Abstract
While treatment of serious infectious diseases may require high-dose amoxicillin, continuous infusion may be limited by lack of knowledge regarding the chemical stability of the drug. Therefore, we have performed a comprehensive study so as to determine the chemical stability of high-dose amoxicillin solutions conducive to safe and effective continuous intravenous administration using portable elastomeric pumps. First, amoxicillin solubility in water was assessed within the range of 25 to 300 mg/mL. Then, amoxicillin solutions were prepared at different concentrations (25, 50, 125, 250 mg/mL) and stored in different conditions (5±2°C, 25±1°C, 30±1°C and 37±1°C) to investigate the influence of concentration and temperature on the chemical stability of amoxicillin. Finally, its stability was assessed under optimized conditions using a fully validated HPLC-UV stability-indicating method. Degradation products of amoxicillin were investigated by accurate mass determination using high-resolution mass spectrometry. Amoxicillin displayed limited water solubility requiring reconstitution at concentrations below or equal to 150 mg/mL. Amoxicillin degradation were time, temperature as well as concentration-dependent, resulting in short-term stability, in particular at high concentrations. Four degradation products of amoxicillin have been identified. Among them, amoxicilloic acid and diketopiperazine amoxicillin are at risk of allergic reaction and may accumulate in the patient. Optimized conditions allowing for continuous infusion of high-dose amoxicillin has been determined: amoxicillin should be reconstituted at 25 mg/mL and stored up to 12 hours at room temperature (22 ± 4°C) or up to 24 hours between 4 and 8°C.
Introduction
The management of several serious infectious diseases such as bone and joint infections as well as infective endocarditis, requires intravenous administration of high-dose amoxicillin (100–300 mg/kg/day) over a prolonged period, ranging from a few weeks to several months [1–4]. Amoxicillin is a β-lactam antibiotic. β-Lactams are time-dependent antibiotics, meaning that their efficacy depends on the time that free serum concentrations remain above the minimal inhibitory concentration (MIC) during the dosing interval [5]. It has been demonstrated that continuous infusion maintains concentrations above the MIC for a longer period of time within the dosing interval [6]. Moreover, mounting evidence from clinical studies indicates that continuous infusion of time-dependent β-lactam antibiotics may improve clinical success [7–9]. Therefore it may be well-founded to administrate β-Lactams using continuous infusion in patients suffering from serious infectious disease. However, despite the possible clinical benefits of this mode of administration, one of the practical concerns related to continuous infusion is the limited stability of certain antibiotic agents. Indeed, stability issues have to be taken into consideration when implementing drug administration, in order to ensure drug efficacy and safety. Regarding β-lactam antibiotics, not knowing how long they remain stable during infusion may be a limiting factor for continuous administration [10,11].
Furthermore, for several decades, intravenous antimicrobials have been administered increasingly in outpatient settings, in particular thanks to the use of portable devices [12,13]. Outpatient parenteral antimicrobial therapy (OPAT) allows for early hospital discharge, and further reduces costs with fewer nursing and clinic visits [14]. Moreover, OPAT improves quality of life, and portable elastomeric pumps gives patients more flexibility and control over their treatment [15]. Among the important aspects described in OPAT practice guidelines, drug stability has been underlined as a crucial point to be taken into consideration to ensure efficacy and safety of antimicrobial therapy [12,16,17]. In a recent survey, osteomyelitis, prosthetic joint infections and endocarditis were the most commonly reported indications for OPAT [18]. In treatment of these infectious diseases, continuous infusion of high-dose amoxicillin may be limited by lack of knowledge of the chemical stability of the drug. Indeed, very few studies are available in the literature regarding the stability of amoxicillin in aqueous solution for intravenous administration and results regarding long-term stability have been inconsistent [19–23].
The aim of this work was to propose safe and effective conditions for continuous intravenous administration of high-dose amoxicillin using portable elastomeric pumps. For that purpose we performed a comprehensive study designed to determine the chemical stability of high-dose amoxicillin solutions.
Materials and methods
Chemicals and infusion devices
Amoxicillin powder used for calibration of the method was purchased from Sigma-Aldrich (Sigma-Aldrich, France) while amoxicillin sodium powder for solution for injection, equivalent to amoxicillin 2 g, was used for the pharmaceutical preparation (Panpharma, France). HPLC-grade methanol was obtained from Carlo Erba (Carlo Erba, France) and ultrapure water was provided using a Millipore Direct-Q 3 UV water purification system (MerckMillipore, France). Sterile water and 0.9% sodium chloride for injection were obtained from B.Braun (B.Braun, France).
Portable elastomeric pumps Infusor (48 mL, 2 mL/h) and FOLFusor (240 mL, 10 mL/h), were obtained from Baxter (Baxter, France) and portable elastomeric pumps Accufuser (480 mL, 20 mL/h) from Wym (Wym, France). The Infusor and FOLFusor reservoirs are made of synthetic polyisoprene and the Accufuser reservoir is made of medical silicone.
