Abstract
The aim of this study was to conduct a prospective analysis of the association between negative Life events (NLEs) and respiratory infections in children genetically at risk for islet autoimmunity (IA) and type 1 diabetes (T1D). Long- and short-term temporal associations between NLEs and rate of respiratory infectious episodes (RIEs) in 5618 children in the TEDDY study for at least 1 up to 4 years were analyzed. All models were adjusted for demographic, day care, season of infection and psychosocial factors associated with the rate of child RIEs between study visits. The rate of child RIEs was 26% higher in Europe (Sweden, Finland, Germany) than in the US (RR=1.26, p <0.001). However, the percentage of child NLEs (OR=1.18, p<0.001) and mother NLEs (OR=1.83, p<0.001) was higher in the US compared to Europe. In both continents (Europe, RR=1.12, p<0.001; US, RR=1.07, p=0.006), high child Cumulative NLEs (>1 NLE per year since study inception) was significantly associated with an increased rate of child RIEs. This large-scale prospective study confirms observations that stress may increase the susceptibility for infections in pediatric populations and suggests at least one mechanism by which stress could increase risk for IA and T1D in genetically at-risk children.
Keywords: stress, negative life events, respiratory childhood infections, longitudinal study, autoimmunity, type 1 diabetes
1. Introduction
The incidence of Type 1 diabetes (T1D) among children has increased globally during recent decades with an annual rate of increase of 3.9% reported in Europe (Patterson, Dahlquist, Gyürüs, Green, Soltesz, & EURODIAB Study, 2009) and 5.3% in North America (The Diamond Study Group, 2006). The rate of increase is of particular concern among children younger than 5 years of age, in whom the number of affected individuals is expected to double by 2020 (Patterson et al., 2009).
T1D is an autoimmune disorder in genetically predisposed individuals (Castano, & Eisenbarth, 1990) and is characterized by destruction of the insulin-producing cells of the pancreas. The disease onset is preceded by a pre-clinical period of islet autoimmunity (IA) (Atkinson, & Eisenbarth, 2001; Ziegler, & Nepom, 2010). Both IA and T1D onset have been associated with respiratory infections and certain specific viruses, such as enteroviruses. Evidence from epidemiological studies, biomedical studies detecting enteroviruses from the blood, pancreas, and gut of T1D patients, and animal studies suggest that enterovirus infections may accelerate the pathogenesis of T1D in susceptible individuals (Peng, & Hagopian, 2006; Christen, & von Herrath, 2005; Christen, Bender, & von Herrath, 2012; Salvatoni, Baj, Bianchi, Federico, Colombo, & Toniolo, 2013; Yeung, Rawlinson, & Craig, 2011; Beyan, Wen, & Leslie, 2012; Laitinen, et al., 2014; Hober, Sane, Jaidane,Riedweg, Goffard, & Desailloud, 2012; Davies-Richardson, & Triplett, 2015; Dotta, & Sebastian, 2014; Beyerlein, Wehweck, Ziegler, & Pflüger, 2013; de Beek, & Eizirik, 2016). The molecular mimicry theory suggests that epitope mimicry between a virus and human proteins can activate autoimmune diseases like T1D (de Oliveira Anrade, Vinhaas Bittencourt, da Silva Almeida, Sodre Bispo Junior, Silva Fonseca, & Santana de Melo, 2016). Respiratory infections were reported to increase the risk for T1D autoimmunity in the BABYDIET [Beyerlein et al., 2013; de Beek, & Eizirik, 2016), MIDIA (Rasmussen, Witso, Tapia, Stene, & Ronnigen, 2011), ABIS (Wahlberg, Vaarala, & Ludvigsson, 2011), and TEDDY (Lönnrot et al., 2017) studies.
Stress is considered a potential trigger of IA and T1D. A review of 11 studies concluded that there was strong evidence for a link between psychological stress or stressful negative life events and increased risk of IA and T1D (Sepa, & Ludvigsson, 2006). Stressful experiences may affect physical and psychological health as well as immune function (Wyman et al., 2007; Faresjo, 2015; Nygren, Carstensen, Koch, Ludvigsson, & Frostell, 2015). While stress could directly affect autoimmunity contributing to IA and T1D (Littorin, Sundkvist, & Nystrom, 2001; Sepa, Frodi, & Ludvigsson, 2005; Lundgren, Ellström, Larsson, for the DiPiS study group, 2018), stress could also lower resistance to infections, leading to greater susceptibility to illness which, in turn, may increase the risk of IA and T1D (Steptoe, Hamer, & Chida, 2007; Segerstrom, & Miller, 2004; Guo, Du, & Wang, 2015; Oh, Jerman, Marques, Koita, Boparai, Harris, & Bucci, 2018). Support for this hypothesis comes from experimental studies linking stressful life events and respiratory infections in healthy young adults in The Pittsburgh Common Cold Study (Cohen, & Williamson, 1991; Cohen, Tyrrell, & Smith, 1993; Cohen, Marick, Rodriguez, Feldman, Rabin, & Manuck, 2002; Cohen, 2005; Cohen, Janicki-Deverts, & Miller, 2007) as well as observational longitudinal studies in children (Turner-Cobb, & Steptoe, 1998; Oh et al., 2018). To date the majority of scientific studies on childhood adversity and biological health outcomes have focused on adults. Furthermore, most pediatric studies have been cross-sectional in nature. A recent systematic review (Oh et al., 2018) examined the link between childhood adversity and pediatric health outcomes – infections and illnesses - in longitudinal studies (Caserta et al., 2008; Wyman et al., 2007; Flaherty et al., 2009; Flaherty et al.,2013) and asthma (Kozyrsky, Mai, McGrath, Hayglass, Becker, & Macnell, 2008; Lange, Bunyavanich, Silberg, Canino, Rosner, & Celedon, 2011; Lanier, Janson-Reid, Stahlschmidt, Drake, & Constantino, 2010, Wolf, Miller, & Chen, 2008). However, no prior longitudinal study has examined the possible link between NLEs and respiratory infections in children at risk for IA and T1D.
Significant life events are viewed as a stressor which requires adaptation; they may be positive or negative (Holmes, & Rahe, 1967; Luhmann, Eid, Hofmann, & Lucas, 2011). In the framework of IA and T1D only adverse or negative life events were considered in this study. The role of stressful life events has been investigated retrospectively, from one week to one year or longer after diagnosis of T1D (Sepa, Frodi, & Ludvigsson, 2005; Sepa, & Ludvigsson, 2006; Nygren, Carstensen, Koch, Ludvigsson, & Frostell, 2015; Littorin et al., 2001; Lundgren et al., 2018) or the onset of infections and illnesses (Turner-Cobb, & Steptoe, 1998; Cohen et al., 2002; Cohen, 2005; Cohen et al., 2007; Cosgrove, 2004; Faresjo, 2015; Segerström, & Miller, 2004; Steptoe, Hamer, & Chida, 2007; Wyman et al., 2007). However, retrospective reports of this type raise serious concerns of reporting bias. The Environmental Determinants of Diabetes in the Young (TEDDY) study is a longitudinal observational study designed to identify environmental triggers of IA and T1D in children genetically at risk for TID; all TEDDY children entered the study before 3.5 months of age. Information about life events and infections are collected every 3 months during the first four years of the child’s life and twice a year thereafter, permitting an unbiased prospective assessment of any possible link between infections, stress and the development of IA and T1D. In a prospective analysis, TEDDY investigators have documented a link between recent respiratory infections and an increased risk of IA in the first four years of a TEDDY child’s life (Lönnrot et al., 2017).
The goal of this paper was to conduct a prospective analysis of the association between stressful NLEs and the development of respiratory infections during the same interval – the first four years of a TEDDY child’s life. Any link between NLEs and respiratory infections confirmed in a prospective analysis would provide one mechanism by which life stress could increase a child’s risk of IA and T1D (Lönnrot et al., 2017).
2. METHOD
2.1. The TEDDY Study
TEDDY is a prospective multinational (Finland, Sweden, Germany, USA) cohort study investigating the environmental determinants of T1D. Both infants from the general population with no family history of T1D and infants with a first degree T1D relative were screened at birth for genetic predisposition for T1D by analyzing their human leukocyte antigen (HLA-DR and DQ genotypes). Parents of infants who met the study’s genetic eligibility requirements were invited to take part in TEDDY; all families had to join the study before the infant was 3.5 months of age. Characteristics of families who were enrolled or refused to be enrolled are described elsewhere (Lernmark et al., 2011; Baxter et al., 2012). Between September 2004 and March 2010, 8676 children were enrolled and are being followed for environmental exposures potentially associated with IA and T1D (The TEDDY Study Group, 2007; The TEDDY Study Group, 2008).
