Abstract
Rationale:
The neuropeptide oxytocin (OXT) has emerged as a potential therapeutic intervention in the treatment of both alcohol use disorder (AUD) and stress-related psychiatric illnesses.
Objectives:
The present study evaluates the effects of systemically administered (intraperitoneal, i.p.) OXT treatment on alcohol relapse-like behavior in male and female mice.
Methods:
Adult male and female C57BL/6J mice were trained to lever respond in operant conditioning chambers for alcohol in daily self-administration sessions. Once lever responding and alcohol intake stabilized mice were tested under extinction conditions for 14 days before reinstatement testing. All mice underwent stress-induced reinstatement testing using either predator odor (2,3,5-Trimethyl-3-thiazoline; TMT) or the α−2 adrenergic receptor agonist yohimbine. In Study 1, mice were exposed to TMT for 15 min and then immediately placed into operant conditioning chambers to examine alcohol-seeking behavior under extinction conditions. At 30 min prior to test session, separate groups of mice were injected with vehicle or OXT (0.1, 0.5, 1 mg/kg). In Study 2, mice were injected with yohimbine (0.3, 0.625 mg/kg) 1 hr prior to reinstatement testing. At 30 min post-yohimbine injection, mice are injected (ip.) with vehicle or OXT (1 mg/kg).
Results:
OXT attenuated alcohol-seeking behavior in a dose-related manner in male and female mice in response to acute challenge with a predator odor. Additionally, OXT administration produced a similar decrease in alcohol relapse-like behavior triggered by the pharmacological stressor yohimbine in both sexes.
Conclusions:
Systemic oxytocin administration attenuates stress-induced reinstatement of alcohol seeking in male and female mice.
Keywords: Oxytocin, Alcohol, Stress, Relapse, Self-Administration, Mouse
INTRODUCTION
The compulsive nature of alcohol seeking and use, along with high rates of relapse present a major challenge in the treatment of alcohol addiction. Stress is a common trigger for relapse, yet mechanisms by which stress provokes relapse and promotes excessive levels of alcohol consumption are not fully understood. Thus, understanding brain mechanisms underlying relapse and the influence of stress on drinking has emerged as a central issue in addiction research.
Oxytocin (OXT) is an endogenous neuropeptide that has been implicated in various processes associated with addiction, including reward, tolerance, memory and stress responses (Lee et al. 2016). OXT is synthesized in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus and released by the posterior pituitary into peripheral circulation. In addition, OXT is released by neurons in the PVN that project to numerous extrahypothalamic regions in the brain (e.g., cortical, limbic, basal ganglia structures) where it mediates an array of behavioral effects via interaction with G(q)-coupled OXT receptors (Lee et al. 2016). Aside from its known hormonal role in parturition and maternal behaviors, OXT also regulates a number of behaviors that involve social interaction (e.g., pair-bonding, social reward processing, aggression) and nonsocial behaviors, including anxiety and response to stress (Baskerville and Douglas 2010; Burkett et al. 2016; Neumann and Landgraf 2012). Through interactions with brain reward and stress systems, OXT is known to play a role in several neuropsychiatric disorders that involve social deficits, including drug and alcohol addiction (Baskerville and Douglas 2010; Lee et al. 2016).
Understandably, the ability of this nonapeptide to modulate both stress and motivational processes has generated growing interest in its potential as a treatment for substance use disorders. For example, in a recent clinical study, intranasal OXT was shown to alleviate alcohol withdrawal symptoms in treatment-seeking subjects (Pedersen et al. 2013). Additional support comes from a growing number of studies showing that systemic administration of OXT reduces alcohol consumption in rodents (Bowen et al. 2011; MacFadyen et al. 2016; McGregor and Bowen 2012). Further, direct brain (intracerebroventricular) infusion of OXT, or manipulation of OXT receptor expression in brain reduced alcohol reward and consumption (Bahi 2015; Bahi et al. 2016; Peters et al. 2017). Recent work in our laboratory demonstrated that systemic administration of OXT reduced binge-like alcohol drinking and oral self-administration of alcohol using operant conditioning procedures in male C57BL/6J mice in a dose-related manner (King et al. 2017). Importantly, OXT reduced alcohol consumption at doses that did not significantly alter sucrose intake. Taken together, accumulating evidence suggests that the OXT system may represent a promising target for the treatment of alcohol use disorders.
While evidence supports a role for OXT in regulating alcohol consumption, few studies have examined the effects of OXT on alcohol relapse-like behavior. The present study aimed to examine the ability of systemic oxytocin to attenuate stress-induced reinstatment of alcohol-seeking behavior in male and female mice. Two different stress procedures were employed: brief exposure to a predator odor and acute challenge with the pharmacological stressor yohimbine. Given that OXT is know to function as an anti-stress neuropeptide (Landgraf and Neumann 2004; Windle et al. 2004), it was predicted that systemic OXT administration would attenuate stress-induced reinstatement of alcohol-seeking behavior in a dose-related manner in male and female mice. Further, since there is some evidence to suggest that gonadal hormones can influence the functional activity of OXT (Ivell and Walther 1999), it was predicted that females would exhibit a greater response to OXT treatment compared to males.
MATERIALS AND METHODS
Subjects
Adult male and female C57BL/6J mice (25 to 30 g) were obtained from Jackson Laboratories (Bar Harbor, ME) at 9 to 10 weeks of age and were acclimated to the experimental housing rooms for a minimum of 1 week prior to the start of experiments. Male and female mice were group housed (4 per cage) in the same colony room, but with males and females on separate racks. The animals were maintained under a 12-hour reversed light/dark cycle in an AAALAC-accredited facility. All testing was conducted during the dark phase of the circadian cycle. Mice were provided free access to food and water, except at the start of operant oral self-administration training. All experimental protocols were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee and consistent with guidelines of the NIH Guide for the Care and Use of Laboratory Animals.
