Abstract
Purpose:
While older adults with cancer are more likely to develop chemotherapy-induced peripheral neuropathy (CIPN), the study aimed to determine if patient-reported and objective measures of CIPN differ by age among cancer survivors.
Methods:
Cancer survivors with persistent CIPN after completion of platinum and/or taxane chemotherapy completed CIPN questionnaires (severity, interference with activities, sensory and motor symptoms) and objective testing (light touch, vibration, pain, cold sensation). CIPN measures were compared by age group (<65 n=260 versus ≥65 n=165) using parametric and nonparametric tests.
Results:
Among 425 cancer survivors with CIPN, mean age was 60.9 (SD 10.5). CIPN location did not differ by age (overall 68% hands and feet, 27% only feet, 5% only hands). For patient-reported measures, older survivors reported less severe pain in the hands and feet than younger survivors. In addition, older survivors reported lower interference with general activity, routine activities, normal work, enjoyment of life, sleep, mood, relations with other people, and sexual activity. No age differences in sensory and motor symptom scores were found. In contrast, for objective measures, older survivors had worse light touch and cold sensations in their feet and worse vibration detection in their hands and feet.
Conclusions:
Despite having worse light touch, cold, and vibration sensations, older cancer survivors with CIPN reported less severe pain and interference with activities. This discordance highlights the importance of including both patient-reported and objective measures to assess CIPN in cancer survivors to better evaluate this clinical condition.
Keywords: chemotherapy-induced peripheral neuropathy, age, cancer survivor, chemotherapy, patient-reported outcomes
INTRODUCTION
As the incidence of cancer among adults age ≥65 increases to 2.3 million by 2030 [1], the number of cancer survivors who are age ≥65 will increase to 19.1 million by 2040 [2]. As a result, it is critically important to characterize the symptom experience of older cancer survivors, particularly persistent treatment toxicities. Chemotherapy-induced peripheral neuropathy (CIPN) is one of the most prevalent neurologic complications of cancer treatment and can persist for more than ten years after completion of chemotherapy (CTX) [3, 4]. CIPN can precipitate functional decline, falls, and decreased quality of life [5, 6], particularly in older cancer survivors who have pre-existing functional impairments.
Retrospective pooled analyses of cancer clinical trials found that older age is associated with an increased risk of developing moderate to severe CIPN [7, 8]. However, these studies relied on clinician-reported CIPN severity and did not include patient-reported (e.g., pain intensity, interference with activities) or objective (e.g., light touch, vibration) measures of CIPN. While a small prospective study of patients diagnosed with lung or breast cancer included both patient-reported and objective measures of CIPN [9], this study included only 17 older patients. With the increasing recognition of clinically meaningful differences between clinician- and patient-reported outcomes [10, 11] and the clinical benefit of using patient-reported outcomes to identify treatment toxicities sooner [12], it is important to characterize CIPN using both patient-reported and objective measures.
Therefore, the objective of this study was to compare patient-reported and objective measures of CIPN in younger (age <65) and older (age ≥65) cancer survivors with persistent CIPN in the hands and/or feet at least three months after completion of platinum and/or taxane CTX.
METHODS
Patients and Settings
This analysis is part of a larger study, funded by the National Cancer Institute, that evaluated cancer survivors with and without CIPN. The methods for the larger study are described in detail elsewhere [13]. In brief, cancer survivors were recruited from throughout the San Francisco Bay Area. Survivors with CIPN met the following criteria: age ≥18 years; completed platinum and/or taxane CTX ≥3 months prior to enrollment; had changes in sensation and/or pain in their hands and/or feet of ≥3 months duration after completion of CTX; had a rating of ≥3 on a 0 to 10 numeric rating scale (NRS) for any of the Pain Quality Assessment Scale sensations (i.e., numb, tender, shooting, sensitive, electrical, tingling, radiating, throbbing, cramping, itchy, unpleasant) [14]; if they had pain associated with CIPN, had an average pain intensity score in their hands and/or feet of ≥3 on a 0 to 10 NRS; had a Karnofsky Performance Status (KPS) score of ≥50; and were able to read, write, and understand English.
Survivors were excluded if they had diabetic neuropathy, peripheral vascular disease, vitamin B12 deficiency, thyroid dysfunction, HIV neuropathy, another painful condition that was difficult for them to distinguish from their CIPN, a hereditary sensory or autonomic neuropathy, and/or a hereditary mitochondrial disorder. Of the 1450 survivors who were screened, 754 were enrolled, and 623 completed the self-report questionnaires and the study visit. For this analysis, only survivors with CIPN (n=425) were included.
