Abstract
Background:
Open radical cystectomy (ORC) has proven to be an important component in the treatment of high-risk bladder cancer (BCa). ORC surgical morbidity remains high; therefore, minimally invasive surgical techniques have been introduced in an attempt to improve patient outcomes.
Objective:
To compare cancer outcomes in BCa patients managed with ORC or robotic-assisted radical cystectomy (RARC).
Design, setting, and participants:
A prospective, randomized trial was completed between 2010 and 2013. Patients were randomized to ORC/pelvic lymphadenectomy (PLND) or RARC/PLND, with all undergoing open/extracorporeal urinary diversion. Surviving patients were followed for at least 2 yr.
Outcome measurements and statistical analysis:
Secondary outcomes to the trial included recurrence-free, cancer-specific, and overall survival.
Results and limitations:
The trial randomized 118 patients who underwent RC/PLND and urinary diversion. Sixty were randomized to RARC and 58 to ORC. Four RARC-assigned patients refused randomization and received ORC; however, an intention to treat analysis was performed. No differences in recurrence or cancer-specific survival were observed (hazard ratio [HR]: 1.27; 95% confidence interval [CI]: 0.69–2.36; p = 0.4 and p = 0.4, respectively). No difference in overall survival was observed (p = 0.08). However, the pattern of first recurrence demonstrated a nonstatistically significant increase in metastatic sites for those undergoing ORC (sub-HR [sHR]: 2.21; 95% CI: 0.95–5.11; p = 0.064) and a greater number of local/abdominal sites in the RARC-treated patients (sHR: 0.34; 95% CI: 0.12–0.93; p = 0.035). The major limitation to this study is that the trial was not powered to determine differences in cancer recurrences, survival outcomes, or patterns of recurrence.
Conclusions:
The secondary outcomes from our randomized trial did not definitively demonstrate differences in cancer outcomes in patients treated with ORC or RARC. However, differences in observed patterns of first recurrence highlights the need for future studies.
Patient summary:
Of 118 patients randomly assigned to undergo radical cystectomy/pelvic lymphadenectomy and urinary diversion, half were assigned to open surgery and half to robot-assisted techniques. We found no difference in risk of recurring or dying of bladder cancer between the two groups.
In this secondary analysis of cancer outcomes from our randomized controlled trial, we did not find a difference in overall recurrence rates and cancer-specific survival between open radical cystectomy and robot-assisted radical cystectomy for high-risk bladder cancer. Variations in patterns of recurrence require further study.
1. Introduction
Radical cystectomy (RC) with regional pelvic lymphadenectomy (PLND) is the established standard of care for treating high-risk bladder cancer (BCa) [1,2]. BCa is common in older individuals and strongly linked to smoking exposure. Performing extensive pelvic surgery and reconstruction of the urinary system in an elderly, comorbid population carries significant surgical risks. The development of minimally invasive surgical techniques has been widely used in a variety of surgical procedures. One major goal associated with the adaptation of minimally invasive techniques is to minimize surgical morbidity and improve recovery.
Robot-assisted RC (RARC) was introduced in hopes of decreasing the substantial morbidity following standard of RC and urinary diversion. Several retrospective series had reported perioperative outcomes including complications following RARC with either open or intracorporeal techniques [3–6]. We reported our results of a randomized controlled study designed to compare complications between open RC (ORC) and RARC [7,8]. In that trial, we reported no large differences in 90-d overall complications, high-grade complications, or hospital length of stay. Pathologic outcomes including positive soft tissue margin (PSTM) rates and lymph node yield were similar between open and robotic techniques. Margin rates and lymph node yields were similar to previously reported benchmarks.
Long-term oncologic outcomes following RARC have not been well documented in the reported literature. Here, we describe the oncologic outcomes from our ORC versus RARC randomized controlled trial, including the secondary endpoints of recurrence-free survival, overall survival, and patterns of first recurrence.
