Abstract
Neuroticism is associated with greater reactivity to stress and lifetime psychopathology. In the present study we examined the association between neuroticism and regional and total cortical thickness (CT) across the lifespan, accounting for gender. We also assessed interactions among these factors. 450 subjects between 19 and 80 years were included. Participants completed the International Personality Item Pool and a structural MRI scan. Total CT and the mean values of CT in five regions of interest were examined. We also investigated the interaction effect among age, gender and neuroticism on CT. There was no significant association between neuroticism and regional/total CT. A significant interaction between neuroticism, age, and gender on the thickness of the anterior cingulate was found. Women high in neuroticism showed a thinner anterior cingulate cortex than women low in neuroticism, with increasing age. In contrast, men high in neuroticism had a thicker anterior cingulate cortex compared to men low in neuroticism, with increasing age. Overall, high neuroticism was associated with differential cortical thickness in the anterior cingulate among men and women with increasing age.
Keywords: Aging, Gyrus cinguli, Magnetic resonance imaging, Personality, Psychopathology
1. Introduction
Neuroticism is the only personality trait that has been found to be highly associated with future development of psychopathology (Jeronimus et al., 2016; Krueger et al., 1996). It is characterized by emotional instability, self-consciousness, anxiety, negative affect, and sensitivity to negative environmental stimuli (Izard et al., 1993; Larsen and Ketelaar, 1991; Rusting and Larsen, 1997). Disorders commonly associated with high neuroticism include depression, anxiety, eating disorders, substance abuse, schizophrenia, and Alzheimer’s Disease (AD) (Clark et al., 1994; Davis, 1997; Jylhä and Isometsä, 2006; Kilbey et al., 1992; Ormel et al., 2004; Prescott et al., 1997; Van Os and Jones, 2001; Wilson et al., 2006).
The literature on brain imaging of neuroticism and aging has been scarce. One twin study also showed that differences in levels of neuroticism associated with genetics become more substantial with age (Eaves and Eysenck, 1976).
Decreasing cortical thickness (CT) occurs during normal aging in the prefrontal cortex, but there are regional and age-related differences in heritability of cortical thickness (DeCarli et al., 1994; Jernigan et al., 2001; Lenroot et al., 2009; Raz et al., 1997; Sowell et al., 2003). Studies of the association between neuroticism and cortical thickness (CT) in specific brain regions have shown mixed results. A positive correlation was found between neuroticism and CT in the left parietal lobe in young women (Privado et al., 2017). In contrast, a study with a larger sample of adults over 20 years old of both genders found no relationship between neuroticism and CT (Bjørnebekk et al., 2013).
Additional studies point out the need to consider factors such as gender and age when investigating the relationship between neuroticism and regional CT. Wright et al. (2006) initially found an inverse relationship between neuroticism and orbitofrontal CT, but when gender was included as a covariate, these differences became non-significant. Another study found neuroticism was positively correlated with CT in the subgenual anterior cingulate cortex in females whereas the relationship was negative in males (Blankstein et al., 2009).
Other studies suggest that there could be differences in the relationship between neuroticism and regional CT as a function of age. In young subjects, neuroticism was found to be associated with thinner left orbitofrontal cortex (Wright et al., 2006), while in another study of elders, an association between neuroticism and thinner right superior frontal cortex and inferior frontal cortex was found (Wright et al., 2007). There have not yet been any studies comparing young and elderly subjects in terms of neuroticism and regional cortical thickness. Given that neuroticism remains stable throughout the lifespan, since it is a personality trait, its possible role in changes in brain morphology is lacking investigation, especially because it has been shown to be related to differences in CT and rates of future development of psychopathology.
There is currently no existing research on interactions among neuroticism, age, and gender on regional cortical thickness. Further research including a broader age range and taking account of gender, could better clarify the influence of these variables on the relationship between neuroticism and regional CT. Additionally, since CT may be affected by aging, investigation into differences across the lifespan may offer insight into cumulative effects of personality differences over the lifespan. The potentially complex interplay of these factors could help elucidate underlying etiology of many neurological and psychological disorders.
