Abstract
Aims
Riata® implantable cardioverter-defibrillator (ICD) leads from St. Jude Medical are prone to malfunction. This study aimed to describe the rate of this lead's malfunction in a very long-term follow-up.
Methods
This single-centre observational study included 50 patients who received a Riata 7Fr dual-coil lead between 2003 and 2008. Follow-up was conducted both in person and remotely, and analysed at 8-month intervals. We evaluated the rates of cable externalization (CE), electrical failure (EF), and the interaction of these two complications. Structural lead failure was defined as radiographic CE. Oversensing of non-cardiac signal or sudden changes in impedance, sensing, or pacing thresholds constituted EF.
Results
During a mean follow-up of 10.2 ± 2.9 years, 16 patients (32%) died. We observed lead malfunction in 13 patients (26%): three (23%) due to CE, six (46%) to EF and four (31%) to both complications. Of the malfunctioning leads, 77% failed after seven years of follow-up. The incidence rate (IR) of overall malfunction per 100 patients per year was 0.9 during the first seven years post-implantation, increased to 7.0 after the 7th year and more than doubled (to 16.7) after 10 years. Beyond seven years post-implantation, IR per 100 patient-years increased in both EF and CE (from 0.6 to 5.6 vs. 0.3 to 4.2, respectively). Presence of CE was associated with a 4-fold increase in the proportion of EF.
Conclusion
The incidence of Riata ICD lead malfunction, both for EF and CE, increased dramatically after seven years and then more than doubled after 10 years post-implantation.
Keywords: Implantable cardioverter-defibrillator, Long-term follow-up, Malfunction, Cable externalization, Electrical failure
Abbreviations
- CAD
coronary artery disease
- CE
cable externalization
- EF
electrical failure
- Fr
French
- ICD
implantable cardioverter-defibrillator
- IR
incidence rate
- IE
infective endocarditis
- PY
patients per year
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- SVC
superior vena cava
1. Introduction
Since 2007, when the Riata® implantable cardioverter-defibrillator (ICD) leads were first associated with significantly more cardiac perforations [1,2], many cases related to Riata lead malfunction have been reported. In 2011, the United States Food and Drug Administration (FDA) issued a class I advisory for the Riata and Riata ST ICD leads [3]. This advisory described design problems in silicone-coated Riata leads, resulting in conductor cable externalization (CE) [4,5]. An increased rate of electrical failure (EF) also has been observed [4]. Although most observational studies have demonstrated a significantly increased rate of EF in the setting of CE (between 3 and 6 times higher when CE was present, compared to leads without externalization), this specific association remains unclear [4,6,7]. The use of routine radiographic screening for CE has accordingly been questioned, in particular because the incidence of EF without externalization is not trivial and lead revision (and especially lead extraction) has been associated with a higher rate of major complications [4,5].
Cable externalization is the primary cause of malfunction, with a prevalence ranging from 11 to 43% [6,[8], [9], [10]]. The overall estimated proportion of EF is 6.3%, but the rate is 17.3% in the presence of CE and just 2.7% without externalization [6]. It is important to emphasize that EF rates do not appear to differ between lead subtypes. Despite the reported association between CE and EF and a higher percentage of CE occurring with 8 French (Fr) leads compared with 7Fr leads, similar proportions of EF have been noted for both 7Fr and 8Fr leads [4,6]; this suggests that the smaller calibre 7Fr lead might be more susceptible to outside-in abrasion, rather than inside-out abrasion [11].
Several studies have focused on the short-term performance of the Riata ICD lead; however, only two studies [5,12] have investigated the associated long-term complications, with a mean follow-up duration of 8.9 and 7 years, respectively.
The aim of this study was to describe, over the very long term (>10 years), the malfunction rate and reliability of the Riata 7Fr dual-coil lead by examining the rates of EF, CE, and the interaction of these two complications.
2. Methods
This single-centre observational study included all patients who underwent Riata ICD lead implantation between 2003 and 2008 using a 7Fr model with a dual-coil design.
The primary outcome measures included rates of lead failure (structural lead failure and/or EF) and time to any malfunction. Follow-up included routine clinical visits for device interrogation (45 days post-implantation and at 8-month intervals) and remote monitoring (Merlin.net™) when available. All death events were strictly verified for precise measurement of survival from the date of implantation.