Operant Conditioning Chambers
Mice were tested in standard operant conditioning chambers configured with 2 retractable levers, a centrally located well for liquid reinforcers, house light, a tone generator, and a stimulus light above the well. The chambers were situated in sound-attenuating cubicles with a ventilation fan (Med Associates; Fairfax, VT). One lever was assigned as the “active” lever, with scheduled responses activating an infusion pump that delivered alcohol (12% v/v ethanol) reinforcement (20 μl) into the well along with activating a light and a tone stimulus (80 dB). Responses on the “inactive lever” were recorded, but did not result in any stimulus consequences or reinforcement delivery. The position of the “active” lever (left vs. right of well) was counterbalanced across subjects. Stimulus events and responses were controlled and monitored using Med PC, Version IV software (Med Associates). At the end of each session, any residual fluid left in the well was collected with a pipette, measured, and subtracted from the total volume of alcohol delivered. The corrected volume was used to calculate g/kg alcohol intake during the 20-min daily sessions. Male and female mice were tested in separate chambers throughout the experiments (8 chambers/sex).
Operant Alcohol Self-Administration
Mice were trained to self-administer alcohol using standard operant conditioning procedures, as previously described (King et al. 2017). Briefly, mice were first trained to respond for alcohol reinforcement under a fixed ratio-1 (FR1) schedule. Response requirements were gradually increased until mice achieved stable lever responding under a FR4 schedule of reinforcement (<15% variability across 3 consecutive daily sessions). Once stable baseline alcohol responding/intake was established, mice entered into the extinction phase of the study. During daily extinction sessions, responding on the “active” lever no longer had stimulus consequences and alcohol reinforcement was no longer delivered. After 14 days of extinction (when responding has reached <15% of baseline responding), all mice were tested for stress-induced reinstatement of alcohol responding.
Study 1: Predator Odor-Induced Reinstatement Testing
Mice were transferred to a chamber and exposed to a predator odor (2,3,5-Trimethyl-3-thiazoline; TMT) for 15 min. This stressor, a synthetically derived component of fox feces, was selected because of its ethological relevance and validity (Janitzky et al. 2015), as well as the fact that we have successfully used TMT to produce robust relapse-like behavior in our preliminary studies. TMT (0.03 mL; 1% v/v) was placed on a gauze pad in a weigh boat in the chamber such that mice could not manipulate it. After the 15-min exposure period, mice were immediately placed into self-administration chambers to examine alcohol-seeking behavior under extinction conditions. At 30 min prior to the reinstatement test session (15 min prior to TMT exposure), separate groups of mice were injected (ip.) with vehicle (saline) or OXT (0.1, 0.5, 1 mg/kg) (N= 8–10/group).
Study 2: Yohimbine-Induced Reinstatement Testing
Mice were injected with yohimbine (0.3, 0.625 mg/kg; ip.) 60 min prior to the reinstatement test session. At 30 min post-yohimbine injection, mice are injected (ip.) with vehicle (saline) or OXT (1 mg/kg). Reinstatement testing was conducted under extinction conditions 30 min after OXT injection. Results from a series of pilot studies indicated that the 60 min pretreatment time for yohimbine administration was optimal for triggering reinstatement responding (unpublished data). In this case, oxytocin was administered after yohimbine treatment to keep the pretreatment time for the neuropeptide consistent with that used in the TMT study (Study 1). Each mouse received both yohimbine doses in a counterbalanced order with one week of extinction responding between tests. Male and female cohorts were tested separately (N= 7–10/group/sex).
Drugs
Synthetic human oxytocin (CellSciences, Canton, MA) and yohimbine (Tocris Bioscience, Minneapolis, MN), were dissolved in 0.9% saline, which served as the vehicle. Injections were administered intraperitoneally in a volume of 0.01 ml/g body weight in all experiments. Alcohol (95% ethanol) was obtained from AAPER (Shelbyville, KY) and diluted with tap water to the appropriate concentration. The alcohol solution was prepared daily and presented at room temperature.
Statistical Analysis
The primary dependent variable analyzed was lever responses during different phases of the studies. Lever responses were averaged over the last three daily sessions of baseline alcohol self-administration and the last 3 days of extinction testing prior to reinstatement testing. Number of reinforcers earned and amount of alcohol intake (g/kg) averaged over the last three self-administration sessions were also analyzed. For Study 1, lever responses, reinforcers earned, and alcohol intake in male and female mice were analyzed by t-tests. Responses during reinstatement testing were analyzed by ANOVA, with Sex and Dose as between-subject factors. For Study 2, since male and female mice were not tested simultaneously, reinstatement responding for males and females was separately analyzed by ANOVA, with oxytocin Dose as a between-subject factor and yohimbine Treatment as a repeated measure. Initial analyses also included order of yohimbine dose as a factor but since this variable did not significantly interact with the other variables, data were presented and analyzed as collapsed over this factor. Posthoc analyses (Newman-Keuls) were conducted when appropriate. For all analyses, significance
RESULTS
Study 1: Oxytocin Effects on TMT-Induced Reinstatement of Alcohol Seeking in Male and Female Mice
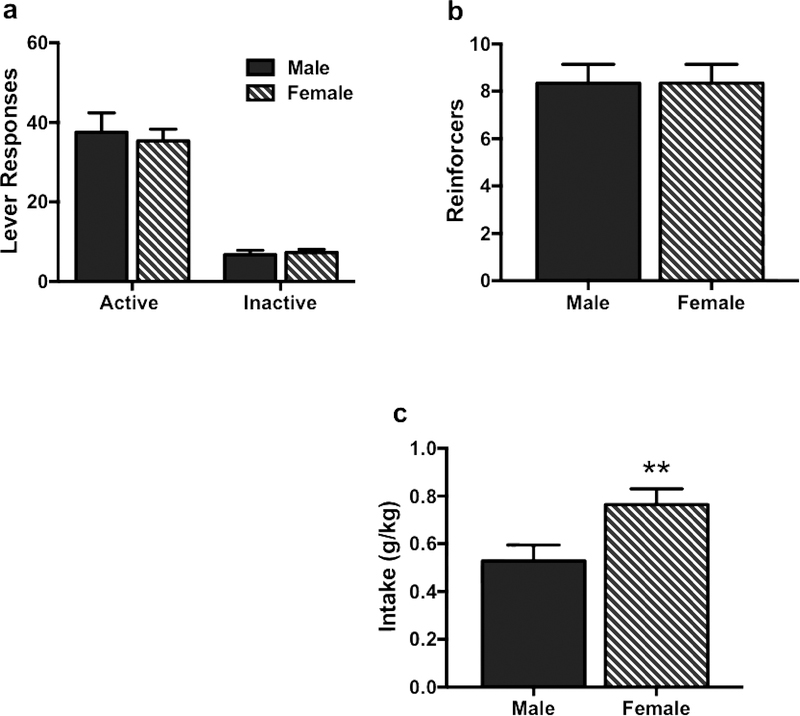
All animals demonstrated stable and preferential responding on the active lever. Males and females did not significantly differ in responding on the active lever [t(66)= 0.08, p> 0.1] and inactive lever [t(66)= 0.95, p> 0.1] (Figure 1a), and there was no sex-related difference in the number of alcohol reinforcers earned [t(66)= 0.25, p> 0.1] (Figure 1b). However, average alcohol intake was significantly higher in females compared to males [t(66)= 2.87, p< 0.01] due to differences in body weight (Figure 1c).