2. Patients and methods
2.1. Patients
Patients with BCa scheduled to undergo RC and PLND were recruited from the urology clinics at Memorial Sloan Kettering Cancer Center (MSKCC) between March 2010 and March 2013. The study protocol was approved by the Institutional Review Board, and all patients were required to provide written consent prior to enrollment and surgery. Patients were randomized 1:1 to undergo RARC or ORC using MSKCC’s Clinical Research Database, a secure system that ensures allocation concealment.
Eligible patients were medically fit for RC, aged ≥18 yr, and had clinical stage Ta-T3/N0–3/M0 BCa. Patients with a prior history of pelvic radiation, clinical stage T4, prior extensive open abdominal surgery, or any clinical contraindication to minimally invasive surgery were excluded. Postoperatively all patients were followed every 3–6 mo with routine history and physical exams, diagnostic imaging of the chest/abdomen/pelvis, urine cytology, and complete blood work. Additional diagnostic imaging or investigations were conducted at the discretion of the surgical team.
2.2. Surgical intervention
Complete details regarding the surgical intervention have been previously described [7]. Men underwent removal of the prostate, and women underwent hysterectomy and bilateral salpingo-oophorectomy if these organs were present. The extent of PLND was left to the discretion of the surgeon based on clinician preference, and judgment and was determined prior to randomization. At a minimum, the primary drainage regions (hypogastric, obturator, external iliac) were removed bilaterally. Extended dissections removed at least the lymph nodes overlying the aortic bifurcation and continued to the take-off of the inferior mesenteric artery.
2.3. Statistical analysis
Details of the trial design and randomization have been previously described [7]. The date of the first documented BCa recurrence was identified from clinical records, radiological findings, or pathological specimens. Cancer-specific mortality and all-cause mortality were recorded from institutional and clinical records.
Kaplan–Meier methods were used to estimate recurrence and survival probabilities after RC on an intention to treat basis, and the log-rank test was used to compare differences in recurrence and cancer death rates between the RARC and ORC groups. Greenwood’s variance estimates were used to calculate confidence intervals (CIs) for differences in recurrence and cause-specific death rates after RC. Recurrent BCa was characterized by the site of disease detection in one of the three categories: (1) local pelvic recurrence (soft tissue recurrences within or surrounding the cystectomy bed, pelvis, rectum, or pelvic nodal regions), (2) abdominal recurrence (abdominal wall, peritoneal carcinomatosis, or other intraperitoneal implants within the abdominal cavity), and (3) distant recurrence (lung, liver, bone, extrapelvic lymph nodes). New urothelial-based tumors (urethra, ureters) were evaluated separately. Based on observed recurrences, we undertook an analysis that was not prespecified in the study protocol to test for a pattern in the site of the first recurrence by treatment arm. Using competing risks methods, we compared local pelvic recurrence, abdominal recurrence, and distant recurrence in three separate analyses. We also grouped local pelvic recurrence and abdominal recurrence into a single local/regional recurrence group and repeated the competing risk analysis. For each of these analyses, the competing events were death from another case or recurrence to another site.
3. Results
3.1. Patient population
Patient demographics and disease characteristics of the two groups were similar (Table 1). Pathologic staging of the two groups was not significantly different (Table 1). Pathologic stage T4 was found in five patients (8.3%) undergoing RARC and four (6.9%) undergoing ORC. There was no difference in the lymph node yield based on the extent of dissection and PSTM rate between RARC (3.6%) and ORC (4.8%).
