Abstract
Objectives:
To compare safety and efficacy outcomes between the cyclophosphamide (CYC) arms of Scleroderma Lung Study (SLS) I and II.
Methods:
Participants enrolled in the CYC arms of SLS I (N=79) and II (N=73) were included. SLS I and II randomized participants to oral CYC for 1 year and followed patients for an additional year off therapy (In SLS II, patients received placebo in year two). Eligibility criteria for SLS I and II were nearly identical. Outcomes included the FVC%-predicted and DLCO%-predicted (measured every 3 months) and quantitative radiographic extent of ILD (QILD) (measured at 1 and 2 years for SLS I and SLS II, respectively). Joint models were created to evaluate the treatment effect on the course of the FVC/DLCO over 2-years while controlling for baseline disease severity.
Results:
SLS I and II-CYC participants had similar baseline characteristics. After adjusting for baseline disease severity, there was no difference in the course of the FVC%-predicted (P=0.535) nor the DLCO%-predicted (P=0.172) between the SLS I and II-CYC arms. In both groups, treatment with CYC led to a significant improvement in the FVC%-predicted from 3–12 months, but no significant improvement beyond this point. Treatment with CYC had no effect on the DLCO for either group.
Conclusion:
Treatment with 1 year of oral CYC led to similar improvements in lung function in both SLS I and II, although the effects were not sustained following cessation of CYC. These results suggest that increasing the duration of ILD therapy may improve outcomes for SSc-ILD patients.
Keywords: Systemic sclerosis, interstitial lung disease, cyclophosphamide
INTRODUCTION
Interstitial lung disease (ILD) is the leading cause of death in patients with systemic sclerosis (SSc)[1, 2]. Randomized controlled trials have favored the use of CYC for treating SSc-ILD [3, 4, 5]. Compared with placebo, one year of CYC improved lung function in patients with SSc-ILD in SLS I [3]. However, the effects of CYC waned after monitoring patients for an additional year off therapy [6]. In SLS II (comparing CYC and mycophenolate [MMF]), treatment with one year of CYC appeared to have a more sustained effect on lung function over two years [5]. Following the publication of SLS II, a number of SSc experts have questioned whether the CYC arm of SLS II performed better than the CYC arm of SLS I.
However, because SLS I [3] and II [5] used different analytic approaches, comparing efficacy outcomes reported in these publications has inherent limitations. In SLS I [3], the analysis of the primary endpoint (forced vital capacity [FVC]) was based on a longitudinal model that included terms for treatment and time and an interaction term [3]. In contrast, in SLS II [5], the analysis of the primary endpoint (FVC) was performed using an inferential joint model combining a mixed effects model for longitudinal outcomes and a survival model to handle non-ignorable missing data [7,8].
To further understand the effects of CYC on SSc-ILD outcomes, the present study directly compared efficacy outcomes between the CYC arms of SLS I and II using an inferential joint model approach. In using a uniform analysis approach, the present analysis aimed to test the hypothesis that the CYC arm of SLS I and II had similar clinical outcomes in terms of the course of the FVC and DLCO. This study also aimed to compare the safety profiles for patients in these two groups.
MATERIALS AND METHODS
Study participants
All participants enrolled in the CYC arm of SLS I [3] and II [5] were included in this analysis. Participating centers, investigators, and eligibility criteria were similar for both trials [3,5]. The protocol was approved by a Data and Safety Monitoring Board (DSMB) constituted by the National Heart, Lung and Blood Institute, National Institutes of Health. The institutional review board (IRB) at the main coordinating center, University of California, Los Angeles, approved this study (11–002659-CR-00005). In addition, each of the participating centers (N=12 for SLS I and N=14 for SLS II) had IRB approval to conduct this study. All participants gave written informed consent. Please see the prior SLS publications for inclusion and exclusion criteria [3,5].
SLS I and II Study Design
SLS I consisted of 162 participants randomized between September 2000 and January 2004 to receive either oral CYC (titrated to 2.0 mg/kg once daily) or matching placebo for one year, followed by an additional year of observation off-treatment as previously published [3]. In SLS II, 142 patients were randomized between September 2009 and December 2012 and assigned to receive either MMF (titrated as tolerated to 3.0 gm/day in divided doses) for 2 years or oral CYC (titrated as tolerated to 2 mg/kg one daily) for 1 year followed by an additional year on placebo [5].
SLS I and II Assessment Measurement
Completed pulmonary function tests were performed at baseline. The FVC (primary SLS I/II endpoint) and DLCO (secondary SLS I/II endpoint) were measured every 3 months during the trials. Dyspnea was assessed using the Mahler Dyspnea Index at baseline (BDI) and every 3 months thereafter for SLS I and every 6 months thereafter for SLS II using the Transition Dyspnea Index (TDI) [9,10]. In SLS I, an interview-administered paper version of the BDI/TDI was used [9] while in SLS II a self-administered computer-assisted version of the BDI/TDI was used [10]. The Modified Rodnan Skin Score (MRSS) [11] was used to assess cutaneous sclerosis. The MRSS [11] was performed every 3 months in SLS II and every 6 months in SLS I.
