]
- 2.Bow H, Hwang LS, Schildhaus N, Xing J, Murray L, Salditch Q, Ye X, Zhang Y, Weingart J, Brem H, and Tyler B, Local delivery of angiogenesis-inhibitor minocycline combined with radiotherapy and oral temozolomide chemotherapy in 9L glioma. J Neurosurg, 2014. 120(3): p. 662–9. [DOI] [PubMed] [Google Scholar]
- 3.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, and Ram Z, A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol, 2003. 5(2): p. 79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manome Y, Kobayashi T, Mori M, Suzuki R, Funamizu N, Akiyama N, Inoue S, Tabata Y, and Watanabe M, Local delivery of doxorubicin for malignant glioma by a biodegradable PLGA polymer sheet. Anticancer Res, 2006. 26(5A): p. 3317–26. [PubMed] [Google Scholar]
- 5.Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, and Sawaya RE, Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol, 2014. 32(8): p. 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorger M, Tumor microenvironment in the brain. Cancers (Basel), 2012. 4(1): p. 218–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mason WP and Cairncross JG, Drug Insight: temozolomide as a treatment for malignant glioma--impact of a recent trial. Nat Clin Pract Neurol, 2005. 1(2): p. 88–95. [DOI] [PubMed] [Google Scholar]
- 8.Chang CH, Horton J, Schoenfeld D, Salazer O, Perez-Tamayo R, Kramer S, Weinstein A, Nelson JS, and Tsukada Y, Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer, 1983. 52(6): p. 997–1007. [DOI] [PubMed] [Google Scholar]
- 9.Medical Research Council Brain Tumor Working, P., Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: a Medical Research Council trial. J Clin Oncol, 2001. 19(2): p. 509–18. [DOI] [PubMed] [Google Scholar]
- 10.Darefsky AS, King JT Jr., and Dubrow R, Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer, 2012. 118(8): p. 2163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamstra DA, Moffat BA, Hall DE, Young JM, Desmond TJ, Carter J, Pietronigro D, Frey KA, Rehemtulla A, and Ross BD, Intratumoral injection of BCNU in ethanol (DTI-015) results in enhanced delivery to tumor--a pharmacokinetic study. J Neurooncol, 2005. 73(3): p. 225–38. [DOI] [PubMed] [Google Scholar]
- 12.Yimam MA, Bui T, and Ho RJ, Effects of lipid association on lomustine (CCNU) administered intracerebrally to syngeneic 36B-10 rat brain tumors. Cancer Lett, 2006. 244(2): p. 211–9. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Marijnissen JC, and Wang CH, Microparticles developed by electrohydrodynamic atomization for the local delivery of anticancer drug to treat C6 glioma in vitro. Biomaterials, 2006. 27(17): p. 3321–32. [DOI] [PubMed] [Google Scholar]
- 14.Chaichana KL, Pinheiro L, and Brem H, Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther Deliv, 2015. 6(3): p. 353–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou J, Atsina KB, Himes BT, Strohbehn GW, and Saltzman WM, Novel delivery strategies for glioblastoma. Cancer J, 2012. 18(1): p. 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang C, Wang K, Stephen ZR, Mu Q, Kievit FM, Chiu DT, Press OW, and Zhang M, Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl Mater Interfaces, 2015. 7(12): p. 6674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker SD, Wirth M, Statkevich P, Reidenberg P, Alton K, Sartorius SE, Dugan M, Cutler D, Batra V, Grochow LB, Donehower RC, and Rowinsky EK, Absorption, metabolism, and excretion of 14C-temozolomide following oral administration to patients with advanced cancer. Clin Cancer Res, 1999. 5(2): p. 309–17. [PubMed] [Google Scholar]
- 18.Zhang D, Tian A, Xue X, Wang M, Qiu B, and Wu A, The effect of temozolomide/poly(lactide-co-glycolide) (PLGA)/nano-hydroxyapatite microspheres on glioma U87 cells behavior. Int J Mol Sci, 2012. 13(1): p. 1109–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kou JH, Emmett C, Shen P, Aswani S, Iwamoto T, Vaghefi F, Cain G, and Sanders L, Bioerosion and biocompatibility of poly(d,llactic-co-glycolic acid)implants in brain. J Control. Release, 1997. 43: p. 123–130. [Google Scholar]
- 20.Menei P, Daniel V, Montero-Menei C, Brouillard M, Pouplard-Barthelaix A, and Benoit JP, Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials, 1993. 14(6): p. 470–8. [DOI] [PubMed] [Google Scholar]
- 21.Lee CY, Strategies of temozolomide in future glioblastoma treatment. Onco Targets Ther, 2017. 10: p. 265–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi M, Kretlow JD, Nguyen A, Young S, Scott Baggett L, Wong ME, Kasper FK, and Mikos AG, Antibiotic-releasing porous polymethylmethacrylate constructs for osseous space maintenance and infection control. Biomaterials, 2010. 31(14): p. 4146–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arya N, Chakraborty S, Dube N, and Katti DS, Electrospraying: a facile technique for synthesis of chitosan-based micro/nanospheres for drug delivery applications. J Biomed Mater Res B Appl Biomater, 2009. 88(1): p. 17–31. [DOI] [PubMed] [Google Scholar]
