Abstract
Background.
Alcohol use disorders is a serious illness marked by uncontrollable drinking and a negative withdrawal state when not using. Alcohol is one of the most commonly used drugs among adolescent populations. Given that adolescence is a unique developmental stage during which alcohol has long-term effects on future drug-taking behavior; it is essential to understand how early exposure to ethanol during adolescence may affect the abuse liability of the drug later in life. Our studies focused on characterizing how exposure to alcohol in adolescence alters later adult alcohol dependence behaviors, by using well-established mouse models of ethanol drinking. We hypothesized that early exposure to ethanol leads to increased ethanol intake in adults and other behavioral phenotypes that may lead to dependence.
Methods.
We investigated the impact of ethanol drinking in early adolescent C57BL/6J mice using a modified Drinking in the Dark (DID) model.
Results.
Our results showed that exposure to ethanol during adolescence enhanced ethanol intake in adulthood in the DID, and the 2-bottle choice drinking paradigms. In contrast, adult exposure of alcohol did not enhance later alcohol intake. We also conducted tests for ethanol behavioral sensitivity such as loss of righting reflex and anxiety-related behaviors to further elucidate the relationship between adolescent ethanol exposure and enhanced ethanol intake in adult mice.
Conclusions.
Overall, our results suggest that adolescence is a critical period of sensitivity and binge drinking that can lead to lasting changes in ethanol intake in adulthood. Further research will be required in order to more fully examine the neurochemical mechanisms underlying the lasting changes in adulthood.
Keywords: adolescence, adulthood, alcohol, mice, DID, intake
Introduction
Alcohol use disorder (AUD) and alcohol abuse, characterized by the compulsive and uncontrolled consumption of alcohol, is a global problem that results in the death of 2.5 million people in the world annually and causes illness and injury to millions more (World Health Organization (WHO) 2014). Social, environmental, and genetic factors play significant roles in the initiation of alcohol use and contribute to progression from use to abuse and/or addiction. Alcohol use often begins during the teenage years and the majority of alcohol consumed by teens is in the form of binge-drinking episodes (SAMHSA 2014). Binge drinking is defined as consuming enough ethanol to achieve blood ethanol levels greater than 80 mg/dl (NIAAA 2004). Interestingly, prior experience with binge ethanol is a strong predictor of alcohol dependence later in life. Teens who began drinking before age 14 were 47% more likely to be diagnosed with alcohol dependence in their lifetime as compared to those who started drinking after age 21 (Hingson et al., 2006). In adolescence, alcohol consumption can have enduring behavioral, social and neurobiological consequences since adolescence is a critical period characterized by significant transformations in brain development, cognitive capacities, personalities, and frontal cortical executive functions (Crews et al., 2000, Spear 2000b, Spear 2018).
In addition to neural and hormonal changes, increases in social activity, novelty seeking, impulsivity, and risk-taking are frequently reported during the transitional phase from adolescence to adult (Spear 2000a). Consistent with human epidemiological data, exposure to binge ethanol during the developmental period of adolescence in mice is associated with increased ethanol intake in adulthood (Pascual et al., 2007; Moore et al., 2010; Strong et al.,2010; Fabio et al., 2013). Although this is not always found in all drinking paradigms or both sexes (Strong et al. 2010, Broadwater et al., 2011). Excessive ethanol intake during adolescence can be particularly devastating with some behavioral effects persisting into adulthood. For example, adolescent ethanol increases ethanol intake (Strong et al., 2010; Fabio et al., 2013; Cox et al., 2013) and risk-taking preference (Spear 2000a, Spear 2018), as well as impairing conditioned discrimination, memory (Pascual et al., 2007; Montesinos et al., 2014), reversal learning (Coleman et al., 2011; Coleman et al., 2014) and changes in alcohol-induced withdrawal (Lee et al., 2016; 2017). These impairments were more robust when pretreatment occurred during adolescence rather than in adulthood (White et al., 2000; Sircar et al., 2005; Bergstrom et al., 2006). Furthermore, adolescent binge ethanol increased behavioral sensitivity and decreased ethanol’s aversive effects which can contribute to increased drinking (Spear 2000a). Importantly, Metten et al. have shown ethanol access restricted to the early-mid adolescence is critical for ethanol intake increases in adulthood (Metten et al., 2011).
In the current study, we used the Drinking in the Dark (DID) procedure, an intake paradigm where mice consume large amounts of ethanol reaching blood ethanol concentrations (BEC) 80 mg/dl in a 2-h period (Rhodes et al., 2005), typically over 100 mg/dl, to model binge ethanol consumption and determine whether different ethanol exposure patterns during early adolescence would affect the subsequent ethanol behavioral responses in adulthood. While adolescence in human occurs between the ages of approximately 11-21 years, adolescence in rodents ranges from postnatal days (PNDs) 28-42 (Spear et al., 2000a; Spear 2010; Laviola et al., 2003). In particular, we used daily or intermittent DID ethanol exposure for 9 days during early adolescence and tested for binge-like drinking (DID test) and relapse-like drinking in adulthood. We also assessed whether adolescent binge ethanol alters ethanol sensitivity (anxiolytic-like effects and loss of righting reflex) in adult mice, since a low level of response to acute ethanol is a strong predictor of risk for developing an alcohol use disorder (Heath et al., 1999; Schuckit et al., 2001; Schuckit et al., 2004). Together, these data provide further evidence that adolescence is a critically sensitive period for ethanol’s insults which can produce lasting behavioral changes in adulthood.
Materials and Methods:
Animals
Adolescent (arriving at PND 21) and adult (arriving at PND 67) male and female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME, USA) were housed individually in standard Plexiglas cages (12×18×28 cm) with corn-cob bedding in a temperature- and humidity-controlled room (22±2°C, 50±5%) under a 12-hour inverted light/dark cycle (lights on at 6PM). Mice had food (Teklad, Envigo, Madison, WI; cat. #7012) and water available ad libitum, except where noted during DID sessions. Cages contained small cardboard tubes and a cotton nestlet (5×5 cm) for the purpose of environmental enrichment. Mice were acclimated to the vivarium for one week while water intake and body weight were measured. Different sets of mice were used in the different experiments. On PND 28 and PND 72, three hours into the dark cycle, adolescent and adult mice began the DID protocol. Institutional Animal Care and Use Committee of Virginia Commonwealth University approved the study. All studies were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1: Drinking in the dark (DID) during adolescence
We used a slightly modified mouse DID model (Rhodes et al., 2005). Male and female C57BL/6J adolescent mice (n=8/group) were exposed to one of two single-bottle (25 ml sipper tubes) conditions for a 4-hour period from PND 28 to PND 36 beginning 3 hours after lights out: (1) nine days consecutive daily access to 20% ethanol in tap water or (2) water only. In all cases, after the 4-hour period, bottles were replaced with water only bottles. On day nine, immediately after measuring ethanol intake, blood samples were collected via submandibular bleed. Mice remained singly housed for 36 days with access ad libitum to food and water until they reached adulthood.
