Abstract
Background
Contact-force sensing catheter is widely used for catheter ablation, however, it did not take account of radiofrequency power. Ablation index (AI) is a novel marker incorporating contact force-time-power, was shown to be reliable in predicting lesion size and depth for radiofrequency delivery. We aimed to assess the latest evidence on ablation index guided procedure versus conventional ablation procedure.
Methods
We performed a comprehensive search on topic that assesses ablation index guided procedure versus conventional procedures from inception up until February 2019 through PubMed, EuropePMC, EBSCOhost, Cochrane Central Database, and ClinicalTrials.gov.
Results
A total of 1727 subjects from five studies were included. 12 months’ incidence of AF/AT/AFL was lower in ablation index guided with an OR of 0.35 [0.17, 0.73], p = 0.005; I2 58%. Upon sensitivity analysis by removing a study, heterogeneity decreased to 0% with OR of 0.26 [0.15, 0.46], p < 0.001. First-pass isolation has a pooled OR of 11.29 [4.68, 27.20], p < 0.001; I2 58%. Pooled OR for acute pulmonary vein reconnection was 0.43 [0.29, 0.64], p < 0.001; I2 46%. AI group has a shorter fluoroscopy time of MD -1.62 [-2.62, −0.62] minutes, p = 0.001; I2 51% and total ablation time MD -9.96 [-17.16, −2.76] minutes, p < 0.001; I2 95%. Total procedural time and complication rate were similar.
Conclusion
Ablation index guided procedure resulted in a significantly lower incidence of AF/AT/AFL, shorter fluoroscopy time, and total ablation time. First-pass isolation was higher in AI group and acute PVR was lower in AI group. Ablation-index guided procedure has a similar safety profile to conventional ablation.
Keywords: Ablation index, Contact force, Catheter ablation, Atrial fibrillation, Pulmonary vein isolation
1. Introduction
The rate of atrial fibrillation (AF) recurrences after ablation remained high despite advances in technology and the use of contact-force (CF) and Force-Time integral (FTI). Contact-force sensing catheter is widely used for catheter ablation; however, CF did not take account of radiofrequency (RF) power [1]. Insufficient RF delivery may result in suboptimal lesion formation, and excessive RF delivery may cause complications such as cardiac tamponade [2]. Force-Time integral showed a directly proportional relationship between CF and time although the CF and RF power varies between procedures [3]. Hence, the need for a new delivery strategy remains.
Ablation index (AI) is a novel marker incorporating contact force, time, and power in a weighted formula. Ablation index was shown to be reliable in predicting lesion size, and depth for RF delivery [4]. Pulmonary vein reconnection (PVR) was low in AI group, and AI group showed better freedom from atrial tachyarrhythmias [5,6].
We aimed to assess the latest evidence comparing the clinical outcomes and procedural duration of subjects undergoing ablation index guided procedure versus conventional ablation procedure.
2. Methods
2.1. Search strategy
We performed a comprehensive search on topic that assesses ablation index guided procedure versus conventional procedures from inception up until February 2019 through PubMed, EuropePMC, EBSCOhost, Cochrane Central Database, and ClinicalTrials.gov. A broad strategy to maximise the initial scope of research with keyword [“Ablation Index”] to ensure broad amount of records searched. The records were then systematically evaluated using inclusion and exclusion criteria. We also hand-sample from references of the included studies and abstracts from conference proceedings. Two researchers (R.P and I.H) independently performed an initial search, discrepancies were resolved by discussion. (A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the literature search strategy of studies investigating the ablation index was presented in Fig. 1.
Fig. 1.
PRISMA study flowchart.
2.2. Selection criteria
The inclusion criteria for this study were all studies that compared the ablation index guided procedure versus conventional procedures for pulmonary vein isolation in patients with AF. The primary outcome measured was 12 months incidence of atrial arrhythmia including atrial fibrillation(AF)/atrial flutter (AFL)/atrial tachycardia(AT), first-pass isolation, acute pulmonary reconnection (PVR), and procedure-related complications. The secondary outcome is the fluoroscopy time, total ablation time, and total procedural time. We include all related clinical researches/original articles including conference proceedings, and exclude case reports, review articles, and non-English language articles.
