Abstract
Conceptual knowledge allows us to comprehend the multisensory stimulation impinging on our senses. Its representation in the anterior temporal lobe is a subject of considerable debate, with the “enigmatic” temporal pole (TP) being at the center of that debate. The controversial models of the organization of knowledge representation in TP range from unilateral to fully unified bilateral representational systems.
To address the multitude of mutually exclusive options, we developed a novel cross-modal approach in a multifactorial brain imaging study of the blind, manipulating the modality (verbal vs pictorial) of both the reception source (reading text/verbal vs images/pictorial) and the expression (writing text/verbal vs drawing/pictorial) of conceptual knowledge. Furthermore, we also varied the level of familiarity. This study is the first to investigate the functional organization of (amodal) conceptual knowledge in TP in the blind, as well as, the first study of drawing based on the conceptual knowledge from memory of sentences delivered through Braille reading.
Through this paradigm, we were able to functionally identify two novel subdivisions of the temporal pole - the TPa, at the apex, and the TPdm - dorso-medially. Their response characteristics revealed a complex interplay of non-visual specializations within the temporal pole, with a diversity of excitatory/inhibitory inversions as a function of hemisphere, task-domain and familiarity, which motivate an expanded neurocognitive analysis of conceptual knowledge.
The interplay of inter-hemispheric specializations found here accounts for the variety of seemingly conflicting models in previous research for conceptual knowledge representation, reconciling them through the set of factors we have investigated: the two main knowledge domains (verbal and pictorial/sensory-motor) and the two main knowledge processing modes (receptive and expressive), including the level of familiarity as a modifier. Furthermore, the interplay of these factors allowed us to also reveal for the first time a system of complementary symmetries, asymmetries and unexpected anti-symmetries in the TP organization. Thus, taken together these results constitute a unifying explanation of the conflicting models in previous research on conceptual knowledge representation.
Introduction
Conceptual knowledge allows us to comprehend the multisensory stimulation impinging on our senses; semantic representations allow us to both generalize and express knowledge appropriately over a wide variety of both verbal and non-verbal task domains. For example, knowledge can be expressed by naming and verbal definitions (i.e., verbally), as well as, by drawing and object use (nonverbally), (Lambon Ralph et al, 2009).
How is such conceptual knowledge represented in the brain? The vast interest in conceptual knowledge – both theoretical and clinical, particularly because of semantic dementia – led to the accumulation of a highly significant body of neuroimaging data, in spite of that, however, its neural representation is not well understood. Presently, there is considerable debate about its neural substrate. The anterior temporal lobe - and the “enigmatic” temporal pole (TP) in particular - are at the center of that debate (e.g., Olson et al., 2007). A recent large-scale meta-analysis (Grace et al., 2015) evaluated four most prominent theories: i) The “ATL hub-and-spoke” account proposes that the right and left ATLs represent conceptual knowledge in a unified manner as part of a bilateral, coupled system [thereby promoting robust representations: see Schapiro et al. (2013)]; ii) An extreme version of this account would predict no differences between the hemispheres; iii) A more nuanced position holds that graded hemispheric specialization emerges as a consequence of differential connectivity (Lambon Ralph et al. 2001; Binney et al. 2012; Schapiro et al. 2013); iv) Conversely, a greater degree of specialization between the right and left ATLs has been proposed as well, reflecting the modality of stimulus input (Gainotti 2007, 2013), the involvement of word retrieval or visual recognition in the task (Damasio et al. 2004), or the social content of the stimulus (Olson et al. 2007; Zahn et al. 2007).
Until recently, the TP has been considered both structurally and functionally homogeneous. However, it has now been demonstrated that the TP has rich cortical and subcortical connections (e.g., Fan et al., 2014). Because of its extensive connectivity with diverse modality-specific regions, the TP is ideal for forming amodal semantic representations, and it has been suggested as a key “amodal convergence hub”. TP is capable of complex multisensory integration, but is also involved in various high-order cognitive functions, including semantic memory, high-level language processing, empathy, emotions, social and abstract semantic cognition, etc. When damaged, as in semantic dementia, a wide variety of semantically demanding tasks – both receptive and expressive – are affected. Thus, conceptual knowledge representations allow us not only to be the recipient of but to also express knowledge in a wide variety of domains; Furthermore, our semantic representations allow us to generalize knowledge across exemplars (Lambon Ralph and Patterson 2008).