Table 1 –
Patient characteristics
| RARC (n = 60) | ORC (n = 58) | |
|---|---|---|
| Age (yr), median (IQR) | 66 (60–71) | 65 (58–69) |
| Male sex, n (%) | 51 (85) | 42 (72) |
| ASA score, n (%) | ||
| 2 | 17 (28) | 12 (21) |
| ≥3 | 43 (72) | 46 (79) |
| Clinical stage, n (%) | ||
| ≤T1 | 30 (52) | 24 (42) |
| T2 | 24 (41) | 28 (49) |
| T3 | 4 (6.8) | 5 (8.8) |
| T4 | 1 (1.7) | 0 (0) |
| Neoadjuvant chemotherapy, n (%) | 19 (32) | 26 (45) |
| Pathologic stage, n (%) | ||
| T0 | 13 (22) | 7 (12) |
| Tis | 14 (23) | 11 (19) |
| Ta | 1 (1.7) | 3 (5.2) |
| T1 | 7 (12) | 11 (19) |
| T2 | 8 (13) | 7 (12) |
| T3 | 12 (20) | 15(26) |
| T4 | 5 (8.3) | 4 (6.9) |
| Histology, n (%) | ||
| Urothelial cell carcinoma | 57 (95) | 55 (95) |
| Small cell carcinoma | 1 (1.7) | 1 (1.7) |
| Small cell plus TCC | 1 (1.7) | 0 (0) |
| Squamous cell carcinoma | 1 (1.7) | 1 (1.7) |
| Adenocarcinoma | 0 (0) | 1 (1.7) |
| Lymph node yield, mean (SD) | ||
| Extended dissection | 31.9 (12) | 30 (12) |
| Standard dissection | 19.5 (10) | 18.9 (10) |
| Lymph node-positive patients, n (%) | 10 (18) | 9 (15) |
| Positive surgical margin, n (%) | 2 (3.6) | 3 (4.8) |
ASA = American Society of Anesthesiologists; IQR = interquartile range; ORC = open radical cystectomy; RARC = robot-assisted radical cystectomy; SD = standard deviation; TCC = transitional cell carcinoma.
Among the 118 enrolled patients, the median follow-up was 4.9 (IQR: 3.9–5.9) yr after surgery among surviving patients. Overall, there were 45 patients who experienced recurrences. A total of 36 deaths were observed, 19 of which were from BCa. One patient who underwent ORC died from surgical complication 3 mo after RC.