To determine the impact of early exposure on adult ethanol consumption, all groups of mice (from PND 72 to PND 80) had daily exposure to ethanol (20%) for 4 hours during PND 28-36. Three hours after lights out, home cage water 25ml tubes were replaced with sipper tubes containing a 20% ethanol in tap water and mice were allowed to drink for 4 hours for nine consecutive days. As spillage and evaporation controls, average volume depleted from tubes in control cages without mice was subtracted from individual drinking values each day.
Experiment 2: DID in adulthood
In order to determine if the enhancement of ethanol intake is unique to the adolescent period, adult male and female C57BL/6J mice (PND 67, n = 7/group) who were housed individually in the same testing room since adolescence age, were exposed to ethanol or water for nine days at PND 72 using the daily DID procedure as described above with adolescent animals. Thirty-six days later, on PND 108, all groups were tested for 20% ethanol consumption in the DID procedure for nine days.
Experiment 3: Two bottle choice drinking and alcohol deprivation effect (ADE)
In order to determine the impact of adolescent binge drinking history on adult ethanol consumption and relapse-like drinking, two studies were conducted:
Exp. 3.1.
C57BL/6J male adolescent (PND 28-36 days) mice were exposed to DID daily ethanol (20%, v/v) or water. When they reached their adulthood period (PND 72), ethanol exposed (EE) group as well as water expose (WE) group were given a choice of ethanol or water in a two-bottle choice paradigm. The ethanol concentration was ramped up in a stepwise manner (5, 10, and 15%, v/v) for three consecutive days at each concentration. The average of three consecutive days’ results was recorded per concentration. The bottle positions were alternated every day to control for the development of a side preference. The choice of ethanol concentrations in this procedure did not match the DID one.
Exp. 3.2.
To study the impact on adolescent ethanol on relapse-like behavior, we used the alcohol deprivation effect (ADE) paradigm. The ADE has generally referred to an increase in alcohol intake seen after a short period of alcohol deprivation during a continuous intake study (Vengeliene et al., 2014). To evaluate ADE on ethanol intake and preference, mice underwent two-bottle choice paradigm using ethanol (15%) vs. water for additional five days after completing above mentioned experiments in Exp. 3.1. (total of fourteen days continuous two-bottle choice access, last eight days with 15% ethanol continuous two-bottle choice). The last measurement of daily ethanol intake was recorded as pre-ADE. Then, ethanol was taken from the cages, and mice underwent a-week duration of alcohol deprivation. At the end of deprivation, mice were re-exposed to ethanol (15%) vs. water in 2-bottle choice paradigm for 24-hour. Ethanol intake was reported as g/kg of body weight and ethanol preference was reported as the ratio between ethanol intake and the total fluid intake. Subjects were weighed daily to accurately calculate ethanol intake.
Experiment 4: Light-dark box (LDB) and Elevated plus maze (EPM) tests of anxiety-like behavior
The LDB test is based upon a conflict between the innate aversion to brightly illuminated areas and spontaneous exploratory activity (Crawley and Goodwin, 1980). The test was adapted as previously described (Wilkerson et al., 2016) with minor modifications. Male C57BL/6J (n=8/group) adolescent mice (PND28) were exposed to alcohol in the DID procedure as described above. At PND 72, mice were given at least 30 min to acclimate to the testing room. Then, mice were injected with either 2 g/kg (20% v/v, i.p.) ethanol or saline and 15 min later they were placed in the light compartment of the LDB (Med-Associates, St. Albans, VT) which consisted of a small, enclosed dark arena (36 × 10 × 34 cm) with a passage way (6 × 6 cm) leading to a larger, light arena (36 × 21 × 34 cm) and allowed to explore the apparatus for 5 min. The number of entries into the light compartment and the total time spent (sec) in the light compartment were recorded by a video monitoring system and measured by ANY-MAZE software (Stoelting Co., Wood Dale, IL).
A similar procedure was followed for the EPM test where reduction in anxiety-like behavior induced by ethanol was assessed in a different cohort of mice. This is an elevated platform consisting of two crossbars that create four arms. Two of these arms have walls (closed arms) and the other two arms are exposed (open arms). Because mice commonly display an innate fear of open, elevated places, an increase in the amount of time spent in the open arms is thought to represent a reduction in anxiety-like behavior. At PND 72, mice were given at least 30 minutes to acclimate to the testing room. Then, mice were injected with either 2 g/kg (20% v/v, i.p.) ethanol or saline and 15 min later, they were placed briefly in a plastic container and transferred to the center of the maze. The subject was allowed to freely explore the apparatus for 5 minutes, with time starting immediately after placement in the center of the maze. Data (mean ± SEM) were expressed as the total time spent in the open arms in seconds. The number of crossovers was also recorded to document any changes in locomotor activity.
Experiment 5: Loss of Righting Reflex (LORR)
The sedative-hypnotic effects of ethanol were measured using the loss of LORR. Male C57BL/6J mice were exposed to adolescent DID for 20% ethanol (n=10/group) or water (n=10/group) as above from PND 28 to PND 36 for a 4-hour period each day. As adults, at PND72, both groups were injected with 3.5 g/kg ethanol (20%v/v, i.p.). Mice were placed in a supine position in a V-shaped trough and monitored for the onset of LORR, defined by the inability to right themselves 3 times in 30 seconds after being in the supine position. Time from ethanol injection to LORR was recorded as LORR onset. Mice taking longer than 5 min to experience LORR were eliminated from the study due to the possibility of misplaced injection (one mouse was eliminated). Immediately after the onset of LORR, a 20 μl blood sample was collected from the periorbital sinus. The time taken to recover from LORR, LORR duration, was defined as the time required for the subject to right itself three times within 30 seconds. A second 20 μl blood sample was collected from the periorbital sinus at this time. Care was taken to collect all blood samples within 10 seconds of each behavioral endpoint so as to minimally affect the true relationships between BEC and LORR onset or recovery. Acute functional tolerance (AFT) was calculated by subtracting the BEC at LORR onset from the BEC at recovery.
Blood Ethanol Concentration (BEC) Determination
Blood was collected via cheek bleed using 5 mm Lancets (Medipoint, Inc., Minenola, NY) or via the periorbital sinus. Blood was stored in heparinized BD microtainers and analyzed for ethanol content using gas chromatography similar to a previously described procedure (Gallaher et al. 1996). Animals were injected with saline (s.c) to avoid hypovolemia and received food/water ad libitum after blood collection.