Thus, the aim of the present study was to examine the relationship between self-reported neuroticism and regional/total CT, in a large sample of adults, across the lifespan. Neuroticism was used as a predictor of CT since personality traits are stable throughout the lifespan, thus it could hold implications for morphological differences in aging or vulnerability to various neurological and psychiatric disorders. We further investigated this relationship by examining the potential interactions among the influence of age, gender, and neuroticism on regional CT.
2. Methods
2.1. Participants
The participating pool was from two large ongoing studies, the Reference Ability Neural Network (RANN) study and the Cognitive Reserve (CR) study. The RANN study examines the neural basis of age-related changes in four cognitive abilities. The CR study investigates differences in cognitive processes or neural networks that allow some people with brain damage to perform better than others. More details can be found in previous publications (Habeck et al., 2016; Stern, 2009; Stern et al., 2014).
Four hundred and fifty subjects were included in the study. The demographics and characteristics of the sample are shown in Table 1. Participants were native English speakers, strongly right-handed, and had at least a fourth-grade reading level. They had a mean age of 53 with a standard deviation of 17 years and ranged from age 19 to 80. The average length of education was 16 years with a standard deviation of 2.5 years. The sample was 55% female. Participants were recruited by market mailing or referrals from enrolled participants. Participants were included if they were English speakers, strongly right-handed according to the Edinburgh Handedness Index, and had at least a 4th grade reading level (WRAT-3 reading subtest), a score of at least 130 on the Mattis Dementia Rating Scale, and normal or corrected-to-normal vision (Lucas et al., 1998). Exclusion criteria included contraindications for MRI or any major medical, neurological, or psychiatric conditions. Thus, all participants had to be cognitively normal, without a diagnosis of Mild Cognitive Impairment (MCI) or dementia at baseline. The study was approved by the Institutional Review Board (IRB) of the College of Physicians and Surgeons of Columbia University. All participants provided written informed consent.
Table 1.
Demographic and clinical characteristics of our sample.
| Characteristics | Total |
|---|---|
| Total, N | 450 |
| Age, mean (SD) | 53 (17) |
| Range | 19–80 |
| Education, years, mean (SD) | 16 (2.5) |
| Females, N (%) | 247 (55) |
| Race, N (%) | |
| Asian | 32 (5.1) |
| Black/African American | 171 (27.1) |
| Hispanic/Spanish | 1 (0.2) |
| NA | 2 (0.3) |
| Other | 61 (9.6) |
| Pacific Islander | 5 (0.8) |
| White | 363 (57.1) |
| Anterior cingulate cortical Thickness, mean (SD) | 5.487 (0.424) |
| Neuroticism, mean (SD) | 2.421 (0.783) |
2.2. Materials
2.2.1. Personality measurements
The 50-item International Personality Item Pool (IPIP) was administered by self-report. The IPIP measures the Big-Five factors of extraversion, agreeableness, conscientiousness, neuroticism, and intellect/imagination (Donnellan et al., 2006). For each personality domain the scale ranged from 1 to 5, with higher scores indicating higher levels of the trait. Reliability, as measured by Cronbach’s alpha, is 0.864 for neuroticism (Socha et al., 2010). To create the graphs depicting the three-way interaction, those participants who scored above the mean (2.4) on the Neuroticism scale of the IPIP were considered “high neuroticism” and those with scores below the median were considered as “low neuroticism”.
2.3. Procedure
2.3.1. Structural MRI scan and image processing
MRI images were acquired on a 3.0T Philips Achieva Magnet. Each scan used 240 mm field of view. The parameters for EPI acquisition were TE/TR (ms) 20/2000; Flip Angle 72°; In-plane resolution (voxels) 112 × 112; Slice thickness/gap (mm) 3/0; Slices 41. T1 scans for each participant were reconstructed with FreeSurfer (v5.1.0) software for human brain imaging analysis (http://surfer.nmr.mgh.harvard.edu). Cortical thickness in the regions was computed by standard FreeSurfer parcellation (Desikan et al., 2006). Reconstructions were checked, and if needed, manual control points and repeated reconstruction were used. CT values for regions, as well as the total CT were computed for each subject. FreeSurfer’s subcortical segmentation and cortical parcellation has been shown to have comparable accuracy to manual labeling (Fischl et al., 2002, 2004).
