Abstract
Rationale:
No pharmacotherapies are approved for cocaine use disorder. Phendimetrazine, a prodrug of the monoamine releaser phenmetrazine, attenuates the reinforcing effects of cocaine in preclinical models, has minimal abuse potential and is safe when combined with cocaine.
Objectives:
This study determined the influence of phendimetrazine maintenance on the reinforcing effects of cocaine (i.e., choice to self-administer cocaine), along with the subjective, performance and physiological effects of cocaine. We hypothesized that phendimetrazine would attenuate the reinforcing effects of cocaine.
Methods:
Twenty-nine subjects with cocaine use disorder completed this within-subject, inpatient study. Subjects were maintained on placebo and 210 mg phendimetrazine in a counterbalanced order. After at least 7 days of maintenance on the target dose, subjects completed experimental sessions in which the effects of single doses of 0, 20, 40 and 80 mg of intranasal cocaine were determined.
Results:
Cocaine functioned as a reinforcer, producing significant dose related increases in self-administration. Cocaine increased prototypic effects (e.g., ratings of Stimulated and blood pressure). Phendimetrazine attenuated ratings on a select set of subjective outcomes (e.g., ratings of Talkative/Friendly), but failed to reduce the reinforcing effects of cocaine or a majority of positive subjective cocaine effects. Phendimetrazine increased heart rate, indicating a physiologically active dose was tested, but heart rate increases were not clinically significant.
Conclusions:
These results indicate that although phendimetrazine can safely be combined with cocaine, it does not attenuate the abuse related effects of cocaine. It is unlikely, then, that phendimetrazine will be an effective standalone treatment for cocaine use disorder.
Keywords: Humans, Self-Administration, Behavioral Economic Demand, Pharmacotherapy, Phendimetrazine, Cocaine
Introduction
Cocaine use disorder remains a significant and persistent public health problem. Over two million Americans were current (i.e., past month) cocaine users in 2017 and approximately one million met cocaine use disorder criteria that same year (Substance Abuse and Mental Health Services Administration 2018). Cocaine use produces multiple health problems and is associated with numerous negative health consequences (Butler et al. 2017; Havakuk et al. 2017; Rounsaville et al. 1991). Further, a recent study found that cocaine was the most common contributor to unintentional overdose in Black men and women between 2000 and 2015 in the United States (Shiels et al., 2018). These overdose rates due to cocaine were similar to those for opioid overdose rates in non-Black men and women during that same time period. In fact, elevated cocaine overdose rates were observed in Black men and women for approximately eight years before elevated opioid overdose rates were observed in non-Black men and women, suggesting that cocaine overdose is an ongoing, but overlooked, public health concern (see Jalal et al., 2018 for further discussion regarding cocaine overdose).
Over 60 putative pharmacotherapies have been tested as cocaine use disorder treatments in more than 100 clinical trials (Czoty et al. 2016b). But despite being a research priority for nearly three decades (Schuster and Snyder 1989), the Food and Drug Administration has yet to approve any medications for treating cocaine use disorder. One reason for the failure to bring a cocaine pharmacotherapy to market could be the lack of a “pipeline” approach to identifying and screening these medications (Czoty et al. 2016b). Specifically, a majority of drugs tested in clinical trials had not been screened in either preclinical or human laboratory experiments; even fewer have first been tested in both preclinical and human laboratory studies, notwithstanding the precedent for other approved pharmacotherapies to be moved through such a screening pipeline (Czoty et al. 2016b).
Phendimetrazine (Bontril®), a DEA schedule III medication in the United States, is used to treat obesity. Phendimetrazine is a weak monoamine releaser but is metabolized into phenmetrazine, a more potent norepinephrine and dopamine releaser (Chait et al. 1987; Corwin et al. 1987; Rothman et al. 2002). Phendimetrazine shares discriminative stimulus effects with d-amphetamine and cocaine (Banks et al. 2013a; de la Garza and Johanson 1987; Evans and Johanson 1987), but it is a relatively weak reinforcer with limited abuse potential in non-human primates and human subjects reporting cocaine use (Bolin et al. 2016; Corwin et al. 1987; Griffiths et al. 1979). As a first step in the screening pipeline for evaluating phendimetrazine as a putative cocaine pharmacotherapy, preclinical research demonstrated that phendimetrazine reduced cocaine self-administration in monkeys (Banks et al. 2013b, 2013c; Czoty et al. 2016a). As the next step in the screening pipeline, a study from our laboratory demonstrated the safety and tolerability of cocaine during phendimetrazine maintenance in humans (Stoops et al. 2016). This series of findings indicated that phendimetrazine was worthy of advancement along the development continuum to a randomized, controlled study evaluating whether phendimetrazine impacted cocaine self-administration in humans. Such a design is crucial to informing a go/no-go decision about whether to advance phendimetrazine to a larger clinical trial (Czoty et al. 2016b). To this end, the experiment reported here determined how maintenance on placebo and phendimetrazine (210 mg/day) influenced cocaine self-administration (i.e., the reinforcing effects of cocaine) using a sophisticated human laboratory design. Subjective, performance and physiological effects of cocaine were also evaluated as a function of phendimetrazine maintenance. We hypothesized that phendimetrazine would attenuate the reinforcing effects of cocaine.