2.3. Data extraction
Data extraction and quality assessment was done by two independent authors (R.P and R.V) using standardized extraction form which includes authors, year of publication, sample size, AI target, controls, atrial arrhythmia evaluated by the study, 12 months atrial arrhythmia incidence, first-pass isolation, acute PVR, complications, total fluoroscopy time, total ablation time, total procedural time, and follow-up.
2.4. Statistical analysis
To perform the meta-analysis, we used RevMan version 5.3 software (Cochrane Collaboration). We used the odds ratio (OR) and a 95% CI as a pooled measure for dichotomous data. We used mean difference (MD) and its standard deviation (DS) as a pooled measure for the continuous data. Inconsistency index (I [2]) test which ranges from 0 to 100% was used to assess heterogeneity across studies. A value above 50% or p < 0.05 indicates statistically significant heterogeneity. We used the Mantel-Haenzsel method (for OR), and the Inverse Variance method (for MD) with a fixed-effect model for meta-analysis and a random-effect model was used in case of heterogeneity. All P values were two-tailed with a statistical significance set at 0.05 or below.
3. Results
3.1. Literature search
We found a total of 98 results and acquired three additional records from hand-search for abstracts. There were 48 records after removing duplicates. We screened 41 title/abstracts, removing 7 records from clinicaltrials.gov because the trials were yet to begin. Six were relevant titles/abstract, and we assessed four full-text for eligibility; we excluded one full-text because of no control for the study. We included five studies (four full paper and one abstract) in the qualitative synthesis and meta-analysis (Fig. 1).
3.2. Study characteristics
A total of 1727 subjects from five studies were included, 1081 (62.6%) underwent ablation-index guided ablation (interventional group), and 746 (37.4%) underwent conventional ablation (control group) [[6], [7], [8], [9], [10]]. The incidence of paroxysmal AF was 49.8%, and persistent AF was 51.2%. After omitting one study that only provided data on procedural time and complications, 62.4% had paroxysmal AF in which two studies enrolled 100% paroxysmal AF patients. Hence, in the outcome measuring atrial arrhythmia, first-pass isolation, and acute PVR were 62.4% paroxysmal AF patients. The AI target varies between studies and ranges from 330 to 550 depending on the locations. Solimene et al. study have the lowest AI target among the studies. Most of these studies followed up the subjects for 12 months, Solimene et al. reported a 14 months follow-up (Table 1).
Table 1.
Summary of the key findings the included studies.
| Study | Design | Samples (n) | AF (Paroxysmal vs Persistent; n) | Intervention vs Control (n) | AI Target | Control (Without AI) | Study Definition of Atrial Arrhythmias | 12 months Atrial Arrhythmia Incidence (%) | Time AI vs Control (Fluoroscopy/Ablation/Procedural) | 1st Pass Isolation (%) | Acute PVR (%) | Complications (AI/Control; n) | Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dhillon 2018 | Prospective Observational | 100 | 100/0 | 50/50 | 350–450 | CF | AF/AT | 10.8/13.3; p = 0.001 |
|
82/34; <0.001 | 14/24 | 0/3(1 pericarditis, 2 venous hematoma) | 12 |
| Futing 2018 | Retrospective Observational | 1073 | 440/633 | 387/686 | N/A | Non-CF 236; CF 450 | N/A | N/A |
|
N/A | N/A | 7(CT)/17(CT) | N/A |
| Hussein 2017 | Retrospective Observational | 178 | 88/90 | 89/89 | 400–550 | CF | AF/AT/AFL | 17/37; p = 0.002 |
|
87/84; <0.001 | 6/11 | 0/2(1 Phrenic Nerve Palsy, 1 retroperitoneal hematoma) | 12 |
| Phlips 2018 | Prospective Observational | 100 | 100/0 | 50/50 | ≥400-550 | CF | AF/AT/AFL | 6/20; p = 0.048 |
|
98/54; <0.001 | 3/18 | 0/1(CT) | 12 |
| Solimene 2018 | Prospective Observational | 276 | 132/24 | 156/120 | 330-400 → 350–450 (after 17 patients) | CF | AF/AT | 10.8/13.3; p = 0.09 (14 Months) |
|
N/A | N/A | 3 (1 pericardial effusion, 2 groin hematomas)/0 | 14 ± 6/12 ± 5 |
*indicates statistically significant results. Description: AF = Atrial Fibrillation, AFL = Atrial Flutter, AT = Atrial Tachycardia, CF=Contact Force (Conventional), CT=Cardiac Tamponade, N/A = Not Available/Applicable, OR=Odds Ratio (95% Confidence Interval).