Statistical Analysis
The data were analyzed using the GraphPad Prism software, version 6.0 (GraphPad Software, Inc., La Jolla, CA) and expressed as the mean ± S.E.M. Statistical analysis was done using the 2-way analysis of variance test (ANOVA) and t test. In experiments 1 and 2, two-way ANOVA followed by the Bonferroni’s post hoc multiple comparisons test was used with ethanol treatment and sex as factors. To determine if there was a treatment (ethanol or water) or exposure type (continuous vs. intermittent) effect in drinking studies, and ratio of second exposure were compared to the each other with an unpaired, two-tailed t-test. The ratio of adult EE vs WE for each day was calculated by dividing the average daily EE intake by the average daily WE intake of the second exposure and assessed by a two-way ANOVA followed by Bonferroni’s post hoc test. To assess the effect of ethanol concentration in EE and WE exposure, a two-way ANOVA followed by the Bonferroni’s post hoc multiple comparisons test was also used. In experiment 3, t-tests were used to determine the alcohol deprivation effect. In experiments 4 and 5, the effect of ethanol treatment on anxiety-like behavior and on LORR was assessed by one-way ANOVA followed by Tukey’s post hoc. Comparisons were considered statistically significant when p < 0.05.
Results
The impact of adolescent’s binge drinking on consumption later on life.
DID is a limited-exposure model of acute binge ethanol drinking in which animals are briefly exposed to one bottle of ethanol at a high concentration (20% v/v) (Rhodes et al., 2005). Here, we employed a slightly modified version of the standard DID procedure in adolescent male and female C57BL/6J mice, which resulted in alcohol intakes between 6.42-7.85 g/kg ethanol (Suppl. Fig. 1) in a 4-hour period, and yielded blood ethanol concentration (BEC) greater than 80 mg/dL at PND 36 (day 9 of the adolescent DID exposure), immediately after recording the 4-hour intake (males: 107 mg/dL ± 6; females: 110 mg/dL ± 8 after alcohol exposure). There were no significant differences between female and male mice (t=0.2555, df=12; p>0.05).
During adolescence, mice were exposed to daily ethanol for 9 days (PND28 to PND36). As seen in Supplementary Figure 1, access to ethanol provided a relatively stable ethanol consumption in both adolescent male and female mice [Ftime (8, 96) = 3.641, p < 0.001]. A main effect of sex was found with females having greater intake than males [Fsex(1, 12) = 10.78, p < 0.01]. No significant interactions between sex and day were found [Finteraction (8, 96) = 1.207, p > 0.05].
We then investigated the impact of adolescent (PND28-PND36) ethanol DID drinking exposure on later adult ethanol consumption (PND72-PND80). Adolescent ethanol-exposed (EE group) and water-exposed (WE group) mice were tested in adulthood using the same drinking paradigm employed in adolescence. In males, daily adolescent binge drinking increased adult DID ethanol intake (Figure 1A). A subsequent two-way ANOVA revealed a significant effect of treatment [Ftreatment(1,13) =19.51, p < 0.001] and time, [Ftime (8, 104) = 4.753, p < 0.001] and no significant interaction [Finteraction (8, 104) = 1.745, p > 0.05]. Post-hoc analysis revealed a significant difference of treatment on all days except for day 3 (p<0.05). In females, ethanol exposure resulted higher intake of ethanol in all days for the group EE that was exposed to alcohol during early adolescence [(Ftreatment (1, 13) = 29.13, p < 0.001] (Figure 1C). A main effect of time [Ftime (8, 104) = 4.299, p < 0.001] but no significant interaction [Finteraction (8, 104) = 1.242, p > 0.05] was found. The average intake over 9 days of drinking showed that adolescent ethanol exposure resulted in a higher intake of ethanol in adulthood in both male [t=4.426, df=8, p<0.002; Figure 1B] and female [t=7.920, df=16, p< <0.0001; Figure 1D] mice.
Figure 1: Exposure to ethanol during early adolescence enhances ethanol intake later in adulthood.
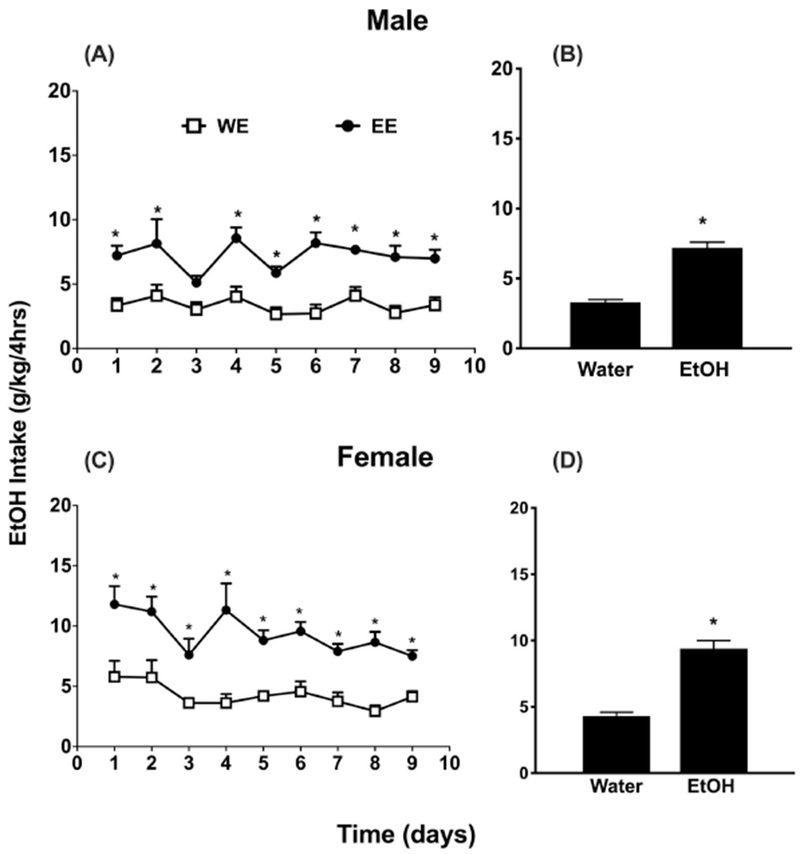
Mice exposed to DID 20% v/v ethanol (EE) vs. water only (WE) consumption during adolescence showed higher daily ethanol (EtOH) intake profiles in the adult phase of exposure (PND72 to PND80). Panels A and C depict PND 72-80 ethanol intake (g/kg/4 hours) in a daily exposure DID procedure for 9 days in male and female C57BL/6J respectively, whereas Panels B and D depict the average ethanol intake for the 9 days period. * Denotes p<0.05 vs. WE group. Each point represents the mean± SEM of 8 animals per group and sex. W= water; E = ethanol.