Method
Subjects
In order to be eligible for the study, subjects had to be healthy and without contraindications to cocaine and phendimetrazine. Subjects also had to report recent use of cocaine, meet diagnostic criteria for a cocaine use disorder (i.e., abuse or dependence) according to a computerized Structured Clinical Interview for DSM-IV (SCID) that was reviewed by a psychiatrist or psychologist and provide a benzoylecgonine positive urine sample during screening to verify current cocaine use status. Cocaine metabolites can be detected in urine approximately 80–90 hours after cessation in chronic users (Preston et al., 2002). Screening procedures for all subjects included a medical history questionnaire, laboratory chemistries (e.g., blood chemistry screen, complete blood count and urinalysis), electrocardiogram and a brief psychiatric examination. Subjects were excluded from participation if a study physician deemed the screening results to be abnormal (e.g., if the electrocardiogram was determined to be outside normal limits). Subjects with histories of serious physical disease, current physical disease or current or past histories of serious psychiatric disorder, including current or past histories of other substance abuse or dependence, that in the opinion of a study physician would have interfered with study participation (e.g., physiologic dependence on opioids, alcohol or benzodiazepines; schizophrenia; major depression; bipolar disorder) were also excluded. Decisions to exclude subjects on these grounds were based on review of screening materials and/or history and physical examination conducted by the study physician. Female subjects had to be using an effective form of birth control (e.g., birth control pills, IUD, condoms or abstinence) in order to participate.
A total of twenty-nine subjects (8 women; 6 White, 21 Black, 1 Hispanic/Latino, 1 biracial) provided sober, written informed consent to participate and completed this within-subjects, placebo-controlled, inpatient study. Subjects weighed 82 ± 20 kg on average (± SD), had BMIs of 28 ± 5, were 46 ± 4 years of age at screening and had 12 ± 1 years of education. Drug Abuse Screening Test (Skinner 1982) scores were 8 ± 5. Twenty-eight subjects reported current smoked cocaine use; the one subject who did not smoke cocaine reported intranasal powdered cocaine use. Subjects reported using cocaine 17 ± 9 days in the month prior to screening. Subjects also reported experience with other drugs of abuse. Twenty-seven subjects were daily cigarette smokers (11 ± 6 cigarettes/day). Twenty-five subjects reported weekly alcohol use (13 ± 9 standard drinks/week). In the month prior to screening, subjects reported cannabis use (n=20), opioid use (n=3) and benzodiazepine use (n=2). Aside from meeting criteria for cocaine abuse or dependence, some subjects also met criteria for alcohol dependence (n=1; not physiologically dependent) and alcohol abuse (n=3). All subjects were paid for their participation. The Medical Institutional Review Board of the University of Kentucky approved this study, which was conducted in accordance with all relevant guidelines, including the 1964 Declaration of Helsinki.
General Procedures
Subjects were enrolled as inpatients at the University of Kentucky Chandler Medical Center inpatient Clinical Services Core (CSC) for up to 33 days and completed one drug-free practice and eight experimental sessions. During inpatient admission, subjects received standard caffeine-free hospital meals. Urine samples were collected daily and expired breath samples were collected prior to each session to confirm drug and alcohol abstinence, respectively. Pregnancy tests were conducted daily on urine samples from the female subjects. All pregnancy tests were negative throughout their participation. When not in session, subjects could smoke cigarettes ad libitum as long as CSC staff was available to escort them to the designated smoking area.
Drug Maintenance Days.
Drug maintenance began on the day immediately following the practice session and continued throughout the protocol. Placebo or phendimetrazine was administered orally at 0700 and 1900 h. Subjects were maintained on placebo or phendimetrazine in a counterbalanced order. During phendimetrazine maintenance, subjects were maintained on 35 mg phendimetrazine twice daily (total daily dose=70 mg) for two days. They were then maintained on 70 mg phendimetrazine twice daily (total daily dose=140 mg) for two more days. After this, subjects were maintained on the target dose of 105 mg phendimetrazine twice daily (total daily dose=210 mg) for seven days. Placebo maintenance also lasted eleven days before experimental sessions commenced.
After eleven days of maintenance on the first assigned condition, subjects completed a block of four experimental sessions, described below. Maintenance on the assigned condition continued across experimental session days. Upon completion of these four experimental sessions, subjects began maintenance on the opposite condition, which also lasted eleven days before the second block of four experimental sessions was completed. After completion of this second block, subjects were discharged from the study.
Experimental Sessions.
Subjects received the appropriate maintenance dose at 0700 h on the morning of all experimental sessions. Subjects were allowed to smoke a cigarette prior to experimental sessions that started at 0900 h and were not allowed to smoke again until the session ended approximately 7.5 h later. Sessions consisted of a Sampling Phase and a Self-Administration Phase, which were separated by an approximately three-hour break in which lunch was available.