Figure 1:
Lever responding, alcohol reinforcers and alcohol Intake during oral selfadministration of alcohol using operant conditioning procedures. Males and females did not significantly differ in responding on the active lever (a), and there was no sex-related difference in the number of alcohol reinforcers (b). Average alcohol intake was significantly higher in females compared to males (c). Values are mean ± s.e.m. (N= 8–10/group). Significantly differs compared to males: ** (p< 0.01)
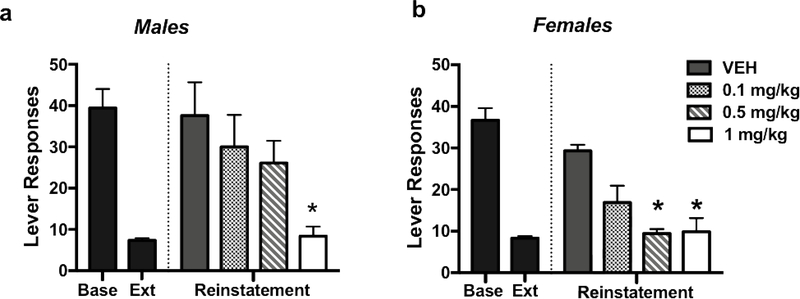
During reinstatement testing, TMT exposure significantly increased alcohol-seeking behavior, and oxytocin treatment significantly reduced this stress-induced relapse-like responding in both male and female mice in a dose-related manner (Figure 2). ANOVA revealed a significant main effect of Dose [F(3,63)= 8.39, p< 0.001], indicating that oxytocin was effective in reducing alcohol responding in both males and females. While it appears that females may have been more sensitive to the effects of oxytocin, this was not supported by the analysis, as the Dose x Sex interaction did not achieve statistical significance.
Figure 2:
Effects of oxytocin on TMT-induced reinstatement of alcohol-seeking behavior. Systemic OXT attenuates TMT-induced reinstatement of alcohol seeking behavior in a dose-related manner in male (left) and female (right) mice. Values are mean ± s.e.m. (N= 10/group). Significantly differs from vehicle condition: * (p< 0.05)
Separate analysis of data from males revealed a significant effect of Dose [F(3,35)= 5.22, p< 0.01] and post-hoc comparisons indicated that the highest oxytocin dose tested (1 mg/kg) reduced responding compared to vehicle (p< 0.05). In females, a main effect of Dose [F(3,34)= 4.54, p< 0.01] and post-hoc analysis indicated that both 0.5 and 1 mg/kg doses of oxytocin significantly reduced alcohol seeking behavior compared to vehicle (ps< 0.05).
Study 2A: Oxytocin Effects on Yohimbine-Induced Reinstatement of Alcohol Seeking in Male Mice
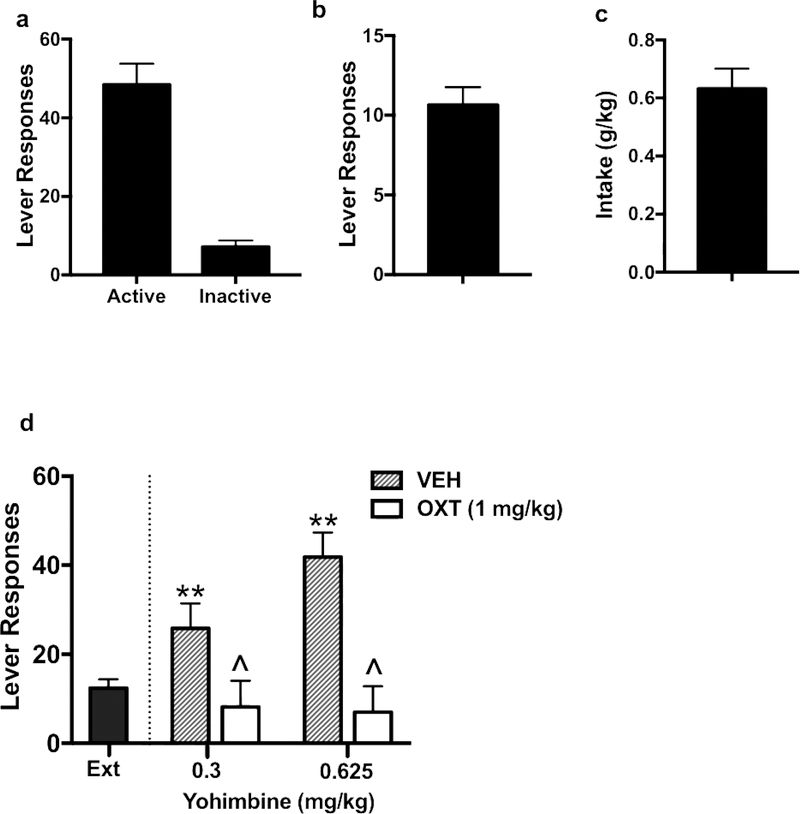
Active and inactive lever responses, number of alcohol reinforcers earned, and alcohol intake over the last 3 days of oral alcohol self-administration in males are shown in Figure 3a, 3b, and 3c, respectively. Yohimbine treatment increased alcohol responding during reinstatement testing and this effect was blocked by pretreatment with oxytocin (1 mg/kg) (Figure 3d). Increased reinstatement responding following injection of yohimbine did not significantly differ as a function of dose of the drug. ANOVA revealed a significant main effect of oxytocin Dose [F(1,17)= 22.15, p< 0.001], but the yohimbine Treatment x oxytocin Dose interaction was not significant. This indicates that systemic administration of oxytocin (1 mg/kg) reduced alcohol seeking behavior provoked by both yohimbine doses to a similar extent.