Alcohol exposure in adulthood did not result an increase of alcohol intake later in life.
In experiment 2, in order to determine if ethanol exposure during the adolescent period uniquely increases ethanol intake later in life, we performed an initial DID ethanol exposure in adult mice (PND 73-81) and determined whether this modulated ethanol intake later in adulthood (PND 117-125), after a similar time interval (36 days) as with the adolescent exposure model in experiment 1. In the first phase of ethanol exposure on PND 73-81 (Suppl. Fig. 2), two way repeated measures ANOVA revealed significant effect of sex, [Fsex(1, 12) = 11.91, p < 0.01], time [Ftime(8, 96) = 7.428, p < 0.0001] and an interaction between sex and time [Finteraction(8, 96) = 4.665, p < 0.001]. Bonferroni’s post-hoc analysis revealed a significant difference of ethanol intake between males and females on day 6 and 7 (p<0.05), where females consumed more than males. However, when the animals were exposed to the ethanol in the second phase later in adulthood (Fig. 2), 36 days following the first exposure, neither males nor females showed a significant enhancement of ethanol intake from prior ethanol exposure (EE vs. WE) [males: [Ftreatment (1, 12) = 0.3167, p > 0.05], [Ftime (8, 96) = 3.914, p <0.001], [Finterraction (8, 96) = 1.573, p > 0.143; Fig. 2A] and females: [Ftreatment (1, 11) = 4.545, p > 0.05], [Ftime (8, 88) = 3.573, p < 0.01, Finteraction (8, 88) = 1.213, p > 0.05; Fig. 2B]. Despite obvious qualitative differences in ethanol consumption between males and females, pre-exposure to ethanol in adulthood did not increase intake for either sex later in life.
Figure 2: Alcohol exposure in adulthood did not result an increase of alcohol intake later in life.
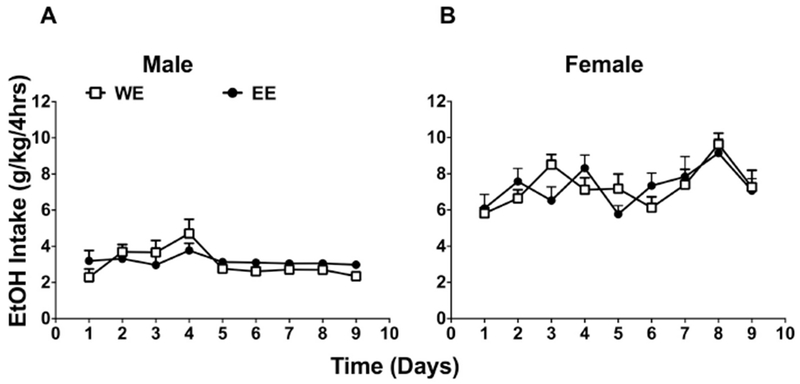
Adult mice exposed to DID 20% v/v ethanol (EE) vs. water only (WE) during adulthood (PND72 to PND80) showed similar daily ethanol (EtOH) intake profiles later in life (starting PND 108) in both A (male) and B (female) mice. Each point represents the mean± SEM of 8 animals per group. W= water; E = ethanol.
Adolescence alcohol exposure resulted in an increase of alcohol intake later in life using the two-bottle choice paradigm.
To assess whether adolescent DID exposure also increased free-choice ethanol access in adult animals, experiment 3 determined adult male ethanol intake in a 24-hour 2-bottle choice assay following adolescent exposure to DID ethanol. Ethanol concentrations (5-15% v/v) were escalated with 3 days at each concentration for the 2-bottle choice model. Two-way ANOVA revealed a main significant effect of ethanol concentration [Fconcentration (2, 36) = 11.47, p < 0.001], and adolescence exposure [Fexposure (1, 36) = 16.42, p < 0.001] but no interaction [Finteraction(2, 36) = 0.1176, p > 0.05, Fig. 3A]. Although ethanol consumption was higher in the EE males than the WE males at all concentrations, ethanol intake only reached significance at the 10% concentration (p<0.05, Fig. 3A). Ethanol preference was similar to ethanol intake where EE males had higher preference than WE males. Two-way ANOVA revealed a significant main effect of concentration [Fconcentration (2, 36) = 5.01, p < 0.05], and adolescence exposure [Fexposure(1, 36) = 19.21, p < 0.001] but no interaction [Finteraction(2, 36) = 0.445, p > 0.05] in ethanol preference (Figure 3B). While ethanol preference was higher in EE group, only the 5% ethanol concentration was significantly higher in EE group (p<0.05, Fig. 3B).
Figure 3: Adolescent mice with a history of binge ethanol increase adult intake in a 2-bottle choice paradigm.
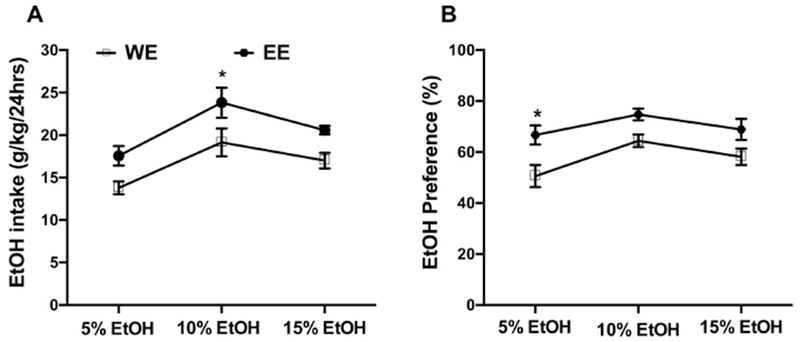
Male adolescent (PND 28-36 days) mice were exposed to DID ethanol (20%, v/v). When they reached adulthood (PND72), they were given a choice of ethanol or water in 2-bottle choice paradigm (EE group). In addition, a water control group (during adolescence period) received ethanol when they were adult (WE group). The 2-bottle choice drinking assay employed escalating ethanol concentration (5%, 10% and 15%) every three days. The average of three consecutive days was recorded for per concentration. The EE mice consumed more ethanol in the 2-bottle choice paradigm compared to WE group. Panel (A) depicts ethanol intake, whereas Panel (B) depicts ethanol preference. Each point represents the mean± SEM of 7 animals per group. * p<0.05. W= water; E = ethanol.