Sampling Phase.
Subjects completed a sampling phase in each experimental session to acquaint them with the effects of the cocaine dose available during that session. Baseline subjective and physiological measures were completed at approximately 0900 h. At approximately 0930 h, the intranasal cocaine dose (0, 20, 40 or 80 mg) available during that session was administered. Cocaine dosing order was determined randomly for each subject. Immediately after dosing and at 15-minute intervals for the next hour, subjective, performance and physiological measures were completed. The sampling phase ended at approximately 1030 h.
Self-Administration Phase.
The self-administration phase began at approximately 1330 h. During this phase, subjects were given 10 opportunities to choose between 1/10th of the cocaine dose insufflated during the sampling session and USD $0.25 on a progressive ratio task (see below). At each of the 10 opportunities, subjects were forced to make a choice for either 1/10th of the cocaine dose or money (i.e., the sum of cocaine and money choices in each session was always 10). Following completion of the task, the drug dose earned and tokens representing the amount of money earned were presented to subjects. Subjects then insufflated the earned dose, at approximately 1430 h, and completed subjective and physiological measures at 15-minute intervals for 60 minutes. Session ended at approximately 1530 h.
Measures
The reinforcing effects of intranasal cocaine during maintenance on placebo and phendimetrazine were assessed using a progressive ratio task. Subjects were able to earn drug doses or money by responding on a computer mouse according to a progressive ratio schedule. Cocaine and money were available on concurrent, independent progressive ratio schedules as described previously (Stoops et al., 2012b). The initial ratio to obtain a reinforcer was 400 clicks. The response requirement for each subsequent choice of that specific reinforcer increased by 100 (i.e., 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300 responses if a subject responded exclusively for cocaine or money). The dependent measure for this task was number of drug choices, out of a maximum of 10 (or 100% of the sampling dose).
Subjective measures were an Adjective Rating Scale (Oliveto et al. 1992) and a locally developed Drug-Effect Questionnaire (Rush et al. 2003). Psychomotor performance was assessed with a computerized version of the Digit-Symbol-Substitution Test (DSST) (McLeod et al. 1982). The dependent measure was the percent of geometric patterns the subject entered correctly. Physiological measures (i.e., heart rate and blood pressure) were recorded using a digital monitor. Temperature was measured using a digital oral thermometer.
Subjects also completed a hypothetical cocaine purchase task (Bruner and Johnson 2014; Stoops et al. 2016) during the practice session and the morning of the first cocaine self-administration session of each phendimetrazine maintenance dose condition (i.e., sessions 1 and 5). A standard instructional vignette was provided in which subjects were instructed they would consume all purchases in a single day, could not get the commodity from another source or stockpile, had no commodity available from previous days, and had not used cocaine or other drugs prior to this decision. The commodity available was 0.1 g of cocaine (Strickland et al. 2016). Consumption was evaluated across 16 increments from $0.00 [free] to $1000/hit, sequentially (full range: $0.00 [free], $0.01, $0.05, $0.10, $0.25, $0.50, $1, $2.50, $5, $10, $25, $50, $100, $250, $500, $1000). All choices were hypothetical and were not purchased or administered. The dependent measures for this task were demand intensity and demand elasticity (see Data Analysis).
Drug Administration
All drugs were administered in a double-blind fashion. Only study investigators and the Investigational Drug Service staff had access to dose orders in order to maintain the blind. These individuals did not interact with subjects during experimental sessions, nor did they collect experimental data. Immediate release phendimetrazine doses (35, 70 and 105 mg b.i.d.; KVKTech Inc., Newtown, PA, NDC: 10702-077-01) were prepared from commercially available drug in a gelatin capsule backfilled with cornstarch. Placebo capsules contained only cornstarch. The seven-day minimum maintenance period on the target dose with twice daily dosing was selected to reach steady state phendimetrazine levels based upon its published half-life of approximately 9 h (Müller et al. 1975). The highest target dose of 210 mg phendimetrazine was selected because it is the maximum daily recommended dose (Cash and Glass, 2014).
Cocaine sampling doses (0, 20, 40 and 80 mg) were prepared by combining the appropriate amount of cocaine HCl (Medisca Inc., Plattsburgh, NY, NDC:38779-0723-03) with lactose to equal a total of 120 mg powder. For self-administration sessions, each potential cocaine amount subjects could earn (e.g., 4, 8, 12, 16, 20, 24, 28, 32, 36 and 40 mg for the 40 mg dose) was admixed with lactose to equal a total of 120 mg powder. The research assistant communicated the appropriate blinded dose code to the nursing staff (e.g., 4 mg in the 40 mg dose condition was labeled Dose 1), who then administered that dose to the subject. During each cocaine administration, a nurse presented the subject with the powder, a mirror and a standard razor blade. The subject was instructed to divide the powder into two even “lines” and insufflate one line of powder through each nostril using a 65-mm plastic straw within 2 min.