Solubility study
Amoxicillin solubility was assessed within the range 25 to 300 mg/mL by dissolving a vial of amoxicillin sodium, equivalent to amoxicillin 2 g, in adequate volume of sterile water for injection. The vial was vortexed for 10 min, centrifugated at 3500 G for 10 min and the amoxicillin concentration was determined in the supernatant. Each experiment was conducted in triplicate.
Stability study
To determine the optimal volume of dilution for amoxicillin reconstitution, we investigated the influence of the concentration on the chemical stability of amoxicillin. Amoxicillin solutions were prepared at different concentrations in various infusion devices (Table 1). The filled elastomeric pumps were stored at 25 ± 1°C for 24 hours in a climate chamber without humidity control (Air concept, FirLabo, France). Samples (n = 3) were collected at different times over the 24-hour storage period and determination of the amoxicillin concentration was performed immediately. Each experiment was conducted in triplicate.
Table 1. Amoxicillin solutions prepared to investigate the influence of concentration on the chemical stability of amoxicillin.
| Amoxicillin concentration (mg/mL) |
Amoxicillin (g) |
Solvent | Solvent volume (mL) | Infusion device: nominal volume, flow rate |
|---|---|---|---|---|
| 250 | 12 | Sterile water for injection | 48 | Infusor: 48 mL, 2 mL/h |
| 125 | 6 | Sterile water for injection | 48 | Infusor: 48 mL, 2 mL/h |
| 50 | 12 | Sterile water for injection | 240 | FOLfusor: 240 mL, 10mL/h |
| 25 | 6 | Sterile water for injection : 0.9% sodium chloride (50:50, v:v) | 240 | FOLfusor: 240 mL, 10mL/h |
To determine the best storage conditions during amoxicillin infusion we investigated the influence of temperature on the chemical stability of amoxicillin. Amoxicillin solutions were prepared at a concentration of 125 mg/mL (6 g of amoxicillin were reconstituted with 48 ml of sterile water for injection), in order to fill an Infusor (nominal volume of 48 mL, flow rate of 2 mL/h). The filled elastomeric pumps were then stored for 24 hours at different temperature conditions: 5 ± 2°C in a refrigerated chamber (Precision, Thermo Scientific, France), 25 ± 1°C, 30 ± 1°C and 37 ± 1°C, in a climate chamber (Air concept, FirLabo, France). Samples (n = 3) were collected at different times over the 24-hour storage period and determination of the amoxicillin concentration was performed immediately. Each experiment was conducted in triplicate.
Finally, the stability of amoxicillin was assessed under optimized conditions. For this purpose, amoxicillin solutions were prepared at a concentration of 25 mg/mL (12 g of amoxicillin were reconstituted using 240 ml of sterile water for injection and 240 mL of 0.9% sodium choride for injection), in order to fill elastomeric pumps (Accufuser, nominal volume of 480 mL, flow rate of 20 mL/h). The filled elastomeric pumps were then stored for 24 hours at room temperature (22 ± 4°C) or between 4 and 8°C in a refrigerated bag. Samples (n = 3) were collected at different times over the storage period and determination of the amoxicillin concentration was performed immediately. Each experiment was conducted in triplicate.
The degradation rate was estimated using the slope of the linear regression curve corresponding to amoxicillin remaining (% of initial concentration) versus time profile.
Stability was defined as less than 10% disappearance of the amoxicillin concentration, in compliance with the provisions of the US Pharmacopoeia concerning the acceptable limit of content of drug preparation settled at 90% [24], and with the European Pharmacopoeia, requiring that β-Lactams solutions always contain at least 90% of intact molecule [25].
Amoxicillin determination
All samples were diluted to a concentration of 100 μg/mL with ultrapure water and assayed for amoxicillin concentration using a high-performance liquid chromatography method coupled to UV detection (HPLC-UV). The Elite LaChrom system (VWR, France) included a binary pump (Primaid 1110) used in isocratic mode, a single wavelength ultraviolet detector (L-2400), and an autosampler (L-2200) and was controlled using EZChrom Elite 3.3 software. Separation was performed using a Nucleosil C8 analytical column (5 μm, 150 x 4.6 mm, VWR, France). Mobile phase consisted of 20% methanol and 80% ultrapure water; flow rate was set at 1 mL/min and 10 μL of the diluted sample were injected onto the column. Quantification was performed by integration of the peak at a detection wavelength of 225 nm. The stability-indicating HPLC-UV method was validated in accordance with the guidelines of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Q2(R1) [26]. Briefly, the method was linear over the range 0–200 μg/mL (r2 > 0.9993) and the limit of quantification of amoxicillin was equal to 12.5 μg/mL. Precision of the method was high, according to intra-day and inter-day coefficients of variation, calculated at low and high concentrations, equal to or less than 4.4% and to the trueness, assessed using the bias, ranging from 88 to 108%. To ensure that the method could be regarded as suitably stability-indicating, we checked to be sure that the decomposition products obtained from amoxicillin solution subjected to severe stress (90°C, pH 1, pH10) did not coelute with the intact drug.