HRCT thoracic imaging was obtained at baseline and at 12 and 24 months in SLS I and II, respectively. Both studies used similar HRCT acquisition and analysis methods [12,13] except that, in SLS I, non-volumetric CT scans of 1–2 mm slice thickness were acquired at 10 mm increments, while in SLS II, volumetric CT scans of 1–1.5mm slice thickness were acquired contiguously. We report the quantitative lung fibrosis (QLF) score, representing the percentage of counts with reticular opacity with architectural distortion, and the Quantitative ILD (QILD) score, representing the sum of all abnormally classified scores, including scores for fibrosis, ground glass opacity and honeycombing. Scores were summated for the whole lung (WL) and for the one zone of maximal involvement (ZM).
Statistical Analysis
Baseline characteristics
Summary statistics were generated for baseline characteristics from the two cohorts. Group comparisons were performed using a two-sample t-test, Wilcoxon ranksum test, and a Chi-square test.
Primary outcome: FVC%-Predicted
An intention-to-treat principle was applied to all analyses using an inferential joint model consisting of a mixed effects model for longitudinal outcomes and a survival model to handle non-ignorable missing data due to study dropout, treatment failure or death [7,8]. The joint model was used as our primary inferential approach because it can provide unbiased and efficient estimates when there are non-ignorable missing data in the outcomes due to dropouts, treatment failures and deaths. Consistent with the intention-totreat principle, treatment failures and others who prematurely withdrew from the doubleblind treatment phase were encouraged to return for monitoring.
Repeated measurements of the FVC %-predicted were characterized by a linear mixed effects sub-model in the joint model, and intra-subject data correlation among multiple measurements over time was accounted for by random intercept and random time trend. Fixed effects were pre-specified covariates for the primary outcome including baseline FVC %-predicted, baseline QILD-WL, a time trend, treatment assignment, treatment-time trend interactions, and treatment-QILD interaction. The time trend was modeled by linear splines with knots at 12 and 21 months. The location of knots was determined by preliminary examination of the data using descriptive statistics. Treatment assignment was coded as a binary variable with SLSI-CYC group as the reference. Thus, the model estimates three piece-wise linear trends for the SLS I-CYC group in 3 – 12 months, 12 – 21 months, and 21 – 24 months, and change in these time trends in the SLS I-CYC group compared with the SLS II-CYC group.
Secondary outcomes: DLCO%-Predicted, TDI, MRSS, and Safety
Secondary efficacy endpoints were also analyzed using a joint model with no adjustment for multiple comparisons. For safety analyses, descriptive statistics were used to compare the incidence of adverse events (AEs) and serious adverse events (SAEs) between treatment arms. The definitions of specific AEs (i.e. leukopenia, anemia, etc.) were identical between SLS I and SLS II [3,5].
All tests were 2-sided. Group comparisons of baseline characteristics were performed using SAS 9.4 (SAS Institute; Cary, NC). The joint modeling analysis was implemented in C.
RESULTS
Baseline Characteristics
Patients assigned to CYC in SLS I and II exhibited similar baseline demographic features except for a slight difference in age (Table 1). The FVC%-predicted, disease duration, and MRSS were similar for the CYC arms of each trial. Patients assigned to CYC in SLS I had a lower DLCO%-predicted and a trend for more extensive QILD than patients assigned to CYC in SLS II. Moreover, the BDI was lower in SLS I-CYC compared with SLS II-CYC, which may have been due to discrepancies in the mode of administration of the BDI in SLS I and II as described in the Methods.
Table 1.
Baseline characteristics of participants assigned to CYC in SLS I and SLS II.*
| SLS I-CYC | SLS II-CYC | P-value | |
|---|---|---|---|
| Characteristics | (N=79) | (N=69) | |
| Age (yr) | 0.040δ | ||
| Mean | 48.4±12.3 | 52.2±9.6 | |
| Range | 28–81 | 28–71 | |
| Female sex (%) | 76.0 | 74.6 | 0.853 σ |
| Duration of scleroderma (yr)† | 0.308 Ψ | ||
| Median (IQR) | 2.4 (1.3, 4.6) | 1.9 (1.2, 4.0) | |
| Limited/Diffuse (%) | 38.0/62.0 | 46.2/53.9 | 0.322 σ |
| Race | 0.220 σ | ||
| White (%) | 67.1 | 67.7 | |
| African American (%) | 15.2 | 23.1 | |
| Asian (%) | 3.8 | 4.6 | |
| Other (%) | 13.9 | 4.6 | |
| FVC (% of predicted) | 67.6±11.4 | 66.9±9.9 | 0.704δ |
| DLCO (% of predicted) | 47.2±13.7 | 54.5±14.6 | 0.002 δ |
| Mahler Dyspnea Index (Focal score) | 5.6±1.8 | 7.1±2.4 | 0.0002 δ |
| Skin-thickness score (MRSS) | |||
| All patients | 0.302 Ψ | ||
| Median (IQR) | 12 (7, 22) | 12 (5, 20) | |
| Range | 2–51 | 2–46 | |
| Patients with dcSSc | 0.545 Ψ | ||
| Median (IQR) | 21 (14, 25) | 19 (12, 26) | |
| Range | 7–51 | 6–46 | |
| Patients with lcSSc | 0.894 Ψ | ||
| Median (IQR) | 5 (3, 9) | 5 (2, 8) | |
| Range | 2, 16 | 2, 18 | |
| QLF-WL, Median (IQR) | 7.5 (2.8, 12.8) | 7.4 (3.1, 13.1) | 0.816 Ψ |
| QLF-ZM, Median (IQR) | 23.5 (6.8, 46.0) | 18.2 (6.3, 34.3) | 0.244 Ψ |
| QILD-WL | 35.8±17.1 | 30.6±14.2 | 0.066 δ |
| QILD-ZM | 58.1±22.3 | 51.1±20.0 | 0.055 δ |
| Positive anti-nuclear antibody | Not available | 65/70 (92.9%) | |
| Positive anti-centromere antibody | Not available | 2/70 (2.9%) | |
| Positive anti-Scl-70 antibody | Not available | 31/70 (44.3%) | |
| Positive RNA Polymerase III antibody | Not available | 9/70 (12.6%) |
Values are mean ± standard deviation, unless otherwise noted.