Doses were not administered if a subject’s heart rate was ≥100 bpm, systolic pressure was ≥150 mmHg or diastolic pressure was ≥100 mmHg or if clinically significant and/or prolonged ECG abnormalities were detected. Two subjects were discharged by medical staff prior to study completion due to experiencing elevated vital signs. No clinically significant or prolonged ECG abnormalities were detected during any subject’s participation.
Data Analysis
Progressive ratio task data were analyzed as number of drug choices using a two-factor repeated General Linear Model (GLM) with Phendimetrazine Dose (0 and 210 mg/day) and Cocaine Dose (0, 20, 40 and 80 mg) as the factors (SPSS, IBM Corporation, Armonk, NY). F values from the GLM were used to determine statistical significance using Geisser-Greenhouse corrected degrees of freedom and a threshold of p < 0.05. Post hoc comparisons (i.e., Fisher’s test) were conducted between active cocaine doses and placebo under placebo maintenance when statistically significant outcomes were observed. Subjective, performance and physiological measures were analyzed as peak effect (i.e., the maximum score observed in the 60 min following administration of the cocaine sampling dose) in the same fashion as data from the progressive ratio task. Due to a data collection error, subjective data for one subject were not collected at the 15-minute post sampling time point for the 80 mg cocaine/0 mg phendimetrazine condition. This data point was imputed as the average of all other subject responses for this dose condition and time point on each measure.
Cocaine purchase task data were first evaluated for non-systematic responding using standardized criteria (Stein et al. 2015). Four plots were removed for this reason (one from placebo and three from phendimetrazine maintenance). Data from systematic curves were analyzed using the exponentiated demand equation (Koffarnus et al. 2015):
where Q = consumption; Q0 = derived demand intensity; k = a constant related to consumption range (a priori set to two); C = commodity price; and α = derived demand elasticity. Demand intensity refers to cocaine consumption at a unit price of zero. Demand elasticity refers to the sensitivity of cocaine consumption to changes in cocaine price. Analyses focused on intensity and elasticity given that prior factor analytic studies have demonstrated that these two measures reflect a two-factor structure underlying purchase task data (Bidwell et al. 2012; Mackillop et al. 2009). Intensity and elasticity were log-transformed to achieve normality. Additional analyses removing or recoding potential outlier values revealed similar results; therefore, original data are presented here. Mixed-effect models evaluated the effects of phendimetrazine dose on purchase task outcomes to account for the within-subject structure and missing values from non-systematic curves.
Results
Progressive Ratio Task
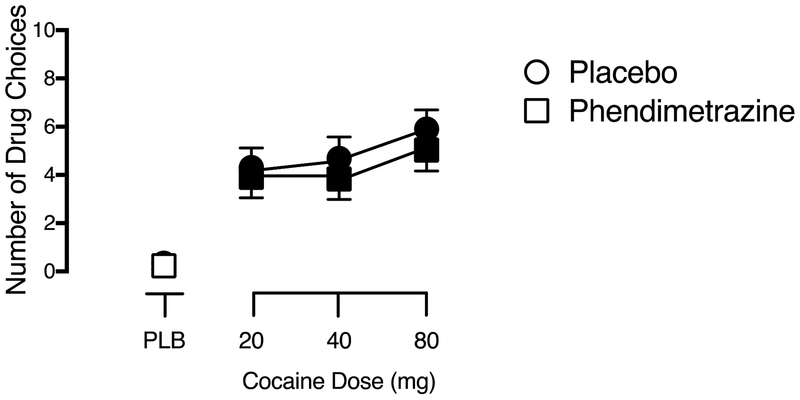
A significant main effect of Cocaine Dose was observed for Number of Drug Choices on the Progressive Ratio Task (F3,84 = 23.30, p < .001, ηp2 = .45). There was no effect of Phendimetrazine Dose, nor was there an interaction of Phendimetrazine Dose and Cocaine Dose, on this outcome. As shown in Figure 1, all active cocaine doses were self-administered to a significantly greater degree than placebo, regardless of phendimetrazine dose.
Fig. 1.
Dose-response function for cocaine following maintenance on placebo (circles) and 210 mg phendimetrazine (squares) for Number of Drug Choices on the Progressive Ratio Task. X-axis: Intranasal cocaine dose. Brackets indicate 1 S.E.M. Filled symbols indicate a significant difference from placebo during placebo maintenance.