The TEDDY protocol is demanding with diet records, stool samples, toenail clippings, parental questionnaires and study visits for blood draws, nasal swabs, height and weight measurements and parental interviews scheduled every 3 months during the first four years of the child’s life. Parents keep detailed records of the child’s illnesses, life stresses, and other environmental exposures in the TEDDY book, a diary given to the parents to keep at home and return to study nurses at each TEDDY clinic visit. Demographic variables as well as maternal lifestyle behaviors and measures of the mother’s understanding of and anxiety about the child’s T1D risk are collected as part of the TEDDY study; these factors were taken into consideration when examining the relationship between stressful negative life events and respiratory illnesses.
In all countries, the TEDDY study was approved by the respective Institutional Review Board or Research Ethics Committee, and a written consent from all participating families.
2.2. Study Population
A study population of 6985 TEDDY children followed a minimum of 12 months and a maximum of 48 months who had not develop IA or T1D was identified. Children who missed two or more consecutive visits were excluded (N = 1367) in order to limit the possibility of recall bias and to avoid low count of RIEs due to long recall intervals between study visits, leaving a final study sample of 5618 TEDDY children.
2.3. Measures of Socio-Demographic Variables, Maternal Lifestyle Behaviors, Maternal Risk Perception, Maternal Anxiety, Day Care, and Season
Since all child illness records were provided by the child’s mother, we considered it important to statistically adjust for factors that might be associated with mother-reported illnesses in the child.
Socio-demographic measures.
These included TEDDY site (Finland, Germany, Sweden, Colorado, Seattle, Georgia/Florida); child’s gender (male, female); child’s ethnic minority status (US: the TEDDY child’s mother’s first language is not English or the mother was not born in the US or the child is a member of an ethnic minority group – yes/no; Europe: the child’s mother’s first language or country of birth is other than that of the TEDDY country in which the child resides – yes/no); child has a first degree relative (FDR) with T1D (yes/no); child is an only child (yes/no); maternal age at child’s birth; mother’s education (1 – basic primary education includes primary school through some trade school, 2 – graduated trade school or some college/university, 3 – higher education includes graduated university/college or higher); parent’s marital status (married or living together vs. single parent); and crowding (number of rooms in the household).
Maternal lifestyle behaviors.
Since smoking in the home can be associated with increased respiratory illness in children (Gergen, Fowler, Maurer, Davis, & Overpeck, 1998; Jarvie & Malone, 2008), we assessed maternal smoking at 9 months and annually thereafter (yes/no). At the same time, we also collected information about whether the mother was working outside the home (yes/no) since that could influence child daycare placement, the amount of time mothers had to observe their children and record illness episodes in the TEDDY book, as well as how attentive mothers might be to potential illness symptoms in the child.
Maternal risk perception.
As part of the TEDDY informed consent process, all parents are informed of the child’s increased risk for T1D which is communicated both orally and in writing (Swartling, Lynch, Smith, Johnson, and the TEDDY Study Group, 2016). Parent risk perception accuracy is assessed by the following item at the 3, 6, 15 months and annually thereafter: “Compared to other children, do you think your child’s risk for developing diabetes is (mark only one answer) - much lower, somewhat lower, about the same, somewhat higher, much higher.” Mothers answering “much lower,” “somewhat lower,” or “about the same” were classified as inaccurate since all TEDDY children are at increased risk for T1D. Mothers answering “somewhat higher” or “much higher” were classified as accurate.
Maternal anxiety about the child’s T1D risk.
Maternal anxiety was measured by a six-item scale (State Anxiety Inventory - SAI) adapted from the 20-item state component of the State Trait Anxiety Inventory (Spielberger, Edwards, Montuori & Lushene, 1970). Mothers were asked to respond to each item while thinking specifically about their child’s risk for T1D; their 6-item score was then converted to a score comparable to that obtained from the full 20 item scale. The scale is highly reliable in the TEDDY population (Coefficient α= .90) (Johnson et al., 2016) and has been used in numerous studies of children or adults at high risk for T1D (Hummel, Ziegler & Roth, 2004; Johnson, Baughcum, Carmichael, She, & Schatz, 2004; Johnson, 2011; Johnson, Lynch, Roth, Schatz, and the TEDDY Study Group, 2017).
Day care.
Date of the child’s entry into a social group and child care is carefully recorded in TEDDY and was considered in the analysis as acute upper respiratory and gastrointestinal infections are common in children who attend child care settings (Roberts, Smith, Jorm, patel, Douglas, & McGilchrist, 2000) and school (Sandora, Shih, & Goldman, 2008); children cared for in the home have fewer bacterial and viral infections (Louhiala, Jaakkola, Ruosalainen, & Jaakkola, 1995).
Season.
Infections are known to increase during the fall and winter months (Winther, Hayden, & Hendley, 2006; Lee et al., 2012; Carlsson, Vissing, Sevelsted, Johnston, Bonnelykke, & Bisgaard, 2015), a finding replicated in TEDDY (Lönnrot et al., 2015). Consequently, season at the time illness events were recorded was included in the analysis.
2.4. Infections
Mothers are asked to record all symptoms of illness in the child in a diary called the TEDDY book which is brought in and reviewed at each TEDDY visit. The mother is encouraged to report all illnesses including the common cold, stomach upset, headaches, asthma, allergies, pinworms etc. A trained study nurse then translates all reports of child illness into the World Health Organization’s International Classification of Disease (ICD-10). For analysis purposes, the ICD-10 codes were further organized into four categories: (1) respiratory infections, (2) gastrointestinal infections, (3) other infections, and (4) unknown febrile infections (Lönnrot et al., 2015). An infection episode approach was used in order to reduce the possibility of overestimation of infections. ICD-10 codes within the respiratory category are merged into one single episode if they are reported within seven days. To be counted as a separate infection episode, a minimum of six days without symptoms must occur (Lönnrot et al., 2015). Only episodes of respiratory infections (common cold, laryngitis and tracheitis, influenza, enterovirus, respiratory syncytial virus infection, tonsillitis or streptococcal pharyngitis, sinusitis, infection of the middle ear and mastoid process, bronchitis and lower respiratory infections, conjunctivitis, other bacterial diseases of the respiratory tract not elsewhere classified, with any of the above fever, gastroenteritis symptoms) were considered in the current study (Lönnrot et al., 2015).
2.5. Stressful Negative Life Events
At each TEDDY visit, mothers are given a list of life events that might have happened in the three month period since their last TEDDY visit (serious illness/injury, hospitalization, family member/close friend died, separation/divorce, marriage, victim of violence, quit/lost job, started new job, serious conflicts, legal conflicts, financial difficulties, moved, and changed family composition) and a list of life events that might have happened to their child (illness/injury, hospitalization, family member/friend/pet died, separation from parent, moved, new sibling, started daycare, changed daycare, and new step-parent). If the mother indicated she or her child had experienced any of the events, she was asked to rate the event’s impact as very bad, bad, good, or no impact. Mothers are also asked to describe any other life events not on the lists and to rate their impact. Only adverse events with very bad or bad impact were considered. The total number of negative life events in each 3-month study window was recorded for mother and child separately.
2.6. Statistical Analyses
To limit the possibility of recall bias and to avoid low count of RIEs due to long intervals between visits, families missing two or more consecutive study visits at any time during the first four years of life were excluded (n = 1367). In order to identify possible differences between participants included and excluded from our subsequent analyses, we compared the two groups using multiple regression.
The number of Respiratory Infectious Episodes (RIEs) and Negative Life Events (NLEs) experienced by the child and the number of NLEs experienced by the mother since the last study visit was recorded at each 3-month study visit. For the purposes of this study, the number of RIEs was presented as a rate per person year. The resulting history of RIE rate and NLEs in the final study sample consisted of a 3-month time series from enrollment up to 48 months.
NLEs experienced by the child and by the mother were separately examined in relation to the RIE rate calculated at each study visit. We eliminated all child illness related NLEs from the analysis to avoid any potentially spurious association between a child’s infection episode and the report of a child NLE. At each study time point, NLE rates were bimodally distributed with most participants having no NLEs. Consequently, we treated NLEs as a categorical variable in the analysis (no NLE, ≥1 NLEs).
Given the longitudinal nature and non-normal distribution of the count data, Generalized Estimating Equation (GEE) methodology with log link was used to linearly regress NLEs on the number of RIEs. The time period (log years) between visits was included as an offset in the model allowing for RIE rates to be calculated. All GEE models assumed exchangeable correlation structure. The empirical based estimates were compared to the model-based estimates to ensure the working correlation was reasonable. Rate of RIEs among participants with a NLE relative to participants without a NLE were presented as rate ratios (95% CI) and were tested for significance using Wald tests.