Figure 3:
Effects of oxytocin on yohimbine-induced reinstatement in male mice. Active and inactive lever responses (a), number of alcohol reinforcers earned (b), and alcohol intake (c) over the last 3 days of oral alcohol self-administration. Yohimbine treatment increased alcohol responding during reinstatement testing and this effect was blocked by pretreatment with OXT (1 mg/kg) (D). Values are mean ± s.e.m. (N= 7–10/group). Significantly differs from average last 3 days of extinction (ext): ** (p< 0.01). Significantly differs from vehicle condition: ^ (p< 0.05).
Study 2B: Oxytocin Effects on Yohimbine-Induced Reinstatement of Alcohol Seeking in Female Mice
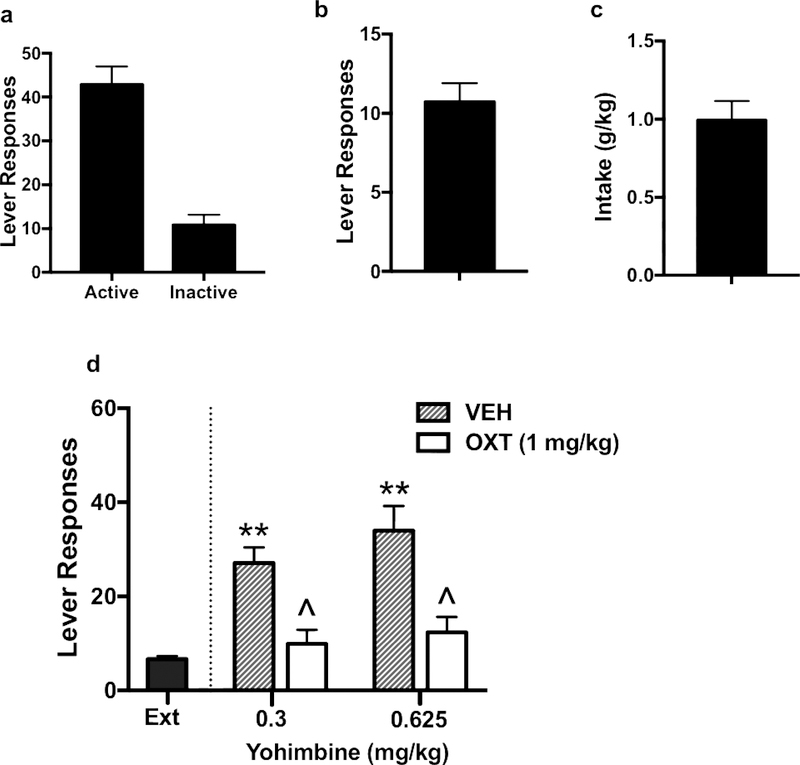
Active and inactive lever responses, number of alcohol reinforcers earned, and alcohol intake over the last 3 days of oral alcohol self-administration in females are shown in Figure 4a, 4b, and 4c, respectively. As in males, yohimbine treatment increased alcohol responding during reinstatement testing and this effect was blocked by pretreatment with oxytocin (1 mg/kg) (Figure 4d). ANOVA indicated that both doses of yohimbine produced a similar increase in alcohol responding [F(1,26)= 1.62, p> 0.1], and oxytocin blocked this increase in responding [F(1,26)= 23.52, p< 0.001]. The lack of a significant yohimbine Treatment x oxytocin Dose interaction indicates that oxytocin was effective in reducing elevated alcohol seeking produced by both doses of yohimbine.
Figure 4:
Effects of oxytocin on yohimbine-induced reinstatement in female mice. Active and inactive lever responses (a), number of alcohol reinforcers earned (b), and alcohol intake (c) over the last 3 days of oral alcohol self-administration. Similar to males, yohimbine treatment increased alcohol responding during reinstatement testing and this effect was attenuated by pretreatment with OXT (1 mg/kg) (d). Values are mean ± s.e.m. (N= 7–10/group). Significantly differs from average last 3 days of extinction (ext): ** (p< 0.01). Significantly differs from vehicle condition: ^ (p< 0.05).
DISCUSSION
Results from the present study demonstrate that systemic administration of oxytocin reduces stress-induced reinstatement of alcohol-seeking behavior. In Study 1, oxytocin attenuated predator odor (TMT)-induced reinstatement in a dose-related manner in both male and female mice. In Study 2, oxytocin attenuated increased alcohol-seeking following administration of the pharmacological stressor yohimbine in both sexes. Although oxytocin has been shown to effectively reduce cue- and drug-primed reinstatement to alcohol and other drugs of abuse (Baracz et al. 2015; Baracz et al. 2016; Cox et al. 2017; Ferland et al. 2016; Hansson et al. 2018; Kohtz et al. 2018; Weber et al. 2018), to our knowledge, this is the first report to demonstrate that oxytocin attenuates stress-induced alcohol relapse-like behavior in mice.
Our finding that oxytocin reduces stress-induced alcohol reinstatement responding is consistent with other studies that have shown OXT decreases stress-related relapse-like behavior to other drugs of abuse. Systemic administration of OXT decreased methamphetamine-seeking behavior following predator odor exposure (Ferland et al. 2016) and yohimbine administration (Cox et al. 2013) in rats. In mice, centrally administered OXT (icv.) attenuated reinstatement of methamphetamine conditioned place preference (CPP) induced by restraint stress (Qi et al. 2009), and systemic administration of the oxytocin analog, carbetocin, reduced the effects of forced-swim stress on reinstatement of morphine-induced CPP (Zanos et al. 2014). Collectively, results from the present study and related reports in the literature indicate that oxytocin treatment is effective in attenuating relapse-like behavior provoked by various stress procedures.