3.3. 12 Months incidence of atrial fibrillation/atrial tachycardia/atrial flutter
Dhillon et al. and Solimene et al. assessed for AF and AT as the atrial arrhythmia outcome. Hussein et al. and Phlips et al. included AFL in addition to AF and AT to atrial arrhythmia outcome. Solimene et al. reported a non-significant difference on a follow-up of 14 ± 6 months and 12 ± 5 months in their study. Dhillon et al., Hussein et al., and Phlips et al. reported a statistically significant difference of 10.8% vs 13.3%, 17% vs 37%, and 6% vs 20% respectively at 12 months follow-up.
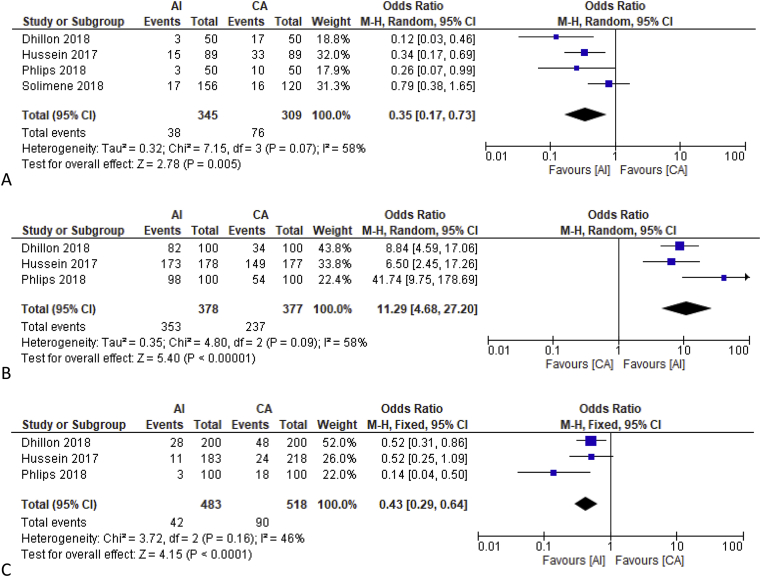
12 months’ incidence of AF/AT/AFL was lower in ablation index guided with an OR of 0.35 [0.17, 0.73], p = 0.005; heterogeneity I [2] 58%, p = 0.07 (Fig. 2A). Upon sensitivity analysis by removing Solimene et al. study, heterogeneity decreased to 0% with OR of 0.26 [0.15, 0.46], p < 0.001.
Fig. 2.
Comparison between Ablation Index and Conventional Ablation on Clinical Outcome. Fig. 2A showed the use of AI guided procedure was better compared to CA (Mostly contact-force ablation) on the 12 months atrial arrhythmia incidence including Atrial Fibrillation/Atrial Flutter/Atrial Tachycardia. Fig. 2B showed a higher incidence of first pass isolation in AI vs CA. Fig. 2C showed that acute pulmonary vein reconnection was higher in CA. Fig. 2D demonstrated no difference in rate of complications between AI and CA group. Description: AI = Ablation Index Guided, CA=Conventional Ablation.
3.4. First-pass isolation
First-pass isolation was shown to be higher in the ablation index guided in three studies. Dhillon et al. reported 82% vs 34%. Hussein et al. reported 87% vs 84% and Phlips et al. reported 98% vs 54%. Pooled analysis of these three studies was an OR of 11.29 [4.68, 27.20], p < 0.001; heterogeneity I [2] 58%, p = 0.09 (Fig. 2B). Upon sensitivity analysis and removal of Phlips et al. study, OR was 8.04 [4.66, 13.86], p < 0.001; heterogeneity I [2] 0%, p = 0.61.
3.5. Acute pulmonary vein reconnection
Dhillon et al. reported an acute PVR of 14% vs 24%, significantly lower in AI group. Hussein et al. and Phlips et al. reported similar results of 6% vs 11% and 3% vs 18% respectively. Pooled odds ratio for acute PVR was 0.43 [0.29, 0.64], p < 0.001; heterogeneity I [2] 46%, p = 0.16 (Fig. 2C). On sensitivity analysis by removing Phlips et al. study, the heterogeneity was reduced to 0%.