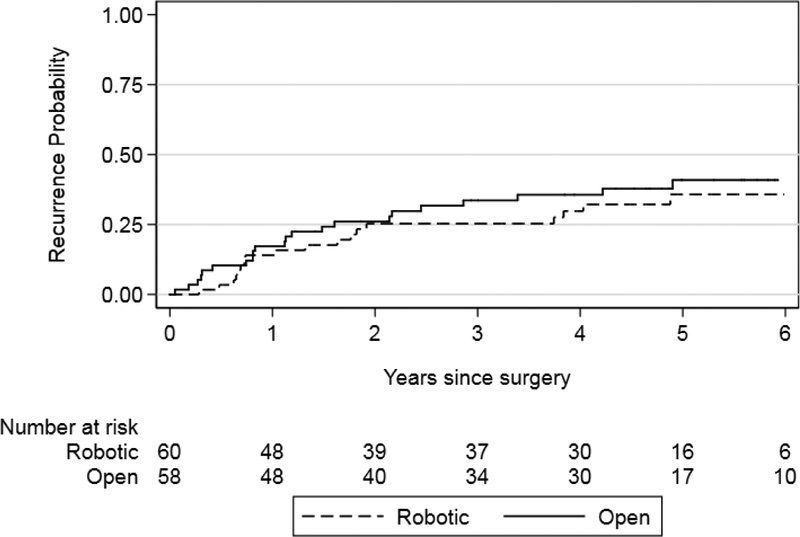
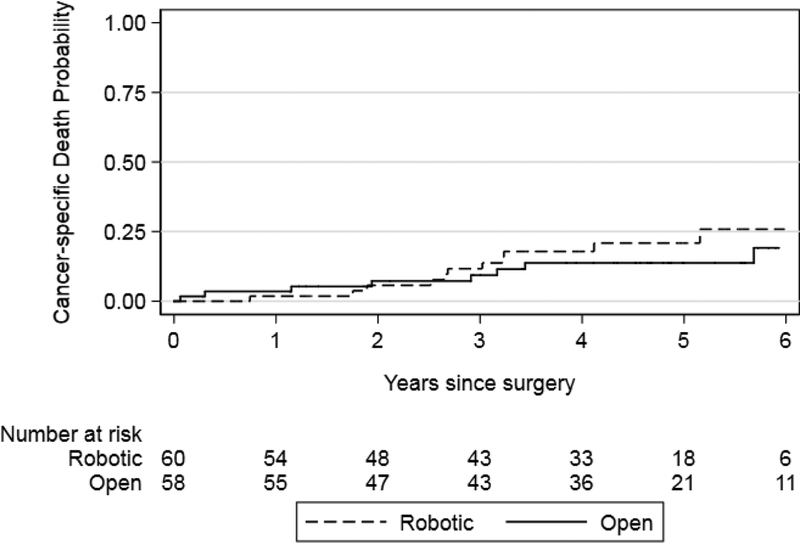
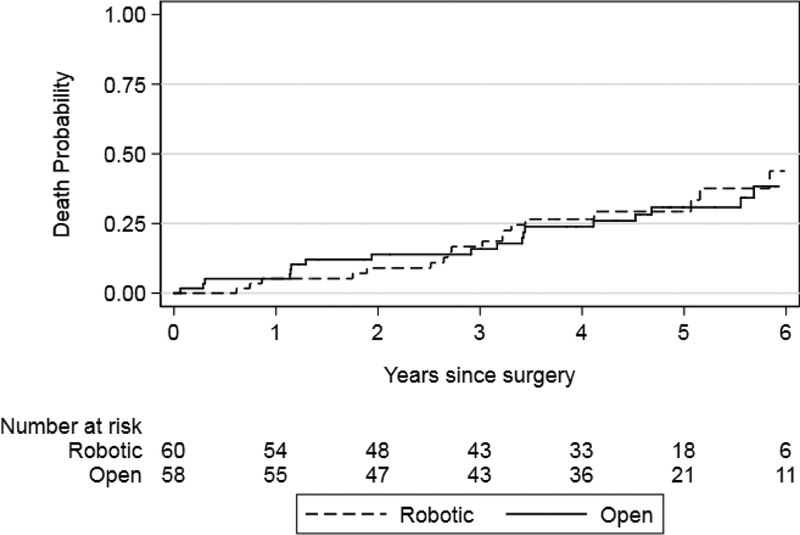
We found that recurrence-free survival and BCa-specific survival were similar between the robot and open surgery arms (p = 0.4 and p = 0.4, respectively; Figs. 1 and 2). Results were similar for risk estimates of all cause mortality after surgery in the RARC and ORC arms (p = 0.8; Fig. 3). Risk of recurrence at 5 yr was 36% and 41% for RARC and ORC, respectively (difference: −5.2%; 95% CI: −25−14). The wide CIs around the difference in recurrence risk preclude us from making conclusions regarding oncologic equivalence of the surgical modalities.
Fig. 1 –
Kaplan–Meier risk estimates for recurrence after radical cystectomy for the robotic and open surgery arms. Log-rank p = 0.4.
Fig. 2 –
Kaplan–Meier risk estimates for death from bladder cancer after radical cystectomy for the robotic and open surgery arms. Log-rank p = 0.4.
Fig. 3 –
Kaplan–Meier risk estimates for death after radical cystectomy for the robotic and open surgery arms. Log-rank p = 0.8.
3.2. Patterns of first recurrence
3.2.1. Distant
Twenty-five patients experienced a distant recurrence as their site of first recurrence. Patients in the ORC arm had a trend toward higher overall rates of distant recurrence as their first site of recurrence, including nine with lung metastases compared to one in the RARC arm. However, the difference was not statistically significant (sub-hazard ratio [sHR]: 2.21; 95% CI: 0.95–5.11; p = 0.064). During the entire follow-up period, distant metastatic sites of involvement were found in 17 RARC patients and 22 ORC patients.