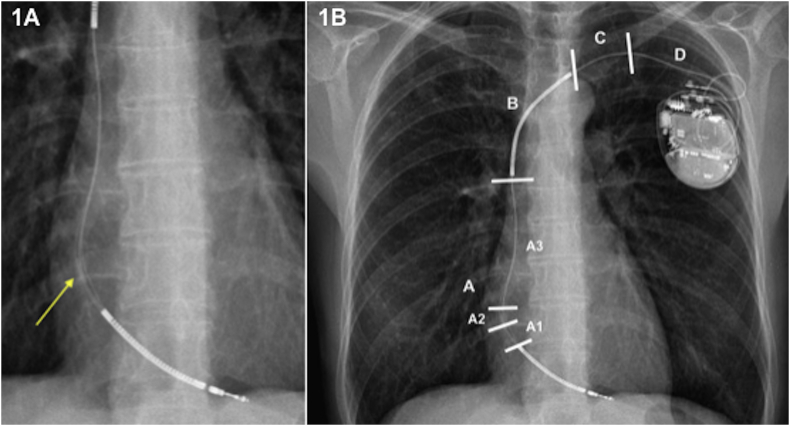
Structural lead failure was defined as a lead presenting an externalization of conductors, insulation defect, or fracture [13]. Externalization was defined as conductor(s) visible outside the lead body on chest X-ray or cinefluoroscopy, as reviewed by radiologists and/or electrophysiologists (Fig. 1A). We sub-classified CE into four groups according to the topography of the mechanical malfunction: A, distal to superior vena cava (SVC)-coil; B, distal to clavicle including SVC-coil; C, near clavicle; and D, clavicle to pulse generator (Fig. 1B) [14].
Fig. 1.
A. Cable externalization between both coils (zones A1-A2). Yellow arrow indicates the affected area. Fig. 1B. Cable externalization sub-classified according to the topography of the mechanical dysfunction: D, clavicle to pulse generator; C, near clavicle; B, distal to clavicle including SVC-coil; A, distal to SVC-coil. In turn, that area between both coils is divided into: SVC-coil to the tricuspid valve annulus (A3); just in the tricuspid valve annulus level (A2); tricuspid valve annulus to the right ventricle-coil (A1) [14]. SVC, superior vena cava.
Electrical failure was defined as one or more of the following lead malfunctions: 1) Oversensing of non-physiological electrical noise artefacts; 2) conductor impedance out of range (<200 Ω or >2000 Ω) or a sudden change (>100% increase or > 50% decrease) in the stable baseline; 3) a change in the high-voltage impedance (>200 Ω or < 50 Ω); 4) an abrupt increase in capture threshold (>100% increase over baseline); and 5) an unexpected decrease in R-wave sensing (>50% decrease from baseline) [14]. Lead dislodgments and physiological oversensing were not considered EF.
A proactive lead strategy was implemented that consisted of systematic lead extraction or lead abandonment (especially in patients with multiple comorbidities) in the case of battery replacement and radiographic detection of CE, regardless of electrical lead integrity. Subsequently, a new electrode was implanted during the replacement of the generator.
2.1. Statistical analysis
Categorical variables, represented as proportions, were compared using the chi-square test. Continuous variables were expressed as means, and their standard deviation (SD) and comparisons between groups were made using the Student t-test.
The incidence rate (IR) per 100 patients per year (PY) for lead malfunction was calculated considering the follow-up duration and then specifically for follow-up after the 7th and the 10th year post-implantation. Failure-free survival in the long term was calculated using the Kaplan-Meier method. For each time interval, the probability of failure-free survival was calculated as the number of Riata malfunction-free surviving patients divided by the number of patients at risk. Patients who had died and those with a new lead implanted for causes other than lead failure were considered “censored” and were not counted in the denominator.
All of the tests were two-sided. A p-value <0.05 was considered to be statistically significant. Statistical analysis was conducted using IBM SPSS Statistics v19.0 (Chicago, Illinois, USA).
3. Results
From 2003 through 2008, 50 patients underwent Riata ICD lead implantation in our unit (Fig. 2). The mean follow-up duration was 10.2 ± 2.9 years. No patients were lost to follow-up. All the leads were Riata 7Fr, dual-coil, passive-fixation. The primary patient characteristics of the study population are listed in Table 1. During follow-up, all living patients who still had the Riata lead underwent battery replacement. Moreover, remote monitoring was initiated at the time of replacement (n = 29, 58%).