3.6. Procedural time
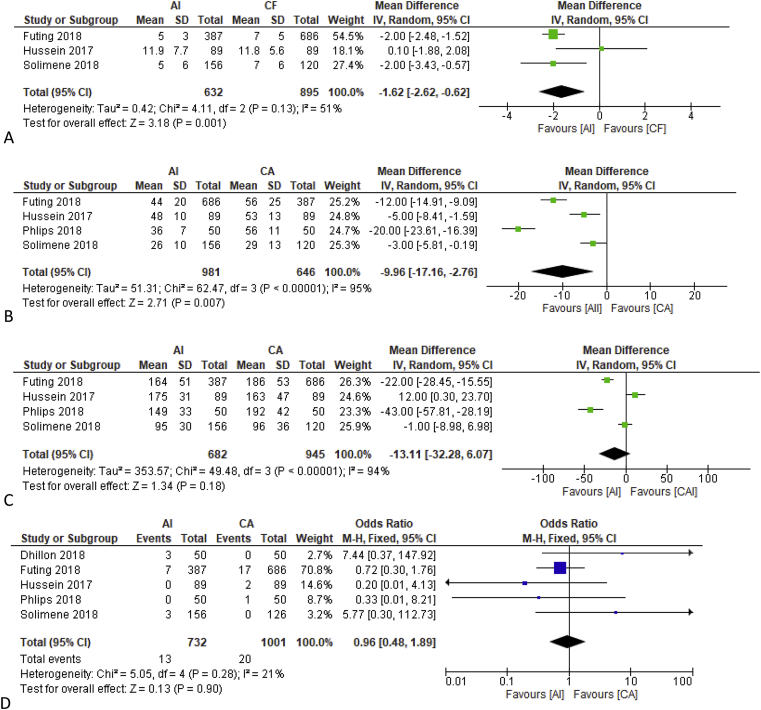
Two studies reported that the AI group has faster fluoroscopy time and two other studies showed a non-significant difference. One study did not report fluoroscopy time. On pooled analysis AI group has a shorter fluoroscopy time of MD -1.62 [-2.62, −0.62] minutes, p = 0.001; heterogeneity I [2] 51%, p = 0.13 (Fig. 3A).
Fig. 3.
Mean Difference in Procedural Duration of Ablation Index Guided and Conventional Ablation. Fig. 3A showed the use of mean difference of fluoroscopy time between AI guided procedure and CA, in which AI had a significantly shorter fluoroscopy time. Fig. 3B showed a shorter total ablation time in AI guided procedure. Fig. 3C showed no significant difference in mean total procedural time between the two procedure. Description: AI = Ablation Index Guided, CA=Conventional Ablation.
All five studies reported a faster total ablation time in the AI group compared to the conventional group. On pooled analysis AI group has a shorter total ablation time MD -9.96 [-17.16, −2.76] minutes, p < 0.001; heterogeneity I [2] 95%, p < 0.001 (Fig. 3B).
Three studies reported a faster total procedural time in the AI group while two did not report any statistically significant difference. On pooled analysis AI group did not differs statistically from the conventional group (Fig. 3C).
On sensitivity analysis by removing one study at a time did not reduce heterogeneity of pooled total ablation time and total procedural time.
3.7. Complications
Four studies reported that complications occurred more frequently in the conventional ablation group, but none was statistically significant. Dhillon et al. reported one pericarditis and two femoral venous hematomas in the conventional group. Futing et al. reported 7 and 17 cardiac tamponades in AI and conventional group respectively. Hussein et al. reported two major complications, which were one phrenic nerve palsy and one retroperitoneal hematoma occurring in the conventional group. Phlips et al. reported an incidence of cardiac tamponade requiring pericardiocentesis and prolonged hospitalization in the conventional group. One study reported a more frequent incidence of complication in the AI group (one pericardial effusion and two groin hematomas requiring no interventions) but they were not statistically significant on meta-analysis (Fig. 2D). On sensitivity analysis by removing Futing et al. study who did not specify their AI target and also included non-contact force, the complication rate did not differ statistically.