The present study demonstrated oxytocin was effective in reducing the ability of two different stress procedures (predator odor (TMT) exposure and systemic administration of the alpha-2 noradrenergic antagonist yohimbine) to reinstate alcohol-seeking behavior. While both stressors produced a significant increase in alcohol-seeking behavior, the use of TMT and yohimbine as tools to induce stress and fear responses are controversial (Blanchard et al. 2003; Chen et al. 2015; Endres and Fendt 2007; Fendt and Endres 2008; Mantsch et al. 2016; McGregor et al. 2002). In rodents, TMT exposure reliably produces an elevation in plasma corticosterone levels (Morrow et al. 2000), and elicits a number of autonomic and behavioral changes that are indicative of fear and anxiety (Endres et al. 2005; Fendt et al. 2005). However, it has been suggested that TMT is not a fear-inducing stimulus but rather a generalized noxious odor (Fendt et al. 2005; McGregor et al. 2002). Studies investigating the neural basis of TMT effects on rodents demonstrate activation of the HPA-axis (Day et al. 2004; Myers and Rinaman 2005) and increases in c-fos expression in brain regions shown to be important in fear processing and stress-related responses, such as the amygdala, bed nucleus of the stria terminalis, lateral septum, and hypothalamus (Day et al. 2004; Janitzky et al. 2015). Thus, there is ample neural, physiological, and behavioral evidence indicating that TMT exposure produces a stress-like state that may trigger alcohol-seeking behavior in the operant conditioning reinstatement model.
The use of yohimbine as a stressor in this relapse model also has been called into question (Chen et al. 2015). It is suggested that yohimbine may not be exerting its effects on alcohol/drug seeking behavior by producing an aversive (stress) state but, rather, merely potentiating cue-related responding. Further, yohimbine was not found to induce conditioned place aversion, suggesting a lack of stress-like effects (Chen et al. 2015). However, evidence from several studies indicate that yohimbine engages critical components of stress circuitry that are known to mediate stress responses, including reinstatement of alcohol/drug seeking behavior (Buffalari and See 2011; Le et al. 2013; Shaham et al. 2003; Shalev et al. 2010). Further, human studies indicate that yohimbine induces both physiological and psychological stress-like responses (Greenwald et al. 2013) and alcohol craving (Umhau et al. 2011). Taken together, there is sufficient evidence indicating that both stress procedures employed in the present study produced an aversive state, and oxytocin was effective in attenuating their ability to provoke alcohol relapse-like behavior in male and female mice.
While results from the present study demonstrated that oxytocin is effective in attenuating stress-induced alcohol relapse-like behavior in both male and female mice, it is difficult to conclude whether a sex-related difference in sensitivity to this effect exists. Results from Study 1 suggest that females may exhibit greater sensitivity to OXT than males (shift to the left in the dose-effect function). Unfortunately, only a single dose was used in Study 2 and this dose (1 mg/kg OXT) was equally effective in reducing stress-induced reinstatement of alcohol seeking in both females and males. Other studies have provided evidence for sex-related differences in sensitivity to OXT. For example, lower doses of OXT were more effective in reducing cue-induced reinstatement of cocaine (Kohtz et al. 2018) and sucrose (Zhou et al. 2015) responding in female rats compared to their male counterparts. Further, sexually dimorphic expression of OXT receptors in the brain has been reported in several strains of rats and mice (Dumais et al. 2016; Dumais et al. 2013; Dumais and Veenema 2016), although no such sex differences were found in a study with C57BL/6J mice (Hammock and Levitt 2013). Oxytocin expression and signaling in the brain has been shown to be modulated by gonadal hormones (Dumais and Veenema 2016; Ivell and Walther 1999; Patchev et al. 1993). Fluctuations in estrogen and progesterone levels across the estrous cycle may influence sensitivity to OXT in females (Dumais et al. 2013; Dumais and Veenema 2016). Although we did not assess estrus status in our studies, other reports have indicated that the effects of OXT on relapse-like behavior are not dependent on phase of estrus at the time of testing (Cox et al. 2013; Leong et al. 2017; Leong et al. 2016). Thus, possible sex-related differences in sensitivity to OXT as well as the role of sex hormones influencing effects of OXT on alcohol relapse-like behavior remain to be determined.
The mechanism by which OXT decreased stress-induced re-invigoration of alcohol responding is not well understood. Previous work in our laboratory demonstrated that oxytocin doses less than 3 mg/kg reduced alcohol self-administration in C57BL/6J mice without altering general locomotor activity (King et al., 2017). Thus, it is unlikely that oxytocin reduced alcohol relapse responding provoked by TMT or yohimbine exposure due to a nonspecific sedative effect.
Oxytocin is known to exert anti-stress and anxiolytic effects (Jurek et al. 2015; Peters et al. 2014; Slattery and Neumann 2010). Oxytocin interacts with corticotrophin-releasing hormone (CRF) neurons and the peptide has been shown to dampen stress-induced HPA-axis activation (elevated corticosterone levels), as well as reduce stress-related behavioral responses in animal models of anxiety and depression (Neumann et al. 2000; Windle et al. 2004). Further, high levels of OXT and OXT receptor mRNA expression are localized in forebrain regions such as the extended amygdala, where OXT signaling can play a significant role in the regulation of anxiety, stress, and reward-related behaviors (Dabrowska et al. 2011; Gimpl and Fahrenholz 2001; Martinon and Dabrowska 2018; Veinante and Freund-Mercier 1997).