3.2.2. Local and abdominal recurrences:
Local recurrences were observed as the first site of recurrence in 14 patients, including 10 in the RARC arm and four in the open arm. Abdominal recurrences were identified in seven patients including five RARC patients and two ORC patients. RARC-associated abdominal wall recurrences were not at previous port sites. The difference in local recurrence rates did not meet conventional levels of significance (sHR: 0.36; 95% CI: 0.11–1.12, p = 0.077). Similarly, the difference in the rate of abdominal recurrence did not reach statistically significance (sHR: 0.38; 95% CI: 0.07, 1.96; p = 0.2). However, when the pelvic and abdominal recurrences were combined into a single group representing local/regional recurrence, the ORC group showed significantly less local/regional recurrence compared to RARC (sHR: 0.34; 95% CI: 0.12–0.93; p = 0.035). Three RARC patients with local recurrences had soft tissue disease with direct rectosigmoid invasion. This pattern of pelvic recurrence was not identified in the ORC arm. All five RARC patients who relapsed within the abdomen demonstrated invasion of the abdominal wall with synchronous bowel implants, another pattern not identified in those undergoing ORC.
4. Discussion
RC and PLND have become the established gold standard for managing nonmetastatic high-risk BCa. The critical surgical tenants of this procedure have been developed over decades and focus on wide resection of the bladder, surrounding tissues, and adjacent organs as well as avoidance of entry into the bladder and thoroughness of PLND to optimize local and regional disease control. Benchmarks for recurrence-free survival following RC using standard open surgical techniques is strongly affected by the stage of disease with a 75%, 60%, and 30% long-term disease control rate for organ confined, extravesical, and lymph node positive disease, respectively [1,2]. Although an RC is an effective local-regional option for controlling high-risk BCa, it is associated with a significant perioperative morbidity [9]. Minimally invasive surgical approaches have been introduced to a variety of surgical procedures for both benign and malignant conditions in the hope that complications and recovery could be improved. Increasingly, robot-assisted minimally invasive surgical techniques have been used to perform RC with or without intracorporeal urinary diversion.
This report presents the oncologic outcomes, which were secondary endpoints of a randomized controlled study comparing ORC versus RARC. In 118 randomized patients with a median follow-up approaching 5 yr, we observed no significant differences in cancer-free or overall survival between ORC and RARC. Both treatment arms were matched with respect to pathologic stage, lymph node positive rates, and PSTM. The percentage of patients with pT3 or pT4 disease in this trial suggests a skewed selection of patients with earlier stage disease compared to previous reports of our institutional RC experience [9]. Recurrence rates and PSTM rates were similar to previously published benchmarks for ORC [1,2]. Oncologic outcomes following RC have been closely associated with tumor characteristics such as stage of disease and status of the regional lymph nodes [1,2]. Important pathologic and surgical features such as PSTMs and extent of PLND have also been associated with recurrence-free and overall survival after RC [10–12]. In retrospective RARC series or prospective series without adequate controls, patient selection introduces bias, which has made it difficult to directly compare cancer outcomes between these two surgical techniques. Even in the current trial, it appeared that the patient population was skewed toward earlier stage disease given the percentage of patients with organ confined disease included for randomization.