Subjective Measures
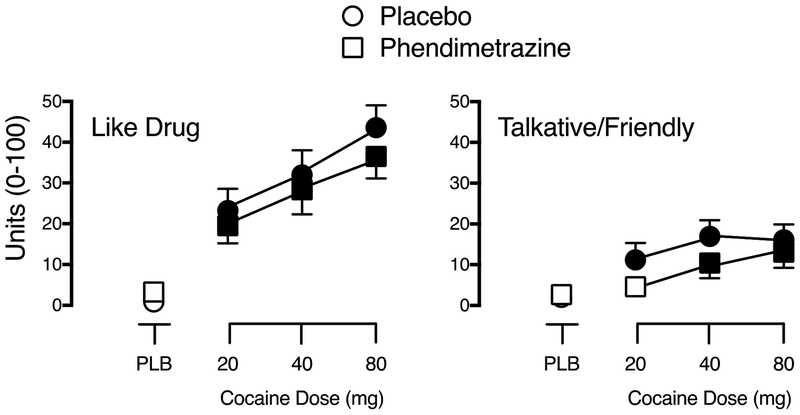
Significant main effects of Phendimetrazine Dose and Cocaine Dose were observed for the Stimulant Subscale of the Adjective Rating Scale and ratings of Performance Impaired and Talkative/Friendly on the Drug-Effect Questionnaire (F3,84 values > 3.19 for Cocaine Dose, p values < .05, ηp2 > .10; F1,28 values > 4.77 for Phendimetrazine Dose, p values < .05, ηp2 > .15). Cocaine produced increases on these items that were significantly attenuated by phendimetrazine (see Figure 2 for the representative item: Talkative/Friendly). A significant main effect of Phendimetrazine Dose was also observed for ratings of Bad Effects reflecting lower ratings on this measure during phendimetrazine maintenance (F1,28 = 4.60, p = .04, ηp2 = .14).
Fig. 2.
Peak effect dose-response functions for cocaine following maintenance on placebo (circles) and 210 mg phendimetrazine (squares) for subjective ratings of Like Drug (left graph) and Talkative/Friendly (right graph). X-axis: Intranasal cocaine dose. Brackets indicate 1 S.E.M. Filled symbols indicate a significant difference from placebo during placebo maintenance.
Significant main effects of Cocaine Dose were observed on sixteen additional items from the Drug-Effect Questionnaire (Active, Alert, Energetic; Any Effect; Euphoric; Good Effects; High; Irregular or Racing Heartbeat; Like Drug; Nauseated; Nervous/Anxious; Performance Improved; Restless; Rush; Shaky/Jittery; Stimulated; Willing to Pay For and Willing to Take Again; F3,84 values > 3.03, p values < .05, ηp2 > .10). There was no effect of Phendimetrazine Dose, nor was there an interaction of Cocaine Dose and Phendimetrazine Dose, on these outcomes. No other statistically significant effects were observed. Figure 2 shows representative data for Like Drug, indicating that active cocaine doses produced increases on these subjective measures, regardless of phendimetrazine maintenance condition.
Performance Measure
A significant interaction of Phendimetrazine Dose and Cocaine Dose was observed for Percent Correct on the DSST (F3,84 = 4.77, p = .01, ηp2 = .15). During placebo maintenance, cocaine produced a bi-tonic dose response curve on this measure such that performance was best after administration of 40 mg intranasal cocaine, with worse relative performance after administration of 0, 20 and 80 mg of intranasal cocaine. During phendimetrazine maintenance, this effect was reversed, such that performance was worst after administration of 40 mg intranasal cocaine, with better relative performance after administration of 0, 20 and 80 mg of intranasal cocaine. Overall percent of correct entries ranged between 98.85 and 99.80 percent.
Physiological Measures
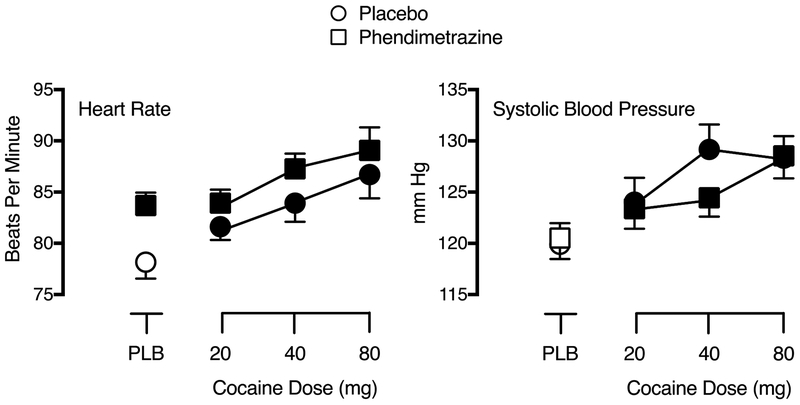
Significant main effects of Phendimetrazine Dose (F1,28 = 8.53, p .007, ηp2 = .23) and Cocaine Dose (F3,84 = 11.46, p < .001, ηp2 = .29) were observed for heart rate. As shown in Figure 3, cocaine increased peak heart rate. Phendimetrazine further increased peak heart rate under all cocaine dose conditions but average peak heart rate did not exceed 90 beats per minute (bpm). Significant main effects of Cocaine Dose were also observed on systolic (F3,84 = 20.21, p < .001, ηp2 = .42) and diastolic (F3,84 = 6.67, p = .001, ηp2 = .19) pressure, but there was no effect of phendimetrazine on these measures. Cocaine increased systolic (Figure 3) and diastolic pressure compared to placebo during placebo maintenance. There were no significant effects observed for temperature nor were there any significant interactions for any of the measures.