2.4. Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) 23 (SPSS, Chicago, Illinois). Nominally significant alpha values were defined as p < 0.05. All of the variables were normally distributed, according to descriptive tests of asymmetry and kurtosis and visual inspection of histograms in SPSS.
Multiple linear regression models including neuroticism, age, gender, and their interactions were used to examine the association between each regional CT and neuroticism.
Based on the existing literature examining the relationship between neuroticism and regional CT, the following regions were included (a priori) in the analyses: frontal, temporal, parietal, anterior cingulate, and orbitofrontal (Bjørnebekk et al., 2013; Blankstein et al., 2009; Privado et al., 2017; Wright et al., 2007, 2006). Total CT was also included. Neuroticism, age, intracranial volume (ICV), education level (years), and regional and total CT were used as continuous variables.
3. Results
There were no significant associations between neuroticism and CT in the frontal (B = −0.023, p = 0.459), temporal (B = −0.042, p = 0.425), parietal (B = −0.031, p = 0.328), anterior cingulate (B = −0.002, p = 0.930), or orbitofrontal (B = −0.200, p = 0.580) regions according to a multiple linear regression analysis which used neuroticism, age, gender, and their interactions to predict regional CT and total CT. The results for each region are shown in Table 2. The results were also non-significant for total CT (B = 0.006, p = 0.723) (Table 2). This indicates that no significant relationship was found between neuroticism and the selected regions and total CT.
Table 2.
Adjusted model (ICV, education).
| Region of interest | Neuroticism effect | Interaction Age x Neuroticism | Interaction Gender x Neuroticism | Interaction Age x Gender x Neuroticism |
|---|---|---|---|---|
| Frontal |
F = 22.335, p = 0.000 B = −0.023, p = 0.459 |
B = 0.001, p = 0.708 | B = −0.035, p = 0.262 | B = 0.000, p = 0.882 |
| Temporal |
F = 20.049, p = 0.000 B = −0.042, p = 0.425 |
B = −0.001, p = 0.667 | B = 0.011, p = 0.829 | B = −0.002, p = 0.580 |
| Parietal |
F = 9.300, p = 0.000 B = −0.031, p = 0.328 |
B = 0.001, p = 0.694 | B = 0.01, p = 0.762 | B = 0.000, p = 0.895 |
| Orbitofrontal |
F = 14.530, p = 0.000 B = −0.200, p = 0.580 |
B = −0.001, p = 0.771 | B = −0.034, p = 0.345 | B = 0.000, p = 0.820 |
| Anterior cingulate |
F = 3.566, p = 0.001 B = −0.002, p = 0.930 |
B = −0.002, p = 0.283 | B = 0.036, p = 0.199 | B = 0.004, p = 0.012 |
| Total CT |
F = 1.314, p = 0.236 B = 0.006, p = 0.723 |
B = 0.000, p = 0.673 | B = −0.002, p = 0.901 | B = 0.000, p = 0.761 |
Significance level p ≤ .05.
Next, we investigated the interaction effects for the relation of neuroticism, gender, and age to regional and total CT. Both the two-way interactions among age and neuroticism as well as gender and neuroticism were tested. The two-way interactions in the model were not significant in any brain region nor in total CT (Table 2). However, there was a significant interaction effect among age, gender, and neuroticism on anterior cingulate CT (B = 0.004, p = 0.012). Table 2 shows the interaction among age, neuroticism, and anterior cingulate CT for males and for females. The presence of only a three-way interaction indicates that probably, the three variables act together on the anterior cingulate, but neither age nor gender alone interacts with neuroticism to produce any effect. The three-way interaction among age, gender, and neuroticism is such that women high in neuroticism had thinner cortex in the anterior cingulate with increasing age in comparison to women with low neuroticism. For men, those high in neuroticism had thicker cortex in the same area with increasing age, compared to men with low neuroticism.
The three-way interaction is shown in Fig. 1. The sample was split at the mean for age and neuroticism in order to visualize the three-way interaction by using two endpoints for each line depicting high or low neuroticism. No statistical analyses were done with the split sample.
Fig. 1.