Early reports of oncologic outcomes following RARC were encouraging; however, short follow-up, small number of patients, and potential patient selection bias limited the ability to generalize these outcomes [13,14]. In 2015, a consensus panel of experts evaluated the state of the literature reporting oncologic outcomes following RARC and found that due to patient selection, lack of randomized data, and relatively short-term follow-up, it was difficult to definitely compare RARC outcomes to those obtained by ORC [15]. More recent reports from single or combined institutional RARC-treated patients are reporting cancer-specific outcomes in patients with intermediate or longer length follow-up [13,16,17]. Most previous reports on RARC-treated patients have focused on pathologic outcomes including soft tissue margin status and lymph node yields [15,17–20]. Outcomes suggested that lymph node yields were similar for any given extent of dissection, which was consistent with findings from randomized studies [7,19]. The importance of PSTM status has been identified as a strong independent predictor of disease recurrence following RC [10,21,22]. Positive margins following open surgery are largely related to stage of disease, lymph node status, and whether variant cell types are present. PSTM rates below 5% have been reproducibly obtained in large, nonselected group of patients undergoing ORC [10,11]. With rare exceptions, positive margins are not seen in patients with organ confined disease, whereas about 9% of patients with extravesical tumors will have a PSTM [10]. We found a 3.6% and 4.8% PSTM rate in our RARC and ORC patients, respectively. Previously published RARC series have reported overall PSTM rates ranging from 0% to 26% [14,23–26]. Raza et al [17] reported one of the larger RARC patient series from the International Robotic Cystectomy Consortium (IRCC) surgical registry [17]. They observed an 8% PSTM rate. The IRCC previously reported on the surgeon’s learning curve to assess the minimum number of cases necessary to obtain overall PSTM rates in the 5% range. They noted that a minimum of 30 RARC cases would need to be performed by any given surgeon before their overall PSTM rates would drop this range. Nonetheless, the concerns regarding PSTMs were reinforced from recently presented data from a large randomized ORC versus RARC study that included over 300 BCa patients noting a significantly greater risk of a PSTM in patients treated with robotic techniques (10.5% vs 4%) (RAZOR trial data J Urol, 2017).
The patterns of recurrence following either ORC or RARC have been reported in retrospective series. The majority of recurrences after RARC occur at distant sites and reported pelvic recurrence rates appear qualitatively similar to previous open experience [16,17,22]. However, some series have reported a concerning pattern of intraperitoneal spread. While intraperitoneal dissemination is observed in patients managed with ORC, these recurrences are distinctly rare and associated with high-stage disease or aberrant histologies [27,28]. Nguyen et al [29] observed an increased risk of peritoneal carcinomatosis in RARC-treated patients undergoing RC compared to a contemporary group of ORC patients. In our study, we identified five patients treated by RARC with peritoneal recurrences compared to two patients who underwent ORC. Both ORC patients had pT3N0 or pT4N2 disease (urothelial carcinoma [CA]); three of the five RARC patients had pT3N0–1 (urothelial CA), one with pT2N0 (urothelial CA with plasmacytoid features), and one with pTAN0 (urothelial CA) only disease. All five RARC patients also demonstrated involvement of the abdominal wall including recurrence at a stoma site (not at previous port site). The two ORC patients with peritoneal disease did not demonstrate abdominal wall involvement. Bowel and abdominal wall metastasis from transitional cell carcinoma after RC are rare patterns of disease relapse; however, they have been reported in the contemporary RARC literature. The EAU Robotic Urology Section Scientific Working Group reported that recurrences within the bowel occurred at an estimated rate of 0.3% at 24 mo [30]. Also, in the largest RARC series published by IRCC, there were seven cases of disease relapse in the abdominal wall and 13 with rectal invasion among 1380 who underwent RARC [21].
Limitations of this study are that cancer-specific outcomes and patterns of recurrence were secondary endpoints of the trial; therefore, we cannot say definitively that RARC and ORC are not different with respect to their cancer control outcomes. The patterns of first recurrence analysis was an unplanned evaluation performed based on the observation of the numbers of distant and local/abdominal recurrences in the two surgical arms and, therefore, should be interpreted cautiously. The percentage of patients with extravesical disease in this trial was less than what would be expected in a nonselected group of BCa patients undergoing RC. Therefore, the cancer outcomes in this trial may not represent those observed in a group of higher-staged patients. This trial was performed at a single high-volume cancer center using high-volume robotic and open surgeons. Therefore, these results may not be directly generalizable to the greater community.
5. Conclusions
In this secondary analysis of cancer outcomes from our randomized controlled trial, we did not find a difference in overall recurrence rates and cancer-specific survival between ORC and RARC for BCa. The observed pattern of first recurrence after ORC and RARC in this study suggests an increased risk for local/abdominal recurrences following RARC. Future studies should be designed to definitively assess if alterations in the patterns of recurrence truly exist. Although both groups demonstrated that there were a low number of patients with intraperitoneal recurrences, abdominal wall involvement was a unique pattern observed only in RARC-treated patients. Future properly powered randomized studies comparing ORC to RARC are needed to confirm the equivalence in cancer control.