Degradation product identification
Degradation products of amoxicillin were investigated by accurate mass determination using high-resolution mass spectrometry (HRMS). Briefly, amoxicillin solutions (50 mg/mL, extemporaneous and kept stored 48 hours at 37°C) were injected onto the HPLC system connected to an ultra-high definition quadrupole time-of-flight mass spectrometer (Xevo QTof, Waters, France). The mass spectrometer was equipped with electrospray source, operating in positive ion mode, using the following operating parameters: capillary voltage: 0.5 kV; sample cone voltage: 20 V; source temperature: 120°C; desolvation temperature: 600°C; cone gas flow: 50L/h; desolvatation gas flow: 1000L/h. Accurate mass measurements of main ions (Low energy: 4 eV) and fragments (high-energy ramp: 10 to 40 eV) were used to identify the major degradation products. LC-MS-measured accurate mass spectra were recorded across the range 50–1000 m/z with scan time settled at 0.1 s. The degradation products were identified by structure elucidation using the molecular ion exact mass determination and the collision-induced dissociation fragments obtained using HRMS.
Results
Solubility study
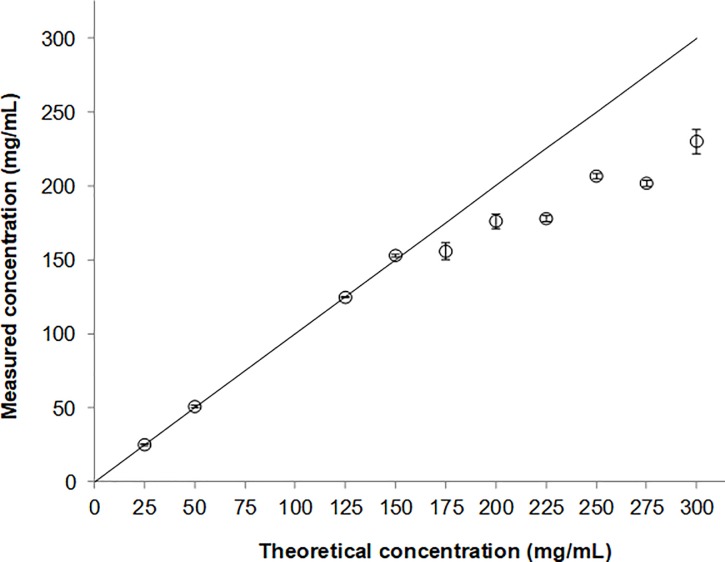
From 0 to 150 mg/mL, measured concentrations of amoxicillin are well-correlated to theoretical concentrations (Fig 1). At concentrations greater than 150 mg/mL, measured amoxicillin concentrations are systematically lower than theoretical values, underlining the incomplete solubility of amoxicillin in water at high concentration.
Fig 1. Solubility of amoxicillin expressed as measured amoxicillin concentrations (○) and expected amoxicillin concentrations (solid line) versus theoretical concentration.
Each value is the mean of three independent determinations ± standard deviation.
Stability study
Whatever the nominal concentration prepared and whatever the storage conditions selected, amoxicillin concentration decreases over time, demonstrating that amoxicillin is unstable in aqueous solution.
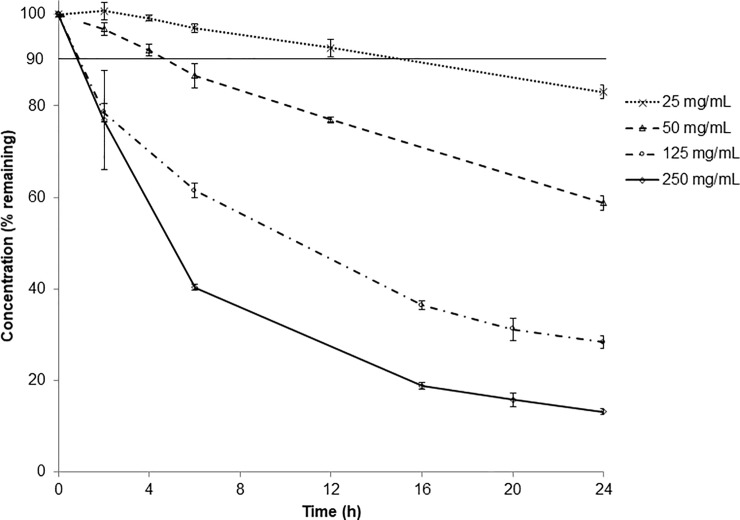
Amoxicillin degradation rate increases depending on the initial concentration: from -0.75%/h to -3.34%/h at 25 mg/mL and 250 mg/mL respectively, leading to concentrations after 24-hour storage ranging from 83% to 13% of the initial concentration (Fig 2). Amoxicillin chemical stability depends on initial concentration: the higher the concentration, the less stable the antibiotic.