4. Discussion
Our study showed that AI-guided procedure resulted in a significantly lower incidence of 12 months AF/AT/AFL. Ablation index guided procedure was also associated with a slightly shorter fluoroscopy time and total ablation time but similar total procedural time. First-pass isolation was higher in AI group and acute PVR was lower in AI group. Ablation-index guided procedure has a similar safety profile to conventional contact-force ablation. Thus the application of Force-Time-Power integral of AI proved to be more effective with similar safety profile. However, since the conventional ablation itself has an excellent safety profile, a larger number of samples are needed to assess complications due to a small number of events. None of the studies reported complications such as esophageal fistula or stroke which is rare in modern AF ablation [9]. Four studies were prospective observational [[6], [7], [8]]. Futing et al. and Hussein et al. studies were retrospectives [9,10]. Hussein et al. had undergone propensity-matching to standardize the patient's baseline characteristics. Futing et al. study were only available in abstract, hence we cannot evaluate the difference in patients' baseline characteristics. Left atrial size was only specified in Hussein et al. and Solimene et al. study. P-wave duration has also been shown to affect AF recurrence and was not yet included in these studies [11,12]. Solimene et al. study lacks a control group; they included patients from another study in which CA was performed by the same two operators. Randomized clinical trials were needed to further establish the efficacy and safety of AI in a more unbiased manner.
These studies also did not provide continuous monitoring, and asymptomatic atrial arrhythmias might be missed, leading to an overestimation of 12 months atrial arrhythmia freedom. The 12 months incidence of arrhythmia was lower in the AI group in three studies, however, a study by Solimene et al. concluded that there is no statistically significant difference [8]. This study caused a significant heterogeneity, during sensitivity analysis the removal of this study reduces heterogeneity to 0%. This heterogeneity might be caused by a lower AI index (330–400) for the first 17 AI patients in their study although the authors suggest a comparable success in low and standard AI index.
Conventional ablation method using contact-force has been widely used, and the operators have more experience using this method [1]. Hussein et al. study considered first 10 patients as a learning curve and excluded them [9]. Learning new procedure took time and despite these measures, the operators may be more fluent in doing CA procedure. The other 4 studies did not explicitly specify these measures. This may underestimate the efficacy of AI ablation on 12 months atrial arrhythmia, first-pass isolation, acute pulmonary vein reconnection, and prolong procedural time. However, since the results favour AI with a pooled OR of 0.35 for the risk of atrial arrhythmia in 12 months, this limitation may highlight the superiority of AI. Fluoroscopy and total ablation time are also shorter despite this limitation, even though the total procedural time stays the same.
First-pass isolation was more frequent in AI group, and acute PVR was lower in AI group which may be due to a more accurate lesion prediction in AI which used Force-Time-Power integral than conventional ablation which used Force-Time integral only [4,9]. Phlips et al. is the main cause of bias of the first-pass isolation outcome, 98% in AI group and only 2 event of no first-pass isolation in AI group leads to a high OR and wide confidence interval. Their study also causes heterogeneity in acute PVR outcome, only two acute PVR in AI group.
Limitation of this systematic review includes potential selection bias because the studies included were not randomized controlled trials. The operator for the procedure also differs from one study to another. There were studies that use another study's sample for control, although the study is performed by the same operator. The number of studies and samples included were small. The AI targets also vary leading to heterogeneity of study result. These studies only addressed a 12 months follow-up, hence, a long term efficacy is unknown at this point.
5. Conclusion
Ablation index guided procedure resulted in a significantly lower incidence of AF/AT/AFL. It is also associated with a shorter fluoroscopy time and total ablation time but similar total procedural time. First-pass isolation was higher in the AI group, and acute PVR was lower in the AI group. Ablation-index guided procedure has a similar safety profile to conventional contact-force ablation. We suggest future studies to use a randomized controlled trial design and considers the effect of the learning curve in those with limited experience in performing AI. Atrial indices that predict the recurrence of AF should be considered. A longer follow-up is also needed to see a long term outcome. A continuous ECG monitoring using a loop-recorder or implantable recorder is also recommended to detect asymptomatic atrial arrhythmias that might be missed. Larger sample size is also required to determine the safety profile of AI sufficiently.