GEE with logit link was also used to test differences in the percentages (odds) of child NLEs or mother NLEs between the United States (US) and European continents. Results were presented as odds ratios (95% CI).
Since there were a number of differences on study variables between the US and European sites, all analyses on RIE rate adjusted for the variables study site, child age, child gender, child ethnic minority, child FDR status, only child, child started daycare, parents married or living together, maternal age at child’s birth, maternal education, maternal perception of child’s T1D risk, maternal anxiety about child’s T1D risk, household size, mother smoked, mother worked outside home and season. Because maternal life style, risk perception, and anxiety are time dependent variables, the value immediately preceding the 3-month between visit window in which an RIE rate was calculated was considered in the analyses. Season is also time dependent. Consequently, season at the time of the RIE recording was used in the analysis.
Four measures of NLE’s were created: (1) Current NLE (yes/no) defined as a NLE occurring in the same ~3 month between visit window for which RIEs were recorded; (2) Previous Year NLE defined as a NLE occurring in the 12 month period prior to, but not including, the 3 month RIE recording window (no NLE; ≥ 1 NLEs); (3) Cumulative NLEs defined as the number of NLEs since study inception up to, but not including, the RIE recording window calculated as a rate per year (< 1 NLE, ≥ 1 NLEs); and (4) for children > 27 months of age, Early Life NLE defined as a NLE occurring in the first 12 months of TEDDY (no NLE, ≥ 1 NLEs). This allowed examination of long- and short-term temporal effects of NLEs on RIE rate in addition to the effect of any NLE during the period RIEs were recorded. Since the correlation of NLEs between consecutive visits was low, Current NLE, Previous Year NLEs, and Early Life NLEs were included in the same model. We subsequently tested for the effect of Cumulative NLEs with Current NLE of the parent and child in the same model. All GEE models were adjusted for demographic, day care, season of infection, and psychosocial factors associated with RIE rates. Statistical analysis was performed using SAS 9.4. P-values less than 0.05 were considered statistically significant unless otherwise stated.
3. RESULTS
3.1. Comparison of Excluded and Included Subjects
Table 1 depicts the results of the multiple regression identifying significant differences between those excluded and included in the analysis. The 1367 subjects excluded from the study because they missed two or more consecutive study visits were more likely to come from the US and Germany (p<0.001). Compared to the study sample, the excluded sample was characterized by lower maternal education (p<0.001), child coming from an ethnic minority (p=0.008), maternal smoking in the first year of the child’s life (p<0.001), multiple children in the family (p<0.001) and younger maternal age (p<0.001). Child NLEs during the first year of the child’s life had no association with exclusion (p=0.31). However, children who were excluded had fewer RIE’s in the first year of life (p<0.001) and their mothers reported more maternal NLEs (p<0.001).
Table 1.
Factors Associated with Exclusion due to Missing ≥2 Consecutive Study Visits
| Factors | Excluded for having more than two consecutive visits missed |
Multivariate regression of factors associated with exclusion |
||
|---|---|---|---|---|
| Variables | No N (% row) |
Yes N (% row) |
OR (95%CI) | p-value |
| Study Site | ||||
| Colorado | 909 (82.3%) | 196 (17.7%) | 1.64 (1.29 – 2.09) | |
| Georgia/Florida | 526 (75.0%) | 175 (25.0%) | 3.16 (2.47 – 4.05) | |
| Washington | 741 (72.1%) | 287 (27.9%) | 3.37 (2.69 – 4.23) | |
| Finland | 1225 (78.7%) | 332 (21.3%) | 2.56 (2.07 – 3.17) | |
| Germany | 305 (65.7%) | 159 (34.3%) | 5.24 (4.01 – 6.84) | <0.001 |
| Sweden | 1912 (89.8%) | 218 (10.2%) | 1 (reference) | |
| Maternal Education | ||||
| Primary School | 1341 (82.3%) | 288 (17.7%) | 1.26 (1.03 – 1.54) | |
| Secondary/Trade school | 1332 (79.0%) | 355 (21.0%) | 1.23 (1.05 – 1.45) | 0.001 |
| College | 2724 (84.7%) | 493 (15.3%) | 1 (reference) | |
| Child Ethnic Minority | ||||
| No | 4727 (83.5%) | 936 (16.5%) | 1 (reference) | |
| Yes | 750 (74.5%) | 257 (25.5%) | 1.23 (1.02 – 1.48) | 0.008 |
| Smoked | ||||
| No | 5108 (83.4%) | 1020 (16.6%) | 1 (reference) | |
| Yes | 476 (73.5%) | 172 (26.5%) | 1.42 (1.15 – 1.74) | <0.001 |
| Only child | ||||
| No | 3187 (81.0%) | 749 (19.0%) | 1 (reference) | |
| yes | 2388 (84.4%) | 443 (15.6%) | 0.68 (0.59 – 0.79) | <0.001 |
| Maternal Age | ||||
| /years | Mean (SD) 30.99 (5.0) |
Mean (SD) 29.6 (5.4) |
0.96 (0.94 – 0.97) | <0.001 |
| Mother NLEs by 12 Months | ||||
| No | 3448 (81.6%) | 777 (18.4%) | 1 (reference) | |
| Yes | 2170 (78.6%) | 590 (21.4%) | 1.34 (1.17 – 1.54) | <0.001 |
| Child NLEs by 12 Months | ||||
| No | 4811 (80.7%) | 1150 (19.3%) | 1 (reference) | |
| Yes | 807 (78.8%) | 217 (21.2%) | 1.10 (092 – 1.31) | 0.31 |
| RIEs by 12 Months | ||||
| /count | Mean (SD) 3.4 (2.1) |
Mean (SD) 2.7 (2.1) |
0.90 (0.87 – 0.93) |
<0.001 |
3.2. Types of Mother and Child NLEs
Tables 2A and 2B display the nature and types of NLEs most commonly reported by mothers for themselves (2A) and for their children (2B). The correlation between the number of mother and child NLEs reported at each study visit for the preceding 3-month period was low, ranging from 0.14 to 0.18. The correlation between mother and child cumulative NLEs was somewhat higher, ranging from 0.20 to 0.30. These very modest associations suggested that mother and child NLEs should be considered separately.
Table 2 A.
Mothers Negative Life Events (NLEs)
| Life category | Negative Life events experienced by the mothers with negative impact |
Count during follow-up | |
|---|---|---|---|
| Description | Total count a N |
Cumulative rate a /100 person years |
|
| Significant Loss | Family member or close friend died, pet died, pet removed from home, other significant loss | 2887 | 14.7 |
| Separation | Mother separated from their spouse or significant other or got a divorce, | 389 | 2.0 |
| Violence | Mother or her family member experienced violence | 230 | 1.2 |
| Job Related | Mother or her spouse/significant other quit or lost your job, or returned to work, or started school or new job, or had job stresses | 3337 | 17.0 |
| Financial Difficulties | Mother or her spouse/significant other had financial difficulties or money problems | 2820 | 14.4 |
| Serious Conflict | Mother got married (negative experience), or mother had serious arguments with spouse, other relatives or friends. | 2509 | 12.8 |
| Legal Conflict | Mother or family member, close friend or relative got in trouble with police/law | 45 | 0.2 |
| Change | Mother moved, household member moved into or out of home, changes in family composition, close friend or relative moved away | 823 | 4.2 |
| Other | 2607 | 13.3 | |
Total person years of follow = 19593 person years by 5618 children.
Table 2 B.
Children Negative Life Events (NLEs)
| Life category | Negative Life Events experienced by the child with negative impact |
Count during follow-up | |
|---|---|---|---|
| Description | Total counta
N |
Cumulative rate a /100 person years |
|
| Significant Loss | Child’s close friend or relative died, pet died, pet removed from the home or other significant loss | 29 | 0.2 |
| Separation | Child was separate from mother or father | 2601 | 13.3a |
| Violence | Experience violence (include physical or sexual abuse) or witness violence | 30 | 0.2 |
| Legal Conflict | Child got in trouble with police | 0 | 0 |
| Change | Child moved, got a new brother or sister, child got a new step-parent, household member moved into or out of home, change in family composition, close friend or relative moved away | 617 | 3.2 |
| Child Care | Child started daycare or school, child changed schools or daycare, new babysitter or childcare worker, regular babysitter left, other changes in childcare not specified | 1628 | 8.3 |
| School | Failed an important exam, not promoted to the next grade, significant damage to child’s school, suspended from school | 6 | 0.0 |
| Other | 1120 | 5.7 | |
Total person years of follow = 19593 person years by 5618 mothers.