Fig 2. Chemical stability of amoxicillin prepared at different concentrations in portable elastomeric pump stored at 25 ± 1°C.
Values are expressed as mean ± standard deviation. The horizontal line indicates the limit set by the Pharmacopoeias (90% of initial drug concentration).
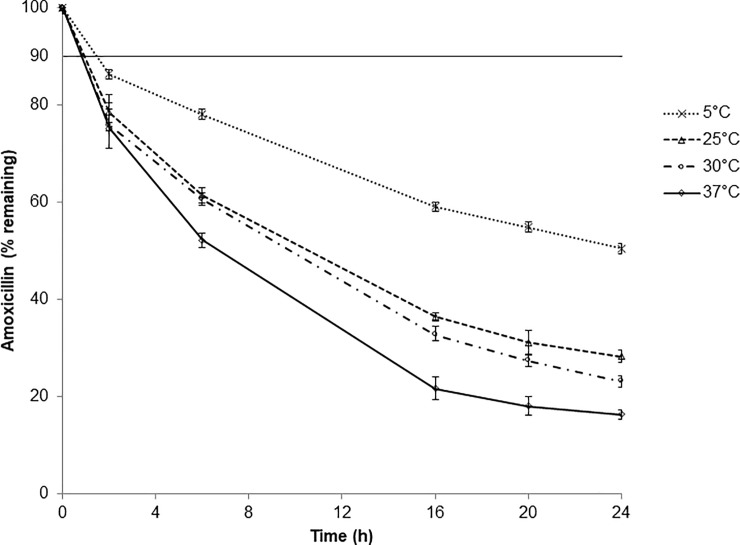
Moreover, amoxicillin degradation rate increases depending on storage temperature: from -1.92%/h to -3.29%/h at 5°C and 37°C mg/mL respectively, leading to concentrations after 24-hour storage ranging from 50% to 16% of the initial concentration (Fig 3). Regarding temperature amoxicillin chemical stability is temperature-dependent: the higher the temperature, the less stable the antibiotic.
Fig 3. Chemical stability of amoxicillin (125 mg/mL) in portable elastomeric pump stored at different temperatures.
Values are expressed as mean ± standard deviation. The horizontal line indicates the limit set by the Pharmacopoeias (90% of initial drug concentration).
Using optimized conditions of administration, the amoxicillin remaining was greater than 90% up to 12 hours of storage at room temperature (22 ± 4°C) and up to 24 hours stored between 4 and 8°C (Table 2).
Table 2. Chemical stability of amoxicillin (25 mg/mL) in portable elastomeric pump stored at room temperature (22 ± 4°C) and under refrigerated conditions (4–8°C).
Values are expressed as mean ± standard deviation.
| Storage conditions | ||
|---|---|---|
| Hours | Room temperature | Refrigerated |
| 0 (mg/mL) |
25.65 ± 1.98 | 25.01 ± 0.58 |
| 2 (% remaining) |
100.78 ± 1.94 | 97.12 ± 4.32 |
| 4 (% remaining) |
99.17 ± 0.66 | 95.92 ± 4.37 |
| 6 (% remaining) |
97.01 ± 0.93 | 95.19 ± 4.29 |
| 12 (% remaining) |
92.63 ± 1.89 | 93.81 ± 3.21 |
| 24 (% remaining) |
83.06 ± 1.43 | 91.24 ± 1.22 |
Degradation product identification
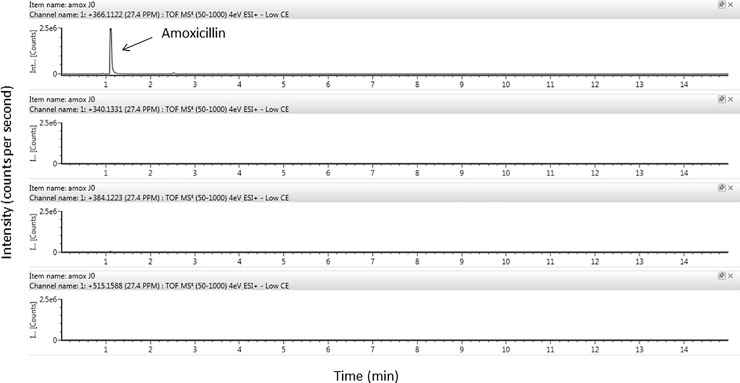
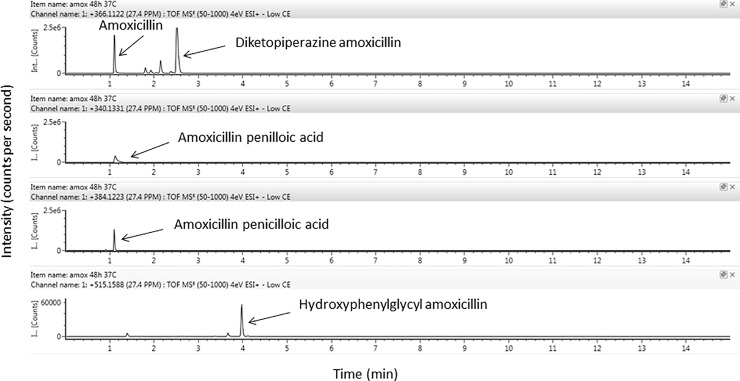
Four degradation products of amoxicillin were identified using HRMS according to their exact mass and pattern of fragmentation (precursor → fragment ions m/z): amoxicillin penilloic acid (340.1331→323.1059/305.0952/189.0691), diketopiperazine amoxicillin (366.1124→160.0426/114.0374), Amoxicillin penicilloic acid (amoxicilloic acid) (384.1223→367.0954/323.1056/305.09520), and hydroxyphenylglycyl amoxicillin (515.1588→498.1361/349.0988/339.0988) (Figs 4 and 5).
Fig 4. Identification of amoxicillin and its degradation products using ion Trap MS/MS analysis of amoxicillin solutions (50 mg/mL) after extemporaneous preparation.
Fig 5. Identification of amoxicillin and its degradation products using ion Trap MS/MS analysis of amoxicillin solutions (50 mg/mL) after 48-hours storage at 37°C.
Discussion
The high doses of amoxicillin required for treatment of severe infections should be administrated over a long period, and preferably by continuous intravenous administration [1–9]. According to the limited volume of medical devices available for continuous drug infusion, particularly the portable devices used for OPAT, highly concentrated solutions of amoxicillin need to be prepared, leading to issues regarding solubility. In this study, we have demonstrated for the first time that amoxicillin