Disease duration based on the onset of the first non-Raynaud’s symptom attributable to SSc.
δ t-est
Chi-square test
Wilcoxon rank-sum test
Definitions of abbreviations: FVC = forced vital capacity; DLCO = diffusing capacity of the lung for carbon monoxide; QLF = quantitative extent of lung fibrosis on HRCT; QILD = quantitative extent of total interstitial lung disease (including fibrosis, honeycomb and ground glass opacity); WL = whole lung; ZM = zone of maximal involvement. Scores for the Mahler Baseline Dyspnea Index (BDI) can range from 0 to 12, with lower scores indicating worse dyspnea. Scores for skin thickening (Modified Rodnan Skin Scores, MRSS) can range from 0 to 51, with higher scores indicating more severe thickening. dcSSc = diffuse cutaneous systemic sclerosis; lcSSc = limited cutaneous systemic sclerosis.
Disposition of Study Participants
In SLS II, 32 (50.8%) CYC patients prematurely withdrew from study drug, 2 failed treatment, and 11 died during the 24-month study period. In SLS I, 25 (31.6%) CYC patients prematurely withdrew from study drug, 4 failed treatment, and 4 died during 24-month study period (Supplemental Figure 1).
Use of Potential Disease Modifying Therapy
Of the 54 CYC arm patients followed during year two of SLS I, 10 began treatment with a glucocorticoid (e.g. prednisone, prednisolone, methylprednisolone) at a dosage of greater than or equal to 10 mg daily (Mean dose 14 mg daily) during this year “off study drug.” None of these patients consumed any other immunosuppressant therapies during this time.
Among the CYC arm patients in SLS II, 32 began treatment with a glucocorticoid at a dosage of greater than or equal to 10 mg daily (Mean dose 18.0 mg) during the 24-month study period. In addition, 10 began treatment with potentially disease-modifying immunosuppressant therapy during year 2 of the study (Azathioprine [N=2], MMF [N=7], CYC [N=1]).
For both studies, the type, duration and dosage of glucocorticoid used varied widely. Moreover, many patients did not receive a stable dosage, frequently stopping and starting glucocorticoid therapy or enduring long or short tapers.
There is No Difference in the Course of the FVC between CYC Arms
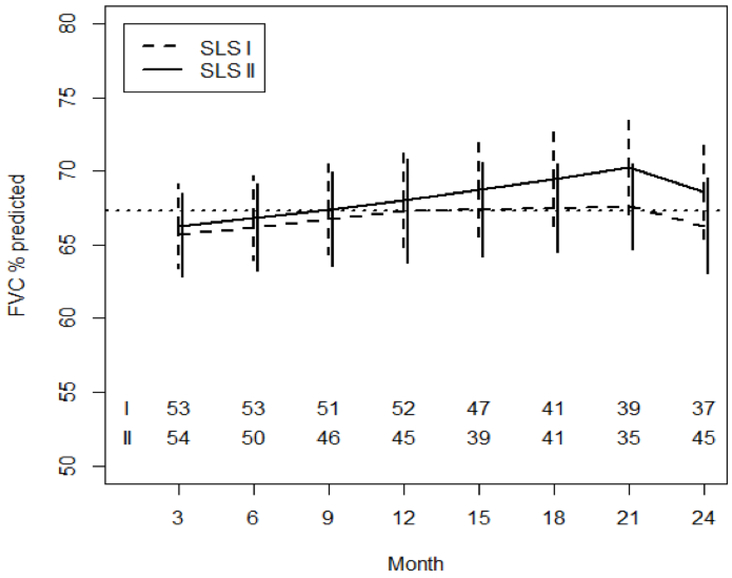
After controlling for baseline FVC%-predicted and baseline QILD-WL, there was no difference in the course of FVC%-predicted over 24 months between the CYC arms of SLS I and II (Table 2; Figure 1). From 3 to 12 months, patients in both CYC arms experienced an increase in the FVC%-predicted with no between arm differences (Figure 1). There appeared to be a persistent increase in the FVC%-predicted from 12 to 21 months in the CYC arm of SLS II; however, there was no significant difference in the course of the FVC%-predicted between CYC arms from 12–21 months, nor from 21–24 months (Table 2). At 24 months, the mean values for the FVC%-predicted were essentially unchanged in the SLS I CYC arm (Figure 1). In contrast, at 24 months, the mean values for the FVC%-predicted in the SLS II CYC arm improved by an average of +2.88 %-predicted for the CYC arm (95% CI 1.19–4.58; p<0.05).