- 24.Acharya G, Shin CS, McDermott M, Mishra H, Park H, Kwon IC, and Park K, The hydrogel template method for fabrication of homogeneous nano/microparticles. J Control Release, 2010. 141(3): p. 314–9. [DOI] [PubMed] [Google Scholar]
- 25.Freiberg S and Zhu XX, Polymer microspheres for controlled drug release. Int J Pharm, 2004. 282(1–2): p. 1–18. [DOI] [PubMed] [Google Scholar]
- 26.Li M, Rouaud O, and Poncelet D, Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm, 2008. 363(1–2): p. 26–39. [DOI] [PubMed] [Google Scholar]
- 27.Saralidze K, Koole LH, and Knetsch MLW, Polymeric microspheres for medical applications. Materials, 2010. 3: p. 3537–3564. [Google Scholar]
- 28.Lee CY and Ooi IH, Preparation of Temozolomide-Loaded Nanoparticles for Glioblastoma Multiforme Targeting-Ideal Versus Reality. Pharmaceuticals (Basel), 2016. 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ananta JS, Paulmurugan R, and Massoud TF, Temozolomide-loaded PLGA nanoparticles to treat glioblastoma cells: a biophysical and cell culture evaluation. Neurol Res, 2016. 38(1): p. 51–9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang YH, Zhang H, Liu JM, and Yue ZJ, Temozolomide/PLGA microparticles: a new protocol for treatment of glioma in rats. Med Oncol, 2011. 28(3): p. 901–6. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen DN, Clasen C, and Van den Mooter G, Pharmaceutical Applications of Electrospraying. J Pharm Sci, 2016. 105(9): p. 2601–2620. [DOI] [PubMed] [Google Scholar]
- 32.Sridhar R and Ramakrishna S, Electrosprayed nanoparticles for drug delivery and pharmaceutical applications. Biomatter, 2013. 3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H and Gao S, Temozolomide/PLGA microparticles and antitumor activity against glioma C6 cancer cells in vitro. Int J Pharm, 2007. 329(1–2): p. 122–8. [DOI] [PubMed] [Google Scholar]
- 34.Bohr A, Kristensen J, Dyas M, Edirisinghe M, and Stride E, Release profile and characteristics of electrosprayed particles for oral delivery of a practically insoluble drug. J R Soc Interface, 2012. 9(75): p. 2437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chew SA, Hinojosa VA, and Arriaga MA, Bioresorbable polymer microparticles in the medical and pharmaceutical fields, in Bioresorbable polymers for biomedical applications, From fundamentals to translational medicine, Perale G and Hilborn J, Editors. 2016, Elsevier. [Google Scholar]
- 36.Qutachi O, Vetsch JR, Gill D, Cox H, Scurr DJ, Hofmann S, Muller R, Quirk RA, ShakeSheff KM, and Rahman CV, Injectable and porous PLGA microspheres that form highly porous scaffolds at body temperature. Acta Biomaterialia, 2014. 10(12): p. 5090–5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devi N and Kakati DK, Smart porous microparticles based on gelatin/sodium alginate polyelectrolyte complex. Journal of Food Engineering, 2013. 117(2): p. 193–204. [Google Scholar]
- 38.Park TG, Bioconjugation of biodegradable poly(lactic/glycolic acd) to protein, petide, and anti-cancer drug: an alternative pathay for achieving controlled release from micro- and nanoparticles, in Polymeric Drugs and Drug Delivery Systems, Ottenbrite RM and Kim SW, Editors. 2001, CRC Press. [Google Scholar]
- 39.Mao S, Xu J, Cai C, Germershaus O, Schaper A, and Kissel T, Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm, 2007. 334(1–2): p. 137–48. [DOI] [PubMed] [Google Scholar]
- 40.Yeo Y and Park K, Control of encapsulation efficiency and initial burst in polymeric microparticle systems. Arch Pharm Res, 2004. 27(1): p. 1–12. [DOI] [PubMed] [Google Scholar]
- 41.Brunner A, Mader K, and Gopferich A, pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm Res, 1999. 16(6): p. 847–53. [DOI] [PubMed] [Google Scholar]
- 42.Li L and Schwendeman SP, Mapping neutral microclimate pH in PLGA microspheres. J Control Release, 2005. 101(1–3): p. 163–73. [DOI] [PubMed] [Google Scholar]
- 43.Duncan G, Jess TJ, Mohamed F, Price NC, Kelly SM, and van der Walle CF, The influence of protein solubilisation, conformation and size on the burst release from poly(lactide-co-glycolide) microspheres. Journal of Controlled Release, 2005. 110(1): p. 34–48. [DOI] [PubMed] [Google Scholar]
- 44.Klose D, Siepmann F, Willart JF, Descamps M, and Siepmann J, Drug release from PLGA-based microparticles: Effects of the “microparticle:bulk fluid” ratio. International Journal of Pharmaceutics, 2010. 383(1–2): p. 123–131. [DOI] [PubMed] [Google Scholar]
- 45.Dunne M, Corrigan I, and Ramtoola Z, Influence of particle size and dissolution conditions on the degradation properties of polylactide-co-glycolide particles. Biomaterials, 2000. 21(16): p. 1659–68. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q, Wang J, Lu Q, Detamore MS, and Berkland C, Injectable PLGA based colloidal gels for zero-order dexamethasone release in cranial defects. Biomaterials, 2010. 31(18): p. 4980–6. [DOI] [PMC free article] [PubMed] [Google Scholar]