Table 2 –
First site of disease recurrence following radical cystectomya
| RARC (n = 60) | ORC (n = 58) | |
|---|---|---|
| Any recurrence (no. of patients) | 20 | 25 |
| Local recurrence (p = 0.077) | ||
| Rectum | 3 | 0 |
| Pelvic lymph nodes | 6 | 2 |
| Pelvic soft tissue | 8 | 3 |
| Abdominal recurrence (p = 0.2) | ||
| Abdominal wall | 5 | 0 |
| Bowel | 5 | 1 |
| Peritoneum (carcinomatosis) | 2 | 2 |
| Soft tissue | 2 | 1 |
| Distant recurrence (p = 0.064) | ||
| Adrenal | 0 | 1 |
| Bone | 2 | 3 |
| Extrapelvic lymph nodes | 5 | 10 |
| Liver | 2 | 4 |
| Lung | 1 | 9 |
| Soft tissue | 2 | 3 |
| Urothelial recurrence | ||
| Upper tract | 5 | 3 |
| Urethra | 1 | 2 |
ORC = open radical cystectomy; RARC = robot-assisted radical cystectomy.
Only one site was counted per patient. For example, if a patient had two bone recurrences on first recurrence, the table only includes one. The p values reflect log-rank analysis for any recurrence and completing risks for sites of first recurrence.
Financial disclosures:
Bernard H. Bochner certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bochner BH, Kattan MW, Vora KC. Postoperative nomogram predicting risk of recurrence after radical cystectomy for bladder cancer. J Clin Oncol 2006;24:3967–72. [DOI] [PubMed] [Google Scholar]
- [2].Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol 2001;19:666–75. [DOI] [PubMed] [Google Scholar]
- [3].Johar RS, Hayn MH, Stegemann AP, et al. Complications after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2013;64:52–7. [DOI] [PubMed] [Google Scholar]
- [4].Nazmy M, Yuh B, Kawachi M, et al. Early and late complications of robot-assisted radical cystectomy: a standardized analysis by urinary diversion type. J Urol 2014;191:681–7. [DOI] [PubMed] [Google Scholar]
- [5].Wechter ME, Mohd J, Magrina JF, et al. Complications in robotic-assisted gynecologic surgery according to case type: a 6-year retrospective cohort study using Clavien-Dindo classification. J Minim Invasive Gynecol 2014;21:844–50. [DOI] [PubMed] [Google Scholar]
- [6].Tan WS, Lamb BW, Tan MY, et al. In-depth critical analysis of complications following robot-assisted radical cystectomy with intracorporeal urinary diversion. Eur Urol Focus 2017;3:273–9. [DOI] [PubMed] [Google Scholar]
- [7].Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur Urol 2015;67:1042–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bochner BH, Sjoberg DD, Laudone VP. A randomized trial of robot-assisted laparoscopic radical cystectomy. N Engl J Med 2014;371:389–90. [DOI] [PubMed] [Google Scholar]
- [9].Shabsigh A, Korets R, Vora KC, et al. Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology. Eur Urol 2009;55:164–74. [DOI] [PubMed] [Google Scholar]
- [10].Dotan ZA, Kavanagh K, Yossepowitch O, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007;178:2308–12. [DOI] [PubMed] [Google Scholar]
- [11].Hadjizacharia P, Stein JP, Cai J, Miranda G. The impact of positive soft tissue surgical margins following radical cystectomy for high-grade, invasive bladder cancer. World J Urol 2009;27:33–8. [DOI] [PubMed] [Google Scholar]
- [12].Koppie TM, Vickers AJ, Vora K, Dalbagni G, Bochner BH. Standardization of pelvic lymphadenectomy performed at radical cystectomy: can we establish a minimum number of lymph nodes that should be removed? Cancer 2006;107:2368–74. [DOI] [PubMed] [Google Scholar]