Conflicts of interest
The authors declare that they have no conflict of interests.
Author's contribution
Raymond Pranata conceived and designed the study and drafted the manuscript. Raymond Pranata and Ian Huang acquired the data and drafted the manuscript. Raymond Pranata, and Rachel Vania extracted the data. Raymond Pranata analyzed the data statistically. All authors contributed to the writing of this manuscript.
Acknowledgements
None.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
Contributor Information
Raymond Pranata, Email: raymond_pranata@hotmail.com.
Rachel Vania, Email: Rachel.vania@gmail.com.
Ian Huang, Email: ianhuang2108@gmail.com.
References
- 1.Chelu M.G., Morris A.K., Kholmovski E.G. Durable lesion formation while avoiding esophageal injury during ablation of atrial fibrillation: lessons learned from late gadolinium MR imaging. J Cardiovasc Electrophysiol. 2018;29(3):385–392. doi: 10.1111/jce.13426. [DOI] [PubMed] [Google Scholar]
- 2.Dagres N., Hindricks G. C. 2009. pp. 1014–1019. (Complications of atrial fibrillation ablation in a high-volume center in 1 , 000 Procedures : still cause for Concern?). March. [DOI] [PubMed] [Google Scholar]
- 3.Münkler P., Kröger S., Liosis S. Ablation index for catheter ablation of atrial fibrillation ― clinical applicability and comparison with force-time integral ―. Circ J. 2018;82(11):2722–2727. doi: 10.1253/circj.CJ-18-0361. [DOI] [PubMed] [Google Scholar]
- 4.Koruth J.S., Iwasawa J., Enomoto Y. Chamber-specific radiofrequency lesion dimension estimation using novel catheter-based tissue interface temperature sensing. JACC Clin Electrophysiol. 2017;3(10):1092–1102. doi: 10.1016/j.jacep.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Hussein A., Das M., Riva S. Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients. Circ Arrhythm Electrophysiol. 2018;11(9) doi: 10.1161/CIRCEP.118.006576. [DOI] [PubMed] [Google Scholar]
- 6.Phlips T., Taghji P., El Haddad M. Improving procedural and one-year outcome after contact force-guided pulmonary vein isolation: the role of interlesion distance, ablation index, and contact force variability in the ‘CLOSE’-protocol. EP Eur. 2018;1–9 doi: 10.1093/europace/eux376. January. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon G., Ahsan S., Honarbakhsh S. A multicentered evaluation of ablation at higher power guided by ablation index: establishing ablation targets for pulmonary vein isolation. J Cardiovasc Electrophysiol. 2018:13813. doi: 10.1111/jce.13813. jce. [DOI] [PubMed] [Google Scholar]
- 8.Solimene F., Schillaci V., Shopova G. Safety and efficacy of atrial fibrillation ablation guided by Ablation Index module. J Interv Card Electrophysiol. 2018:9–15. doi: 10.1007/s10840-018-0420-5. [DOI] [PubMed] [Google Scholar]
- 9.Hussein A., Das M., Chaturvedi V. Prospective use of Ablation Index targets improves clinical outcomes following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2017;28(9):1037–1047. doi: 10.1111/jce.13281. [DOI] [PubMed] [Google Scholar]
- 10.Füting A., Ruprecht U., Buchholz J., Buschmeier F., Reinsch N., Neven K. Use of Ablation Index significantly reduces the incidence of cardiac tamponade complicating pulmonary vein isolation for atrial fibrillation. Clin Res Cardiol. 2018;107(Suppl 3) https://dgk.org/kongress_programme/ht2018/aP559.html [Google Scholar]
- 11.Mugnai G., Chierchia G.B., De Asmundis C. P-wave indices as predictors of atrial fibrillation recurrence after pulmonary vein isolation in normal left atrial size. J Cardiovasc Med. 2016;17(3):194–200. doi: 10.2459/JCM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 12.Jadidi A., Müller-Edenborn B., Chen J. The duration of the amplified sinus-P-wave identifies presence of left atrial low voltage substrate and predicts outcome after pulmonary vein isolation in patients with persistent atrial fibrillation. JACC Clin Electrophysiol. 2018;4(4):531–543. doi: 10.1016/j.jacep.2017.12.001. [DOI] [PubMed] [Google Scholar]