Being so integral to our everyday lives, any impairments of semantic memory are extremely debilitating. That is why, the question of where in the brain conceptual knowledge is represented and what the underlying mechanisms are, is of key importance to neuroscience.
However, as seen above, the structural organization of knowledge representation is highly controversial, with proposed models ranging from a unilateral specialization (typically, leftlateralization) to a graded or fully unified bilateral TP representational system.
To address these mutually exclusive options, we have developed a novel cross-modal approach in a multi-factorial brain imaging study, comparing several modalities of reception and expression of conceptual knowledge through Braille reading, Braille writing, and drawing, including the level of familiarity as a modifier. Furthermore, we were able to achieve a functional parcellation of the temporal pole in the context of conceptual knowledge.
Methods
Experimental Design
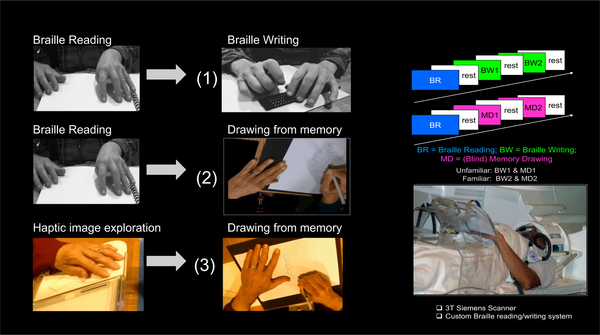
A set of verbal descriptions of objects, faces and scenes were presented through tactile (Braille) text, to form comprehension-based non-visual memory in the blind reader, which was then expressed either through (i) memory-writing in Braille (MemoryWritingFromBraille, BW) or ii) blind memory-drawing, also guided solely by the memory from the Braille reading (MemoryDrawingFromBraille, MD).
The blind MemoryDrawingFromBraille task wouldn’t be possible without first employing our unique Cognitive-Kinesthetic Drawing Training (e.g., Likova, 2012, 2013) that allows us to achieve rapid behavioral and brain plasticity effects. Over only 5 sessions of 2 hr/day, blind participants learn to explore raised-line drawings so as to form precise and robust memory of the explored images, which subsequently guides the freehand drawing of these images without vision or any further tactile input (Likova, 2014, 2015). This training thus makes it possible for blind people to perform two different forms of drawing in the scanner, i) one based on pictorial-type reception (drawing guided by the pictorial memory of explored raised-line images; MemoryDrawingFromPictorial), and ii) another one based on verbal-type reception (memory from Braille-reading guiding the drawing hand; MemoryDrawingFromBraille).
The experimental design for functional Magnetic Resonance Imaging (fMRI) during the Braille-involving tasks was as in Likova et al (2016). Each sample of Braille text was used in two sequential scans. In the first scan, after it was read (Braille reading, BR), it was followed by two repetitions of Braille writing from memory (BW1 and BW2) reproducing the description as understood and memorized from the preceding Braille-reading. In the second scan, the Braille reading was followed by two repetitions of expressing the memory through non-visual drawing (MD1 and MD2). The tasks (20 sec each), were interleaved with 20 sec baseline/rest periods (rest).
Figure 1 illustrates the experimental tasks (left panel), the experimental sequence in the Braille involving tasks (upper right panel), and our custom MR-compatible lectern that makes it possible to run these complex non-visual tasks, each involving a precise motor control component (bottom right panel).
Figure 1. Experimental design:
Experimental tasks (left panel), the fMRI sequence in the Braille involving tasks (upper right panel), and our custom MR-compatible lectern that makes possible to run these complex non-visual tasks, each of which involves a precise motor control component (bottom right panel).