Study Procedures
Research nurses screened and consented the survivors by phone. Survivors completed questionnaires prior to their study visit. At the in-person visit, written informed consent was obtained, questionnaires were reviewed for completeness, and objective measurements were performed.
Study Measures
Demographic and Clinical Characteristics
Survivors provided demographic information and completed the KPS scale [15] and Self-Administered Comorbidity Questionnaire [16]. Clinical information including cancer diagnosis, CTX regimen and doses, and time since CTX completion were obtained through medical record review.
Subjective Measures
A detailed history of CIPN in the hands and/or feet was obtained using a pain questionnaire that was used in our previous [17, 18] and ongoing studies. This questionnaire obtained information on duration of CIPN and current, average daily, and worst amount of pain or changes in sensation using 0 (none) to 10 (excruciating) NRSs.
Sensory, motor, and autonomic CIPN symptom severity was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire CIPN-20 (EORTC QLQ CIPN-20) [19]. Each item was measured on a 1 (not at all) to 4 (very much) Likert scale. The sensory, motor, and autonomic neuropathy subscales are the cumulative scores for 9 sensory, 8 motor, and 3 autonomic items, respectively.
The Brief Pain Inventory Pain Interference Scale [20] was used to assess how CIPN interfered with activities (e.g., walking, work, sleep) during the past week. For our study, we added items on interference with routine activities (e.g., dressing, toileting, typing) to assess upper extremity interference from CIPN, balance to assess lower extremity interference from CIPN, and sexual activity given the prevalence of problems with sexual interest or activity in cancer survivors [21]. Interference with routine activities was assessed separately for CIPN in the hands and feet using 0 (does not interfere) to 10 (completely interferes) NRSs.
Objective measures
Details for each objective measure are described elsewhere [13]. Light touch was evaluated using Semmes Weinstein monofilaments [22]. Vibration threshold was assessed using a biothesiometer [23]. Pain sensation was evaluated using the Neurotip [24]. Cold sensation was evaluated using the Tiptherm Rod [25]. For all objective measures of sensation, both the upper and lower extremities on the dominant side were tested.
Data Analysis
Descriptive statistics and frequency distributions were calculated for survivors’ demographic and clinical characteristics; CIPN pain characteristics; EORTC QLQ-CIPN20 sensory, motor, and autonomic subscales; and pain interference scores. For the four objective measures of sensation, composite scores were created to summarize results from all tested sites on the dominant upper and lower extremities. For light touch, pain, and cold sensations, the number of sites with loss of each sensation was summed. For light touch, loss of protective sensation was defined as the inability to feel the 4.56 size monofilament (4 g) in each of the upper extremity and 5.07 size monofilament (10 g) in each of the lower extremity locations [22]. For vibration, the mean vibration threshold across the sites was calculated. Differences between age groups (i.e., age <65 versus ≥65) in demographic and clinical characteristics and subjective and objective measures of CIPN were evaluated using Independent sample t-tests, Chi-square analyses, and Mann-Whitney U tests. A P-value of <0.05 was considered statistically significant. Data were analyzed using Stata/SE 15.1 (College Station, TX).
RESULTS
Demographic and clinical characteristics
As shown in Table 1, 39.0% of our cancer survivors were age ≥65. Older survivors with CIPN were more likely to be White, live alone, and have a history of ever smoking. Older cancer survivors were less likely to be employed or have breast cancer. Among patients who received both platinum and taxane CTX, older survivors received a higher mean cumulative taxane dose than younger survivors (P=0.04). Mean time since cancer diagnosis was 5.72 years (SD 5.07) among the older survivors compared to 4.27 years (SD 4.61) among the younger survivors (P=0.003). No significant differences were found in KPS score, comorbidity score, receipt of prior surgery or radiation, type of CTX regimen received, dose reduction or delay due to neuropathy, or locations of CIPN. The most common location for CIPN was in both the hands and feet (63.6% among older survivors, 70.8% among younger survivors).
Table 1.