Table 2.
Results of Joint Model Analysis Comparing the Course of the FVC%-predicted between patients assigned to CYC in SLS I and II (N=111).
| Covariate | Estimated Effect | Std Err | P-value |
|---|---|---|---|
| Time (3–12 months)* | 0.251 | 0.111 | 0.024 |
| Time (12–21 months)* | 0.034 | 0.114 | 0.766 |
| Time (21–24 months)* | −0.434 | 0.348 | 0.212 |
| Baseline FVC%-Predicted | 1 | 0.053 | <.0001 |
| Baseline QILD-WL | 0.026 | 0.048 | 0.588 |
| Treatment Arm Assignment† | 1.980 | 3.185 | 0.534 |
| Treatment Arm Assignment x Time Interaction (3–12 months) δ |
−0.012 | 0.172 | 0.944 |
| Treatment Arm Assignment x Time Interaction (12–21 months) δ |
0.214 | 0.171 | 0.211 |
| Treatment Arm Assignment x Time Interaction (21–24 months) δ |
−0.104 | 0.624 | 0.868 |
| Treatment Arm Assignment x Baseline QILD-WL |
0.023 | 0.071 | 0.746 |
The reference group is the CYC arm of SLS I; therefore, these time trends represent the trends observed in the CYC arm of SLS I. From 3–12 months, there was an increase in the FVC%predicted in the CYC arm of SLS I (Estimated effect −0.49), although this was not statistically significant.
This represents the estimate for baseline differences in FVC%-predicted by treatment arm.
These time trends represent the trends observed in the CYC arm of SLS II compared with the CYC arm of SLS I. There were no significant between group differences in these trends over the course of 24 months.
Figure 1. Course of the FVC% from 3 to 24 Months in CYC Patients in SLS I and II Using Joint Model Analysis.
Pre-specified covariates for this model included the baseline FVC%-predicted and baseline QILD-WL. The dotted line represents the mean baseline value for the entire cohort.
The joint model also revealed that patients with a higher FVC%-predicted at baseline had an improved course of FVC%-predicted over 24 months (Table 2). Baseline QILD-WL score was not associated with the course of the FVC%-predicted.
There is No Difference in the Course of the DLCO between CYC Arms
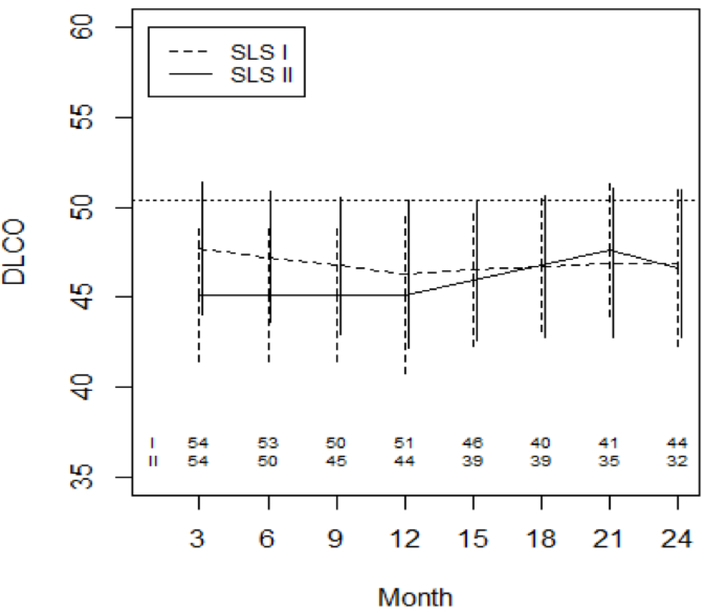
After controlling for baseline DLCO%-predicted and baseline QILD-WL, there was no difference in the course of DLCO%-predicted over 24 months between the CYC arms of SLS I and II (Supplemental Table 1; Figure 2). In SLS I, the DLCO%-predicted declined from baseline to 12 months and subsequently stabilized in the following 12 months (Figure 2). In SLS II, the DLCO%-predicted appeared to increase from 12–21 months (Supplemental Table 1), but there was no difference in the course of the DLCO%-predicted between CYC study arms from 3–12 months, 12–21 months, or 21–24 months. Of note, one developed pulmonary hypertension (PH) requiring therapy in the CYC arm of II; no patients developed PH requiring therapy in the CYC arm of SLS I during the 24-month study period.
Figure 2. Course of the DLCO% from 3 to 24 Months in SLS II Patients Assigned to MMF versus SLS I Patients Assigned to Placebo Using Joint Model Analysis.
Prespecified covariates for this model included the baseline DLCO%-predicted and baseline QILD-WL. The dotted line represents the mean baseline value for the entire cohort.
