The Oman Medical Specialty Board (OMSB) sponsors graduate medical education programs that comprise 560 residents and approximately 600 faculty, distributed among 6 major centers and multiple local health centers (such as family medicine centers) that are located within 15 kilometers of the sponsoring institution's site. After running structured residency training programs for 6 years, OMSB leadership thought it was time to explore international accreditation opportunities.1 Other educational institutions in the region were moving toward Accreditation Council for Graduate Medical Education International (ACGME-I) due to advantages in enhancing education and making graduates eligible for fellowship training in the United States. Graduates of ACGME-I programs with advanced specialty accreditation are able to apply for US ACGME fellowship positions, except in neurological surgery.2
In this perspective, we describe how OMSB sponsoring institution leadership and staff and stakeholders were enaged in the process of preparing for the accreditation site visits. This involved multiple activities, meetings, and tools, including an electronic residency management system. The international accreditation process comes with unique experiences, benefits, challenges, and lessons learned.3–5 Practical approaches to integrate the ACGME-I requirements and the Omani health care delivery system had to be developed to overcome challenges. Our experience may assist interested parties to understand unique features of ACGME-I site visits and identify ways to prepare for and navigate the ACGME-I institutional and program site visits. This has allowed OMSB to succeed; the current accreditation status of our programs is provided as supplemental online information.
Phase 1: Previsit Approaches and Challenges
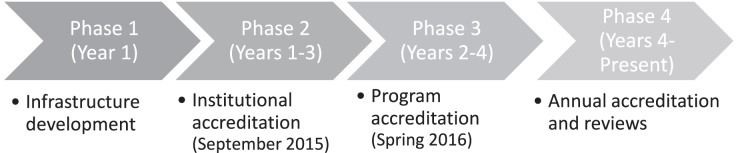
The groundwork toward signing the agreement with ACGME-I took 2 years, and involved multiple negotiation meetings between OMSB and ACGME-I leadership, which culminated in a 4-year agreement in 4 phases signed in 2014 (see the Figure).
Figure.
Phases of Obtaining Institutional and Program Accreditation by ACGME-I
One of the unique features of our setting is that, while OMSB provides oversight, training occurs in centers that are not under OMSB jurisdiction. Each program is managed by an education committee that is responsible for day-to-day operations in the training for centers and for ensuring OMSB quality standards are met.6 Standards for this oversight were developed by the OMSB local accreditation committee in 2007 and revised by a quality assurance committee in 2012. Strong coalitions with training sites are vital to ensure support for education and the eventual success of the accreditation process.
Structural Changes
OMSB deliberately reviewed the requirements and international best practices to identify a graduate medical education (GME) structure for our setting that would have a positive impact on accreditation decisions. Considering the multiple stakeholders in the GME community, OMSB leadership made structural changes to ensure roles are aligned and tasks are assigned and completed.4 This included appointing a designated institutional official (DIO) with the responsibility for program oversight and to lead the newly created GME office. Because of the novelty of the international accreditation process, the DIO had to have strong leadership skills and prior medical education experience. The vice president for academic affairs, who had previous experience as a program director with the ACGME, was selected. Due to OMSB's use of multiple training centers as participating sites, it was important to ensure communication with these sites. Therefore, a postgraduate medical education office (PGME) and committee (PGMEC) were established in each training center, and associate DIOs were appointed in major training centers. Representatives from these PGMECs comprised membership of the newly formed graduate medical education committee (GMEC), along with representatives from the GME programs, residents, and selected OMSB departments and committees. The GMEC and PGMEC members, for example, played a role in addressing the lack of hospital accreditation, which was a previous area of non-compliance. OMSB leadership also reorganized existing education subcommittees into a Clinical Competency Committee and a Program Evaluation Committee to meet requirements and address their roles.
Graduate Medical Education Activities
To ensure progress toward accreditation, the DIO conducted numerous one-on-one meetings with program directors every 2 months to review requirements and prepare for the site visits, and also communicated with DIOs in the United States and internationally to learn from their experiences. Thorough review of the programs' curricula and transformation of the competencies from the CanMEDs model to the ACGME 6 competencies took place. This step was necessary to avoid possible confusion as the application forms request detailed information about teaching and assessment of physician competencies. An update of policies, procedures, and guidelines took place to ensure alignment with ACGME-I required policies, including vendor interactions, disaster management, and program reduction or closure. The GME office monitored the status of all program letters of agreement (PLA) using a template, and collated curricula information and faculty lists in collaboration with individual programs. This served as a challenge and a learning experience, as the GME office keeps records of approximately 200 PLAs for the 16 programs.
Institutional and Program Mock Site Visit
Upon completion of these preparation activities, OMSB underwent mock site visits for the sponsoring institution and 4 programs. To prepare, the GME office and the programs thoroughly reviewed applicable requirements and frequently asked questions to identify areas of non-compliance to make necessary changes prior to the mock visits. An advantage of the mock visits was the opportunity for proactive engagement and involvement of multiple stakeholders. Mock visits helped participants understand how actual institutional and program visits feel, reduce knowledge and preparedness gaps, and increase self-confidence. Despite prior experience with local accreditation committee visits,4 the mock site visits offered added insight about preparing and undergoing international accreditation. Preparation for the mock visits was similar to that for the actual accreditation site visits.