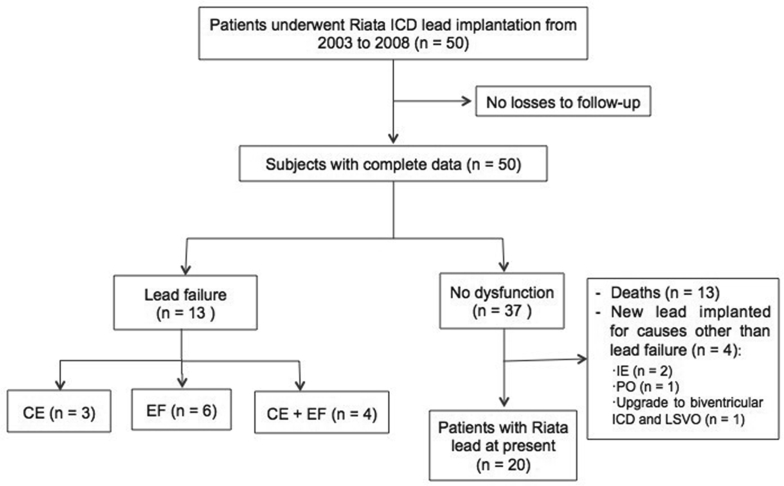
Fig. 2.
This flow diagram illustrates the number of patients included in this study. ICD, implantable cardioverter-defibrillator; CE, cable externalization; EF, electrical failure; IE, infective endocarditis; PO, physiological oversensing; LSVO, left subclavian vein occluded.
Table 1.
Baseline characteristics. SD, standard deviation; CAD, coronary artery disease; HCM, hypertrophic cardiomyopathy; BrS, Brugada syndrome; LQTS, long QT syndrome; ARVD, arrhythmogenic right ventricular dysplasia; LVEF, left ventricular ejection fraction; ICD, implantable cardioverter-defibrillator.
| Variables at baseline | All (n = 50) |
|---|---|
| Sex, male, n (%) | 43 (86) |
| Age at implantation, years, mean ± SD | 59 ± 14 |
| Underlying cardiac disease | |
| CAD, n (%) | 27 (54) |
| Non-ischemic cardiomyopathy, n (%) | 10 (20) |
| HCM, n (%) | 6 (12) |
| BrS, n (%) | 4 (8) |
| LQTS, n (%) | 2 (4) |
| ARVD, n (%) | 1 (2) |
| LVEF, mean ± SD | 41 ± 16 |
| Atrial fibrillation, n (%) | 4 (8) |
| Secondary prevention | 28 (56) |
| Type of ICDs | |
| Single-chamber | 35 (70) |
| Dual-chamber | 9 (18) |
| Biventricular | 6 (12) |
| Pacemaker dependency, n (%) | 2 (4) |
| Left-sided implant, n (%) | 49 (98) |
| Follow-up time, years, mean ± SD | 10.2 ± 2.9 |
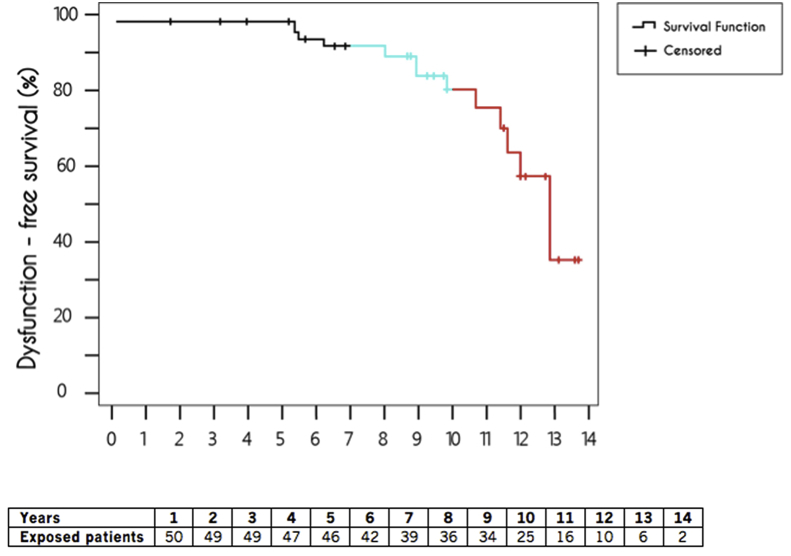
Overall, we detected lead malfunction in 13 patients: three due to CE, six to EF and four to both complications. The IR, considering an absolute malfunction rate of 26% involving 13 leads and 469.8 PY of post-implantation follow-up, was 2.7 per 100 PY. Of the 13 malfunctioning leads, 10 (77%) failed after 7 years of follow-up. Thus, during the first 7 years, the malfunction rate was 0.9 per 100 PY. However, this rate drastically increased to 7.0 after the seventh year and to 16.7 per 100 PY after 10 years post-implantation. The overall Kaplan-Meier survival curve for the Riata leads declined sharply after seven years, from 93% to just 34% after 14 years (Fig. 3).