Another potential mechanism by which oxytocin decreases reinstatement of alcohol-seeking behavior may be through direct influence on reward signaling. Oxytocin receptors have been identified in a number of brain regions known to significantly contribute to drug-related reward and relapse-like behavior. This includes the nucleus accumbens core (NAcc) (Tan et al., 2017; Dolen et al., 2013), ventral tegmental area (VTA) (Peris et al., 2017) and medial prefrontal cortex (mPFC) (Li et al, 2016; Nakajima et al., 2014). Intra-NAcc infusion of oxytocin has been shown to attenuate drug primed- (Baracz et al. 2015; Baracz et al. 2016) and cue- (Bernheim et al., 2017) induced reinstatement of methampethamine seeking. Additionally, Leong et al. (2017) demonstrated that elevated Fos expression in the mPFC, NAcc, and subthalamic nucleus (STN) in response to cocaine associated cues was normalized by a systemic injection of oxytocin in rats. Interestingly, direct infusion of oxytocin into the NAcc and STN reduced whereas intra-PFC oxytocin increased cue-induced reinstatement of cocaine-seeking behavior (Weber et al., 2018; Leong et al., 2018). A few studies have examined the influence of oxytocin on alcohol-related reward effects. For example, acute intracerebroventricular infusion of oxytocin reduced alcohol consumption and elevated dopamine release in the NAc in rats (Peters et al., 2017). Viral-mediated overexpression of oxytocin receptors in the NAc was shown to reduce alcohol drinking and alcohol-induced conditioned place preference (Bahi, 2015; Bahi et al., 2016). Hansson et al. (2018) demonstrated increases in oxytocin receptor mRNA and protein levels in prefrontal cortex, striatal, amygdala, and hippocampal regions in alcohol dependent rats after 3 weeks of abstinence, implicating similar brain regions as the aforementioned studies. Thus, there is evidence to suggest that the effects of oxytocin within brain reward circuitry may not only contribute to its ability to reduce alcohol self-administration, but also reduce alcohol relapse-like behavior. Unfortunately, there are no studies investigating the effects of site-specific administration of oxytocin on reinstatement of alcohol-seeking behavior using operant conditioning procedures. Future studies are needed to investigate specific brain regional changes that mediate the ability of oxytocin to blunt alcohol relapse-like behavior.
Systemic administration of OXT raises the issue of blood-brain barrier penetrance (Lee et al. 2018). A number of studies have recapitulated effects of systemic OXT administration with direct (icv. or site-specific) intracranial infusions across a number of behavioral tasks (Cox et al. 2017; Lee et al. 2018; Love 2014; Ring et al. 2006; Slattery and Neumann 2010; Windle et al. 1997). Additionally, peripheral administration of OXT has been shown to induce Fos expression in OXT neurons in the PVN (Carson et al. 2010; Leong et al. 2017), suggesting that peripheral administration may induce endogenous central release. However, Lee et al. (2018) demonstrated that while OXT administered through intranasal and intravenous routes of administration increased OXT levels in cerebral spinal fluid, it did not activate a feed forward mechanism to elevate endogenous oxytocin. Thus, the mechanism by which exogenous OXT delivered in the periphery activates OXT signaling in the brain remains unclear. Further studies are needed to address this issue, as it is relevant to the potential for oxytocin to serve as a therapeutic for alcohol/drug addiction.
In conclusion, our findings reveal that systemic oxytocin administration attenuates stress-induced reinstatement of alcohol seeking in male and female mice. Exogenous OXT decreased alcohol-seeking behavior in a dose-related manner in response to acute challenge with a predator odor. The reduction in reinstatement responding was similar between males and females, though females showed a decrease in responding at lower doses of OXT compared to males. Additionally, OXT administration produced a similar decrease in alcohol relapse-like behavior triggered by the pharmacological stressor yohimbine in both sexes. Taken together, these results indicate that OXT may be potential therapeutic target for mitigating relapse initiated by stress in both males and females.
Acknowledgements
Supported by grants from the National Institute on Alcohol Abuse and Alcoholism (U01 AA014095, U24 AA020929, P50 AA0107061, F31 AA026483) and VA Medical Research (I01BX000813).
Footnotes
Conflict of Interest: All authors declare that they do not have any conflicts of interest to report in connection with this manuscript.
REFERENCES
- Bahi A (2015) The oxytocin receptor impairs ethanol reward in mice Physiol Behav 139:321–327 doi: 10.1016/j.physbeh.2014.11.046 [DOI] [PubMed] [Google Scholar]
- Bahi A, Al Mansouri S, Al Maamari E (2016) Nucleus accumbens lentiviral-mediated gain of function of the oxytocin receptor regulates anxiety- and ethanol-related behaviors in adult mice Physiol Behav 164:249–258 doi: 10.1016/j.physbeh.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, Cornish JL (2015) The Involvement of Oxytocin in the Subthalamic Nucleus on Relapse to Methamphetamine-Seeking Behaviour PLoS One 10:e0136132 doi: 10.1371/journal.pone.0136-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz SJ, Everett NA, McGregor IS, Cornish JL (2016) Oxytocin in the nucleus accumbens core reduces reinstatement of methamphetamine-seeking behaviour in rats Addict Biol 21:316325 doi: 10.1111/adb.12198 [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Douglas AJ (2010) Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders CNS Neurosci Ther 16:e92–123 doi: 10.1111/j.1755-5949.2010.00154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ (2003) Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion Prog Neuropsychopharmacol Biol Psychiatry 27:1177–1185 doi: 10.1016/j.pnpbp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- Bernheim A, Leong KC, Berini C, Reichel CM (2017) Antagonism of mGlu2/3 receptors in the nucleus accumbens prevents oxytocin from reducing cued methamphetamine seeking in male and female rats Pharmacol Biochem Behav 161:13–21 doi: 10.1016/j.pbb.2017.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen MT, Carson DS, Spiro A, Arnold JC, McGregor IS (2011) Adolescent oxytocin exposure causes persistent reductions in anxiety and alcohol consumption and enhances sociability in rats PLoS One 6:e27237 doi: 10.1371/journal.pone.0027237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE (2011) Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine Psychopharmacology (Berl) 213:19–27 doi: 10.1007/s00213-010-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Andari E, Johnson ZV, Curry DC, de Waal FB, Young LJ (2016) Oxytocin-dependent consolation behavior in rodents Science 351:375–378 doi: 10.1126/science.aac4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson DS et al. (2010) Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus Addict Biol 15:448–463 doi: 10.1111/j.1369-1600.2010.00247.x [DOI] [PubMed] [Google Scholar]