Differences in demographic and clinical characteristics among cancer survivors with CIPN by age group (N = 425).
| Characteristic | Age <65 (n=260, 61.0%) (1) |
Age >65 (n=165, 39.0%) (2) |
pa |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age, years | 54.52 (8.01) | 70.90 (4.48) | <0.0001 |
| Education, years | 16.07 (3.27) | 16.32 (3.41) | 0.47 |
| Body mass index (kg/m2) | 26.54 (5.61) | 26.49 (5.34) | 0.93 |
| Karnofsky Performance Status score | 80.10 (15.38) | 83.18 (17.43) | 0.06 |
| Number of comorbidities | 1.90 (1.47) | 2.07 (1.91) | 0.31 |
| Self-Administered Comorbidity Questionnaire score | 4.07 (3.41) | 4.24 (3.65) | 0.64 |
| Years since cancer diagnosis | 4.27 (4.61) | 5.72 (5.07) | 0.003 |
| Number of prior cancer treatments | 3.18 (0.98) | 3.02 (0.94) | 0.11 |
| Dose of platinum for patients who received only a platinum (mg/m2) | 704.83 (556.52) | 705.03 (388.64) | 1.00 |
| Dose of taxane for patients who received only a taxane (mg/m2) | 738.28 (290.96) | 805.02 (1090.65) | 0 53 |
| Doses for patients who received both a platinum and a taxane compound | |||
| Platinum dose (mg/m2) | 1715.22 (788.25) | 1882.87 (793.25) | 0.25 |
| Taxane dose (mg/m2) | 818.21 (459.00) | 995.00 (447.69) | 0.04 |
| n (%) | n (%) | ||
| Female | 226 (87.3) | 141 (85.5) | 0.60 |
| Race/ethnicity | 0.001 | ||
| White | 184 (70.8) | 145 (87.9) | 1 < 2 |
| Asian/Pacific Islander | 23 (8.9) | 7 (4.2) | |
| Black | 17 (6.5) | 5 (3.0) | |
| Hispanic/Mixed/Other | 36 (13.9) | 8 (4.9) | 1 > 2 |
| Married/partnered | 154 (61.4) | 98 (60.5) | 0.86 |
| Lives alone | 64 (25.3) | 58 (35.4) | 0.03 |
| Employed | 141 (54.2) | 38 (23.2) | <0.0001 |
| Annual household income | |||
| <$30,000 | 57 (23.4) | 34 (22.7) | |
| $30,000 - $69,999 | 47 (19.3) | 36 (24.0) | 0.44 |
| $70,000 - $99,999 | 38 (15.6) | 26 (17.3) | |
| >$100,000 | 102 (41.8) | 54 (36.0) | |
| Ever smoker | 83 (32.1) | 77 (47.2) | 0.002 |
| Type of cancer | 0.04 | ||
| Breast | 158 (60.8) | 75 (45.5) | 1 > 2 |
| Colon | 22 (8.5) | 21 (12.7) | |
| Lung | 4 (1.5) | 4 (2.4) | |
| Ovarian | 26 (10.0) | 24 (14.6) | |
| Other | 50 (19.2) | 41 (24.9) | |
| Metastatic disease | 149 (58.0) | 103 (63.6) | 0.25 |
| Prior surgery | 238 (91.5) | 156 (95.1) | 0.16 |
| Prior radiation | 156 (60.2) | 94 (57.7) | 0.60 |
| Chemotherapy regimen | |||
| Only a platinum compound | 55 (21.2) | 40 (24.2) | |
| Only a taxane compound | 131 (50.4) | 68 (41.2) | 0.18 |
| Both a platinum and a taxane compound | 74 (28.5) | 57 (34.6) | |
| Patients who had a dose reduction or delay due to neuropathy | 35 (14.0) | 20 (13.0) | 0.77 |
| CIPN in both hands and feet | 184 (70.8) | 105 (63.6) | 0.09 |
| CIPN in only feet | 61 (23.5) | 54 (32.7) | |
| CIPN in only hands | 15 (5.8) | 6 (3.6) | |
Abbreviations: CIPN, chemotherapy-induced peripheral; kg, kilogram; mg, milligram; m2, square meter; neuropathy; SD, standard deviation.
P values were calculated using t-tests (continuous variables), Chi-square tests (categorical variables), and Mann-Whitney U test (ordinal household income variable).
Patient-reported CIPN pain characteristics
Older cancer survivors reported a longer duration of CIPN in the lower extremity compared to younger survivors (4.85 vs 3.37 years, P=0.0006) but not in the upper extremity (Table 2). In both the hands and feet, older survivors reported lower mean scores for their current pain at its worst compared to younger survivors. No age-related differences were found in the patient-reported days per week or hours per day in pain. No age-related differences were found in the mean sensory, motor, or autonomic subscale scores of the EORTC QLQ CIPN-20 (Table 2).