The fMRI experimental design for the blind memory-drawing, guided by the pictorial memory of the explored raised-line images, MemoryDrawingFromPictorial, was as in Likova (2012). The drawing (20 sec) followed a 20 sec of exploration and memorization of presented raised-line images. The two tasks were separated by a 20 sec rest period, and followed by a 20 sec control task (Scribble).
General Methods
Equipment
Braille conditions
Braille writing was accomplished via the use of a standard slate and stylus system, as in Likova et al. (2016). The slate consisted of two pieces of plastic held together by a hinge, designed to hold the paper on which the participant wrote. The lower piece was solid with slight indentations for each of the 6 raised dots within each 2×3 Braille cell, and the upper piece had rectangular slots corresponding to each Braille cell. The stylus was a blunted aluminum point with a plastic handle.
To use this slate-and-stylus system, a sheet of paper was placed within the slate, and the stylus was used to puncture dots within each Braille cell outlined by the slate to create the desired characters. In the scanner, the MRI-compatible slate and stylus were positioned on top of our custom MRI-compatible lectern (Likova, 2012), providing both for haptic exploration of the Braille text during reading, and for Braille writing on a slate resting a two-slot (reading/writing) plexiglass table extending across the participant’s lap. Auditory cues were presented through Resonance Technologies earphones (Resonance Technologies, Salem, MA).
Drawing conditions
The custom drawing lectern was used for the drawing conditions as well. In the case of raised-line pictorial stimulus, each stimulus was positioned in the left slot of the lectern, where it was explored with left hand and memorized, then drawn with a stylus in the right slot exclusively with the right hand. When the Braille text was the stimulus, it was placed in the left slot, read with left hand and memorized, then drawn from memory in the right slot with the right hand.
Functional MRI Acquisition and Analyses
Data were collected on a Siemens Trio 3T magnet equipped with a 12-channel head coil. BOLD responses were obtained using an EPI acquisition (TR = 2 s, TE = 28 ms, flip angle = 80°, voxel size = 3.0 × 3.0 × 3.5) consisting of 35 axial slices extending across the whole brain. Pre-processing was conducted using FSL (Analysis Group, FMRIB, Oxford, UK) and included slice-time correction and twophase motion correction, consisting of both within-scan and between-scan 6-parameter rigid-body corrections. To facilitate segmentation and registration, a whole-brain high-resolution T1-weighted anatomical scan was also obtained for each participant (voxel size = 0.8 × 0.8 × 0.8 mm). White matter segmentation in this T1 scan was conducted using FreeSurfer (Martinos Center for Biomedical Imaging, Massachusetts General Hospital) and Gray matter was identified with the mrGray function in the mrVista software package (Stanford Vision and Imaging Science and Technology).
To obtain estimates of neural activation amplitudes for each task, a general linear model (GLM) was fit to the acquired BOLD data for each three-task sequence. The GLM model consisted of a 3 separate 20-s boxcar predictors representing the 3 task activations plus an auditory predictor consisting of sequence of 1-s impulses corresponding to the 6 auditory cues. Each predictor was convolved with an estimated hemodynamic response function (HRF) derived from the whole cortical manifold averaged over the most activated voxels by filtering the 3-cycle sequence at a high activation threshold, and a 4th-order polynomial to account for low-frequency baseline fluctuations. For each task, statistical parametric maps (SPMs) were generated based on the estimated activation amplitudes from the above GLM in each voxel that exceeded the noise threshold defined by the variability in the residual. Note that the first stimulus presentations or task performances were designated as ‘unfamiliar’, while their repeats as ‘familiar’.