The joint model also revealed that patients with a higher DLCO%-predicted at baseline had an improved course of DLCO%-predicted over 24 months (Supplemental Table 1). Baseline QILD-WL score was not associated with the course of the DLCO%-predicted. Regardless of the baseline severity in the interstitial diseases measured by QILD-WL, subjects who were treated by CYC improved in FVC and DLCO % predicted values.
Treatment with CYC is Associated with Improved Course of MRSS in both CYC arms
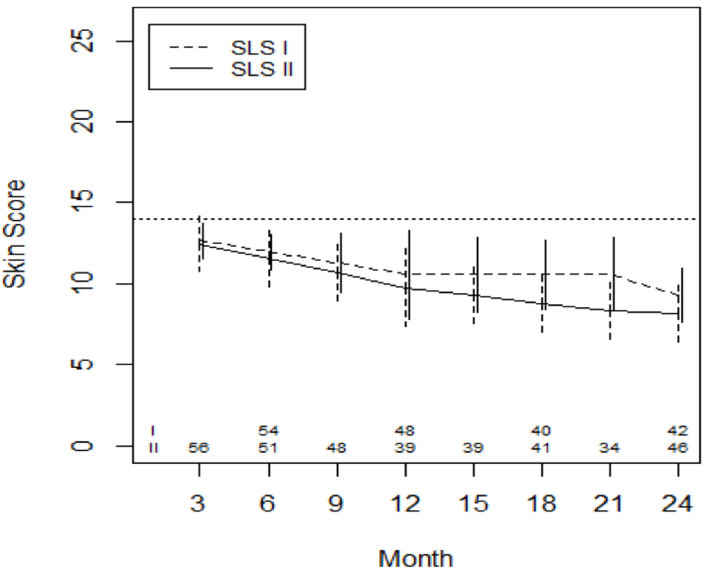
In all patients (those with diffuse and limited cutaneous SSc combined), after adjusting for baseline MRSS, there was a steady decline in the MRSS over 24 months, (Figure 3; Supplemental Table 2). There was no significant difference in the course of the MRSS between CYC arms from 3–12 months, 12–21 months, or 21–24 months (Supplemental Table 2).
Figure 3. Course of the MRSS from 3 to 24 Months in in CYC Patients in SLS I and II Using Joint Model Analysis.
Pre-specified covariates for this model included the baseline MRSS. The dotted line represents the mean baseline value for the entire cohort.
In patients with diffuse cutaneous disease (N= 49 for SLS I; N= 35 for SLS II), the rate of decline of the MRSS was greater within the first 12 months for both groups (Supplemental Figure 2; Supplemental Table 3). There was no significant difference in the course of the MRSS between CYC arms from 3–12 months, 12–21 months, or 21–24 months among patients with diffuse cutaneous disease (Supplemental Table 3).
In patients with limited cutaneous disease (N= 32 for SLS I; N= 30 for SLS II), the MRSS did not substantially change in either group (Supplemental Figure 3). Furthermore, there was no significant difference in the course of the MRSS between CYC arms from 3–12 months, 12–21 months, or 21–24 months among patients with diffuse cutaneous disease (Supplemental Table 4).
Not surprisingly, patients with a higher MRSS at baseline had a greater improvement in the course of the MRSS over 24 months for all patients (P=0.017) and for patients with diffuse cutaneous disease (P=0.032)
Safety Analysis
In terms of pre-defined AEs that would warrant clinical intervention and a change in therapy, there were similar rates of neutropenia (SLS I: 7; SLS II: 5) and pneumonia (SLS I: 6; SLS II: 4) between the CYC arms of SLS I and II (Table 3). However, hematuria occurred in numerically more CYC patients in SLS I compared with SLS II (SLS I: 10; SLS II: 4), while leukopenia (SLS I:19; SLS II: 30) and anemia (SLS I: 4; SLS II: 13) occurred in numerically more CYC patients in SLS II compared with SLS I. The majority of the anemia (70%) and leukopenia (91%) in CYC-SLS II patients occurred during the first year of the study.
Table 3.
Number of patients with adverse events and serious adverse events from baseline to 24 months.
| SLS I-CYC (N=79) |
SLS II-CYC (N=69) |
|
|---|---|---|
| Adverse event (AE)* | ||
| Leukopenia | 19 | 30 |
| Neutropenia | 7 | 5 |
| Anemia | 4 | 13 |
| Hematuria | 10 | 2 |
| Pneumonia | 6 | 4 |
| Serious adverse event (SAE) | ||
| Number of patients with SAEs | 47 | 22 |
| Related to treatment† | 13 | 8 |
| Not related to treatment† | 34 | 16 |
| Death | 6 | 11 |
Pre-defined by protocol as likely to be related to study drug and to warrant protocoldefined management (except for pneumonia): anemia = Hgb <10 gm/dl or <9 for those with Hgb <11 at enrollment; leukopenia = WBC <2500; neutropenia = neutrophils
<1000; thrombocytopenia = platelets <100,000; hematuria = >25 red blood cells (or 1015 red blood cells on more than one urinalysis) in absence of urinary tract infection or menses.
According to consensus classification by Morbidity and Mortality Committee.