Table 2.
Differences in CIPN pain characteristics of cancer survivors by age group (N = 465).
| Characteristic | Age <65 (n=260, 61.0%) |
Age >65 (n=165,39.0%) |
Pa |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Pain Characteristics - Upper Extremity | |||
| Duration of CIPN, years | 3.30 (3.86) | 4.23 (4.41) | 0.06 |
| Pain now | 2.88 (2.18) | 2.56 (1.83) | 0.20 |
| Average pain | 3.17 (2.19) | 2.99 (2.05) | 0.48 |
| Worst pain | 4.90 (2.78) | 4.20 (2.37) | 0.03 |
| Days per week in pain | 3.78 (2.93) | 3.28 (3.14) | 0.17 |
| Hours per day in pain | 12.30 (9.84) | 14.14 (9.77) | 0.14 |
| Pain Characteristics - Lower Extremity | |||
| Duration of CIPN, years | 3.37 (3.83) | 4.85 (4.69) | 0.0006 |
| Pain now | 3.69 (2.33) | 3.46 (2.18) | 0.31 |
| Average pain | 4.07 (2.17) | 3.84 (2.02) | 0.28 |
| Worst pain | 6.31 (2.52) | 5.62 (2.53) | 0.008 |
| Days per week in pain | 3.78 (2.96) | 3.37 (3.12) | 0.19 |
| Hours per day in pain | 14.45 (9.72) | 15.77 (9.02) | 0.18 |
| EORTC QLQ-CIPN20 | |||
| Sensory score | 33.1 (18.5) | 34.1 (16.9) | 0.60 |
| Motor score | 23.0 (18.7) | 20.9 (15.7) | 0.25 |
| Autonomic score | 16.4 (20.0) | 13.0 (16.7) | 0.07 |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; EORTC QLQ, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; SD, standard deviation.
P values were calculated using t-tests.
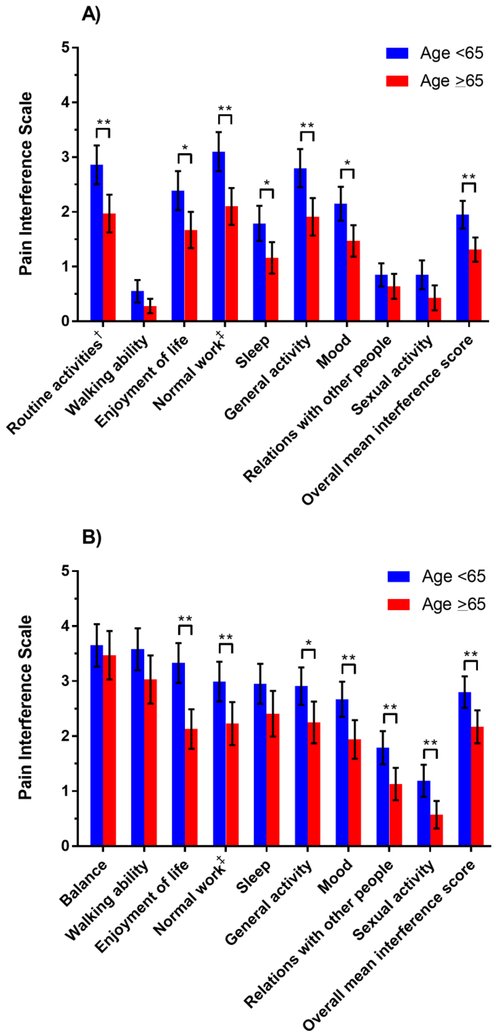
Older survivors consistently reported lower overall mean CIPN interference scores in both the hands (Figure 1A, mean 1.31 [SD 1.44] vs 1.95 [SD 2.06]; P=0.004) and feet (Figure 1B, mean 2.17 [SD 1.95] vs 2.80 [SD 2.35]; P=0.005). In both the hands and feet, older age was associated with lower mean interference scores for enjoyment of life, normal work (includes both work outside the home and housework), general activity, and mood. In the hands only, older age was associated with lower mean interference scores for routine activities (e.g., dressing, toileting, typing) and sleep. In the feet only, older age was associated with lower mean interference for relations with other people and sexual activity.
Figure 1.