has to be reconstituted at concentrations below or equal to 150 mg/mL to achieve complete solubility, which is mandatory in order to ensure accurate drug dosage and efficacy when using intravenous administration mode. Moreover, incomplete solubility of a drug may lead to particles in solutions, which may induce serious harmful effects such as pulmonary embolism or systemic inflammatory response syndrome [27–29]. Therefore, an appropriate volume of solvent is required for the reconstitution of amoxicillin to ensure safe administration, excluding the use of low-volume devices such as syringes or small volume portable pumps, when high doses of amoxicillin are to be administrated.
This work confirms that amoxicillin degradation is time, temperature and concentration-dependent, resulting in short-term stability of amoxicillin solutions for infusion, particularly at high concentrations. Consequently, continuous infusion of the high-dose amoxicillin recommended to treat serious infectious diseases requires applying rigorous conditions of administration especially for OPAT. Elastomeric pumps are commonly used in the setting of OPAT and are available in different volumes and flow rates to allow proper administration of a drug [30]. Therefore, we have investigated different volumes and flow rates through the use of different devices in order to determine which one best helps to achieve amoxicillin concentration-dependent stability. Regarding storage temperature, elastomeric pumps may be used in different conditions, refrigerated or not. Moreover, it has been demonstrated that the temperature of drug solutions in elastomeric pumps may dramatically increase over the day, especially for continuous infusion, reaching a maximum near body temperature [31]. In this context, we have investigated the impact of potentially different storage temperatures on amoxicillin stability in order to determine the optimal conditions for its administration.
Few previous studies have investigated amoxicillin stability in solutions, using varying solvents and concentrations, and different temperatures and containers for storage [19–23]. Most of them report limited stability unsuitable for continuous infusion over a long period. One study has assessed amoxicillin stability in elastomeric pumps and reports results contradictory to ours concerning amoxicillin degradation [21]. Indeed, the authors claim a stability of amoxicillin for 24h-48h up to 40 mg/ml, when stored at 20°C or 35°C. However, these conclusions do not comply with international guidelines regarding the acceptable drug degradation limit of less than 10% [24,25]. Furthermore, even under the same conditions, the degradation rates of amoxicillin reported in this paper are lower than ours, as well as those reported by others under similar conditions [20,22,23]. In fact, it should be noted that the analytical method proposed in this study was not stability-indicating since they did not use a separation method, leading to potential confusion between amoxicillin and its degradation products [32]. This major flaw in the method is likely to explain the underestimated amoxicillin degradation rates reported by these authors.