- Chen YW, Fiscella KA, Bacharach SZ, Tanda G, Shaham Y, Calu DJ (2015) Effect of yohimbine on reinstatement of operant responding in rats is dependent on cue contingency but not food reward history Addict Biol 20:690–700 doi: 10.1111/adb.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Bentzley BS, Regen-Tuero H, See RE, Reichel CM, Aston-Jones G (2017) Oxytocin Acts in Nucleus Accumbens to Attenuate Methamphetamine Seeking and Demand Biol Psychiatry 81:949–958 doi: 10.1016/j.biopsych.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox BM, Young AB, See RE, Reichel CM (2013) Sex differences in methamphetamine seeking in rats: impact of oxytocin Psychoneuroendocrinology 38:2343–2353 doi: 10.1016/j.psyneuen.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J et al. (2011) Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect Psychoneuroendocrinology 36:1312–1326 doi: 10.1016/j.psyneuen.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HE, Masini CV, Campeau S (2004) The pattern of brain c-fos mRNA induced by a component of fox odor, 2,5-dihydro-2,4,5-trimethylthiazoline (TMT), in rats, suggests both systemic and processive stress characteristics Brain Res 1025:139–151 doi: 10.1016/j.brainres.2004.07.079 [DOI] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin Nature 501:179–184 doi: 10.1038/nature12518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Alonso AG, Bredewold R, Veenema AH (2016) Role of the oxytocin system in amygdala subregions in the regulation of social interest in male and female rats Neuroscience 330:138–149 doi: 10.1016/j.neuroscience.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH (2013) Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways Horm Behav 64:693–701 doi: 10.1016/j.yhbeh.2013.08.012 [DOI] [PubMed] [Google Scholar]
- Dumais KM, Veenema AH (2016) Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior Front Neuroendocrinol 40:1–23 doi: 10.1016/j.yfrne.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres T, Apfelbach R, Fendt M (2005) Behavioral changes induced in rats by exposure to trimethylthiazoline, a component of fox odor Behav Neurosci 119:1004–1010 doi: 10.1037/0735-7044.119.4.1004 [DOI] [PubMed] [Google Scholar]
- Endres T, Fendt M (2007) Conditioned behavioral responses to a context paired with the predator odor trimethylthiazoline Behav Neurosci 121:594–601 doi: 10.1037/0735-7044.121.3.594 [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T (2008) 2,3,5-T rimethyl-3-thiazoline (TMT), a component of fox odor - just repugnant or really fear-inducing? Neurosci Biobehav Rev 32:1259–1266 doi: 10.1016/j.neubiorev.2008.05.010 [DOI] [PubMed] [Google Scholar]
- Fendt M, Endres T, Lowry CA, Apfelbach R, McGregor IS (2005) TMT-induced autonomic and behavioral changes and the neural basis of its processing Neurosci Biobehav Rev 29:1145–1156 doi: 10.1016/j.neubiorev.2005.04.018 [DOI] [PubMed] [Google Scholar]
- Ferland CL, Reichel CM, McGinty JF (2016) Effects of oxytocin on methamphetamine-seeking exacerbated by predator odor pre-exposure in rats Psychopharmacology (Berl) 233:1015–1024 doi: 10.1007/s00213-015-4184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation Physiol Rev 81:629–683 doi: 10.1152/physrev.2001.81.2.629 [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL (2013) Yohimbine increases opioid-seeking behavior in heroin-dependent, buprenorphine-maintained individuals Psychopharmacology (Berl) 225:811–824 doi: 10.1007/s00213-012-2868-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock EA, Levitt P (2013) Oxytocin receptor ligand binding in embryonic tissue and postnatal brain development of the C57BL/6J mouse Front Behav Neurosci 7:195 doi: 10.3389/fnbeh.2013.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC et al. (2018) Oxytocin Reduces Alcohol Cue-Reactivity in Alcohol-Dependent Rats and Humans Neuropsychopharmacology 43:1235–1246 doi: 10.1038/npp.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R, Walther N (1999) The role of sex steroids in the oxytocin hormone system Mol Cell Endocrinol 151:95–101 [DOI] [PubMed] [Google Scholar]
- Janitzky K, D’Hanis W, Krober A, Schwegler H (2015) TMT predator odor activated neural circuit in C57BL/6J mice indicates TMT-stress as a suitable model for uncontrollable intense stress Brain Res 1599:1–8 doi: 10.1016/j.brainres.2014.12.030 [DOI] [PubMed] [Google Scholar]
- Jurek B et al. (2015) Oxytocin Regulates Stress-Induced Crf Gene Transcription through CREB-Regulated Transcription Coactivator 3 J Neurosci 35:12248–12260 doi: 10.1523/JNEUROSCI.1345-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CE, Griffin WC, Luderman LN, Kates MM, McGinty JF, Becker HC (2017) Oxytocin Reduces Ethanol Self-Administration in Mice Alcohol Clin Exp Res 41:955–964 doi: 10.1111/acer.13359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtz AS, Lin B, Smith ME, Aston-Jones G (2018) Attenuated cocaine-seeking after oxytocin administration in male and female rats Psychopharmacology (Berl) 235:2051–2063 doi: 10.1007/s00213-018-4902-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID (2004) Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication Front Neuroendocrinol 25:150–176 doi: 10.1016/j.yfrne.2004.05.001 [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Coen K, Li Z, Shaham Y (2013) Role of corticotropin-releasing factor in the median raphe nucleus in yohimbine-induced reinstatement of alcohol seeking in rats Addict Biol 18:448–451 doi: 10.1111/j.1369-1600.2011.00374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Rohn MC, Tanda G, Leggio L (2016) Targeting the Oxytocin System to Treat Addictive Disorders: Rationale and Progress to Date CNS Drugs 30:109–123 doi: 10.1007/s40263-016-0313-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR et al. (2018) Oxytocin by intranasal and intravenous routes reaches the cerebrospinal fluid in rhesus macaques: determination using a novel oxytocin assay Mol Psychiatry 23:115–122 doi: 10.1038/mp.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Cox S, King C, Becker H, Reichel CM (2018) Oxytocin and Rodent Models of Addiction Int Rev Neurobiol 140:201–247 doi: 10.1016/bs.irn.2018.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Freeman LR, Berini CR, Ghee SM, See RE, Reichel CM (2017) Oxytocin Reduces Cocaine Cued Fos Activation in a Regionally Specific Manner Int J Neuropsychopharmacol 20:844–854 doi: 10.1093/ijnp/pyx058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KC, Zhou L, Ghee SM, See RE, Reichel CM (2016) Oxytocin decreases cocaine taking, cocaine seeking, and locomotor activity in female rats Exp Clin Psychopharmacol 24:55–64 doi: 10.1037/pha0000058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Nakajima M, Ibanez-Tallon I, Heintz N (2016) A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors Cell 167:60–72 e11 doi: 10.1016/j.cell.2016.08.