Following an additional 8 days of 15% ethanol (v/v) 2-bottle choice access, animals were deprived of ethanol for 7 days and then re-exposed to 15% v/v 2-bottle choice ethanol to assess whether prior adolescent ethanol exposure altered the alcohol deprivation effect (ADE). Mice exposed to ethanol during adolescence (EE) showed significantly higher ethanol intake [F (1, 36) = 17.07, p<0.05; Fig. 4A] and preference [F (1,36) = 7.679; p<0.05, Fig. 4B] than those who did not (WE), except for preference in pre-deprivation groups (Fig. 4B). However, the ADE was only significant (pre- vs. post-deprivation in Figs. 4A–B) in the adolescent ethanol exposed animals (EE), for both intake and preference.
Figure 4: Adolescent binge ethanol enhances the alcohol deprivation effect (ADE) in males.
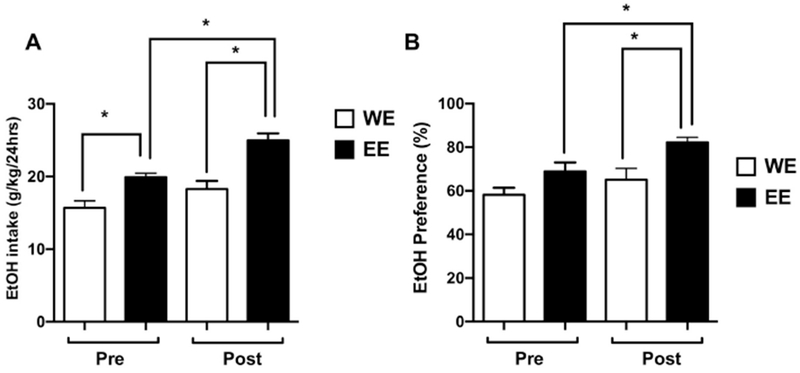
After completing the experiments in Figure 3, all mice underwent 2-bottle choice paradigm using ethanol (15%) vs water for additional 5 days. The last measurement of daily ethanol intake was recorded as pre-ADE. After recording last measurement, ethanol was taken from the cages, and mice underwent a-week duration of alcohol deprivation. Then, mice were re-exposed to ethanol (15%) vs water in 2-bottle choice paradigm. Mice which exposed to DID ethanol during adolescence (EE) showed an increase of ethanol intake (A) and ethanol preference (B) as compared to mice which only exposed to 2-bottle choice ethanol during adulthood (WE). Data are presented as the mean± SEM of 7 animals per group. *p<0.05. W= water; E = ethanol.
Prior exposure to ethanol during adolescence does not alter ethanol-induced anxiolytic-like activity later in adulthood.
In experiment 4, male mice were exposed to ethanol or water using the DID paradigm during adolescence, then tested for acute ethanol-induced anxiolysis using the light-dark box. In saline treated adult mice, both latency to enter the dark zone (Fig. 5A) and time spent on the light side of the light-dark box (Fig. 5B) were not different between mice exposed to ethanol (EE) or water (WE) during adolescence (p>0.05). Likewise, we noted a similar effect when adult mice were injected with acute ethanol (2 g/kg; p>0.05). Ethanol treatment did significantly induce anxiolytic-like activity in both groups. One way ANOVA showed a significant effect of treatment on latency to enter the dark zone [Ftreatment (3,36)=8.091, p<0.001; Figure 5A] and % time spent in light side [Ftreatment (3,36) = 21.97, p<0.0001; Fig. 5B].
Figure 5: The impact of alcohol exposure during adolescence on ethanol-induced anxiolysis-like behaviors in adulthood.
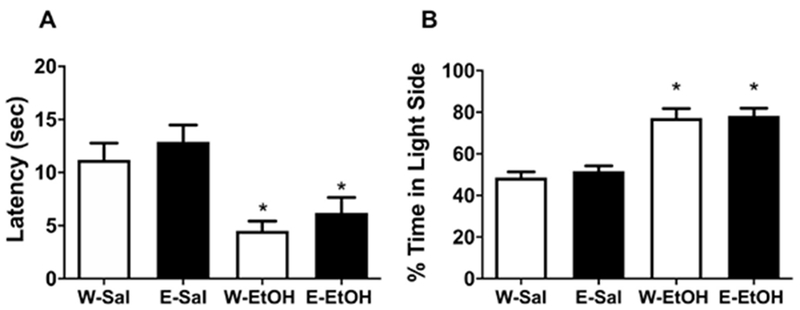
Mice were exposed to 20% v/v ethanol (E) vs. water (W) in the DID paradigm during adolescence and then tested at PND 72 with saline (Sal) or ethanol (EtOH) at 2 g/kg, i.p. and then tested 15 min later in the LDB assay. Panel (A) depicts the latency to enter the dark zone in the light-dark box. Panel (B) depicts % time spent in light side of the light-dark box. *Denotes p<0.05 vs. saline control group. Data represents the mean± SEM of 8 animals per group.
The elevated plus maze (EPM) was used as an additional test to measure the impact of adolescent ethanol exposure on acute ethanol-induced anxiolysis in adulthood. Similar to the light-dark box results, no significant differences were observed in the time spent in the open arms between mice that received ethanol or tap water during adolescence and then injected with saline during adulthood (p>0.05). No differences were found between groups in mice treated with 2 g/kg ethanol and tested on the EPM (p>0.05). However, ethanol injection in adulthood significantly increased the % time spent in the open arms in both groups [Suppl. Fig. 3A, Ftreatment(3,24) = 2.59, p<0.05]. There were no significant differences in the number of crossovers between open and light arms [Suppl. Fig. 3B, Ftreatment (3,24) = 2.858, p>0.05].
The impact of alcohol exposure during adolescence on ethanol-induced LORR in adulthood.
In experiment 5, we assessed the impact of ethanol exposure during adolescence on ethanol-induced LORR in adulthood by measuring the onset and the duration of LORR. LORR onset [t=2.697, df=21, p<0.05, Fig. 6A] and duration [t=3.016, df=21, p<0.01, Fig. 6B] were significantly higher in the EE group compared to WE group. Importantly, BEC (Fig. 6C) was similar between treatment groups at the onset of LORR or loss of function (LOF) (p>0.05), but at recovery from LORR BEC was significantly higher in the WE group compared to EE group (p<0.05). Two-way ANOVA analysis showed [Fexposure(1,24)=8.813, p<0.01]; [FLOF (1,24)=9.59, p<0.01] ; [Finteraction(1,24)=2.566, p>0.05]. Finally, we calculated acute functional tolerance (AFT) by subtracting BEC at LORR onset from the BEC at recovery. There was significant difference in AFT between treatments where WE mice displayed AFT that was absent in EE mice [t=3.273, df=11, p<0.01, Fig. 6D].
Figure 6: The impact of ethanol exposure during adolescence on ethanol LORR in adulthood.