Mean pain interference scores with 95% confidence intervals for chemotherapy-induced peripheral neuropathy in the (A) upper and (B) lower extremities according to age group.
*P<0.05
**P<0.01
†Routine activities such as dressing, toileting, and typing.
‡Normal work includes both work outside the home and housework.
Objective sensory measures of CIPN
For light touch, no age-related differences were found in the mean number of upper extremity sites with loss of protective sensation (Table 3). However, in the lower extremity, older survivors had loss of protective sensation in an average 2.95 (SD 2.50) lower extremity sites out of 9 compared to 1.63 (SD 1.99) sites among younger survivors (P <0.0001). For vibration, the mean detection threshold was higher in both the upper and lower extremities for older survivors. To illustrate, in the 4 upper extremity sites, older survivors detected vibration at an average threshold of 9.95 volts (SD 4.20) while younger survivors detected vibration at an average threshold of 8.48 volts (SD 4.63), P <0.0001. In the three lower extremity sites, older survivors detected vibration at an average threshold of 32.44 volts (SD 11.47), while younger survivors detected vibration at an average threshold of 23.33 volts (10.82), P <0.0001. For cold sensation, older survivors had loss of cold sensation in more upper (P=0.03) and lower extremity sites (P <0.0001) than younger survivors. No age-related differences in pain sensation were found in the upper or lower extremities.
Table 3.
| Characteristic | Age <65 (n=258, 61.1%) |
Age >65 (n=164, 38.9%) |
Pc |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Light touch | |||
| No. of upper extremity sites (out of 7)d with loss of protective sensation | 0.17 (0.77) | 0.23 (0.84) | 0.18 |
| No. of lower extremity sites (out of 9)e with loss of protective sensation | 1.63 (1.99) | 2.95 (2.50) | <0.0001 |
| Vibration | |||
| Mean vibration threshold (in volts) at 4 upper extremity sitesf | 8.48 (4.63) | 9.95 (4.20) | <0.0001 |
| Mean vibration threshold (in volts) at 3 lower extremity sitesg | 23.33 (10.82) | 32.44 (11.47) | <0.0001 |
| Pain sensation | |||
| No. of upper extremity sites (out of 7)d with loss of pain sensation | 1.16 (1.45) | 1.15 (1.43) | 0.87 |
| No. of lower extremity sites (out of 9)e with loss of pain sensation | 3.23 (2.10) | 3.71 (2.24) | 0.06 |
| Cold sensation | |||
| No. of upper extremity sites (out of 4)f with loss of cold sensation | 0.75 (0.96) | 0.94 (1.01) | 0.03 |
| No. of lower extremity sites (out of 4)i with loss of cold sensation | 2.05 (1.17) | 2.58 (1.18) | <0.0001 |
Abbreviations: CIPN, chemotherapy-induced peripheral neuropathy; No., number; SD, standard deviation.
Changes in sensation are reported for the dominant extremity.
Three cancer survivors (two age <65, one age ≥65) did not have the objective measures of CIPN and were not included in this analysis.
Mann-Whitney U test
Upper extremity sites for light touch and pain were: pad of thumb, thumb web space, tip of index finger, tip of little finger, midway base of palm, one third up anterior arm, two thirds up anterior arm.
Lower extremity sites for light touch and pain were: pad of great toe, pad of 3rd toe, pad of 5th toe, base of heel, metatarsophalangeal (MP) joint of great toe, MP joint of 3rd toe, MP joint of 5th toe, midway along tibia, patella.
Upper extremity sites for vibration were: dorsal interphalangeal (IP) joint of thumb, dorsal IP joint of index finger, ulnar prominence, lateral epicondyle.
Lower extremity sites for vibration were: dorsal IP joint of great toe, medial malleolus, patella.
Upper extremity sites for cold were: pad of index finger, pad of 5th finger, dorsal metacarpal area of the hand, dorsal wrist.
Lower extremity sites for cold were: top of great toe at 1st MP joint, pad of great toe, dorsum of foot midpoint, medial malleolus.
DISCUSSION
This study is the first to evaluate for age-related differences in CIPN using both detailed patient-reported and objective measures. Despite having worse objective light touch and cold sensations in the lower extremities and worse vibration sensation in the upper and lower extremities, older cancer survivors reported lower pain severity scores and less interference with common activities. This discordance highlights the importance of using both patient-reported and objective measures to assess CIPN. Using both types of measures may capture older survivors who may have more loss of sensation in their hands and feet than their descriptions suggest as well as younger survivors who may experience more interference with activities than their objective sensory losses suggest.