Fig. 3.
Peak effect dose-response functions for cocaine following maintenance on placebo (circles) and 210 mg phendimetrazine (squares) for Heart Rate (left graph) and Systolic Blood Pressure (right graph). X-axis: Intranasal cocaine dose. Brackets indicate 1 S.E.M. Filled symbols indicate a significant difference from placebo during placebo maintenance.
Cocaine Purchase Task
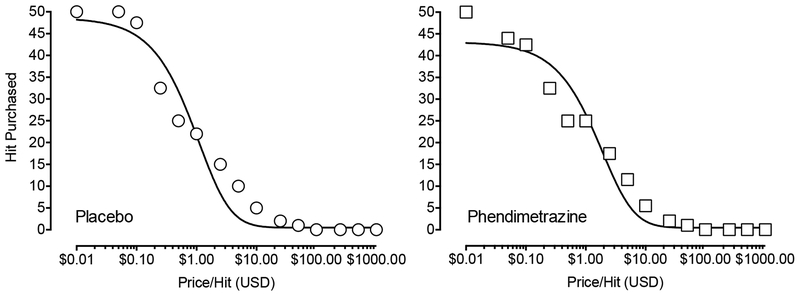
The exponentiated demand equation provided an excellent fit to individual data that did not differ as a function of phendimetrazine dose (median R2 = .96 placebo, R2 = .97 phendimetrazine; see group plots in Figure 4). Demand intensity (Q0), p = .91, and elasticity (α), p = .12, did not differ significantly by dose condition.
Fig. 4.
Economic demand curves for cocaine (0.1 g) following maintenance on placebo (circles; left graph) and 210 mg phendimetrazine (squares; right graph). X-axis: Price paid/0.1 g cocaine dose in U.S. dollars. Representative curves were fit to median data using the exponentiated demand equation (Koffarnus et al. 2015).
Discussion
The results of this experiment indicate that maintenance on the highest daily recommended dose of phendimetrazine does not influence the reinforcing effects of cocaine or hypothetical purchases on a cocaine purchase task. Phendimetrazine maintenance also failed to change a majority of subjective cocaine effects and did not exacerbate blood pressure increases caused by cocaine dosing. An elevation in heart rate was observed during phendimetrazine maintenance, indicating that a physiologically active dose was tested, but average peak heart rate did not approach tachycardia (i.e., above 100 bpm) for any cocaine-phendimetrazine dose combination.
Cocaine produced prototypic effects, maintaining self-administration above placebo levels, engendering positive and stimulant-like subjective effects, improving performance and increasing heart rate and blood pressure. The constellation and magnitude of effects observed here are comparable to those seen in previous research with intranasal cocaine (Foltin and Haney 2004; Greenwald et al. 2010; Stoops et al. 2012a, 2012b, 2016). It is important to note that for the self-administration and subjective measures, average outcomes were in the middle of the scales (e.g., for the 80 mg dose, subjects earned 5–6 cocaine doses out of 10 and peak Like Drug ratings were approximately 50 out of 100), allowing for observation of a reduction or enhancement on these items. Phendimetrazine maintenance neither reduced nor enhanced the majority of these measures, which stands in contrast to preclinical findings (Banks et al. 2013b, 2013c; Czoty et al. 2016a). These results are not necessarily unexpected because the abuse related effects of cocaine are difficult to alter using a pharmacological agent alone in humans (e.g., Foltin et al. 2015). Furthermore, phendimetrazine did not change subjective responses in our previous safety and tolerability study (Stoops et al., 2016).
Phendimetrazine also did not change hypothetical economic demand outcomes for standardized 0.1 g hits on the cocaine purchase task, which is concordant with results previously published from our laboratory (Stoops et al. 2016). The findings of this study extend those of previous work by demonstrating a concordance, albeit negative, between the purchase task and self-administration outcomes (e.g., see Bujarski et al. 2012 for data regarding a positive concordance between purchase task and self-administration outcomes). More work is necessary to further validate the predictive validity of the hypothetical cocaine purchase task, but it is possible that using such a task can provide information about behavioral mechanisms underlying changes in substance use as well as proxy data when drug self-administration is not ethically or medically feasible (e.g., in treatment seeking subjects; Moeller and Stoops 2015).
Phendimetrazine maintenance attenuated subjective responses on several measures. These measures captured stimulant-like (i.e., ratings of Talkative/Friendly; scores on the Stimulant Subscale of the Adjective Rating Scale) or negative (i.e., ratings of Bad Effect and Performance Impaired) effects of cocaine. When considering that reductions were observed in a small minority of subjective outcomes, and that the reductions were observed on measures that assess potentially aversive effects of cocaine, these changes are unlikely to be beneficial or to translate to efficacy of a cocaine pharmacotherapy.