Fig. 3.
Kaplan-Meier survival curve. The incidence of lead malfunctions dramatically increased after the 7th year post-implantation (blue line) and was especially evident after 10 years (red line).
All-cause mortality was 16/50 (32%), with no deaths attributed to lead failure (Table 2). In addition to the patients with lead malfunction, four patients required implantation of new non-Riata leads: two due to infective endocarditis (IE), one because of an upgrade to biventricular ICD in a patient with left subclavian venous thrombosis, and one due to physiological oversensing. In total, we removed the lead in six patients (35%): two with IE and four with lead failure. In the other 11 patients (65%) who received a new implant (nine due to lead malfunction, one to physiological oversensing, and one to a need for biventricular ICD), the cable was abandoned without detecting any associated complications.
Table 2.
Causes of death in our cohort.
| Cause of death | n = 16 |
|---|---|
| Cardiovascular diseases, n (%) | 6 (37,5) |
| Cardiogenic shock, n (%) | 3 (19) |
| Heart failure, n (%) | 3 (19) |
| Septic shock, n (%) | 2 (12,5) |
| Cerebrovascular disease, n (%) | 3 (19) |
| Ischemic stroke, n (%) | 1 (6) |
| Intracerebral haemorrhage | 1 (6) |
| Glioblastoma, n (%) | 1 (6) |
| Respiratory diseases | 3 (19) |
| Pneumonia, n (%) | 2 (12,5) |
| Lung cancer, n (%) | 1 (6) |
| Digestive diseases | 2 (12,5) |
| Intestinal ischemia, n (%) | 1 (6) |
| Peritonitis, n (%) | 1 (6) |
3.1. Electrical failure
During complete follow-up, 10 patients (20%) presented with EF: four cases were associated with CE. The mean time to appearance was 10.0 ± 2.9 years. Eight leads (80%) failed after seven years of follow-up, increasing the IR in patients with EF from 0.6 to 5.6 per 100 PY after seven years post-implantation. Univariate analysis showed that none of the baseline characteristics was a predictor of EF.
The main mode of EF presentation was oversensing of non-physiological noise on the ventricular sensing channel (8 patients, 80%). In two of the 10 patients, EF was detected by remote monitoring and in five patients it was diagnosed at the on-site hospital visit. In one case, EF occurred after the application of a programmed 10 J shock in a patient with known CE (previously revealed by X-ray). The two remaining patients with EF were treated at the emergency department due to inappropriate ICD shocks. In one of these patients, several inappropriate ICD shocks were due to non-physiological noise following an appropriate shock because of ventricular tachycardia.
3.2. Cable externalization
Radiographic data were available on 45 (90%) patients. We found CE in seven (14%) patients; mean time to appearance was 11 ± 2.5 years. The IR of CE increased from 0.3 to 4.2 per 100 PY after seven years post-implantation. The most common location of externalization was zone A (n = 7, 100%), described by Demirel et al. [14] as that area between both coils: 1 (14%) was between the SVC-coil and the tricuspid valve annulus (A3), 4 (57%) were just at the tricuspid valve annulus level (A2) and 2 (29%) were between the tricuspid valve annulus and the right-ventricle coil (A1) (Fig. 1B).
3.3. Interaction between electrical failure and cable externalization
No differences were found between EF and CE rates: 10 cases vs. 7 cases, respectively, during the whole follow-up period (p = 0.42); 6 vs. 5 after the 10th year (p = 0.73). The presence of CE was associated with a 4-fold increase in the proportion of EF (4/7 (57%) vs. 6/43 (14%) with no CE, p = 0.008).
4. Discussion
The main finding of this study was that the malfunction rate of 7Fr dual-coil Riata ICD leads increased for both EF and CE over very long-term follow-up, especially after 10 years post-implantation. The 14-year lead survival rate was just 34%, compared to 93% at seven-year follow-up. The presence of CE was associated with a four-fold increase in the proportion of EF, compared to no CE. Lead abandonment rather than extraction was not associated with a higher rate of adverse consequences.