While older adults with cancer have a higher risk of developing CIPN [7, 8], our findings suggest that among those who developed CIPN, older survivors experienced less pain at its worst than younger survivors. This difference in CIPN pain was found in both the hands and feet. Our findings are consistent with previous reports that found that older cancer patients on average report less pain than younger patients [26–28]. Reports of decreased pain intensity among older cancer patients may be due to age-related differences in how cancer treatment is adjusted in response to increasing symptoms. However, in our study, no differences in CTX dose reductions or delays due to CIPN were found between older and younger survivors that could account for the differences in pain intensity scores. Age differences may be related to how patients adapt to cancer-related pain and how patients perceive symptoms, often referred to as a response shift [29]. For example, in a mixed methods study of cancer-related pain [30], older patients were “living despite pain,” more accepting of pain, and modified activities to maximize their participation. In contrast, younger patients were more likely to be “waiting to live” with their lives and activities on hold until complete pain relief was achieved [30].
Overall, in both age groups, CIPN in the feet interfered with activities more than CIPN in the hands. Consistent with reports of an increased risk of falls in cancer patients with CIPN [5, 31, 32], the worst interference scores were for balance and walking ability. However, no age-related differences were found with this outcome. While in one study CIPN and older age were both independent risk factors for falls [32], our findings suggest that both age groups experience problems with balance and walking ability. Future studies need to evaluate for age differences in objective measures of balance.
Among the other activities assessed, older survivors consistently reported less interference from CIPN. While prior studies have evaluated how CIPN interferes with common activities [33, 34], none have examined age differences. Our finding that younger survivors report more interference with activities from CIPN identifies a potential opportunity to study interventions to minimize the impact of CIPN on common activities in this population.
A major strength of our study is that we assessed CIPN using objective measures of sensation in addition to patient-reported outcomes. Of note, older survivors on average had greater loss of protective sensations in the hands and feet than their descriptions suggested. In contrast to guideline-recommended care for patients at risk of diabetic neuropathy [35], the assessment of patients receiving neurotoxic CTX does not routinely include objective measures of sensation. Our findings suggest that monofilament testing be used to assess patients at risk for CIPN to detect loss of protective sensation that may not be recognized based on patient-report alone [36–38]. Future studies need to assess for additional adverse effects associated with neurotoxic CTX (e.g., audiovestibular).
Several limitations warrant consideration. Given the cross-sectional design of our study, prospective longitudinal studies that assess CIPN during and after completion of cancer treatment are warranted to characterize how different measures of CIPN change over time among older and younger survivors. In addition, our study included only cancer survivors who received platinum and/or taxane CTX, so our results may not generalize to survivors with CIPN from other neurotoxic cancer treatments. Finally, detailed information was not obtained on the survivors’ use of supportive care strategies over the duration of their CIPN.
In summary, our study identified age-related differences in CIPN with older cancer survivors reporting less pain and interference with activities while having objectively worse measures of sensation. This information can enhance patient education with careful attention to interference with activities if CIPN develops. Furthermore, this information can help clinicians more thoroughly evaluate CIPN severity among older survivors who may report moderate CIPN symptoms and better support younger survivors who may be experiencing significant interference with activities.
Acknowledgements:
This study was funded by the National Cancer Institute (NCI, CA151692). Dr. Miaskowski is supported by the American Cancer Society and NCI (CA168960). Dr. Wong is supported by the Conquer Cancer Foundation of the American Society of Clinical Oncology (ASCO)/Vicky Merryman Women Who Conquer Cancer Young Investigator Award, National Institute on Aging (AG044281), and National Center for Advancing Translational Sciences (NCATS, KL2TR001870). This project was supported by NCATS through UCSF Clinical and Transformational Science Institute (CTSI) (UL1TR000004). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Recruitment was facilitated by Dr. Susan Love Research Foundation’s Army of Women Program.
Footnotes
CONFLICT OF INTEREST
Dr. Wong has reported a conflict of interest outside of the submitted work (immediate family member is an employee of Genentech with stock ownership). The remaining authors have no conflicts to report.