Several limitations to the present study should be acknowledged. First, we evaluated the effects of intranasal cocaine in a population that largely reported smoking cocaine as their primary route of administration. Second, we did not collect or analyze blood samples for cocaine and/or phendimetrazine blood levels. Without such pharmacokinetic data we cannot make inferences about whether phendimetrazine changed cocaine bioavailability. A final limitation is that the self-administration outcomes could be operating under a ceiling effect (e.g., subjects are only willing to work for 5–6 cocaine doses at the highest dose tested, rather than all 10), which could obscure the ability of phendimetrazine to change cocaine self-administration. However, in presented, but unpublished, research that has used identical procedures and doses, we have observed near maximal cocaine taking under placebo maintenance conditions (Rush et al., 2017; 2018). The reason that higher levels of cocaine self-administration was not observed in this study is unknown, but may be due to the inclusion of data from several subjects who did not self-administer cocaine under any condition.
This randomized, placebo-controlled, within-subjects human laboratory experiment was designed as a step in the screening process of developing phendimetrazine as a treatment for cocaine use disorder. The project expanded on positive preclinical work (Banks et al. 2013b, 2013c; Czoty et al. 2016a) and an initial safety and tolerability study from our laboratory (Stoops et al. 2016) to rigorously evaluate a range of pharmacodynamic effects of cocaine, including the reinforcing effects of cocaine, during phendimetrazine maintenance. The ability of a pharmacotherapy to reduce the reinforcing effects of cocaine in humans has strong predictive validity for clinical efficacy (Comer et al. 2008; Czoty et al. 2016b; Haney 2009; Haney and Spealman 2008). Attenuating the subjective effects of cocaine, reducing withdrawal or remediating cognitive impairment (Rush and Stoops 2012; Sofuoglu et al 2013) may also be mechanisms by which a medication can effectively treat cocaine use disorder. The results of this study indicate that, despite testing a physiologically active dose in a relatively large sample, phendimetrazine failed to consistently change any effects of cocaine that might indicate clinical efficacy. Thus, these data indicate that phendimetrazine should not be advanced along the cocaine medication development pipeline to a Phase II or III clinical trial as a standalone treatment for cocaine use disorder.
Acknowledgments:
The authors gratefully acknowledge the staff of the University of Kentucky Laboratory of Human Behavioral Pharmacology for technical assistance, the staff of the University of Kentucky Center for Clinical and Translational Science Clinical Research Services Core for medical assistance and the University of Kentucky Investigative Drug Service for preparation of study medications. This study complied with all laws of the United States of America.
Footnotes
Conflict of Interest Statement: The authors declare no relevant conflicts of interest. The authors gratefully acknowledge research support from the National Institute on Drug Abuse (R01DA036553; T32DA035200) and from the National Center for Advancing Translational Sciences (UL1TR001998) of the National Institutes of Health as well as grant 1247392 from the National Science Foundation. These funding agencies had no role in study design, data collection or analysis, or preparation and submission of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
References
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS (2013a) Role of phenmetrazine as an active metabolite of phendimetrazine: evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend 130:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS (2013b) Effects of phendimetrazine treatment on cocaine vs food choice and extended-access cocaine consumption in rhesus monkeys. Neuropsychopharmacology 38:2698–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Negus SS (2013c) Effects of 14-day treatment with the schedule iii anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend 131:204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwell LC, MacKillop J, Murphy JG, Tidey JW, Colby SM (2012) Latent factor structure of a behavioral economic cigarette demand curve in adolescent smokers. Addict Behav 37:1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin BL, Stoops WW, Sites JP, Rush CR (2016) Abuse potential of oral phendimetrazine in cocaine-dependent individuals: implications for agonist-like replacement therapy. J Addict Med 10:156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner NR, Johnson MW (2014) Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology 231:889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, MacKillop J, Ray LA (2012) Understanding naltrexone mechanism of action and pharmacogenetics in Asian Americans via behavioral economics: a preliminary study. Exp Clin Psychopharmacol 20:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AJ, Rehm J, Fischer B (2017) Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depend 80:401–416. [DOI] [PubMed] [Google Scholar]
- Cash JC, Glass CA (2014) Family Practice Guidelines, Third Edition Springer, New York. [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE (1987) Reinforcing and subjective effects of several anorectics in normal human volunteers. J Pharmacol Exp Ther 242:777–783. [PubMed] [Google Scholar]
- Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL (2008) The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE (1987) Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res 7:351–361. [PubMed] [Google Scholar]
- Czoty PW, Blough BE, Fennell TR, Snyder RW, Nader MA (2016a). Attenuation of cocaine self-administration by chronic oral phendimetrazine in rhesus monkeys. Neuroscience 324:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Stoops WW, Rush CR (2016b) Evaluation of the “pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev 68:533–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE (1987) Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther 243:955–962. [PubMed] [Google Scholar]