Several studies [5,12] have found an exponential increase in the malfunction rate, especially in leads surviving more than five years. Our study, with a mean follow-up longer than 10 years, confirmed that exponential increase and showed an even sharper curve after 10 years. Thus, while the IR increased to 7.0 per 100 PY after the seventh year, it more than doubled (16.7 per 100 PY) after 10 years post-implantation. During the entire follow-up period, 13 lead failures were observed, accounting for an IR of 2.7 per 100 PY. The difference between this finding and the IR of 1.17 per 100 PY reported in the meta-analysis by Providencia et al. [13] is likely due to our longer follow-up: if only the first 10 years of follow-up were considered, both IRs would be quite similar (1.4 per 100 PY). Indeed, 10 of the 13 leads (77%) failed after seven years of follow-up. On the other hand, we analysed a 7Fr Riata model. Some studies have suggested that thinner leads may be associated with an increased likelihood of malfunction [13,15], while other researchers report no differences based on lead caliber [12].
No differences were found between EF and CE rates during the whole follow-up period nor after the 10th year. We observed an exponential increase over time in both EF and CE (0.6–5.6 per 100 PY vs. 0.3 to 4.2 PY, respectively). Although the cumulative incidence of EF was similar to previous studies at 8 years (4% in our cohort vs. 5.2% in the registry of Parkash et al. [5]), during the complete follow-up it rose to 20%. On the other hand, over the whole follow-up period the 14% CE rate was within the lower range of previous studies (11%–43%) [6] but was only 2% at eight years. This low percentage could be related to the 7Fr lead calibre, which has been associated with a lower proportion of CE [6,16]. We found a significantly increased incidence of EF in the setting of CE (57% vs 14%, respectively, p = 0.008). Our data are consistent with those of Steinberg et al. [17] (25% vs. 4.7%), Zeitler E [6] et al. (17.3% vs. 2.7%) and Larsen et al. [7] (19.2% vs. 4.9%). It should be mentioned that we implanted a new lead in patients with structural lead failure, which could have increased the difference observed between the two types of malfunction. Similar to previous reports [12], oversensing of non-physiological noise was the main mode of presentation of EF.
Regarding the decision to extract or abandon a malfunctioning lead, Maytin M and colleagues [17] point out that extraction of the Riata leads could be performed safely by experienced operators. However, in other series lead extraction has been related to a high risk of complications (18.6% vs. 5.2% for abandonment, p < 0.0001) [5]. In our study, the most common strategy was abandonment of the lead (65% vs. 35%), which did not lead to any complications.
Given the high failure rate of Riata ICD leads more than 10 years post-implantation, remote monitoring could be recommended to improve early detection of EF. In addition, it could be useful to perform serial radiographic exams, especially after 10 years post-implantation, given the likely association between EF and CE.
5. Limitations
The present study has several limitations that must be acknowledged. Results were obtained in a single centre, therefore, the generalizability of our results should be viewed with caution. The length of time to CE could not be determined with precision because radiographic exams were performed according to physician discretion rather than annually. As a consequence, the EF rate might have been overestimated compared to that of CE. Nevertheless, since all living patients underwent battery replacement, results of one or more radiographic exams were available for each patient over a long follow-up period.
6. Conclusion
The incidence of 7Fr dual-coil Riata ICD lead malfunctions dramatically increased following the seventh year post-implantation and more than doubled after 10 years post-implantation in both EF and CE cases. A combination of remote monitoring and regular radiographic follow-up, particularly after 10 years, could help with appropriate decision making.
Conflict of interest statement
LM declares consulting services, and advisory boards to St. Jude Medical (Now Abbott), Medtronic, Biotronik, Boston Scientific, Livanova and Johnson&Johnson. JMT declares consulting services, and advisory boards to St. Jude Medical (Now Abbott), Medtronic, Biotronik and Boston Scientific. All other authors declare no financial involvement with any organization having a financial conflict with the subject matter discussed in the manuscript.
Funding
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement 633196 (CATCH ME project); from Instituto de Salud Carlos III — Fondo de Investigaciones Sanitarias (PI16/00703 and PI16/00435); Agència de gestió d’Ajuts Universitaris i de Recerca (AGAUR; 2017_SGR_1548); and La MARATÓ-TV3 (20152730).