3.3. US and European Differences in NLEs and RIE rate
The age specific RIE rate among children as well as the age specific percentage of children and their mothers having a NLE since the last visit are shown in Figure 1 for Europe and the US. As we have previously noted (Lönnrot et al., 2015), the reported RIE rate among children was higher in Europe than the US (1.26, 95% CI = 1.23 – 1.30, p<0.001). In contrast, the percentage (odds) of mothers reporting a NLE (OR = 1.83, 95% CI = 1.71 – 1.96, p<0.0001) or their child having a NLE (OR = 1.18, 95% CI = 1.09 – 1.28, p<0.0001) were both lower in Europe compared to the US although the difference in child NLE between continents was less pronounced (see Figure 1).
Figure 1:
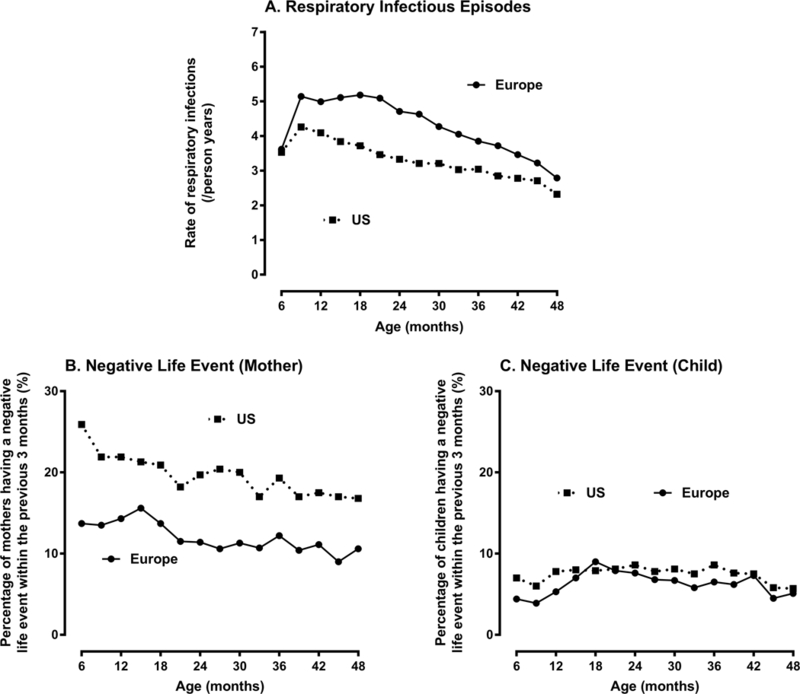
Rate of Respiratory Infection Episodes (RIEs) and Negative Life Events (NLEs) by Age of Child and Continent of Residence
3.4. Factors Associated with the Number of Mother-Reported Child RIEs
Table 3 shows the factors associated with the number of mother-reported child RIEs. In addition to large site differences - with higher RIE rates reported among children living in Europe than in the US - season (p<0.001) was significantly associated with the RIE rate among children, with higher rates reported in the fall/winter months. Male gender (p<0.001), placement in daycare (p<0.001), parents married or living together (p=0.03), higher maternal education (p<0.001) and accurate maternal perception of the child’s T1D risk (p<0.001), were all associated with an increase in mother-reported RIE rate among the children. In contrast, older child age (p<0.001), female gender (p<0.001), ethnic minority status (p<0.001), only child status (p<0.001), mother working outside the home (p=0.02), and maternal smoking (p<0.001) were all associated with a lower rate of mother-reported RIE in their children (see Table 1). FDR status, maternal age and anxiety about T1D risk, and crowding were not associated with RIE rate (p values >0.10).
Table 3.
Factors Associated with the Rate of Respiratory Infectious Episodes (RIE)
| Factors Associated with Rate of Respiratory Infectious Episodes |
Sample N (%) or Mean (SD) at 1 year of age |
Association with RIE rate | ||||
|---|---|---|---|---|---|---|
| RIE Rate Ratio+ |
95% CI | p-value | ||||
| Socio-demographic Measures | ||||||
| Study Site | Colorado | 909 (16.2) | 1.00 | reference | ||
| Georgia/Florida | 526 (9.4) | 1.14 | 1.08 | 1.20 | <0.001 | |
| Washington | 741 (13.2) | 1.04 | 0.99 | 1.09 | 0.12 | |
| Finland | 1225 (21.8) | 1.43 | 1.37 | 1.50 | <0.001 | |
| Germany | 305 (5.4) | 1.21 | 1.13 | 1.30 | <0.001 | |
| Sweden | 1912 (34.0) | 1.27 | 1.22 | 1.32 | <0.001 | |
| Child Age | Years | 1 | 0.86 | 0.85 | 0.87 | <0.001 |
| Child Gender | Boy | 2844 (50.6) | 1.00 | reference | ||
| Girl | 2774 (49.4) | 0.95 | 0.93 | 0.97 | <0.001 | |
| Child Ethnic Minority | No | 4727 (86.3) | 1.00 | reference | ||
| Yes | 750 (13.7) | 0.89 | 0.86 | 0.93 | <0.001 | |
| Only Child | No | 3187 (57.2) | 1.00 | reference | ||
| Yes | 2388 (42.8) | 0.96 | 0.94 | 0.98 | <0.001 | |
| Parents married or living together | No | 210 (3.8) | 1.00 | reference | ||
| Yes | 5367 (96.2) | 1.07 | 1.01 | 1.13 | 0.03 | |
| Maternal Education | Primary school | 1035 (18.5) | 1.00 | reference | ||
| Secondary/trade school | 1303 (23.3) | 1.05 | 1.01 | 1.10 | 0.01 | |
| College | 3249 (58.2) | 1.14 | 1.10 | 1.18 | <0.001 | |
| Started Day Care | No | 1150 (20.5) | 1.00 | reference | ||
| Yes | 4468 (79.5) | 1.26 | 1.23 | 1.31 | <0.001 | |
| Season | Spring/Summer | 2852 (50.8) | 1.00 | reference | ||
| Fall/Winter | 2766 (49.2) | 1.30 | 1.28 | 1.31 | <0.001 | |
| Maternal Lifestyle Behaviors | ||||||
| Smoked | No | 5108 (91.5) | 1.00 | reference | ||
| Yes | 476 (8.5) | 0.91 | 0.87 | 0.95 | <0.001 | |
| Worked Outside Home | No | 3596 (64.7) | 1.00 | reference | ||
| Yes | 1963 (35.3) | 0.97 | 0.95 | 1.00 | 0.02 | |
| Maternal Perception of Child’s T1D Risk | ||||||
| Accurate | No | 2102 (37.5) | 1.00 | reference | ||
| Yes | 3500 (62.5) | 1.06 | 1.04 | 1.09 | <0.001 | |
First degree relative (FDR) status, mothers age at child’s birth, maternal anxiety about child’s T1D risk, and household size were not associated with rate of mother reported child RIEs (p values >0.10). Note:
Rate Ratio (RR) = RIE rate relative to RIE rate in reference group; RR >1 indicates higher rate relative to reference group, RR <1 lower rate.
3.5. The Association Between NLE and Child RIEs
All GEE models examining the association between mother and child NLEs and child RIEs adjusted for all factors in Table 3. Table 4 depicts the association between Current, Previous Year and Early Life (in those ≥ 27 months) NLEs and child RIEs. The presence of a child Concurrent NLE (RIE rate ratio =1.07, p<0.001), but not a mother Concurrent NLE (RIE rate ratio =1.01, p=0.26), during the same period of recorded RIEs was significantly associated with an increase in the RIE rate among children compared to the absence of a Concurrent NLE. The associations were consistent in younger (≤27 months) and older (>27 months) children. In contrast, the presence of a mother or child Previous Year NLE were both associated with an increase in child RIE rate in both younger and older children. Child – but not mother – Early Life NLEs occurring in the child’s first year of life were also associated with an increase in the child’s rate of RIEs.
Table 4.