In terms of SAEs, 47 patients experienced an SAE in the SLS I CYC arm compared with 22 patients in the SLS II CYC arm. In both CYC arms, the majority of these SAEs were judged by the Morbidity and Mortality Committee to not be related to treatment (72.3% for SLS I and 75.0% for SLS II) as described in Table 3. The number of deaths was greater in the SLS II-CYC patients (N=11) compared with the SLS I-CYC patients (N=4).
DISCUSSION
Historically, CYC was the treatment of choice for progressive ILD in patients with SSc. Two RCTs, SLS I and SLS II, evaluated the effects of one year of CYC therapy compared with placebo (SLS I) and mycophenolate (SLS II). In SLS II, the results seemed to suggest that CYC use was associated with a more sustained effect on treatment outcomes compared with SLS I. However, the analytic approaches employed in these two studies differed. The present study is the first to provide an in-depth analysis of outcomes for patients in the CYC arms from these two studies using a uniform analytic approach. The results reported herein demonstrate that efficacy outcomes were similar for both CYC arms.
Patients assigned to CYC in both SLS I and II experienced an improvement in the course of the FVC%-predicted during the first year of therapy with no between study arm differences (Figure 1). Following cessation of CYC, the FVC%-predicted appeared to continue to increase from 12–21 months in the CYC-SLS II arm; however, there was no significant difference in the course of the FVC%-predicted between CYC arms from 12–21 months. The mean values for the FVC%-predicted were unchanged from baseline to 24 months in the CYC-SLS I arm, while there was a slight improvement in the FVC%-predicted in the CYC-SLS II arm of 2.88%, although it is unknown whether this small change represents a clinically meaningful improvement.
The use of potentially disease modifying therapies in the second year of the study may have influenced the course of the FVC%-predicted during this time frame. For example, in SLS I, other than prednisone, no CYC participants started immunosuppressant therapy during year two [6]. However, 10 patients in the CYC-SLS II arm began treatment with immunosuppressant therapy during year two, and the majority started MMF [5]. It is possible that continued immunosuppression may have led to improved outcomes, although the paucity of patients receiving continued immunosuppression limits our ability to perform any meaningful analyses on this small subgroup. Furthermore, there is likely a strong selection bias as patients were probably more likely to receive continued immunosuppression if they experienced progression of their ILD.
In addition to the FVC%-predicted, there was no difference in the course of the DLCO%-predicted between the CYC arms after controlling for baseline disease severity (Figure 2). In both studies, the DLCO%-predicted remained essentially unchanged from baseline to 24 months. Very few patients in either study developed PH during the two-year study period.
In both joint models, higher baseline FVC and higher baseline DLCO were associated with an improved course of the FVC and DLCO, respectively. While the radiographic extent of ILD for the whole lung (QILD-WL) was associated with the course of the FVC and DLCO in univariate analysis. QILD-WL was not significantly associated with the course of the FVC and DLCO in the multivariate joint model analysis when the baseline FVC and DLCO measures were included as co-variates. This may have resulted from collinearity since the QILD scores correlated with the FVC and DLCO.
In terms of cutaneous sclerosis, the MRSS declined to a similar degree in both CYC arms over two years (Figure 3). Patients with diffuse cutaneous sclerosis experienced the greatest decline in the MRSS (Supplementary Figures 2 and 3). Unlike the lung parameters, the MRSS continued to decline in both CYC groups during the second year of the study off treatment, an observation that is consistent with the natural of history of cutaneous sclerosis in SSc [14]. Taken together, these findings suggest that continued immunosuppression (beyond one year) among patients with SSc-ILD may be beneficial for ILD outcomes, but may not be necessary for skin disease.
From a safety and tolerability standpoint, we observed differences in the rates of specific AEs in the CYC arms from both studies. For example, hematuria occurred in numerically more CYC patients in SLS I compared with SLS II, while leukopenia and anemia occurred in numerically more CYC patients in SLS II compared with SLS I. The reasons for these disparities are unclear as the dosage of CYC used in both studies were similar. However, the number of patients who experienced these AEs was relatively small in both studies; thus, these differences may be due to chance alone.
One striking observation was that over twice as many patients in the SLS I CYC arm experienced an SAE compared with the SLS II CYC arm. The definitions for SAEs were identical for both studies. A possible explanation for this observation is that more patients withdrew from study drug in the CYC arm of SLS II compared with SLS I, and perhaps these SLS II-CYC withdrawals occurred in patients who may have developed an SAE had they not withdrawn.
Interestingly, substantially more deaths occurred in the SLS II-CYC arm compared with the SLS I-CYC arm. Age may have contributed to the difference in death rates as patients in SLS II were older than patients in SLS I. However, given that this is an analysis of patients enrolled in two different studies, it is impossible to discern the exact reason for the observed differences in SAE and death rates. Overall, the safety and tolerability results from these two trials suggest that CYC does not appear to be well tolerated and is associated with a high number of SAEs, including death.
There are important limitations. First, comparing cohorts from two different trials can introduce bias. Time-period bias is one potential source of bias as enrollment for SLS I and II concluded in 2004 and 2012, respectively. Furthermore, without a randomization process, one cannot adequately control for differences in those unknown baseline features, which may affect ILD progression. Fortunately, the CYC arms from these two studies appeared relatively similar in terms of their key baseline features. Moreover, the patients for these two studies were recruited from similar academic centers (9 centers were the same for both trials) and were often treated by the same principal investigators [3,5].