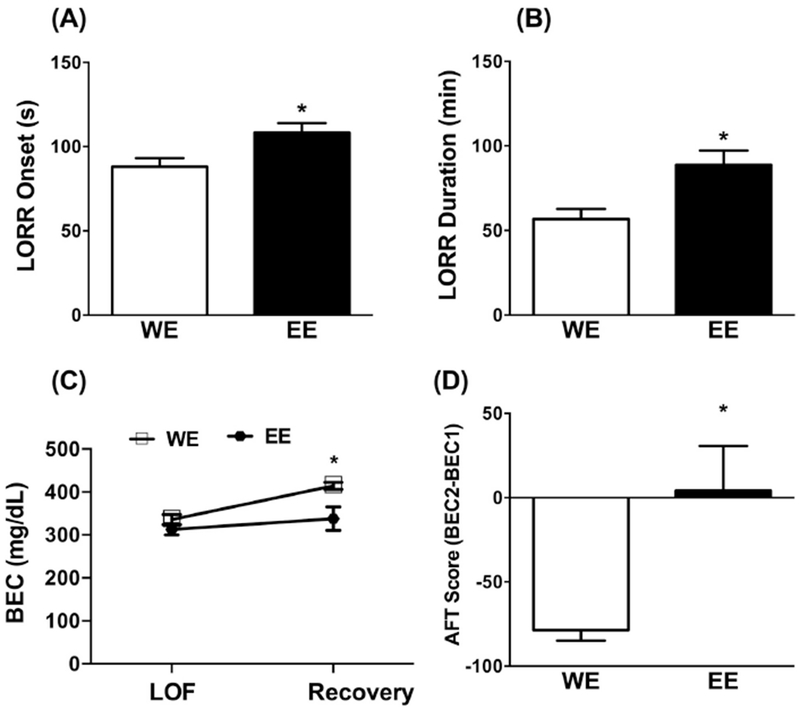
Mice exposed to 20% v/v ethanol (EE) vs. water only (WE) in the DID paradigm during adolescence and then tested at PND 72 with 3.5g/kg ethanol (20%v/v, i.p.) and monitored for the onset and duration of loss of righting reflex (LORR). Panel (A) depicts latency for the onset of LORR in sec. Panel (B) depicts LORR duration in min. Panel (C) depicts blood ethanol concentration (BEC) at the loss of function (LOF) and LORR recovery. Panel (D) depicts acute functional tolerance (AFT) score calculated as the difference between BEC at LORR recovery (BEC2) versus BEC at LORR onset (BEC1). * p<0.05 vs. WE group. Data represents the mean± SEM of 10 animals per group. W= water; E = ethanol.
Discussion
The aim of this study was to model binge ethanol exposure using a modified DID protocol in adolescent C57BL/6J mice to determine behavioral effects that persist into adulthood. We hypothesized that binge drinking in adolescence would increase ethanol intake and reduce sensitivity to ethanol in adulthood when compared to animals who are first exposed to ethanol in adulthood. We demonstrate that binge drinking during early adolescence increased ethanol 4 hour DID and 24 hour 2-bottle choice drinking in adulthood, which was affected by the duration and pattern of administration, and age of exposure. This increase in ethanol intake was also associated with an increase in relapse drinking via the alcohol deprivation effect and a reduction of ethanol’s sedative effects such as initial ethanol sensitivity in the LORR test, but also an increase in duration of LORR that is possibly due to diminution of acute functional tolerance. Interestingly, protracted abstinence in adults that were pre-exposed to ethanol in adolescence did not alter anxiety-like behaviors in two independent tasks. Together, these data show that early life/adolescent exposure to binge alcohol persistently increases ethanol consumption, relapse drinking, and initial sensitivity in adulthood and supports the level of response model for increased AUD risk (Schuckit et al., 2004).
Using a modified DID paradigm as a limited-exposure model of acute binge drinking (Rhodes et al. 2005), we found that 4-hour exposure every day to ethanol in adolescence is necessary to robustly increase adult intake. This is consistent with previous studies that showed that early alcohol experience predisposes animals to consume higher alcohol amount in their adulthood stage (Cox et al., 2013; Lee et al., 2017). Intermittent (every-other-day) 4 hour DID exposure moderately increased adult ethanol intake but only on a few days of the drinking sessions. Importantly, mice in the daily 4 hours exposure paradigm had four more drinking sessions than mice in the intermittent exposure paradigm. This raises the possibility that a minimum number of ethanol exposures in adolescence may be needed to reliable increase adult drinking. Indeed, in outbred HS/Npt mice 2 weeks of DID exposure did not increase ethanol intake as adults, whereas mice exposed for 4 or 8 weeks did show increases (Metten et al. 2011). As predicted by studies in adult mice and rats (Hopf et al. 2010, Hwa et al. 2011), ethanol intake in the intermittent treatment adolescent group did escalate over the treatment period (PND 28-36), modeling a dependence phenotype and suggesting that adolescent mice may find ethanol rewarding even though their adult intake did not escalate as robustly as with daily access. Cox et al., in 2013 also found that repeated cycles of binge-like ethanol drinking during adulthood increased subsequent voluntary ethanol intake, and this effect become more robust with increasing DID cycles. Interestingly, contrary to the present work, mice given DID exposure during adulthood, showed increases in subsequent voluntary ethanol intake (Cox et al. 2013). The difference may result from different exposure protocols used in our studies. More generally, the fact that different approaches and routes of adolescent exposure to alcohol are reported in the literature, this may influence adult intake behavioral measures later in life.
We also report that the adolescent period is especially sensitive to binge ethanol since adult exposure did not alter ethanol intake three weeks after ethanol abstinence. Our findings are in line with several previous studies, showing adolescence is a critical period for ethanol induced risk for increased adult intake (Pascual et al. 2007, Moore et al. 2010, Strong et al. 2010, Fabio et al. 2014). Interestingly, Cox et al. found that repeated cycles of binge-like ethanol drinking during adulthood increased subsequent voluntary ethanol intake, and this effect become more robust with increasing DID cycles (Cox et al. 2013). Importantly, we show that 4 hours DID increase adult ethanol intake in two drinking paradigms, both 4 hours DID and 24-hour access to 2-bottle choice. Others have shown that adolescent binge ethanol only reliably increases adult intake in an unlimited 24-hour access model but not in a shorter 2-hour access model (Strong et al. 2010). Differences in the number and length of ethanol exposure likely account for these adult behavioral differences. Mice in the Strong et al. study was exposed to ethanol in the scheduled high alcohol consumption procedure with only 30 minutes of ethanol exposure per day, while mice here had 4 hours of exposure (Strong et al. 2010). While adolescent and adult females consumed more ethanol than adult males, both males and females similarly increased ethanol intake in adulthood. The relative difference in ethanol intake between males and females was not as robust as 24-hour access models, likely due to the abbreviated 4-hour exposure in our model. Finally, it is important to note that age difference in testing could have possibly accounted for the drinking differences seen in our studies.