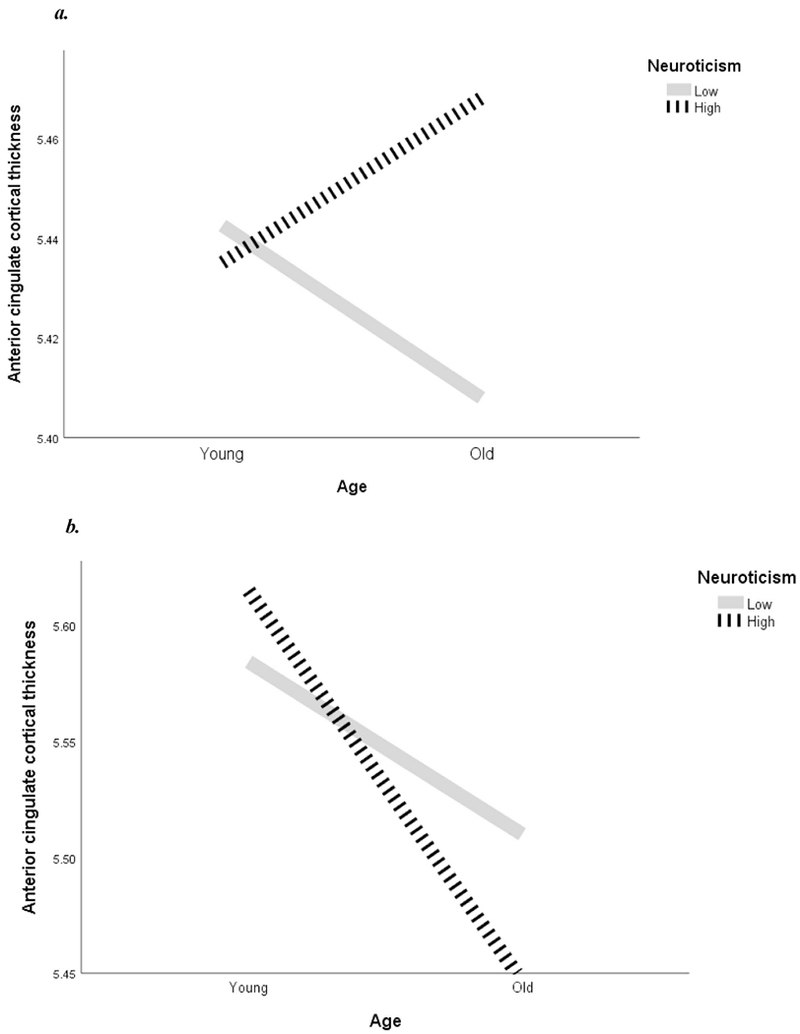
(a) Interaction among neuroticism, gender, and age in males, (b) Interaction among neuroticism, gender, and age in females.
In conclusion, our hypothesis for an effect of neuroticism on the selected brain regions and total CT was not confirmed; however, we did find a three-way interaction among neuroticism, age, and gender, as predicted (p = 0.012). Thus, the objective of the study, to examine the relationship among neuroticism and cortical thickness in several brain regions was met with the consideration of additional factors in their relationship.
4. Discussion
The current study investigated the associations between neuroticism and regional CT. There were no significant associations between neuroticism and total CT, or frontal, temporal, parietal, anterior cingulate, or orbitofrontal CT. There was a significant interaction between the effects of age, gender, and neuroticism on anterior cingulate CT. Analysis of the interaction effect indicates that high neuroticism in females is associated with a thinner anterior cingulate cortex with increasing age. In contrast, in males, high neuroticism was associated with a thicker anterior cingulate cortex as age increased. This suggests that neuroticism may moderate age-related differences on anterior cingulate CT, depending on gender.
No significant associations between neuroticism and regional or total CT were found. Although the sample was large, the effect may be too small to have been detected in this sample. Alternatively, given the interaction between age, gender, and neuroticism, the effect of neuroticism on CT may be obscured by the opposing effects of gender and age. Since older men high in neuroticism had greater CT in the anterior cingulate than older women high in neuroticism, when the sample was not split by gender, the effects would be opposite and appear insignificant overall when the sample is combined.