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27: 2758–2765. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH (2016) Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 25: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer JR, Morrison G, Dolan ME, Fleming GF (2016) Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol 140: 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beijers AJM, Mols F, Vreugdenhil G (2014) A systematic review on chronic oxaliplatin-induced peripheral neuropathy and the relation with oxaliplatin administration. Support Care Cancer 22: 1999–2007. [DOI] [PubMed] [Google Scholar]
- 5.Tofthagen C, Overcash J, Kip K (2012) Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Cancer 20: 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L (2014) Chemotherapy-induced peripheral neuropathy and its association with quality of life: A systematic review. Support Care Cancer 22: 2261–2269. [DOI] [PubMed] [Google Scholar]
- 7.Karavasilis V, Papadimitriou C, Gogas H, Kouvatseas G, Pentheroudakis G, Koutras A, Christodoulou C, Bafaloukos D, Samantas E, Pisanidis N, Papakostas P, Aravantinos G, Karanikiotis C, Kosmidis P, Pectasides D, Dimopoulos MA, Fountzilas G (2016) Safety and tolerability of anthracycline-containing adjuvant chemotherapy in elderly high-risk breast cancer patients. Clin Breast Cancer 16: 291–298. [DOI] [PubMed] [Google Scholar]
- 8.Hershman DL, Till C, Wright JD, Awad D, Ramsey SD, Barlow WE, Minasian LM, Unger J (2016) Comorbidities and risk of chemotherapy-induced peripheral neuropathy among participants 65 years or older in Southwest Oncology Group Clinical Trials. J Clin Oncol 34: 3014–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyriou AA, Polychronopoulos P, Koutras A, Iconomou G, Gourzis P, Assimakopoulos K, Kalofonos HP, Chroni E (2006) Is advanced age associated with increased incidence and severity of chemotherapy-induced peripheral neuropathy? Support Care Cancer 14: 223–229. [DOI] [PubMed] [Google Scholar]
- 10.Basch E, Iasonos A, McDonough T, Barz A, Culkin A, Kris MG, Scher HI, Schrag D (2006) Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: Results of a questionnaire-based study. Lancet Oncol 7: 903–909. [DOI] [PubMed] [Google Scholar]
- 11.Blagden SP, Charman SC, Sharples LD, Magee LRA, Gilligan D (2003) Performance status score: Do patients and their oncologists agree? Br J Cancer 89: 1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basch E, Deal AM, Dueck AC, Scher HI, Kris MG, Hudis C, Schrag D (2017) Overall survival results of a trial assessing patient-reported outcomes for symptom monitoring during routine cancer treatment. JAMA 318: 197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miaskowski C, Mastick J, Paul SM, Topp K, Smoot B, Abrams G, Chen LM, Kober KM, Conley YP, Chesney M, Bolla K, Mausisa G, Mazor M, Wong M, Schumacher M, Levine JD (2017) Chemotherapy-induced neuropathy in cancer survivors. J Pain Symptom Manage 54: 204–218 e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS (2008) The dimensions of pain quality: Factor analysis of the Pain Quality Assessment Scale. Clin J Pain 24: 550–555. [DOI] [PubMed] [Google Scholar]
- 15.Karnofsky D, Abelmann WH, Craver LV, Burchenal JH (1948) The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1: 634–656. [Google Scholar]
- 16.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN (2003) The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research. Arthritis Rheum 49: 156–163. [DOI] [PubMed] [Google Scholar]
- 17.Posternak V, Dunn LB, Dhruva A, Paul SM, Luce J, Mastick J, Levine JD, Aouizerat BE, Hammer M, Wright F, Miaskowski C (2016) Differences in demographic, clinical, and symptom characteristics and quality of life outcomes among oncology patients with different types of pain. Pain 157: 892–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langford DJ, Schmidt B, Levine JD, Abrams G, Elboim C, Esserman L, Hamolsky D, Mastick J, Paul SM, Cooper B, Kober K, Dodd M, Dunn L, Aouizerat B, Miaskowski C (2015) Preoperative breast pain predicts persistent breast pain and disability after breast cancer surgery. J Pain Symptom Manage 49: 981–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. European Journal of Cancer 41: 1135–1139. [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Ryan KM (1994) Pain assessment: Global use of the Brief Pain Inventory. Ann Acad Med Singapore 23: 129–138. [PubMed] [Google Scholar]