Prior experience with ethanol also significantly increased ethanol intake following a period of ethanol abstinence and this effect was greater in mice with an adolescent history of binge ethanol. The alcohol deprivation effect is a temporary increase in ethanol intake after a forced period of ethanol abstinence (Sinclair et al. 1968) which has been routinely used to model relapse drinking in rodents (Vengeliene et al. 2014). This may be the first report in mice to show that adolescent binge ethanol can enhance the alcohol deprivation effect in adulthood. This effect is similar to the one reported in rats by Gilpin et al., 2012 that shows that voluntary binge drinking early in adolescence augments relapse-like drinking in adult male rats. These results add to the evidence that adolescence is critically sensitive for increased risk to developing alcohol abuse tendencies.
In our studies, binge ethanol drinking in adolescence did not lead to changes in ethanol-induced anxiolysis in adult animals as seen in LDB and EPM assay, 36 days after alcohol removal. Relative to saline-treated mice, ethanol significantly decreased anxiety-like behavior in the light-dark model and the elevated plus maze. But no differences were found between mice with a history of binge ethanol or ethanol naive mice. In addition, binge alcohol-drinking did not elicit an increase in basal anxiety-like behaviors 36 days following the alcohol removal. Our findings are in line with a previous report in DBA/2J mice (Wolstenholme 2017) which also did not report a lasting effect on ethanol anxiolysis in adults with a history of binge ethanol. Similarly, when assayed 24 hours after alcohol exposure, adolescent C57BL/6J mice that consumed alcohol-for 2 weeks did not show withdrawal-induced anxiety in the marble burying test (Lee et al., 2016). In contrast, adolescent mice in protracted withdrawal and adult mice in early withdrawal from alcohol drinking exhibited hyper-anxiety-like behaviors in the marble burying (Lee et al., 2017). This increased anxiety-like behavior during alcohol withdrawal in mice with a history of binge-drinking may result from the changes in mGlu5 function and modulation in the brain (Lee et al., 2017; Lee et al., 2018). Similarly, (Pandey et al., 2015) reported increased anxiety-like behaviors after both early and protracted alcohol withdrawal in adolescent rats. We do not believe that motor function impairment has affected our results in the LDB and EPM assays since the total number of crossovers between arms/boxes is not significantly changed by alcohol injection. A limitation of our study with alcohol’s anxiolytic effects is the use of one dose alcohol challenge in adulthood.
The effects of adolescent binge ethanol on adult ethanol sensitivity in the LORR assay were not straight forward. Adolescent binge ethanol exposure increased LORR onset and decreased AFT in adulthood, suggesting long-lasting alterations in ethanol sensitivity. Conversely, the duration of LORR was significantly greater in mice pre-exposed to binge ethanol in adolescence, similar to previous reports (Wolstenholme 2017) and suggesting these mice are more sensitive to ethanol. To estimate potential changes in acute functional tolerance, we calculated the change in BEC when each mouse regained the righting reflex from its LORR onset. Ethanol naïve mice had a significantly greater change in BEC than mice with a history of binge ethanol, indicating that mice with adolescent history of binge drinking had to achieve a lower BEC in order to recover the righting reflex relative to ethanol naive mice. This suggests that adolescent mice with a binge-drinking history do not display acute functional tolerance unlike the ethanol naive mice. Clearly, adolescent binge ethanol has lasting effects on adult ethanol sensitivity, at least at high, sedative doses, and may decrease the development of acute functional tolerance to ethanol. Interestingly, acute functional tolerance and DID ethanol intake were significantly correlated to each other in inbred mouse strains (Radcliffe et al. 2013), where decreased sensitivity was associated with increased ethanol intake. These data lend support to the level of response model (Schuckit 2004) where less intense effects of high doses of ethanol (here, initial sensitivity at LORR onset and AFT) are associated with increased ethanol drinking and ultimately increased risk for developing an AUD.
The mechanisms underlying the effect of “binge drinking” experience during adolescence to increase subsequent ethanol intake during adulthood are not known. A possible explanation for the elevated consumption in mice subsequent to adolescent “binge drinking” experience is that age-related differences in the neurobiological effects of ethanol differ in adolescent versus adult animals. The neurochemistry and structure of the brain is still developing in adolescent mice (Spear 2000b), which makes adolescents acutely sensitive to ethanol’s insults. Delay or damage to the developing nervous system can lead to the behavioral differences we report in adulthood such as increased intake, heightened ADE response, and altered sensitivity in LORR. This altered brain development may also explain the disconnect between ethanol sensitivity and intake. Future studies using this model can aid in understanding these mechanisms by interrogating the neurochemical changes in adolescent brains following binge ethanol.
Supplementary Material
Highlights.
Ethanol exposure early in adolescent mice increases drinking and relapse in adulthood.
Ethanol exposure early in adolescent mice did not reduce alcohol-induced anxiety-like behavior in adulthood.
Ethanol exposure early in adolescent mice did increase the loss of righting reflex induced by alcohol in adulthood
Acknowledgments
This work was supported by NIAAA [grant number P50AA022537] and NIDA [grant number P30DA033934].