The anterior cingulate is implicated in numerous psychological and neurological disorders so an interaction in this region may warrant further investigation due to its relevance to psychopathology (Cotter et al., 2001; Etkin et al., 2010; Pizzagalli et al., 2001; Rosenberg et al., 2004; Tekin et al., 2001). The region has previously been found to be associated with anxiety and mood disorders, with those higher in neuroticism showing greater activation in the region during emotional conflict (Haas et al., 2007). In addition, another study found late-onset depression to be associated with a thinner anterior cingulate cortex compared to controls (Lim et al., 2012). Lastly, a thinner cortex in the anterior cingulate has been found in individuals with AD in comparison with controls (Lerch et al., 2004). Such findings highlight the importance of this region in psychopathology and aging.
Numerous studies have found cortical thinning to occur during aging, but there is a pattern of thinning that spares certain brain regions (Fjell et al., 2009; Salat et al., 2004). In terms of the cingulate gyrus, the extant research is sparse and mixed. Two studies found thinning in the region, while another found thickening with age (Fjell et al., 2009; Salat et al., 2004; Thambisetty et al., 2010). The present study found that gender and level of neuroticism is related to the direction and magnitude of the CT difference across ages. Females high in neuroticism showed a thinner anterior cingulate cortex than females low in neuroticism with increasing age. Males high in neuroticism showed the opposite pattern, with a thicker cortex in this region with age while males low in neuroticism showed a thinner cortex with increasing age. Therefore, it appears that age-related differences in CT in the anterior cingulate might be at least partly driven by gender and level of neuroticism.
Gender, age, and neuroticism have all independently been found to be associated with various psychopathologies, but no studies have investigated the effect of an interaction among these factors on brain physiology. To our knowledge, there is also no existing research on interactions involving neuroticism on CT. One previous study found an interaction between neuroticism and age on cerebral white matter volume, with individuals higher in neuroticism having thinner cortex in this region with increasing age (Jackson et al., 2011). Although the present study focused on CT, interactions affecting white matter volume may be relevant since CT is one component of volume.
There are no existing studies examining interactions with gender on regional cortical thickness. However, a number of differences between the genders may account for the opposite pattern found between men and women in this study. The discrepancy may be partially explained by existing differences in CT between genders. Females appear to have a thicker cortex in numerous brain regions, with the cingulate gyrus having one of the largest differences in thicknesses between genders (Luders et al., 2006; Sowell et al., 2006). This pre-existing difference in cortical thickness could impact the magnitude and direction of CT across age.
Finally, these results may also provide insight into gender differences in the prevalence of certain mental illnesses. Depression and anxiety disorders are more common in women, while substance use and suicide are more common in men (Baum and Grunberg, 1991; Boyd and Weissman, 1981; Canetto, 1991; Cleary, 1987). Since the effect of high neuroticism on CT in the anterior cingulate across ages affects men and women differently, it is possible that this is related to the differential rates of specific mental illnesses by gender. These results suggest that further investigation into the links among neuroticism, gender, age, and psychopathology may be warranted.
The current study has some limitations that should be noted. This study had a cross-sectional design so changes in CT during aging were not able to be investigated. There may also be cohort effects due to this design. For instance, one study showed that neuroticism scores increased between 1953 and 1993 (Twenge, 2000). These differences are hypothesized to relate to environmental and sociocultural factors, which could result in a cohort effect where some subjects of similar ages had different environmental experiences from other subjects that influence neuroticism scores (Twenge, 2000). Additionally, the IPIP is a self-reported measure.
This study, however, also has several strengths. The study’s large sample size allowed for a more powerful analysis. It also had one of the largest age ranges among CT and neuroticism studies. Furthermore, it used more accurate imaging methods to investigate CT as a more specific measure of physiological differences. Lastly, it used a cognitively healthy group of participants.
Future research should focus on identifying additional brain regions that may be affected by neuroticism or its interaction with other factors. Lastly, longitudinal research on the relationship between the interaction among age, gender, and neuroticism with psychopathology could determine causation and direction of the effect.
In summary, the interaction between age, gender, and neuroticism in the anterior cingulate may have important implications for psychopathology research. It also could play a role in rectifying existing discrepancies in the literature on CT differences due to age and personality. It appears that there is a complex relationship among neuroticism, age, gender, and regional CT.