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM (2014) Oxytocin, motivation and the role of dopamine Pharmacol Biochem Behav 119:49–60 doi: 10.1016/j.pbb.2013.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen K, Loveless R, DeLucca B, Wardley K, Deogan S, Thomas C, Peris J (2016) Peripheral oxytocin administration reduces ethanol consumption in rats Pharmacol Biochem Behav 140:27–32 doi: 10.1016/j.pbb.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y (2016) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress Neuropsychopharmacology 41:335–356 A doi: 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon D, Dabrowska J (2018) Corticotropin-Releasing Factor Receptors Modulate Oxytocin Release in the Dorsolateral Bed Nucleus of the Stria Terminalis (BNST) in Male Rats Front Neurosci 12:183 doi: 10.3389/fnins.2018.00183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT (2012) Breaking the loop: oxytocin as a potential treatment for drug addiction Horm Behav 61:331–339 doi: 10.1016/j.yhbeh.2011.12.001 [DOI] [PubMed] [Google Scholar]
- McGregor IS, Schrama L, Ambermoon P, Dielenberg RA (2002) Not all ‘predator odours’ are equal: cat odour but not 2,4,5 trimethylthiazoline (TMT; fox odour) elicits specific defensive behaviours in rats Behav Brain Res 129:1–16 [DOI] [PubMed] [Google Scholar]
- Morrow BA, Redmond AJ, Roth RH, Elsworth JD (2000) The predator odor, TMT, displays a unique, stress-like pattern of dopaminergic and endocrinological activation in the rat Brain Res 864:146–151 [DOI] [PubMed] [Google Scholar]
- Myers EA, Rinaman L (2005) Trimethylthiazoline supports conditioned flavor avoidance and activates viscerosensory, hypothalamic, and limbic circuits in rats Am J Physiol Regul Integr Comp Physiol 288:R1716–1726 doi: 10.1152/ajpregu.00479.2004 [DOI] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors Trends Neurosci 35:649–659 doi: 10.1016/j.tins.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Nakajima M, Gorlich A, Heintz N (2014) Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons Cell 159:295–305 doi: 10.1016/j.cell.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R (2000) Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus J Neuroendocrinol 12:235–243 [DOI] [PubMed] [Google Scholar]
- Patchev VK, Schlosser SF, Hassan AH, Almeida OF (1993) Oxytocin binding sites in rat limbic and hypothalamic structures: site-specific modulation by adrenal and gonadal steroids Neuroscience 57:537–543 [DOI] [PubMed] [Google Scholar]
- Pedersen CA et al. (2013) Intranasal oxytocin blocks alcohol withdrawal in human subjects Alcohol Clin Exp Res 37:484–489 doi: 10.1111/j.1530-0277.2012.01958.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, Slattery DA, Uschold-Schmidt N, Reber SO, Neumann ID (2014) Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice Psychoneuroendocrinology 42:225–236 doi: 10.1016/j.psyneuen.2014.01.021 [DOI] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG (2017) Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets J Comp Neurol 525:1094–1108 doi: 10.1002/cne.24116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters ST, Bowen MT, Bohrer K, McGregor IS, Neumann ID (2017) Oxytocin inhibits ethanol consumption and ethanol-induced dopamine release in the nucleus accumbens Addict Biol 22:702–711 doi: 10.1111/adb.12362 [DOI] [PubMed] [Google Scholar]
- Qi J, Yang JY, Wang F, Zhao YN, Song M, Wu CF (2009) Effects of oxytocin on methamphetamine-induced conditioned place preference and the possible role of glutamatergic neurotransmission in the medial prefrontal cortex of mice in reinstatement Neuropharmacology 56:856–865 doi: 10.1016/j.neuropharm.2009.01.010 [DOI] [PubMed] [Google Scholar]
- Ring RH et al. (2006) Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications Psychopharmacology (Berl) 185:218–225 doi: 10.1007/s00213-005-0293-z [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, de Wit H, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings Psychopharmacology (Berl) 168:3–20 doi: 10.1007/s00213-002-1224-x [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y (2010) Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking Brain Res 1314:15–28 doi: 10.1016/j.brainres.2009.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID (2010) Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats Neuropharmacology 58:56–61 doi: 10.1016/j.neuropharm.2009.06.038 [DOI] [PubMed] [Google Scholar]
- Tan Y, Singhal S, Hiller H, Harden S, Nguyen D, Colon-Perez C, Febo M, Wang L, Cahill K, de Kloet A, Frazier C, Krause E (2017) Optogenetic excitation of neurons in the prefrontal cortex that express oxytocin receptors eliminates preference for social novelty in male mice. Society for Neuroscience Abstract No. 78312, 2017. [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M (2011) Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate Neuropsychopharmacology 36:1178–1186 doi: 10.1038/npp.2010.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinante P, Freund-Mercier MJ (1997) Distribution of oxytocin- and vasopressin-binding sites in the rat extended amygdala: a histoautoradiographic study J Comp Neurol 383:305–325 [PubMed] [Google Scholar]
- Weber RA, Logan CN, Leong KC, Peris J, Knackstedt L, Reichel CM (2018) Regionally Specific Effects of Oxytocin on Reinstatement of Cocaine Seeking in Male and Female Rats Int J Neuropsychopharmacol 21:677–686 doi: 10.1093/ijnp/pyy025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Kershaw YM, Shanks N, Wood SA, Lightman SL, Ingram CD (2004) Oxytocin attenuates stress-induced c-fos mRNA expression in specific forebrain regions associated with modulation of hypothalamo-pituitary-adrenal activity J Neurosci 24:2974–2982 doi: 10.1523/JNEUROSCI.3432-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD (1997) Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats Endocrinology 138:2829–2834 doi: 10.1210/endo.138.7.5255 [DOI] [PubMed] [Google Scholar]
- Zanos P, Georgiou P, Wright SR, Hourani SM, Kitchen I, Winsky-Sommerer R, Bailey A (2014) The oxytocin analogue carbetocin prevents emotional impairment and stress-induced reinstatement of opioid-seeking in morphine-abstinent mice Neuropsychopharmacology 39:855–865 doi: 10.1038/npp.2013.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, See RE, Reichel CM (2015) Oxytocin differentially affects sucrose taking and seeking in male and female rats Behav Brain Res 283:184–190 doi: 10.1016/j.bbr.2015.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]