Rate of Respiratory Infectious Episodes (RIE) Between Visits (Average 3-Months) Reported by Those with ≥1 Negative Life Events (NLEs) Relative to Those with No NLE
| Age of Child in Months |
Recording Period of NLEs Relative to the 3-month Period of Recorded Child RIEs |
Mother Negative Life Events | Child Negative Life Events | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RIE Rate Ratio |
95% CI | p-value | RIE Rate Ratio |
95% CI | p-value | ||||
| 3 – 27 | Current NLEs+ | ||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.02 | 1.00 | 1.05 | 0.07 | 1.08 | 1.04 | 1.12 | <0.001 | |
| Previous Year NLEs# | |||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.03 | 1.01 | 1.06 | 0.01 | 1.12 | 1.09 | 1.15 | <0.001 | |
| 27 – 48 | Current NLEs+ | ||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLE | 1.01 | 0.98 | 1.05 | 0.57 | 1.06 | 1.01 | 1.11 | 0.01 | |
| Previous Year NLEs# | |||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.05 | 1.02 | 1.08 | 0.001 | 1.06 | 1.03 | 1.10 | <0.001 | |
| Early Life NLEs§ | |||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.04 | 1.00 | 1.07 | 0.08 | 1.09 | 1.04 | 1.14 | <0.001 | |
Note: NLEs adjusted for all factors in table 1.
Current NLEs: NLEs occurring in the same 3-month window for which RIEs were recorded;
Previous Year NLEs: NLEs occurring in the 12-month period prior to, but not including, the 3-month RIEs recording window (no NLE; ≥ 1 NLEs);
Early Life NLEs: for children > 27 months of age, NLEs occurring in the first 12 months of life
Because both mother and child Previous Year NLEs and child Early Life NLEs were all associated with the rate of the child’s RIE over and above the effect of Current NLEs, we next examined the independent association of mother and child Cumulative NLE on the RIE rate among children, controlling for presence of a mother or child Current NLE (see Table 5). Current child NLEs was significantly associated with RIE rate overall and in Europe but not in the US while mother Current NLEs was not associated with the RIE rate among children in either continent. Children with child Cumulative NLEs of 1 or more per year had a significantly higher rate of respiratory infections compared to children having less than 1 NLE per year (rate ratio = 1.10, 95% CI = 1.06 – 1.14, p <0.001). This difference was consistent across continent: US (rate ratio=1.07, 95% CI =1.01 – 1.14, p =0.026) and Europe (RIE rate ratio=1.12, 95% CI = 1.07 – 1.17, p <0.001). Mother Cumulative NLEs of 1 or more per year compared to less than 1 per year was also significantly associated with the RIE rate among children from either continent but its effect was small and not significant for Europe when the sample was divided by continent (see Table 5). Figure 2 illustrates the age and continent specific rate of RIEs for children with and without high rates of Cumulative NLEs (≥ 1 per year). The impact of high Cumulative NLEs on the RIE rate among children was markedly greater for Child Cumulative NLEs compared to Mother Cumulative NLEs (Figure 2A compared to 2B). When European children experienced a high rate (≥ 1 per year) of Cumulative NLEs beforehand, the age specific RIE rate was close to six per year until two years of age. After two years of age, these children had more than one additional respiratory infection per year over children who had low child Cumulative NLEs. In the US, the differences in RIE rates between those children with high and low Cumulative NLEs were substantial but somewhat lower compared to European children (Figure 2B).
Table 5.
Rate of Child Respiratory Infectious Episodes (RIEs) by Child and Mother Current Negative Life Events and Cumulative Negative Life Events (NLEs) for the Whole Study Cohort and for Europe and the United States Separately
| Overall | United States | Europe | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recording Period of Mother or Child NLEs Relative to the 3-month Period of Recorded Child RIEs |
RIE rate ratio |
95% CI | p- value |
RIE rate ratio | 95% CI | p- value |
RIE rate ratio | 95% CI | p- value |
|||
| Mother Current NLEs§ | ||||||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.01 | 0.99 | 1.03 | 0.33 | 1.02 | 0.98 | 1.05 | 0.36 | 1.01 | 0.98 | 1.04 | 0.56 |
| Mother Cumulative NLEs+ | ||||||||||||
| <1 NLE per year | 1.00 | ref | ref | 1.00 | ref | ref | 1.00 | ref | ref | |||
| ≥1 NLEs per year | 1.03 | 1.01 | 1.06 | 0.007 | 1.05 | 1.01 | 1.09 | 0.007 | 1.02 | 0.99 | 1.06 | 0.19 |
| Child Current NLEs§ | ||||||||||||
| No NLE | 1.00 | ref | ref | 1.00 | ref | ref | 1.00 | ref | ref | |||
| Yes, NLEs | 1.06 | 1.03 | 1.09 | <0.001 | 1.02 | 0.97 | 1.08 | 0.35 | 1.08 | 1.05 | 1.12 | <0.001 |
| Child Cumulative NLE+ | ||||||||||||
| <1 NLE per year | 1.00 | ref | Ref | 1.00 | ref | ref | 1.00 | ref | ref | |||
| ≥1 NLEs per year | 1.10 | 1.06 | 1.14 | <0.001 | 1.07 | 1.01 | 1.14 | 0.02 | 1.12 | 1.07 | 1.17 | <0.001 |
Note: Mother and child Current and Cumulative NLEs included in same multivariate GEE model and adjusted for all factors in table.1
Current NLEs: NLEs occurring in the same 3-month window for which RIEs were recorded;
Cumulative NLEs: number of NLEs since study inception up to, but not including, the RIE recording window calculated as a rate per year (< 1NLE, ≥ 1 NLEs).
Figure 2:
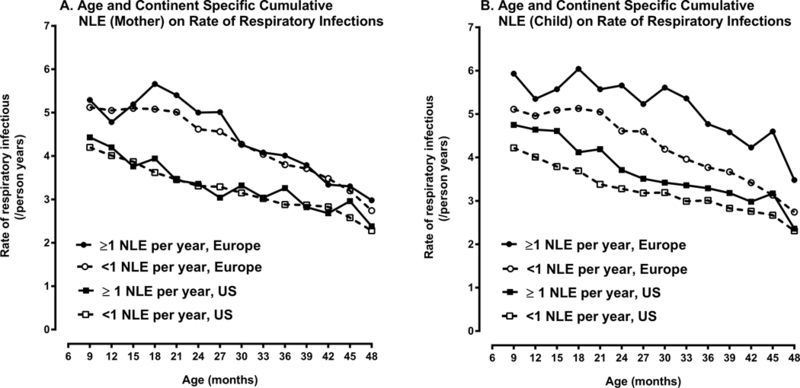
Rate of Respiratory Infection Episodes (RIEs) by Age of Child, Continent of Residence and Mother and Child Cumulative Negative Life Events (NLEs).
4. DISCUSSION
Respiratory infections are a leading cause of illness, outpatient physician visits and hospitalizations among children (Jensen-Fangel, Mohey, Johnsen, Andersen, Sorensen, & Ostergaard; Nair et al., 2013). In TEDDY, child RIEs were more commonly reported in Europe than in the US, a finding consistent with global comparisons conducted by the World Health Organization (2015). The current study also replicated the seasonal distribution of RIEs previously reported (Winther et al., 2006; Lee et al., 2012; Carlsson et al., 2015; Lönnrot et al., 2015) and the association between day care and an increased frequency of RIEs (Jackson, Mathews, Pulanic, Falconer, Rudan, Campell, & Nair, 2013; Roberts et al., 2000).
In the present study, a number of sociodemographic and maternal psychosocial lifestyle factors were associated with the frequency of mother-reported child RIEs. Boys were reported to have more RIEs than girls did, and children with siblings were reported to have more infections than only children, findings that are consistent with the prior literature (Jensen-Fangel et al., 2004; Nair et al., 2010; Jackson et al., 2013; Grüber et al., 2008; Simoes, 2003; Shi et al., 2015). We also found that more educated mothers and those with more accurate perceptions about their child’s T1D risk, reported more RIEs. In contrast, TEDDY moms who worked, smoked, and whose child was an ethnic minority reported fewer RIEs. Several previous studies have suggested that more educated mothers at higher socioeconomic status may be more compliant or attentive to requests to document RIEs in their child leading to higher reports (Grüber et al., 2008; Simoes, 2003; Shi et al., 2015; Bergmann et al., 2002; Zoch, Karch, Dreesman, Monazahian, Baillot, & Mikolajczyk, 2015). Previous studies have also documented that more concerned parents tend to report more RIEs in the child (Andre, Hedin, Hakansson, Molstad, Rodhe, & Petersson, 2007; Van der Gaag, & Van Droffelaar, 2012). Mothers who work may be more absent from home and have less time to observe and document illness symptoms of their children. Although some studies report parental smoking is associated with increased reports of RIEs in the child (Jackson et al., 2013), we did not find this be the case. Grüber et al. (2008) also found no association between parental smoking and child respiratory infections using a diary method to report respiratory infections. The authors suggest that tobacco smoke may contribute to the severity of respiratory infections, but might not enhance susceptibility to respiratory infections per se. Smokers may also be less health conscious and less likely to note mild respiratory symptoms in a child.