Unreliable data regarding stability of such antibiotics may have serious consequences since clinicians may use these results to promote continuous infusion over a prolonged period. This issue raises the need for clinicians to seek pharmaceutical expertise in order to assess the level of proof of the analytical methods used to provide data having clinical impact [33].
Despite the lack of consistent data on amoxicillin stability, some authors have investigated the suitability of continuous intravenous administration of high-dose amoxicillin in patients [23,34]. However, while Verdier et al. demonstrated that continuous infusion of amoxicillin provides fewer low concentrations than intermittent administration, they reported highly inconstant amoxicillin plasma concentrations using this administration mode, despite adapted doses [34]. Moreover, despite continuous administration of high-doses amoxicillin (12 g/day), Arensdorff et al. observed, very low amoxicillin plasma concentrations (0.9 and 2.4 mg/mL) in two out of the nine patients enrolled [23]. In both these studies, amoxicillin unstability may have resulted in inaccurate dose administration, leading to suboptimal or ineffective concentrations in treated patients, with respect to the MIC of the bacteria involved in the infection. Clearly, while the great variability in amoxicillin plasma levels is due to many factors, unstability issue should not be ruled out as a causal factor.
Last but not least, drug degradation may lead to toxic byproducts [17]. Regarding amoxicillin we have identified four degradation products, Among them, amoxicilloic acid and diketopiperazine amoxicillin have been identified as the two major degradation products of amoxicillin. Hydroxyphenylglycyl amoxicillin acid and amoxicillin penilloic acid have also been described by others [35,36]. None of these degradation products have antimicrobial activity, but amoxicilloic acid and diketopiperazine amoxicillin are at risk of allergic reaction, of the same order of magnitude as those observed with amoxicillin [37,38]. In addition, it has been demonstrated that the amoxicilloic acid concentrations measured in pig kidney after intravenous administration are higher than amoxicillin concentrations and decrease more slowly, meaning that this degradation product may accumulate in the patient [39,40]. This issue could partly explain the side effects reported in patients treated with high-dose amoxicillin.
In conclusion, according to the experiments carried out in this work, the optimal conditions for the administration of continuous infusion of high-dose amoxicillin using portable elastomeric pumps have been defined. First of all, amoxicillin has to be diluted in order to obtain a final concentration of 25 mg/ml, allowing for complete dissolution and long-term stability. Dilution should be performed in sterile water for injection combined with 0.9% sodium choride for injection (50:50, v:v) in order to maintain osmolarity at a level (approximately 280 mOsm/L, data no shown) suitable for intravenous infusion using peripheral access [16]. Dilution in dextrose should be avoided since lower stability using this solvent has been demonstrated [20]. Then, under these optimized conditions, the elastomeric pump may be stored at ambient temperature (22 ± 4°C) for 12 hours, requiring filling an elastomeric pump every 12 hours for continuous infusion. In addition, it is important to store the elastomeric pump at a temperature below 26°C, which implies not holding the device near the body or under clothing during the day, and keeping it beside the head, outside of the blankets when the patient is bedridden. Finally, amoxicillin may remain stable over the entire day under the same conditions, provided that the elastomeric pump is stored between +4°C and +8°C throughout the administration, using for instance a refrigerated bag; however this can lead to potential intolerance due to infusion of cold solution.
Acknowledgments
We wish to thank Jeffrey Arsham, an American medical translator, for his highly helpful reading of our original text.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36: 3075–3128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Papalia R, Zampogna B, Torre G, Albo E, Denaro V. The management of osteomyelitis in the adult. The Surgeon. 2016;14: 345–360. 10.1016/j.surge.2015.12.005 [DOI] [PubMed] [Google Scholar]
- 3.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, et al. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015;132: 1435–1486. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 4.Everts RJ, Chambers ST, Murdoch DR, Rothwell AG, McKie J. Successful antimicrobial therapy and implant retention for streptococcal infection of prosthetic joints. ANZ J Surg. 2004;74: 210–214. 10.1111/j.1445-2197.2004.02942.x [DOI] [PubMed] [Google Scholar]