Results
Functional Parcellation of the Temporal Pole
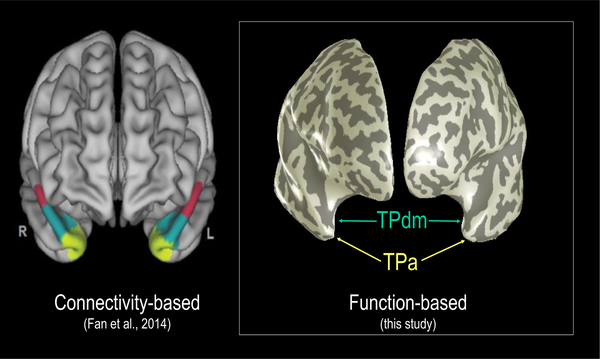
The fMRI analyses revealed two adjacent functional subdivisions within TP (Figure 2). These subdivisions - the apex (TPa) and a dorso-medial region (TPdm) – were differentiated on the basis of their contrasting behavior as a function of the three experimental variable of task-domain, hemisphere and familiarity.
Figure 2.
Temporal pole parcellation. Left panel: Connectivity-based (Fan et al., 2014). Right panel: Function-based subdivisions (this study).
Task-Dependent Hemispheric Specialization
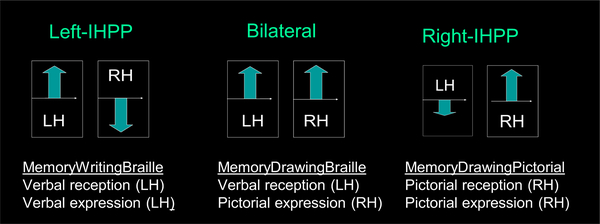
We will refer to tasks with same modality of reception (i.e., input of the information to be memorized) and expression (output) as ‘within-domain tasks’. These are i) the MemoryWritingFromBraille, which had ‘verbal reception/verbal expression’, and ii) the MemoryDrawingFromPictorial, which had ‘pictorial reception/pictorial expression’.
The MemoryDrawingFromBraille, on the other hand, is a ‘cross-domain task’ as it is of a mixed modality by having a verbal input but the pictorial expression.
Different patterns of interhemispheric relationships were revealed as a function of the modalities of both the reception and of the expression of that memorized information.
Temporal pole apex (TPa)
Remarkably, for within-domain tasks, each subdivision showed previously unreported interhemispheric anti-symmetries such as reciprocal inter-hemispheric suppression.
The cross-domain MemoryDrawingFromBraille task, however, showed symmetrical bilateral activation, implying transformation of the conceptual information from the receptive format into the format of the expressive domain (e.g., from verbal into pictorial), before the expressive performance itself. Granger causality analysis differentiated the respective source and target networks involved (not included here).
Familiarity restricted hemispheric specialization patterns in dorsomedial temporal pole (TPdm)
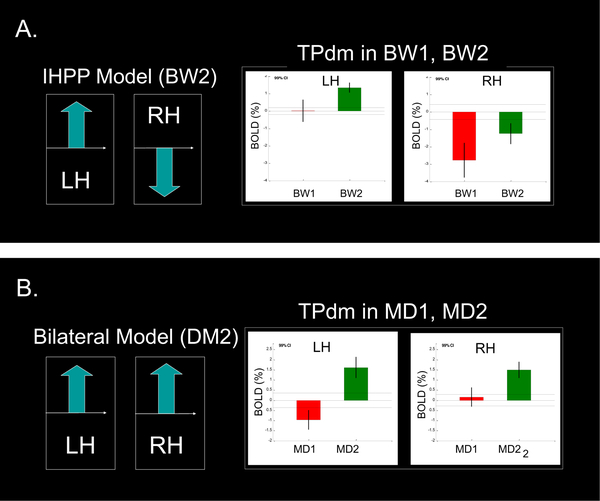
Although, analogous types of hemispheric specialization patterns were observed in TPdm, they were manifested in the phase of familiarity only, i.e., only after task repetition or training (Fig. 4).
Figure 4.
Analogous types of hemispheric specialization patterns were observed in TPdm. TPdm was involved, however, in the familiarity phase only of each task.
Moreover, the TPdm subdivision manifested a remarkable inversion of the familiarity effect (increase instead of decrease with familiarity). This inversion effect was strongly expressed in the familiarity phase (BW2, MD2) of both tasks (see Figure 5). In the unfamiliar phase, TPdm was either not significantly activated or was even suppressed. In the within-domain verbal/verbal task of MemoryWritingFromBraille (Figure 5, A) this effect was exhibited in the left hemisphere, while it was bilateral in the cross-domain verbal/pictorial task of MemoryDrawingFromBraille (Figure 5, B).