Acknowledgements
We are indebted to Neus Portella for secretarial assistance and to Elaine Lilly, Ph.D., for English language editing.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Danik S.B., Mansour M., Singh J. Increased incidence of subacute lead perforation noted with one implantable cardioverter-defibrillator. Heart Rhythm. 2007;4(4):439–442. doi: 10.1016/j.hrthm.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 2.Vlay S.C. Concerns about the Riata ST (St. Jude medical) ICD lead. Pacing Clin Electrophysiol. 2008;31(1):1–2. doi: 10.1111/j.1540-8159.2007.00916.x. [DOI] [PubMed] [Google Scholar]
- 3.US Food and Drug Administration . 2011. FDA classifies voluntary physician advisory letter on Riata and Riata ST silicone defibrillation leads as class I recall (urgent medical device advisory) [Google Scholar]
- 4.Voigt A.H., Saba S. Don't just do something, stand there?. Unraveling the complexities of Riata. Circ Arrhythm Electrophysiol. 2016;9(10) doi: 10.1161/CIRCEP.116.004648. [DOI] [PubMed] [Google Scholar]
- 5.Parkash R., Thibault B., Mangat I. Canadian registry of implantable electronic device outcomes. Circ: Arrhythm Electrophysiol. 2016;9(10) doi: 10.1161/CIRCEP.116.004282. [DOI] [PubMed] [Google Scholar]
- 6.Zeitler E.P., Pokorney S.D., Zhou K. Cable externalization and electrical failure of the Riata family of implantable cardioverter-defibrillator leads: a systematic review and meta-analysis. Heart Rhythm. 2015;12:1233–1240. doi: 10.1016/j.hrthm.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Larsen J.M., Nielsen J.C., Johansen J.B. Prospective nationwide fluoroscopic and electrical longitudinal follow-up of recalled Riata defibrillator leads in Denmark. Heart Rhythm. 2014;11:2141–2147. doi: 10.1016/j.hrthm.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Kubala M., Traulle S., Leborgne L. Progressive decrease in amplitude of intracardiac ventricular electrogram and higher left ventricular ejection fraction are associated with conductors' externalization in Riata leads. Europace. 2013;15:1198–1204. doi: 10.1093/europace/eut015. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg C., Sarrazin J.F., Philippon F. Detection of high incidence of Riata lead breaches by systematic postero-anterior and lateral chest X-ray in a large cohort. Europace. 2013;15:402–408. doi: 10.1093/europace/eus339. [DOI] [PubMed] [Google Scholar]
- 10.Abdelhadi R.H., Saba S.F., Ellis C.R. Independent multicenter study of Riata and Riata ST implantable cardioverter-defibrillator leads. Heart Rhythm. 2013;10:361–365. doi: 10.1016/j.hrthm.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 11.Hayes D., Freedman R., Curtis A.B. Prevalence of externalized conductors in Riata and Riata ST silicone leads: results from the prospective, multicenter Riata Lead Evaluation Study. Heart Rhythm. 2013;10:1778–1782. doi: 10.1016/j.hrthm.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Ströker E., de Asmundis C., Vanduynhoven P. Long-term performance of the Riata/ST implantable cardioverter defibrillator lead. Am J Cardiol. 2016;117:807–812. doi: 10.1016/j.amjcard.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Providência R., Kramer D.B., Pimenta D. Transvenous implantable cardioverter-defibrillator (ICD) lead performance: a meta-analysis of observational studies. J Am Heart Assoc. 2015;4(11) doi: 10.1161/JAHA.115.002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demirel F., Adiyaman A., Delnoy P.P.H. Mechanical and electrical dysfunction of Riata implantable cardioverter-defibrillator leads. Europace. 2014;16(12):1787–1794. doi: 10.1093/europace/euu079. [DOI] [PubMed] [Google Scholar]
- 15.Rordorf R., Poggio L., Savastano S. Failure of implantable cardioverter-defibrillator leads: a matter of lead size? Heart Rhythm. 2013;10:184–190. doi: 10.1016/j.hrthm.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg C., Sarrazin J.F., Philippon F. Longitudinal follow-up of Riata leads reveals high annual incidence of new conductor externalization and electrical failure. J Intervent Card Electrophysiol. 2014;41(3):217–222. doi: 10.1007/s10840-014-9951-6. [DOI] [PubMed] [Google Scholar]
- 17.Maytin M., Wilkoff B.L., Brunner M. Multicenter experience with extraction of the Riata/Riata ST ICD lead. Heart Rhythm. 2014;11:1613–1618. doi: 10.1016/j.hrthm.2014.05.014. [DOI] [PubMed] [Google Scholar]