- Evans SM, Johanson CE (1987) Amphetamine-like effects of anorectics and related compounds in pigeons. J Pharmacol Exp Ther 241:817–825. [PubMed] [Google Scholar]
- Foltin RW, Haney M (2004) Intranasal cocaine in humans: Acute tolerance, cardiovascular and subjective effects. Pharmacol Biochem Behav 78:93–101. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M, Rubin E, Reed SC, Vadhan N, Balter R, Evans SM (2015) Development of translational preclinical models in substance abuse: Effects of cocaine administration on cocaine choice in humans and non-human primates. Pharmacol Biochem Behav 134:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL (2010) Sustained release d-amphetamine reduces cocaine but not ‘speedball’-seeking in buprenorphine-maintained volunteers: a test of dual-agonist pharmacotherapy for cocaine/heroin polydrug abusers. Neuropsychopharmacology 35:2624–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Bradford LD, Brady JV (1979) Predicting the abuse liability of drugs with animal drug self-administration procedures: psychomotor stimulants and hallucinogens In: Thompson T, Dews PB (ed) Advances in behavioral pharmacology: Vol. 2 Academic Press, New York: pp 164–208. [Google Scholar]
- Haney M (2009) Self-administration of cocaine, cannabis and heroin in the human laboratory: benefits and pitfalls. Addict Biol 14:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Spealman R (2008) Controversies in translation research: drug self-administration. Psychopharmacology 199:403–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havakuk O, Rezkalla SH, Kloner RA (2017) The cardiovascular effects of cocaine. J Am Coll Cardiol 70:101–113. [DOI] [PubMed] [Google Scholar]
- Jalal H, Buchanich JM, Roberts MS, Balmert LC, Zhang K, Burke DS (2018) Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science EPub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, Bickel WK (2015) A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 23:504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackillop J, Murphy JG, Tidey JW, Kahler CW, Ray LA, Bickel WK (2009) Latent structure of facets of alcohol reinforcement from a behavioral economic demand curve. Psychopharmacology 203:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE Yingling J (1982) An automated version of the digit symbol substitution test (DSST). Behav Res Methods Instrum 4:433–436. [Google Scholar]
- Moeller SJ, Stoops WW (2015) Cocaine choice procedures in animals, humans and treatment seekers: Can we bridge the divide? Pharmacol Biochem Behav 138:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller OF, Hundt HK, Gosling JA (1975) Availability of phendimetrazine from sustained and non-sustained action formulations. S Afr Med J 49:135–9. [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, Fenwick JW (1992) Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther 261:885–894. [PubMed] [Google Scholar]
- Preston KL, Epstein DH, Cone EJ, Wtsadik AT, Huestis MA, Moolchan ET (2002) Urinary elimination of cocaine metabolites in chronic cocaine users during cessation. J Anal Toxicol 26:393–400. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, Baumann MH (2002) Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol 447:51–57. [DOI] [PubMed] [Google Scholar]
- Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F (1991) Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry 48:43–51. [DOI] [PubMed] [Google Scholar]
- Rush CR, Bolin BL, Rayapati AO, Hays LR, Lile JA (2017) Bupropion-Naltrexone combinations as a pharmacotherapy for cocaine dependence Presented at the College on Problems of Drug Dependence, Montreal, QC. [Google Scholar]
- Rush CR, Reynolds AR, Stoops WW, Lile JA, Rayapati AO, Hays LR (2018) Topiramate-Phentermine combinations as a pharmacotherapy for cocaine dependence Presented at the College on Problems of Drug Dependence, San Diego, CA. [Google Scholar]
- Rush CR, Stoops WW (2012) Agonist replacement therapy for cocaine dependence: A Translational review. Future Med Chem 4:245–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PEA, Hays LS (2003) Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther 306:195–204. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Snyder M (1989) NIDA’s medication development program. NIDA Res Monogr 95:64–73. [PubMed] [Google Scholar]
- Skinner HA (1982) The drug abuse screening test. Addict Behav 7:363–371. [DOI] [PubMed] [Google Scholar]
- Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A (2018) Trends in US drug overdose deaths in non-Hispanic black, Hispanic, and non-Hispanic white persons, 2000–2015. Ann Intern Med 168:453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, Carroll KM (2013) Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64:452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Koffarnus MN, Snider SE, Quisenberry AJ, Bickel WK (2015) Identification and management of nonsystematic purchase task data: Toward best practice. Exp Clin Psychopharmacol 23: 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2012a) Alternative reinforcer response cost impacts cocaine choice in humans. Prog Neuropsychopharm Biol Psychiatry 36:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2012b) Influence of acute bupropion pre-treatment on the effects of intranasal cocaine. Addiction 107:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Strickland JC, Hays LR, Rayapati AO, Lile JA, Rush CR (2016) Safety and tolerability of intranasal cocaine during phendimetrazine maintenance. Psychopharmacology 233: 2055–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Lile JA, Rush CR, Stoops WW (2016) Comparing exponential and exponentiated models of drug demand in cocaine users. Exp Clin Psychopharmacol 24:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2018) Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication No. SMA 18–5068, NSDUH Series H-53). Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/ on 27 November 2018. [Google Scholar]