Because of the numerous factors associated with RIE reporting, all were adjusted for in the analysis of the association between NLEs and RIEs. A clear positive relationship between NLEs and RIEs was observed. This was particularly true when the NLE occurred in the same time frame as the RIE recording. However, Previous Year NLE, Early Life NLE and Cumulative NLEs all showed significant associations with RIEs in addition to and independent of the effect of Current NLE. Child NLEs appeared to be more strongly linked to child RIEs than mother NLEs despite the fact that child NLEs occurred less frequently. This pattern of results appeared to be consistent across continents.
The observed association between NLEs, particularly child NLEs, and child RIEs is important because it replicates prior cross-sectional studies linking stress to infectious episodes (Wyman et al., 2007; Cohen et al., 2002; Cohen, 2005; Turner-Cobb, & Steptoe, 1998; Caserta et al., 2008; Cohen, & Williamson, 1991; Drummond, & Hewson-Bower, 1997) using a longitudinal prospective design with a very large sample of young children. The fact that this association was found not only when NLEs and RIEs were recorded in the same time frame (Current NLE) but more so when NLEs was measured in time periods prior to the report of RIEs, adds further weight to the finding. Documenting this association in the TEDDY population supports the hypothesis that cumulative stress may influence the development of IA and T1D by increasing the child’s risk for respiratory infection which in turn increases the child’s risk of IA and T1D (Lönnrot et al., 2017).
Most previous studies examining the association between life stress and IA or T1D have not differentiated between parent and child life events (Sepa, & Ludvigsson, 2006, Sepa et al., 2005; Littorin et al., 2001). Our findings suggest that such a distinction may be important. We found that mother and child NLEs in this sample of very young children were not highly correlated and that child NLEs showed a stronger association to child RIEs than mother NLEs.
A study limitation is its dependence on self-report of both NLEs and RIEs. The usage of a diary and the regular collection of these data every three months about a narrow (3 month) time frame was designed to minimize recall bias. Mothers were also prompted by the study nurses with a list of possible life events and they were asked about illnesses. However, it is certainly possible that reporting bias existed, with some mothers having a tendency to report more events regardless of content. We attempted to adjust for these biases by eliminating all reports of child illness from our measures of NLEs and by controlling for numerous factors (study site, child’s age, gender, ethnic minority, siblings, daycare attendance, mother’s family status, education and lifestyle behaviors as smoking and working outside and maternal perception of child’s T1D risk) associated with RIE reporting in our statistical models. The fact that Prior Year, Early Life, and Cumulative NLEs occurring in time frames prior to the RIE recording period were all associated with an increase in RIE also suggests that the findings are not spurious products of self-report bias.
The TEDDY population is a highly motivated sample of educated mothers who have volunteered to participate in a longitudinal study with a demanding protocol. We do not get income data in TEDDY, but we do measure the socioeconomic background according to maternal education, marital status, crowding and ethnic minority status. All of these factors – except crowding which was not associated with maternal reports of child RIE – were included as control variables in the analysis. Further, those mothers included in the current study were particularly conscientious about coming to study visits where they regularly provided information on mother and child NLEs and child RIEs. Because we were concerned with reporting bias and underestimation of RIEs due to exceeding long periods between study visits, we excluded participants who missed two or more consecutive study visits. We have previously shown that NLEs is not associated with drop-out in TEDDY (Johnson et al, 2016). However, in this study we found study participants who were less compliant with study visits and therefore excluded from the analysis differed from those included in several important ways. These less compliant mothers were younger and less educated, were more likely to have an ethnic minority child and have multiple children in the family, and were more likely to report smoking in the first year of the child’s life. Excluded mothers did not differ from included mothers in their reports of child NLEs in the first year of the child’s life but they did report more mother NLEs and fewer child RIEs in that time frame. Because the excluded and included samples did not differ on child NLEs in the first year of the child’s life, we believe that our findings of an association between child NLE and child RIEs can be generalized to the full TEDDY cohort. However, the fact that mothers who report more mother NLEs in the first year of the child’s life were less compliant and therefore more likely to be excluded from the analysis, suggests that we may have underestimated any association between maternal NLEs and child RIEs.
Another limitation of our findings is that the investigation was carried out in a genetically at risk for T1D population. That limits the generalizability of the finding, which required replication in unselected cohorts.
5. CONCLUSION
Prior Year, Early Life, and Cumulative NLEs occurring in time frames prior to the RIE recording period were all associated with an increase in RIEs and showed a stronger association between child NLEs and RIEs than mothers NLEs and RIEs. Biomarkers of both NLE and RIE would provide an additional test of the association. Fortunately, some biomarkers of RIE are available in TEDDY which can be used to further elucidate this association in subsequent studies. However, the current study’s findings suggest one mechanism by which averse negative life stress may lead to the development of IA and T1D in children.
Acknowledgement
Funded by U01 DK63829, U01 DK63861, U01 DK63821, U01 DK63865, U01 DK63863, U01 DK63836, U01 DK63790, UC4 DK63829, UC4 DK63861, UC4 DK63821, UC4 DK63865, UC4 DK63863, UC4 DK63836, UC4 DK95300, UC4 DK100238, UC4 DK106955, and Contract No. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Child Health and Human Development (NICHD), National Institute of Environmental Health Sciences (NIEHS), Juvenile Diabetes Research Foundation (JDRF), and Centers for Disease Control and Prevention (CDC). This work supported in part by the NIH/NCATS Clinical and Translational Science Awards to the University of Florida (UL1 TR000064) and the University of Colorado (UL1 TR001082).
Footnotes
The authors have no conflicts of interest to disclose.
The datasets generated and analyzed during the current study will be made available in the NIDDK Central Repository at https://www.niddkrepository.org/studies/teddy
6. REFERENCES
- Andre M, Hedin K, Hakansson A, Molstad S, Rodhe N, & Petersson C (2007). More physician consultations and antibiotic prescriptions in families with high concern about infectious illness - adequate response to infection-prone child or self-fulfilling prophecy? The Journal of Family Practice, 24(4), 302–307. doi: 10.1093/fampra/cmm016. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, & Eisenbarth GS (2001). Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet, 358(9277), 221–229. 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- Baxter J, Vehik K, Johnson SB, Lermark B, Roth S Simell T, and the TEDDY Study Group (2012) Differences in recruitment and early retention among ethnic minority participants in a large pediatric cohort: the TEDDY Study. Contemporary Clinical Trials, 33(4), 633–640. doi: 10.1016/j.cct.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann RL, Diepgen TL, Kuss O, Bergmann KE, Kujat J Dudenhausen JW., … and the MAS‐study group (2002). Breastfeeding duration is a risk factor for atopic eczema. Clin Exp Allergy, 32(2), 205–209. 10.1046/j.1365-2222.2002.01274.x. [DOI] [PubMed] [Google Scholar]
- Beyan H, Wen L, & Leslie RD (2012). Guts, germs, and meals: the origin of type 1 diabetes. Current diabetes reports, 12(5), 456–462. 10.1007/s11892-012-0298-z. [DOI] [PubMed] [Google Scholar]
- Beyerlein A, Wehweck F, Ziegler AG, & Pflueger M (2013). Respiratory infections in early life and the development of islet autoimmunity in children at increased type 1 diabetes risk: evidence from the BABYDIET study. JAMA Pediatric, 167(9), 800–807. doi: 10.1001/jamapediatrics.2013.158. [DOI] [PubMed] [Google Scholar]