Acknowledgments
Funding: This research was funded by the National Institute on Aging (RO1AG038465 and ROIAG026158, both to Dr. Stern). The sponsors had no role in the study design, data collection, analysis, interpretation, writing of the report, or decision to submit the article for publication.
Footnotes
Competing interests
The authors declare no competing interests.
References
- Baum A, Grunberg NE, 1991. Gender, stress, and health. Health Psychol. 10, 80–85. [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A, Fjell AM, Walhovd KB, Grydeland H, Torgersen S, Westlye LT, 2013. Neuronal correlates of the five factor model (FFM) of human personality: multimodal imaging in a large healthy sample. Neuroimage 65, 194–208. [DOI] [PubMed] [Google Scholar]
- Blankstein U, Chen JY, Mincic AM, McGrath PA, Davis KD, 2009. The complex minds of teenagers: neuroanatomy of personality differs between sexes. Neuropsychol 47, 599–603. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Weissman MM, 1981. Epidemiology of affective disorders: a reexamination and future directions. Arch. Gen. Psychiatry 38, 1039–1046. [DOI] [PubMed] [Google Scholar]
- Canetto SS, 1991. Gender roles, suicide attempts, and substance abuse. J. Psychol 125, 605–620. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Mineka S, 1994. Temperament, personality, and the mood and anxiety disorders. J. Abnorm. Psychol 103, 103. [PubMed] [Google Scholar]
- Cleary PD, 1987. Gender differences in stress-related disorders. Gend. Stress 39–72. [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I, 2001. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry 58, 545–553. [DOI] [PubMed] [Google Scholar]
- Davis C, 1997. Normal and neurotic perfectionism in eating disorders: an interactive model. Int. J. Eat. Disord 22, 421–426. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Murphy D, Gillette J, Haxby J, Teichberg D,, Schapiro M, Horwitz B, 1994. Lack of age-related differences in temporal lobe volume of very healthy adults. Am. J. Neuroradiol 15, 689–696. [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. [DOI] [PubMed] [Google Scholar]
- Donnellan MB, Oswald FL, Baird BM, Lucas RE, 2006. The mini-IPIP scales: tinyyet-effective measures of the Big Five factors of personality. Psychological assessment 18, 192. [DOI] [PubMed] [Google Scholar]
- Eaves L, Eysenck H, 1976. Genotype x age interaction for neuroticism. Behav. Genet 6, 359–362. [DOI] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF, 2010. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am. J. Psychiatry 167, 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, 2002. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, 2004. Automatically parcellating the human cerebral cortex. Cereb. Cortex 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, 2009. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex 19, 2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T, 2007. Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci 121, 249. [DOI] [PubMed] [Google Scholar]
- Habeck C, Gazes Y, Razlighi Q, Steffener J, Brickman A, Barulli D, Salthouse T, Stern Y, 2016. The Reference Ability Neural Network Study: life-time stability of reference-ability neural networks derived from task maps of young adults. Neuroimage 125, 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izard CE, Libero DZ, Putnam P, Haynes OM, 1993. Stability of emotion experiences and their relations to traits of personality. J. Personal. Soc. Psychol 64, 847. [DOI] [PubMed] [Google Scholar]
- Jackson J, Balota DA, Head D, 2011. Exploring the relationship between personality and regional brain volume in healthy aging. Neurobiol. Aging 32, 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J , Hesselink JR, 2001. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol. Aging 22, 581–594. [DOI] [PubMed] [Google Scholar]
- Jeronimus B, Kotov R, Riese H, Ormel J, 2016. Neuroticism’s prospective association with mental disorders halves after adjustment for baseline symptoms and psychiatric history, but the adjusted association hardly decays with time: a meta-analysis on 59 longitudinal/prospective studies with 443 313 participants. Psychol. Med 46, 2883–2906. [DOI] [PubMed] [Google Scholar]
- Jylhä P, Isometsä E, 2006. The relationship of neuroticism and extraversion to symptoms of anxiety and depression in the general population. Depress. Anxiety 23, 281–289. [DOI] [PubMed] [Google Scholar]