List of the abbreviations:
- AUD
alcohol use disorder
- DID
drinking in the dark
- BEC
blood ethanol concentration
- PND
postnatal day
- CA
consecutive access
- IA
intermittent access
- ADE
alcohol deprivation effect
- EE
ethanol exposed
- WE
water exposed
- LDB
light dark box
- EPM
elevated plus maze
- LORR
loss of righting reflex
- AFT
acute functional tolerance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Administration, SAMHSA (2014). Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings Rockville, MD, Substance Abuse and Mental Health Services Administration; NSDUH Series H-48. [Google Scholar]
- Alcoholism, NIAAA (2004). NIAAA Council Approves Definition of Binge Drinking. NIAAA Newsletter. 3. [Google Scholar]
- Bergstrom HC, McDonald CG and Smith RF (2006). Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long-Evans rats. Physiology & behavior 88(4-5), 466–72. 10.1016/j.physbeh. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI and Spear LP (2011). Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcoholism, clinical and experimental research 35(8), 1392–403. 10.1111/j.1530-0277.2011.01474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., He J, Lee J, Styner M and Crews FT (2011). Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcoholism, clinical and experimental research 35(4), 671–88. 10.1111/j.1530-0277.2010.01385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG Jr., Liu W, Oguz I, Styner M and Crews FT (2014). Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacology, biochemistry, and behavior 116, 142–51. 10.1016/j.pbb.2013.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BR, Olney Jeffrey J, Lowery-Gionta Emily G, Sprow Gretchen M, Rinker Jennifer A, Navarro Montserrat, Kash Thomas L, and Thiele Todd E (2013). Repeated Cycles of Binge-Like Ethanol (EtOH)-Drinking in male C57BL/6J Mice Augments Subsequent Voluntary EtOH Intake but not other Dependence-Like Phenotypes. Alcoholism: Clinical and Experimental Research. 37(10), 1688–1695. 10.1111/acer.12145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Goodwin FK (1980). Preliminary report of a simple animal behavior model for anxiolytic effects of benzodiazepines. Pharmacology, biochemistry, and behavior 13: 167–170 [DOI] [PubMed] [Google Scholar]
- Crews FT, CJ Braun, Hoplight B, Switzer RC and Knapp DJ (2000). Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats. Alcoholism: Clinical and Experimental Research 24(11), 1712–23 [PubMed] [Google Scholar]
- Fabio MC, March SM, Molina JC, Nizhnikov ME, Spear NE and Pautassi RM (2013). Prenatal ethanol exposure increases ethanol intake and reduces c-Fos expression in infralimbic cortex of adolescent rats. Pharmacology, biochemistry, and behavior 103(4), 842–52. 10.1016/j.pbb.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio MC, Nizhnikov ME, Spear NE and Pautassi RM (2014). Binge ethanol intoxication heightens subsequent ethanol intake in adolescent, but not adult, rats. Dev Psychobiol 56(3), 574–83. 10.1002/dev.21101. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7(2):e31466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB and Martin NG (1999). Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med 29(5), 1069–81 [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T and Winter MR (2006). Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Arch Pediatr Adolesc Med 160(7), 739–46. 10.1001/archpedi.160.7.739 [DOI] [PubMed] [Google Scholar]
- Hopf FW, Chang SJ, Sparta DR, Bowers MS and Bonci A (2010). Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol selfadministration. Alcoholism, clinical and experimental research 34(9), 1565–73. 10.1111/j.1530-0277.2010.01241.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF and Miczek KA (2011). Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcoholism, clinical and experimental research 35(11), 1938–47. 10.1111/j.1530-0277.2011.01545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, and Adriani W (2003). Risktaking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci. Biobehav. Rev 27, 19–31. doi: 10.1016/S0149-7634(03)00006-X [DOI] [PubMed] [Google Scholar]
- Lee KM., Coelho MA, McGregor HA, Solton NR, Cohen M, and Szumlinski KK. (2016). Adolescent mice are resilient to alcohol withdrawal induced anxiety and changes in indices of glutamate function within the nucleus accumbens. Front. Cell. Neurosci 10:265. doi: 10.3389/fncel.2016.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KM, Coelho Michal A, Class MacKayla A, Szumlinskim Karen K, (2017). Glu5-dependent modulation of anxiety during early withdrawal from binge-drinking in adult and adolescent male mice. Drug and Alcohol Dependence. 184, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metten P, Brown LL and Crabbe JC (2011). Limited access ethanol drinking in the dark in adolescent and adult mice. Pharmacology, biochemistry, and behavior 98(2), 279–85. 10.1016/j.pbb.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Pla A, Maldonado C, Rodriguez-Arias M, Minarro J and Guerri C (2014). TLR4 elimination prevents synaptic and myelin alterations and long-term cognitive dysfunctions in adolescent mice with intermittent ethanol treatment. Brain, behavior, and immunity. 10.1016/j.bbi.2014.11.015 [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC and Boehm SL 2nd (2010). Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcoholism, clinical and experimental research 34(4), 734–42. 10.1111/j.1530-0277.2009.01143.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J and Guerri C (2007). Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European journal of neuroscience 25(2), 541–50. 10.1111/j.1460-9568.2006.05298.x [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Larson C and Bennett B (2013). Genetic studies of acute tolerance, rapid tolerance, and drinking in the dark in the LXS recombinant inbred strains. Alcoholism, clinical and experimental research 37(12), 2019–28. 10.1111/acer.12188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA and Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & behavior 84(1), 53–63. 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Wilkerson Richard Gentry, Kim Hong K, Windsor Thomas Andrew, Mareiniss Darren P.(2016). The Opioid Epidemic in the United States. [DOI] [PubMed]
- Schuckit MA, Edenberg HJ, Kalmijn J, Flury L, Smith TL, Reich T, Bierut L, Goate A and Foroud T (2001). A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res 25(3), 323–9 [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Anderson KG and Brown SA (2004). Testing the level of response to alcohol: social information processing model of alcoholism risk--a 20-year prospective study. Alcohol Clin Exp Res 28(12), 1881–9 [DOI] [PubMed] [Google Scholar]
- Sinclair JD and Sente RJr (1968). Development of an alcohol-deprivation effect in rats. Q J Stud Alcohol 29(4), 863–7 [PubMed] [Google Scholar]
- Sircar R and Sircar D (2005). Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcoholism, clinical and experimental research 29(8), 1402–10 [DOI] [PubMed] [Google Scholar]
- Spear LP (2000a). The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews 24, 417–63 [DOI] [PubMed] [Google Scholar]
- Spear LP (2000b). Modeling adolescent development and alcohol use in animals. Alcohol Res. Health 24, 115–123. [PMC free article] [PubMed] [Google Scholar]
- Spear LP (2010). The Behavioral Neuroscience of Adolescence, 1st Edn. New York, NY: W. W. Norton. [Google Scholar]
- Spear LP (2018). Effects of adolescent alcohol consumption on the brain and behaviour. Nat Rev Neurosci 19(4), 197–214. doi: 10.1038/nrn.2018.10. [DOI] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA and Finn DA (2010). “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones and behavior 58(1), 82–90. 10.1016/j.yhbeh.2009.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, et al. (2005). Homer2 is necessary for EtOH-induced neuroplasticity. J. Neurosci 25, 7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, and Bowers MS (2007). Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology 190, 415–431. doi: 10.1007/s00213-006-0641-7 [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, and Kippin TE (2008). Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology 33, 1365–1378. doi: 10.1038/sj.npp.1301473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A and Spanagel R (2014). The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol 48(3), 313–20. 10.1016/j.alcohol.2014.03.002 [DOI] [PubMed] [Google Scholar]
- White AM, AJ Ghia, Levin ED and Swartzwelder HS (2000). Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcoholism, clinical and experimental research 24(8), 1251–6. [PubMed] [Google Scholar]
- Wolstenholme JT, Mahmood T, Harris GM, Abbas S,and Miles MF (2017). Intermittent Ethanol during Adolescence Leads to Lasting Behavioral Changes in Adulthood and Alters Gene Expression and Histone Methylation in the PFC. Frontiers in Molecular Neuroscience, 10, 10.3389/fnmol.2017.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) Global status report on alcohol and health. 2014 http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