Figure 5. Inversed familiarity effect in the dorso-medial subdivision of the temporal pole (TPdm):
A familiarity effect, inversed in comparison with TPa (and PRC), i.e., an increase instead of a decrease with familiarity, was observed in both BW2 and MD2. The inversed effect was left-hemispheric in the MemoryWritingFromBraille (see panel A), while, it was bilateral in MemoryDrawingFromBraille (see panel B).
Discussion & Conclusions
Taken together the results from our multimodal paradigm shed new light on the path towards an explanation of current contradictions in the field of conceptual knowledge representation in the temporal poles of the two hemispheres of the brain. Also, we were able to functionally identify two specialized subdivisions of the ‘enigmatic’ temporal pole: the TPa, at the apex, and the dorso-medial TPdm. Additionally, an unexpected novel form of profound push-pull interactions was revealed, acting both inter-hemispherically (left vs right hemisphere) and inter-regionally (TPa vs TPdm). We also note that this is the first study of drawing based on conceptual knowledge from memory of sentences delivered through Braille reading, as well as, the first study to investigate the functional organization of (amodal) conceptual knowledge in TP in the blind.
Although, our results are generally in support of the third of the theoretical accounts reviewed above – that of a greater degree of specialization (GDS) between the right and left ATLs - their implications go beyond that account. The main proposals within GDS are restricted to either i) the modality of stimulus input (Gainotti 2007, 2013), ii) the involvement of word retrieval or visual recognition in the task (Damasio et al. 2004), or iii) the social content of the stimulus (Olson et al. 2007; Zahn et al. 2007).
To address these proposals, our multidimensional study has included not only a passive task (BrailleReading) but also three active expression tasks, such as MemoryWritingFromBraille, MemoryDrawingFromBraille and MemoryDrawingFromPictorial. Moreover, we have varied the modality (verbal vs pictorial) of both the reception source (reading text/verbal vs images/pictorial), and of the expressive output (writing text/verbal vs drawing/pictorial). We have also manipulated the level of familiarity.
Task-domain and familiarity
As a whole, the results reveal a complex interplay of non-visual hemispheric specializations for conceptual knowledge representation and expression within the temporal pole. The two subdivisions exhibited a diversity of excitatory/inhibitory inversions as a function of brain hemisphere, task-domain and familiarity, providing data for an expanded neurocognitive analysis of conceptual knowledge. Both direct and inverse familiarity effects were observed.
The same-modality task of memory writing from Braille text activated the left temporal pole only, while – unexpectedly - it strongly suppressed the right temporal pole. We call this unobserved previously behavior ‘inter-hemispheric push-pull model’.
In contrast, the mixed modality - or cross-domain - task of memory drawing from Braille text fully conformed to a bilateral temporal pole model for conceptual knowledge representation in both TPa and TPm subdivisions. It was, however, a subject to a strong TPa/TPdm push-pull interaction driven by familiarity.
The task of memory drawing from pictorial input activated the right temporal pole only.
These differences in temporal pole lateralization above suggest that the left hemisphere component of the bilateral drawing activation in the cross-domain Braille memory drawing derives from the verbal nature of the receptive phase when the memory was formed from reading Braille text, while its right component derives from the pictorial nature of the expression phase.
In summary, these data show that, in the verbal input or expression mode, the left TP is activated; pictorial input or expression involves the right TP, and a mixed form input/expression (verbal and pictorial) gives a bilateral TP activation.