- 5.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis Off Publ Infect Dis Soc Am. 1998;26: 1–10; quiz 11–12. [DOI] [PubMed] [Google Scholar]
- 6.MacVane SH, Kuti JL, Nicolau DP. Prolonging β-lactam infusion: a review of the rationale and evidence, and guidance for implementation. Int J Antimicrob Agents. 2014;43: 105–113. 10.1016/j.ijantimicag.2013.10.021 [DOI] [PubMed] [Google Scholar]
- 7.Teo J, Liew Y, Lee W, Kwa AL-H. Prolonged infusion versus intermittent boluses of β-lactam antibiotics for treatment of acute infections: a meta-analysis. Int J Antimicrob Agents. 2014;43: 403–411. 10.1016/j.ijantimicag.2014.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Chant C, Leung A, Friedrich JO. Optimal dosing of antibiotics in critically ill patients by using continuous/extended infusions: a systematic review and meta-analysis. Crit Care Lond Engl. 2013;17: R279 10.1186/cc13134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdul-Aziz MH, Lipman J, Mouton JW, Hope WW, Roberts JA. Applying Pharmacokinetic/Pharmacodynamic Principles in Critically Ill Patients: Optimizing Efficacy and Reducing Resistance Development. Semin Respir Crit Care Med. 2015;36: 136–153. 10.1055/s-0034-1398490 [DOI] [PubMed] [Google Scholar]
- 10.Charmillon A, Novy E, Agrinier N, Leone M, Kimmoun A, Levy B, et al. The ANTIBIOPERF study: a nationwide cross-sectional survey about practices for β-lactam administration and therapeutic drug monitoring among critically ill patients in France. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2016;22: 625–631. 10.1016/j.cmi.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 11.Longuet P, Lecapitaine AL, Cassard B, Batista R, Gauzit R, Lesprit P, et al. Preparing and administering injectable antibiotics: How to avoid playing God. Med Mal Infect. 2016;46: 242–268. 10.1016/j.medmal.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 12.Seaton RA, Barr DA. Outpatient parenteral antibiotic therapy: Principles and practice. Eur J Intern Med. 2013;24: 617–623. 10.1016/j.ejim.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 13.Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46: 307–312. 10.1016/j.ijantimicag.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 14.MacKenzie M, Rae N, Nathwani D. Outcomes from global adult outpatient parenteral antimicrobial therapy programmes: A review of the last decade. Int J Antimicrob Agents. 2014;43: 7–16. 10.1016/j.ijantimicag.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 15.Durojaiye OC, Bell H, Andrews D, Ntziora F, Cartwright K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): a decade of Sheffield (UK) OPAT service. Int J Antimicrob Agents. 2018;51: 26–32. 10.1016/j.ijantimicag.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 16.Norris AH, Shrestha NK, Allison GM, Keller SC, Bhavan KP, Zurlo JJ, et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;68: 1–4. 10.1093/cid/ciy867 [DOI] [PubMed] [Google Scholar]
- 17.Steffens E, Quintens C, Derdelinckx I, Peetermans WE, Van Eldere J, Spriet I, et al. Outpatient parenteral antimicrobial therapy and antibiotic stewardship: opponents or teammates? Infection. 2019;47: 169–181. 10.1007/s15010-018-1250-1 [DOI] [PubMed] [Google Scholar]
- 18.Muldoon EG, Switkowski K, Tice A, Snydman DR, Allison GM. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT). Infect Dis Lond Engl. 2015;47: 39–45. 10.3109/00365548.2014.967290 [DOI] [PubMed] [Google Scholar]
- 19.Drug_Stability_Easypump_II_012018_EN.pdf [Internet]. Available: https://www.bbraun.nl/content/dam/b-braun/nl/website/Drug_Stability_Easypump_II_012018_EN.pdf.bb-.97401460/Drug_Stability_Easypump_II_012018_EN.pdf
- 20.Cook B, Hill SA, Lynn B. The stability of amoxycillin sodium in intravenous infusion fluids. J Clin Hosp Pharm. 1982;7: 245–250. [DOI] [PubMed] [Google Scholar]
- 21.Arlicot N, Marie A, Cade C, Laffon M, Antier D. Stability of amoxicillin in portable pumps is drug concentration dependent. Pharm. 2011;66: 631–632. [PubMed] [Google Scholar]
- 22.Bernard L, Sautou V, Bourdeaux D, Chopineau J. [Stability and compatibility of acetaminophen, ketoprofen and amoxicillin in a fail-safe intravenous administration set]. Ann Fr Anesth Reanim. 2012;31: 47–52. 10.1016/j.annfar.2011.08.019 [DOI] [PubMed] [Google Scholar]
- 23.Arensdorff L, Boillat-Blanco N, Decosterd L, Buclin T, de Vallière S. Adequate plasma drug concentrations suggest that amoxicillin can be administered by continuous infusion using elastomeric pumps. J Antimicrob Chemother. 2017;72: 2613–2615. 10.1093/jac/dkx178 [DOI] [PubMed] [Google Scholar]
- 24.United States Pharmacopeial Convention. The United States Pharmacopeia 2018: USP 41 ; The national formulary : NF 36. 2017.