Phases 2 and 3: During and Following the Site Visits
The GME office played a role in preparing for the actual accreditation site visits, including facilitating visa processing for site visitors, booking meeting rooms, communicating with hospital directors to release faculty for meetings, and arranging hospital tours if requested. The GME office also worked closely with the programs to ensure preparedness and assist with logistics, including reviews of documents at least 2 weeks prior to the site visits to ensure availability of requested documents. Logistically, arranging for release of residents and faculty for the visits was difficult especially due to service commitments of both.
After the site visits, the GME office used detailed reflection and discussion of activities to ensure the process for preparing and undergoing ACGME-I institutional and program accreditation was properly documented to formalize, learn from, and improve these processes for the future. Preparation for future accreditation applications began almost immediately after completion of the previous site visits. Overall, preparation for the site visits took about 4 months.
Strengths and Challenges
OMSB had implemented some ACGME-I requirements prior to the formal shift to international accreditation.4 Upon reflection, we feel that this made the shift more manageable and the transition smoother.
Making the required structural changes was one of the challenges faced by OMSB. We overcame this challenge by forming powerful coalitions of multiple stakeholders, such as PGMECs, and communicating the vision for change through multiple meetings and awareness campaigns. The PGMEC involved administrators, faculty, and residents from different institutions, and served as a catalyst for change. This degree of stakeholder engagement was new at OMSB, and encouraged dialogue between stakeholders and a venue to discuss common challenges and suggest solutions. Stakeholders were kept in the loop. The awareness campaigns allowed for open informal discussions between OMSB leaders and stakeholders that built confidence in the ACGME-I accreditation process.
Other challenges arose when comparing international requirements and country-specific regulations and practices. Some related to different approaches for organizing health care service structures or in meeting educationally focused requirements, such as the standard for autopsies, geriatrics, and adolescent medicine.4 Programs are working on implementing changes to meet these requirements utilizing the best resources available. Exemptions were not granted by ACGME-I, and programs have innovated solutions to overcome cultural and health system delivery impediments through didactics, simulations, and gradual introduction of services. Discussions by the GMEC and PGMEC about citations related to these areas raised awareness of these issues and allowed for brainstorming solutions.
Completing ACGME required data, such as faculty information and curricula vitae (CVs) required extensive reviews, collection of data from multiple faculty, and understanding the requirements related to faculty qualifications and scholarly activities. These were addressed by explaining the requirements, and expanding faculty development activities to add a focus on preparing faculty to understand requirements and the new educational terminology.7
Practical Approaches, Tools, and Moving Forward
In the Box, we provide practical tips that may assist institutions and programs considering international accreditation. Additional detailed guidance for all 3 phases of the process is provided as supplemental material.
Box Practical Tips and Tools.
Ensure proper process documentation throughout the accreditation journey.
Make use of a resident management software for documentation and data collection about programs, residents, and faculty.
Hire a reliable graduate medical education office team and ensure clear roles and distribution of tasks.
-
Ensure the team possesses these crucial skills, including:
Organization skills
Proofreading skills
Negotiation skills
Communication skills
Documentation and software use
Use help from a local accreditation/internal review committee to assist with mid-cycle and mock reviews.
-
Communicate:
With international DIOs and Associate DIOs (if applicable)
With program directors (individually and through a program director forum)
With residents (individually and through a resident forum)
Create coalitions with training centers (participating sites or hospital sites used for training of residents and fellows and affiliated with OMSB) through the training centers' PGMECs.
Abbreviations: DIO, designated institutional official; OMSB, Oman Medical Specialty Board; PGMEC, postgraduate medical education committee.
The journey to ACGME-I accreditation has been an exhilarating experience for OMSB. The process and experience has evolved over time and has become easier as many lessons were learned. Different phases of the process have come with their own challenges. We believe these are possible to overcome by having strong leadership, collaboration and teamwork, and effective organization of tasks.
Supplementary Material
References
- 1.Burney IA, Al-Lamki N. Accreditation of graduate medical education programmes: one size fits all-or does it? Sultan Qaboos Univ Med J. 2013;13(2):198–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Accreditation Council for Graduate Medical Education. ACGME Common Program Requirements (Fellowship) 20182019 http://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/CPRFellowship2019.pdf Accessed May 22.
- 3.Accreditation Council for Graduate Medical Education–International. Accreditation process. Steps in the accreditation process. 2019 https://www.acgme-i.org/Accreditation-Process/Overview Accessed May 22.
- 4.Al-Lamki N, Al-Lamki L. International accreditation of postgraduate medical education: whither its role in Oman? Oman Med J. 2016;31(1):1–4. doi: 10.5001/omj.2016.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accreditation Council for Graduate Medical Education–International. International benchmarking through accreditation drives Oman Medical Specialty Board (OMSB) to meet worldwide standards. 2019 https://www.acgme-i.org/What-is-Accreditation/Accreditation-Stories/Accreditation-Story-Details/ArticleID/3 Accessed May 22.
- 6.QA PGME Committee; Oman Medical Specialty Board. “P” and “T” standards for OMSB Residency Program. June 2015. 2019 http://www.omsb.org/File_Publications/QA Booklet_4TH EDITION_14NOV2015 - sent to printer.pdf Accessed May 22.
- 7.Yin CC, Yin OJ, Min BCS, Ong SAK, Al Ling Y, Moh CO. Implementing residency programs and its challenges. Innov Glob Heal Prof Educ. 2015;5:1–7. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.