- Kilbey MM, Breslau N, Andreski P, 1992. Cocaine use and dependence in young adults: associated psychiatric disorders and personality traits. Drug Alcohol Depend. 29, 283–290. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Caspi A, Moffitt TE, Silva PA, McGee R, 1996. Personality traits are differentially linked to mental disorders: a multitrait-multidiagnosis study of an adolescent birth cohort. J. Abnorm. Psychol 105, 299. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T, 1991. Personality and susceptibility to positive and negative emotional states. J. Personal. Soc. Psychol 61, 132. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN, 2009. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum. Brain Mapp 30, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC, 2004. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb. Cortex 15, 995–1001. [DOI] [PubMed] [Google Scholar]
- Lim HK, Jung WS, Ahn KJ, Won WY, Hahn C, Lee SY, Kim I, Lee CU, 2012. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology 37, 838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC, 1998. Normative data for the Mattis dementia rating scale. J. Clin. Exp. Neuropsychol 20, 536–547. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, DeLuca H, Jancke L, Toga AW, 2006. Gender effects on cortical thickness and the influence of scaling. Hum. Brain Mapp 27, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Rosmalen J, Farmer A, 2004. Neuroticism: a non-informative marker of vulnerability to psychopathology. Soc. Psychiatry Psychiatr. Epidemiol 39, 906–912. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Davidson RJ, 2001. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am. J. Psychiatry 158, 405–415. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Neale MC, Corey LA, Kendler KS, 1997. Predictors of problem drinking and alcohol dependence in a population-based sample of female twins. J. Stud. Alcohol 58, 167–181. [DOI] [PubMed] [Google Scholar]
- Privado J, Román FJ, Saénz-Urturi C, Burgaleta M, Colom R, 2017. Gray and white matter correlates of the Big Five personality traits. Neurosci 349, 174–184. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD, 1997. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb. cortex 7, 268–282. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, Bhandari R, Rose M, Ivey J, Boyd C, 2004. Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J. Am. Acad. Child Adolesc. Psychiatry 43, 1146–1153. [DOI] [PubMed] [Google Scholar]
- Rusting CL, Larsen RJ, 1997. Extraversion, neuroticism, and susceptibility to positive and negative affect: a test of two theoretical models. Personal. Individ. Differ 22, 607–612. [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B, 2004. Thinning of the cerebral cortex in aging. Cerebr. Cortex 14, 721–730. [DOI] [PubMed] [Google Scholar]
- Socha A, Cooper CA, McCord DM, 2010. Confirmatory factor analysis of the M5-50: an implementation of the International Personality Item Pool item set. Psychol. Assess 22, 43. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW, 2006. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cerebr. Cortex 17, 1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW, 2003. Mapping cortical change across the human life span. Nat. Neurosci 6, 309. [DOI] [PubMed] [Google Scholar]
- Stern Y, 2009. Cognitive reserve. Neuropsychology 47, 2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Habeck C, Steffener J, Barulli D, Gazes Y, Razlighi Q, Shaked D, Salthouse T, 2014. The Reference Ability Neural Network Study: motivation, design, and initial feasibility analyses. Neuroimage 103, 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters HV, Cummings JL, 2001. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann. Neurol 49, 355–361 [PubMed] [Google Scholar]
- Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM, 2010. Longitudinal changes in cortical thickness associated with normal aging. Neuroimage 52, 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, 2000. The age of anxiety? The birth cohort change in anxiety and neuroticism, 1952–1993. J. Personal. Soc. Psychol 79, 1007–1021. [DOI] [PubMed] [Google Scholar]
- Van Os J, Jones PB, 2001. Neuroticism as a risk factor for schizophrenia. Psychol. Med 31, 1129–1134. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA, 2006. Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology 27, 143–153. [DOI] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D, 2007. Neuroanatomical correlates of personality in the elderly. Neuroimage 35, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Williams D, Feczko E, Barrett LF, Dickerson BC, Schwartz CE, Wedig MM, 2006. Neuroanatomical correlates of extraversion and neuroticism. Cerebr. Cortex 16, 1809–1819. [DOI] [PubMed] [Google Scholar]