- 25.9789287181336: Pharmacopée européenne : 3 volumes, suppléments 9.0, 9.1 et 9.2—AbeBooks—Conseil de l’Europe: 9287181330 [Internet]. [cited 28 Feb 2019]. Available: https://www.abebooks.fr/9789287181336/Pharmacop%C3%A9e-europ%C3%A9enne-volumes-suppl%C3%A9ments-9.0-9287181330/plp
- 26.Abraham J. International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. In: Brouder A, Tietje C, editors. Handbook of Transnational Economic Governance Regimes. Netherlands: Brill; 2009. pp. 1041–1054. 10.1163/ej.9789004163300.i-1081.897 [DOI]
- 27.Puntis JW, Wilkins KM, Ball PA, Rushton DI, Booth IW. Hazards of parenteral treatment: do particles count? Arch Dis Child. 1992;67: 1475–1477. 10.1136/adc.67.12.1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McNearney T, Bajaj C, Boyars M, Cottingham J, Haque A. CASE REPORT: Total Parenteral Nutrition Associated Crystalline Precipitates Resulting in Pulmonary Artery Occlusions and Alveolar Granulomas. Dig Dis Sci. 2003;48: 1352–1354. 10.1023/a:1024119512162 [DOI] [PubMed] [Google Scholar]
- 29.Lehr H-A, Brunner J, Rangoonwala R, James Kirkpatrick C. Particulate Matter Contamination of Intravenous Antibiotics Aggravates Loss of Functional Capillary Density in Postischemic Striated Muscle. Am J Respir Crit Care Med. 2002;165: 514–520. 10.1164/ajrccm.165.4.2108033 [DOI] [PubMed] [Google Scholar]
- 30.Skryabina EA, Dunn TS. Disposable infusion pumps. Am J Health-Syst Pharm AJHP Off J Am Soc Health-Syst Pharm. 2006;63: 1260–1268. 10.2146/ajhp050408 [DOI] [PubMed] [Google Scholar]
- 31.Voumard R, Van Neyghem N, Cochet C, Gardiol C, Decosterd L, Buclin T, et al. Antibiotic stability related to temperature variations in elastomeric pumps used for outpatient parenteral antimicrobial therapy (OPAT). J Antimicrob Chemother. 2017;72: 1462–1465. 10.1093/jac/dkw582 [DOI] [PubMed] [Google Scholar]
- 32.Trissel LA. Avoiding common flaws in stability and compatibility studies of injectable drugs. Am J Hosp Pharm. 1983;40: 1159–1160. [PubMed] [Google Scholar]
- 33.Jenkins A, Hills T, Santillo M, Gilchrist M. Extended stability of antimicrobial agents in administration devices. J Antimicrob Chemother. 2017;72: 1217–1220. 10.1093/jac/dkw556 [DOI] [PubMed] [Google Scholar]
- 34.Verdier MC, Tribut O, Tattevin P, Michelet C, Bentué-Ferrer D. Assessment of Interindividual Variability of Plasma Concentrations after Administration of High Doses of Intravenous Amoxicillin or Cloxacillin in Critically Ill Patients. J Chemother. 2011;23: 277–281. 10.1179/joc.2011.23.5.277 [DOI] [PubMed] [Google Scholar]
- 35.Nägele E, Moritz R. Structure elucidation of degradation products of the antibiotic amoxicillin with ion trap MSn and accurate mass determination by ESI TOF. J Am Soc Mass Spectrom. 2005;16: 1670–1676. 10.1016/j.jasms.2005.06.002 [DOI] [PubMed] [Google Scholar]
- 36.Hirte K, Seiwert B, Schüürmann G, Reemtsma T. New hydrolysis products of the beta-lactam antibiotic amoxicillin, their pH-dependent formation and search in municipal wastewater. Water Res. 2016;88: 880–888. 10.1016/j.watres.2015.11.028 [DOI] [PubMed] [Google Scholar]
- 37.Torres MJ, Ariza A, Fernández J, Moreno E, Laguna JJ, Montañez MI, et al. Role of minor determinants of amoxicillin in the diagnosis of immediate allergic reactions to amoxicillin. Allergy. 2010;65: 590–596. 10.1111/j.1398-9995.2009.02245.x [DOI] [PubMed] [Google Scholar]
- 38.Rico AG. Drug residues in animals [Internet]. Academic; 1986. Available: http://bases.bireme.br/cgi-bin/wxislind.exe/iah/online/?IsisScript=iah/iah.xis&src=google&base=REPIDISCA&lang=p&nextAction=lnk&exprSearch=116951&indexSearch=ID
- 39.Reyns T, Cherlet M, De Baere S, De Backer P, Croubels S. Rapid method for the quantification of amoxicillin and its major metabolites in pig tissues by liquid chromatography-tandem mass spectrometry with emphasis on stability issues. J Chromatogr B. 2008;861: 108–116. 10.1016/j.jchromb.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 40.Reyns T, De Boever S, De Baere S, De Backer P, Croubels S. Tissue Depletion of Amoxicillin and Its Major Metabolites in Pigs: Influence of the Administration Route and the Simultaneous Dosage of Clavulanic Acid. J Agric Food Chem. 2008;56: 448–454. 10.1021/jf072398p [DOI] [PubMed] [Google Scholar]