Relevance to models of conceptual representation
Importantly, the interplay of inter-hemispheric specializations found here accounts for the variety of conflicting models in previous research for knowledge representation. The multitude of seemingly contradictory findings in the literature, can be reconciled and now logically explained as a function of the set of factors we have investigated: the two main knowledge domains (verbal and pictorial/sensory-motor), the two main knowledge processing modes (receptive/input and expressive), with the level of familiarity as a modifier. Furthermore, varying these factors allowed us to also reveal for the first time a system of complementary symmetries, asymmetries and unexpected anti-symmetries in the TP functional organization relative to the left vs right hemisphere, activation vs suppression, and cooperation vs competition. Thus, taken together these results delineate a unifying explanation of the conflicting models in previous research on conceptual knowledge representation.
Figure 3.
Temporal pole apex (TPa) responses: 1: Comprehension of the Braille text expressed through the MemoryWritingFromBraille task produced neither bilateral nor left-hemisphere-only response, but a previously unobserved interhemispheric push-pull (IHPP) behavior with a strong left-lateralized response, combined with extensive contralateral suppression. 2: Braille-text comprehension expressed through the blind MemoryDrawingFromBraille task fully conformed to the bilateral TP model for conceptual knowledge representation, both in the unfamiliar and in the familiar phase after repetition. Interestingly, the familiarity effect is manifested as a reduction (rather than enhancement) of the response, similarly to what we have already observed in the perirhinal cortex of the blind in the pictorial memory drawing task after the Likova Cognitive-Kinesthetic training (Cacciamani & Likova, 2016). 3: Memory drawing guided by pictorial memory (from raised-line image exploration) eliminated the left, and conformed to the right TPa only.
Table 1.
Hemispheric engagement as a function of the receptive/expressive modality combination.
| EXPRESSIVE MODALITY (Task) | |||
|---|---|---|---|
| RECEPTIVE MODALITY (Stimulus Input) | Verbal (Reading Braille) | Verbal/Motor (Memory Writing Braille) | Pictorial/Motor (Memory Drawing) |
| Verbal (Braille Text) | Left TP | Left TP | Left TP + Right TP |
| Pictorial (Raised-line Images) | N/A | N/A | Right TP |
Acknowledgements
NEI/NIH Grant R0IEY024056 to Lora Likova. Acknowledgements to Spero Nicholas for his help with data preprocessing, Kristyo Mineff helping with subject training, and Christopher W. Tyler providing valuable feedback on the manuscript.
Author Biography
Dr. Likova is the Director of Brain Plasticity, Learning & Neurorehabilitation Lab at Smith-Kettlewell Eye Research Institute. Based on her background in magnetic physics, cognitive neuroscience science and computer science, she has brought together a collaborative team focusing on the enhancement of brain plasticity for the rehabilitation of blind and low vision individuals. Through her unique Cognitive-Kinesthetic training regimen of less than a week’s duration, her lab has been able to drive brain plasticity and achieve major enhancements in both spatial memory and fine spatiomotor skills. Dr. Likova is on the HVEI Organizing Committee.
References
- Acres K, Taylor KI, Moss HE, Stamatakis EA, Tyler LK. (2009) Complementary hemispheric asymmetries in object naming and recognition: a voxel-based correlational study. Neuropsychologia 47:1836–1843. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, & Lambon Ralph MA. (2010) The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion corrected fMRI, rTMS, and semantic dementia. Cereb Cortex 20(11), 2728e2738 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Parker GJ, & Lambon Ralph MA. (2012) Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion weighted imaging probabilistic tractography. J Cogn Neurosci 24(10), 1998e2014 10.1162/jocn_a_00263. [DOI] [PubMed] [Google Scholar]
- Butler CR, Brambati SM, Miller BL, Gorno-Tempini ML. (2009) The neural correlates of verbal and nonverbal semantic processing deficits in neurodegenerative disease. Cogn Behav Neurol. 22:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciamani L, Likova LT. (2016) Tactile Object Familiarity in the Blind Brain Reveals the Supramodal Perceptual-Mnemonic Nature of the Perirhinal Cortex. Front Human Neurosci 2016;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. (2004) Neural systems behind word and concept retrieval. Cognition 92:179–229. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, Silbergeld DL, Miller JW, Voets NL, Saindane AM, et al. (2013) Famous face identification in temporal lobe epilepsy: support for a multimodal integration model of semantic memory. Cortex 49(6):1648–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Miller JW, Tranel D. (2008) Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia 46:1242–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G (2007). Different patterns of famous people recognition disorders in patients with right and left anterior temporal lesions: a systematic review. Neuropsychologia 45:1591–1607. [DOI] [PubMed] [Google Scholar]