- 21.Jackson SE, Wardle J, Steptoe A, Fisher A (2016) Sexuality after a cancer diagnosis: A population‐ based study. Cancer 122: 3883–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell-Krotoski JA (2002) Sensibility testing with Semmes-Weinstein monofilaments In: Hunter JM, Mackin EJ, Callahan ED (eds) Rehabilitation of the Hand and Upper Extremity. Mosby, Inc., St. Louis. [Google Scholar]
- 23.Duke J, McEvoy M, Sibbritt D, Guest M, Smith W, Attia J (2007) Vibrotactile threshold measurement for detecting peripheral neuropathy: Defining variability and a normal range for clinical and research use. Diabetologia 50: 2305–2312. [DOI] [PubMed] [Google Scholar]
- 24.Papanas N, Ziegler D (2011) New diagnostic tests for diabetic distal symmetric polyneuropathy. J Diabetes Complications 25: 44–51. [DOI] [PubMed] [Google Scholar]
- 25.Viswanathan V, Snehalatha C, Seena R, Ramachandran A (2002) Early recognition of diabetic neuropathy: Evaluation of a simple outpatient procedure using thermal perception. Postgrad Med J 78: 541–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krok JL, Baker TA, McMillan SC (2013) Age differences in the presence of pain and psychological distress in younger and older cancer patients. J Hosp Palliat Nurs 15: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohile SG, Heckler C, Fan L, Mustian K, Jean-Pierre P, Usuki K, Sprod L, Janelsins M, Purnell J, Peppone L, Palesh O, Devine KA, Morrow G (2011) Age-related differences in symptoms and their interference with quality of life in 903 cancer patients undergoing radiation therapy. J Geriatr Oncol 2: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cataldo JK, Paul S, Cooper B, Skerman H, Alexander K, Aouizerat B, Blackman V, Merriman J, Dunn L, Ritchie C, Yates P, Miaskowski C (2013) Differences in the symptom experience of older versus younger oncology outpatients: A cross-sectional study. BMC Cancer 13: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MA, Fayers PM (2006) The clinical significance of adaptation to changing health: A meta-analysis of response shift. Qual Life Res 15: 1533–1550. [DOI] [PubMed] [Google Scholar]
- 30.Gagliese L, Jovellanos M, Zimmermann C, Shobbrook C, Warr D, Rodin G (2009) Age-related patterns in adaptation to cancer pain: A mixed-method study. Pain Medicine 10: 1050–1061. [DOI] [PubMed] [Google Scholar]
- 31.Ward PR, Wong MD, Moore R, Naeim A (2014) Fall-related injuries in elderly cancer patients treated with neurotoxic chemotherapy: A retrospective cohort study. J Geriatr Oncol 5: 57–64. [DOI] [PubMed] [Google Scholar]
- 32.Kolb NA, Smith AG, Singleton JR, Beck SL, Stoddard GJ, Brown S, Mooney K (2016) The association of chemotherapy-induced peripheral neuropathy symptoms and the risk of falling. JAMA Neurol 73: 860–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JH, Lee KM, Jeon MJ, Seol ME, Lee SH, Park J (2013) Symptom and interference of activities of daily living of chemotherapy-induced peripheral neuropathy in patients receiving taxanes and platinums. Asian Oncol Nurs 13: 145–151. [Google Scholar]
- 34.Tofthagen C, McAllister RD, Visovsky C (2013) Peripheral neuropathy caused by paclitaxel and docetaxel: An evaluation and comparison of symptoms. J Adv Pract Oncol 4: 204–215. [PMC free article] [PubMed] [Google Scholar]
- 35.American Diabetes Association (2018) 10. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2018. Diabetes Care 41: S105–s118. [DOI] [PubMed] [Google Scholar]
- 36.da Silva Simao DA, Teixeira AL, Souza RS, de Paula Lima ED (2014) Evaluation of the Semmes-Weinstein filaments and a questionnaire to assess chemotherapy-induced peripheral neuropathy. Support Care Cancer 22: 2767–2773. [DOI] [PubMed] [Google Scholar]
- 37.Griffith KA, Dorsey SG, Renn CL, Zhu S, Johantgen ME, Cornblath DR, Argyriou AA, Cavaletti G, Merkies IS, Alberti P, Postma TJ, Rossi E, Frigeni B, Bruna J, Velasco R, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galie E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey DJ, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG (2014) Correspondence between neurophysiological and clinical measurements of chemotherapy-induced peripheral neuropathy: Secondary analysis of data from the CI-PeriNomS study. J Peripher Nerv Syst 19: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyette-Davis JA, Eng C, Wang XS, Cleeland CS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Zhang H, Dougherty PM (2012) Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res 18: 3180–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]