- Carlsson CJ, Vissing NH, Sevelsted A, Johnston SL, Bonnelykke K, & Bisgaard H (2015). Duration of wheezy episodes in early childhood is independent of the microbial trigger. Journal of Allergy Clinical Immunology, 136(5),1208–1214. 10.1016/j.jaci.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta MT, O’Connor TG, Wyman PA, Wang H, Moynihan J, Cross W, … Xia J (2008). The associations between psychosocial stress and the frequency of illness, and innate and adaptive immune function in children. Brain Behavior Immunology, 22(6), 933–940. doi: 10.1016/j.bbi.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castano L, & Eisenbarth GS (1990). Type-I diabetes: a chronic autoimmune disease human, mouse, and rat. Annual Review of Immunology, 8, 647–679. 10.1146/annurev.iy.08.040190.003243. [DOI] [PubMed] [Google Scholar]
- Christen U, & von Herrath MG. (2005). Infections and autoimmunity - good or bad? Journal of Immunology, 174(12), 7481–7486. doi: 10.4049/jimmunol.174.12.7481. [DOI] [PubMed] [Google Scholar]
- Christen U, Bender C, & von Herrath MG (2012). Infection as a cause of type 1 diabetes? Current Opinion in Rheumatology, 24(4), 417–423. doi: 10.1097/BOR.0b013e3283533719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S (2005). Keynote Presentation at the Eight International Congress of Behavioral Medicine: the Pittsburgh common cold studies: psychosocial predictors of susceptibility to respiratory infectious illness. International Journal of Behavior Medicine, 12(3), 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Williamson GM (1991). Stress and infectious disease in humans. Psychological Bulletin, 109(1), 5–24. 10.1037/0033-2909.109.1.5. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, & Miller GE (2007). Psychological stress and disease. JAMA, 298(14), 1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- Cohen S, Tyrrell DA, & Smith AP (1993). Negative life events, perceived stress, negative affect, and susceptibility to the common cold. Journal of Personality and Social Psychology, 64(1), 131–140. 10.1037/0022-3514.64.1.131. [DOI] [PubMed] [Google Scholar]
- Cohen S, Hamrick N, Rodriguez MS, Feldman PJ, Rabin BS, & Manuck SB (2002). Reactivity and vulnerability to stress-associated risk for upper respiratory illness. Psychosomatic Medicine, 64(2), 302–310. [DOI] [PubMed] [Google Scholar]
- Cosgrove M (2004). Do stressful life events cause type 1 diabetes? Occupational Medicine (Lond), 54(4), 250–254. 10.1093/occmed/kqh047. [DOI] [PubMed] [Google Scholar]
- Davis-Richardson AG, & Triplett EW (2015). A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia, 58(7), 1386–1393. DOI 10.1007/s00125-015-3614-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beeck AO, & Eizirik DL (2016). Viral infections in type 1 diabetes mellitus - why the beta cells? Nature Review Endocrinology, 12(5), 263–273. doi: 10.1038/nrendo.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Andrade LJ, Vinhaaes Bittencourt AM, da Silva Almeida R, Sodre Bispo Junior W, Silva Fonseca BK, & Santana de Melo PR (2016). Type 1 diabetes and viral infections: Similarities among human glutamic acid decarboxylase-65 (gad65), human insulin and H1N1 influenza a virus. Brazilian Journal of Medicine and Human Health, 4, 5–12. DOI: 2317-3386bjmhh.v4i1.754. [Google Scholar]
- Dotta F, & Sebastiani G (2014). Enteroviral infections and development of type 1 diabetes: The Brothers Karamazov within the CVBs. Diabetes, 63(2), 384–386. DOI: 10.2337/db13-1441. [DOI] [PubMed] [Google Scholar]
- Drummond PD, & Hewson-Bower B (1997). Increased psychosocial stress and decreased mucosal immunity in children with recurrent upper respiratory tract infections. Journal of Psychosomatic Research, 43(3), 271–278. 10.1016/S0022-3999(97)00002-0. [DOI] [PubMed] [Google Scholar]
- Faresjo M (2015). The Link between Psychological Stress and Autoimmune Response in Children. Critical Review of Immunology, 35(2), 117–134. DOI: 10.1615/CritRevImmunol.2015013255. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thomson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, … Everson MD. (2009). Adverse childhood exposures and reported child health at age 12. Academic Pediatric, 9(3), 150–156. 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Dubowitz H,Harvey EM, English DJ, Proctor LJ, & Runyan DK (2013). Adverse childhood experiences and child health in early adolescence. JAMA Pediatrics, 167(7), 622–629. 10.1001/jamapediatrics.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen PJ, Fowler JA, Maurer KR, Davis WW, & Overpeck MD (1998). The burden of environmental tobacco smoke exposure on the respiratory health of children 2 months through 5 years of age in the United States: Third National Health and Nutrition Examination Survey, 1988 to 1994. Pediatrics, 101(2), e8 DOI: 10.1542/peds.101.2.e8. [DOI] [PubMed] [Google Scholar]
- Grüber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V, the MAS‐90 Study Group (2008). History of respiratory infections in the first 12 yrs among children from a birth cohort. Pediatric Allergy and Immunology, 19(6), 505–512. 10.1111/j.1399-3038.2007.00688.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Du Y, & Wang J (2015). Network analysis reveals a stress affected common gene module among seven stress-related diseases/systems which provides potential targets for mechanism research. Science Report, 5, 129–39. doi: 10.1038/srep12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hober D, Sane F, Jaidane H, Riedweg K, Goffard A, & Desailloud R (2012). Immunology in the clinic review series; focus on type 1 diabetes and viruses: role of antibodies enhancing the infection with Coxsackievirus-B in the pathogenesis of type 1 diabetes. Clinical & Experimental Immunology, 168(1), 47–51. doi: 10.1111/j.1365-2249.2011.04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, & Rahe RH (1967). The Social Readjustment Rating Scale. Journal of Psychosomatic Research, 11, 213–218. Doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Hummel M, Ziegler AG & Roth R (2004). Psychological impact of childhood autoantibody testing in families participating in the BABYDIAB study. Diabetic Medicine, 21(4), 324–328. 10.1111/j.1464-5491.2004.01142.x. [DOI] [PubMed] [Google Scholar]
- Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, & Nair H (2013). Risk factors for severe acute lower respiratory infections in children: a systematic review and meta-analysis. Croatian Medical Journal, 54(2), 110–121. doi: 10.3325/cmj.2013.54.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvie JA, & Malone RE (2008). Children’s secondhand smoke exposure in private homes and cars: an ethical analysis. American Journal of Public Health, 98(12), 2140–2145. doi: 10.2105/AJPH.2007.130856). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen-Fangel S, Mohey R, Johnsen SP, Andersen PL, Sorensen HT, & Ostergaard L (2004). Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scandinavian Journal of Infection Diseases, 36(1), 31–36. 10.1080/00365540310017618. [DOI] [PubMed] [Google Scholar]
- Johnson SB (2011). Psychological impact of screening and prediction in type 1 diabetes. Current Diabetes Report, 11, 454–59. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Baughcum AE, Carmichael SK, She J-X, & Schatz DA (2004). Maternal anxiety associated with newborn genetic screening for type 1 diabetes. Diabetes Care, 27, 392–97. 10.2337/diacare.27.2.392. [DOI] [PubMed] [Google Scholar]
- Johnson SB, Lynch KF, Baxter J, Lernmark B, Roth R Simell T.,… and the TEDDY Study Group (2016). Predicting Later Study Withdrawal in Participants Active in a Longitudinal Birth Cohort Study for 1 Year: The TEDDY Study. Journal of Pediatric Psychology, 41(3), 373–383. 10.1093/jpepsy/jsv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Lynch KF, Roth R, Schatz D, and the TEDDY Study Group (2017). My Child is Autoantibody Positive: Impact on Parental Anxiety. Diabetes Care. Published online June 29, 2017. 10.2337/dc17-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozyrsky AL, Mai XM, McGrath P, Hayglass KT, Becker AB, & Macnell B (2008). Continued exposure to maternal distress in early life is associated with an increased risk of childhood asthma. American Journal of Respiratory and Critical Care Medicine. 177, 142–147. 10.1164/rccm.200703-381OC. [DOI] [PubMed] [Google Scholar]
- Laitinen OH, Honkanen H, Pakkanen O, Hankaniemi MM, Huhtala H, Ruokoranta T, … Hyöty H (2014). Coxsackievirus B1 is associated with induction of beta-cell autoimmunity that portends type 1 diabetes. Diabetes, 63(2), 446–455. 10.2337/db13-0619. [DOI] [PubMed] [Google Scholar]
- Lanier P, Janson-Reid M, Stahlschmidt MJ, Drake B, Constantino J (2010). Child maltreatment and pediatric health outcomes: a longitudinal study of low-income children. Journal of Pediatric Psychology, 35(5), 511–522. 10.1093/jpepsy/jps086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange NE, Bunyavanich S, Silberg JL, Canino G, Rosner BA, & Celedon JC (2011). Parental psychosocial stress and asthma morbidity in Puerto Rican twins. Journal of Allergy and Clinical Immunology, 127(3), 734–740. 10.1016/j.jaci.2010.11010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WM., Lemanske RF Jr., Evans MD., Vang F., Pappas T., Gangnon P, … Gern JE (2012). Human rhinovirus species and season of infection determine illness severity. American Journal of Respiratory and Critical Care Medicine, 186(9), 886–891. DOI: 10.1164/rccm.201202-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark B, Johnson SB, Vehik K, Smith L, Ballard L, Baxter J, … and the TEDDY Study Group (2011). Enrollment experiences in a pediatric longitudinal observational study: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Contemporary Clinical Trials. 2011;32(4):517–523. DOI: 10.1016/j.cct.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littorin B, Sundkvist G, Nystrom L, Carlson A, Landin-Olson M, Östman J, … Wibell L. (2001). Family characteristics and life events before the onset of autoimmune type 1 diabetes in young adults: a nationwide study. Diabetes Care, 24(6), 1033–1037. 10.2337/diacare.24.6.1033. [DOI] [PubMed] [Google Scholar]