A survival bias may also contribute to the diminished CYC-treatment effect in months 12 to 24. However, as stated in the Methods, our joint model analysis specifically adjusts for non-ignorable missing data due to study dropout, treatment failure or death.
Our study also has important strengths. First, the sample size is relatively large for an SSc-ILD interventional trial. Second, unlike many prior studies in this area, we did not evaluate an outcome measure at a single time point, but instead examined outcomes measured at multiple time points. Measuring ILD-related outcomes at multiple time points is likely a more meaningful reflection of ILD progression than a single outcome measurement.
To conclude, in patients with symptomatic SSc-ILD, treatment with one year of oral CYC is associated with short-term improvements in the FVC%-predicted and the MRSS, but not the DLCO%-predicted. Following treatment cessation, the FVC-related treatment effect diminished in the SLS I CYC arm, and to a lesser degree, in the SLS II CYC arm. As described previously, CYC use in both studies was associated with a number of treatment-related adverse events and serious adverse events, including a high death rate in SLS II. These findings suggest that alternate safe and effective therapy is still needed for SSc-ILD and that continued immunosuppression beyond one year may be necessary to achieve a sustained treatment response in patients with SSc-ILD.
Supplementary Material
ACKNOWLEDGEMENT
We thank the patients, investigators and coordinators who participated in the Scleroderma Lung Study I and II.
The following persons and institutions participated in the Scleroderma Lung Study 1: University of California at Los Angeles (UCLA), Los Angeles: P.J. Clements, D.P. Tashkin, R. Elashoff, J. Goldin, M. Roth, D. Furst, K. Bulpitt, D. Khanna, W.-L.J. Chung, S. Viasco, M. Sterz, L. Woolcock, X. Yan, J. Ho, S. Vasunilashorn, I. da Costa; University of Medicine and Dentistry of New Jersey, New Brunswick: J.R. Seibold, D.J. Riley, J.K. Amorosa, V.M. Hsu, D.A. McCloskey, J.E. Wilson; University of Illinois Chicago, Chicago: J. Varga, D. Schraufnagel, A. Wilbur, D. Lapota, S. Arami, P. Cole-Saffold; Boston University, Boston: R. Simms, A. Theodore, P. Clarke, J. Korn, K. Tobin, M. Nuite; Medical University of South Carolina, Charleston: R. Silver, M. Bolster, C. Strange, S. Schabel, E. Smith, J. Arnold, K. Caldwell, M. Bonner; Johns Hopkins School of Medicine, Baltimore: R. Wise, F. Wigley, B. White, L. Hummers, M. Bohlman, A. Polito, G. Leatherman, E. Forbes, M. Daniel; Georgetown University, Washington, D.C.: V. Steen, C. Read, C. Cooper, S. Wheaton, A. Carey, A. Ortiz; University of Texas at Houston, Houston: M. Mayes, E. Parsley, S. Oldham, T. Filemon, S. Jordan, M. Perry; University of California at San Francisco, San Francisco: K. Connolly, J. Golden, P. Wolters, R. Webb, J. Davis, C. Antolos, C. Maynetto; University of Alabama at Birmingham, Birmingham: B. Fessler, M. Olman, C. Sanders, L. Heck, T. Parkhill; University of Connecticut Health Center, Farmington: N. Rothfield, M. Metersky, R. Cobb, M. Aberles, F. Ingenito, E. Breen; Wayne State University, Detroit: M. Mayes, K. Mubarak, J.L. Granda, J. Silva, Z. Injic, R. Alexander; Virginia Mason Research Center, Seattle: D. Furst, S. Springmeyer, S. Kirkland, J. Molitor, R. Hinke, A. Mondt; Data Safety and Monitoring Board: Harvard Medical School, Boston — T. Thompson; Veterans Affairs Medical Center, Brown University, Providence, R.I. — S. Rounds; Cedars Sinai–UCLA, Los Angeles — M. Weinstein; Clinical Trials Surveys, Baltimore — B. Thompson; Mortality and Morbidity Review Committee: UCLA, Los Angeles — H. Paulus, S. Levy; Johns Hopkins University, Baltimore — D. Martin.