- Gainotti G (2012) The format of conceptual representations disrupted in semantic dementia: a position paper. Cortex 48:521–529. [DOI] [PubMed] [Google Scholar]
- Gainotti G (2013) Laterality effects in normal subjects’ recognition of familiar faces, voices and names. Perceptual and representational components. Neuropsychologia 51:1151–1160. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. (2003) Naming and recognizing famous faces in temporal lobe epilepsy. Neurology 61:81–86. [DOI] [PubMed] [Google Scholar]
- Rice GE, Lambon Ralph MA, Hoffman P. (2015) The roles of left versus right anterior temporal lobes in conceptual knowledge: An ALE metaanalysis of 97 functional neuroimaging studies Cereb Cortex 25 (11): 4374–4391: bhv024v1-bhv024. doi: 10.1093/cercor/bhv024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA. (2014) Neurocognitive insights on conceptual knowledge and its breakdown. Philos Trans R Soc Lond B Biol Sci 369: 20120392–20120392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. (2001) No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci 13:341–356. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E (2009) Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex 19:832–838. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA. (2013). Why bilateral damage is worse than unilateral damage to the brain. J Cogn Neurosci 25(12), 2107e2123 10.1162/jocn_a_00441. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K. (2008). Generalization and differentiation in semantic memory. Ann New York Acad Sci 1124(1):61–76. [DOI] [PubMed] [Google Scholar]
- Likova LT. (2012a) Drawing enhances cross-modal memory plasticity in the human brain: a case study in a totally blind adult. Front Human Neurosci 14;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likova LT. (2012b) A cross-modal perspective on the relationships between imagery and working memory. Mental Imagery. 2012:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likova LT (2013) A cross-modal perspective on the relationships between imagery and working memory. Front Psychol 3:561. doi: 10.3389/fpsyg.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likova LT. (2015) Temporal evolution of brain reorganization under crossmodal training: insights into the functional architecture of encoding and retrieval networks In SPIE/IS&T Electronic Imaging 2015 (pp. 939417939417). International Society for Optics and Photonics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likova LT. (2014) Learning-based cross-modal plasticity in the human brain: insights from visual deprivation fMRI. Advanced Brain Neuroimaging Topics in Health and Disease-Methods and Applications. 2014:327–58. [Google Scholar]
- Likova LT, Tyler CW, Cacciamani L, Mineff K, & Nicholas S (2016) The cortical network for Braille writing in the blind. Soc Imag Science Tech doi: 10.2352/ISSN.2470-1173.2016HVEI-095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. (2013) Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 136:601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, McCoy D, Klobusicky E, Ross LA. (2013) Social cognition and the anterior temporal lobes: a review and theoretical framework. Soc Cogn Affect Neurosci 8:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. (2007) The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 130(Pt 7):1718–31. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. (2007) Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci 104:20137–20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. (2010) Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia 48:1336–1342. [DOI] [PubMed] [Google Scholar]
- Schapiro AC, McClelland JL, Welbourne SR, Rogers TT, &Visser M, Embleton KV, Jefferies, Parker GJ, Lambon Ralph MA (2010) The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia 48:1689–1696. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. (2012) Famous people knowledge and the right and left temporal lobes. Behav Neurol 25:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. (2004) Knowledge of famous faces and names in semantic dementia. Brain 127(Pt 4):860–872. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. (1997) A neural basis for the retrieval of conceptual knowledge. Neuropsychologia 35:1319–1327. [DOI] [PubMed] [Google Scholar]
- Wong C, Gallate J. (2012) The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res 1449:94–116. [DOI] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. (2007) Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci USA 104: 6430–6435. [DOI] [PMC free article] [PubMed] [Google Scholar]