- Lönnrot M, Lynch K, Larsson HE, Lernmark A, Rewers M Hagopian W, … and the TEDDY Stuy Group. (2015). A method for reporting and classifying acute infectious diseases in a prospective study of young children: TEDDY. BMC Pediatrics, 15, 24 10.1186/s12887-015-0333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnrot M, Lynch K, Larsson HE, Lernmark A., Rewers MJ., Törn C., … and the TEDDY Study Group. (2017). Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia, 60,1931–1940. DOI: 10.1007/s00125-017-4365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhiala PJ, Jaakkola N, Ruotsalainen R, & Jaakkola JJK (1995). Form of day care and respiratory infections among Finnish children. American Journal of Public Health, 85, 1109–1112. DOI: 10.2105/AJPH.85.8_Pt_1.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann M, Eid M, Hofman W, & Lucas RE (2012). Subjective Well-Being and Adaption to Life Events: A Meta-Analysis. Journal of Personality and Social Psychology, 102(3), 592–615. DOI: 10.1037/a(K)25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren M, Ellström K, Larsson HE, for the DiPiS study group (2018). Influence of early-life parental severe life events on the risk of type 1 diabetes in children: the DiPiS study. Acta Diabetologica, 55, 797–804. 10.1007/s00592-018-1150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, … for the Severe Acute Lower Respiratory Infections Working Group (2013). Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet, 381(9875), 1380–1390. 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygren M, Carstensen J, Koch F, Ludvigsson J, & Frostell A (2015). Experience of a serious life event increases the risk for childhood type 1 diabetes: the ABIS population-based prospective cohort study. Diabetologia, 58(6), 1188–1197. DOI 10.1007/s00125-015-3555-2. [DOI] [PubMed] [Google Scholar]
- Oh DL, Jerman P, Marques SS, Koita K Boparai SKP., Harris NB., & Bucci M. (2018). Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatrics, 18, 83 10.1186/s12887-018-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G, and the EURODIAB Study Group. (2009). Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet, 373(9680), 2027–2033. 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- Peng H, & Hagopian W (2006). Environmental factors in the development of Type 1 diabetes. Reviews in Endocrine and Metabolic Disorder, 7(3), 149–162. 10.1007/s11154-006-9024-y. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Witso E, Tapia G, Stene LC, & Ronningen KS (2011). Self-reported lower respiratory tract infections and development of islet autoimmunity in children with the type 1 diabetes high-risk HLA genotype: the MIDIA study. Diabetes/Metabolic Research and Reviews, 27(8), 834–837. 10.1002/dmrr.1258. [DOI] [PubMed] [Google Scholar]
- Roberts L, Smith W, Jorm L, Patel L, Douglas RM, & McGilchrist C (2000). Effect of infection control measures on the frequency of upper respiratory infection in child care: a randomized, controlled trial. Pediatrics, 105(4), 738–742. doi: 10.1542/peds.105.4.738. [DOI] [PubMed] [Google Scholar]
- Salvatoni A, Baj A, Bianchi G, Federico G, Colombo M, & Toniolo A (2013). Intrafamilial spread of enterovirus infections at the clinical onset of type 1 diabetes. Pediatric Diabetes, 14(6), 407–416. 10.1111/pedi.12056. [DOI] [PubMed] [Google Scholar]
- Sandora TJ, Shih MC, & Goldmann DA (2008). Reducing absenteeism from gastrointestinal and respiratory illness in elementary school students: a randomized, controlled trial of an infection-control intervention. Pediatrics, 121(6), e1555–1562. doi: 10.1542/peds.2007-2597. [DOI] [PubMed] [Google Scholar]
- Segerstrom SC, & Miller GE (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychological Bulletin, 130(4), 601–630. 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepa A, & Ludvigsson J (2006). Psychological stress and the risk of diabetes-related autoimmunity: a review article. Neuroimmunomodulation, 13(5–6), 301–308. 10.1159/000104858. [DOI] [PubMed] [Google Scholar]
- Sepa A, Frodi A, & Ludvigsson J (2005). Mothers’ experiences of serious life events increase the risk of diabetes-related autoimmunity in their children. Diabetes Care, 28(10), 2394–2399. 10.2337/diacare.28.10.2394. [DOI] [PubMed] [Google Scholar]
- Shi T, Balsells E, Wastnedge E, Singleton R, Rasmussen ZA, Zar HJ, … Nair H(2015). Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: Systematic review and meta-analysis. Journal of Global Health, 5(2), 020416. doi: 10.7189/jogh.05.020416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes EA (2003). Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. Journal of Pediatric, 143(5 Suppl), 118–126. 10.1067/S0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Edwards C, Montuori J, & Lushene R (1970). State-Trait Anxiety Inventory. Redwood City, CA: MindGarden, Inc. [Google Scholar]
- Steptoe A, Hamer M, & Chida Y (2007). The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behavior and Immunity, 21(7), 901–912. 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Swartling U, Lynch K, Smith L, Johnson SB, and the TEDDY Study Group. (2016). Parental estimation of their child’s increased type 1 diabetes risk during the first two years of participation in an international observational study: results from the TEDDY study. Journal of Empirical Research on Human Research Ethics, 11(2), 106–114. Doi: 10.1177/1556264616648589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Diamond Study Group (2006). Incidence and trends of childhood Type 1 diabetes worldwide 1990–1999. Diabetes Medicine, 23(8), 857–866. DOI: 10.1111/j.1464-5491.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- The TEDDY Study Group (2007). The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatric Diabetes, 8(5), 286–298. 10.1111/j.1399-5448.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- The TEDDY Study Group (2008). The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Annals of the New York Academy of Science, 1150, 1–13. doi: 10.1196/annals.1447.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Cobb JM, & Steptoe A (1998). Psychosocial influences on upper respiratory infectious illness in children. Journal of Psychosom Res, 45(4), 319–330. DOI: 10.1016/S0022-3999(97)00311-5. [DOI] [PubMed] [Google Scholar]
- Van Der Gaag E, & Van Droffelaar N (2012). Upper respiratory tract infections in children: A normal stage or high parental concern? Open Journal of Pediatrics, 2, 244–249. 10.4236/ojped.2012.23038. [DOI] [Google Scholar]
- Wahlberg J, Vaarala O, Ludvigsson J, for the ABIS Study Group (2011). Asthma and allergic symptoms and type 1 diabetes-related autoantibodies in 2.5-yr-old children. Pediatric Diabetes, 12(7), 604–610. 10.1111/j.1399-5448.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- Winther B, Hayden FG, & Hendley JO (2006). Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: Association with symptomatic illness and effect of season. Journal of Medical Virology, 78(5), 644–650. 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- Wolf JM, Miller GE, & Chen E (2008). Parent psychological states predict changes in inflammatory markers in children with asthma and healthy children. Brain, Behavior and Immunity, 22(4), 433–441. 10.1016/j.bbi.2007.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2015). WHO Statistics 2015. http://www.who.int/gho/publications/world_health_statistics/2015/en/. Accessed June 22, 2016.
- Wyman PA, Moynihan J, Eberly S, Cox C, Cross W, Jin X, & Caserta MT (2007). Association of family stress with natural killer cell activity and the frequency of illnesses in children. Archives of Pediatric and Adolescent Medicine, 161(3), 228–234. doi: 10.1001/archpedi.161.3.228. [DOI] [PubMed] [Google Scholar]
- Yeung WC, Rawlinson WD, & Craig ME (2011). Enterovirus infection and type 1 diabetes mellitus: systematic review and meta-analysis of observational molecular studies. BMJ, 342, d35. doi: 10.1136/bmj.d35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler AG, & Nepom GT (2010). Prediction and pathogenesis in type 1 diabetes. Immunity, 32(4), 468–478. 10.1016/j.immuni.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoch B, Karch A, Dreesman J, Monazahian M, Baillot A, & Mikolajczyk RT (2015). Feasibility of a birth cohort study dedicated to assessing acute infections using symptom diaries and parental collection of biomaterials. BMC Infectous Diseases, 15, 436 10.1186/s12879-015-1189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