- [13].Raza SJ, Al-Daghmin A, Zhuo S, et al. Oncologic outcomes following robot-assisted radical cystectomy with minimum 5-year follow-up: the Roswell Park cancer institute experience. Eur Urol 2014;66:920–8. [DOI] [PubMed] [Google Scholar]
- [14].Xylinas E, Green DA, Otto B, et al. Robotic-assisted radical cystectomy with extracorporeal urinary diversion for urothelial carcinoma of the bladder: analysis of complications and oncologic outcomes in 175 patients with a median follow-up of 3 years. Urology 2013;82:1323–9. [DOI] [PubMed] [Google Scholar]
- [15].Yuh B, Wilson T, Bochner B, et al. Systematic review and cumulative analysis of oncologic and functional outcomes after robot-assisted radical cystectomy. Eur Urol 2015;67:402–22. [DOI] [PubMed] [Google Scholar]
- [16].Gandaglia G, De Groote R, Geurts N, et al. Oncologic outcomes of robot-assisted radical cystectomy: results of a high-volume robotic center. J Endourol 2016;30:75–82. [DOI] [PubMed] [Google Scholar]
- [17].Raza SJ, Wilson T, Peabody JO, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2015;68:721–8. [DOI] [PubMed] [Google Scholar]
- [18].Guru KA, Kim HL, Piacente PM, Mohler JL. Robot-assisted radical cystectomy and pelvic lymph node dissection: initial experience at Roswell Park Cancer Institute. Urology 2007;69:469–74. [DOI] [PubMed] [Google Scholar]
- [19].Nix J, Smith A, Kurpad R, Nielsen ME, Wallen EM, Pruthi RS.. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol 2010;57:196–201. [DOI] [PubMed] [Google Scholar]
- [20].Ng CK, Kauffman EC, Lee MM, et al. A comparison of postoperative complications in open versus robotic cystectomy. Eur Urol 2010;57:274–81. [DOI] [PubMed] [Google Scholar]
- [21].Hussein AA, Saar M, May PR, et al. Early oncologic failure after robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol 197:1427–36. [DOI] [PubMed] [Google Scholar]
- [22].Albisinni S, Rassweiler J, Abbou CC, et al. Long-term analysis of oncological outcomes after laparoscopic radical cystectomy in Europe: results from a multicentre study by the European Association of Urology (EAU) section of Uro-technology. BJU Int 2015;115:937–45. [DOI] [PubMed] [Google Scholar]
- [23].Pruthi RS, Wallen EM. Robotic assisted laparoscopic radical cystoprostatectomy: operative and pathological outcomes. J Urol 2007;178:814–8. [DOI] [PubMed] [Google Scholar]
- [24].Yuh BE, Nazmy M, Ruel NH, et al. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur Urol 2012;62:806–13. [DOI] [PubMed] [Google Scholar]
- [25].Hellenthal NJ, Hussain A, Andrews PE, et al. Surgical margin status after robot assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. J Urol 2010;184:87–91. [DOI] [PubMed] [Google Scholar]
- [26].Phillips EA, Uberoi V, Tuerk IA. Robot-assisted radical cystectomy in octogenarians. J Endourol 2014;28:219–23. [DOI] [PubMed] [Google Scholar]
- [27].Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumor. AJR Am J Roentgenol 2011;196:117–22. [DOI] [PubMed] [Google Scholar]
- [28].Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2–4): an autopsy study on 367 patients. Urol Int 1999;62:69–75. [DOI] [PubMed] [Google Scholar]
- [29].Nguyen DP, Al Hussein Al Awamlh B, Wu X, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol 2015;68:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Collins JW, Hosseini A, Adding C, et al. Early recurrence patterns following totally intracorporeal robot-assisted radical cystectomy: results from the EAU Robotic Urology Section (ERUS) scientific working group. Eur Urol 2017;71:723–6. [DOI] [PubMed] [Google Scholar]