The following persons and institutions participated in the Scleroderma Lung Study 2: University of Boston, Boston: A.C. Theodore, R.W. Simms, E. Kissin, F.Y. Cheong; Georgetown University, Washington, D.C.: V.D. Steen, C.A. Read Jr., C. Fridley, M. Zulmatashvili; Johns Hopkins University, Baltimore: R.A. Wise, F.M. Wigley, L. Hummers, G. Leatherman; Medical University of South Carolina, Charleston: R.M. Silver, C. Strange, F.N. Hant, J. Ham, K. Gibson, D. Rosson; University of California, Los Angeles (UCLA), Los Angeles: D.P. Tashkin, R.M. Elashoff, M.D. Roth, P.J. Clements, D. Furst, E. Volkmann, S. Kafaja, E. Kleerup, D. Elashoff, J. Goldin, E. Ariola, G. Marlis, J. Mason-Berry, P. Saffold, M. Rodriguez, L. Guzman, J. Brook; University of California, San Francisco (UCSF), San Francisco: J. Golden, M.K. Connolly, A. Eller, D. Leong, M. Lalosh, J. Obata; University of Illinois, Chicago: S. Volkov, D. Schraufnagel, S. Arami, D. Franklin; Northwestern University, Chicago: J. Varga, J. Dematte, M. Hinchcliff, C. DeLuca, H. Donnelly, C. Marlin; University of Medicine and Dentistry of New Jersey, New Brunswick: D.J. Riley, V.M. Hsu, D.A. McCloskey; University of Michigan, Ann Arbor: K. Phillips, D. Khanna, F.J. Martinez, E. Schiopu, J. Konkle; University of Texas, Houston: M. Mayes, B. Patel, S. Assassi, F. Tan; National Jewish Health, Denver: A. Fischer, J. Swigris, R. Meehan, K. Brown, T. Warren, M. Morrison; University of Utah, Salt Lake City: M. B. Scholand, T. Frecht, P. Carey, M. Villegas; University of Minnesota, Minneapolis: J. Molitor, P. Carlson.
Funding: This work was supported by grants from the NHLBI/NIH: R01 HL089758 (DPT) and R01 HL089901 (RME), NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124 (NL), the Scleroderma Foundation (ERV), the Rheumatology Research Foundation (ERV).
Footnotes
Competing interests: No non-financial conflicts of interest exist for any of the authors.
Financial conflicts of interest appear below:
PJC, RME, JG, GK, AMHV, MS and NL all report nothing to disclose.
ERV reports personal fees from Boerhinger Ingelheim, Astellas Pharma, and grants from EMD Serono.
DPT reports personal fees from EMD Serono, and non-financial support from Genentech, during the conduct of the study.
MDR reports non-financial support from Hoffmann-La Roche/Genentech, during the conduct of the study.
DEF reports Grant/Research support from AbbVie, Actelion, Amgen, BMS, Gilead, GSK, Novartis, Pfizer, Roche/Genentech,UCB, and consultant work with AbbVie, Amgen, BMS, Cytori, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB Speaker’s Bureau (CME ONLY) AbbVie, Actelion, UCB during the course of the study.
DK reports personal fees from: Actelion, Astra Zeneca, Bayer, Boehringer-Ingelheim, Chemomab, Corbus, Covis, Cytori, Eicos, EMD Sereno, Genentech/Roche, Gilead, GSK, Sanofi-Aventis, UCB Pharma. He reports grants from Bayer, Boehringer-Ingelheim, Genentech/Roche, Pfizer, Sanofi-Aventis.
REFERENCES
- 1.Elhai M, Meune C, Avouac J, Kahan A, Allanore. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatol (Oxford) 2012;51:1017–26. [DOI] [PubMed] [Google Scholar]
- 2.Tyndall AJ, Bannert B, Vonk M, Airo P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 2010;69:1809–15. [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth M, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med 2006;354:2655–66. [DOI] [PubMed] [Google Scholar]
- 4.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum 2006;54:3962–70. [DOI] [PubMed] [Google Scholar]
- 5.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease: Scleroderma lung study II (SLS-II), a double-blind, parallel group, randomised controlled trial. Lancet Respir Med 2016;4:708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. Am J Respir Crit Care Med 2007;176:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Elashoff RM, Li G, Tseng CH. Joint analysis of bivariate longitudinal ordinal outcomes and competing risks survival times with nonparametric distributions for random effects. Stat Med 2012;31:1707–21. [DOI] [PubMed] [Google Scholar]
- 8.Elashoff RM, Li G, Li N. A joint model for longitudinal measurements and survival data in the presence of multiple failure types. Biometrics 2008;64:762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement and physiologic correlates of two new clinical indexes. Chest 1984;85:751–758. [DOI] [PubMed] [Google Scholar]
- 10.Mahler DA, Ward J, Fierro-Carrion G, Waterman LA, Lentine TF, Mejia-Alfaro R, et al. Development of self-administered versions of modified baseline and transition dyspnea indexes in COPD. COPD: J of COPD 2004;1:1–8. [DOI] [PubMed] [Google Scholar]
- 11.Clements PJ, Lachenbruch PA, Seibold JR, Zee B, Steen VD, Brennan P, et al. Skin thickness score in systemic sclerosis (SSc): An assessment of inter-observer variability in three independent studies. J Rheumatol 1993;20:1892–6. [PubMed] [Google Scholar]
- 12.Goldin J, Elashoff R, Kim HJ, Yan X, Lynch D, Strollo D, et al. Treatment of scleroderma-interstitial lung disease with cyclophosphamide is associated with less progressive fibrosis on serial thoracic high-resolution CT scan than placebo: findings from the scleroderma lung study. Chest 2009;136:1333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HJ, Li G, Gjertson D, Elashoff R, Shah SK, Ochs R, et al. Classification of parenchymal abnormality in scleroderma lung using a novel approach to denoise images collected via a multicenter study. Acad Radiol 2008;15:1004–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clements PJ, Medsger TA Jr, Feghali CA. Cutaneous involvement in systemic sclerosis In: Clements PJ, Furst DE, editors. Systemic sclerosis. 2nd Lippincott Williams and Wilkins; 2004. p. 129–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.